false
0001488917
0001488917
2025-02-11
2025-02-11
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event
reported): February 11, 2025
ELECTROMED, INC.
(Exact Name of Registrant as Specified in
Its Charter)
| Minnesota |
001-34839 |
41-1732920 |
|
(State or Other Jurisdiction of
Incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification
Number) |
500 Sixth Avenue NW
New Prague, MN 56071
(Address of Principal Executive Offices)
(Zip Code)
(952) 758-9299
(Registrant’s Telephone Number, Including
Area Code)
Not Applicable
(Former Name or Former Address, if Changed
Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Common Stock, $0.01 par value |
|
ELMD |
|
NYSE American LLC |
| (Title of each class) |
|
(Trading Symbol) |
|
(Name of each exchange on which registered) |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 |
Results of Operations and Financial Condition. |
On February 11, 2025, Electromed, Inc., a Minnesota corporation
(the “Company”), issued a press release announcing its financial results for the second quarter ended December 31,
2024. The full text of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference
into this Item 2.02.
| Item 7.01 |
Regulation FD Disclosure. |
The Company has updated its investor presentation, a copy of
which is furnished as Exhibit 99.2 to this Current Report on Form 8-K and incorporated by reference into this Item 7.01. The Company
intends to use the presentation in whole or in part, in one or more meetings with investors and analysts.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits:
The information contained in this Current Report on Form 8-K,
including Exhibits 99.1 and 99.2 attached hereto, shall not be deemed “filed” for the purposes of Section 18 of the
Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section, and shall not be incorporated
by reference into any registration statement pursuant to the Securities Act of 1933, as amended, except as shall be expressly set
forth by specific reference in such filing.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act
of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
ELECTROMED, INC. |
| |
|
|
| Date: February 11, 2025 |
By: |
/s/ Bradley M. Nagel |
| |
Name: |
Bradley M. Nagel |
| |
Title: |
Chief Financial Officer |
Exhibit 99.1
Electromed,
Inc. Announces Record Financial Performance in Fiscal 2025 Second Quarter
Company maintained
strong momentum to deliver another quarter of record revenue and earnings, while continuing to invest in multiple strategic growth
initiatives
NEW PRAGUE, Minn.--(BUSINESS
WIRE)-- Electromed, Inc. (“Electromed” or the “Company”) (NYSE American: ELMD), a leader in innovative
airway clearance technologies, today announced financial results for the three and six months ended December 31, 2024 (“Q2
FY 2025”).
Q2 FY 2025 Company Highlights
| ● | Net revenue increased 18.7% to a record $16.3 million in Q2 FY 2025, from $13.7 million in the second quarter of the prior
fiscal year. |
| ● | Operating income increased to a record $2.5 million, 15.6% of net revenues and a year-over-year increase of 12.3%. |
| ● | Net income was $2.0 million, or $0.22 per diluted share, compared to $1.7 million, or $0.19 per diluted share in Q2 FY 2024. |
| ● | Continued to deliberately expand the sales force, ending the quarter with 54 reps. |
| ● | Reached over 10,000 clinicians through the “Triple Down on Bronchiectasis” educational
campaign launched in Q1 FY 2025. |
“Our team’s
performance across the board in sales, marketing, manufacturing, and order fulfillment was outstanding,” said Jim Cunniff,
President, and Chief Executive Officer. “The impact of their work is plain to
see, with strong growth in all three of our customer categories, record quarterly revenues and solid operating and net income during
the quarter. A prime example of our sterling performance is our ability to improve our working capital by reducing inventory while
continuing to meet our patients’ therapy needs. This is particularly important for us given our unique direct-to-patient
model. We are also continually seeking ways to improve efficiency across the organization,
and during the quarter we initiated an investment in a new CRM system to further
enhance our commercial team’s productivity.
I am proud of Electromed’s position as a growing and profitable MedTech company, and we expect to report continued improvements
throughout the remainder of fiscal 2025.”
Q2 FY 2025 Results
All amounts below are
for the three months ended December 31, 2024, and compare to the three months ended December 31, 2023 (“Q2 FY 2024”).
Net revenues grew 18.7%
to $16.3 million, from $13.7 million.
Revenue in our direct
homecare business increased year-over-year by 15.2% to $14.6 million, from $12.7 million. The increase in revenue was due to an
increase in referrals and approvals driven by an increase in direct sales representatives, higher net revenues per approval, and
efficiencies within our reimbursement department. Field sales force employees totaled 60 at quarter end, 54 of which were direct
sales representatives. The annualized homecare revenue per weighted average direct sales representative in Q2 was $1,077,000, slightly
higher than Electromed’s target range of $900,000 to $1,000,000.
Gross profit increased
to $12.6 million or 77.7% of net revenues from $10.5 million or 77.0% of net revenues. The increase in gross profit dollars and
percentage were primarily a result of increased volumes and higher average net revenue
per device.
Selling, general and administrative
(“SG&A”) expenses were $9.8 million representing an increase of $1.7 million or 20.3%. The increase in the current
year period was primarily due to the accelerated recognition of non-cash share-based compensation associated with the vesting of
performance-based equity awards, along with increased salaries and incentive compensation related to the higher average number
of sales, sales support, marketing, and reimbursement personnel to process higher patient referrals.
Operating income was a
record $2.5 million, compared to $2.3 million. The increase in operating income was driven primarily by increased revenue and gross
profit.
Net income was a record
$2.0 million, or $0.22 per diluted share, compared to $1.7 million, or $0.19 per diluted share.
As of December 31, 2024,
Electromed had $16.2 million in cash, $22.8 million in accounts receivable and no debt, achieving a working capital of $35.5 million
and total shareholders’ equity of $43.6 million. The cash balance reflects an increase of $0.2 million for the six months
ended December 31, 2024, compared to an increase in cash of $3.1 million in the six months ended December 31, 2023. The increase
in cash for the 6 months ended December 31, 2024, was driven by $5.5 million
of positive operating cash flow, offset by share repurchases of approximately $4.5 million of Electromed common stock and $0.8
million of taxes paid from net share settlement of vested stock.
