0001636651false00016366512023-10-022023-10-02
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): October 02, 2023 |
Ovid Therapeutics Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-38085 |
46-5270895 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
441 Ninth Avenue 14th Floor |
|
New York, New York |
|
10001 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 646 661-7661 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 per share |
|
OVID |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On October 2, 2023, Ovid Therapeutics Inc. (the "Company") presented its corporate presentation, which will be posted to the "Events & Presentations" tab on the Company's website at https://investors.ovidrx.com, following a live presentation.
A copy of the corporate presentation is filed as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibit
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Ovid Therapeutics Inc. |
|
|
|
|
Date: |
October 2, 2023 |
By: |
/s/ Thomas M. Perone |
|
|
|
Thomas M. Perone
General Counsel & Corporate Secretary |

OVID R&D DAY OCTOBER 2, 2023

Dawn of the neurotherapeutics era Dr. Jeremy Levin CEO & Chairman

Forward looking statement This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 including, without limitation, statements regarding the timing and development of Ovid’s product candidate pipeline and achievement of expected near- and long-term milestones; the potential therapeutic benefits of Ovid’s current or future product candidates and pipeline programs; the reporting of data for the potential Phase 1 study in healthy volunteers for OV329; the potential use of OV329 to treat rare and treatment-resistant forms of epilepsy and seizures; the clinical and regulatory development of OV329, including the anticipated timing of clinical trials of OV329; the likelihood that data for OV329 will support future development and therapeutic potential; the potential development of OV350 and other KCC2 compounds in the Company’s library; the potential timing of a potential IND filing for OV350; the suitability of the Company’s library of novel, direct KCC2 transporter activators for a range of formulations and administrations that would make it possible to pursue both chronic and acute epilepsies; the potential development and therapeutic opportunity of OV888 (GV101) and other Rho/Rho associated coiled-coil containing protein kinase 2 inhibitors; and the potential safety, selectivity and potency of OV888 (GV101) and other ROCK2 inhibitors; the potential use of OV888 (GV101) and other ROCK2 inhibitors to treat cavernous cerebral malformations and other rare central nervous system disorders; the potential timing of the pivotal formulation for OV888 (GV101); the potential timing of Phase 1 and 2 clinical studies for OV888 (GV101) and the resulting data; the timing for the completion of Takeda’s two pivotal Phase 3 trials evaluating soticlestat for Lennox-Gastaut and Dravet syndromes; the potential timing of regulatory decisions on soticlestat; the success of Takeda’s trials in soticlestat; the duration of the Company’s cash runway, and the expectation that it will support the advancement of the Company’s pipeline; Ovid’s business development intentions; the success of any licensing or partnering opportunities; the success, timing, ability to attract and maintain strategic collaborations; the clinical and regulatory development and potential commercialization of soticlestat, OV329, OV350, OV888 (GV101) or any of Ovid’s other current or future product candidates and pipeline programs and market opportunities. You can identify forward-looking statements because they contain words such as “will,” “may,” “plan,” “believes,” intends,” “anticipates,” “design,” “advance,” “target,” “seek,” “expects,” “demonstrates,” “observe,” and “potential,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances). Forward-looking statements are based on Ovid’s current expectations and assumptions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that may differ materially from those contemplated by the forward-looking statements, which are neither statements of historical fact nor guarantees or assurances of future performance. Important factors that could cause actual results to differ materially from those in the forward-looking statements include, without limitation, uncertainties inherent in the preclinical and clinical development and regulatory approval processes, risks related to Ovid’s ability to achieve its financial objectives, the risk that Ovid may not be able to realize the intended benefits of its technology or its business strategy, risks related to Ovid’s ability to identify business development targets or strategic partners, to enter into strategic transactions on favorable terms, or to consummate and realize the benefits of any business development transactions and risks to Ovid’s or any of its partners’ abilities to meet anticipated deadlines and milestones. Additional risks that could cause actual results to differ materially from those in the forward-looking statements are set forth under the caption “Risk Factors” in Ovid’s Quarterly Report of Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 4, 2023, and in future filings Ovid makes with the SEC. Any forward-looking statements contained in this press release speak only as of the date hereof, and Ovid assumes no obligation to update any forward-looking statements contained herein, whether because of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.

Our trust 01 Epilepsy burden: The need for new mechanisms & medicines Dr. Imad Najm, Director, Epilepsy Center, Cleveland Clinic, Vice Chair for Strategy & Development, Neurological Inst. 02 Scientific & pipeline strategy Meg Alexander, Chief Strategy Officer 03 ROCK2 inhibition for cerebral cavernous malformations & CNS conditions Dr. Zhong Zhong, Chief Scientific Officer Dr. Connie Lee, Founder & CEO, Alliance To Cure Cavernous Malformations Q&A FOLLOWED BY COFFEE BREAK 04 A next-generation GABA aminotransferase inhibitor Dr. Manoj Malhotra, Chief Medical Officer Dr. Zhong Zhong, Chief Scientific Officer 05 Unlocking a new CNS target: KCC2 direct activation Dr. Stephen Moss, Director, Moss Lab For Neuropharmacology at Tufts University & Professor of Molecular Pharmacology At University College London Dr. Zhong Zhong, Chief Scientific Officer 06 Soticlestat: Anticipated profile Jason Tardio, Chief Operating Officer 07 Milestones & financial strategy Jeff Rona, Chief Business & Financial Officer Q&A & CONCLUDE Jeremy Levin, Chief Executive Officer & Chairman
Epilepsy will be a key to unlocking a revolution of targeted small molecules �for neurological conditions Ovid is part of this revolution.

Today’s news Pipeline programs offer anti-convulsant & broader CNS opportunities New preclinical research offers confidence to accelerate & expand programs 5 clinical milestones expected within the next 12-15 months Strong capital base and financial strategy to propel pipeline to milestones Potential for substantial non-dilutive capital from Takeda* to support pipeline *If soticlestat is approved and commercialized
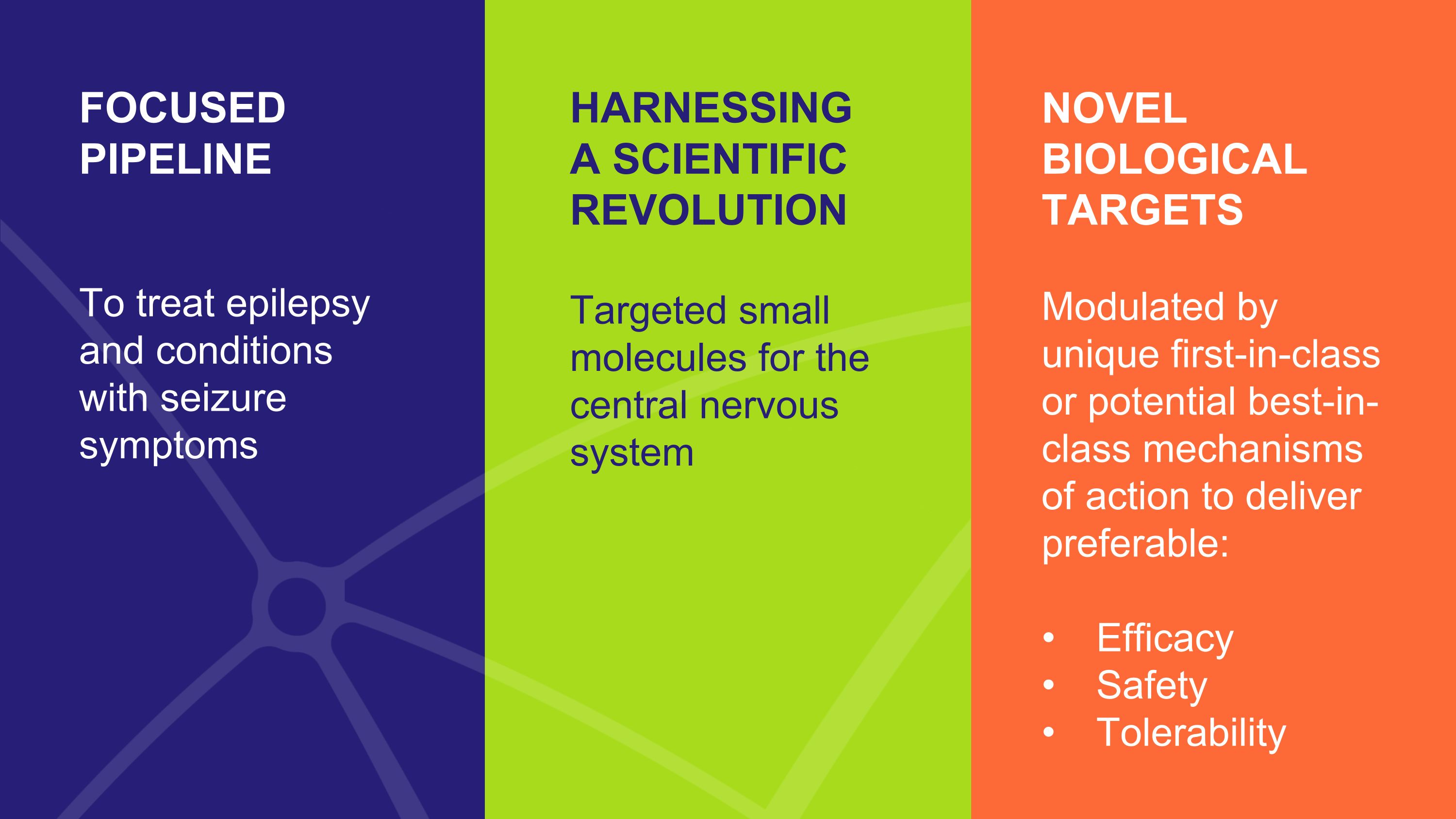
NOVEL BIOLOGICAL TARGETS Modulated by unique first-in-class or potential best-in-class mechanisms of action to deliver preferable: Efficacy Safety Tolerability HARNESSING �A SCIENTIFIC REVOLUTION Targeted small molecules for the central nervous system FOCUSED �PIPELINE To treat epilepsy and conditions with seizure symptoms
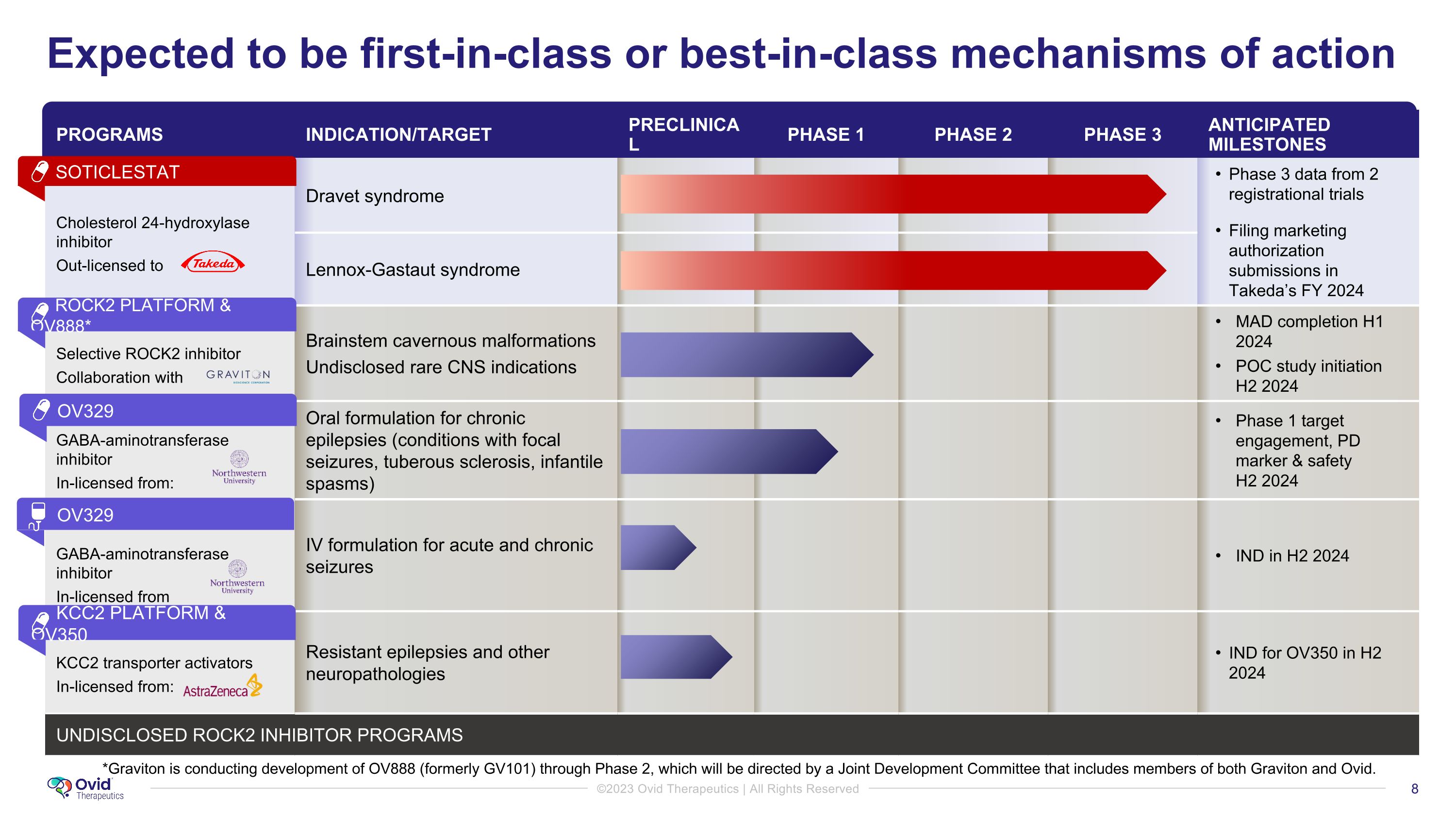
PROGRAMS INDICATION/TARGET PRECLINICAL PHASE 1 PHASE 2 PHASE 3 ANTICIPATED MILESTONES Cholesterol 24-hydroxylase inhibitor Out-licensed to Dravet syndrome Phase 3 data from 2 registrational trials Filing marketing authorization submissions in Takeda’s FY 2024 Lennox-Gastaut syndrome Selective ROCK2 inhibitor Collaboration with Brainstem cavernous malformations Undisclosed rare CNS indications MAD completion H1 2024 POC study initiation H2 2024 GABA-aminotransferase inhibitor In-licensed from: GABA-aminotransferase inhibitor In-licensed from Oral formulation for chronic epilepsies (conditions with focal seizures, tuberous sclerosis, infantile spasms) Phase 1 target engagement, PD marker & safety �H2 2024 IV formulation for acute and chronic seizures IND in H2 2024 KCC2 transporter activators In-licensed from: Resistant epilepsies and other �neuropathologies IND for OV350 in H2 2024 UNDISCLOSED ROCK2 inhibitor programs GENETIC RESEARCH PROGRAMS Expected to be first-in-class or best-in-class mechanisms of action *Graviton is conducting development of OV888 (formerly GV101) through Phase 2, which will be directed by a Joint Development Committee that includes members of both Graviton and Ovid. ROCK2 PLATFORM & OV888* SOTICLESTAT OV329 OV329 KCC2 PLATFORM & OV350
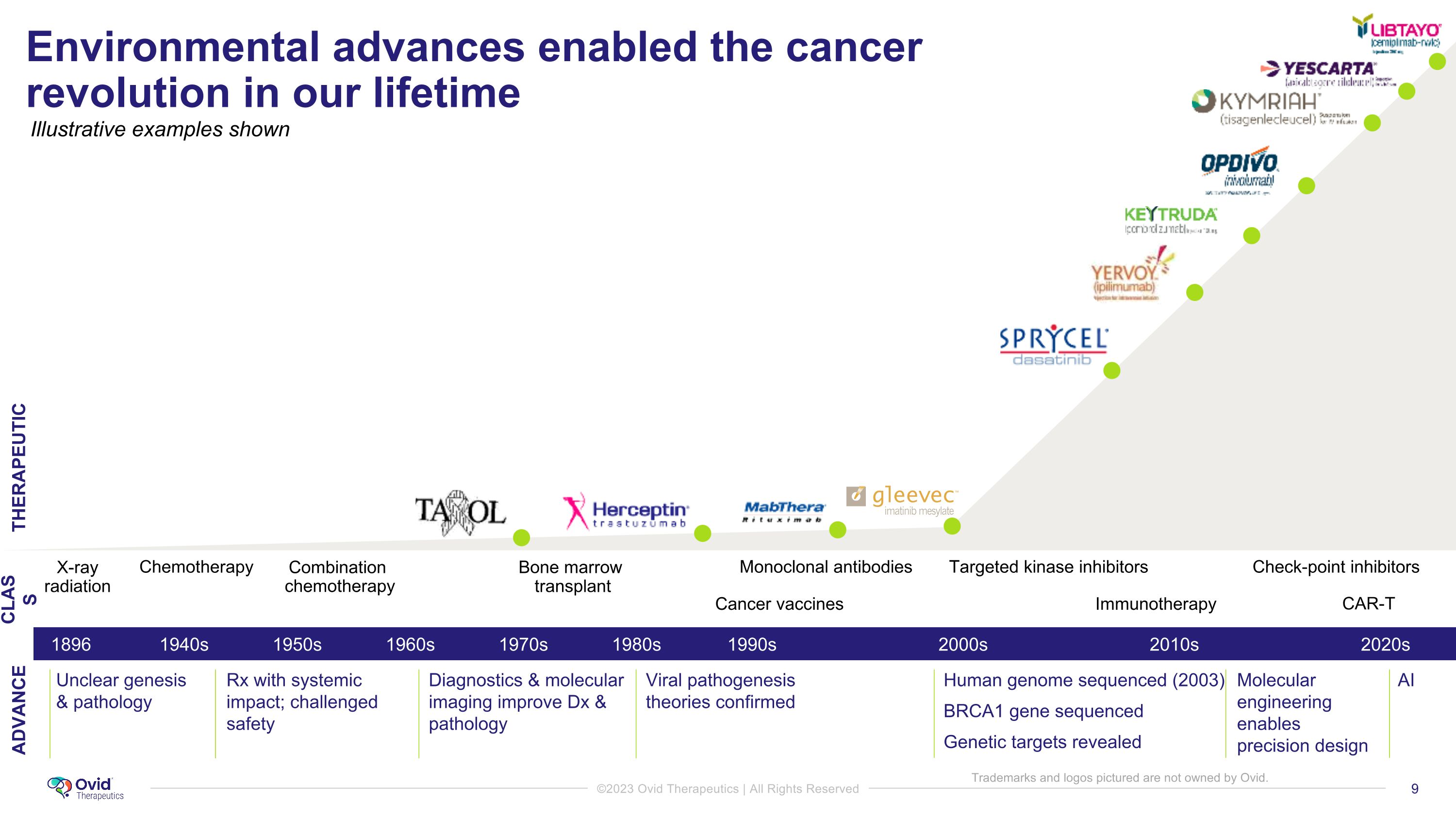
Environmental advances enabled the cancer revolution in our lifetime Unclear genesis & pathology Rx with systemic impact; challenged safety Diagnostics & molecular imaging improve Dx & pathology Viral pathogenesis theories confirmed Human genome sequenced (2003) BRCA1 gene sequenced Genetic targets revealed AI Molecular engineering enables precision design 2000s 2010s 1940s 1896 1990s 1950s 1960s 1980s 2020s 1970s Chemotherapy X-ray�radiation Bone marrow �transplant Combination� chemotherapy Monoclonal antibodies Cancer vaccines Check-point inhibitors Targeted kinase inhibitors Immunotherapy CAR-T CLASS ADVANCE THERAPEUTIC Illustrative examples shown Trademarks and logos pictured are not owned by Ovid.
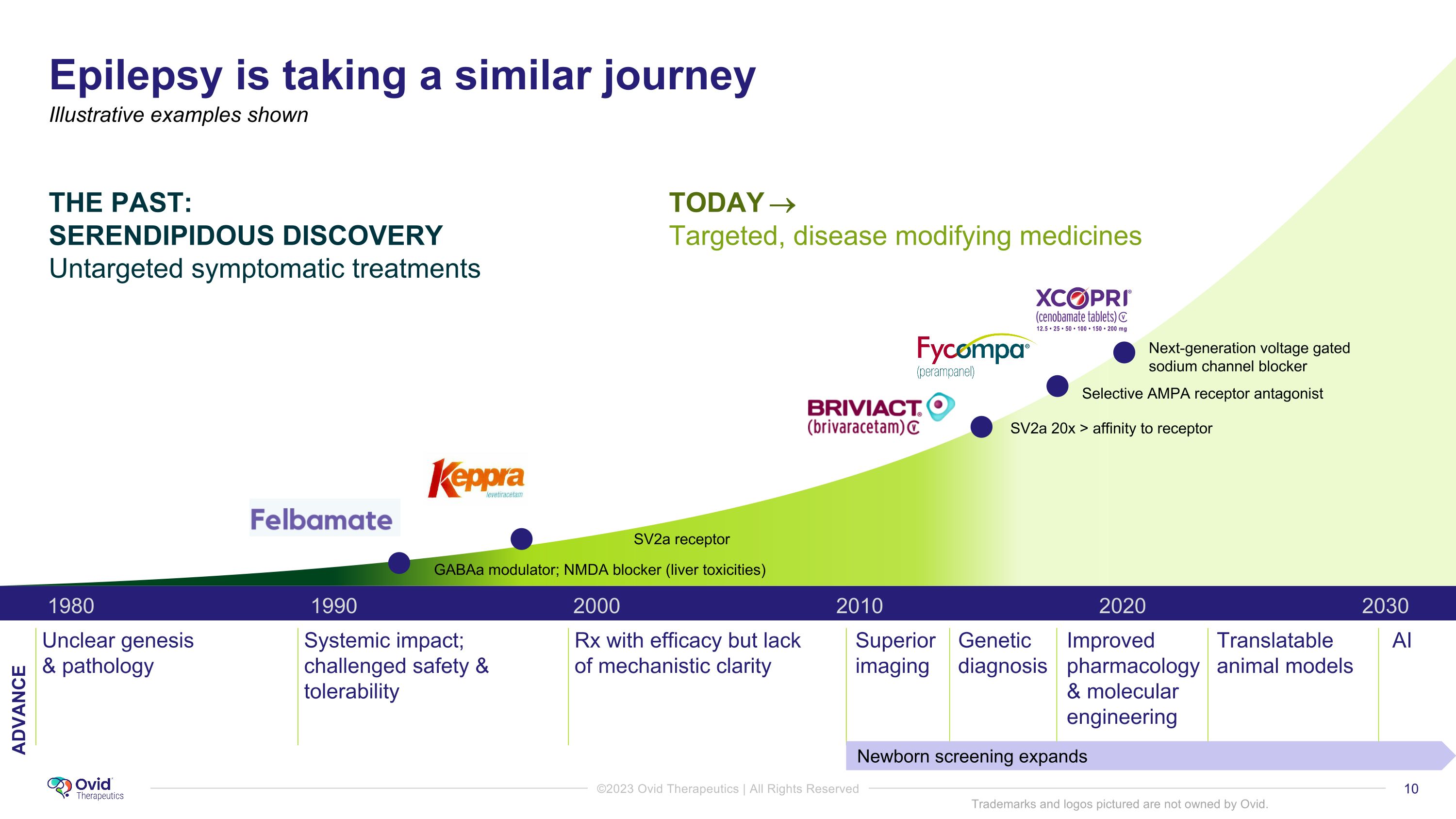
Epilepsy is taking a similar journey 1990 2000 1980 2010 2030 2020 Unclear genesis & pathology Systemic impact; challenged safety & tolerability Rx with efficacy but lack of mechanistic clarity Superior imaging AI Genetic diagnosis Improved pharmacology & molecular engineering Newborn screening expands SV2a receptor SV2a 20x > affinity to receptor Next-generation voltage gated sodium channel blocker GABAa modulator; NMDA blocker (liver toxicities) THE PAST: �SERENDIPIDOUS DISCOVERY Untargeted symptomatic treatments TODAY Targeted, disease modifying medicines Illustrative examples shown Translatable animal models Trademarks and logos pictured are not owned by Ovid. ADVANCE Selective AMPA receptor antagonist

The need for new mechanisms & medicines in epilepsy Dr. Imad Najm Director of Epilepsy Center Vice Chair for Strategy & Development, Cleveland Clinic Neurological Institute

