false 0001227636 0001227636 2024-11-12 2024-11-12
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) November 12, 2024
NEURONETICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-38546 |
|
33-1051425 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
|
|
| 3222 Phoenixville Pike, Malvern, PA |
|
19355 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code (610) 640-4202
(Former name or former address, if changed since last report.) Not applicable.
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol (s) |
|
Name on each exchange on which registered |
| Common Stock ($0.01 par value) |
|
STIM |
|
The Nasdaq Global Market |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
Neuronetics, Inc. (“Neuronetics” or the “Company”) issued a press release on November 12, 2024 announcing its financial results for the three months ended September 30, 2024. A copy of the press release is being furnished to the Securities and Exchange Commission as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference to this Item 2.02.
The information furnished pursuant to Item 2.02 , including Exhibit 99.1, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed to be incorporated by reference into any of the Company’s filings with the Securities and Exchange Commission under the Exchange Act or the Securities Act of 1933, as amended, whether made before or after the date hereof, regardless of any general incorporation language in such a filing, except as expressly set forth by specific reference in such a filing. Except as required by law, the Company undertakes no duty or obligation to publicly update or revise the information so furnished.
| Item 7.01 |
Regulation FD Disclosure. |
On November 12, 2024, Neuronetics released a presentation (the “Presentation”) relating to the Company’s financial results for the three months ended September 30, 2024. A copy of the Presentation in connection therewith is attached hereto as Exhibit 99.2. The information contained in Exhibit 99.2 is incorporated herein by reference.
The information in this report furnished pursuant to Item 7.01, including Exhibit 99.2, shall not be deemed “filed” for the purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed to be incorporated by reference into any of the Company’s filings with the Securities and Exchange Commission under the Exchange Act or the Securities Act of 1933, as amended, whether made before or after the date hereof, regardless of any general incorporation language in such a filing, except as expressly set forth by specific reference in such a filing. Except as required by law, the Company undertakes no duty or obligation to publicly update or revise the information so furnished.
“Safe harbor” statement under the Private Securities Litigation Reform Act of 1995:
Certain statements in this Current Report, include “forward-looking statements” within the meaning of U.S. federal securities laws. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by words or expressions such as “expect”, “anticipate”, “intend”, “plan”, “believe”, “estimate”, “may”, “will”, “project”, “could”, “should”, “would”, “seek”, “forecast”, “expect”, “anticipate”, “predict”, “outlook”, “potential”, or other similar expressions, including without limitation the negative of these terms. Forward-looking statements represent current judgments about possible future events, including, but not limited to statements regarding expectations or forecasts of business, operations, financial performance, prospects, and other plans, intentions, expectations, estimates, and beliefs relating to the proposed transaction between Greenbrook TMS Inc. (“Greenbrook”) and Neuronetics, such as statements regarding the combined operations and prospects of Greenbrook and Neuronetics, estimates of pro forma financial information of the combined company, the current and projected market, growth opportunities and synergies for the combined company, federal and state regulatory tailwinds, the expected cash balance of Greenbrook at the time of the closing of the proposed Arrangement (as such term is defined in the Neuronetics definitive proxy statement), expectations regarding Neuronetics’ ability to leverage Greenbrook’s assets, the expected composition of the management and the board of directors of the combined company, gross margin and future profitability expectations, and the timing and completion of the Arrangement, including the satisfaction or waiver of all the required conditions thereto. These forward-looking statements are based upon the current beliefs and expectations of the management of Neuronetics and are subject to known and unknown risks and uncertainties. Factors that could cause actual events to differ include, but are not limited to:
| |
• |
|
the inherent uncertainty associated with financial or other projections or outlooks, including due to the unpredictability of the underlying assumptions, adjustments and estimates; |
| |
• |
|
Neuronetics’ ability to maintain the listing requirements of Nasdaq; |
| |
• |
|
the total addressable market of Neuronetics’ and Greenbrook’s businesses; |
| |
• |
|
general economic conditions in the markets where Neuronetics and Greenbrook operate; |
| |
• |
|
the expected timing of any regulatory approvals relating to the Arrangement, the businesses of Greenbrook and Neuronetics and of the combined company and product launches of such businesses and companies; |
| |
• |
|
the non-performance of third-party vendors and contractors; |
| |
• |
|
the risks related to the combined company’s ability to successfully sell its products and the market reception to and performance of its products; |
| |
• |
|
Greenbrook’s, Neuronetics’, and the combined company’s compliance with, and changes to, applicable laws and regulations; |
| |
• |
|
the combined company’s limited operating history; |
| |
• |
|
the combined company’s ability to manage growth; |
| |
• |
|
the combined company’s ability to obtain additional or suitable financing; |
| |
• |
|
the combined company’s ability to expand product offerings; |
| |
• |
|
the combined company’s ability to compete with others in its industry; |
| |
• |
|
the combined company’s ability to protect its intellectual property; |
| |
• |
|
the retention of employees of Greenbrook and Neuronetics following the announcement of the Arrangement; |
| |
• |
|
Greenbrook’s, Neuronetics’, and the combined company’s ability to defend against legal proceedings; |
| |
• |
|
the combined company’s success in retaining or recruiting, or changes required in, its officers, key employees or directors; |
| |
• |
|
the combined company’s ability to achieve the expected benefits from the Arrangement within the expected time frames or at all; |
| |
• |
|
the incurrence of unexpected costs, liabilities or delays relating to the proposed Arrangement; |
| |
• |
|
the satisfaction (or waiver) of closing conditions to the consummation of the Arrangement; |
| |
• |
|
the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the Arrangement Agreement (as such term is defined in the Neuronetics definitive proxy statement); |
| |
• |
|
the disruption of the attention of management of Greenbrook and Neuronetics from ongoing business operations due to the Arrangement Agreement; |
| |
• |
|
the outcome of any legal proceedings related to the Arrangement Agreement; |
| |
• |
|
the fact that the trading price of the Greenbrook Shares or the Neuronetics Shares may decline significantly if the Arrangement is not completed; |
| |
• |
|
the effect of the announcement or pendency of the transaction on the combined company’s business relationships, operating results and business generally; and |
| |
• |
|
other economic, business, competitive, and regulatory factors affecting the businesses of the companies generally, including, but not limited to, those set forth in Greenbrook’s filings with the SEC and the Canadian Securities Administrators, including in the “Risk Factors” section of the Greenbrook 10-K and any subsequent filings with the U.S. Securities and Exchange Commission (the “SEC”) and the Canadian Securities Administrators, and those set forth in Neuronetics’ filings with the SEC, including in the “Risk Factors” section of Neuronetics’ Annual Report on Form 10-K filed with the SEC on March 8, 2024 and any subsequent SEC filings. These documents with respect to Greenbrook can be accessed on Greenbrook’s website at https://www.greenbrooktms.com/investor-relations, on Greenbrook’s SEDAR+ profile at www.sedarplus.ca or on Greenbrook’s EDGAR profile at www.sec.gov and these documents with respect to Neuronetics can be accessed on Neuronetics’ website at https://ir.neuronetics.com/ or on Neuronetics’ EDGAR profile at www.sec.gov. |
Readers are cautioned not to place undue reliance on forward-looking statements. It is uncertain whether any of the events anticipated by the forward-looking statements will transpire or occur, or, if any of them do, what impact they will have on the results of operations and financial condition of Greenbrook, Neuronetics or the combined company. Forward-looking statements speak only as of the date they are made, and Greenbrook, Neuronetics and the combined company undertake no obligation to update publicly or otherwise revise any forward-looking statements, whether as a result of new information, future events, or other factors that affect the subject of these statements, except where they are expressly required to do so by law.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
NEURONETICS, INC. |
|
|
|
|
(Registrant) |
|
|
|
|
| Date: November 12, 2024 |
|
|
|
By: |
|
/s/ W. Andrew Macan |
|
|
|
|
Name: |
|
W. Andrew Macan |
|
|
|
|
Title: |
|
EVP, GC & Chief Compliance Officer |
Exhibit 99.1