Conference Call and Webcast Information
The conference call with
members of Electromed management will be held at 5:00 p.m. Eastern Time on Tuesday, February 11, 2025.
Interested parties may participate
in the call by dialing (844) 826-3033 (Domestic) or (412) 317-5185 (International) using passcode 0177798.
The live conference call webcast will
be accessible in the Investor Relations section of Electromed’s website and directly via the following link: https://viavid.webcasts.com/starthere.jsp?ei=1705037&tp_key=8af23b601f.
For those who cannot listen to the
live broadcast, a replay will be available by dialing (844) 512-2921 (Domestic) or (412) 317-6671 (International) and referencing
the replay pin number 10196088. Additionally, an online replay will be available for one
year in the Investor Relations section of Electromed’s web site at: https://investors.smartvest.com/events-and-presentations/default.aspx
About Electromed, Inc.
Electromed, Inc. manufactures, markets,
and sells products that provide airway clearance therapy, including the SmartVest® Airway Clearance System, to patients with
compromised pulmonary function. It is headquartered in New Prague, Minnesota, and was founded in 1992. Further information about
Electromed can be found at www.smartvest.com.
Cautionary Statements
Certain statements in this press release constitute forward-looking
statements as defined in the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be
identified by words such as “anticipate,” “believe,” “continue,” “estimate,” “expect,”
“intend,” “may,” “plan” “potential,” “should,” “will,”
and similar expressions, including the negative of these terms, but they are not the exclusive means of identifying such statements.
Forward-looking statements cannot be guaranteed, and actual results may vary materially due to the uncertainties and risks, known
or unknown associated with such statements. Examples of risks and uncertainties for the Company include, but are not limited to,
competitive nature of our market; changes to Medicare, Medicaid, or private insurance reimbursement policies; changes to state
and federal health care laws; changes affecting the medical device industry; our ability to develop new sales channels for our
products such as the homecare distributor channel; our need to maintain regulatory compliance and to gain future regulatory approvals
and clearances; new drug or pharmaceutical discoveries; general economic and business conditions; our ability to renew our line
of credit or obtain additional credit as necessary; our ability to protect and expand our intellectual property portfolio; the
risks associated with expansion into international markets, as well as other factors we may describe from time to time in the Company’s
reports filed with the Securities and Exchange Commission (including the Company’s most recent Annual Report on Form 10-K,
as amended from time to time, and subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K). Investors should
not consider any list of such factors to be an exhaustive statement of all the risks, uncertainties or potentially inaccurate assumptions
investors should take into account when making investment decisions. Shareholders and other readers should not place undue reliance
on “forward-looking statements,” as such statements speak only as of the date of this press release. We undertake no
obligation to update them in light of new information or future events.
Brad Nagel, Chief Financial Officer
(952) 758-9299
investorrelations@electromed.com
Mike Cavanaugh, Investor Relations
ICR Healthcare
(617) 877-9641
mike.cavanaugh@icrhealthcare.com
Source: Electromed, Inc.
Electromed, Inc.
Condensed Balance Sheets
| | |
December 31, 2024 | | |
June 30, 2024 | |
| | |
(Unaudited) | | |
(Audited) | |
| Assets | |
| | | |
| | |
| Current Assets | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 16,235,000 | | |
$ | 16,080,000 | |
| Accounts receivable (net of allowances for credit losses of $45,000) | |
| 22,775,000 | | |
| 23,333,000 | |
| Contract assets | |
| 997,000 | | |
| 719,000 | |
| Inventories | |
| 3,081,000 | | |
| 3,712,000 | |
| Income taxes receivable | |
| 514,000 | | |
| - | |
| Prepaid expenses and other current assets | |
| 587,000 | | |
| 329,000 | |
| Total current assets | |
| 44,189,000 | | |
| 44,173,000 | |
| Property and equipment, net | |
| 5,216,000 | | |
| 5,165,000 | |
| Finite-life intangible assets, net | |
| 609,000 | | |
| 657,000 | |
| Other assets | |
| 108,000 | | |
| 87,000 | |
| Deferred income taxes | |
| 2,152,000 | | |
| 2,152,000 | |
| Total assets | |
$ | 52,274,000 | | |
$ | 52,234,000 | |
| | |
| | | |
| | |
| Liabilities and Shareholders’ Equity | |
| | | |
| | |
| Current Liabilities | |
| | | |
| | |
| Accounts payable | |
| 1,506,000 | | |
| 1,010,000 | |
| Accrued compensation | |
| 3,623,000 | | |
| 3,893,000 | |
| Income tax payable | |
| - | | |
| 277,000 | |
| Warranty reserve | |
| 1,599,000 | | |
| 1,567,000 | |
| Other accrued liabilities | |
| 1,939,000 | | |
| 930,000 | |
| Total current liabilities | |
| 8,667,000 | | |
| 7,677,000 | |
| Other long-term liabilities | |
| 4,000 | | |
| 12,000 | |
| Total liabilities | |
| 8,671,000 | | |
| 7,689,000 | |
| | |
| | | |
| | |
| Commitments and Contingencies | |
| | | |
| | |
| | |
| | | |
| | |
| Shareholders’ Equity | |
| | | |
| | |
| Common stock, $0.01 par value per share, 13,000,000 shares authorized; | |
| | | |
| | |
| 8,556,844 and 8,637,883 shares issued and outstanding, as of December 31, 2024 and June 30, 2024, respectively | |
| 86,000 | | |
| 87,000 | |
| Additional paid-in capital | |
| 20,940,000 | | |
| 20,790,000 | |
| Retained earnings | |
| 22,577,000 | | |
| 23,668,000 | |
| Total shareholders’ equity | |
| 43,603,000 | | |
| 44,545,000 | |
| Total liabilities and shareholders’ equity | |
$ | 52,274,000 | | |
$ | 52,234,000 | |
Electromed, Inc.