Epilepsy has been known for 3,000 years; �yet remains a major unmet need Up to 1 in 10 humans will experience a seizure in their lifetime1 Living with epilepsy across the globe3 million 50 adults living with epilepsy in the US4 million 3 ≤17 years �have epilepsy4 US children 470,000 Epilepsy. World Health Organization. Updated February 9, 2022. Accessed July 12, 2022. https://www.who.int/news-room/fact-sheets/detail/epilepsy. Terman SW, Aubert CE, Hill CE, et al. Polypharmacy in patients with epilepsy: a nationally representative cross-sectional study. Epilepsy Behav. 2020;111:107261. World Health Organization epilepsy fact sheet updated in February 2023. https://www.who.int/news-room/fact-sheets/detail/epilepsy Epilepsy fast facts. Centers for Disease Contr Easl and Prevention. Updated September 30, 2020. Accessed July 12, 2022. https://www.cdc.gov/epilepsy/about/fast-facts.htm. Who can get epilepsy. Epilepsy Foundation. Updated February 4, 2022. Accessed July 12, 2022. https://www.epilepsy.com/what-is-epilepsy/understanding-seizures/who-gets-epilepsy. Reimbursement & coverage dynamics remain favorable Polypharmacy generally well covered $$ ~47% of epilepsy patients report polypharmacy – taking on average 5 medications2
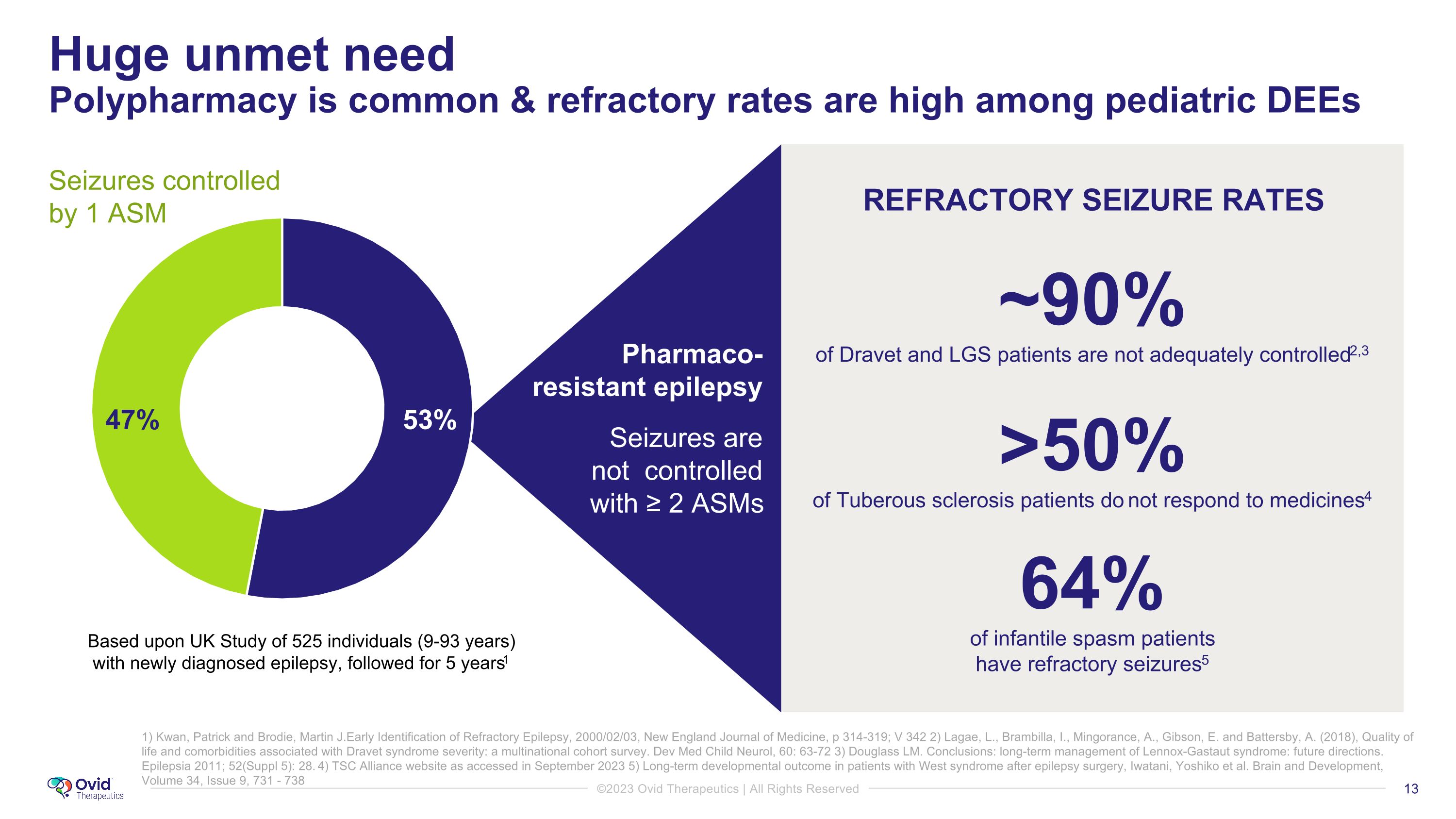
Huge unmet need�Polypharmacy is common & refractory rates are high among pediatric DEEs 1) Kwan, Patrick and Brodie, Martin J.Early Identification of Refractory Epilepsy, 2000/02/03, New England Journal of Medicine, p 314-319; V 342 2) Lagae, L., Brambilla, I., Mingorance, A., Gibson, E. and Battersby, A. (2018), Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol, 60: 63-72 3) Douglass LM. Conclusions: long-term management of Lennox-Gastaut syndrome: future directions. Epilepsia 2011; 52(Suppl 5): 28. 4) TSC Alliance website as accessed in September 2023 5) Long-term developmental outcome in patients with West syndrome after epilepsy surgery, Iwatani, Yoshiko et al. Brain and Development, Volume 34, Issue 9, 731 - 738 REFRACTORY SEIZURE RATES ~90% of Dravet and LGS patients are not adequately controlled2,3 >50% of Tuberous sclerosis patients do not respond to medicines4 64% of infantile spasm patients �have refractory seizures5 Based upon UK Study of 525 individuals (9-93 years) with newly diagnosed epilepsy, followed for 5 years1 Seizures controlled by 1 ASM Pharmaco- resistant epilepsy Seizures are not controlled with ≥ 2 ASMs 53% 47%

Why is this happening? Historically, anti-seizure medicines �have failed to Adequately target the root cause(s) underlying excitability Achieve a maximal effective dose due to side effects or tolerability Avoid “off target” effects causing safety issues
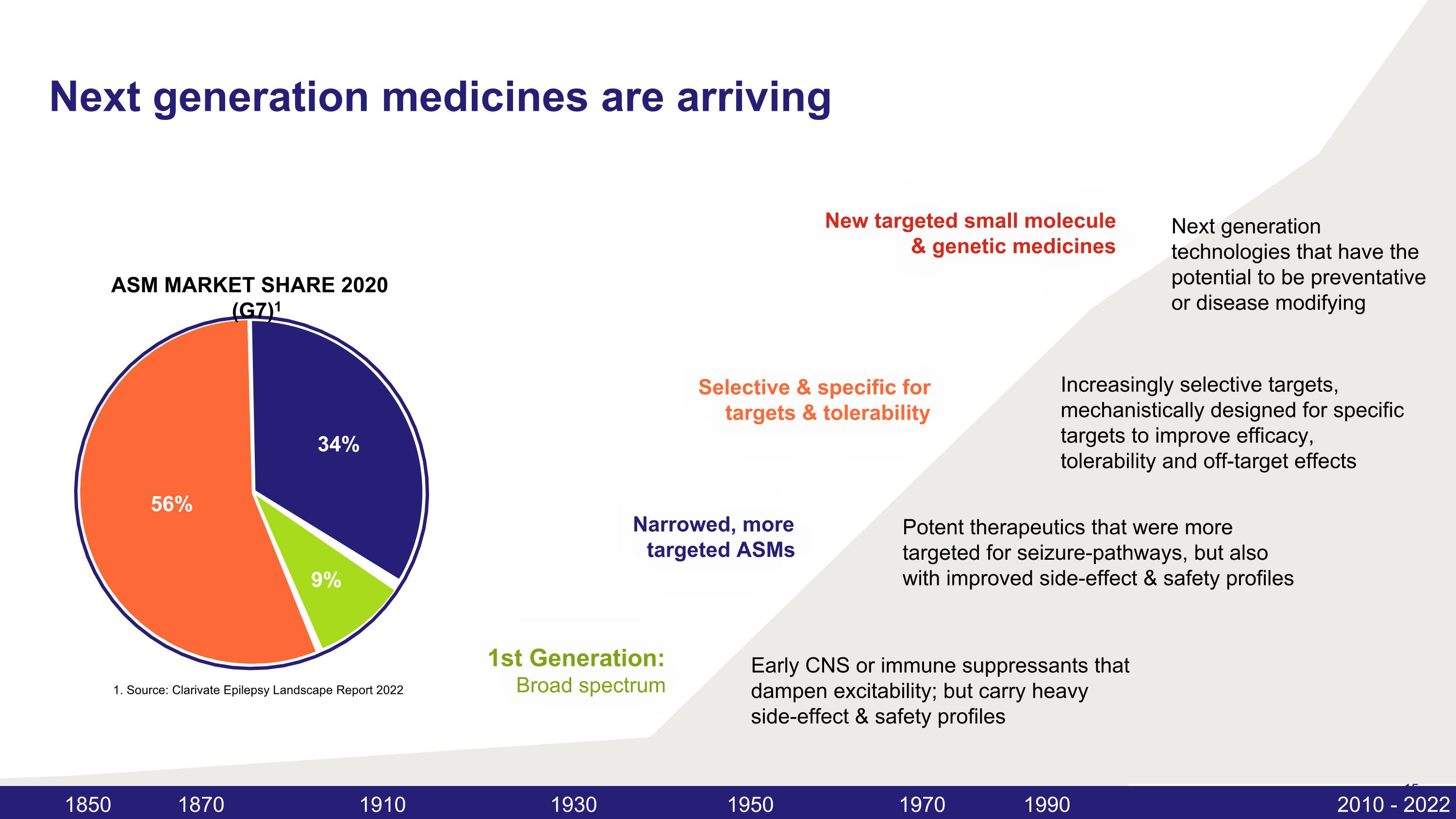
Next generation technologies that have the potential to be preventative or disease modifying New targeted small molecule & genetic medicines 9% Early CNS or immune suppressants that dampen excitability; but carry heavy side-effect & safety profiles 1st Generation: Broad spectrum 34% Potent therapeutics that were more targeted for seizure-pathways, but also with improved side-effect & safety profiles Narrowed, more targeted ASMs 56% Increasingly selective targets, mechanistically designed for specific targets to improve efficacy, tolerability and off-target effects Selective & specific for targets & tolerability 1930 1910 1870 1950 1850 1970 1990 2010 - 2022 ASM MARKET SHARE 2020 (G7)1 1. Source: Clarivate Epilepsy Landscape Report 2022 Next generation medicines are arriving

