false
0001513525
0001513525
2025-01-08
2025-01-08
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (date of earliest event reported):
January 8, 2025
Adial Pharmaceuticals, Inc.
(Exact name of registrant as specified in charter)
Delaware
(State or other jurisdiction of incorporation)
| 001-38323 |
|
82-3074668 |
| (Commission File Number) |
|
(IRS Employer Identification No.) |
4870 Sadler Road, Ste 300
Glen Allen, VA 23060
(Address of principal executive offices and
zip code)
(804) 487-8196
(Registrant’s telephone number including
area code)
(Former Name and Former Address)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbols |
|
Name of each exchange on which registered |
| Common Stock |
|
ADIL |
|
The Nasdaq Stock Market LLC
((Nasdaq Capital Market) |
Indicate by check mark whether the registrant
is an emerging growth company as defined in in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of
the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by checkmark
if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
Adial Pharmaceuticals, Inc. (the “Company”)
has updated its corporate presentation. A copy of the updated corporate presentation is furnished as Exhibit 99.1 to this Current Report
on Form 8-K.
The information in this Item 7.01 and in the corporate
presentation attached as Exhibit 99.1 to this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of
Section 18 of the Securities Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2)
of the Securities Act of 1933, as amended. The information contained in this Item 7.01 and in the corporate presentation attached as Exhibit
99.1 to this Current Report on Form 8-K shall not be incorporated by reference into any filing with the Securities and Exchange Commission
made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
The corporate presentation attached as Exhibit
99.1 to this Current Report on Form 8-K includes “safe harbor” language pursuant to the Private Securities Litigation Reform
Act of 1995, as amended, indicating that certain statements contained therein are “forward-looking” rather than historical.
The Company undertakes no duty or obligation to
update or revise the information contained in this Current Report on Form 8-K, although it may do so from time to time if its management
believes it is appropriate. Any such updating may be made through the filing of other reports or documents with the Securities and
Exchange Commission, through press releases or through other public disclosures.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: January 8, 2025 |
ADIAL PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Cary J. Claiborne |
| |
Name: |
Cary J. Claiborne |
| |
Title: |
President and Chief Executive Officer |