Condensed Statements of Operations
| | |
| | |
| | |
| | |
| |
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
December 31, | | |
December 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
| (Unaudited) | | |
| (Unaudited) | | |
| (Unaudited) | | |
| (Unaudited) | |
| Net revenues | |
$ | 16,255,000 | | |
$ | 13,689,000 | | |
$ | 30,923,000 | | |
$ | 26,013,000 | |
| Cost of revenues | |
| 3,628,000 | | |
| 3,144,000 | | |
| 6,805,000 | | |
| 5,970,000 | |
| Gross profit | |
| 12,627,000 | | |
| 10,545,000 | | |
| 24,118,000 | | |
| 20,043,000 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses | |
| | | |
| | | |
| | | |
| | |
| Selling, general and administrative | |
| 9,834,000 | | |
| 8,175,000 | | |
| 19,221,000 | | |
| 17,325,000 | |
| Research and development | |
| 251,000 | | |
| 107,000 | | |
| 417,000 | | |
| 313,000 | |
| Total operating expenses | |
| 10,085,000 | | |
| 8,282,000 | | |
| 19,638,000 | | |
| 17,638,000 | |
| Operating income | |
| 2,542,000 | | |
| 2,263,000 | | |
| 4,480,000 | | |
| 2,405,000 | |
| Interest income, net | |
| 152,000 | | |
| 96,000 | | |
| 347,000 | | |
| 173,000 | |
| Net income before income taxes | |
| 2,694,000 | | |
| 2,359,000 | | |
| 4,827,000 | | |
| 2,578,000 | |
| | |
| | | |
| | | |
| | | |
| | |
| Income tax expense | |
| 726,000 | | |
| 685,000 | | |
| 1,385,000 | | |
| 749,000 | |
| | |
| | | |
| | | |
| | | |
| | |
| Net income | |
$ | 1,968,000 | | |
$ | 1,674,000 | | |
$ | 3,442,000 | | |
$ | 1,829,000 | |
| | |
| | | |
| | | |
| | | |
| | |
| Income per share: | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | 0.23 | | |
$ | 0.20 | | |
$ | 0.41 | | |
$ | 0.21 | |
| | |
| | | |
| | | |
| | | |
| | |
| Diluted | |
$ | 0.22 | | |
$ | 0.19 | | |
$ | 0.38 | | |
$ | 0.21 | |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted-average common shares outstanding: | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 8,424,534 | | |
| 8,545,120 | | |
| 8,494,511 | | |
| 8,541,254 | |
| Diluted | |
| 8,953,349 | | |
| 8,800,172 | | |
| 8,983,726 | | |
| 8,791,519 | |
Electromed, Inc.
Condensed Statements of Cash Flows
| | |
Six Months Ended December 31, | |
| | |
2024 | | |
2023 | |
| | |
(Unaudited) | | |
(Unaudited) | |
| Cash Flows From Operating Activities | |
| | | |
| | |
| Net income | |
$ | 3,442,000 | | |
$ | 1,829,000 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | |
| | | |
| | |
| Depreciation | |
| 414,000 | | |
| 398,000 | |
| Amortization of finite-life intangible assets | |
| 78,000 | | |
| 25,000 | |
| Share-based compensation expense | |
| 1,652,000 | | |
| 791,000 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| 558,000 | | |
| 1,142,000 | |
| Contract assets | |
| (278,000 | ) | |
| (87,000 | ) |
| Inventories | |
| 500,000 | | |
| (509,000 | ) |
| Prepaid expenses and other assets | |
| (279,000 | ) | |
| 1,104,000 | |
| Income taxes receivable, net | |
| (791,000 | ) | |
| (83,000 | ) |
| Accounts payable and accrued liabilities | |
| 434,000 | | |
| (1,171,000 | ) |
| Accrued compensation | |
| (270,000 | ) | |
| (212,000 | ) |
| Net cash provided by operating activities | |
| 5,460,000 | | |
| 3,227,000 | |
| | |
| | | |
| | |
| Cash Flows From Investing Activities | |
| | | |
| | |
| Expenditures for property and equipment | |
| (270,000 | ) | |
| (180,000 | ) |
| Expenditures for finite-life intangible assets | |
| (25,000 | ) | |
| (40,000 | ) |
| Net cash used for investing activities | |
| (295,000 | ) | |
| (220,000 | ) |
| | |
| | | |
| | |
| Cash Flows From Financing Activities | |
| | | |
| | |
| Issuance of common stock upon exercise of options | |
| 346,000 | | |
| 55,000 | |
| Taxes paid on net share settlement of stock awards | |
| (820,000 | ) | |
| - | |
| Repurchase of common stock | |
| (4,536,000 | ) | |
| - | |
| Net cash (used for) provided by financing activities | |
| (5,010,000 | ) | |
| 55,000 | |
| Net increase in cash | |
| 155,000 | | |
| 3,062,000 | |
| | |
| | | |
| | |
| Cash And Cash Equivalents | |
| | | |
| | |
| Beginning of period | |
| 16,080,000 | | |
| 7,372,000 | |
| End of period | |
$ | 16,235,000 | | |
$ | 10,434,000 | |
Exhibit 99.2

| 1 Investor Presentation Electromed, Inc. Investor Presentation February 11, 2025 NYSE American: ELMD Innovation Leader in Airway Clearance Technologies

| 2 Investor Presentation Forward Looking Statements Certain statements in this press release constitute forward - looking statements as defined in the US Private Securities Litigation Reform Act of 1995 . Forward - looking statements can generally be identified by words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “should,” “will,” and similar expressions, including the negative of these terms, but they are not the exclusive means of identifying such statements . Forward - looking statements cannot be guaranteed, and actual results may vary materially due to the uncertainties and risks, known or unknown associated with such statements . Examples of risks and uncertainties for the Company include, but are not limited to the competitive nature of our market ; changes to Medicare, Medicaid, or private insurance reimbursement policies ; changes to state and federal health care laws ; changes affecting the medical device industry ; our ability to develop new sales channels for our products such as the homecare distributor channel ; our need to maintain regulatory compliance and to gain future regulatory approvals and clearances ; new drug or pharmaceutical discoveries ; general economic and business conditions ; our ability to renew our line of credit or obtain additional credit as necessary ; our ability to protect and expand our intellectual property portfolio ; the risks associated with expansion into international markets, as well as other factors we may describe from time to time in the Company’s reports filed with the Securities and Exchange Commission (including the Company’s most recent Annual Report on Form 10 - K, as amended from time to time, and subsequent Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K) . Investors should not consider any list of such factors to be an exhaustive statement of all of the risks, uncertainties or potentially inaccurate assumptions investors should take into account when making investment decisions . Shareholders and other readers should not place undue reliance on “forward - looking statements,” as such statements speak only as of the date of this press release . We undertake no obligation to update them in light of new information or future events .