Neuronetics Reports Record Third Quarter 2024 Financial and Operating Results
MALVERN, PA., November 12, 2024 – Neuronetics, Inc. (NASDAQ: STIM) (the “Company” or “Neuronetics”) a commercial stage
medical technology company with a strategic vision of transforming the lives of patients whenever and wherever they need help, with the best neurohealth therapies in the world, today announced its financial and operating results for the third
quarter of 2024.
Third Quarter 2024 Highlights
| |
• |
|
Third quarter 2024 revenue of $18.5 million, a 4% increase as compared to the third quarter
2023 |
| |
• |
|
U.S. NeuroStar Advanced Therapy system revenue of $4.1 million in the quarter, representing
48 systems |
| |
• |
|
U.S. treatment session revenue increased by 2% versus the third quarter of 2023 |
Recent Operational and Marketing Highlights
| |
• |
|
Neuronetics stockholders approved the acquisition of Greenbrook TMS on
November 8, 2024 |
| |
• |
|
NeuroStar Oral Presentation at AACAP 2024 Highlights Largest Study Evaluating TMS Efficacy in Adolescents with
Depression |
| |
• |
|
Achieved milestone of over 188,000 global patients treated with 6.9 million treatment
sessions |
“We are very excited about the approval of the acquisition of Greenbrook TMS, which positions Neuronetics to be one
of the largest, and most innovative provider of mental health care in the United States,” said Keith J. Sullivan, President and Chief Executive Officer of Neuronetics. “This strategic combination will significantly enhance our ability to
expand access to mental health solutions through a unified commercial organization, while also creating opportunities to accelerate the rollout of additional services like SPRAVATO® across our
network. By leveraging our complementary strengths—including Greenbrook’s extensive presence in the U.S. and Neuronetics’ industry-leading technology and training programs – we’re building an organization with the unique
ability to increase patient access, improve outcomes, and ultimately, drive value for stockholders.”
Keith J. Sullivan continued, “Beyond the
Greenbrook acquisition, we saw continued progress across our strategic initiatives in the third quarter, highlighted by strong momentum in our adolescent treatment program and encouraging early results from our targeted marketing efforts. We’re
more confident than ever in our ability to create meaningful value for patients, providers, and stockholders while advancing mental health treatment.”
Third Quarter 2024 Financial and Operating Results for the Three Months Ended September 30, 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Revenues by Geography
Three Months Ended September 30, |
|
|
|
|
| |
|
2024 |
|
|
2023 |
|
|
|
|
| |
|
Amount |
|
|
Amount |
|
|
% Change |
|
| |
|
(Unaudited; in thousands, except percentages) |
|
| U.S. |
|
$ |
17,922 |
|
|
$ |
17,211 |
|
|
|
4 |
% |
| International |
|
|
608 |
|
|
|
673 |
|
|
|
(10 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total revenues |
|
$ |
18,530 |
|
|
$ |
17,884 |
|
|
|
4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue for the three months ended September 30, 2024 was $18.5 million, an increase of 4% compared to the
revenue of $17.9 million in the third quarter of 2023. During the quarter, total U.S. revenue increased by 4% and international revenue decreased marginally over the third quarter of 2023. The increase in U.S. revenue was primarily attributable
to an increase in U.S. treatment sessions and U.S. NeuroStar Advanced Therapy System sales period over period.
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
U.S. Revenues by Product Category
Three Months Ended September 30, |
|
|
|
|
| |
|
2024 |
|
|
2023 |
|
|
|
|
| |
|
Amount |
|
|
Amount |
|
|
% Change |
|
| |
|
(Unaudited; in thousands, except percentages) |
|
| NeuroStar Advanced Therapy System |
|
$ |
4,108 |
|
|
$ |
3,597 |
|
|
|
14 |
% |
| Treatment sessions |
|
|
13,326 |
|
|
$ |
13,060 |
|
|
|
2 |
% |
| Other |
|
|
488 |
|
|
$ |
554 |
|
|
|
(12 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total U.S. revenues |
|
$ |
17,922 |
|
|
$ |
17,211 |
|
|
|
4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. NeuroStar Advanced Therapy System revenue for the three months ended September 30, 2024 was $4.1 million, an
increase of 14% compared to $3.6 million in the third quarter of 2023. For the three months ended September 30, 2024, and 2023, the Company shipped 49 and 43 systems, respectively.
U.S. treatment session revenue for the three months ended September 30, 2024 was $13.3 million, an increase of 2% compared to $13.1 million in
the third quarter of 2023.
In the third quarter of 2024, U.S. treatment session revenue per active site was $11,390 compared to $11,917 in the third
quarter of 2023.
Gross margin for the third quarter of 2024 was 75.6%, an increase of approximately 980 basis points from the third quarter of 2023 gross
margin of 65.8%. The increase in gross margin was a result of the change in product mix, the absence of one-time manufacturing cost by our new contract manufacturer and inventory impairment incurred in 2023.
Operating expenses during the third quarter of 2024 were $21.7 million, an increase of $1.1 million, or 5%, compared to $20.6 million in
the third quarter of 2023.
Net loss for the third quarter of 2024 was $(13.3) million, or $(0.44) per share, as compared to $(9.4) million, or $(0.33)
per share, in the third quarter of 2023. Net loss per share was based on 30,267,236 and 28,875,720 weighted average common shares outstanding for the third quarters of 2024 and 2023, respectively.
EBITDA for the third quarter of 2024 was $(11.6) million as compared to the third quarter of 2023 EBITDA of
$(7.7) million. See the accompanying financial table that reconciles EBITDA, which is a non-GAAP financial measure, to net loss.
Cash and cash equivalents were $20.9 million as of September 30, 2024. This compares to cash and cash equivalents of $59.7 million as of
December 31, 2023.
Stockholders Approve Acquisition of Greenbrook TMS
On November 8, 2024, Neuronetics’ stockholders approved the previously announced acquisition of Greenbrook TMS Inc. (“Greenbrook”), in
which Neuronetics will acquire all of the outstanding common shares of Greenbrook in an all-stock transaction. The next step in finalizing the transaction is a hearing in respect of the Final Order pursuant to
the Ontario Business Corporations Act scheduled for November 15, 2024. The transaction will create a vertically integrated organization capable of providing access to mental health treatment with significant scale in the U.S. The transaction
offers multiple strategic benefits for Neuronetics and its customers, including increased brand awareness for NeuroStar, more consistent delivery of best practices, and the ability to offer a variety of positive benefits for all NeuroStar customers.
Beyond the strategic benefits, the transaction is expected to create compelling financial benefits, including increased revenue scale and a strong growth trajectory, material cost synergies, an accelerated path to profitability, and a bolstered
balance sheet.
The Company has outlined several key strategic initiatives, including merging Neuronetics’ sales team with Greenbrook’s regional
area managers to optimize commercial operations, implementing the Better Me Provider Program best practices across all Greenbrook sites, and accelerating the rollout of SPRAVATO® treatment
across the combined network. Through these initiatives and additional identified opportunities to optimize the combined company’s cost structure in connection with the transaction, the Company now expects to achieve cash flow breakeven by the
second quarter of 2025.
NeuroStar TMS Data Presented at Leading Child & Adolescent Psychiatry Conference Shows Strong Efficacy in Treating
Teen Depression
At the 2024 American Academy of Child and Adolescent Psychiatry (“AACAP”) annual meeting, Neuronetics presented data from
the largest study to date evaluating transcranial magnetic stimulation (“TMS”) in adolescents with major depressive disorder. In an oral presentation, Paul E. Croarkin, DO, MS of Mayo Clinic shared compelling results showing
NeuroStar’s strong efficacy in treating Major Depressive Disorder (“MDD”) in adolescent patients, with impressive response and remission rates of 78% and 48% respectively. The findings were similar to those previously reported in
adult populations treated with NeuroStar TMS including clinically meaningful improvement in anxiety symptoms in this MDD population. As the only FDA-cleared TMS device for first-line adjunct treatment of MDD
in patients aged 15-21, these findings further establish NeuroStar’s leadership in providing innovative solutions for adolescent mental health treatment.
Business Outlook
For the fourth quarter of 2024, on a
stand alone basis, the Company expects total worldwide revenue between $19.0 million and $20.0 million.
For the full year 2024, on a stand alone basis the Company now expects total worldwide revenue to be between
$71.0 million and $72.0 million.
For the full year 2024, on a stand alone basis, the Company now expects total operating expenses to be between
$81.0 million and $82.0 million. This forecast excludes pre-closing transaction costs of approximately $2.0 million.
Webcast and Conference Call Information
Neuronetics’ management team will host a conference call on November 12, 2024, beginning at 8:30 a.m. Eastern Time.
The conference call will be broadcast live in listen-only mode via webcast at https://edge.media-server.com/mmc/p/yk5bidgv. To listen to the
conference call on your telephone, you may register for the call here. While it is not required, it is recommended you join 10 minutes prior to the event start.
About Neuronetics
Neuronetics, Inc. believes that mental
health is as important as physical health. As a global leader in neuroscience, Neuronetics is redefining patient and physician expectations with its NeuroStar Advanced Therapy for Mental Health. NeuroStar is a
non-drug, noninvasive treatment that can improve the quality of life for people suffering from neurohealth conditions when traditional medication hasn’t helped. The NeuroStar Advanced Therapy System is
cleared by the U.S. Food and Drug Administration (the “FDA”) for adults with MDD, as an adjunct for adults with obsessive-compulsive disorder, and to decrease anxiety symptoms in adult patients with MDD that may exhibit comorbid anxiety
symptoms (anxious depression), and as a first line adjunct for the treatment of MDD in adolescent patients aged 15-21. NeuroStar Advanced Therapy is the leading TMS treatment for MDD in adults with more than
6.9 million treatments delivered. NeuroStar is backed by the largest clinical data set of any TMS treatment system for depression, including the world’s largest depression outcomes registry. Neuronetics is committed to transforming lives
by offering an exceptional treatment that produces extraordinary results. For safety information and indications for use, visit NeuroStar.com.
“Safe harbor” statement under the Private Securities Litigation Reform Act of 1995:
Statements in the press release regarding the Company that are not historical facts constitute “forward-looking statements” within the meaning of the
Private Securities Litigation Reform Act of 1995. These forward-looking statements may be identified by terms such as “outlook,” “potential,” “believe,” “expect,” “plan,” “anticipate,”
“predict,” “may,” “will,” “could,” “would” and “should” as well as the negative of these terms and similar expressions. These statements include those relating to the Company’s
business outlook and current expectations for upcoming quarters and fiscal year 2024, including with respect to revenue, expenses, growth, and any statements of assumptions underlying any of the foregoing items. These statements are subject to
significant risks and uncertainties and actual results could differ materially from those projected. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this release. These risks and
uncertainties include, without limitation, risks and uncertainties related to: the effect of the Arrangement with Greenbrook, initially announced on August 11, 2024 and approved by Greenbrook’s shareholders and Neuronetics stockholders on
November 8, 2024, on our business relationships, operating results and business generally; the Company’s ability to execute its business strategy; the Company’s ability to achieve or sustain profitable operations due to its history of
losses; the Company’s reliance on the sale and use of its NeuroStar Advanced Therapy system to generate revenues; the scale and efficacy of the Company’s salesforce; the Company’s ability to retain talent; availability of coverage and
reimbursement from third-party payors for treatments using the Company’s products; physician and patient demand for treatments using the Company’s products; developments in competing technologies and therapies for the indications that the
Company’s products treat; product defects; our revenue has been concentrated among a small number of customers; the Company’s ability to obtain and maintain intellectual property protection for its technology; developments in clinical
trials or regulatory review of NeuroStar Advanced Therapy system for additional indications; developments in regulation in the U.S. and other applicable jurisdictions; the terms of our credit facility; our ability to successfully roll-out our Better Me Provider program on the planned timeline; our self-sustainability and existing cash balances; and our ability to achieve cash flow break-even in the third quarter of 2025. For a discussion of
these and other related risks, please refer to the Company’s recent filings with the U.S. Securities and Exchange Commission (the “SEC”), which are available on the SEC’s website at www.sec.gov. These forward-looking statements
are based on the Company’s expectations and assumptions as of the date of this press release. Except as required by law, the Company undertakes no duty or obligation to update any forward-looking statements contained in this press release as a
result of new information, future events, or changes in the Company’s expectations.
Investor Contact:
Mike Vallie or Mark Klausner
ICR Healthcare
443-213-0499
ir@neuronetics.com
Media Contact:
EvolveMKD
646-517-4220
NeuroStar@evolvemkd.com
NEURONETICS, INC.
Statements of Operations
(Unaudited; In thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months ended
September 30, |
|
|
Nine months ended
September 30, |
|
| |
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
| Revenues |
|
$ |
18,530 |
|
|
$ |
17,884 |
|
|
$ |
52,397 |
|
|
$ |
51,034 |
|
| Cost of revenues |
|
|
4,529 |
|
|
|
6,120 |
|
|
|
13,129 |
|
|
|
15,100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gross profit |
|
|
14,001 |
|
|
|
11,764 |
|
|
|
39,268 |
|
|
|
35,934 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sales and marketing |
|
|
11,877 |
|
|
|
12,141 |
|
|
|
35,820 |
|
|
|
35,602 |
|
| General and administrative |
|
|
7,436 |
|
|
|
6,339 |
|
|
|
19,540 |
|
|
|
19,151 |
|
| Research and development |
|
|
2,416 |
|
|
|
2,155 |
|
|
|
6,999 |
|
|
|
7,308 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total operating expenses |
|
|
21,729 |
|
|
|
20,635 |
|
|
|
62,359 |
|
|
|
62,061 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss from operations |
|
|
(7,728 |
) |
|
|
(8,871 |
) |
|
|
(23,091 |
) |
|
|
(26,127 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other (income) expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense |
|
|
1,725 |
|
|
|
1,184 |
|
|
|
5,529 |
|
|
|
3,580 |
|
| Loss on extinguishment of debt |
|
|
4,427 |
|
|
|
— |
|
|
|
4,427 |
|
|
|
— |
|
| Other income, net |
|
|
(539 |
) |
|
|
(664 |
) |
|
|
(2,001 |
) |
|
|
(4,895 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(13,341 |
) |
|
$ |
(9,391 |
) |
|
$ |
(31,046 |
) |
|
$ |
(24,812 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share of common stock outstanding, basic and diluted |
|
$ |
(0.44 |
) |
|
$ |
(0.33 |
) |
|
$ |
(1.04 |
) |
|
$ |
(0.87 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average common shares outstanding, basic and diluted |
|
|
30,267 |
|
|
|
28,876 |
|
|
|
29,931 |
|
|
|
28,505 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NEURONETICS, INC.
Balance Sheets
(Unaudited; In thousands, except per share data)
|
|
|
|
|
|
|
|
|
| |
|
September 30,
2024 |
|
|
December 31,
2023 |
|
| Assets |
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
20,867 |
|
|
$ |
59,677 |
|
| Accounts receivable, net |
|
|
16,825 |
|
|
|
15,782 |
|
| Inventory |
|
|
4,960 |
|
|
|
8,093 |
|
| Current portion of net investments in sales-type leases |
|
|
572 |
|
|
|
905 |
|
| Current portion of prepaid commission expense |
|
|
2,921 |
|
|
|
2,514 |
|
| Current portion of note receivables |
|
|
2,477 |
|
|
|
2,056 |
|
| Prepaid expenses and other current assets |
|
|
4,837 |
|
|
|
4,766 |
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
53,459 |
|
|
|
93,793 |
|
|
|
|
|
|
|
|
|
|
| Property and equipment, net |
|
|
1,639 |
|
|
|
2,009 |
|
| Operating lease
right-of-use assets |
|
|
2,328 |
|
|
|
2,773 |
|
| Net investments in sales-type leases |
|
|
140 |
|
|
|
661 |
|
| Prepaid commission expense |
|
|
8,733 |
|
|
|
8,370 |
|
| Long-term notes receivable |
|
|
2,878 |
|
|
|
3,795 |
|
| Other assets |
|
|
4,940 |
|
|
|
4,430 |
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
$ |
74,117 |
|
|
$ |
115,831 |
|
|
|
|
|
|
|
|
|
|
| Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
3,295 |
|
|
$ |
4,752 |
|
| Accrued expenses |
|
|
11,429 |
|
|
|
12,595 |
|
| Deferred revenue |
|
|
1,311 |
|
|
|
1,620 |
|
| Current portion of operating lease liabilities |
|
|
862 |
|
|
|
845 |
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
16,897 |
|
|
|
19,812 |
|
|
|
|
|
|
|
|
|
|
| Long-term debt, net |
|
|
46,002 |
|
|
|
59,283 |
|
| Deferred revenue |
|
|
4 |
|
|
|
200 |
|
| Operating lease liabilities |
|
|
1,833 |
|
|
|
2,346 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
64,736 |
|
|
|
81,641 |
|
|
|
|
|
|
|
|
|
|
| Commitments and contingencies (Note 18) |
|
|
— |
|
|
|
— |
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
|
| Preferred stock, $0.01 par value: 10,000 shares authorized; no shares issued or outstanding on
September 30, 2024 and December 31, 2023 |
|
|
— |
|
|
|
— |
|
| Common stock, $0.01 par value: 200,000 shares authorized; 30,317 and 29,092 shares issued and
outstanding on September 30, 2024 and December 31, 2023, respectively |
|
|
303 |
|
|
|
291 |
|
| Additional paid-in capital |
|
|
416,205 |
|
|
|
409,980 |
|
| Accumulated deficit |
|
|
(407,127 |
) |
|
|
(376,081 |
) |
|
|
|
|
|
|
|
|
|
| Total Stockholders’ equity |
|
|
9,381 |
|
|
|
34,190 |
|
|
|
|
|
|
|
|
|
|
| Total liabilities and Stockholders’ equity |
|
$ |
74,117 |
|
|
$ |
115,831 |
|
|
|
|
|
|
|
|
|
|
NEURONETICS, INC.
Statements of Cash Flows
(Unaudited; In thousands)
|
|
|
|
|
|
|
|
|
| |
|
Nine months ended September 30, |
|
| |
|
2024 |
|
|
2023 |
|
| Cash flows from Operating activities: |
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(31,046 |
) |
|
$ |
(24,812 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
| Depreciation and amortization |
|
|
1,627 |
|
|
|
1,503 |
|
| Allowance for credit losses |
|
|
1,947 |
|
|
|
369 |
|
| Inventory impairment |
|
|
346 |
|
|
|
1,905 |
|
| Share-based compensation |
|
|
4,320 |
|
|
|
5,693 |
|
| Non-cash interest expense |
|
|
580 |
|
|
|
460 |
|
| Loss on extinguishment of debt |
|
|
4,427 |
|
|
|
— |
|
| Changes in certain assets and liabilities: |
|
|
|
|
|
|
|
|
| Accounts receivable, net |
|
|
(3,834 |
) |
|
|
(7,933 |
) |
| Inventory |
|
|
2,718 |
|
|
|
(2,742 |
) |
| Net investment in sales-type leases |
|
|
854 |
|
|
|
1,092 |
|
| Prepaid commission expense |
|
|
(770 |
) |
|
|
(804 |
) |
| Prepaid expenses and other assets |
|
|
(374 |
) |
|
|
(3,338 |
) |
| Accounts payable |
|
|
(1,524 |
) |
|
|
54 |
|
| Accrued expenses |
|
|
(1,166 |
) |
|
|
(4,801 |
) |
| Deferred revenue |
|
|
(506 |
) |
|
|
(817 |
) |
|
|
|
|
|
|
|
|
|
| Net Cash used in Operating activities |
|
|
(22,401 |
) |
|
|
(34,171 |
) |
|
|
|
|
|
|
|
|
|
| Cash flows from Investing activities: |
|
|
|
|
|
|
|
|
| Purchases of property and equipment and capitalized software |
|
|
(1,377 |
) |
|
|
(1,490 |
) |
| Repayment of notes receivable |
|
|
1,340 |
|
|
|
731 |
|
|
|
|
|
|
|
|
|
|
| Net Cash used in Investing activities |
|
|
(37 |
) |
|
|
(759 |
) |
|
|
|
|
|
|
|
|
|
| Cash flows from Financing activities: |
|
|
|
|
|
|
|
|
| Payments of debt issuance costs |
|
|
(2,188 |
) |
|
|
(863 |
) |
| Proceeds from issuance of long-term debt |
|
|
48,084 |
|
|
|
2,500 |
|
| Proceeds from issuance of warrants |
|
|
1,916 |
|
|
|
— |
|
| Repayment of long-term debt |
|
|
(60,000 |
) |
|
|
(1,200 |
) |
| Payment for debt extinguishment cost |
|
|
(4,185 |
) |
|
|
— |
|
| Proceeds from exercises of stock options |
|
|
1 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Net Cash (used in) provided by Financing activities |
|
|
(16,372 |
) |
|
|
437 |
|
|
|
|
|
|
|
|
|
|
| Net decrease in Cash and Cash equivalents |
|
|
(38,810 |
) |
|
|
(34,493 |
) |
| Cash and Cash equivalents, Beginning of Period |
|
|
59,677 |
|
|
|
70,340 |
|
|
|
|
|
|
|
|
|
|
| Cash and Cash equivalents, End of Period |
|
$ |
20,867 |
|
|
$ |
35,847 |
|
|
|
|
|
|
|
|
|
|
Non-GAAP Financial Measures (Unaudited)
EBITDA is not a measure of financial performance under generally accepted accounting principles in the U.S. (“GAAP”), and should not be construed as
a substitute for, or superior to, GAAP net loss. However, management uses both the GAAP and non-GAAP financial measures internally to evaluate and manage the Company’s operations and to better understand
its business. Further, management believes that the addition of the non-GAAP financial measure provides meaningful supplementary information to, and facilitates analysis by, investors in evaluating the
Company’s financial performance, results of operations and trends. The Company’s calculation of EBITDA may not be comparable to similarly designated measures reported by other companies, because companies and investors may differ as to
what type of events warrant adjustment.
The following table reconciles reported net loss to EBITDA:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months ended
September 30, |
|
|
Nine months ended
September 30, |
|
| |
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
| |
|
(in thousands) |
|
|
(in thousands) |
|
| Net loss |
|
$ |
(13,341 |
) |
|
$ |
(9,391 |
) |
|
$ |
(31,046 |
) |
|
$ |
(24,812 |
) |
| Interest expense, net |
|
|
1,186 |
|
|
|
1,184 |
|
|
|
3,528 |
|
|
|
3,580 |
|
| Income taxes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Depreciation and amortization |
|
|
512 |
|
|
|
500 |
|
|
|
1,627 |
|
|
|
1,503 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| EBITDA |
|
$ |
(11,643 |
) |
|
$ |
(7,707 |
) |
|
$ |
(25,891 |
) |
|
$ |
(19,729 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