Scientific & pipeline strategy Meg Alexander Chief Strategy Officer
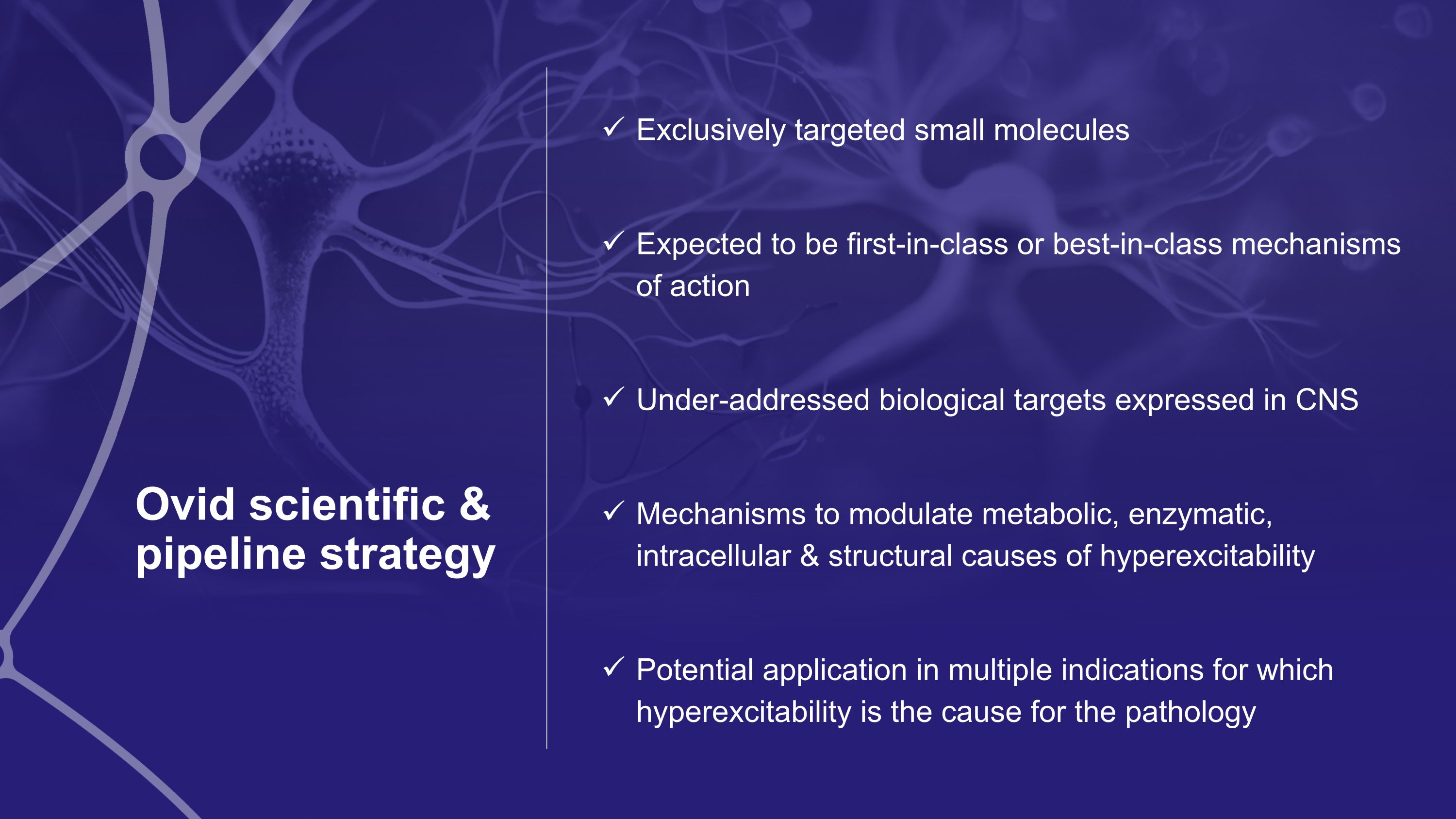
Ovid scientific & pipeline strategy Exclusively targeted small molecules Expected to be first-in-class or best-in-class mechanisms of action Under-addressed biological targets expressed in CNS Mechanisms to modulate metabolic, enzymatic, intracellular & structural causes of hyperexcitability Potential application in multiple indications for which hyperexcitability is the cause for the pathology
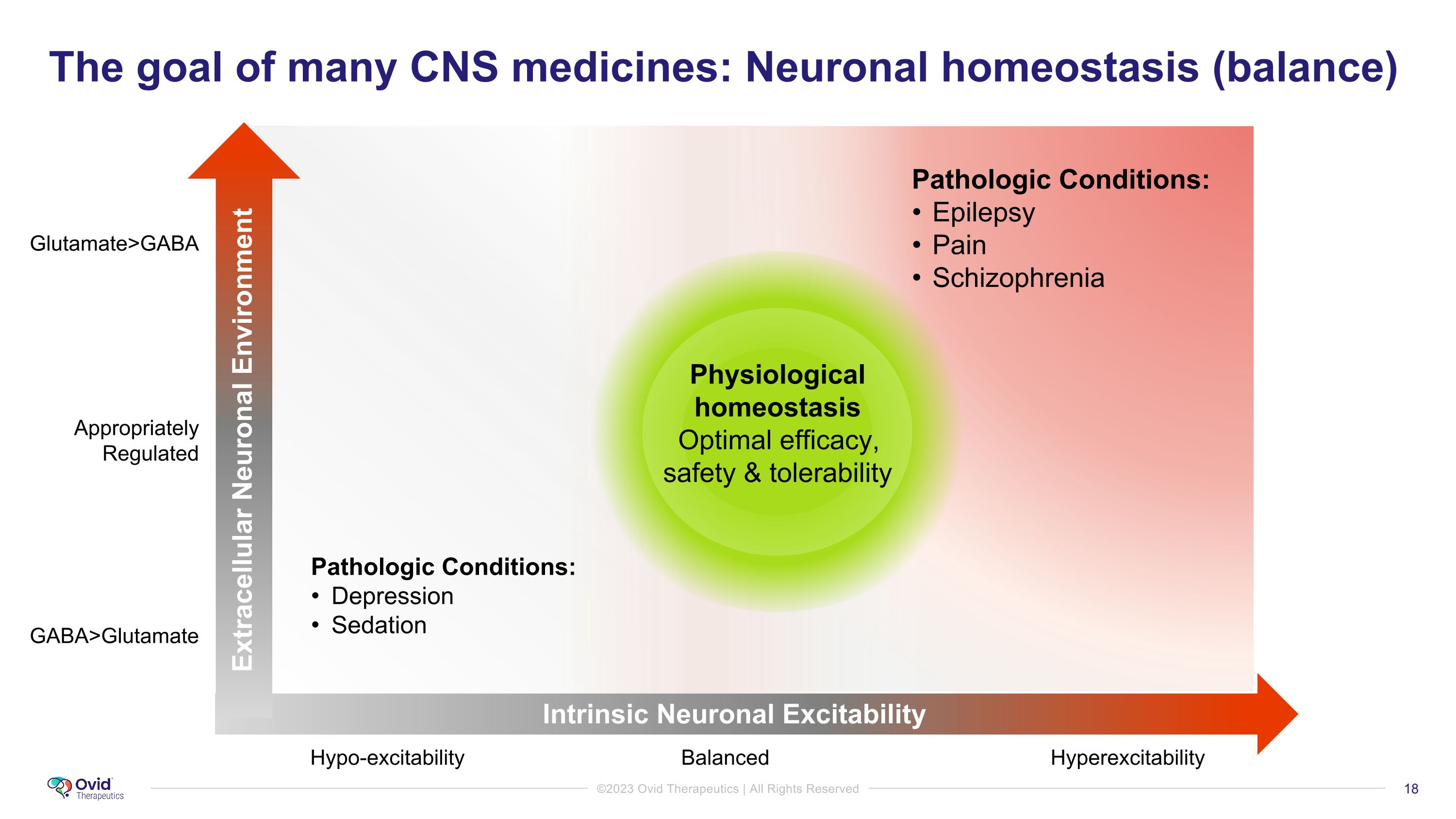
The goal of many CNS medicines: Neuronal homeostasis (balance) GABA>Glutamate Glutamate>GABA Appropriately Regulated Hypo-excitability Balanced Hyperexcitability Intrinsic Neuronal Excitability Extracellular Neuronal Environment Physiological homeostasis Optimal efficacy, safety & tolerability Pathologic Conditions: Epilepsy Pain Schizophrenia Pathologic Conditions: Depression Sedation
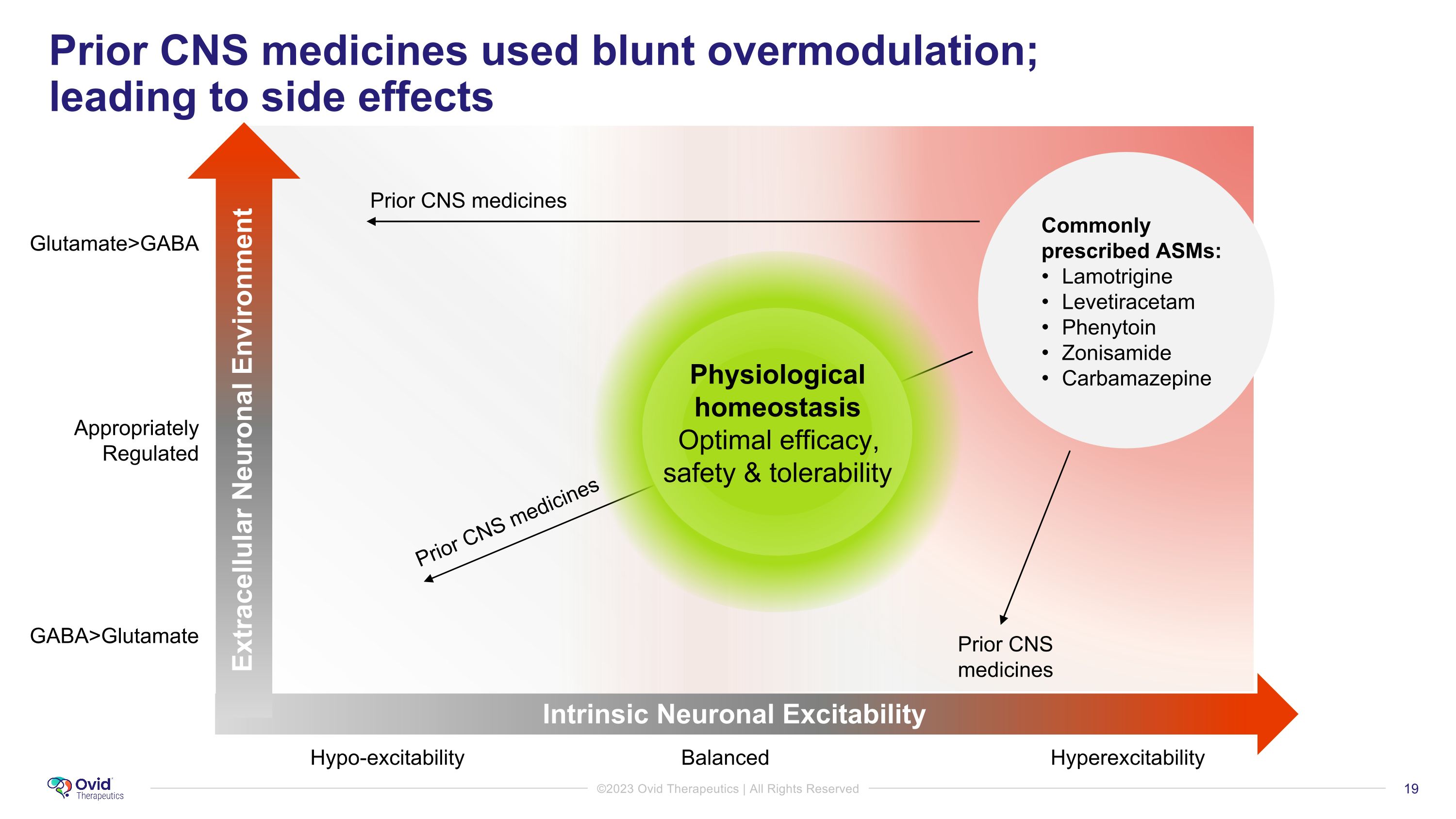
Prior CNS medicines used blunt overmodulation; �leading to side effects Prior CNS medicines Prior CNS medicines GABA>Glutamate Glutamate>GABA Appropriately Regulated Hypo-excitability Balanced Hyperexcitability Commonly prescribed ASMs: Lamotrigine Levetiracetam Phenytoin Zonisamide Carbamazepine Physiological homeostasis Optimal efficacy, safety & tolerability Prior CNS medicines Intrinsic Neuronal Excitability Extracellular Neuronal Environment
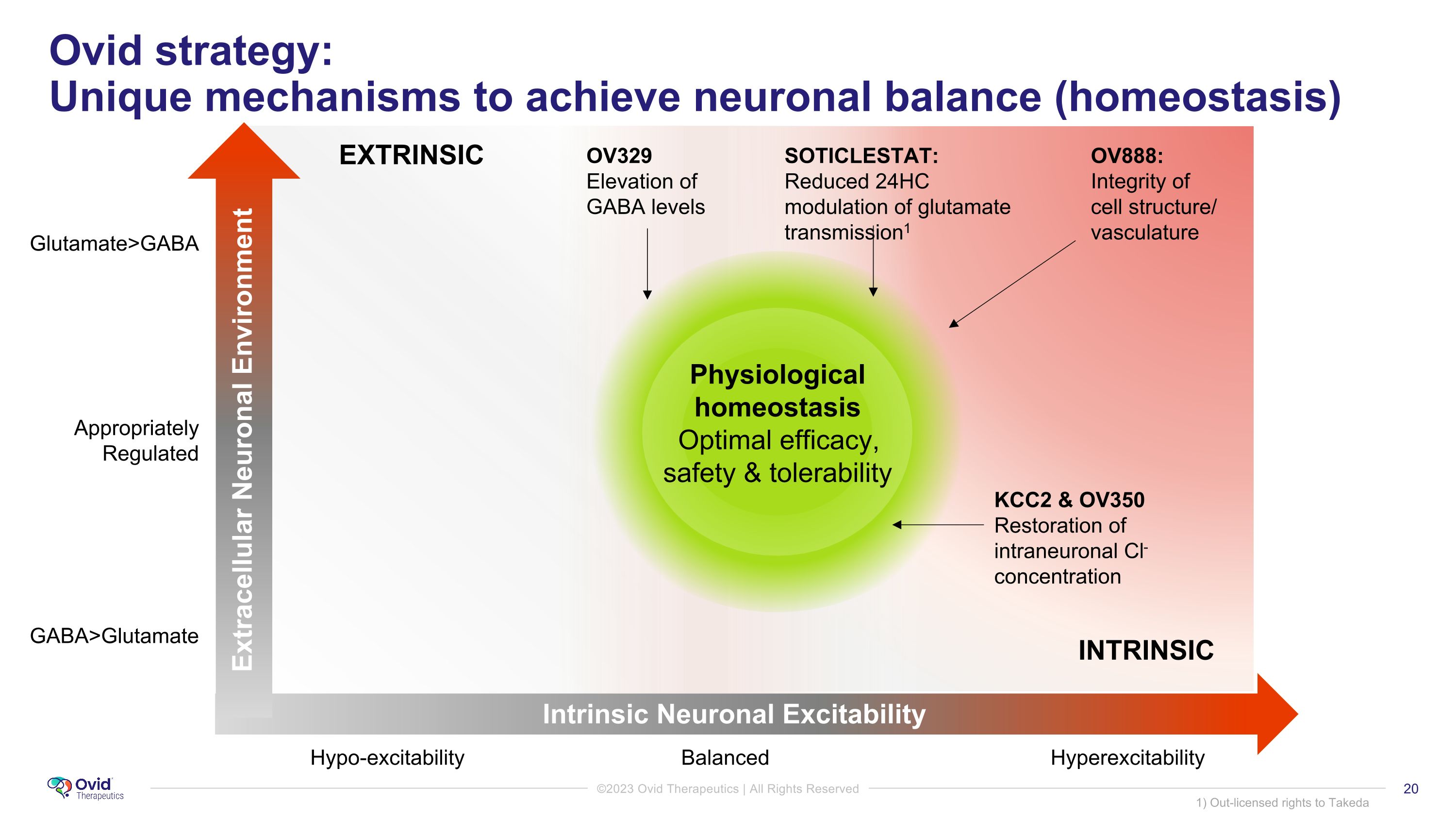
Ovid strategy: �Unique mechanisms to achieve neuronal balance (homeostasis) Physiological homeostasis Optimal efficacy, safety & tolerability OV329 Elevation of GABA levels SOTICLESTAT: Reduced 24HC modulation of glutamate transmission1 KCC2 & OV350 Restoration of intraneuronal Cl-concentration GABA>Glutamate Glutamate>GABA Appropriately Regulated Hypo-excitability Balanced Hyperexcitability OV888: Integrity of �cell structure/ vasculature EXTRINSIC INTRINSIC 1) Out-licensed rights to Takeda Intrinsic Neuronal Excitability Extracellular Neuronal Environment
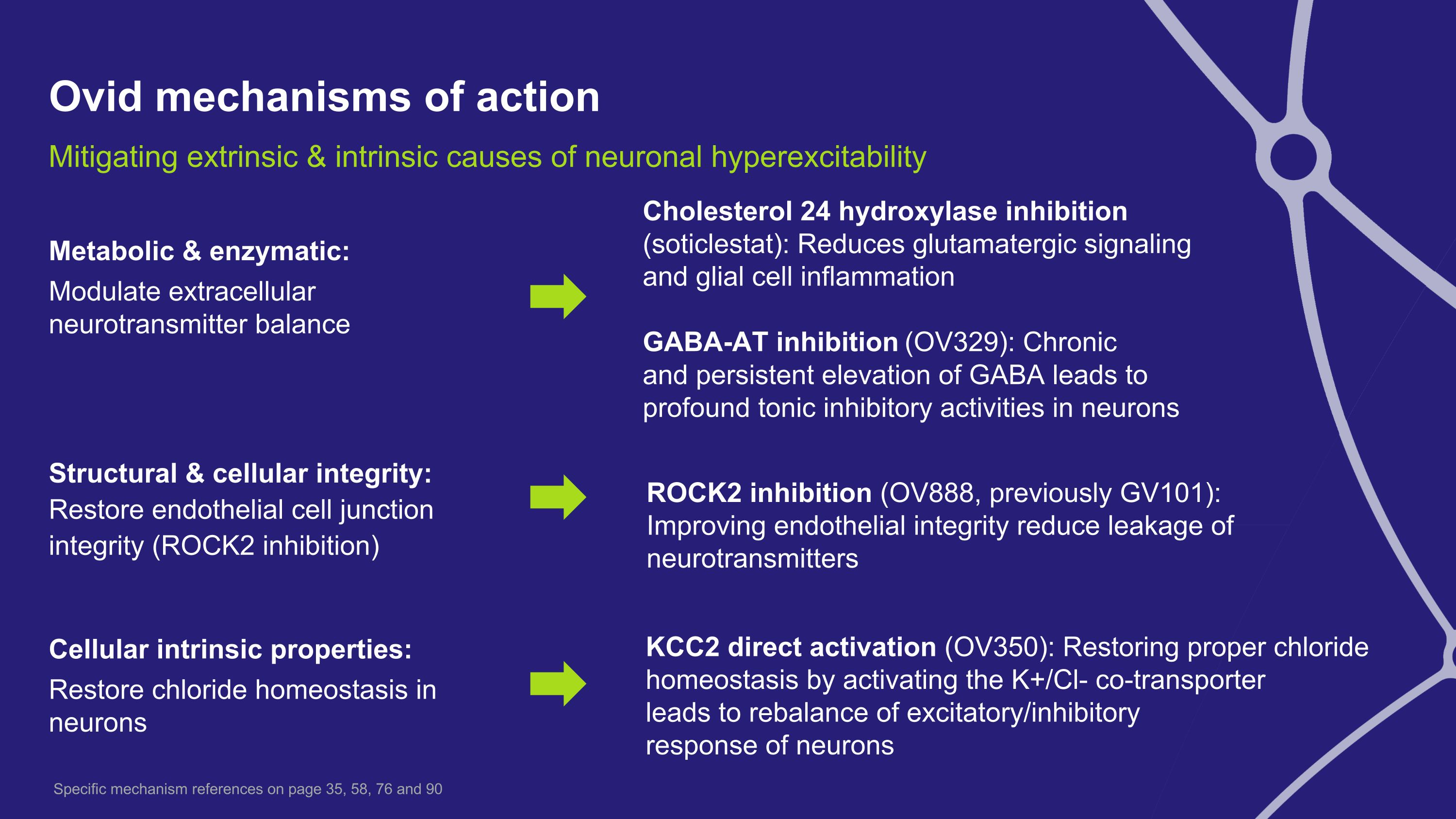
Ovid mechanisms of action Metabolic & enzymatic: Modulate extracellular neurotransmitter balance Structural & cellular integrity: �Restore endothelial cell junction integrity (ROCK2 inhibition) Cellular intrinsic properties: Restore chloride homeostasis in neurons KCC2 direct activation (OV350): Restoring proper chloride homeostasis by activating the K+/Cl- co-transporter leads to rebalance of excitatory/inhibitory response of neurons ROCK2 inhibition (OV888, previously GV101): Improving endothelial integrity reduce leakage of neurotransmitters Mitigating extrinsic & intrinsic causes of neuronal hyperexcitability Cholesterol 24 hydroxylase inhibition (soticlestat): Reduces glutamatergic signaling and glial cell inflammation GABA-AT inhibition (OV329): Chronic �and persistent elevation of GABA leads to profound tonic inhibitory activities in neurons Specific mechanism references on page 35, 58, 76 and 90
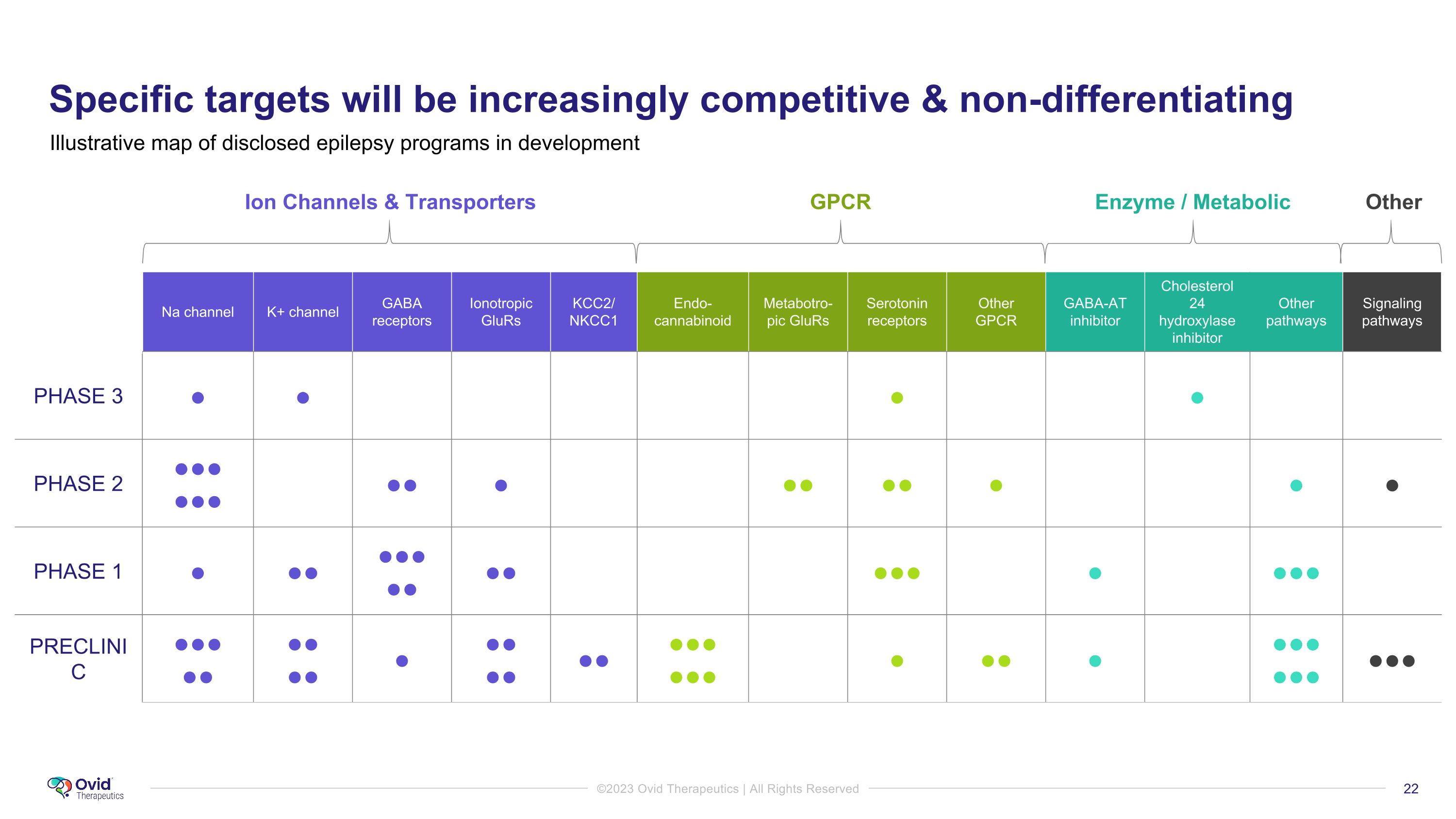
Specific targets will be increasingly competitive & non-differentiating Illustrative map of disclosed epilepsy programs in development Ion Channels & Transporters GPCR Enzyme / Metabolic Other Na channel K+ channel GABA receptors Ionotropic GluRs KCC2/ NKCC1 Endo-cannabinoid Metabotro-pic GluRs Serotonin receptors Other GPCR GABA-AT inhibitor Cholesterol 24 hydroxylase inhibitor Other pathways Signaling pathways PHASE 3 ● ● ● ● PHASE 2 ●●● ●●● ●● ● ●● ●● ● ● ● PHASE 1 ● ●● ●●● ●● ●● ●●● ● ●●● PRECLINIC ●●● ●● ●● ●● ● ●● ●● ●● ●●● ●●● ● ●● ● ●●● ●●● ●●●
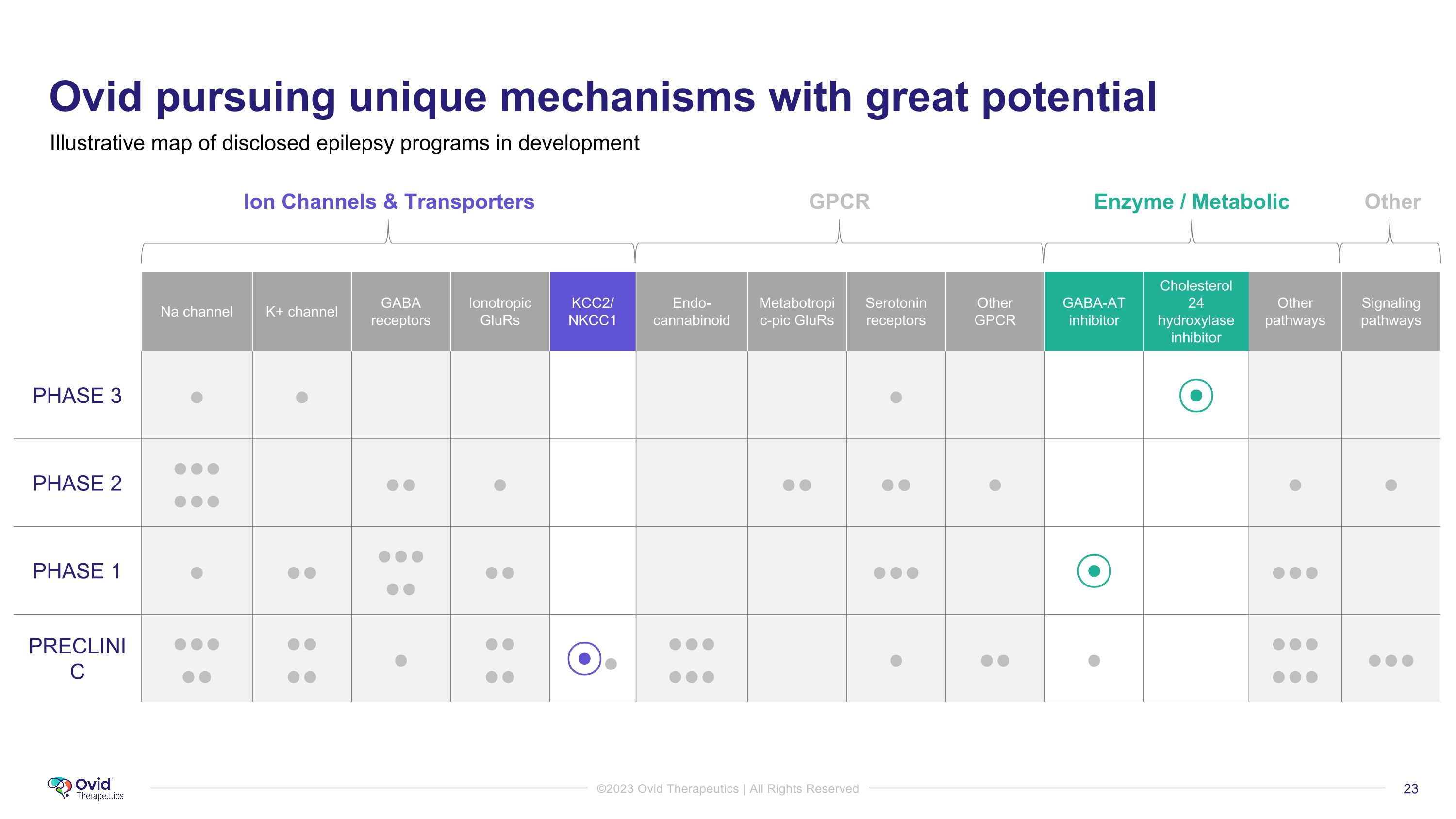
PRECLINIC Ovid pursuing unique mechanisms with great potential Ion Channels & Transporters GPCR Enzyme / Metabolic Other Na channel K+ channel GABA receptors Ionotropic GluRs KCC2/ NKCC1 Endo-cannabinoid Metabotropic-pic GluRs Serotonin receptors Other GPCR GABA-AT inhibitor Cholesterol 24 hydroxylase inhibitor Other pathways Signaling pathways PHASE 3 ● ● ● ⦿ PHASE 2 ●●● ●●● ●● ● ●● ●● ● ● ● PHASE 1 ● ●● ●●● ●● ●● ●●● ⦿ ●●● PRECLINIC ●●● ●● ●● ●● ● ●● ●● ⦿● ●●● ●●● ● ●● ● ●●● ●●● ●●● Illustrative map of disclosed epilepsy programs in development
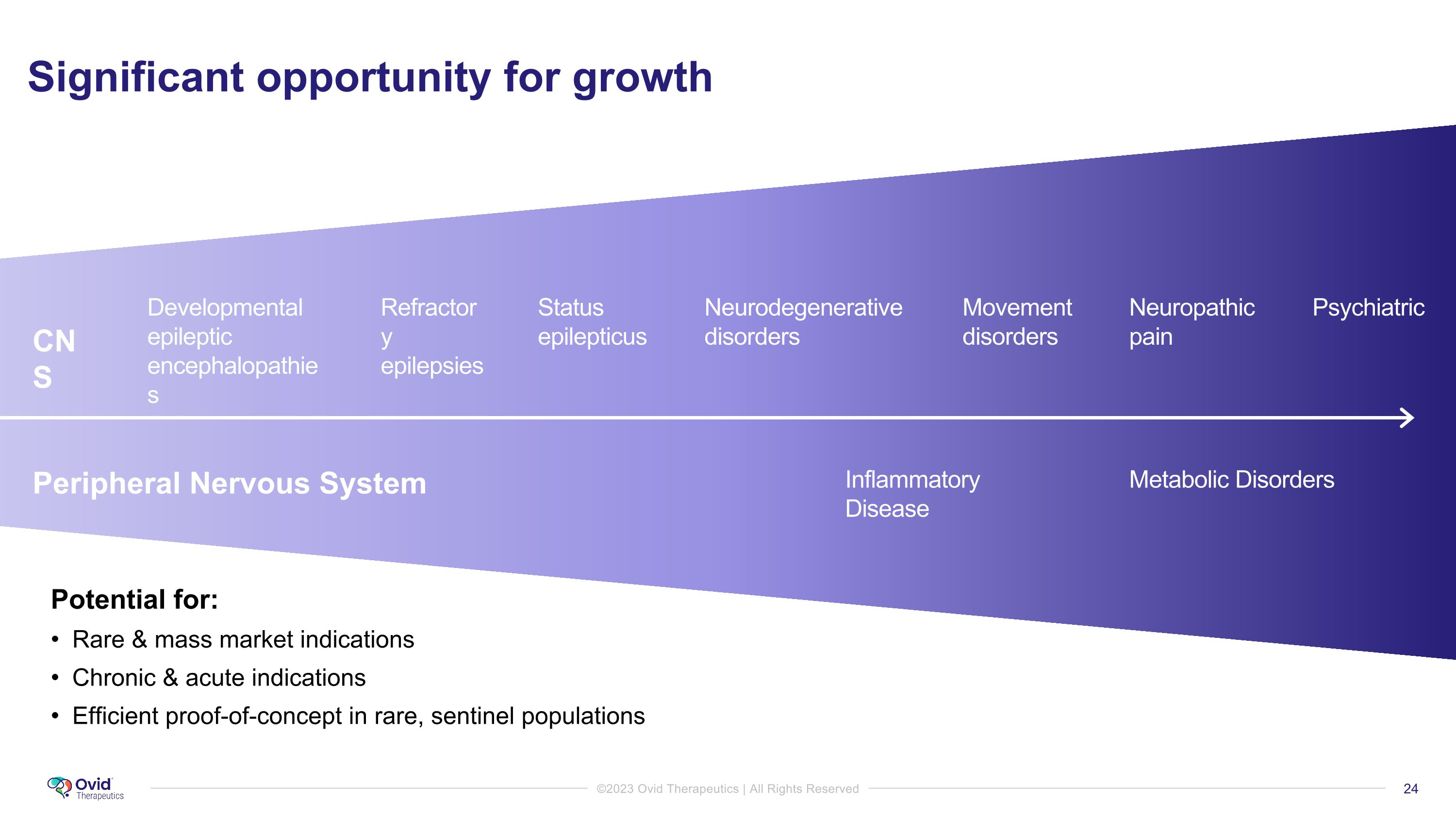
Significant opportunity for growth Potential for: Rare & mass market indications Chronic & acute indications Efficient proof-of-concept in rare, sentinel populations Developmental epileptic encephalopathies Status epilepticus Neurodegenerative disorders Neuropathic pain Psychiatric Refractory epilepsies Movement disorders Metabolic Disorders Inflammatory Disease CNS Peripheral Nervous System
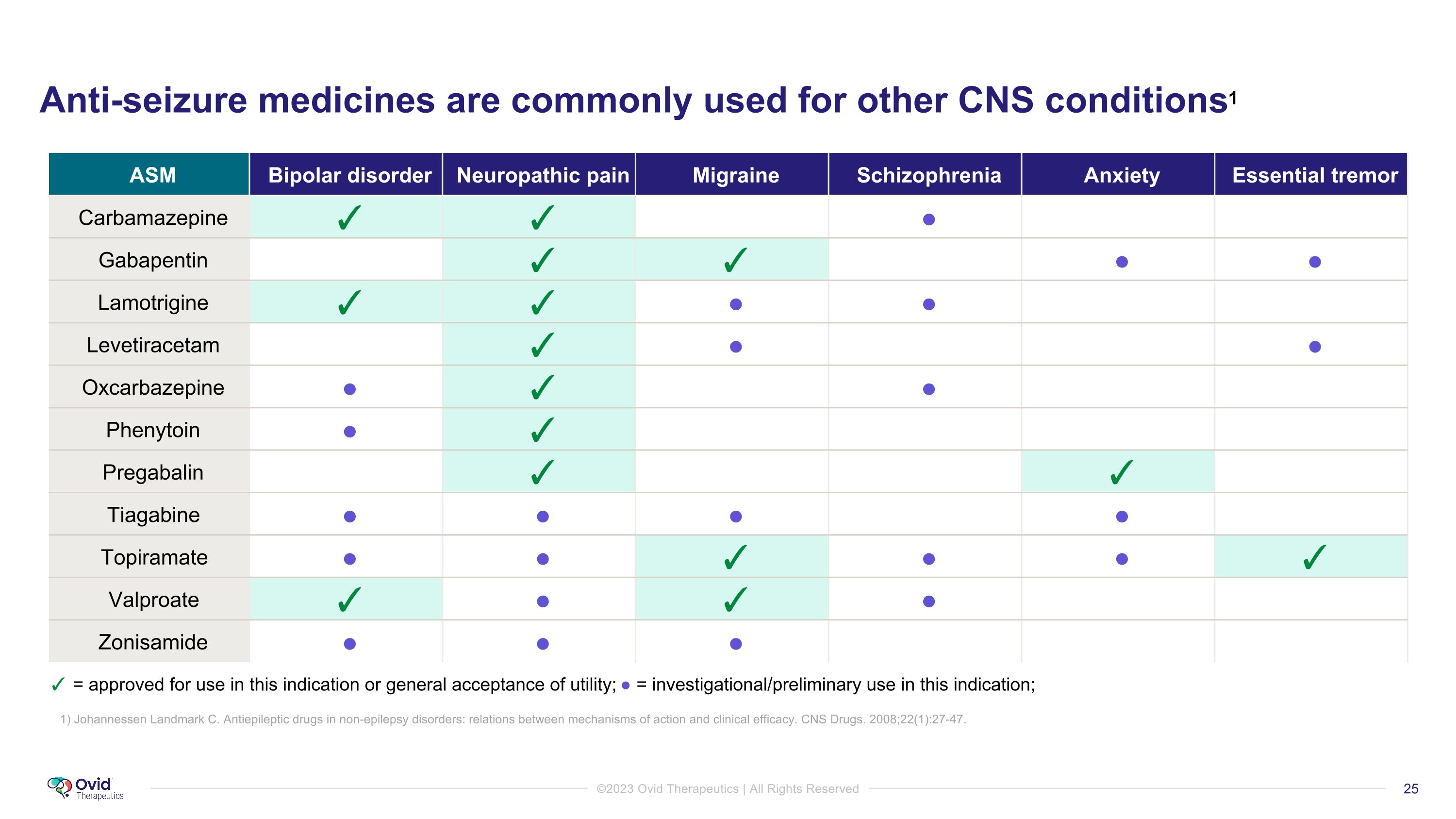
Anti-seizure medicines are commonly used for other CNS conditions1 ASM Bipolar disorder Neuropathic pain Migraine Schizophrenia Anxiety Essential tremor Carbamazepine ✓ ✓ ● Gabapentin ✓ ✓ ● ● Lamotrigine ✓ ✓ ● ● Levetiracetam ✓ ● ● Oxcarbazepine ● ✓ ● Phenytoin ● ✓ Pregabalin ✓ ✓ Tiagabine ● ● ● ● Topiramate ● ● ✓ ● ● ✓ Valproate ✓ ● ✓ ● Zonisamide ● ● ● ✓ = approved for use in this indication or general acceptance of utility; ● = investigational/preliminary use in this indication; 1) Johannessen Landmark C. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.