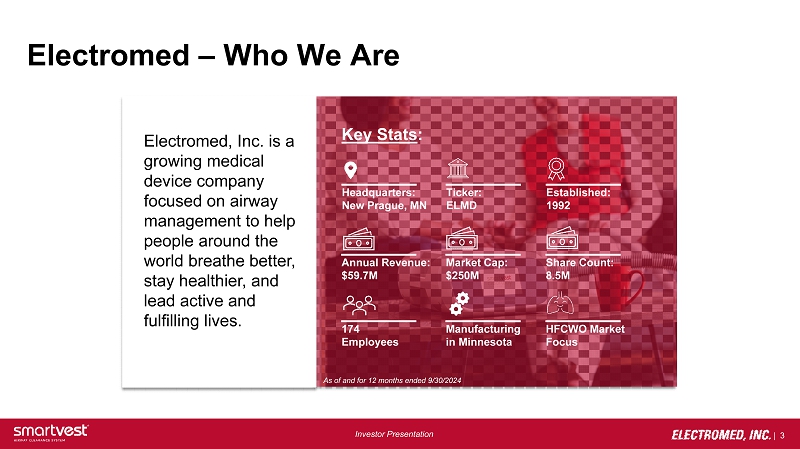
| 3 Investor Presentation Electromed – Who We Are Electromed, Inc. is a growing medical device company focused on airway management to help people around the world breathe better, stay healthier, and lead active and fulfilling lives. As of and for 12 months ended 9/30/2024 Key Stats : Headquarters: New Prague, MN Ticker: ELMD Established: 1992 Annual Revenue: $59.7M Market Cap: $250M Share Count: 8.5M 174 Employees Manufacturing in Minnesota HFCWO Market Focus
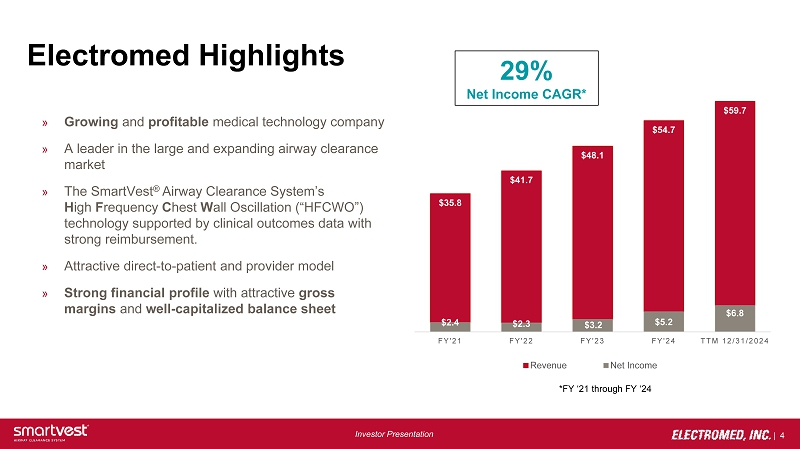
| 4 Investor Presentation Electromed Highlights » Growing and profitable medical technology company » A leader in the large and expanding airway clearance market » The SmartVest ® Airway Clearance System’s H igh F requency C hest W all Oscillation (“HFCWO”) technology supported by clinical outcomes data with strong reimbursement. » Attractive direct - to - patient and provider model » Strong financial profile with attractive gross margins and well - capitalized balance sheet $35.8 $41.7 $48.1 $54.7 $59.7 $2.4 $2.3 $3.2 $5.2 $6.8 FY'21 FY'22 FY'23 FY'24 TTM 12/31/2024 Revenue Net Income 29% Net Income CAGR* *FY ‘21 through FY ‘24
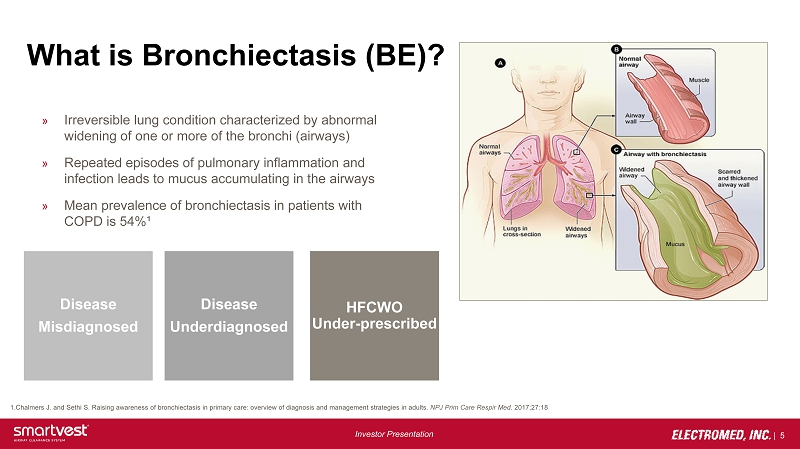
| 5 Investor Presentation What is Bronchiectasis (BE)? » Irreversible lung condition characterized by abnormal widening of one or more of the bronchi (airways) » Repeated episodes of pulmonary inflammation and infection leads to mucus accumulating in the airways » Mean prevalence of bronchiectasis in patients with COPD is 54%¹ 1.Chalmers J. and Sethi S. Raising awareness of bronchiectasis in primary care: overview of diagnosis and management strategi es in adults. NPJ Prim Care Respir Med . 2017;27:18 HFCWO Under - prescribed Disease Underdiagnosed Disease Misdiagnosed