Exhibit 99.2 COMPANY PRESENTATION NASDAQ: STIM November 2024 Now
FDA-Cleared as an Adjunct Therapy for Ages 15 to 21!

Forward Looking Statements This presentation contains estimates and
other statistical data prepared by independent parties and by Neuronetics, Inc. (“Neuronetics” or the “Company”) relating to market size and growth and other data about the industry in which the Company operates. These
estimates and data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates and data. Certain statements in this presentation, including the documents incorporated by reference herein, include
“forward-looking statements” within the meaning of U.S. federal securities laws. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking
statements may be identified by words or expressions such as “expect”, “anticipate”, “intend”, “plan”, “believe”, “estimate”, “may”, “will”,
“project”, “could”, “should”, “would”, “seek”, “forecast”, “expect”, “anticipate”, “predict”, “outlook”, “potential”,
or other similar expressions, including without limitation the negative of these terms. Forward-looking statements represent current judgments about possible future events, including, but not limited to statements regarding expectations or forecasts
of business, operations, financial performance, prospects, and other plans, intentions, expectations, estimates, and beliefs relating to the proposed transaction between Greenbrook TMS Inc. (“Greenbrook” or “Greenbrook TMS”)
and Neuronetics, such as statements regarding the combined operations and prospects of Greenbrook and Neuronetics, estimates of pro forma financial information of the combined company, the current and projected market, growth opportunities and
synergies for the combined company, federal and state regulatory tailwinds, the expected cash balance of Greenbrook at the time of the closing of the proposed Arrangement (as such term is defined in the Neuronetics definitive proxy statement),
expectations regarding Neuronetics’ ability to leverage Greenbrook’s assets, the expected composition of the management and the board of directors of the combined company, gross margin and future profitability expectations, and the
timing and completion of the Arrangement, including the satisfaction or waiver of all the required conditions thereto. These forward-looking statements are based upon the current beliefs and expectations of the management of Neuronetics and are
subject to known and unknown risks and uncertainties. Factors that could cause actual events to differ include, but are not limited to: • the inherent uncertainty associated with financial or other projections or outlooks, including due to the
unpredictability of the underlying assumptions, adjustments and estimates; • Neuronetics’ ability to maintain the listing requirements of Nasdaq; • the total addressable market of Neuronetics’ and Greenbrook’s
businesses; • general economic conditions in the markets where Neuronetics and Greenbrook operate; • the expected timing of any regulatory approvals relating to the Arrangement, the businesses of Greenbrook and Neuronetics and of the
combined company and product launches of such businesses and companies; • the non-performance of third-party vendors and contractors; • the risks related to the combined company’s ability to successfully sell its products and the
market reception to and performance of its products; • Greenbrook’s, Neuronetics’, and the combined company’s compliance with, and changes to, applicable laws and regulations; • the combined company’s limited
operating history; • the combined company’s ability to manage growth; • the combined company’s ability to obtain additional or suitable financing; • the combined company’s ability to expand product offerings;
• the combined company’s ability to compete with others in its industry; • the combined company’s ability to protect its intellectual property; • the retention of employees of Greenbrook and Neuronetics following the
announcement of the Arrangement; 2

Forward Looking Statements (continued) • Greenbrook’s,
Neuronetics’, and the combined company’s ability to defend against legal proceedings; • the combined company’s success in retaining or recruiting, or changes required in, its officers, key employees or directors; • the
combined company’s ability to achieve the expected benefits from the Arrangement within the expected time frames or at all; • the incurrence of unexpected costs, liabilities or delays relating to the proposed Arrangement; • the
satisfaction (or waiver) of closing conditions to the consummation of the Arrangement; • the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the Arrangement Agreement (as such term
is defined in the Neuronetics definitive proxy statement); • the disruption of the attention of management of Greenbrook and Neuronetics from ongoing business operations due to the Arrangement Agreement; • the outcome of any legal
proceedings related to the Arrangement Agreement; • the fact that the trading price of the Greenbrook Shares or the Neuronetics Shares may decline significantly if the Arrangement is not completed; • the effect of the announcement or
pendency of the transaction on the combined company’s business relationships, operating results and business generally; and • other economic, business, competitive, and regulatory factors affecting the businesses of the companies
generally, including, but not limited to, those set forth in Greenbrook’s filings with the SEC and the Canadian Securities Administrators, including in the “Risk Factors” section of the Greenbrook 10-K and any subsequent filings
with the U.S. Securities and Exchange Commission (the “SEC”) and the Canadian Securities Administrators, and those set forth in Neuronetics’ filings with the SEC, including in the “Risk Factors” section of
Neuronetics’ Annual Report on Form 10-K filed with the SEC on March 8, 2024 and any subsequent SEC filings. These documents with respect to Greenbrook can be accessed on Greenbrook’s website at
https://www.greenbrooktms.com/investor-relations, on Greenbrook’s SEDAR+ profile at www.sedarplus.ca or on Greenbrook’s EDGAR profile at www.sec.gov and these documents with respect to Neuronetics can be accessed on Neuronetics’
website at https://ir.neuronetics.com/ or on Neuronetics’ EDGAR profile at www.sec.gov. Readers are cautioned not to place undue reliance on forward-looking statements. It is uncertain whether any of the events anticipated by the
forward-looking statements will transpire or occur, or, if any of them do, what impact they will have on the results of operations and financial condition of Greenbrook, Neuronetics or the combined company. Forward-looking statements speak only as
of the date they are made, and Greenbrook, Neuronetics and the combined company undertake no obligation to update publicly or otherwise revise any forward-looking statements, whether as a result of new information, future events, or other factors
that affect the subject of these statements, except where they are expressly required to do so by law. Projections and estimates used in this presentation are considered forward looking statements. See cautionary statement above regarding
forward-looking statements. Forward-looking information representing post-closing expectations is inherently uncertain. Estimates such as expected accretion, expected future production, internal rate of return, financial flexibility and balance
sheet strength are preliminary in nature. There can be no assurance that the proposed Arrangement will close or that the forward-looking information will prove to be accurate. 3