2
Exhibit
99.1

December 2021 CONFIDENTIAL Medicines f or A ddi c tion Investor Presentation January 2025

This presentation includes statements that are, or may be deemed, ‘‘forward - looking statements . ’’ In some cases, these forward - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “might,” estimates,” “approximately,” “expects,” “anticipates,” “intends,” “estimates,” “plans,” “seeks,” “may,” “should,” “could,” “would,” “will”, “future,” “likely,” “goal,” “continue,” “appears,” “suggests,” “ongoing,” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward - looking statements contain these words . Forward looking statements appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting alcohol addiction, the strength and breadth of our intellectual property, our planned clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us . By their nature, forward - looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated . Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward - looking statements contained in this presentation . In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this presentation, they may not be predictive of results or developments in future periods . Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law . You should read carefully our “Cautionary Note Regarding Forward - Looking Statements” and the factors described in the “Risk Factors” sections of our Annual Report on Form 10 - K for the year ended December 31 , 2023 , and any subsequent reports that have been filed with the Securities and Exchange Commission (the “SEC”) to better understand the risks and uncertainties inherent in our business . . 2 Forward Looking Statements ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108

V ision 3 ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108 Adial is a clinical - stage biopharmaceutical company focused on the treatment and prevention of addictions and other unmet medical needs .

Alcohol Use Disorder is a Major Public Health Problem in the U.S. 4 In the U . S . alone, an estimated 3 0 MILLION people SUFFER FROM AUD , resulting in significant health, social and financial costs Excessive Alcohol Use : • Leading risk factor for death ages 15 – 49 (Globally) • 31 % of driving fatalities due to alcohol use • Contributes to over 200 different diseases • Costs U . S . economy approximately $ 250 billion annually • 50 % increase in prevalence from 2002 to 2013 Despite these enormous costs, just over 7 % seek help, but less than 5 % AUD cases are treated by a health care practitioner 3 0 M people suffer from AUD Sources : NIAAA Alcohol Facts & Statistics . www . cdc . gov/features/costsofdrinking/index . html accessed Sep . 10 . 2017 . NIH study finds alcohol use disorder on the increase, June 3 , 2015 . Failure to help people with AUD is a major health, social and financial problem Sources : SAMHSA, Center for Behavioral Health Statistics and Quality . 2021 National Survey on Drug Use and Health . Tables 5 . 6 A & 5 . 6 B . ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