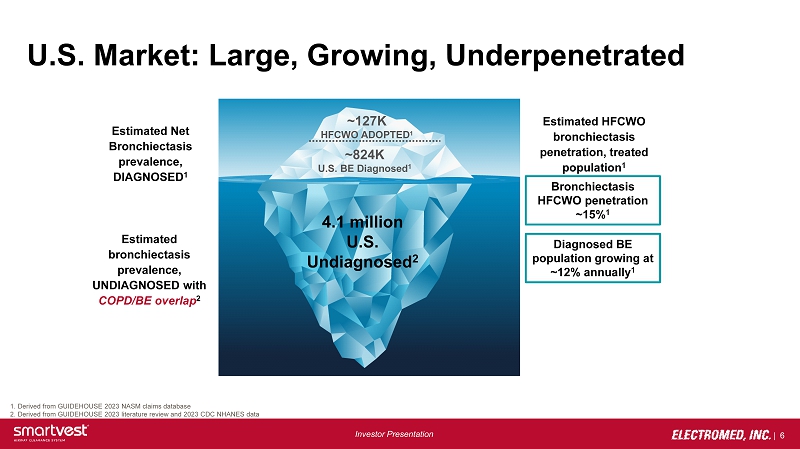
| 6 Investor Presentation U.S. Market: Large, Growing, Underpenetrated 1. Derived from GUIDEHOUSE 2023 NASM claims database 2. Derived from GUIDEHOUSE 2023 literature review and 2023 CDC NHANES data Bronchiectasis HFCWO penetration ~15% 1 Diagnosed BE population growing at ~12% annually 1 Estimated Net Bronchiectasis prevalence, DIAGNOSED 1 Estimated bronchiectasis prevalence, UNDIAGNOSED with COPD/BE overla p 2 Estimated HFCWO bronchiectasis penetration, treated population 1 ~824K U.S. BE Diagnosed 1 4.1 million U.S. Undiagnosed 2 ~127K HFCWO ADOPTED 1
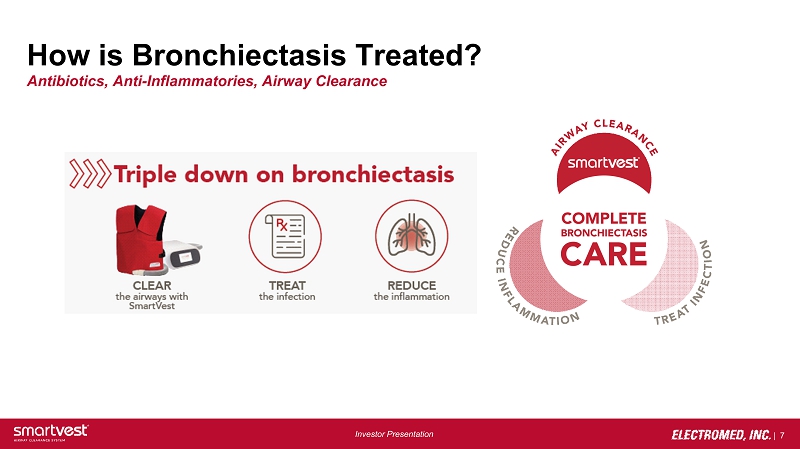
| 7 Investor Presentation How is Bronchiectasis Treated? Antibiotics, Anti - Inflammatories, Airway Clearance
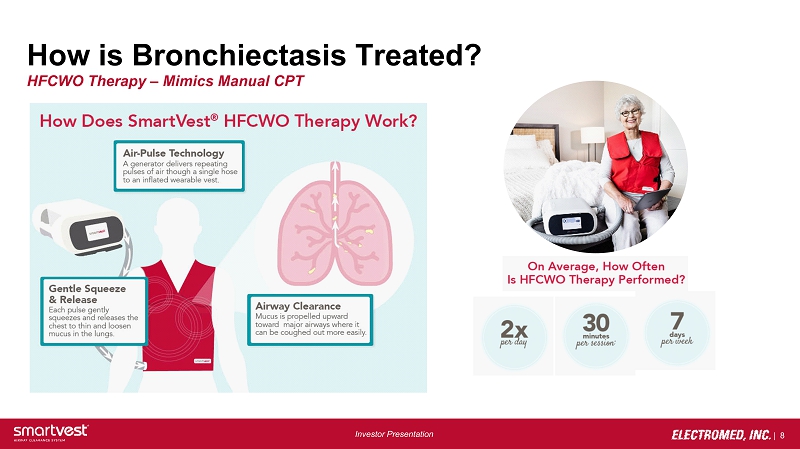
| 8 Investor Presentation How is Bronchiectasis Treated? HFCWO Therapy – Mimics Manual CPT

| 9 Investor Presentation Sleek and light weight generator Intuitive user interface for better patient adherence More portable and easier for travel SmartVest Clearway ® HFCWO Designed with the Patient in Mind An Enhanced Patient Experience SmartVest ® has a well - established reimbursement code from CMS – E0483; Electromed has over 275M contracted lives in the US
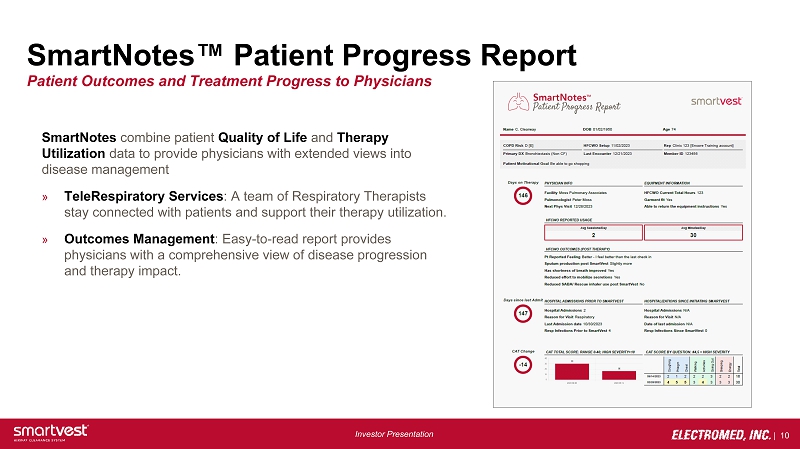
| 10 Investor Presentation SmartNotes™ Patient Progress Report SmartNotes combine patient Quality of Life and Therapy Utilization data to provide physicians with extended views into disease management » TeleRespiratory Services : A team of Respiratory Therapists stay connected with patients and support their therapy utilization. » Outcomes Management : Easy - to - read report provides physicians with a comprehensive view of disease progression and therapy impact. Patient Outcomes and Treatment Progress to Physicians