n e u r o s t a r . c o m Presenters 38+ years of experience 37+ years
of experience Keith Sullivan Steve Furlong President & Executive Vice President, Chief Executive Officer Chief Financial Officer & Treasurer 4
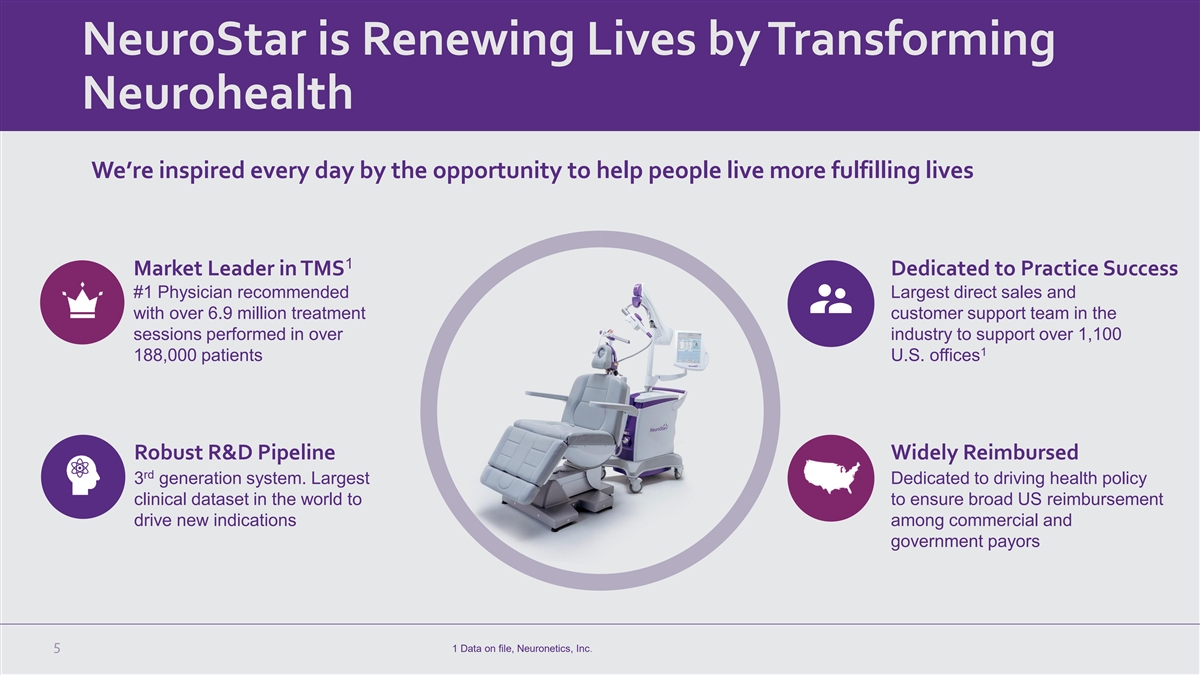
NeuroStar is Renewing Lives by Transforming Neurohealth We’re
inspired every day by the opportunity to help people live more fulfilling lives 1 Market Leader in TMS Dedicated to Practice Success #1 Physician recommended Largest direct sales and with over 6.9 million treatment customer support team in the
sessions performed in over industry to support over 1,100 1 188,000 patients U.S. offices Robust R&D Pipeline Widely Reimbursed rd 3 generation system. Largest Dedicated to driving health policy clinical dataset in the world to to ensure broad
US reimbursement drive new indications among commercial and government payors 5 1 Data on file, Neuronetics, Inc.
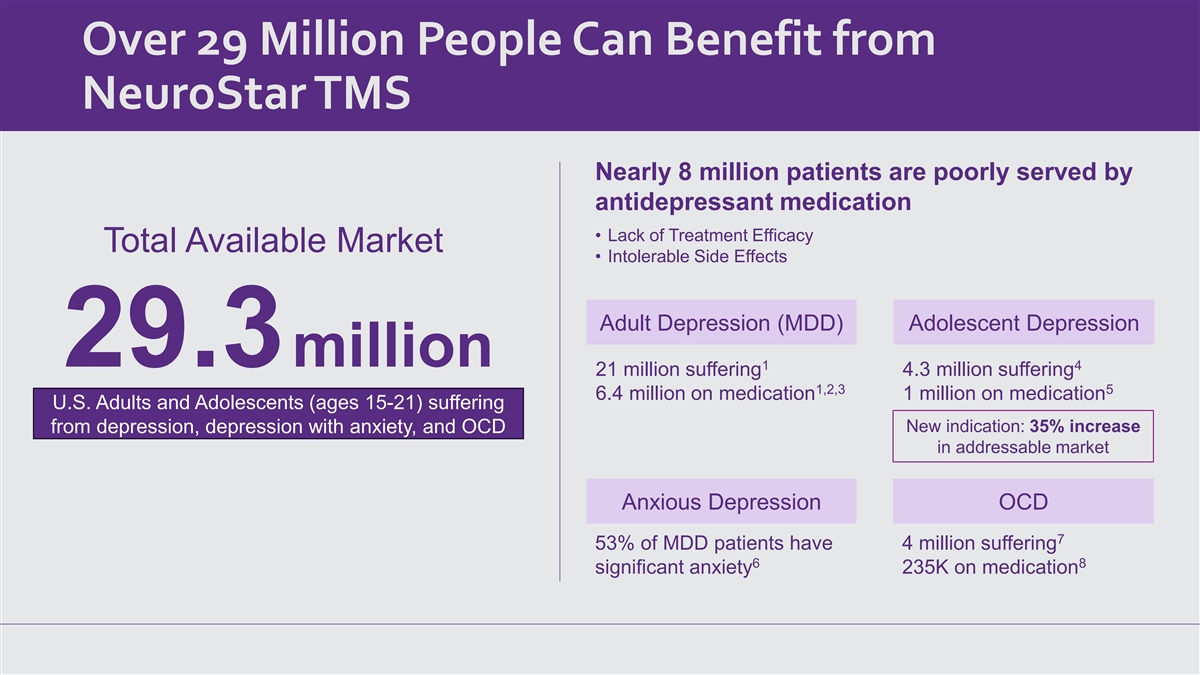
Over 29 Million People Can Benefit from NeuroStar TMS Nearly 8 million
patients are poorly served by antidepressant medication • Lack of Treatment Efficacy Total Available Market • Intolerable Side Effects Adult Depression (MDD) Adolescent Depression 29.3million 1 4 21 million suffering 4.3 million
suffering 1,2,3 5 6.4 million on medication 1 million on medication U.S. Adults and Adolescents (ages 15-21) suffering New indication: 35% increase from depression, depression with anxiety, and OCD in addressable market Anxious Depression OCD 7 53%
of MDD patients have 4 million suffering 6 8 significant anxiety 235K on medication
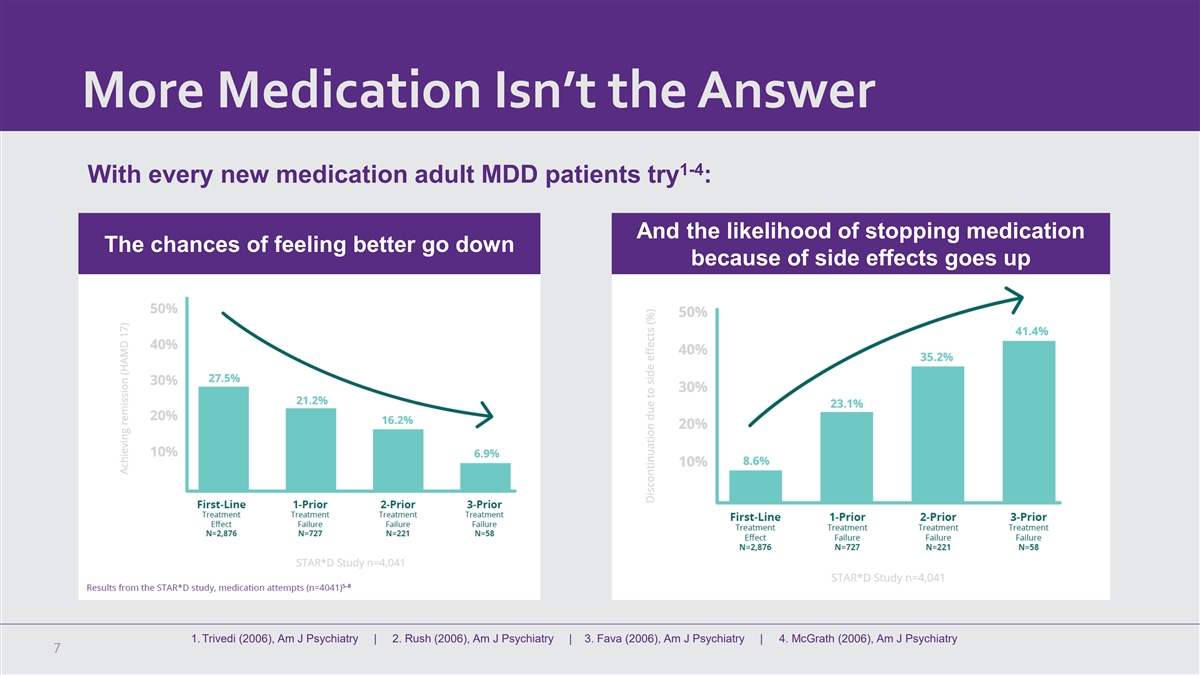
More Medication Isn’t the Answer 1-4 With every new medication
adult MDD patients try : And the likelihood of stopping medication The chances of feeling better go down because of side effects goes up 1. Trivedi (2006), Am J Psychiatry | 2. Rush (2006), Am J Psychiatry | 3. Fava (2006), Am J Psychiatry | 4.
McGrath (2006), Am J Psychiatry 7
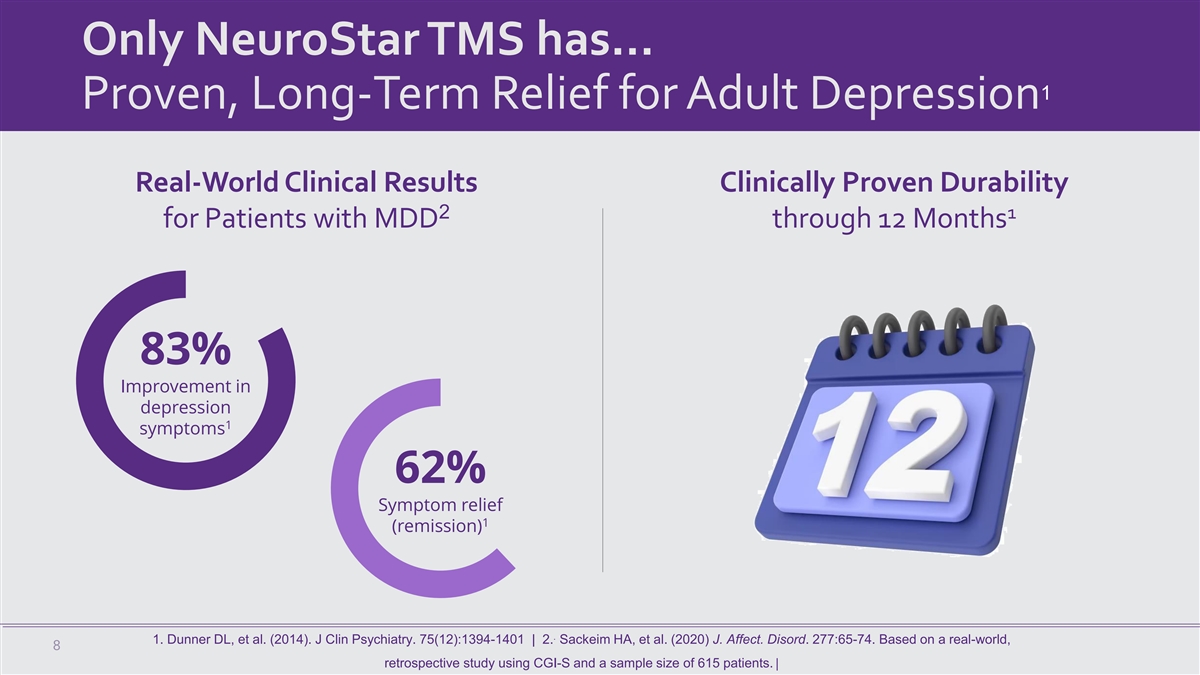
Only NeuroStar TMS has… 1 Proven, Long-Term Relief for Adult
Depression Real-World Clinical Results Clinically Proven Durability 2 1 for Patients with MDD through 12 Months 83% Improvement in depression 1 symptoms 62% Symptom relief 1 (remission) . 1. Dunner DL, et al. (2014). J Clin Psychiatry.
75(12):1394-1401 | 2. Sackeim HA, et al. (2020) J. Affect. Disord. 277:65-74. Based on a real-world, 8 retrospective study using CGI-S and a sample size of 615 patients. |

NeuroStar Clinical Excellence Validated by Extensive Research and
Publications Investigator Initiated Studies Largest Real-World Sample Sizes in TMS 1 Research Expands understanding of TMS and its potential clinical applications 1 6 High Impact NeuroStar Registry 65+ studies with 1,900+ patients 2-7 Publications
9
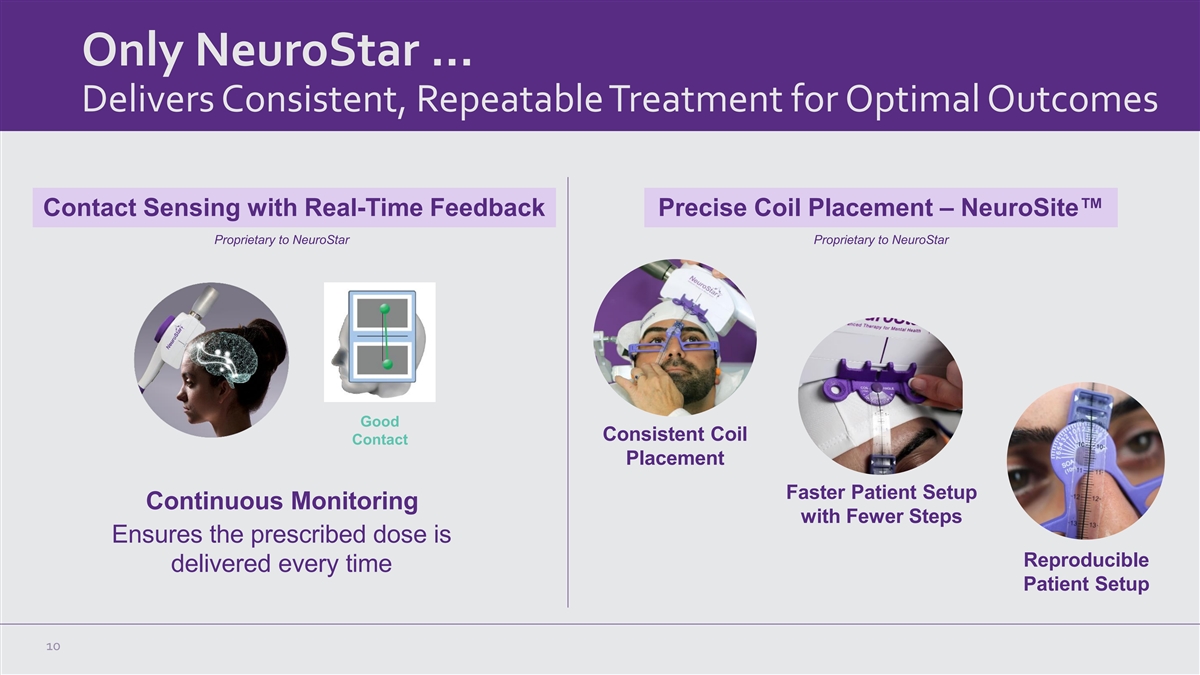
Only NeuroStar … Delivers Consistent, Repeatable Treatment for
Optimal Outcomes Contact Sensing with Real-Time Feedback Precise Coil Placement – NeuroSite™ Proprietary to NeuroStar Proprietary to NeuroStar Good Consistent Coil Contact Placement Faster Patient Setup Continuous Monitoring with Fewer
Steps Ensures the prescribed dose is Reproducible delivered every time Patient Setup 10
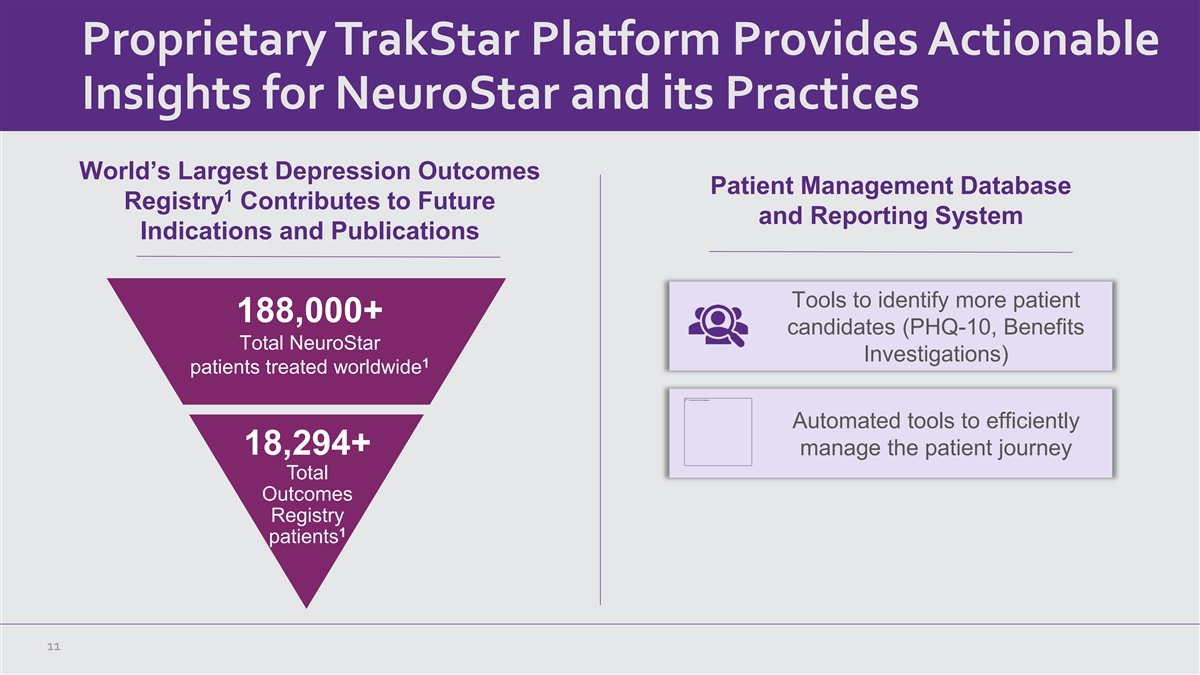
Proprietary TrakStar Platform Provides Actionable Insights for
NeuroStar and its Practices World’s Largest Depression Outcomes Patient Management Database 1 Registry Contributes to Future and Reporting System Indications and Publications Tools to identify more patient 188,000+ candidates (PHQ-10, Benefits
Total NeuroStar Investigations) 1 patients treated worldwide Automated tools to efficiently 18,294+ manage the patient journey Total Outcomes Registry 1 patients 11