ROCK2 inhibition & OV888 Dr. Zhong Zhong Chief Scientific Officer

OV888��A highly selective ROCK2 inhibitor for cerebral cavernous malformations (CCM) & other CNS disorders

Hemorrhage, symptom exacerbation, and seizure can happen at any time. The disease is mercilessly unpredictable. -Dr. Connie Lee, mother of daughter with cavernous malformations & founder of Alliance to Cure Cavernous Malformations “ . ”
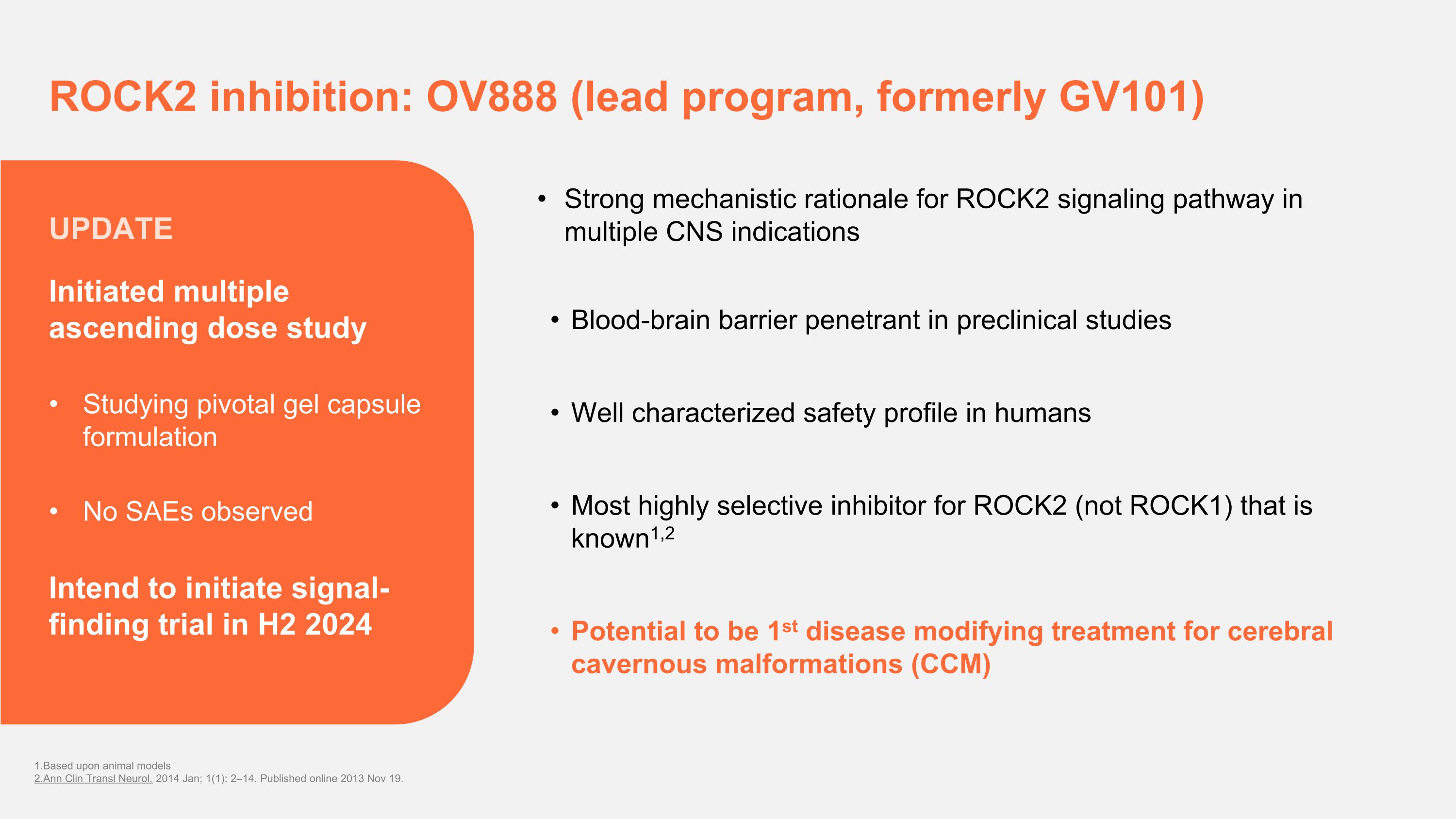
ROCK2 inhibition: OV888 (lead program, formerly GV101) Strong mechanistic rationale for ROCK2 signaling pathway in multiple CNS indications Blood-brain barrier penetrant in preclinical studies Well characterized safety profile in humans Most highly selective inhibitor for ROCK2 (not ROCK1) that is known1,2 Potential to be 1st disease modifying treatment for cerebral cavernous malformations (CCM) 1.Based upon animal models 2.Ann Clin Transl Neurol. 2014 Jan; 1(1): 2–14. Published online 2013 Nov 19. Initiated multiple ascending dose study Studying pivotal gel capsule formulation No SAEs observed Intend to initiate signal- finding trial in H2 2024 UPDATE

EXAMPLE AT CELLULAR LEVEL Wild type Endothelial cytoskeleton abnormalities & weakened cell junctions can lead to lesions and bleeds Relative to wild type (healthy) cells CCM exhibits cellular mis-formation due to stress fibers A. L. Borilkova, et. al (2010) Journal of Biological Chemistry, Vol 285. 11760 and O. Pertz, et al (2006) Nature, Vol 440:1069 Illustrative cerebral cavernous malformation (CCM) brain
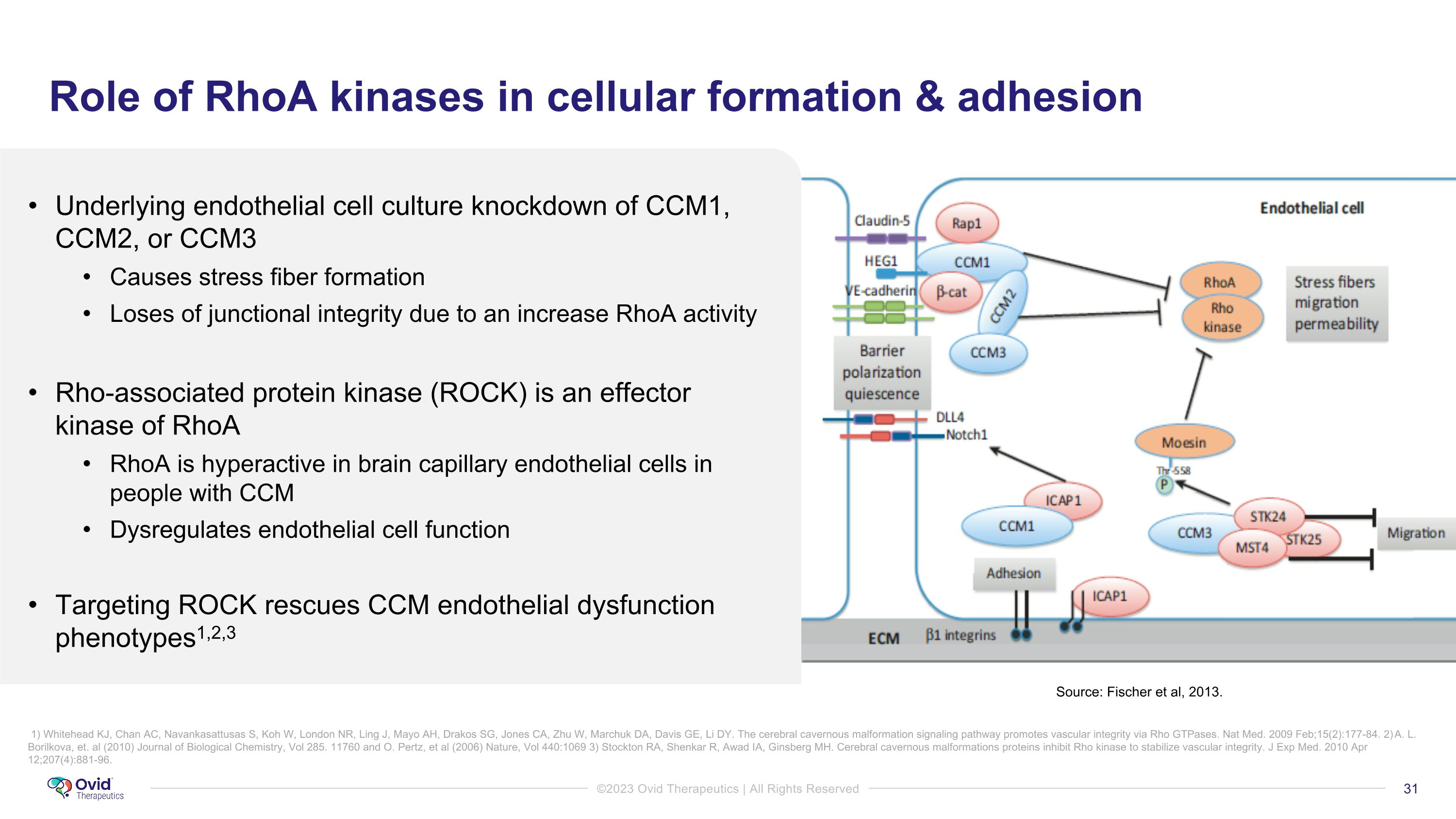
Role of RhoA kinases in cellular formation & adhesion Underlying endothelial cell culture knockdown of CCM1, CCM2, or CCM3 Causes stress fiber formation Loses of junctional integrity due to an increase RhoA activity Rho-associated protein kinase (ROCK) is an effector kinase of RhoA RhoA is hyperactive in brain capillary endothelial cells in people with CCM Dysregulates endothelial cell function Targeting ROCK rescues CCM endothelial dysfunction phenotypes1,2,3 Source: Fischer et al, 2013. 1) Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Jones CA, Zhu W, Marchuk DA, Davis GE, Li DY. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009 Feb;15(2):177-84. 2) A. L. Borilkova, et. al (2010) Journal of Biological Chemistry, Vol 285. 11760 and O. Pertz, et al (2006) Nature, Vol 440:1069 3) Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010 Apr 12;207(4):881-96.
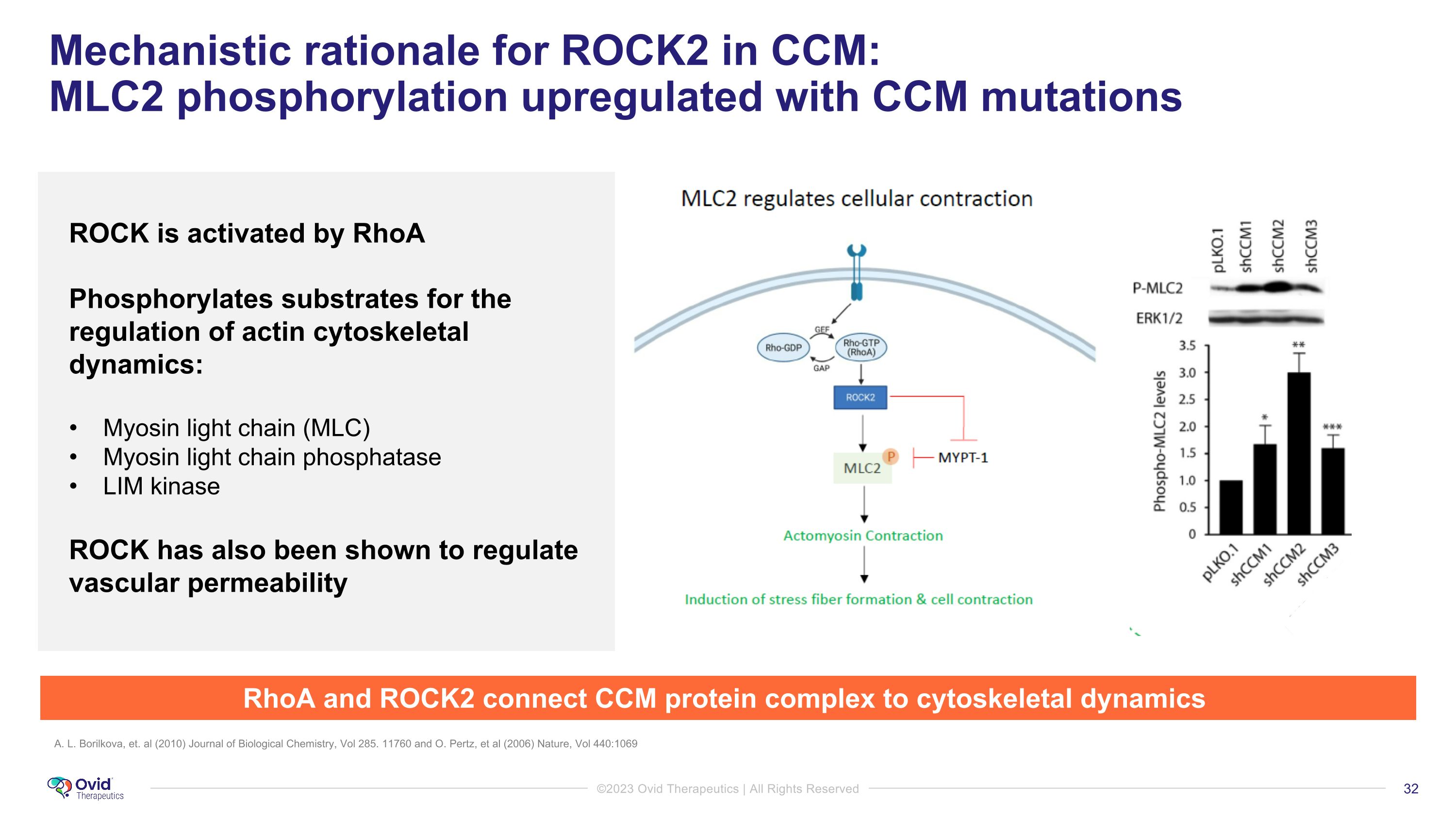
Mechanistic rationale for ROCK2 in CCM: �MLC2 phosphorylation upregulated with CCM mutations WT A. L. Borilkova, et. al (2010) Journal of Biological Chemistry, Vol 285. 11760 and O. Pertz, et al (2006) Nature, Vol 440:1069 ROCK is activated by RhoA Phosphorylates substrates for the regulation of actin cytoskeletal dynamics: Myosin light chain (MLC) Myosin light chain phosphatase LIM kinase ROCK has also been shown to regulate vascular permeability RhoA and ROCK2 connect CCM protein complex to cytoskeletal dynamics
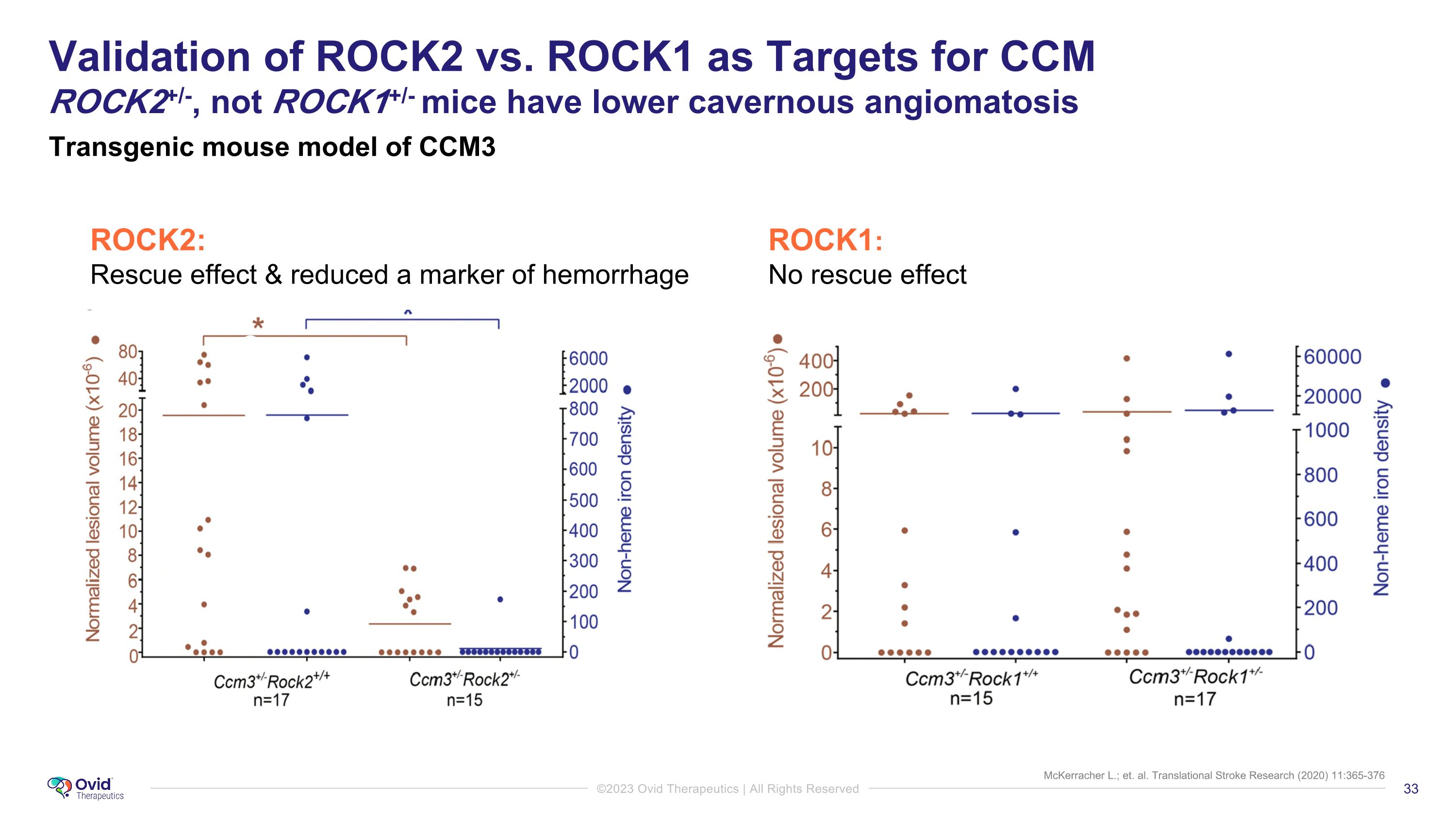
McKerracher L.; et. al. Translational Stroke Research (2020) 11:365-376 Validation of ROCK2 vs. ROCK1 as Targets for CCM�ROCK2+/-, not ROCK1+/- mice have lower cavernous angiomatosis Transgenic mouse model of CCM3 ROCK1: No rescue effect ROCK2: Rescue effect & reduced a marker of hemorrhage

OV888’s potential as a potent, selective, best-in-class ROCK2 inhibitor Molecules with pan-ROCK inhibition have shown off-target effects ROCK2�IC50 (uM) ROCK1 �IC50 (uM) Selectivity (ROCK1/ROCK2) BBB Penetrant Off-Target Effects Indication OV888 OVID/GRAVITON 0.002-0.02 12.1 1071 fold CCM REDX10843 RedX Pharma Plc 0.2 2.25 ~100 fold × Fibrotic disease KD0251 Kadmon Pharmaceuticals 0.105 24 ~80 - 100 fold × Graft vs host disease NRL-10492 (Neurellis) 0.24 – 0.73 3.9 – 10.2 13.9 – 16 fold CCM 1 Ann Clin Transl Neurol. 2014 Jan; 1(1): 2–14. Published online 2013 Nov 19. doi: 10.1002/acn3.19 2 https://patents.google.com/patent/US10106525B2/en Chart based on biochemical assays
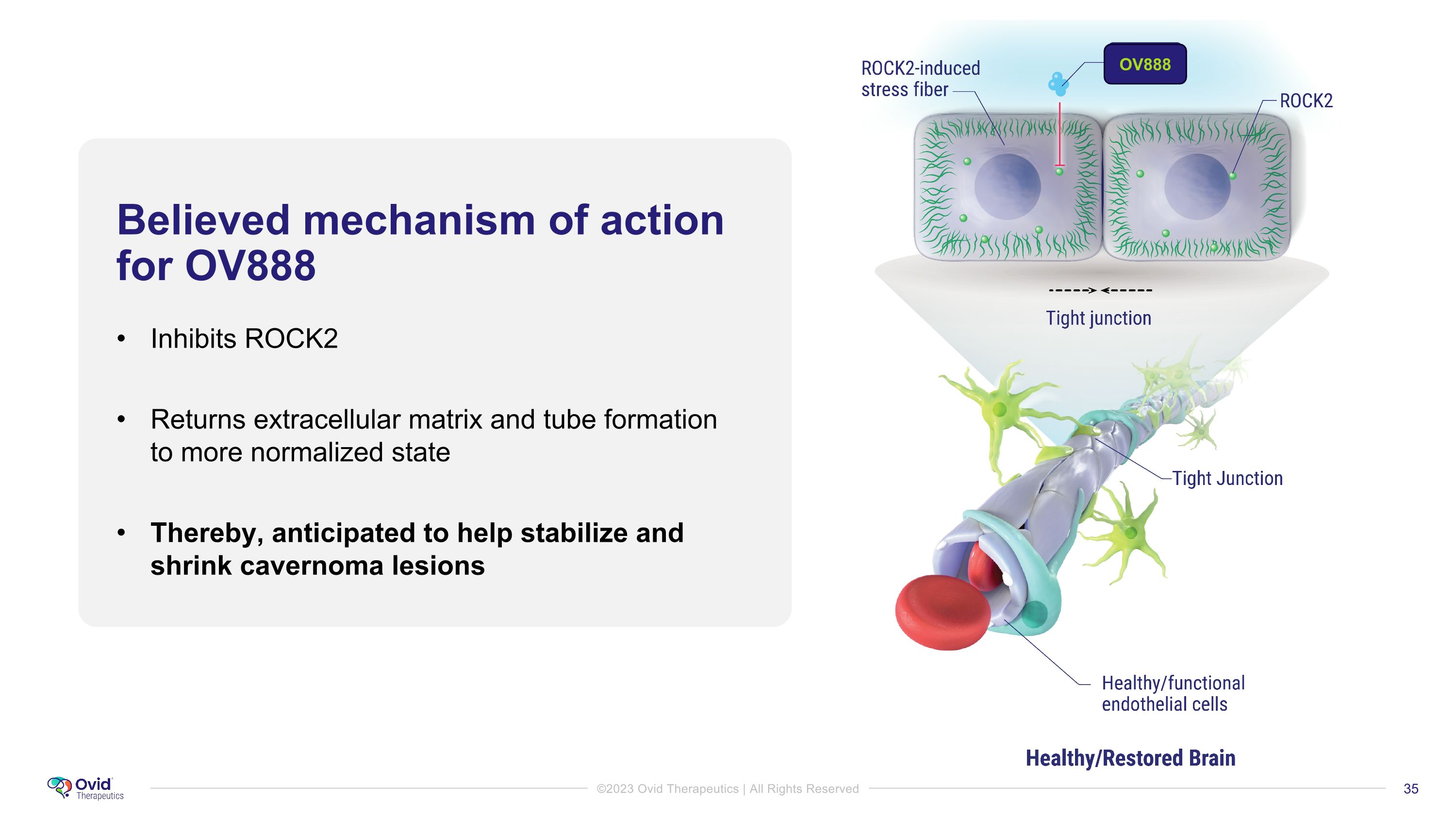
Believed mechanism of action for OV888 Inhibits ROCK2 Returns extracellular matrix and tube formation to more normalized state Thereby, anticipated to help stabilize and shrink cavernoma lesions OV888

Unmet need in cavernous malformations Dr. Connie Lee, President & Founder of the Alliance to Cure Cavernous Malformations