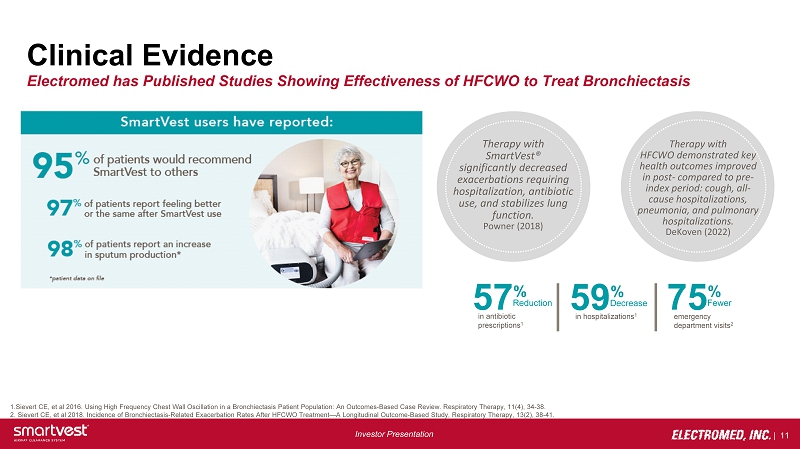
| 11 Investor Presentation Clinical Evidence Electromed has Published Studies Showing Effectiveness of HFCWO to Treat Bronchiectasis 1.Sievert CE, et al 2016. Using High Frequency Chest Wall Oscillation in a Bronchiectasis Patient Population: An Outcomes - Based Case Review. Respiratory Therapy, 11(4), 34 - 38. 2. Sievert CE, et al 2018. Incidence of Bronchiectasis - Related Exacerbation Rates After HFCWO Treatment — A Longitudinal Outcome - B ased Study, Respiratory Therapy, 13(2), 38 - 41. 57 % Reduction in antibiotic prescriptions 1 59 % Decrease in hospitalizations 1 75 % Fewer emergency department visits 2 Therapy with SmartVest® significantly decreased exacerbations requiring hospitalization, antibiotic use, and stabilizes lung function. Powner (2018) Therapy with HFCWO demonstrated key health outcomes improved in post - compared to pre - index period: cough, all - cause hospitalizations, pneumonia, and pulmonary hospitalizations. DeKoven (2022)
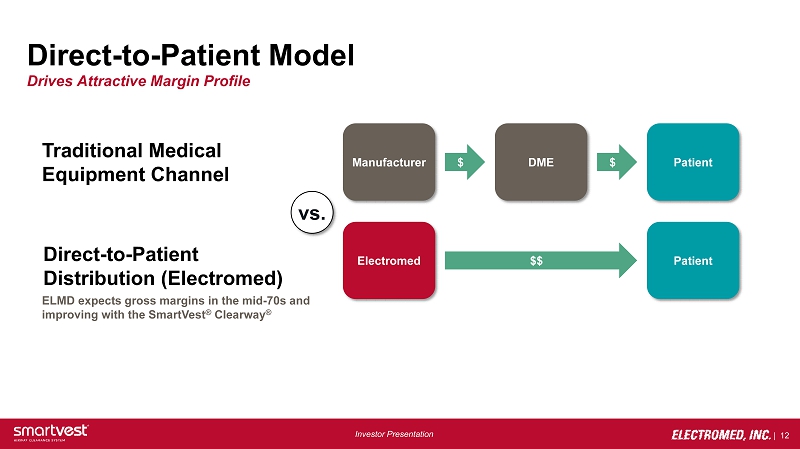
| 12 Investor Presentation Direct - to - Patient Model Drives Attractive Margin Profile Manufacturer DME Patient $ $ vs. Electromed Patient $$ Traditional Medical Equipment Channel Direct - to - Patient Distribution (Electromed) ELMD expects gross margins in the mid - 70s and improving with the SmartVest ® Clearway ®
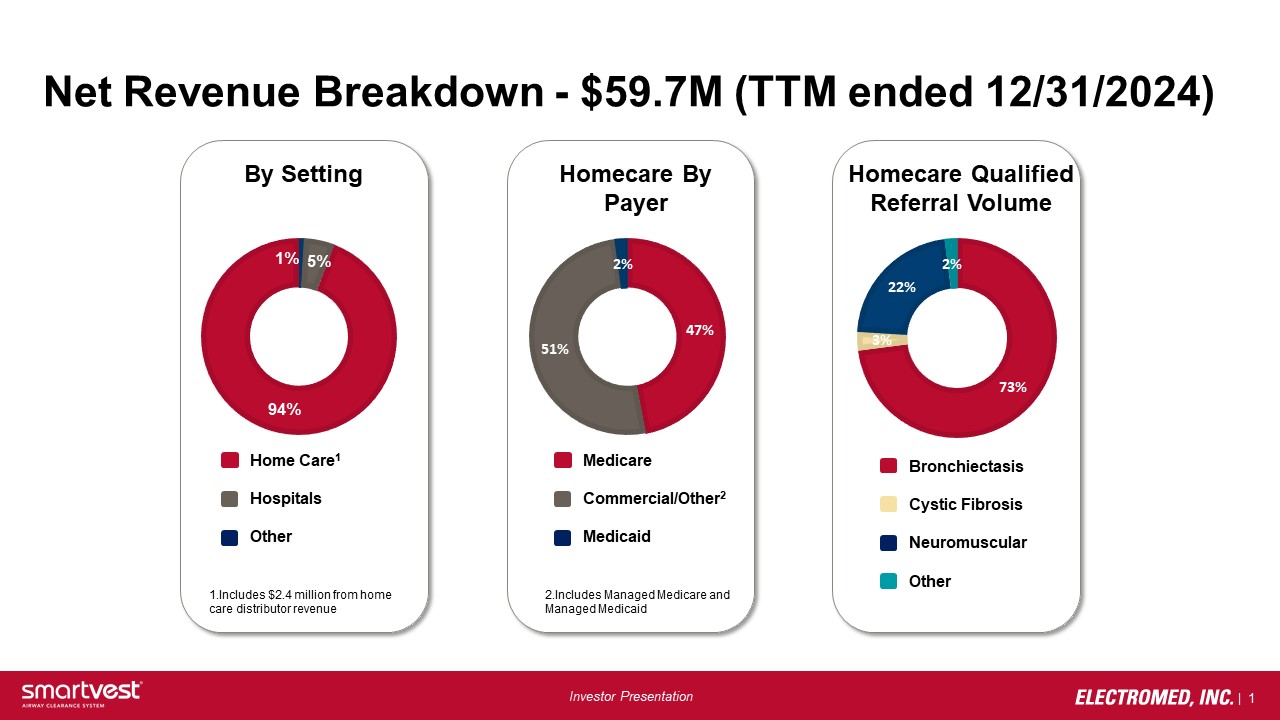