NeuroStar Has one of the Largest Issued Patent Portfolio of All TMS
Companies… Patent Portfolio Contact Sensing MT Assist Iron Core Magnet U.S. patent U.S. patent U.S. patent • 33 US / 53 OUS Issued or allowed patents • 10 US / 6 OUS Pending patent applications …protecting our technical
advantage and ensuring freedom to operate globally 12
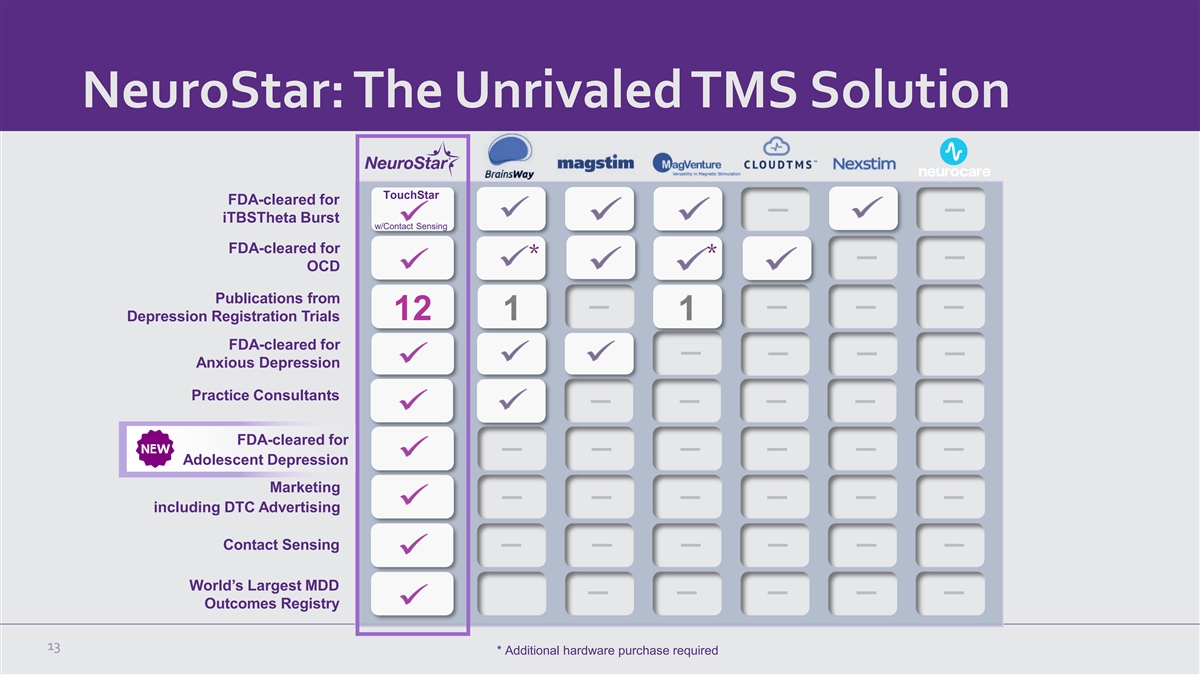
NeuroStar: The Unrivaled TMS Solution TouchStar FDA-cleared for
iTBSTheta Burst w/Contact Sensing FDA-cleared for * * OCD Publications from 12 1 1 Depression Registration Trials FDA-cleared for Anxious Depression Practice Consultants FDA-cleared for Adolescent Depression Marketing including DTC Advertising
Contact Sensing World’s Largest MDD Outcomes Registry 13 * Additional hardware purchase required

TAP INTO A NEW POSSIBILITY for adolescent depression Now FDA-Cleared as
a First-Line, Adjunct Treatment for Ages 15 and Older NEW: FDA-Cleared March 22, 2024

Treating Adolescent Depression Had Two Choices… Until Now Youth
Depression is On the Rise Limited Options for Adolescents • Only 2 antidepressants are FDA-approved • Nearly 1 in 5 US adolescents experience at 1 for use in adolescents: Prozac (fluoxetine) least one major depressive episode each year 3
and Lexapro (escitalopram) • Mental health concerns top parents’ list of 2 • The FDA has issued a “black box” warning worries, even above children’s physical safety indicating the use of these drugs to treat MDD
in adolescents may increase the risk of 4 suicidal ideations and behaviors 1 National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/major-depression#part_2565. | 2. new polling from the Pew Research Center of 3,757 U.S.
parents with children under 18 October 9, 2023 15 | 3 FDA.gov.. https://www.fda.gov/consumers/womens-health-topics/depression-medicines#. 4 AMA Journal of Ethics.
https://journalofethics.ama-assn.org/article/black-box-blues-kids-and-antidepressants/2005

As the Market Leader, NeuroStar is Revolutionizing Mental Health with
New Adolescent Indication NeuroStar is the only FDA-cleared TMS st 1 and Only to Market 1 treatment for adolescent depression For adolescents, NeuroStar can be used as an st add-on treatment, without prior medication 1 Line Treatment failures 1. FDA
Clearance K231926
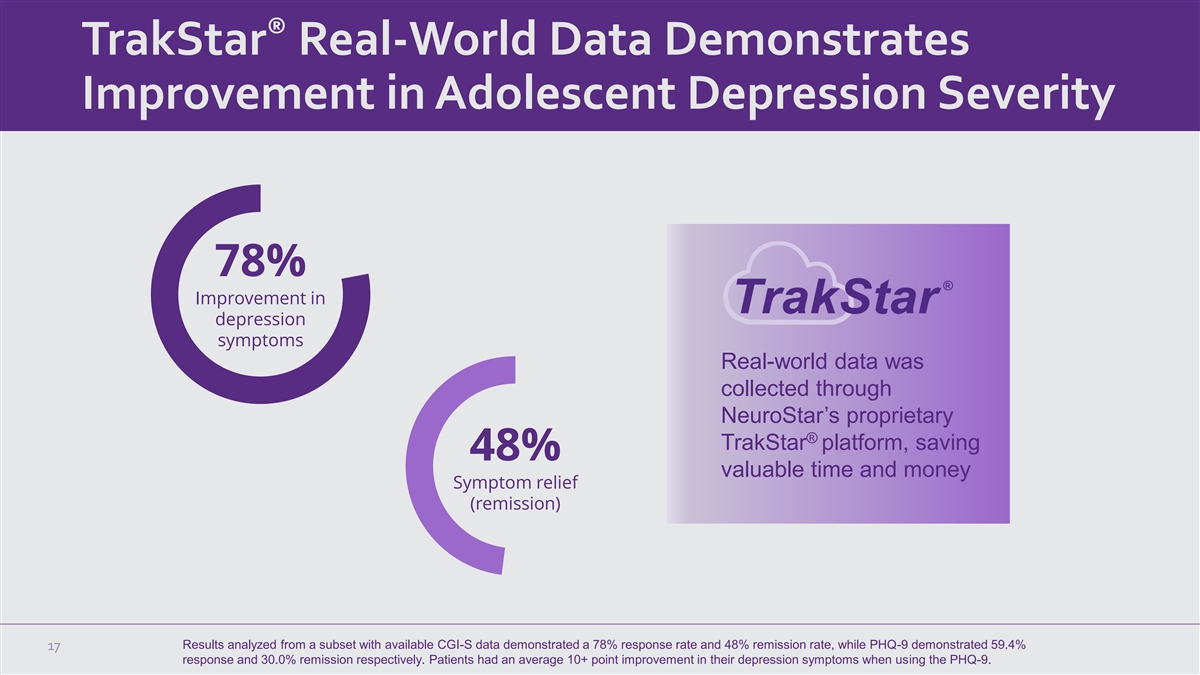
® TrakStar Real-World Data Demonstrates Improvement in Adolescent
Depression Severity 78% Improvement in depression symptoms Real-world data was collected through NeuroStar’s proprietary ® TrakStar platform, saving 48% valuable time and money Symptom relief (remission) Results analyzed from a subset
with available CGI-S data demonstrated a 78% response rate and 48% remission rate, while PHQ-9 demonstrated 59.4% 17 response and 30.0% remission respectively. Patients had an average 10+ point improvement in their depression symptoms when using the
PHQ-9.
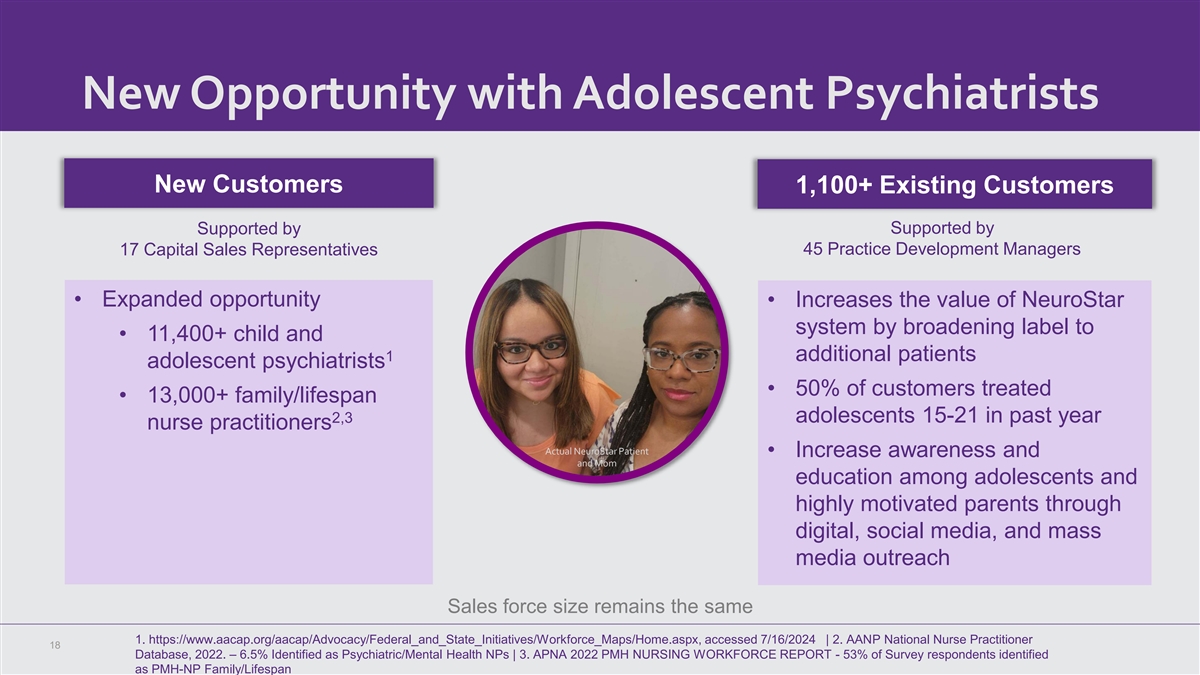
New Opportunity with Adolescent Psychiatrists New Customers 1,100+
Existing Customers Supported by Supported by 17 Capital Sales Representatives 45 Practice Development Managers • Expanded opportunity • Increases the value of NeuroStar system by broadening label to • 11,400+ child and 1 additional
patients adolescent psychiatrists • 50% of customers treated • 13,000+ family/lifespan 2,3 adolescents 15-21 in past year nurse practitioners Actual NeuroStar Patient • Increase awareness and and Mom education among adolescents and
highly motivated parents through digital, social media, and mass media outreach Sales force size remains the same 1. https://www.aacap.org/aacap/Advocacy/Federal_and_State_Initiatives/Workforce_Maps/Home.aspx, accessed 7/16/2024 | 2. AANP National
Nurse Practitioner 18 Database, 2022. – 6.5% Identified as Psychiatric/Mental Health NPs | 3. APNA 2022 PMH NURSING WORKFORCE REPORT - 53% of Survey respondents identified as PMH-NP Family/Lifespan