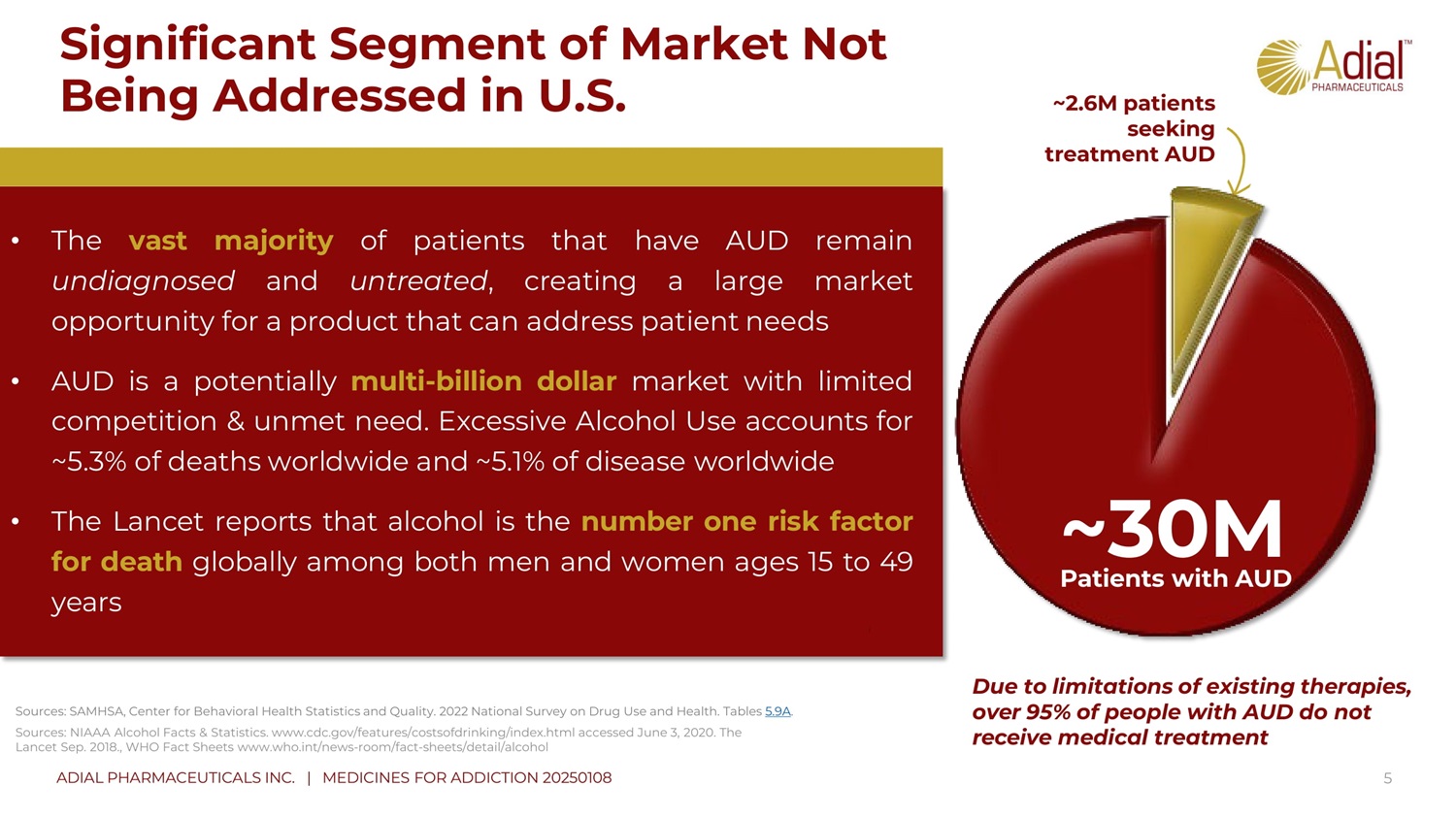
Significant Segment of Market Not Being Addressed in U.S. 5 Due to limitations of existing therapies, over 95% of people with AUD do not receive medical treatment • The vast majority of patients that have AUD remain undiagnosed and untreated , creating a large market opportunity for a product that can address patient needs • AUD is a potentially multi - billion dollar market with limited competition & unmet need . Excessive Alcohol Use accounts for ~ 5 . 3 % of deaths worldwide and ~ 5 . 1 % of disease worldwide • The Lancet reports that alcohol is the number one risk factor for de ath globally among both men and women ages 15 to 49 years Sources : SAMHSA, Center for Behavioral Health Statistics and Quality. 2022 National Survey on Drug Use and Health. Tables 5.9A . ~ 3 0 M Patients with AUD Sources: NIAAA Alcohol Facts & Statistics. www.cdc.gov/features/costsofdrinking/index.html accessed June 3, 2020. The Lancet Sep. 2018., WHO Fact Sheets www.who.int/news - room/fact - sheets/detail/alcohol ~2.6M patients seeking treatment AUD ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
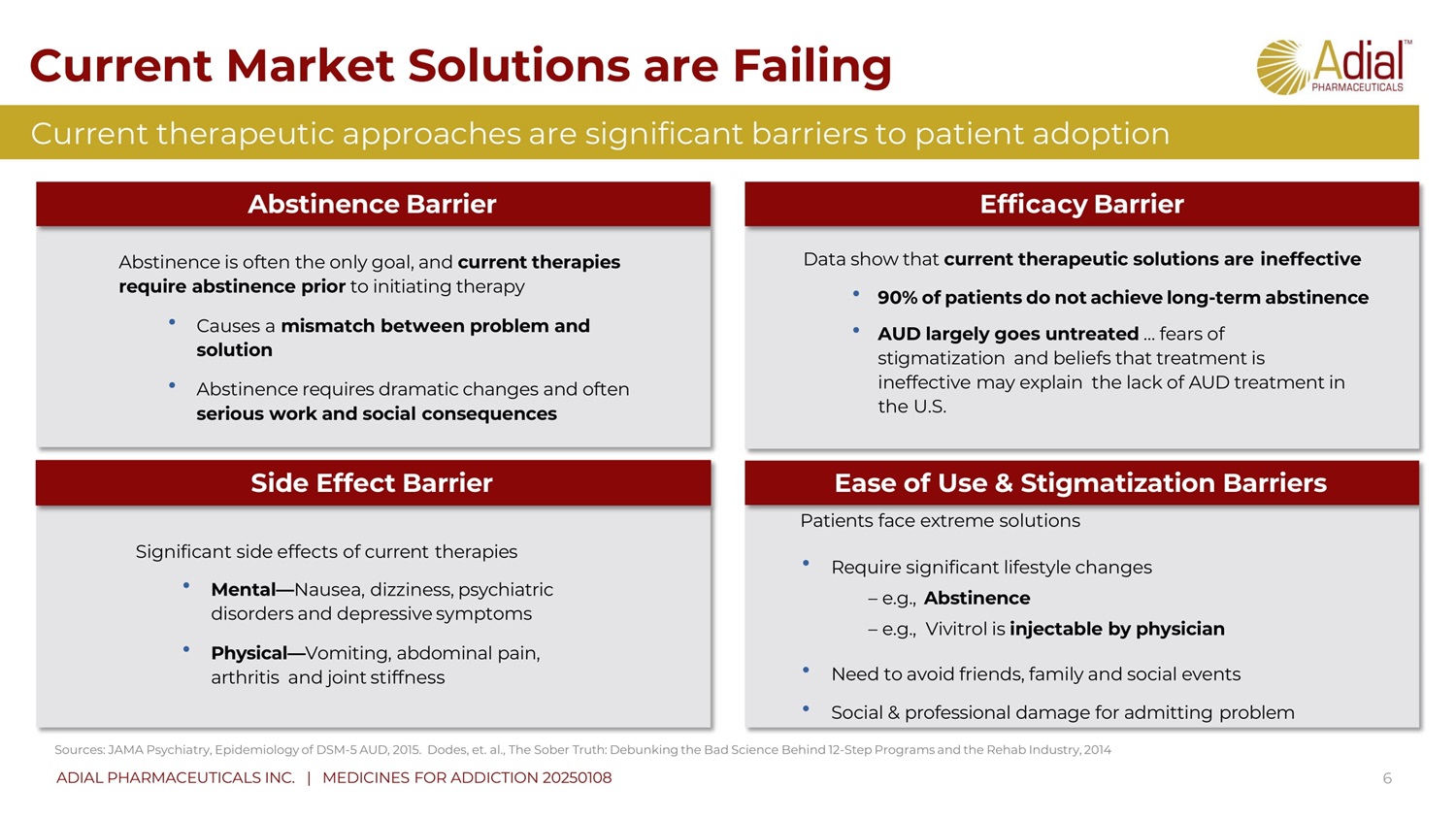
Current Market Solutions are Failing 6 Abstinence is often the only goal, and current therapies require abstinence prior to initiating therapy • Causes a mismatch between problem and solution • Abstinence requires dramatic changes and often serious work and social consequences Patients face extreme solutions • Req uire significant lifestyle changes – e.g., Abstinence – e.g., Vivitrol is injectable by physician • Need to avoid friends, family and social events • Social & professional damage for admitting problem Significant side effects of current therapies • Mental — Nausea, dizziness, psychiatric disorders and depressive symptoms • Physical — Vomiting, abdominal pain, arthritis and joint stiffness Data show that current therapeutic solutions are ineffective • 90% of patients do not achieve long - term abstinence • AUD largely goes untreated … fears of stigmatization and beliefs that treatment is ineffective may explain the lack of AUD treatment in the U.S. Abstinence Barrier Side Effect Barrier Efficacy Barrier Ease of Use & Stigmatization Barriers Sources: JAMA Psychiatry, Epidemiology of DSM - 5 AUD, 2015. Dodes, et. al., The Sober Truth: Debunking the Bad Science Behind 12 - Step Programs and the Rehab Industry, 2014 Current therapeutic approaches are significant barriers to patient adoption ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