| 13 Investor Presentation Net Revenue Breakdown - $59.7M (TTM ended 12/31/2024) 1% [PE RCE NTA GE] 94% By Setting Homecare By Payer Homecare Qualified Referral Volume 73% 3% 22% 2% 47% 51% 2% Home Care 1 Hospitals Other Medicare Commercial/Other 2 Medicaid Bronchiectasis Cystic Fibrosis Neuromuscular Other 1.Includes $2.4 million from home care distributor revenue 2.Includes Managed Medicare and Managed Medicaid
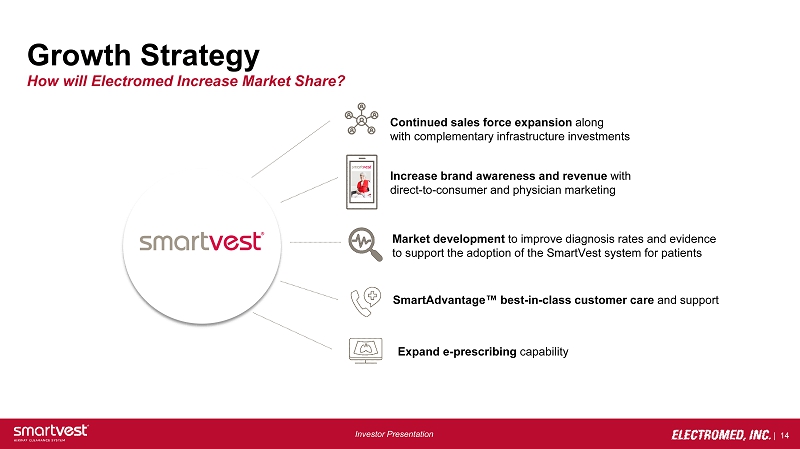
| 14 Investor Presentation Growth Strategy How will Electromed Increase Market Share? Continued sales force expansion along with complementary infrastructure investments Increase brand awareness and revenue with direct - to - consumer and physician marketing Market development to improve diagnosis rates and evidence to support the adoption of the SmartVest system for patients SmartAdvantage ™ best - in - class customer care and support Expand e - prescribing capability
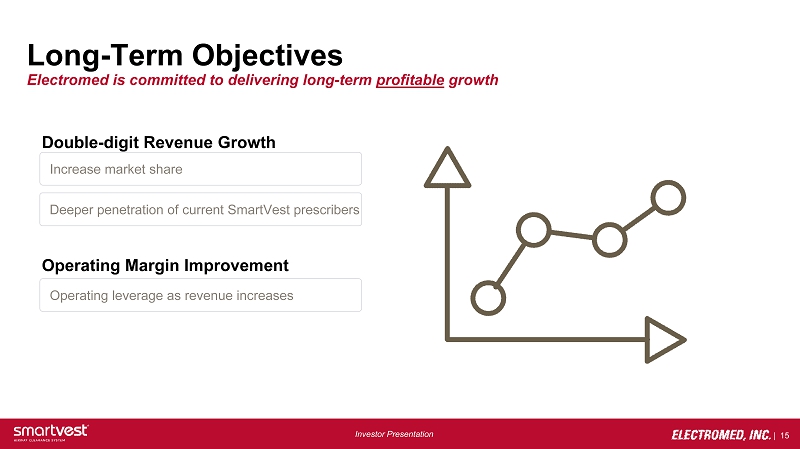
| 15 Investor Presentation Long - Term Objectives Electromed is committed to delivering long - term profitable growth Double - digit Revenue Growth Operating Margin Improvement Increase market share Deeper penetration of current SmartVest prescribers Operating leverage as revenue increases
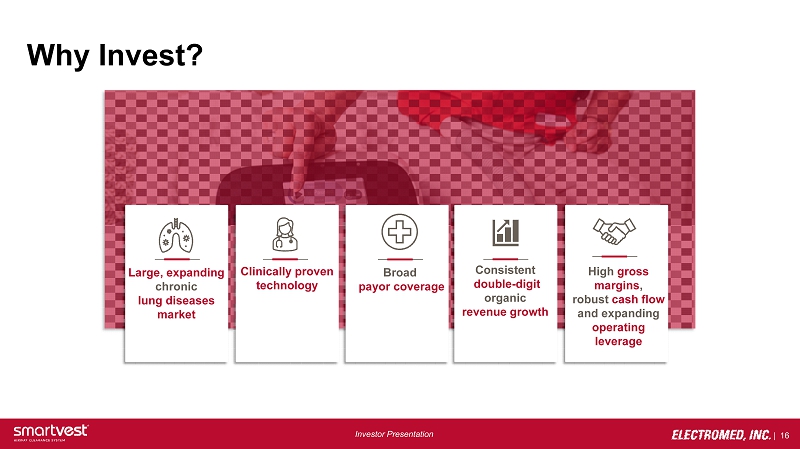
| 16 Investor Presentation Why Invest? Large, expanding chronic lung diseases market Clinically proven technology Broad payor coverage Consistent double - digit organic revenue growth High gross margins , robust cash flow and expanding operating leverage

| 17 Investor Presentation Management Incentives Aligned w/Investors CEO Incentive Management’s Incentive Compensation Reward based on increasing total shareholder return . Focused solely on delivering financial results .