2024 Adolescent Marketing Strategy Focused on Parents and Practices Key
Messages Treats depression at the source | Non-drug, non-invasive | Proven safe and effective Outside the Practice Inside the Practice Parent Awareness & Education Practice Tools & Education Patient Advocate Collaborations
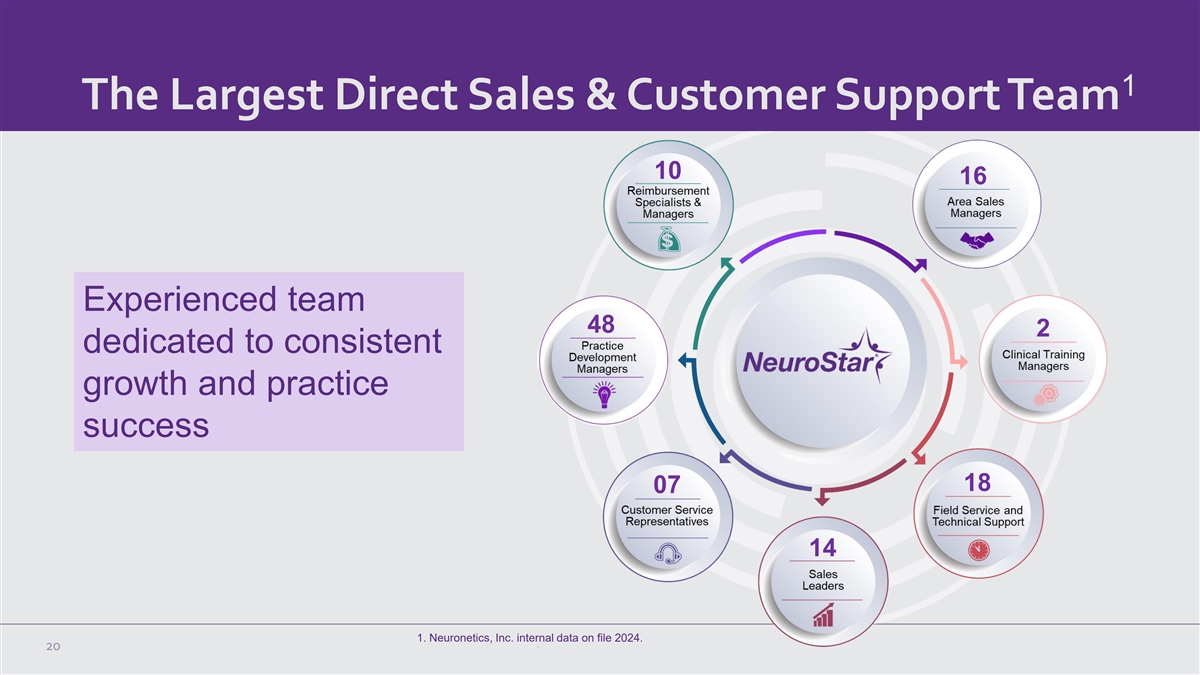
1 The Largest Direct Sales & Customer Support Team 10 16
Experienced team 48 2 dedicated to consistent growth and practice success 18 07 14 1. Neuronetics, Inc. internal data on file 2024. 20
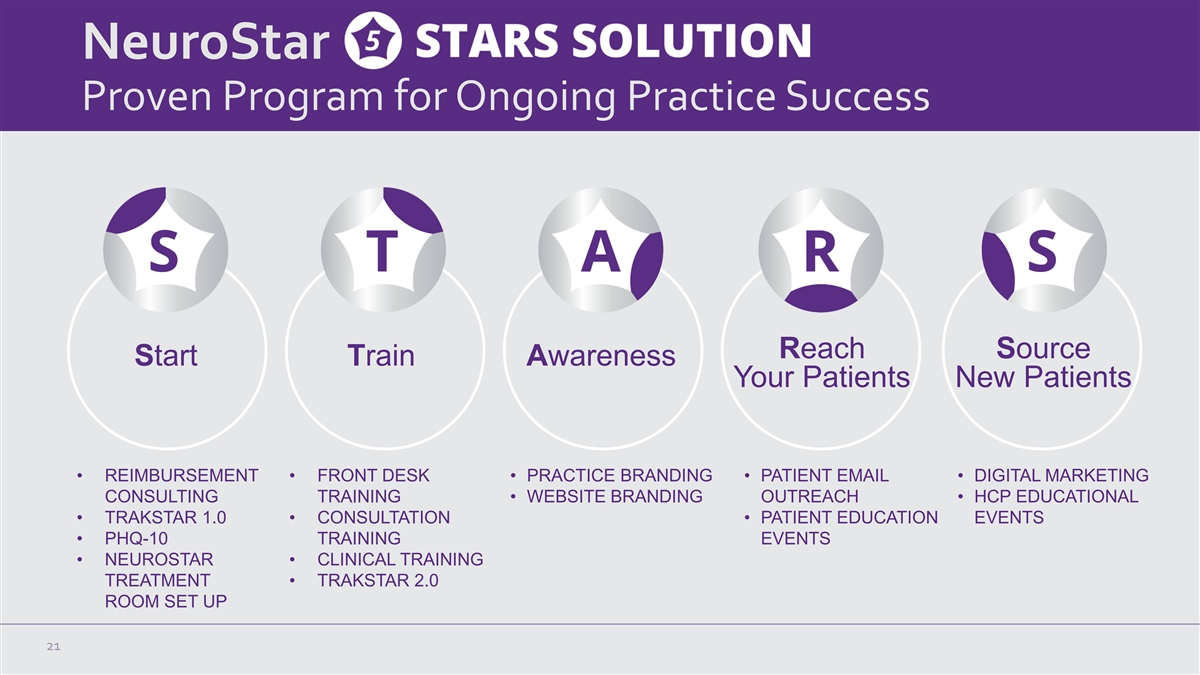
NeuroStar Proven Program for Ongoing Practice Success Reach Source
Start Train Awareness Your Patients New Patients • REIMBURSEMENT • FRONT DESK • PRACTICE BRANDING • PATIENT EMAIL • DIGITAL MARKETING CONSULTING TRAINING • WEBSITE BRANDING OUTREACH • HCP EDUCATIONAL •
TRAKSTAR 1.0 • CONSULTATION • PATIENT EDUCATION EVENTS • PHQ-10 TRAINING EVENTS • NEUROSTAR • CLINICAL TRAINING TREATMENT • TRAKSTAR 2.0 ROOM SET UP 21
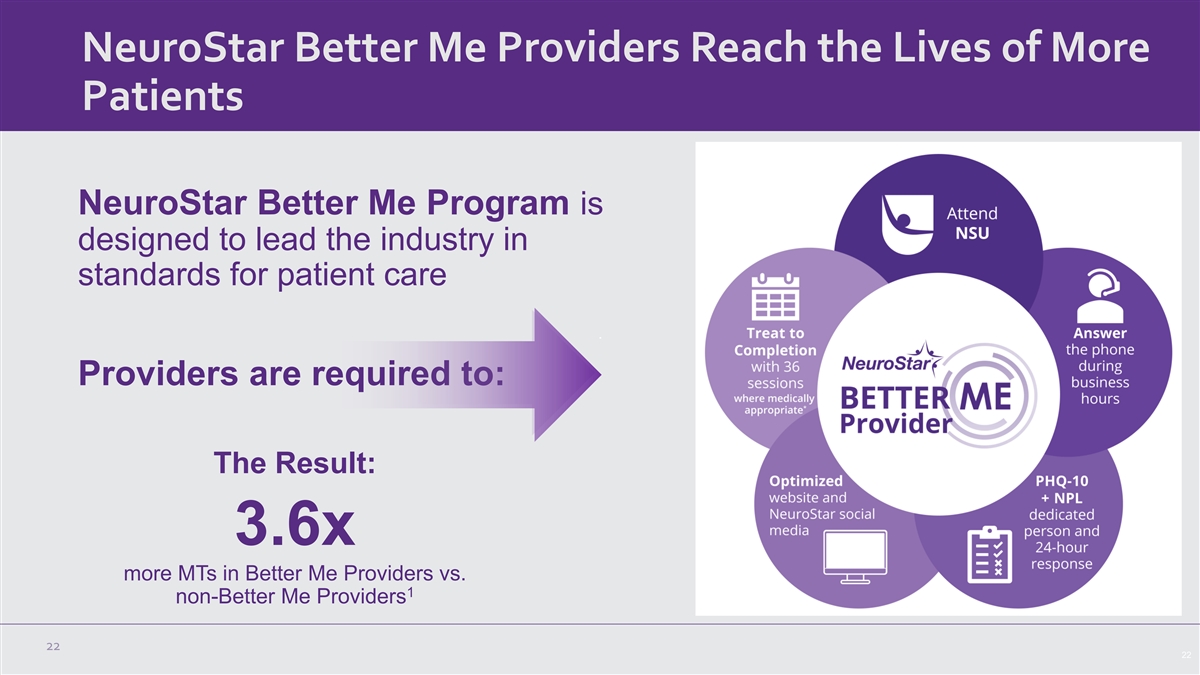
NeuroStar Better Me Providers Reach the Lives of More Patients
NeuroStar Better Me Program is designed to lead the industry in standards for patient care Providers are required to: The Result: 3.6x more MTs in Better Me Providers vs. 1 non-Better Me Providers 22 22
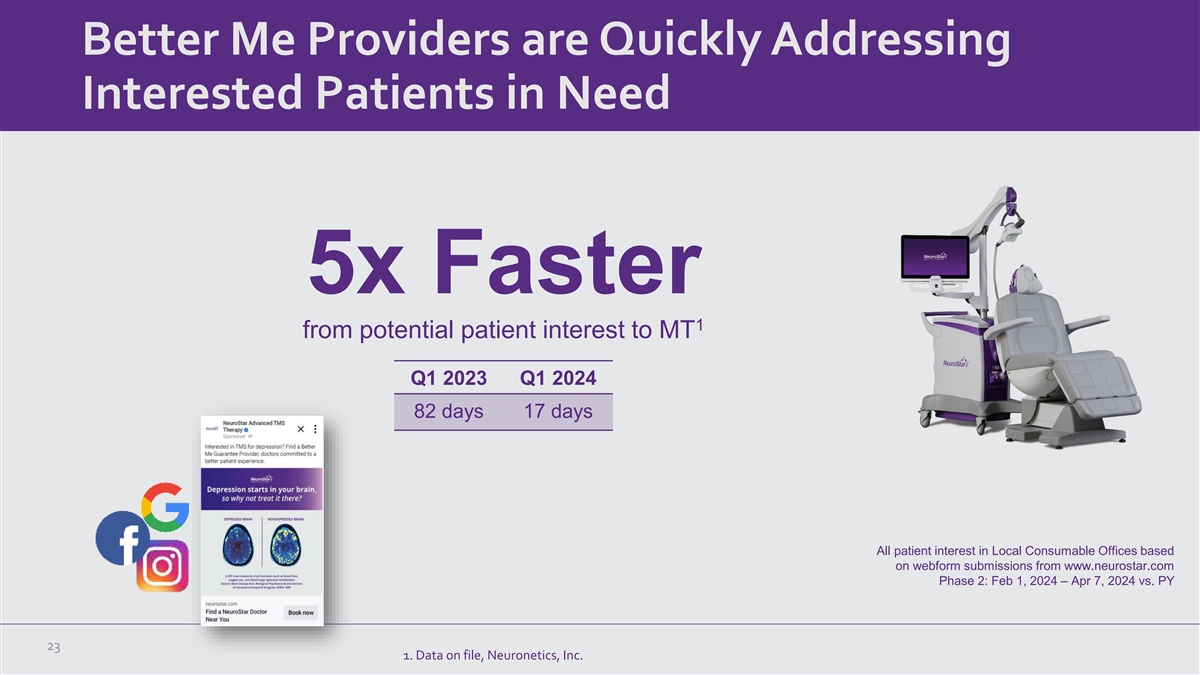
Better Me Providers are Quickly Addressing Interested Patients in Need
5x Faster 1 from potential patient interest to MT Q1 2023 Q1 2024 82 days 17 days All patient interest in Local Consumable Offices based on webform submissions from www.neurostar.com Phase 2: Feb 1, 2024 – Apr 7, 2024 vs. PY 23 1. Data on
file, Neuronetics, Inc.
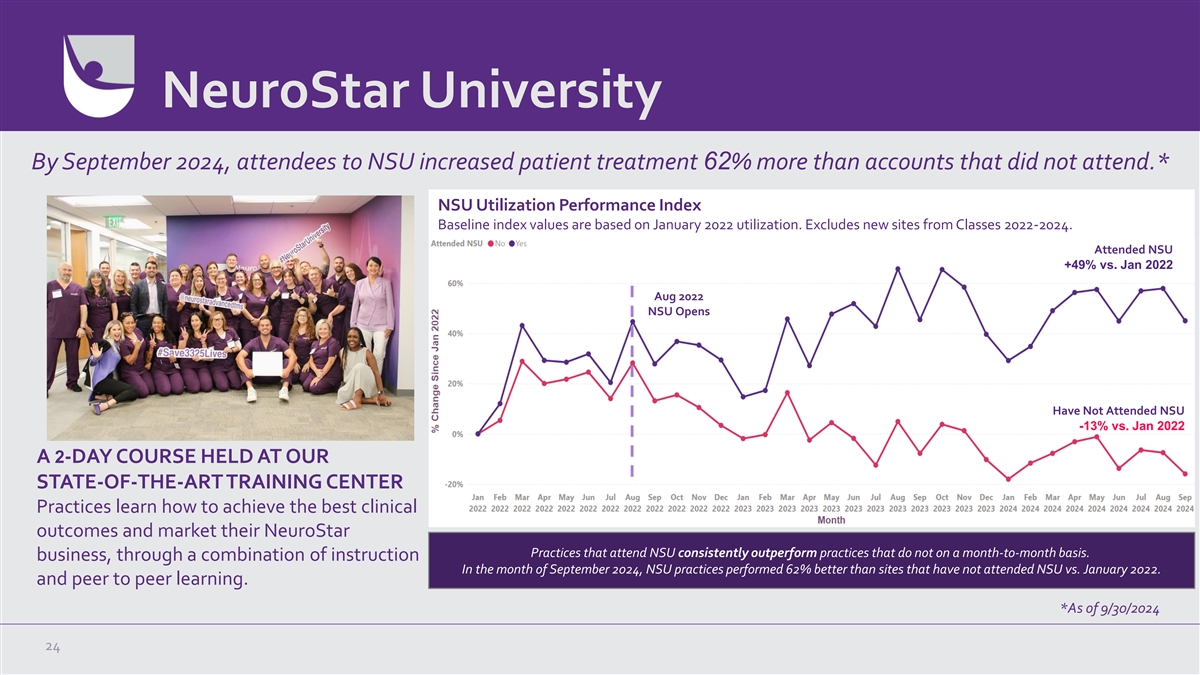
NeuroStar University By September 2024, attendees to NSU increased
patient treatment 62% more than accounts that did not attend.* NSU Utilization Performance Index Baseline index values are based on January 2022 utilization. Excludes new sites from Classes 2022-2024. Attended NSU +49% vs. Jan 2022 Aug 2022 NSU
Opens Have Not Attended NSU -13% vs. Jan 2022 A 2-DAY COURSE HELD AT OUR STATE-OF-THE-ART TRAINING CENTER Practices learn how to achieve the best clinical outcomes and market their NeuroStar Practices that attend NSU consistently outperform
practices that do not on a month-to-month basis. business, through a combination of instruction In the month of September 2024, NSU practices performed 62% better than sites that have not attended NSU vs. January 2022. and peer to peer learning. *As
of 9/30/2024 24
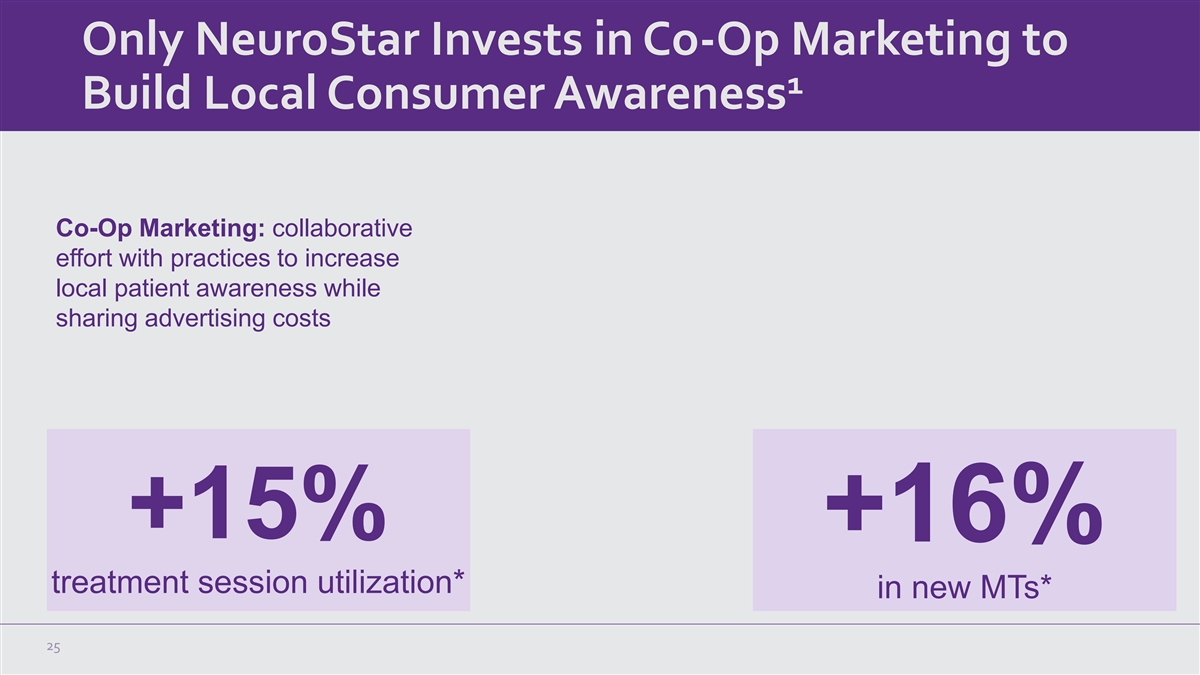
Only NeuroStar Invests in Co-Op Marketing to 1 Build Local Consumer
Awareness Co-Op Marketing: collaborative effort with practices to increase local patient awareness while sharing advertising costs +15% +16% treatment session utilization* in new MTs* 25