Cerebral Cavernous Malformation (CCM) Connie Lee, CEO October 2023
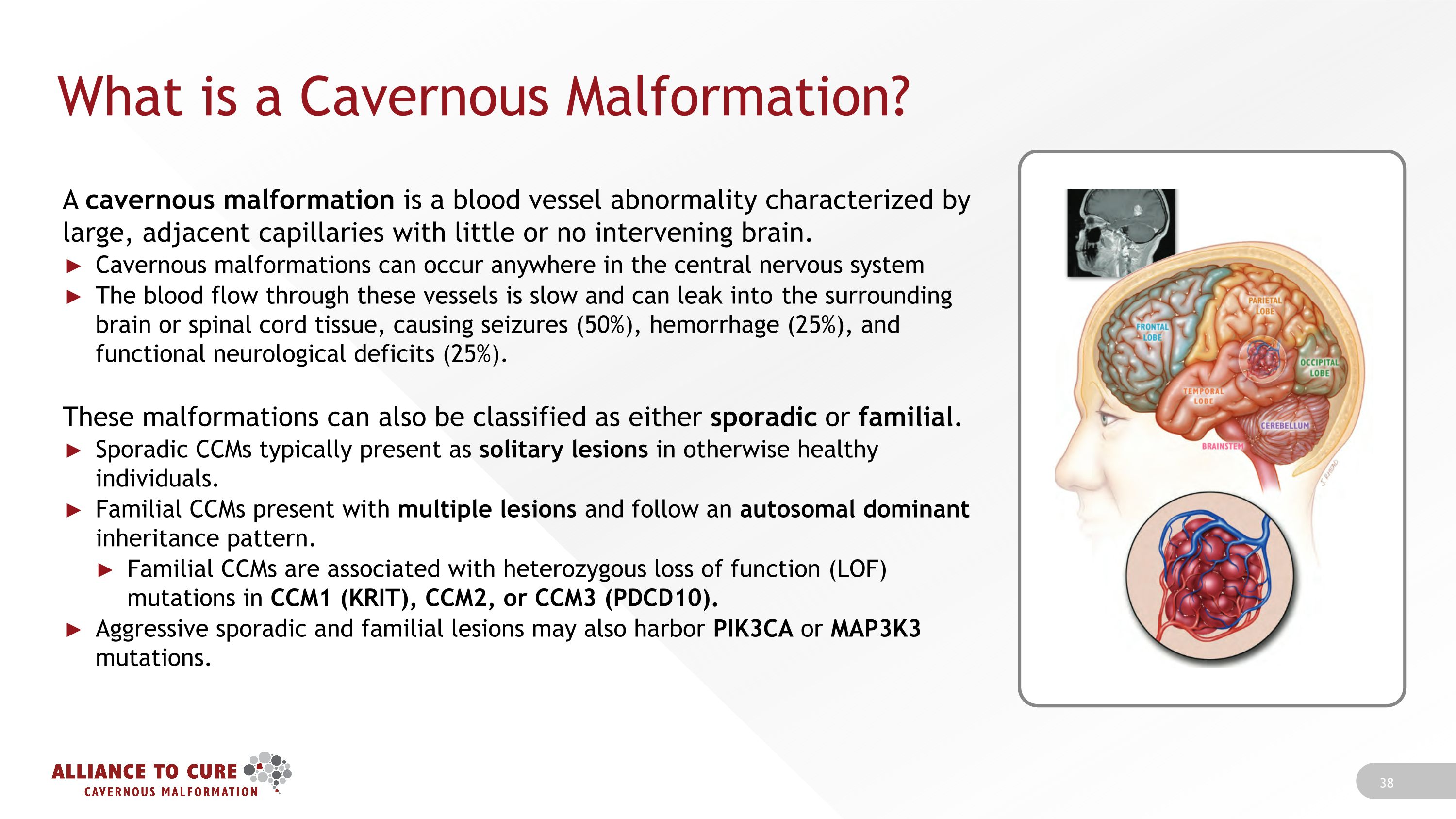
What is a Cavernous Malformation? A cavernous malformation is a blood vessel abnormality characterized by large, adjacent capillaries with little or no intervening brain. Cavernous malformations can occur anywhere in the central nervous system The blood flow through these vessels is slow and can leak into the surrounding brain or spinal cord tissue, causing seizures (50%), hemorrhage (25%), and functional neurological deficits (25%). These malformations can also be classified as either sporadic or familial. Sporadic CCMs typically present as solitary lesions in otherwise healthy individuals. Familial CCMs present with multiple lesions and follow an autosomal dominant inheritance pattern. Familial CCMs are associated with heterozygous loss of function (LOF) mutations in CCM1 (KRIT), CCM2, or CCM3 (PDCD10). Aggressive sporadic and familial lesions may also harbor PIK3CA or MAP3K3 mutations.
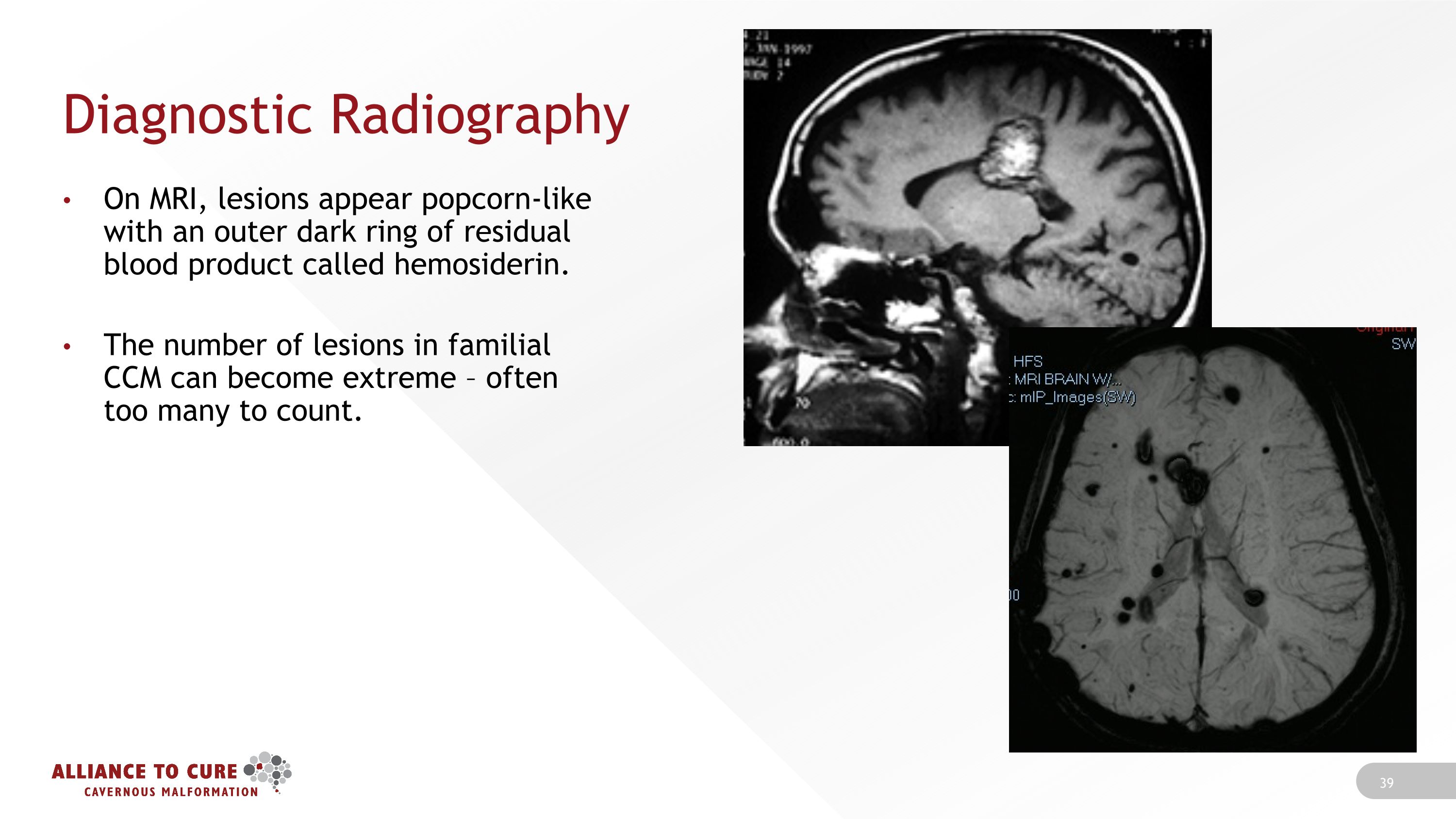
Diagnostic Radiography On MRI, lesions appear popcorn-like with an outer dark ring of residual blood product called hemosiderin. The number of lesions in familial CCM can become extreme – often too many to count.
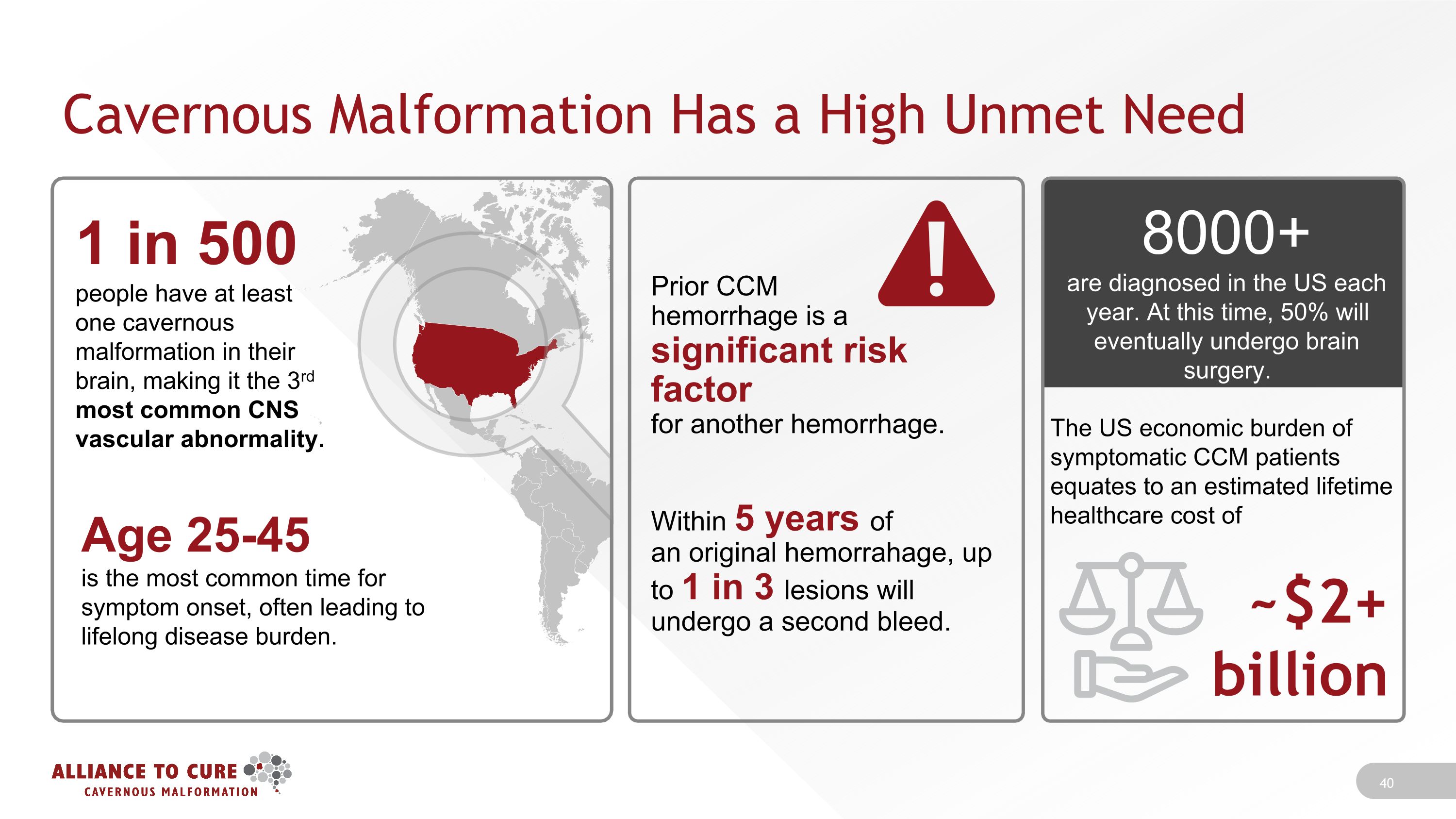
Cavernous Malformation Has a High Unmet Need Prior CCM �hemorrhage is a �significant risk factor �for another hemorrhage. Within 5 years of �an original hemorrahage, up �to 1 in 3 lesions will �undergo a second bleed. 8000+ �are diagnosed in the US each year. At this time, 50% will eventually undergo brain surgery. The US economic burden of symptomatic CCM patients equates to an estimated lifetime healthcare cost of ~$2+ �billion 1 in 500 �people have at least one cavernous malformation in their brain, making it the 3rd most common CNS vascular abnormality. Age 25-45 �is the most common time for symptom onset, often leading to lifelong disease burden.

What Challenges Do Patients Face? Hemorrhage, symptom exacerbation, and seizure can happen at any time. The disease is mercilessly unpredictable. 15-20% of patients in our registry experience serious, lifelong disability. Long-term deterioration from these disabilities can cause premature death. CCM-related seizures can also be fatal. All patients are traumatized. Brain surgery or symptom management remain the only treatment options. Surgery is not an option available to everyone. Because the illness most often strikes young adults who may already be parents, the disease affects the entire family unit.

Thank You! Our families are hopeful for a day when they no longer live in fear of their next hemorrhage, a new onset seizure, or their next life-changing neurological event.

OV888 clinical development update��Dr. Manoj Malhotra Chief Medical Officer

OV888 clinical development update Timing: Initiated multiple ascending dose trial in Q3 Objective: Confirm safety and establish maximum dose tolerated Approach: Randomized, placebo-controlled, double-blind, single-center MAD 8 per dose group; 3:1 randomization 7-day dosing, 72-hour trailing PK data collection Status: No serious adverse events Next steps: Complete multiple ascending dose program Requesting FDA pre-IND meeting Single & multiple ascending dose study in healthy adult volunteers in 2022 Double-blinded, placebo controlled 50 male/female healthy adult volunteers 7-day consecutive dosing: SAD: 400 mg, 800 mg, and 1200 mg MAD: 200 mg and 400 mg Endpoints: Safety & tolerability; PK Findings: 0 SAEs AE of interest (headache (21%), diarrhea (11%), CPK elevations (6%) nausea/vomiting (2%) Similar AUC to U.S. study (AUC 11,600 in 1,200 mg group) PRIOR PHASE 1: NANO-SUSPENSION PROGRESS UPDATE: OV888 CLINICAL FORMULATION
What comes next? Establish proof of concept to “tie” the radiological endpoints to functional & quality of life endpoints Anticipated enrichment strategy Prioritize brainstem and symptomatic patients for whom surgery is not preferred Radiological endpoints to stabilize and shrink the malformation lesion Clinical endpoints to correlate with treatment Open label “signal finding” portion to measure changes at time intervals Targeting “hard” secondary endpoints Radiological scan of a brainstem cavernoma
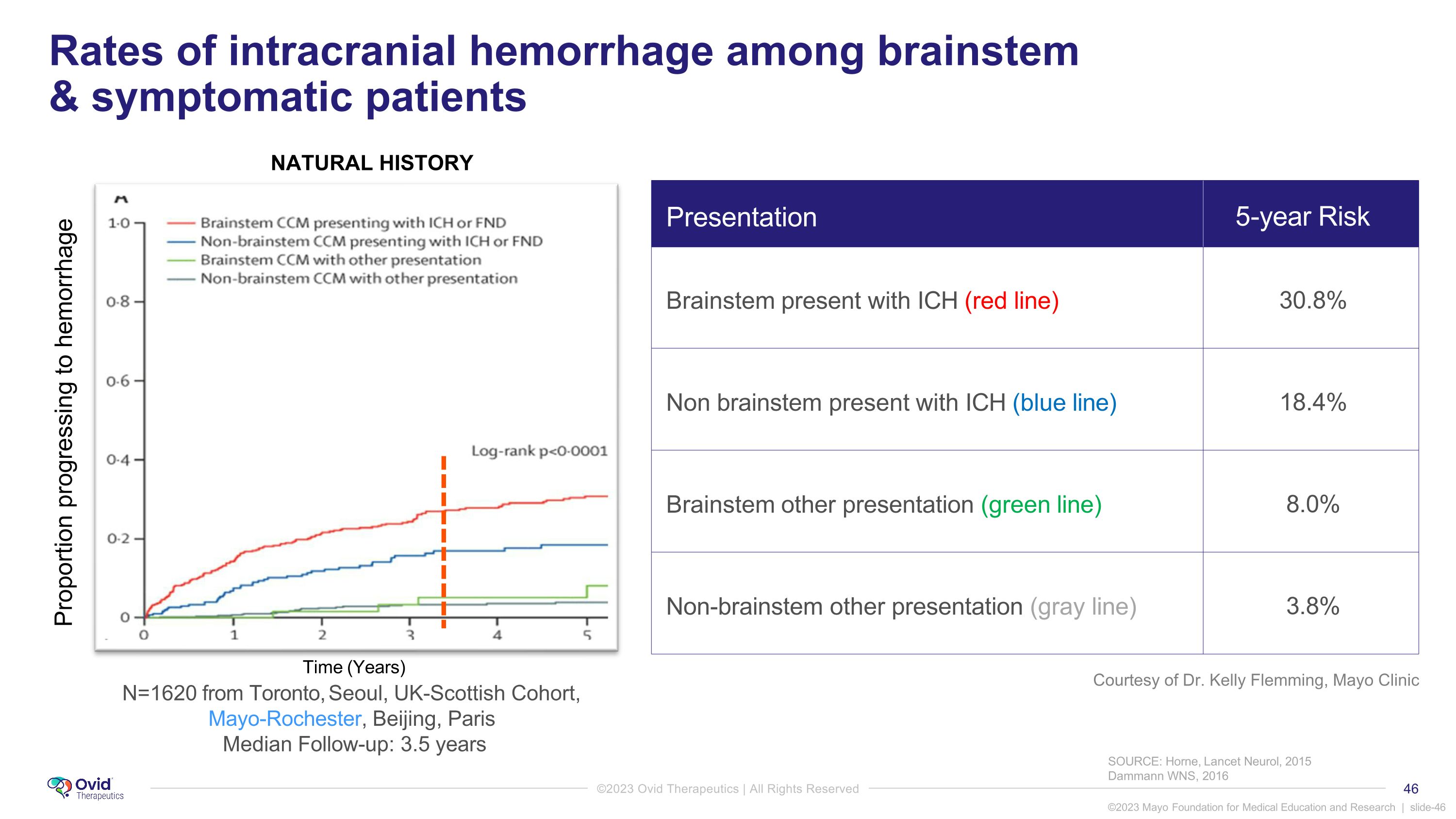
SOURCE: Horne, Lancet Neurol, 2015 Dammann WNS, 2016 Proportion progressing to hemorrhage Presentation 5-year Risk Brainstem present with ICH (red line) 30.8% Non brainstem present with ICH (blue line) 18.4% Brainstem other presentation (green line) 8.0% Non-brainstem other presentation (gray line) 3.8% Rates of intracranial hemorrhage among brainstem & symptomatic patients N=1620 from Toronto, Seoul, UK-Scottish Cohort, Mayo-Rochester, Beijing, Paris Median Follow-up: 3.5 years Time (Years) NATURAL HISTORY Courtesy of Dr. Kelly Flemming, Mayo Clinic

Ovid 2022 Global market research. OV888: An opportunity to bring hope to the CCM community Huge unmet need for an effective pharmacological therapy to address the underlying disease Large market opportunity ~400K Addressable CCM patients (7 major markets) Lesions can result in intracranial hemorrhage, epileptic seizures, or focal neurological deficits, and potentially lead to severe disability No drugs available to prevent and/or reduce the clinical symptoms of CCM Only available curative therapy is surgical resection of the CCM (when possible) Risk/benefit balance of surgical resection remains conflicting; some studies report worsening of symptoms after surgery approximately in 15% of cases and mortality in 1.5% of cases Common intracranial vascular malformation in humans Significant need for a pharmacological therapy