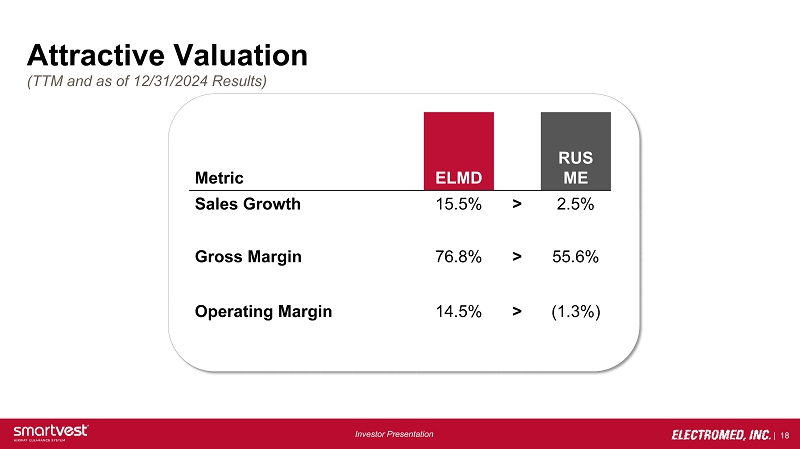
| 18 Investor Presentation Attractive Valuation (TTM and as of 12/31/2024 Results) Metric ELMD RUS ME Sales Growth 15.5% > 2.5% Gross Margin 76.8% > 55.6% Operating Margin 14.5% > (1.3%)

| 19 Investor Presentation Mike Cavanaugh ( 617) 877 - 8641 m ike.cavanaugh@westwicke.com Maren Czura (332) 242 - 4365 maren.czura@westwicke.com Jim Cunniff , President & CEO (952) 758 - 9299 jcunniff @Electromed.com Brad Nagel, CFO (952) 758 - 9299 bnagel@Electromed.com

| 20 Investor Presentation APPENDIX
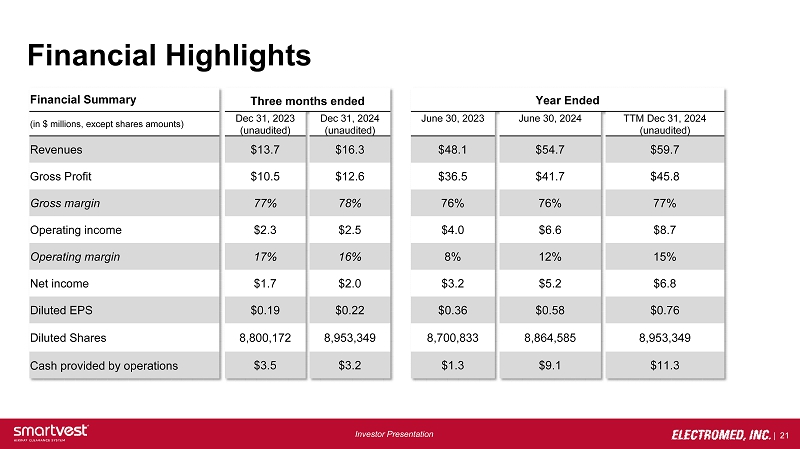
| 21 Investor Presentation Financial Highlights Financial Summary Three months ended Year Ended ( in $ millions, except shares amounts) Dec 31, 2023 (unaudited) Dec 31, 2024 (unaudited) June 30, 2023 June 30, 2024 TTM Dec 31, 2024 (unaudited) Revenues $13.7 $16.3 $48.1 $54.7 $59.7 Gross Profit $10.5 $12.6 $36.5 $41.7 $45.8 Gross margin 77% 78% 76% 76% 77% Operating income $2.3 $2.5 $4.0 $6.6 $8.7 Operating margin 17% 16% 8% 12% 15% Net income $1.7 $2.0 $3.2 $5.2 $6.8 Diluted EPS $0.19 $0.22 $0.36 $0.58 $0.76 Diluted Shares 8,800,172 8,953,349 8,700,833 8,864,585 8,953,349 Cash provided by operations $3.5 $3.2 $1.3 $9.1 $11.3
v3.25.0.1
Cover
|
Feb. 11, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Feb. 11, 2025
|
| Entity File Number |
001-34839
|
| Entity Registrant Name |
ELECTROMED, INC.
|
| Entity Central Index Key |
0001488917
|
| Entity Tax Identification Number |
41-1732920
|
| Entity Incorporation, State or Country Code |
MN
|
| Entity Address, Address Line One |
500 Sixth Avenue NW
|
| Entity Address, City or Town |
New Prague
|
| Entity Address, State or Province |
MN
|
| Entity Address, Postal Zip Code |
56071
|
| City Area Code |
(952)
|
| Local Phone Number |
758-9299
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.01 par value
|
| Trading Symbol |
ELMD
|
| Security Exchange Name |
NYSEAMER
|
| Entity Emerging Growth Company |
false
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Electromed (AMEX:ELMD)
과거 데이터 주식 차트
부터 1월(1) 2025 으로 2월(2) 2025

Electromed (AMEX:ELMD)
과거 데이터 주식 차트
부터 2월(2) 2024 으로 2월(2) 2025