NeuroStar is Available at the Largest National Mental Health Centers
26

Only NeuroStar is Dedicated to Driving Health Policy to Ensure Broad US
Reimbursement NeuroStar also connects our practice partners NeuroStar TMS is covered by most commercial with financing companies to provide options for insurance carriers, all Medicare jurisdictions and many patients who have insurance out-of-pocket
costs or opt to pay for the procedure themselves. state Medicaid programs providing access to more 1 than 300 million people NeuroStar is driving adolescent policy expansion and gaining momentum! 1. Neuronetics, Inc. internal data on file 2024.
27
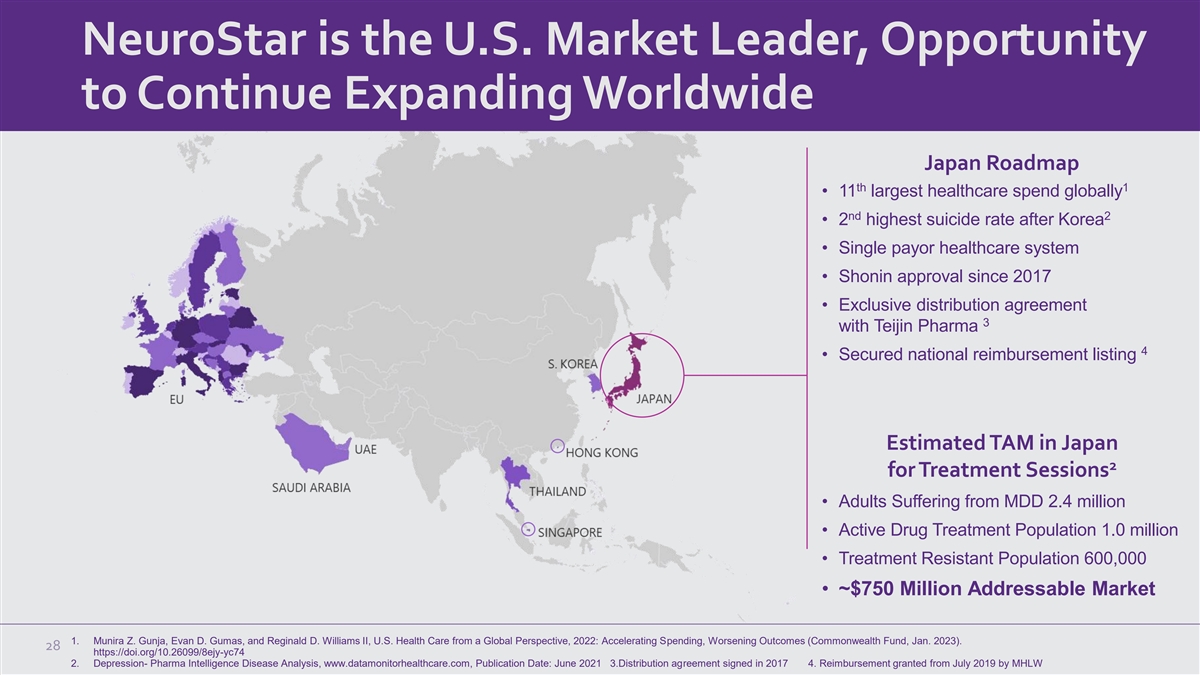
NeuroStar is the U.S. Market Leader, Opportunity to Continue Expanding
Worldwide Japan Roadmap th 1 • 11 largest healthcare spend globally nd 2 • 2 highest suicide rate after Korea • Single payor healthcare system • Shonin approval since 2017 • Exclusive distribution agreement 3 with
Teijin Pharma 4 • Secured national reimbursement listing Estimated TAM in Japan 2 for Treatment Sessions • Adults Suffering from MDD 2.4 million • Active Drug Treatment Population 1.0 million • Treatment Resistant Population
600,000 • ~$750 Million Addressable Market 1. Munira Z. Gunja, Evan D. Gumas, and Reginald D. Williams II, U.S. Health Care from a Global Perspective, 2022: Accelerating Spending, Worsening Outcomes (Commonwealth Fund, Jan. 2023). 28
https://doi.org/10.26099/8ejy-yc74 2. Depression- Pharma Intelligence Disease Analysis, www.datamonitorhealthcare.com, Publication Date: June 2021 3.Distribution agreement signed in 2017 4. Reimbursement granted from July 2019 by MHLW

Keith Sullivan Cory Anderson Steve Furlong Rick Grubbs Sheryl Conley
Rob Cascella Glenn Muir President & CEO SVP, R&D and Clinical EVP, CFO & Treasurer SVP, National Board Chairman Accounts Megan Andrew Macan Rusty Page Lisa Rosas Sara Grubbs Keith Sullivan EVP, GC & Chief SVP, Chief Rosengarten SVP,
Operations SVP, Chief Revenue Officer Compliance Officer & Quality Marketing Officer 29

Financial Overview NeuroStar Advanced Therapy for Mental Health
30
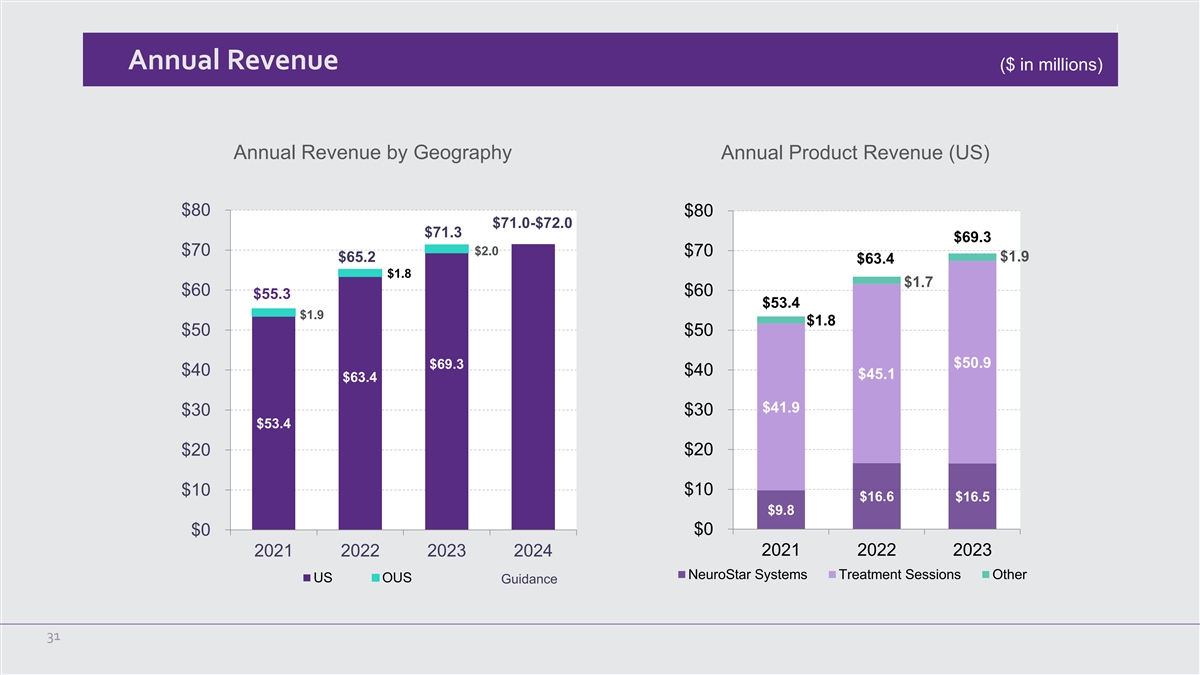
Annual Revenue ($ in millions) Annual Revenue by Geography Annual
Product Revenue (US) $80 $80 $71.0-$72.0 $71.3 $69.3 $2.0 $70 $70 $1.9 $65.2 $63.4 $1.8 $1.7 $60 $60 $55.3 $53.4 $1.9 $1.8 $50 $50 $50.9 $69.3 $40 $40 $45.1 $63.4 $41.9 $30 $30 $53.4 $20 $20 $10 $10 $16.6 $16.5 $9.8 $0 $0 2021 2022 2023 2024 2021
2022 2023 NeuroStar Systems Treatment Sessions Other US OUS Guidance 31
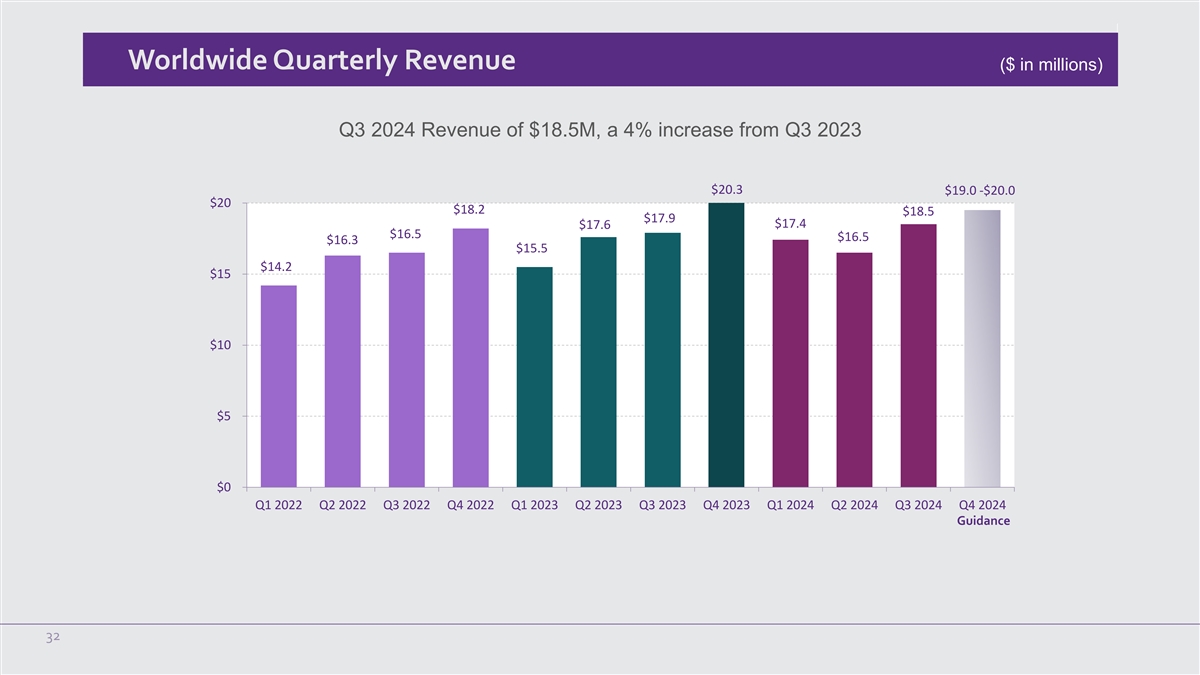
Worldwide Quarterly Revenue ($ in millions) Q3 2024 Revenue of $18.5M,
a 4% increase from Q3 2023 $20.3 $19.0 -$20.0 $20 $18.2 $18.5 $17.9 $17.4 $17.6 $16.5 $16.5 $16.3 $15.5 $14.2 $15 $10 $5 $0 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 Q2 2023 Q3 2023 Q4 2023 Q1 2024 Q2 2024 Q3 2024 Q4 2024 Guidance 32
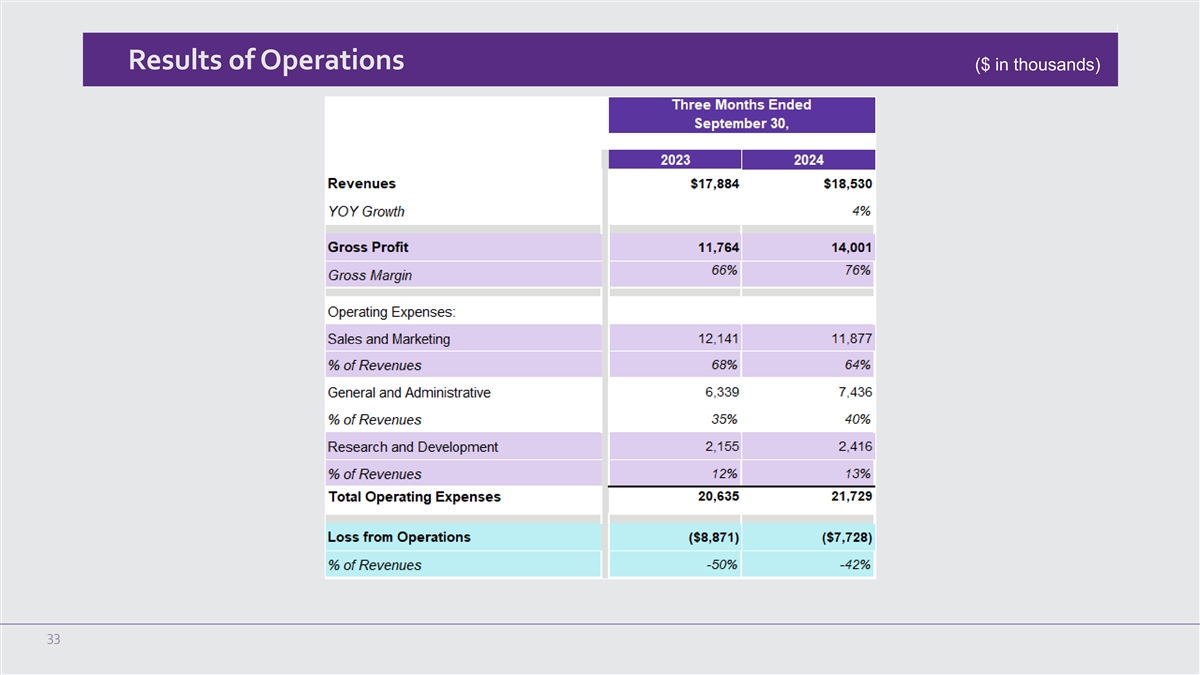
Results of Operations ($ in thousands) 33
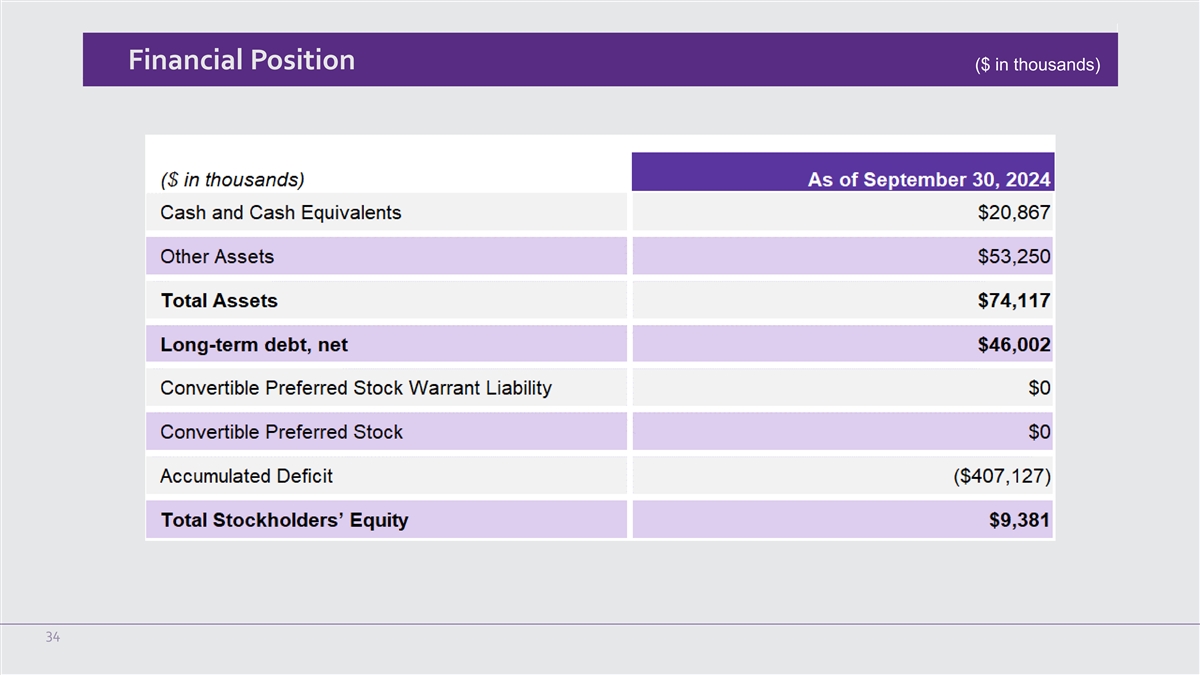
Financial Position ($ in thousands) 34