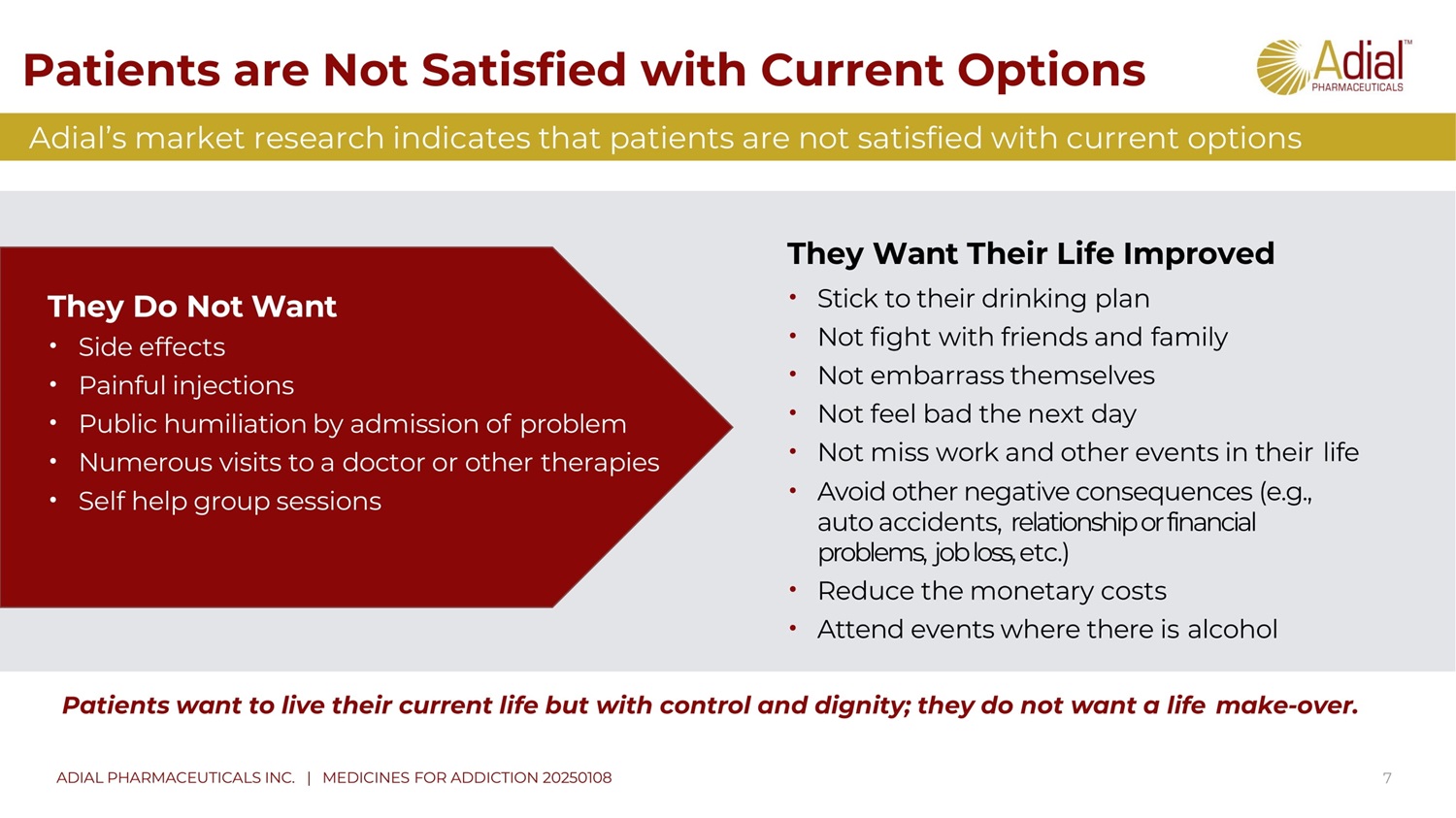
Patients want to live their current life but with control and dignity ; they do not want a life make - over . Patients are Not Satisfied with Current Options 7 They Want Their Life Improved • Stick to their drinking plan • Not fight with friends and family • Not embarrass themselves • Not feel bad the next day • Not miss work and other events in their life • Avoid other negative consequences (e.g ., auto accidents, relationship or financial problems, job loss, etc.) • Reduce the monetary costs • Attend events where there is alcohol Adial’s market research indicates that patients are not satisfied with current options They Do Not Want • Side effects • Painful injections • Public humiliation by admission of problem • Numerous visits to a doctor or other therapies • Self help group sessions ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108

AD04 E ffectively C urb s A lcohol I ntake by Reducing Craving New M echanism Action : Treatment of Alcohol Use Disorder (AUD) 8 AD04 is Designed to Meet the Market Need Management of Heavy Drinking ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108 AD 04 is a Near Micro - Dose ( 0 . 33 mg/tablet) Formulation of Ondansetron . Ondansetron (brand name : Zofran), widely used for nausea since 1991 , is well - characterized, has a good safety profile at high doses (from 4 mg oral to 16 mg i . v . ) . AD 04 is a genetically targeted precision medication intended to reduce drinking in patients with specific genotypes . Clinical Trial Results and Post - Hoc Analysis Demonstrated that ADO 4 Reduces Craving in Heavy Drinkers . AD 04 reduced heavy drinking days (HDDs) by 86 % among heavy drinkers 1 and eliminated HDDs in 48 % of subjects who possessed the AG+ genotype . 2 These results are thought to be due to AD 04 ’s suppression of the craving and pleasure derived from alcohol by affecting the 5 HT 3 system (serotonin signaling and dopamine release) . Parallels Can be Drawn Between Semaglutide and AD 04 Mechanisms of Action . There has been significant adoption of Semaglutide for weight loss, which interacts with the parts of the brain that suppress appetite . Interestingly, Semaglutide and AD 04 share a feature in common, which may promote a better understanding of AD 04 ’s Mechanism of Action on drinking, as they both impact parts of the brain that curb craving . 1. The NIAAA defines Heavy Drinkers as follows: Men who consume 5 or more drinks on any day or 15 or more per week or women who con sume 4 or more on any day or 8 or more per week. 2. Post - Hoc analysis of ONWARD trial