Potential for ROCK2 inhibition in other CNS disorders Dr. Zhong Zhong Chief Scientific Officer
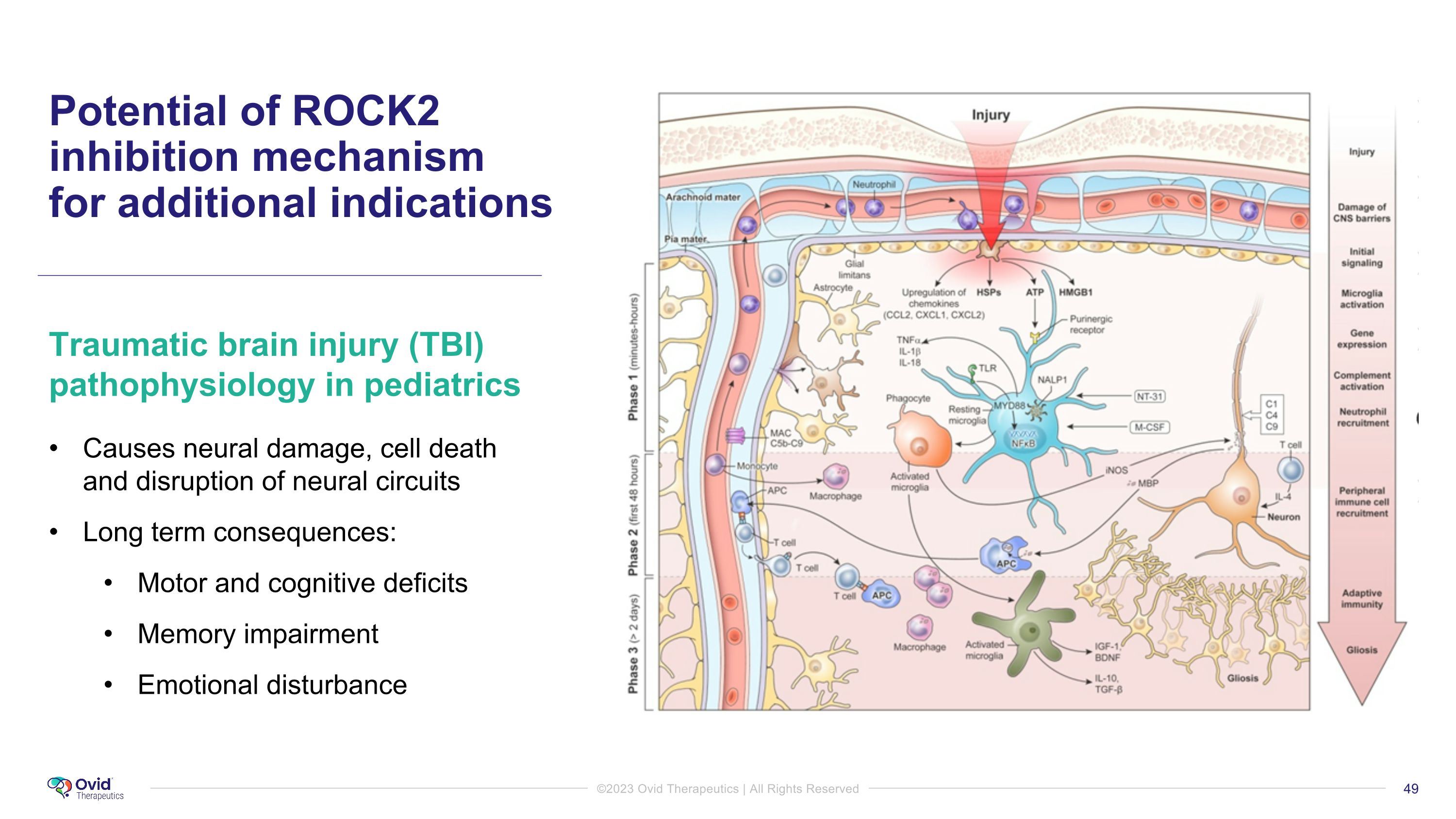
Potential of ROCK2 inhibition mechanism �for additional indications Traumatic brain injury (TBI) pathophysiology in pediatrics Causes neural damage, cell death and disruption of neural circuits Long term consequences: Motor and cognitive deficits Memory impairment Emotional disturbance
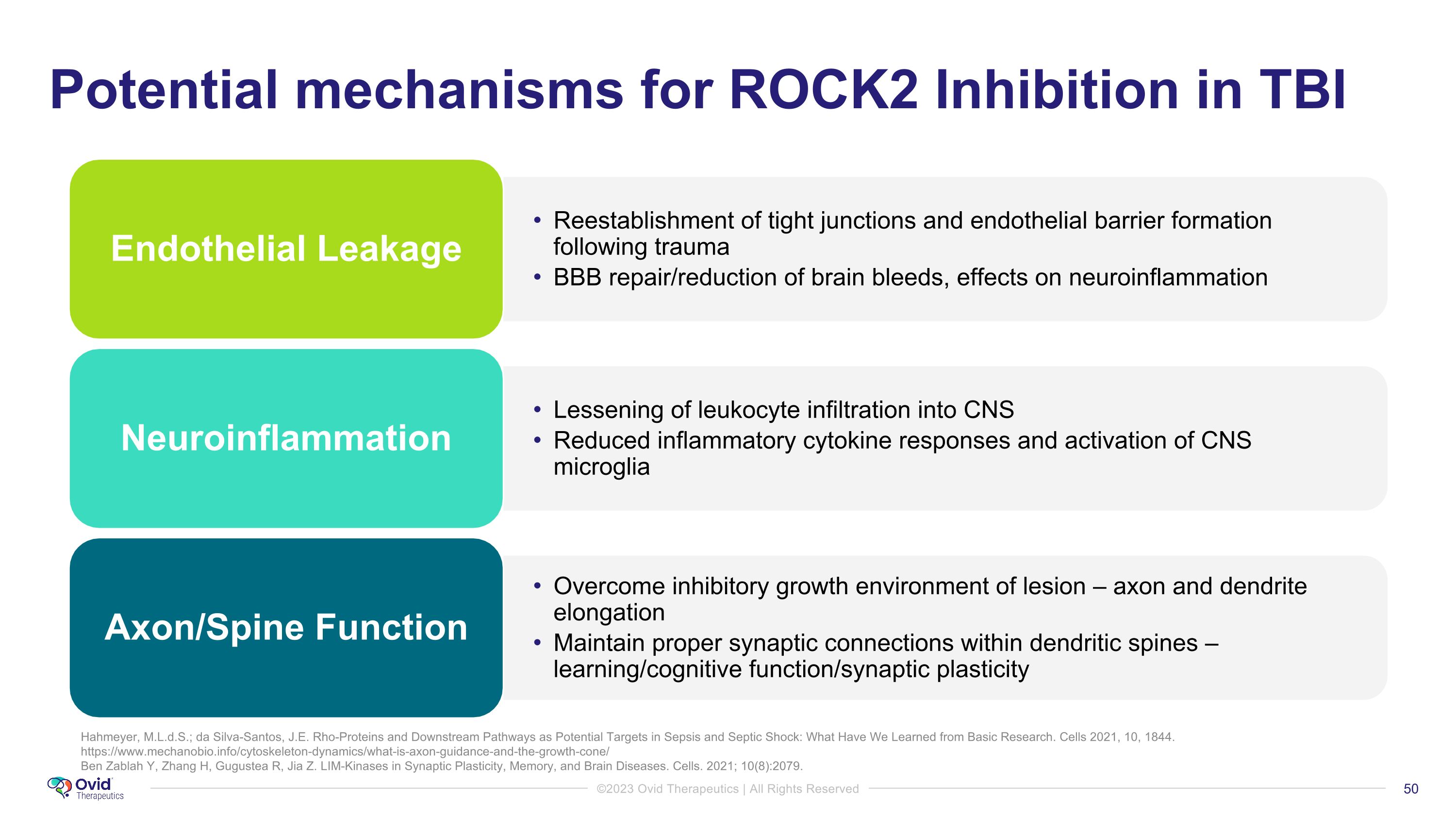
Hahmeyer, M.L.d.S.; da Silva-Santos, J.E. Rho-Proteins and Downstream Pathways as Potential Targets in Sepsis and Septic Shock: What Have We Learned from Basic Research. Cells 2021, 10, 1844. https://www.mechanobio.info/cytoskeleton-dynamics/what-is-axon-guidance-and-the-growth-cone/ Ben Zablah Y, Zhang H, Gugustea R, Jia Z. LIM-Kinases in Synaptic Plasticity, Memory, and Brain Diseases. Cells. 2021; 10(8):2079. Potential mechanisms for ROCK2 Inhibition in TBI Endothelial Leakage Reestablishment of tight junctions and endothelial barrier formation following trauma BBB repair/reduction of brain bleeds, effects on neuroinflammation Neuroinflammation Lessening of leukocyte infiltration into CNS Reduced inflammatory cytokine responses and activation of CNS microglia Axon/Spine Function Overcome inhibitory growth environment of lesion – axon and dendrite elongation Maintain proper synaptic connections within dendritic spines – learning/cognitive function/synaptic plasticity
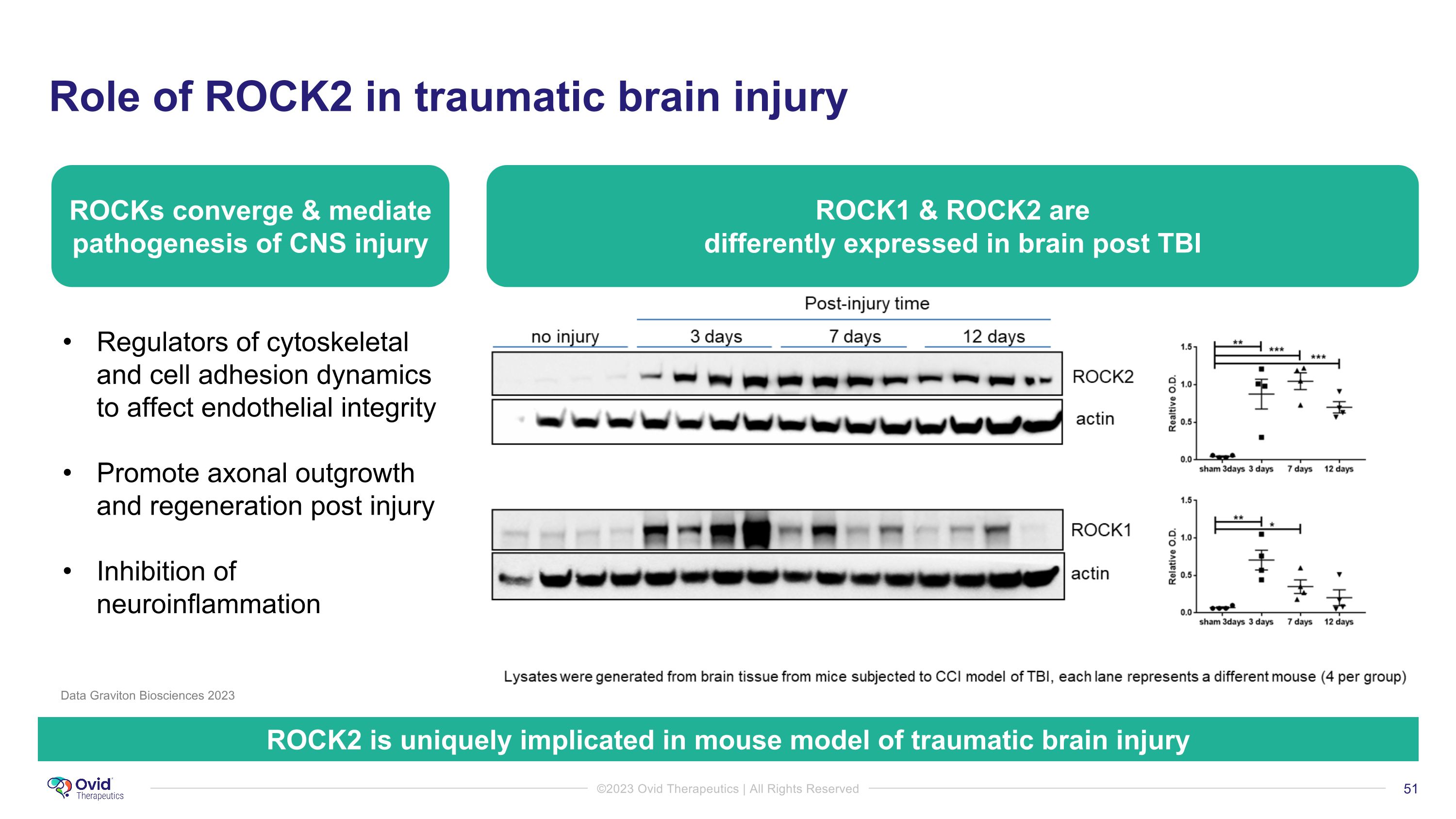
Role of ROCK2 in traumatic brain injury Regulators of cytoskeletal and cell adhesion dynamics to affect endothelial integrity Promote axonal outgrowth and regeneration post injury Inhibition of neuroinflammation ROCK1 & ROCK2 are �differently expressed in brain post TBI ROCK2 is uniquely implicated in mouse model of traumatic brain injury Data Graviton Biosciences 2023 ROCKs converge & mediate pathogenesis of CNS injury
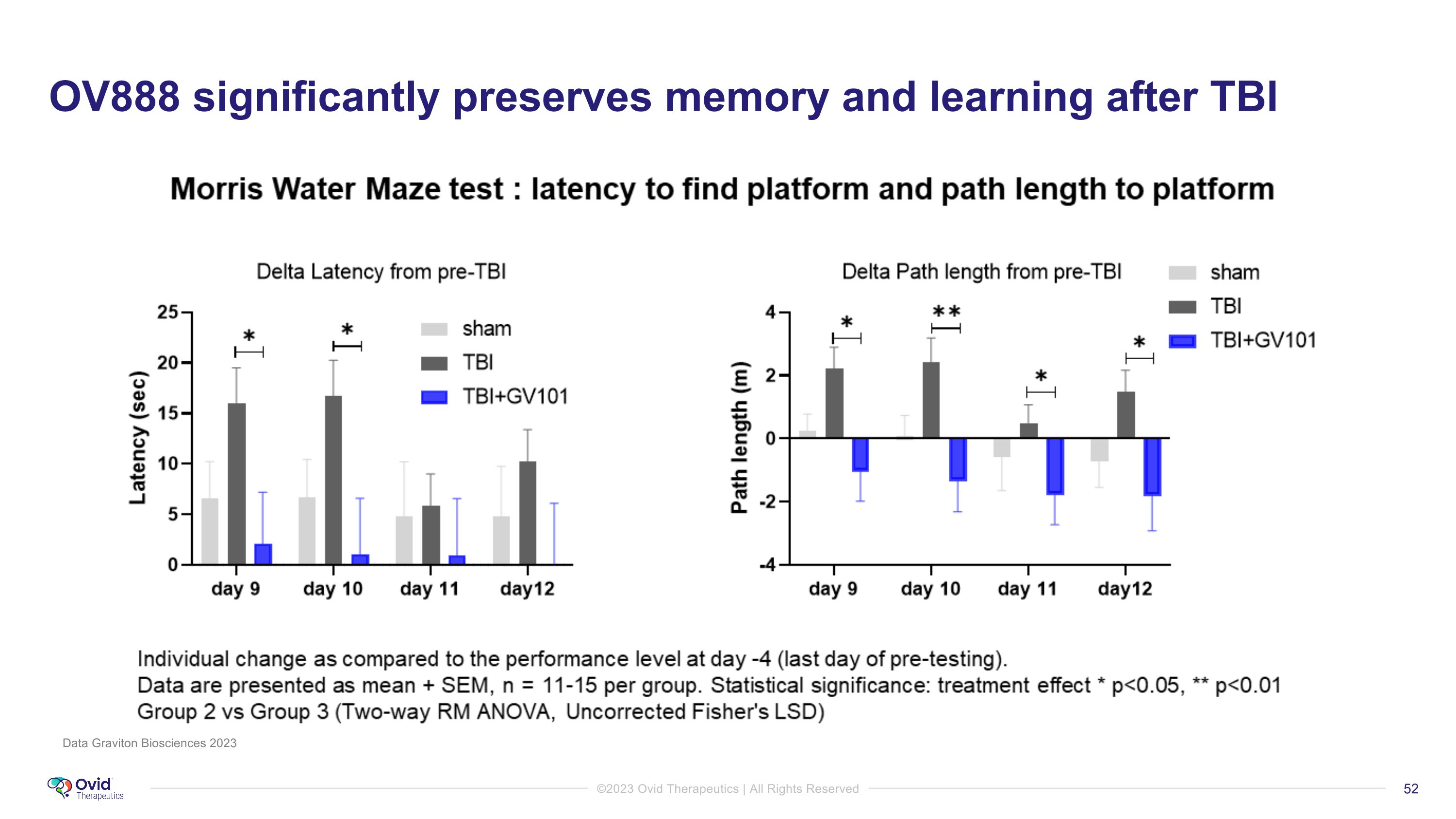
OV888 significantly preserves memory and learning after TBI Data Graviton Biosciences 2023

OV329: Large opportunities��Dr. Manoj Malhotra Chief Medical Officer

OV329��A next-generation GABA- aminotransferase inhibitor intended for treatment resistant seizures

…Pediatricians tell you that you never recover from this disease. - Father of a 10-year-old girl with TSC Beyond the first line benzodiazepine therapy, we do not have one ASM that is clearly best in stopping status epilepticus… …among those tested in randomized clinical trials, ASMs stopped the status in less than half of the patients. - Neurologist, Cleveland Clinic “ Graffigna G, Bosio C, Cecchini I. Assisting a child with tuberous sclerosis complex (TSC): a qualitative deep analysis of parents' experience and caring needs. BMJ Open. 2013 Dec 6;3(12):e003707. ”

OV329: Large opportunities Unique molecule for a validated target, GABA aminotransferase (GABA-AT) Promotes inhibitory neurotransmitter (GABA) to reduce seizures Established therapeutic window1 Prior GABA-AT inhibitor, vigabatrin, had no window Phase 1 study - exploring safety & surrogate biomarker for efficacy New findings support low, repeat dosing:1 Prolonged effects in seizure reduction Ability to maintain optimal levels of GABA Sustained shifts of tonic and phasic current Established a PD marker for human studies OV329 may have utility in acute seizures in addition to chronic Phase 1 SAD (3 cohorts complete) No safety signals Dose escalating Expanding formulations Oral Intravenous Expanding development program Multiple potential indications Chronic Acute UPDATE 1) In animal models
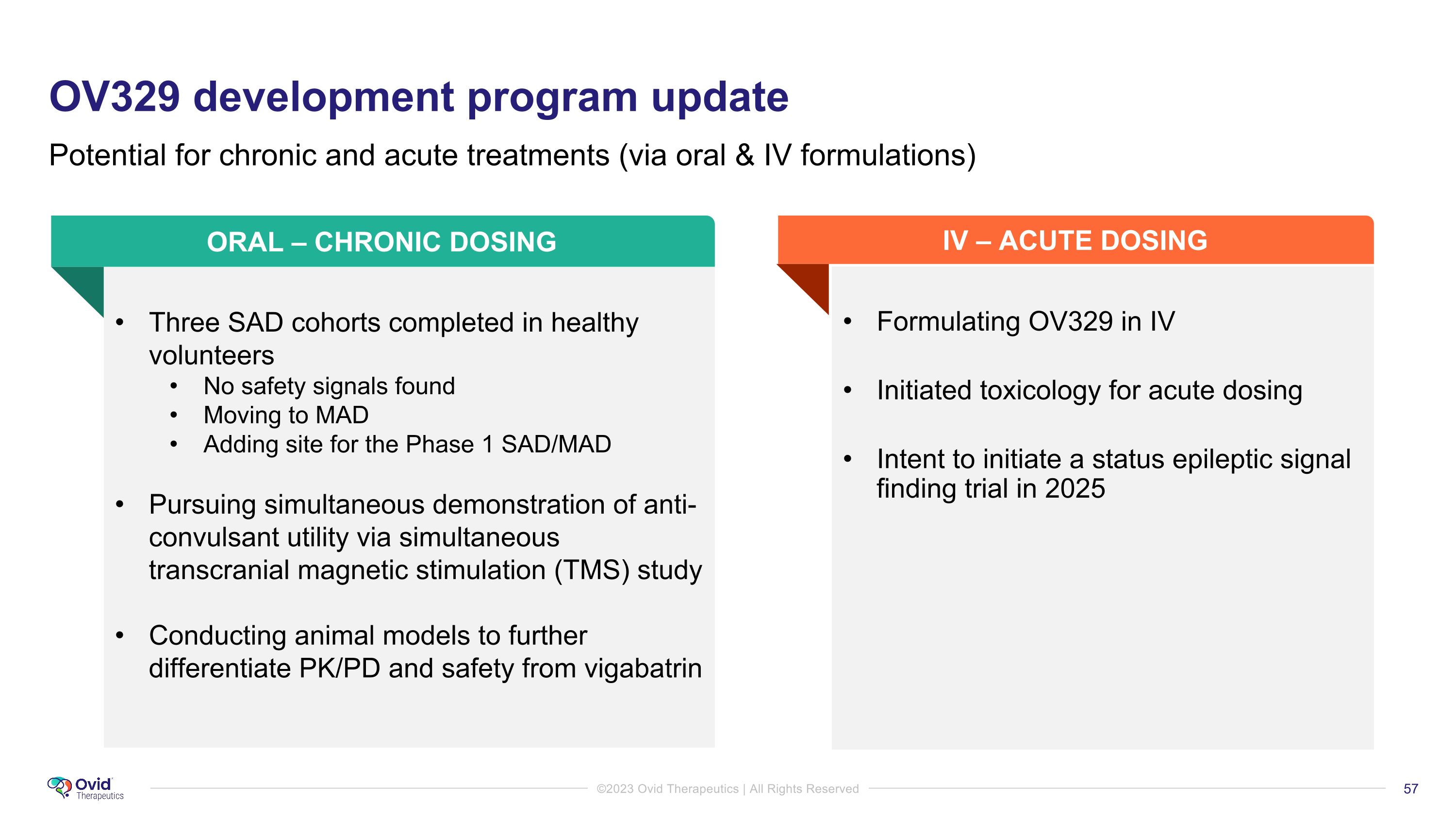
OV329 development program update Potential for chronic and acute treatments (via oral & IV formulations) Three SAD cohorts completed in healthy volunteers No safety signals found Moving to MAD Adding site for the Phase 1 SAD/MAD Pursuing simultaneous demonstration of anti-convulsant utility via simultaneous transcranial magnetic stimulation (TMS) study Conducting animal models to further differentiate PK/PD and safety from vigabatrin Formulating OV329 in IV Initiated toxicology for acute dosing Intent to initiate a status epileptic signal finding trial in 2025 ORAL – CHRONIC DOSING IV – ACUTE DOSING
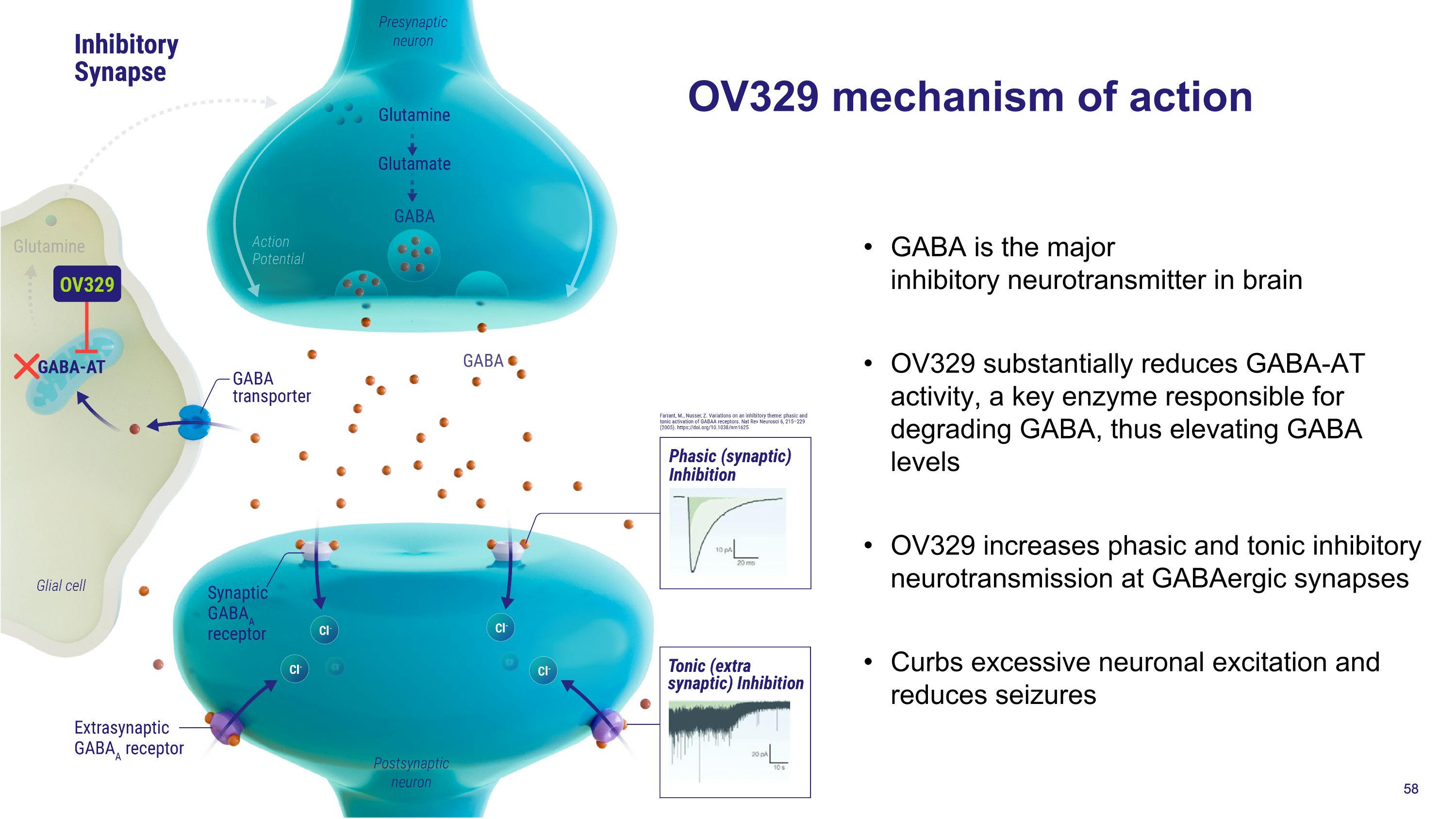
OV329 mechanism of action GABA is the major inhibitory neurotransmitter in brain OV329 substantially reduces GABA-AT activity, a key enzyme responsible for degrading GABA, thus elevating GABA levels OV329 increases phasic and tonic inhibitory neurotransmission at GABAergic synapses Curbs excessive neuronal excitation and reduces seizures
NEW: Preclinical findings Low, multiple day doses of OV329 have a prolonged effect in seizure reduction Animal models demonstrate anti-convulsant properties in chronic and acute seizures OV329 induces profound inhibitory tonic currents in postsynaptic neurons at very low doses Electrophysiology data shows pharmacodynamic effects at low doses & support signal detection efforts in humans Supports repeated low dosing of oral OV329 in humans