Supplemental Information NeuroStar Advanced Therapy for Mental Health
35
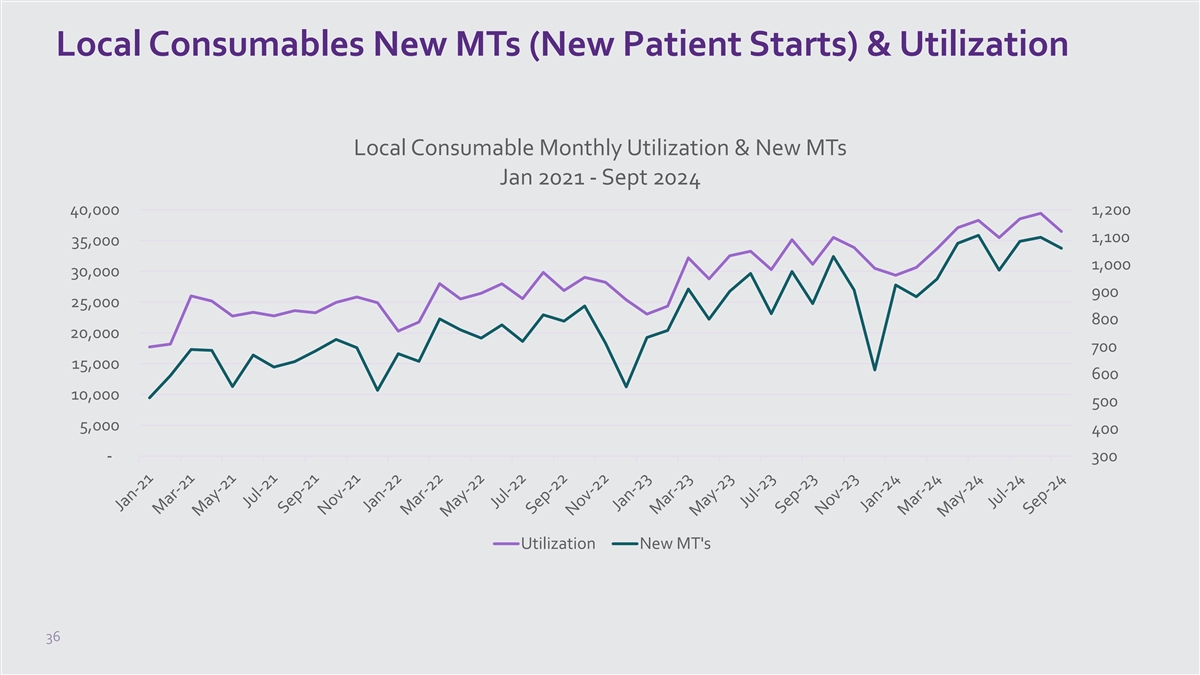
Local Consumables New MTs (New Patient Starts) & Utilization Local
Consumable Monthly Utilization & New MTs Jan 2021 - Sept 2024 40,000 1,200 1,100 35,000 1,000 30,000 900 25,000 800 20,000 700 15,000 600 10,000 500 5,000 400 - 300 Utilization New MT's 36
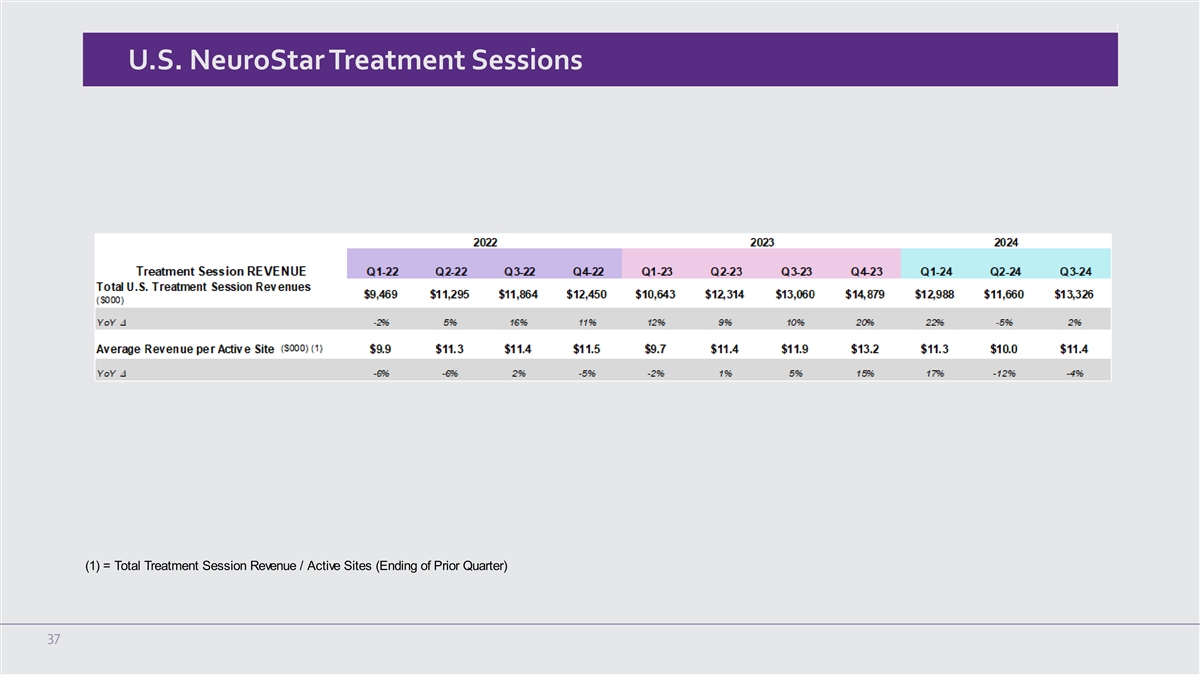
U.S. NeuroStar Treatment Sessions (1) = Total Treatment Session Revenue
/ Active Sites (Ending of Prior Quarter) 37
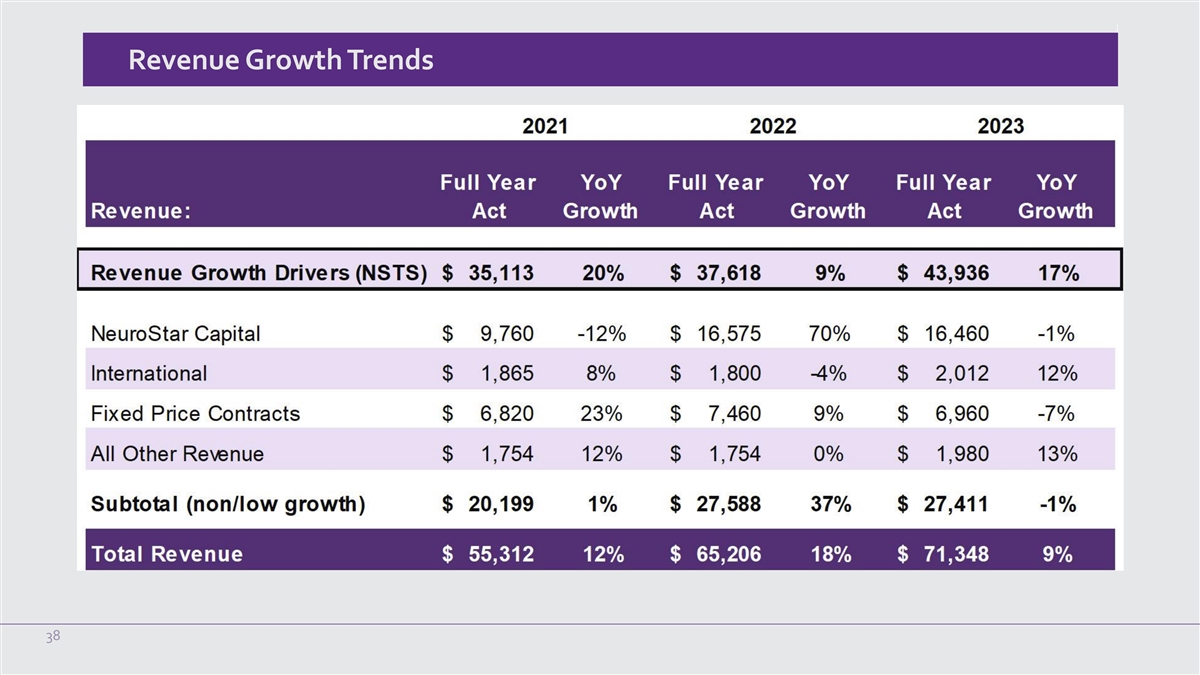
Revenue Growth Trends 38
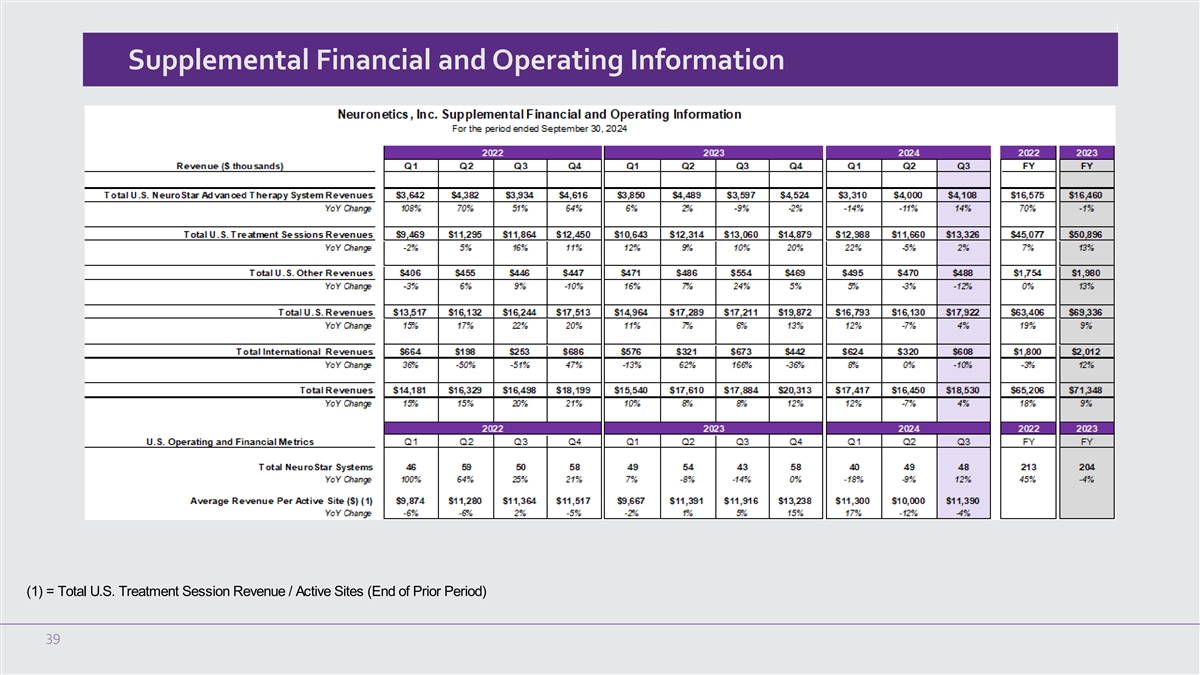
Supplemental Financial and Operating Information (1) = Total U.S.
Treatment Session Revenue / Active Sites (End of Prior Period) 39
v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Neuronetics (NASDAQ:STIM)
과거 데이터 주식 차트
부터 11월(11) 2024 으로 12월(12) 2024

Neuronetics (NASDAQ:STIM)
과거 데이터 주식 차트
부터 12월(12) 2023 으로 12월(12) 2024