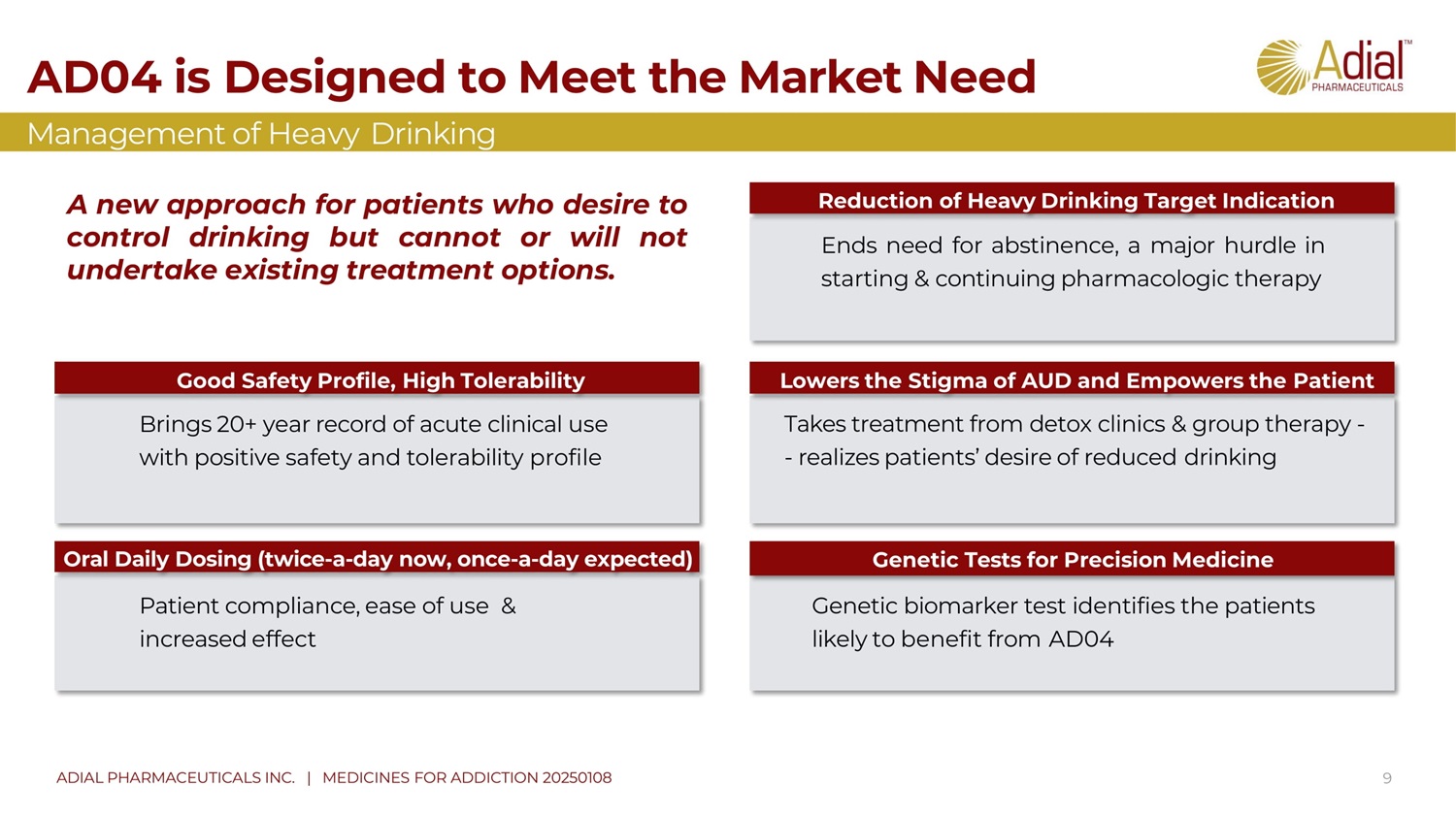
Brings 20+ year record of acute clinical use with positive safety and tolerability profile Patient compliance, ease of use & increased effect Ends need for abstinence, a major hurdle in starting & continuing pharmacologic therapy Takes treatment from detox clinics & group therapy - - realizes patients’ desire of reduced drinking G enetic biomarker test identifies the patients likely to benefit from AD 04 Good S afety P rofile , H igh T olerability Oral D aily D osing (twice - a - day now, once - a - day expected) Reduction of H eavy D rinking T arget I ndication Lowers the S tigma of AUD and E mpowers the P atient Genetic Tests for Precision Medicine 9 AD04 is Designed to Meet the Market Need Management of Heavy Drinking ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108 A new approach for patients who desire to control drinking but cannot or will not undertake existing treatment options .
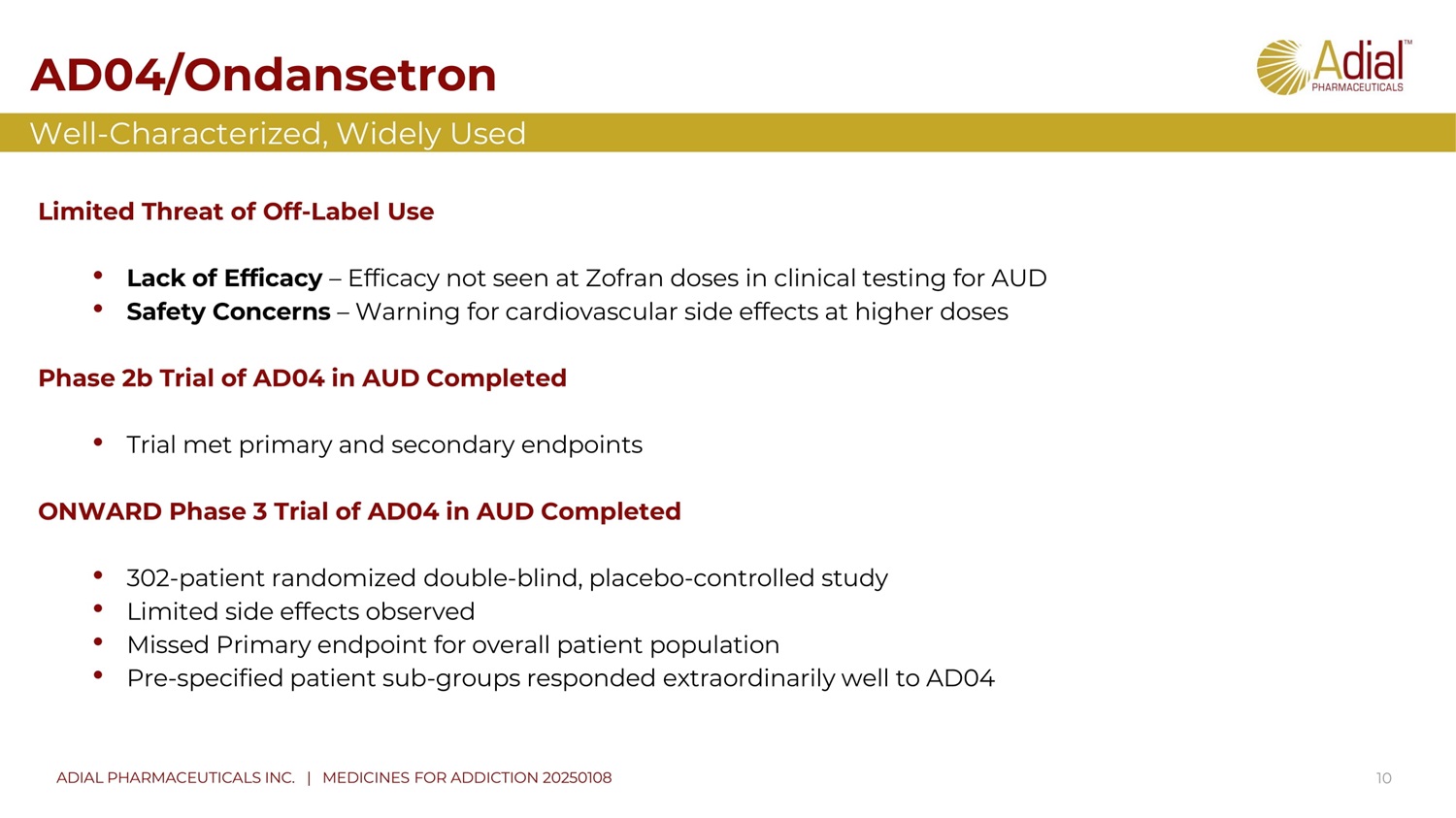
AD04 / Ondansetron 10 Limited T hreat of O ff - L abel U se • Lack of Efficacy – Efficacy not seen at Zofran doses in clinical testing for AUD • Safety Concerns – Warning for cardiovascular side effects at higher doses Phase 2 b Trial of AD 04 in AUD Completed • Trial met primary and secondary endpoints ONWARD Phase 3 Trial of AD 04 in AUD Completed • 302 - patient randomized double - blind, placebo - controlled study • Limited side effects observed • Missed Primary endpoint for overall patient population • Pre - specified patient sub - groups responded extraordinarily well to AD 04 Well - Characterized, Widely Used ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108

Genetic Test Expected to Drive Market Uptake 11 The g enetic test is expected to increase prescription fill rate and compliance . • Physician conversation with patient • First step of a test vs. a drug • Patient buy - in to treatment after positive test • Potential of increased compliance resulting in effective therapeutic Precision Medicine Enables: ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
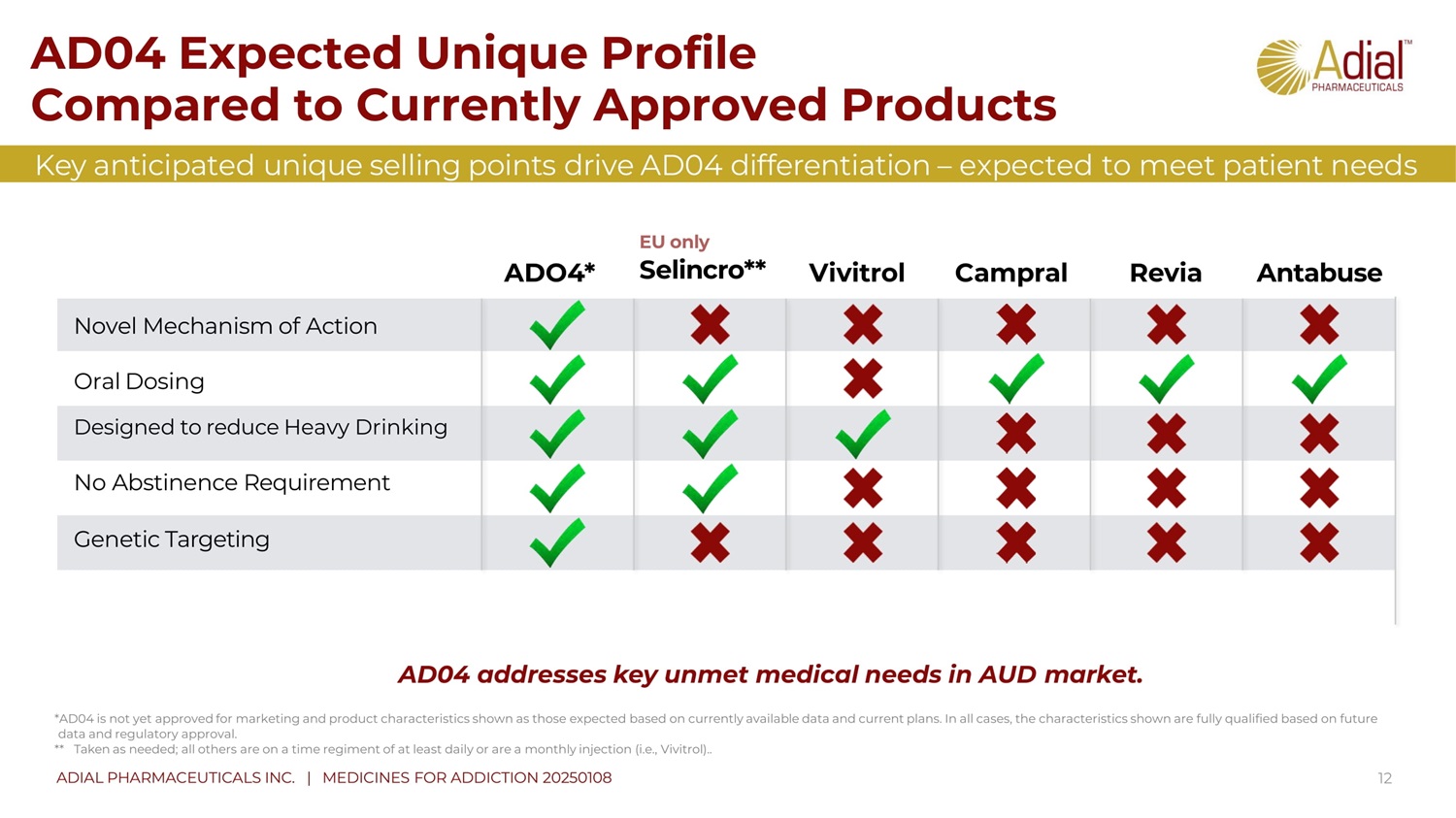
AD04 addresses key unmet medical needs in AUD market . *AD04 is not yet approved for marketing and product characteristics shown as those expected based on currently available data and current plans. In all cases, the characteristics shown are fully qualified based on future data and regulatory approval. ** Taken as needed; all others are on a time regiment of at least daily or are a monthly injection (i.e. , Vivitrol). . A DO 4 * EU only S e l in c r o * * V iv i tr o l C a m p ral R e v i a A nta b us e Novel Mechanism of Action Oral Dosing Designed to reduce Heavy Drinking No Abstinence Requirement Genetic Targeting 12 AD04 Expected Unique Profile Compared to Currently Approved Products Key anticipated unique selling points drive AD04 differentiation – expected to meet patient needs ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