Repeat dosing of OV329 leads to enhanced GABA-AT inhibition A single dose of OV329 leads to long-lasting (>24 hours) inhibition of GABA-AT enzymatic activity Cumulative effect of repeated, low dosing of OV329 was expected and observed Enhancing GABA-AT inhibition as compared to a single dose of OV329 OVID data on file Control OV329 low dose OV329 high dose OV329 repeat low dose Persistent and chronic elevation of GABA is expected to modulate neuronal excitability and thereby reduce seizures
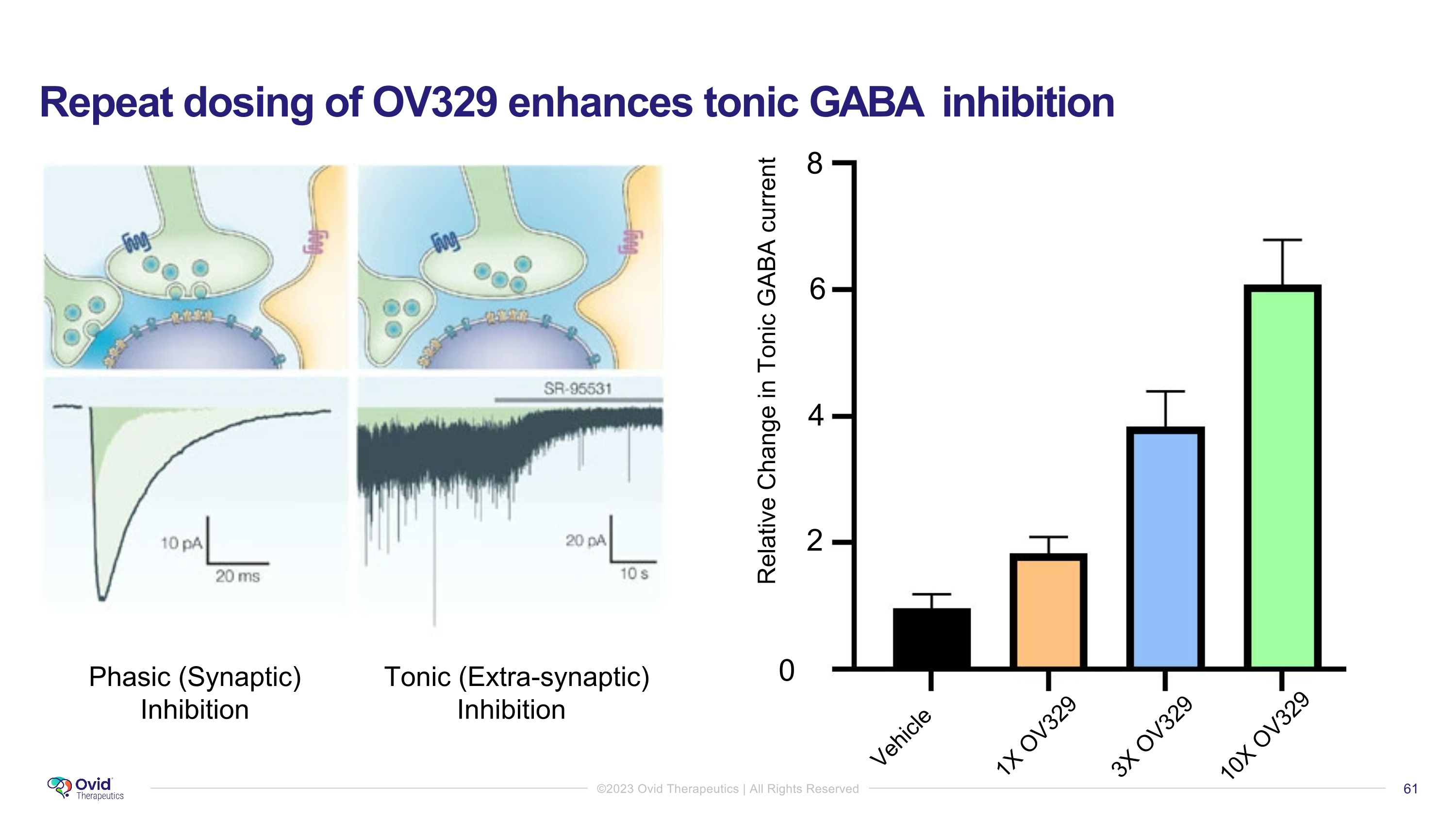
Repeat dosing of OV329 enhances tonic GABA inhibition Phasic (Synaptic) Inhibition Tonic (Extra-synaptic) Inhibition 0 4 2 8 6 Relative Change in Tonic GABA current Vehicle 1X OV329 10X OV329 3X OV329
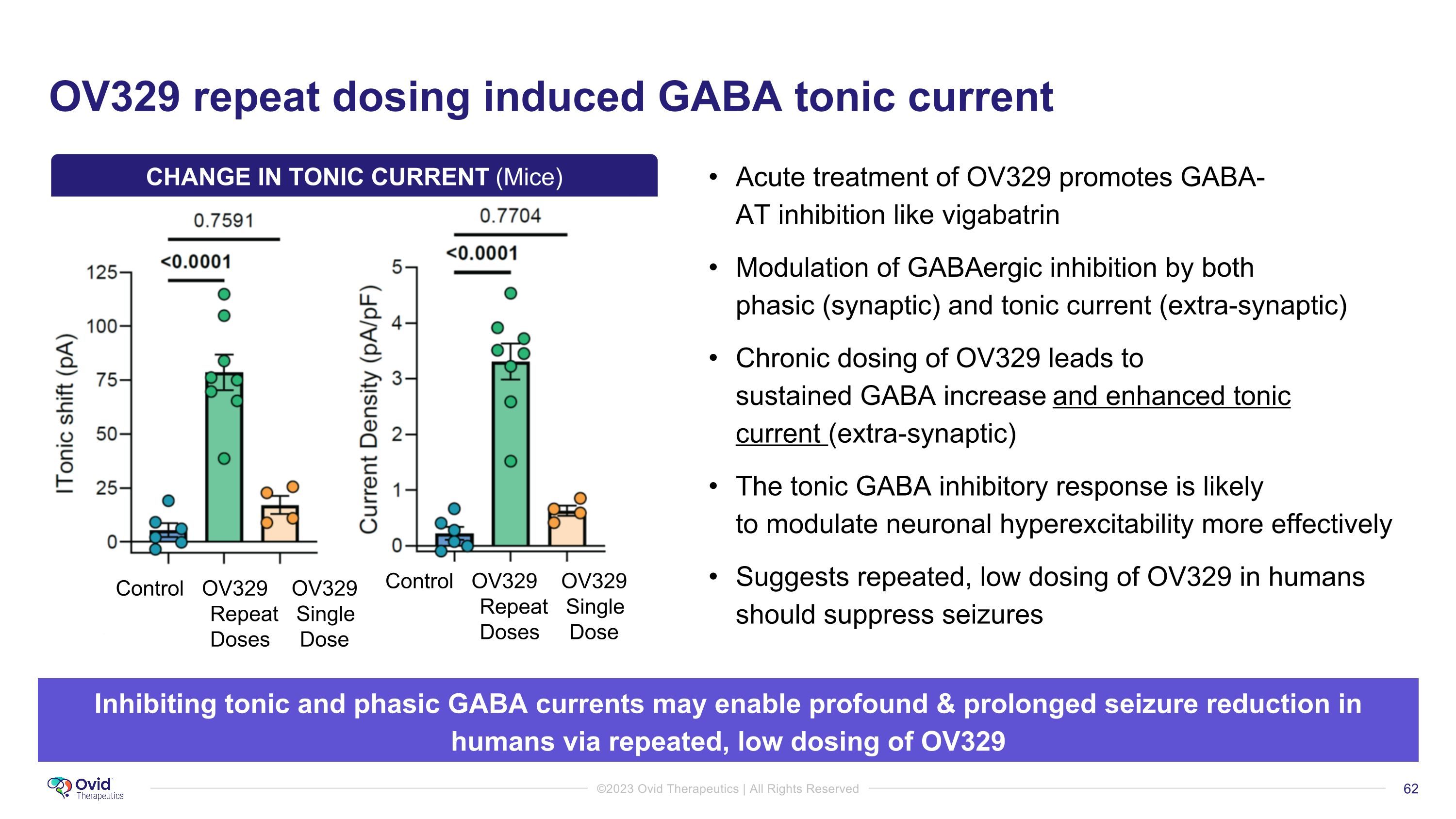
OV329 repeat dosing induced GABA tonic current Acute treatment of OV329 promotes GABA-AT inhibition like vigabatrin Modulation of GABAergic inhibition by both phasic (synaptic) and tonic current (extra-synaptic) Chronic dosing of OV329 leads to sustained GABA increase and enhanced tonic current (extra-synaptic) The tonic GABA inhibitory response is likely to modulate neuronal hyperexcitability more effectively Suggests repeated, low dosing of OV329 in humans should suppress seizures Inhibiting tonic and phasic GABA currents may enable profound & prolonged seizure reduction in humans via repeated, low dosing of OV329 Control OV329 OV329 Repeat Single Doses Dose Control OV329 OV329 Repeat Single Doses Dose CHANGE IN TONIC CURRENT (Mice)
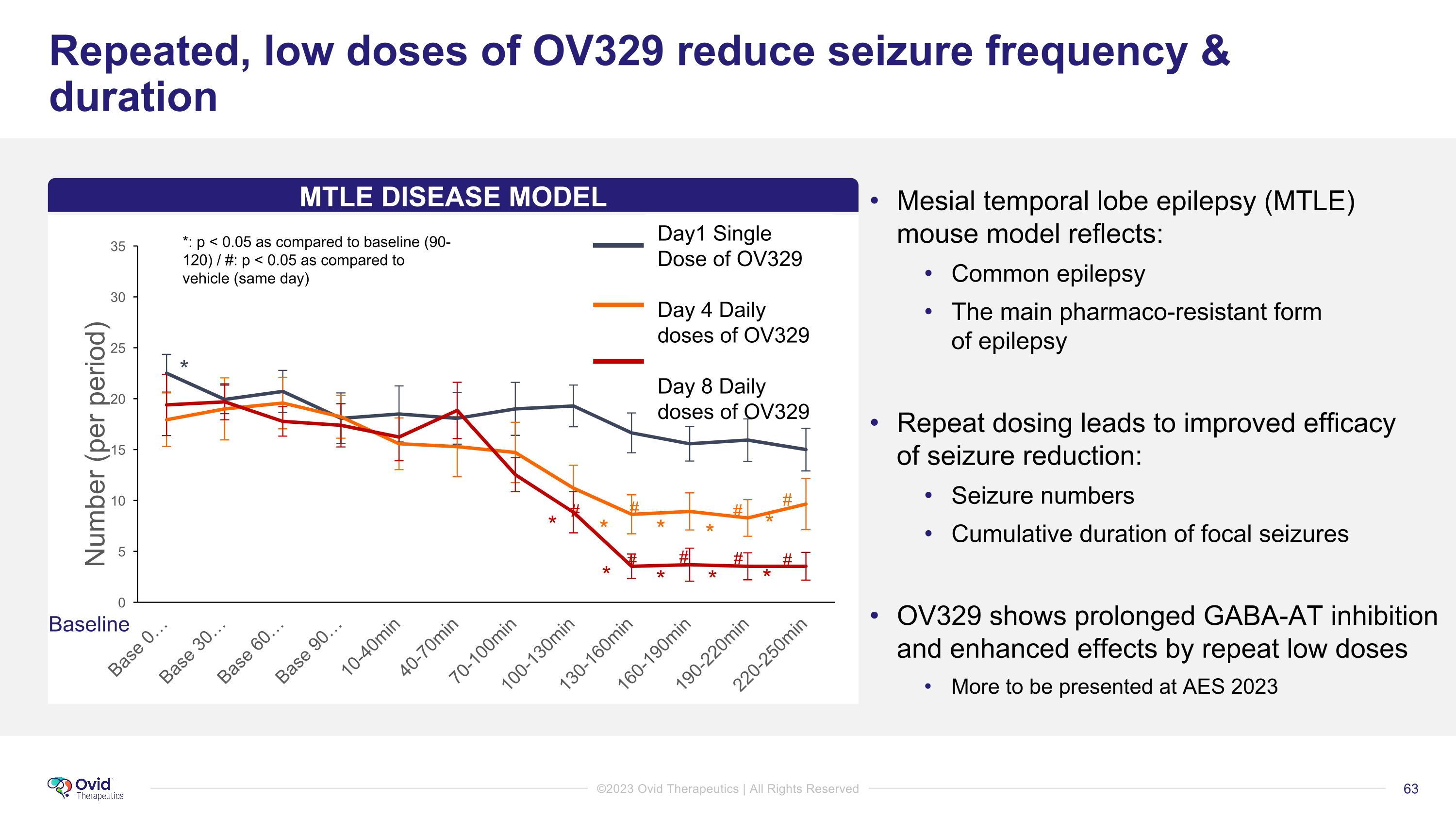
Repeated, low doses of OV329 reduce seizure frequency & duration Mesial temporal lobe epilepsy (MTLE) mouse model reflects: Common epilepsy The main pharmaco-resistant form of epilepsy Repeat dosing leads to improved efficacy of seizure reduction: Seizure numbers Cumulative duration of focal seizures OV329 shows prolonged GABA-AT inhibition and enhanced effects by repeat low doses More to be presented at AES 2023 * * * * * * * * * * # # # # # # # # Baseline D1 D4 - Chronic D8 - Chronic *: p < 0.05 as compared to baseline (90-120) / #: p < 0.05 as compared to vehicle (same day) MTLE DISEASE MODEL Day1 Single Dose of OV329 Day 4 Daily doses of OV329 Day 8 Daily doses of OV329
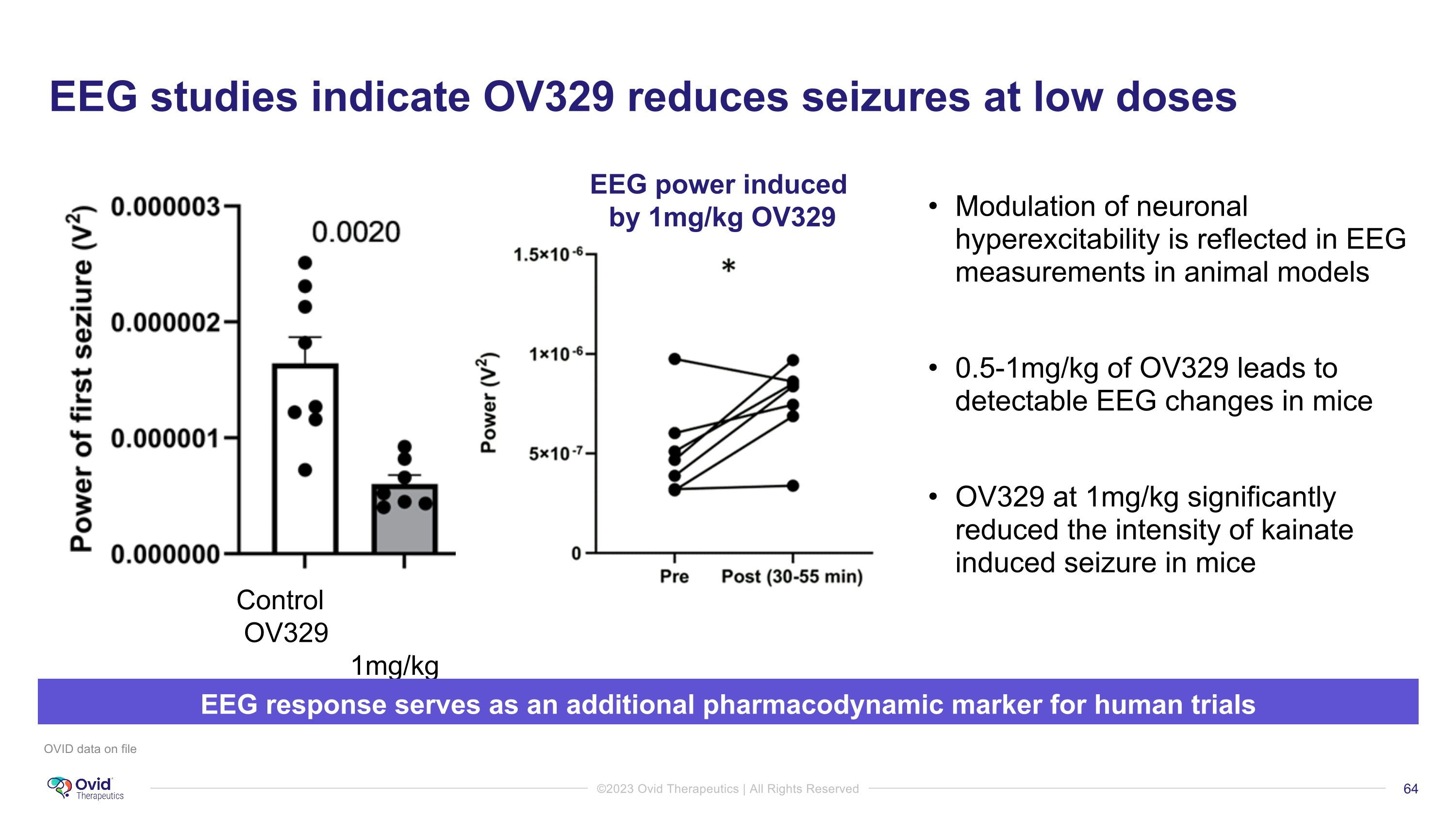
EEG studies indicate OV329 reduces seizures at low doses OVID data on file Control OV329 1mg/kg EEG response serves as an additional pharmacodynamic marker for human trials EEG power induced by 1mg/kg OV329 Modulation of neuronal hyperexcitability is reflected in EEG measurements in animal models 0.5-1mg/kg of OV329 leads to detectable EEG changes in mice OV329 at 1mg/kg significantly reduced the intensity of kainate induced seizure in mice
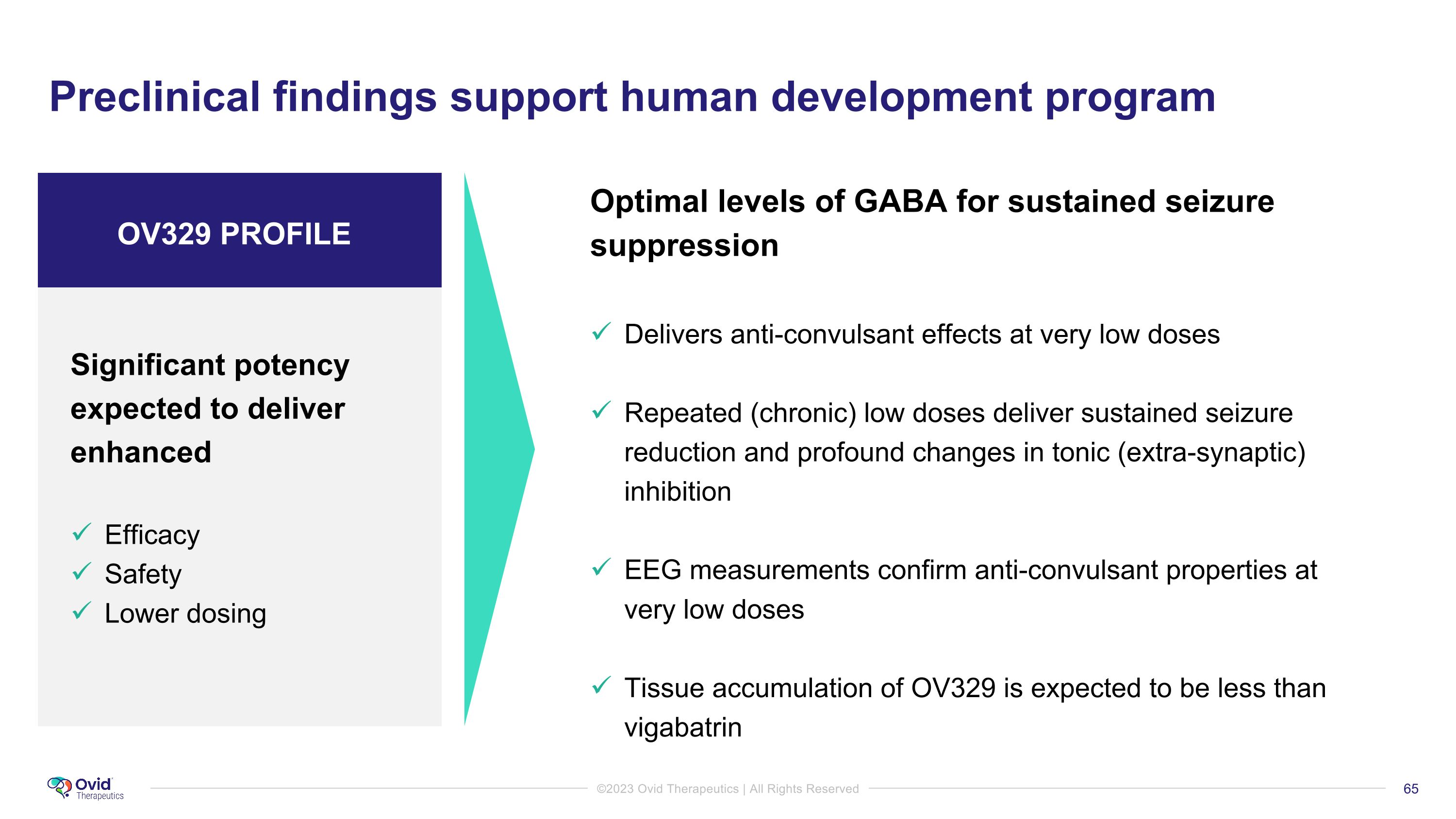
Preclinical findings support human development program Optimal levels of GABA for sustained seizure suppression Delivers anti-convulsant effects at very low doses Repeated (chronic) low doses deliver sustained seizure reduction and profound changes in tonic (extra-synaptic) inhibition EEG measurements confirm anti-convulsant properties at �very low doses Tissue accumulation of OV329 is expected to be less than vigabatrin Significant potency expected to deliver enhanced Efficacy Safety Lower dosing OV329 PROFILE
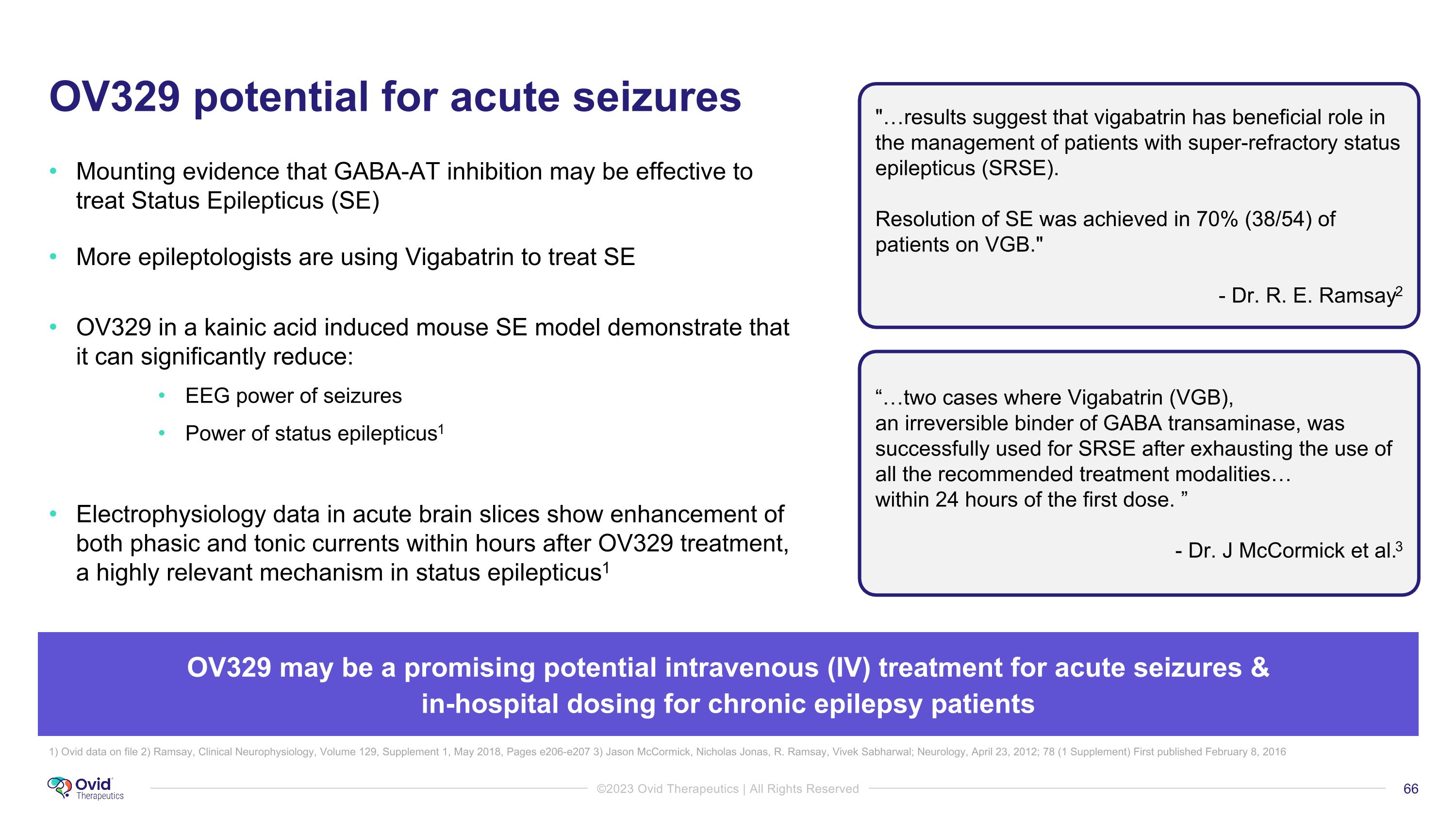
OV329 potential for acute seizures Mounting evidence that GABA-AT inhibition may be effective to treat Status Epilepticus (SE) More epileptologists are using Vigabatrin to treat SE OV329 in a kainic acid induced mouse SE model demonstrate that it can significantly reduce: EEG power of seizures Power of status epilepticus1 Electrophysiology data in acute brain slices show enhancement of both phasic and tonic currents within hours after OV329 treatment, a highly relevant mechanism in status epilepticus1 "…results suggest that vigabatrin has beneficial role in the management of patients with super-refractory status epilepticus (SRSE). Resolution of SE was achieved in 70% (38/54) of patients on VGB." - Dr. R. E. Ramsay2 “…two cases where Vigabatrin (VGB), �an irreversible binder of GABA transaminase, was successfully used for SRSE after exhausting the use of all the recommended treatment modalities… within 24 hours of the first dose. ” - Dr. J McCormick et al.3 1) Ovid data on file 2) Ramsay, Clinical Neurophysiology, Volume 129, Supplement 1, May 2018, Pages e206-e207 3) Jason McCormick, Nicholas Jonas, R. Ramsay, Vivek Sabharwal; Neurology, April 23, 2012; 78 (1 Supplement) First published February 8, 2016 OV329 may be a promising potential intravenous (IV) treatment for acute seizures & �in-hospital dosing for chronic epilepsy patients
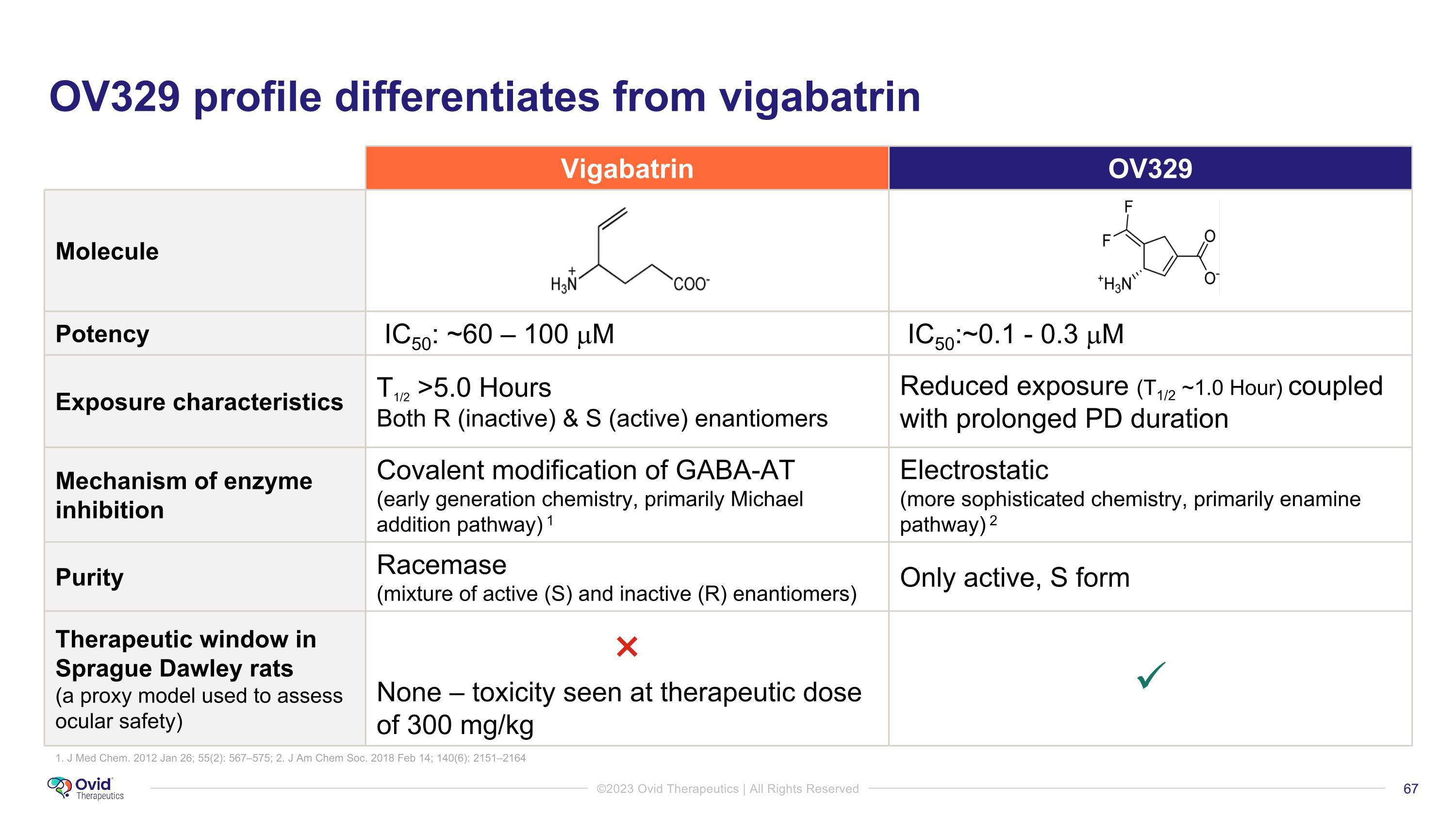
OV329 profile differentiates from vigabatrin Vigabatrin OV329 Molecule Potency IC50: ~60 – 100 mM IC50:~0.1 - 0.3 mM Exposure characteristics T1/2 >5.0 Hours Both R (inactive) & S (active) enantiomers Reduced exposure (T1/2 ~1.0 Hour) coupled with prolonged PD duration Mechanism of enzyme inhibition Covalent modification of GABA-AT (early generation chemistry, primarily Michael addition pathway) 1 Electrostatic �(more sophisticated chemistry, primarily enamine pathway) 2 Purity Racemase (mixture of active (S) and inactive (R) enantiomers) Only active, S form Therapeutic window in Sprague Dawley rats �(a proxy model used to assess ocular safety) × None – toxicity seen at therapeutic dose of 300 mg/kg 1. J Med Chem. 2012 Jan 26; 55(2): 567–575; 2. J Am Chem Soc. 2018 Feb 14; 140(6): 2151–2164