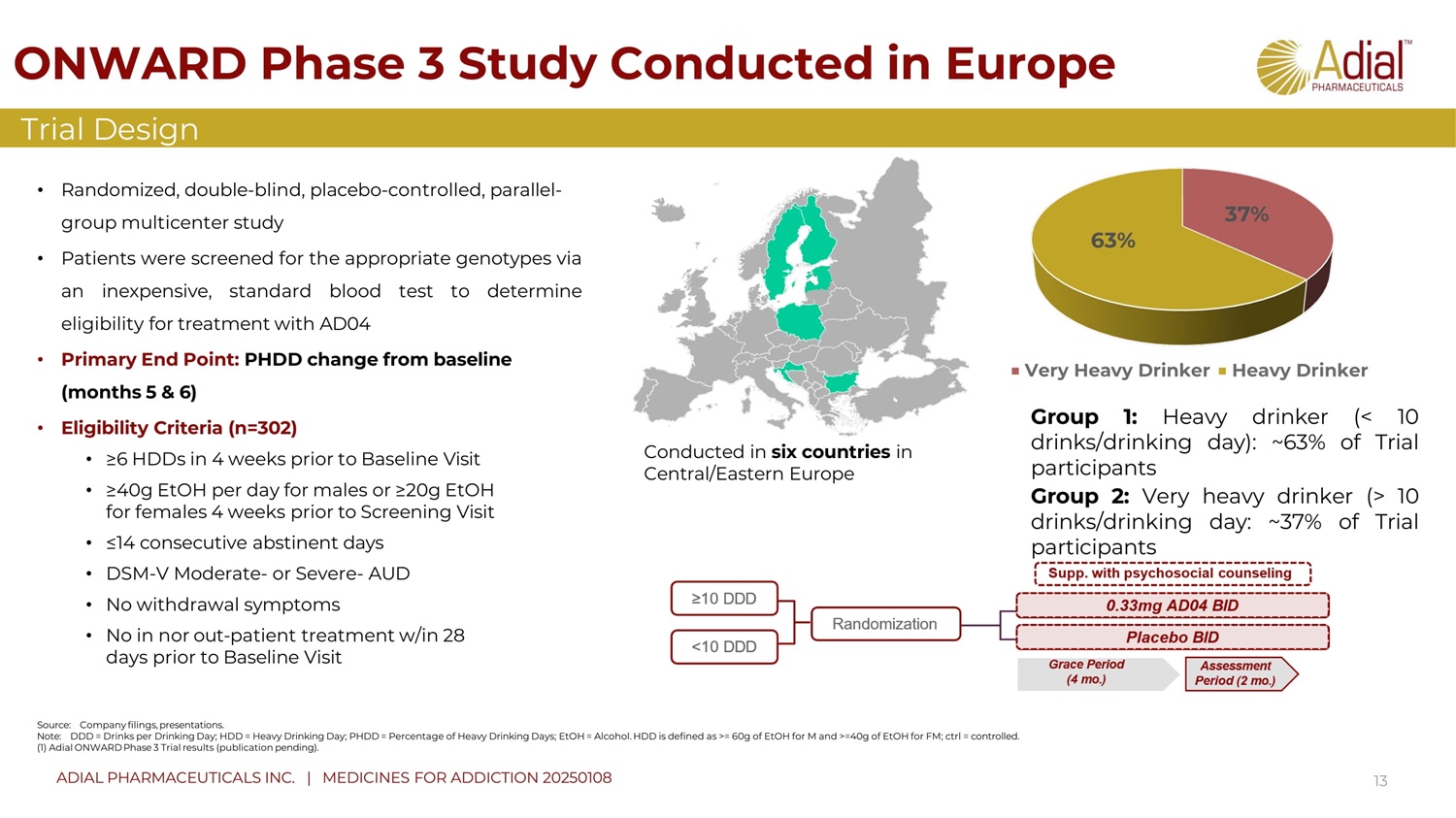
ONWARD Phase 3 Study Conducted in Europe 13 • Randomized, double - blind, placebo - c ontrolled , parallel - group multicenter study • Patients were screened for the appropriate genotypes via an inexpensive, standard blood test to determine eligibility for treatment with AD 04 • Primary End Point: PHDD change from baseline (months 5 & 6) • Eligibility Criteria (n=302) Trial D esign Source: Company filings, presentations. Note: DDD = Drinks per Drinking Day; HDD = Heavy Drinking Day; PHDD = Percentage of Heavy Drinking Days; EtOH = Alcohol. HDD is defined as >= 60g of EtOH for M and >=40g of EtOH for FM; ctrl = controlled. (1) Adial ONWARD Phase 3 Trial results (publication pending). • ≥6 HDDs in 4 weeks prior to Baseline Visit • ≥40g EtOH per day for males or ≥20g EtOH for females 4 weeks prior to Screening Visit • ≤14 consecutive abstinent days • DSM - V Moderate - or Severe - AUD • No withdrawal symptoms • No in n or out - patient treatment w/in 28 days prior to Baseline V i sit Conducted in six countries in Central/Eastern Europe Group 1 : Heavy drinker (< 10 drinks/drinking day) : ~ 63 % of Trial participants Group 2 : Very heavy drinker (> 10 drinks/drinking day : ~ 37 % of Trial participants 37% 63% Very Heavy Drinker Heavy Drinker ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
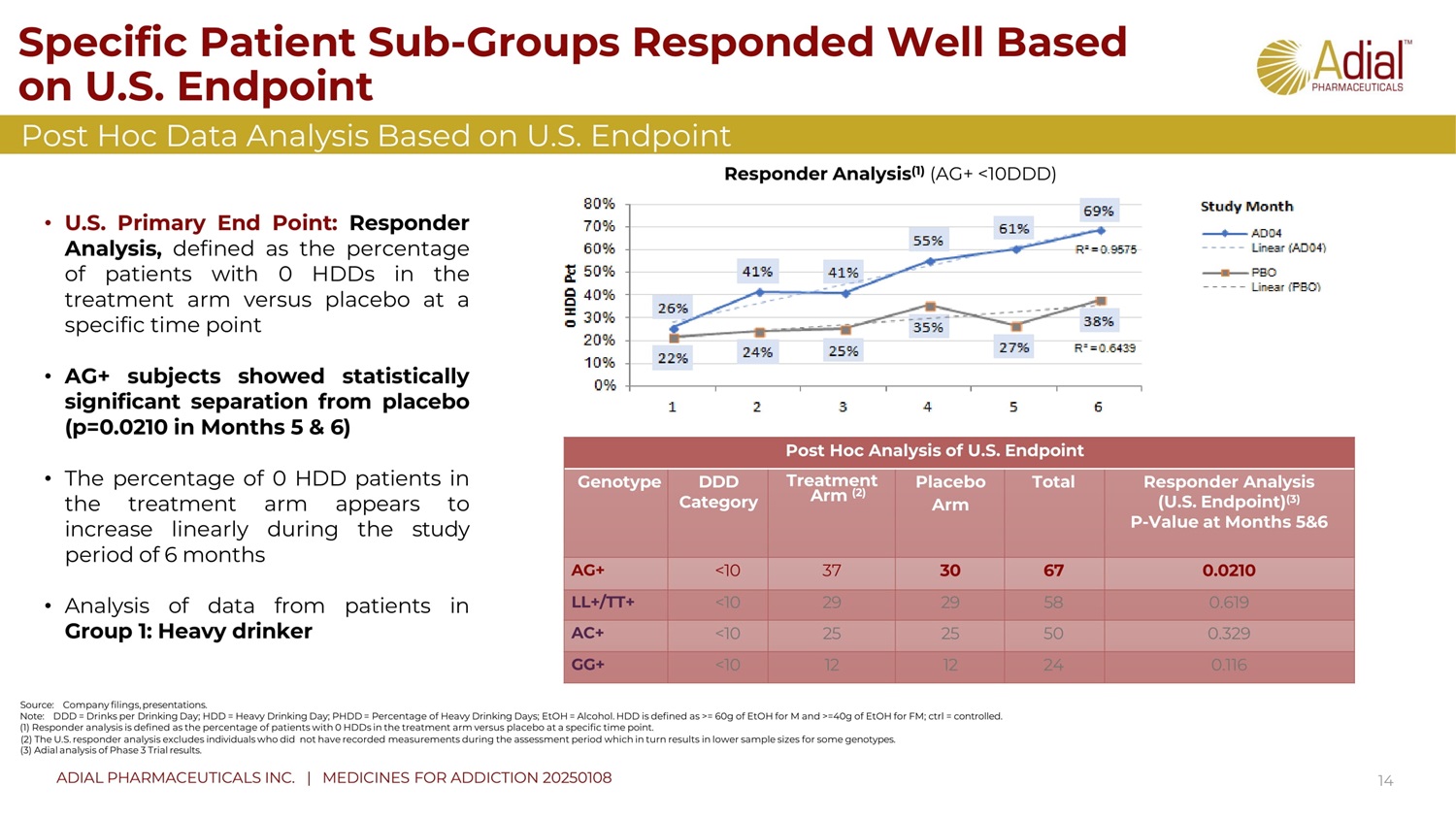
Specific Patient Sub - Groups Responded Well Based on U.S. Endpoint 14 Post Hoc Data Analysis Based on U.S. Endpoint Post Hoc Analysis of U.S. Endpoint Responder Analysis (U.S. Endpoint) (3) P - Value at Months 5&6 Total Placebo Arm Treatment Arm (2) DDD Category Genotype 0.02 1 0 67 30 37 <10 AG+ 0. 619 58 29 29 <10 LL+/TT+ 0. 329 50 25 25 <10 AC+ 0. 116 24 12 12 <10 GG+ Responder Analysis (1) (AG+ <10DDD) • U . S . Primary End Point : Responder Analysis, defined as the percentage of patients with 0 HDDs in the treatment arm versus placebo at a specific time point • AG+ subjects showed statistically significant separation from placebo (p= 0 . 02 1 0 in Months 5 & 6 ) • The percentage of 0 HDD patients in the treatment arm appears to increase linearly during the study period of 6 months • Analysis of data from patients in Group 1 : Heavy drinker Source: Company filings, presentations. Note: DDD = Drinks per Drinking Day; HDD = Heavy Drinking Day; PHDD = Percentage of Heavy Drinking Days; EtOH = Alcohol. HDD is defined as >= 60g of EtOH for M and >=40g of EtOH for FM; ctrl = controlled. (1) R esponder analysis is defined as the percentage of patients with 0 HDDs in the treatment arm versus placebo at a specific time point. (2) The U.S. responder analysis excludes individuals who did not have recorded measurements during the assessment period which in turn results in lower sample sizes for some genotypes. (3) Adial analysis of Phase 3 Trial results. ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
Pharmacokinetics Study for AD04 15 ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108 Objective defined by the FDA to design a precise and informed Phase 3 Trial Adial recently completed a Pharmacokinetics Study , a key component to progress AD 04 ’s clinical development as advised by the FDA . Topline Results indicate that AD 04 displays proportional dose response pharmacokinetic properties and can be taken in fed or fasted states . The study evaluated : Pharmacokinetic variability and dose proportionality between two doses of AD 04 ( 0 . 33 mg and 0 . 99 mg) Relative bioavailability of AD 04 ( 0 . 33 mg) compared to a marketed ondansetron tablet ( 4 mg) The effect of food on the bioavailability of AD 04 ( 0 . 33 mg) The study produced data which will help Adial optimize study design elements needed for the upcoming Phase 3 Clinical Trial of AD 04 . The study validates Adial’s proprietary formulation of AD 04 and the pharmacokinetic properties are being designed to meet FDA requirements . By completing this study, it fulfills a necessary component of Adial’s partnering strategy .
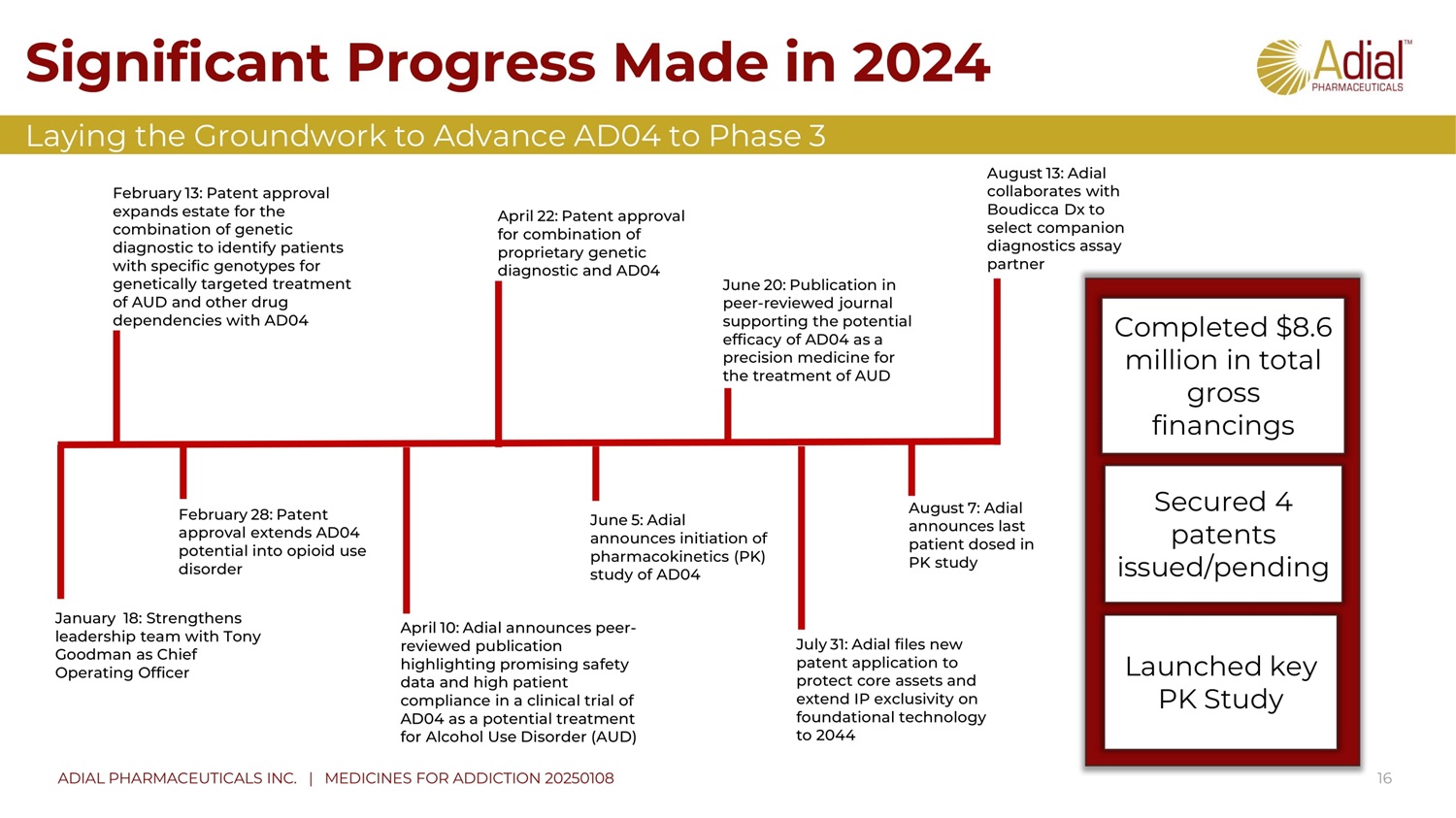
Significant Progress Made in 2024 16 ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108 August 13: Adial collaborates with B oudicca D x to select companion diagnostics assay partner January 18: Strengthens leadership team with Tony G oodman as Chief O perating Officer June 20: Publication in peer - reviewed journal supporting the potential efficacy of AD04 as a precision medicine for the treatment of AUD April 10: Adial announces peer - reviewed publication highlighting promising safety data and high patient compliance in a clinical trial of AD04 as a potential treatment for Alcohol U se D isorder (AUD) June 5: Adial announces initiation of pharmacokinetics (PK) study of AD04 July 31: A dial files new patent application to protect core assets and extend IP exclusivity on foundational technology to 2044 April 22: Patent approval for combination of proprietary genetic diagnostic and AD04 February 28: Patent approval extends AD04 potential into opioid use disorder February 13: Patent approval expands estate for the combination of genetic diagnostic to identify patients with specific genotypes for genetically targeted treatment of AUD and other drug dependencies with AD04 August 7: Adial announces last patient dosed in PK study Completed $8.6 million in total gross financings Secured 4 patents issued/pending Laying the Groundwork to Advance AD04 to Phase 3 Launched key PK Study
Next Steps 17 Finalize plans to achieve potential commercial launch by 2027 Complete Pharmacokinetics Study to Optimize Elements of Phase 3 Studies Finalize Clinical Development Plan Final decision on conducting one or two Trials Finalize Trial design and Costs (current estimate $ 8 - $ 12 million per Trial pending final design and scope) Timeline completion Review Study Design, Protocol and Statistical Analysis Plan with FDA Advancing Discussions with Potential Strategic Partners : Phase 3 clinical program funding Commercialization of AD 04 assuming a successful regulatory outcome ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
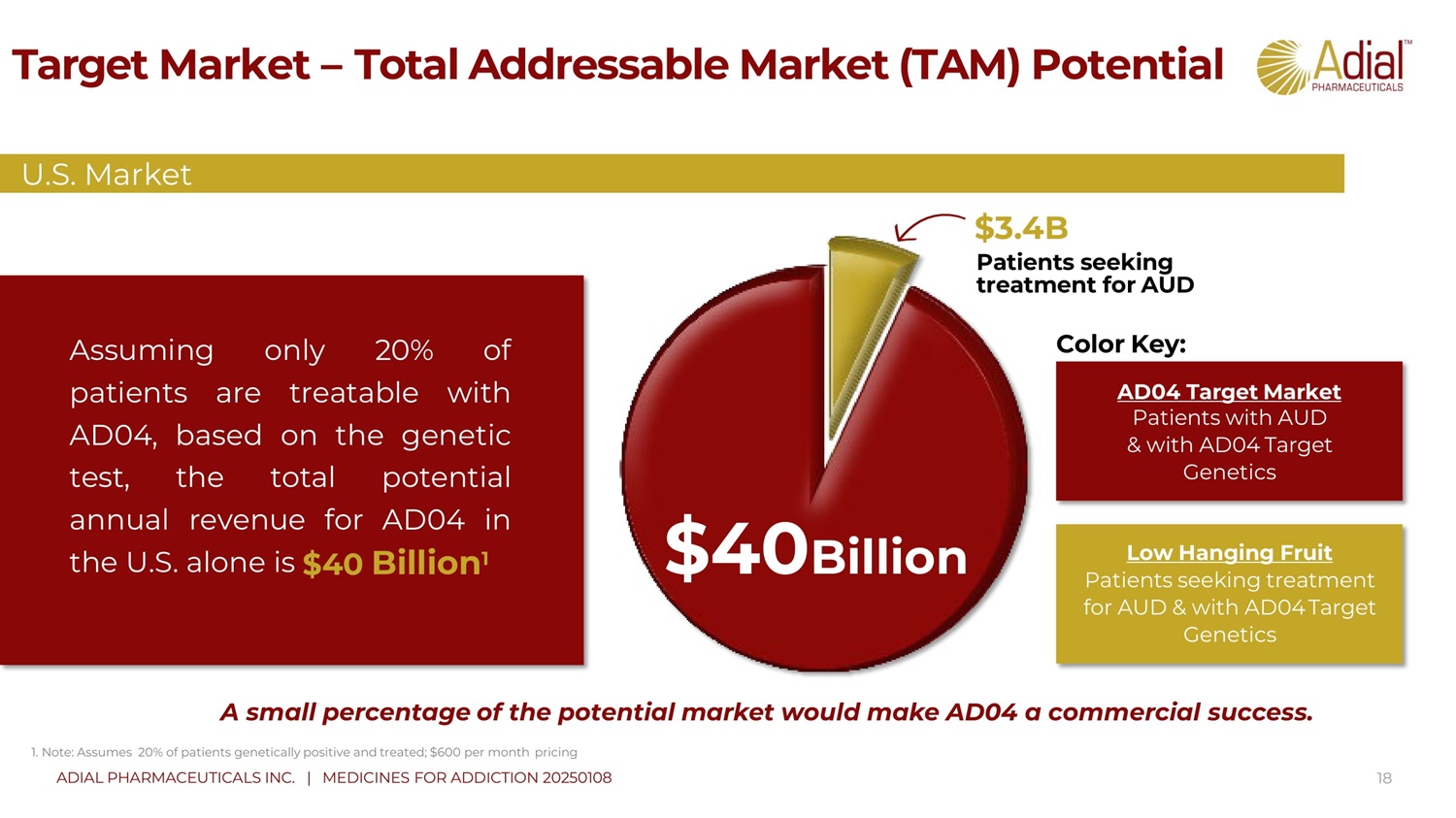
Target Market – Total Addressable Market (TAM) Potential 18 A small percentage of the potential market would make AD04 a commercial success . 1. Note: Assumes 20 % of patients genetically positive and treated; $ 600 per month pricing AD04 Target Market Patients with AUD & with AD04 Target Genetics Low Hanging Fruit Patients seeking treatment for AUD & with AD04 Target Genetics Color Key: Assuming only 20 % of patients are treatable with AD 04 , based on the genetic test, the total potential annual revenue for AD 04 in the U . S . alone is $ 40 Billion 1 $ 3.4 B Patients seeking treatment for AUD $ 40 Billion U.S. Market ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108

Building an Addiction Focused Pharmaceutical Company 19 Lead Product for AUD • Large market with unmet need • Late - stage oral drug (Phase 3 ) • Companion diagnostic designed to identify responders • Seeking 505 (B)( 2 ) path to regulatory approval • Low - cost manufacturing • Licensed patent protection through 2031 Potential Indication Expansion Opportunities for AD04 (opioid use disorder, obesity, others) Experienced and Q ualified Management Team ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108

C o n t a c t : GENERAL INQUIRES : info@adialpharma.com INVESTOR RELATIONS : Crescendo Communications, LLC 405 Lexington Ave 9th Floor, Suite 9034 New York, NY 10174 T: 212 - 671 - 1021 ADIL@crescendo - ir.com F ind U s @ A dial P harma adialpharma.com MEDIA RELATIONS : Russo Partners , LLC 215 Park Ave South Suite 1905 New York, NY 1000 3 T: 817 - 371 - 0654 Adial@RussoPR.com CONFIDENTIAL | 20 ADIAL PHARMACEUTICALS INC. | MEDICINES FOR ADDICTION 20250108
v3.24.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Adial Pharmaceuticals (NASDAQ:ADIL)
과거 데이터 주식 차트
부터 1월(1) 2025 으로 2월(2) 2025

Adial Pharmaceuticals (NASDAQ:ADIL)
과거 데이터 주식 차트
부터 2월(2) 2024 으로 2월(2) 2025