Next steps OV329 Oral formulation OV329 IV formulation Initiating multiple ascending dose cohorts Expect higher dose escalation in humans Concurrent TMS study to demonstrate PD Measuring target engagement surrogate biomarker via MRS (vs VGB) Concurrently assessing back of eye accumulation vs VGB in animals Timing: Full readout H2 2024 Formulating OV329 for IV Conducting supportive animal toxicology & disease models Tuberous sclerosis seizures Infantile spasms Conditions with focal seizures DEVELOPMENT POTENTIAL INDICATIONS Status epilepticus Refractory status epilepticus

Open Q&A Ovid strategy ROCK2 inhibition & OV888 OV329, a next-generation GABA-AT inhibitor
Break �(10 minutes)

KCC2 direct �activator portfolio & OV350��Dr. Zhong Zhong Chief Scientific Officer
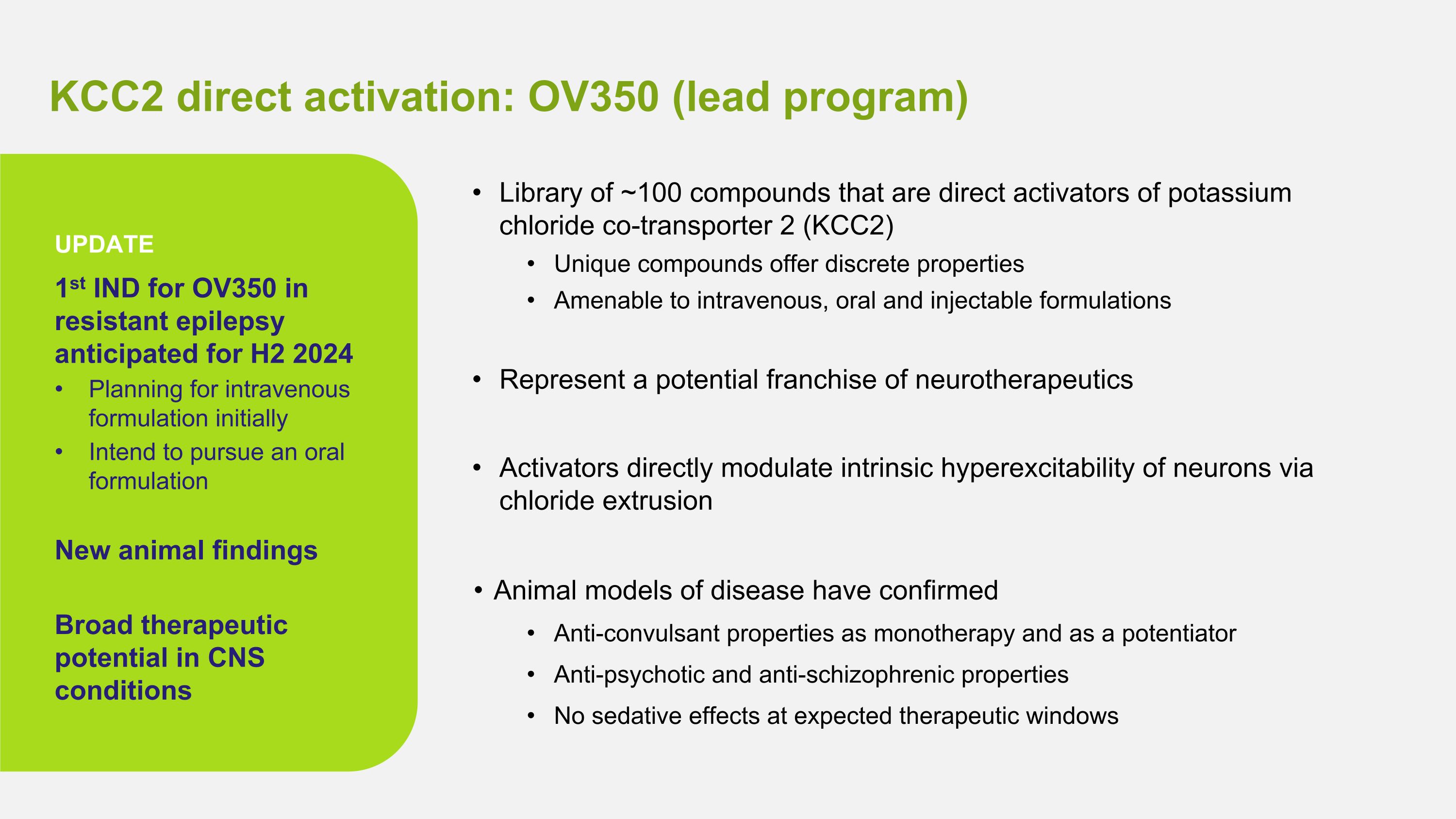
KCC2 direct activation: OV350 (lead program) Library of ~100 compounds that are direct activators of potassium chloride co-transporter 2 (KCC2) Unique compounds offer discrete properties Amenable to intravenous, oral and injectable formulations Represent a potential franchise of neurotherapeutics Activators directly modulate intrinsic hyperexcitability of neurons via chloride extrusion Animal models of disease have confirmed Anti-convulsant properties as monotherapy and as a potentiator Anti-psychotic and anti-schizophrenic properties No sedative effects at expected therapeutic windows 1st IND for OV350 in resistant epilepsy anticipated for H2 2024 Planning for intravenous formulation initially Intend to pursue an oral formulation New animal findings Broad therapeutic potential in CNS conditions UPDATE
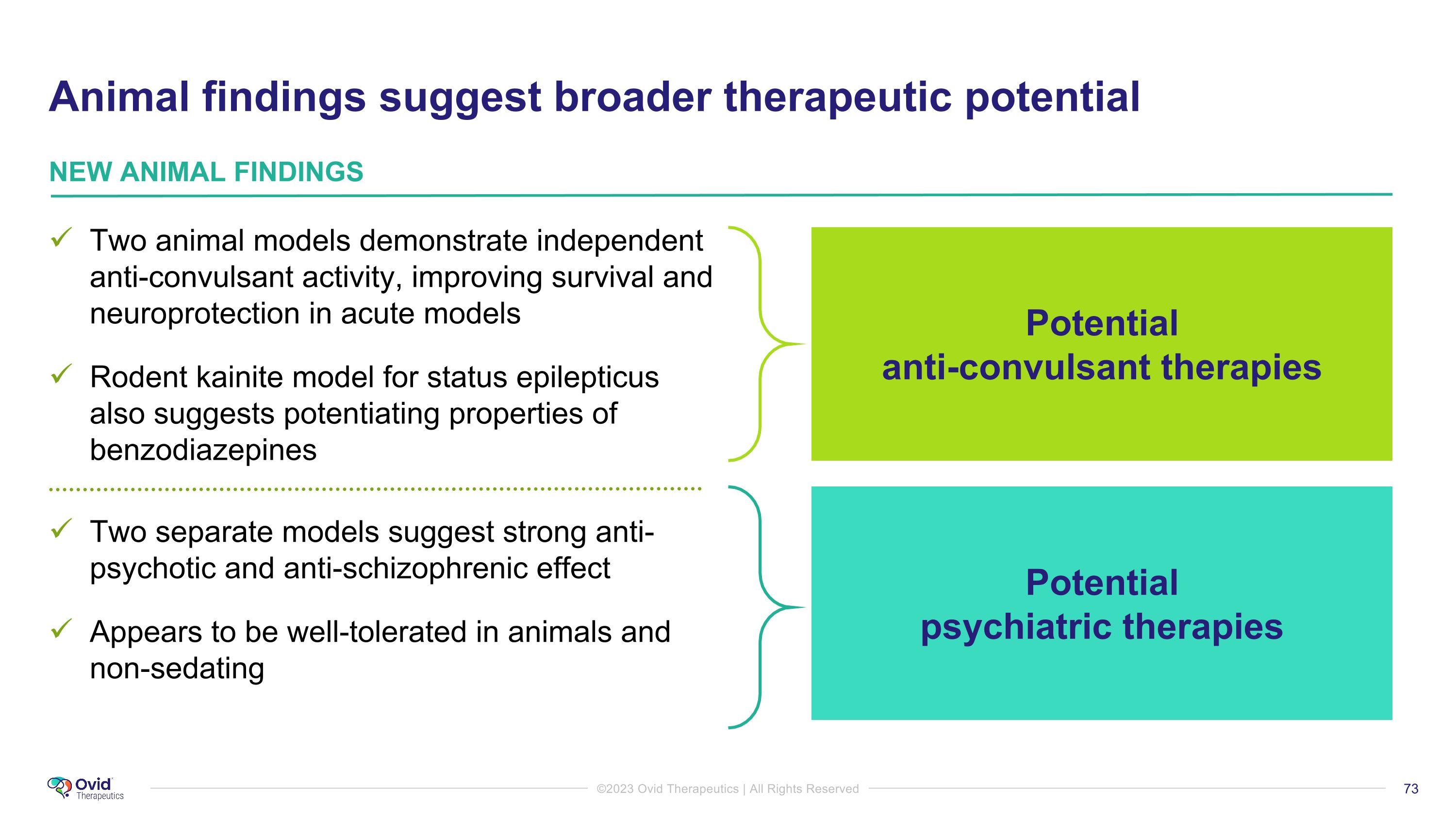
Two animal models demonstrate independent anti-convulsant activity, improving survival and neuroprotection in acute models Rodent kainite model for status epilepticus also suggests potentiating properties of benzodiazepines Two separate models suggest strong anti-psychotic and anti-schizophrenic effect Appears to be well-tolerated in animals and non-sedating Potential �anti-convulsant therapies NEW ANIMAL FINDINGS Potential �psychiatric therapies Animal findings suggest broader therapeutic potential

Unlocking a new CNS target: KCC2 direct activation��Dr. Stephen Moss �Director, Moss Lab for Neuropharmacology at Tufts University Professor of Molecular Pharmacology at University College London
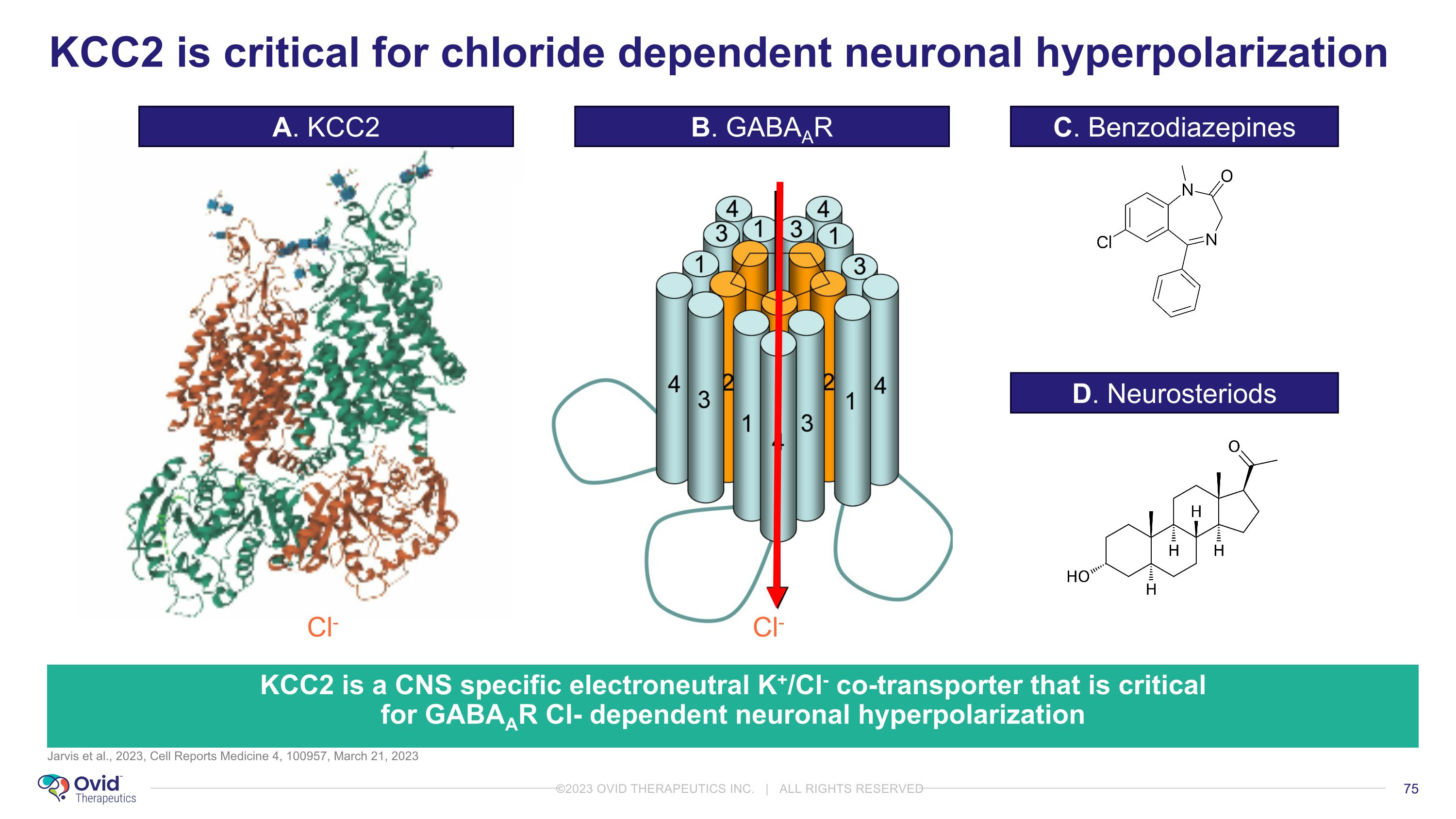
Jarvis et al., 2023, Cell Reports Medicine 4, 100957, March 21, 2023 KCC2 is a CNS specific electroneutral K+/Cl- co-transporter that is critical for GABAAR Cl- dependent neuronal hyperpolarization Cl- Cl- KCC2 is critical for chloride dependent neuronal hyperpolarization A. KCC2 B. GABAAR C. Benzodiazepines D. Neurosteriods
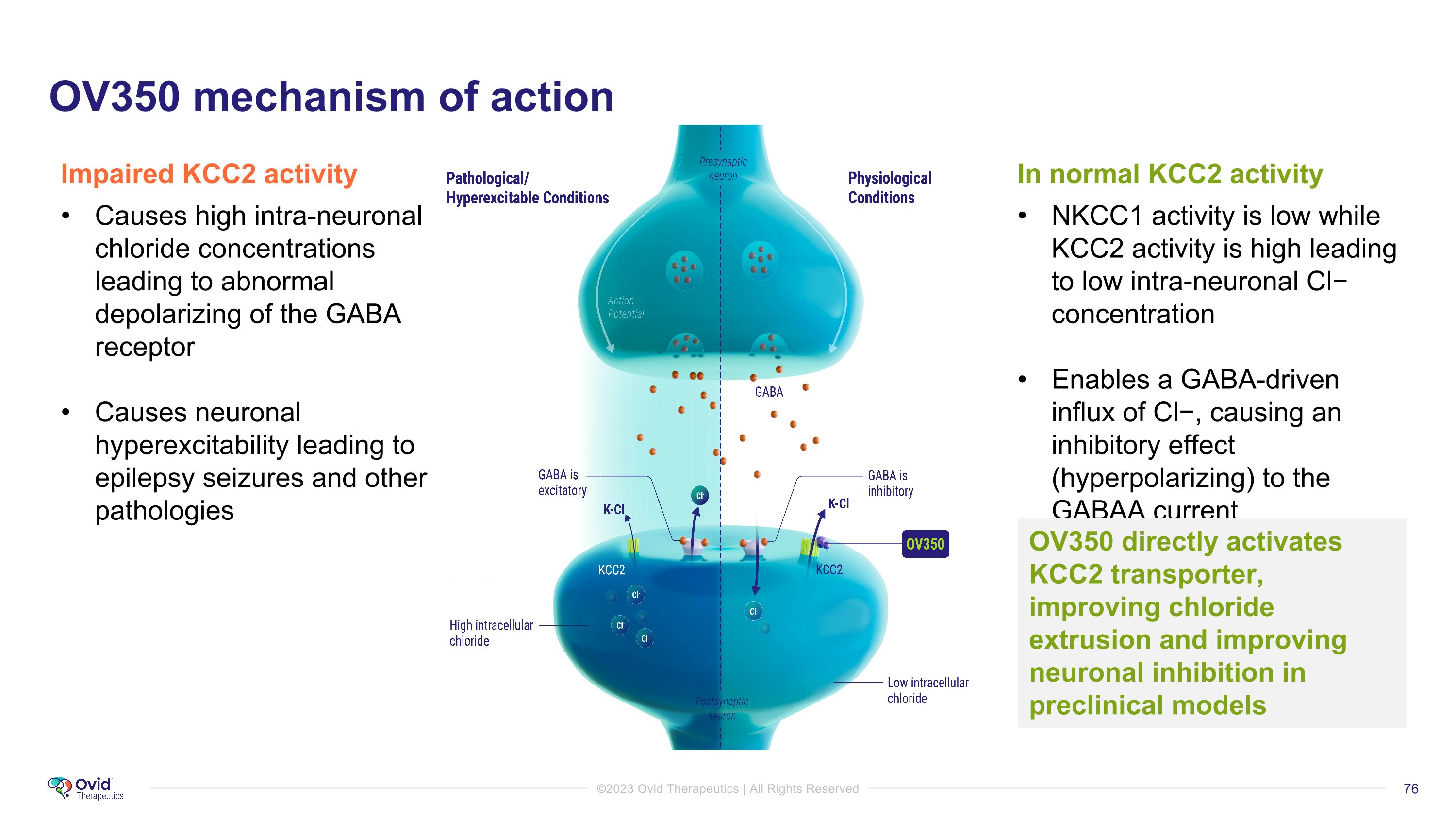
In normal KCC2 activity NKCC1 activity is low while KCC2 activity is high leading to low intra-neuronal Cl− concentration Enables a GABA-driven influx of Cl−, causing an inhibitory effect (hyperpolarizing) to the GABAA current Impaired KCC2 activity Causes high intra-neuronal chloride concentrations leading to abnormal depolarizing of the GABA receptor Causes neuronal hyperexcitability leading to epilepsy seizures and other pathologies OV350 directly activates KCC2 transporter, improving chloride extrusion and improving neuronal inhibition in preclinical models OV350 mechanism of action
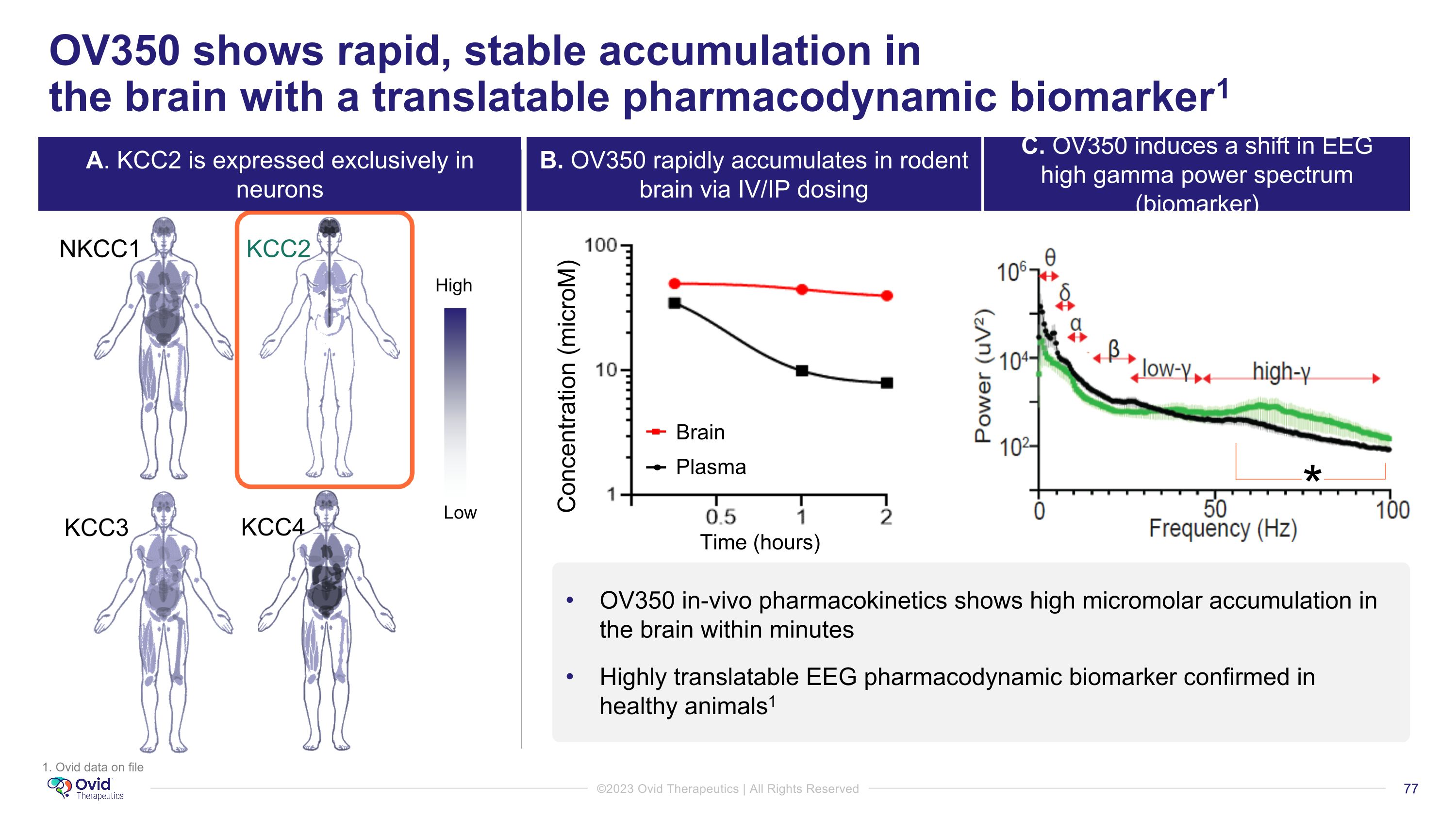
OV350 shows rapid, stable accumulation in �the brain with a translatable pharmacodynamic biomarker1 NKCC1 KCC2 KCC3 KCC4 Low High Concentration (microM) Brain Plasma * OV350 in-vivo pharmacokinetics shows high micromolar accumulation in the brain within minutes Highly translatable EEG pharmacodynamic biomarker confirmed in healthy animals1 Time (hours) 1. Ovid data on file A. KCC2 is expressed exclusively in neurons B. OV350 rapidly accumulates in rodent brain via IV/IP dosing C. OV350 induces a shift in EEG high gamma power spectrum (biomarker)
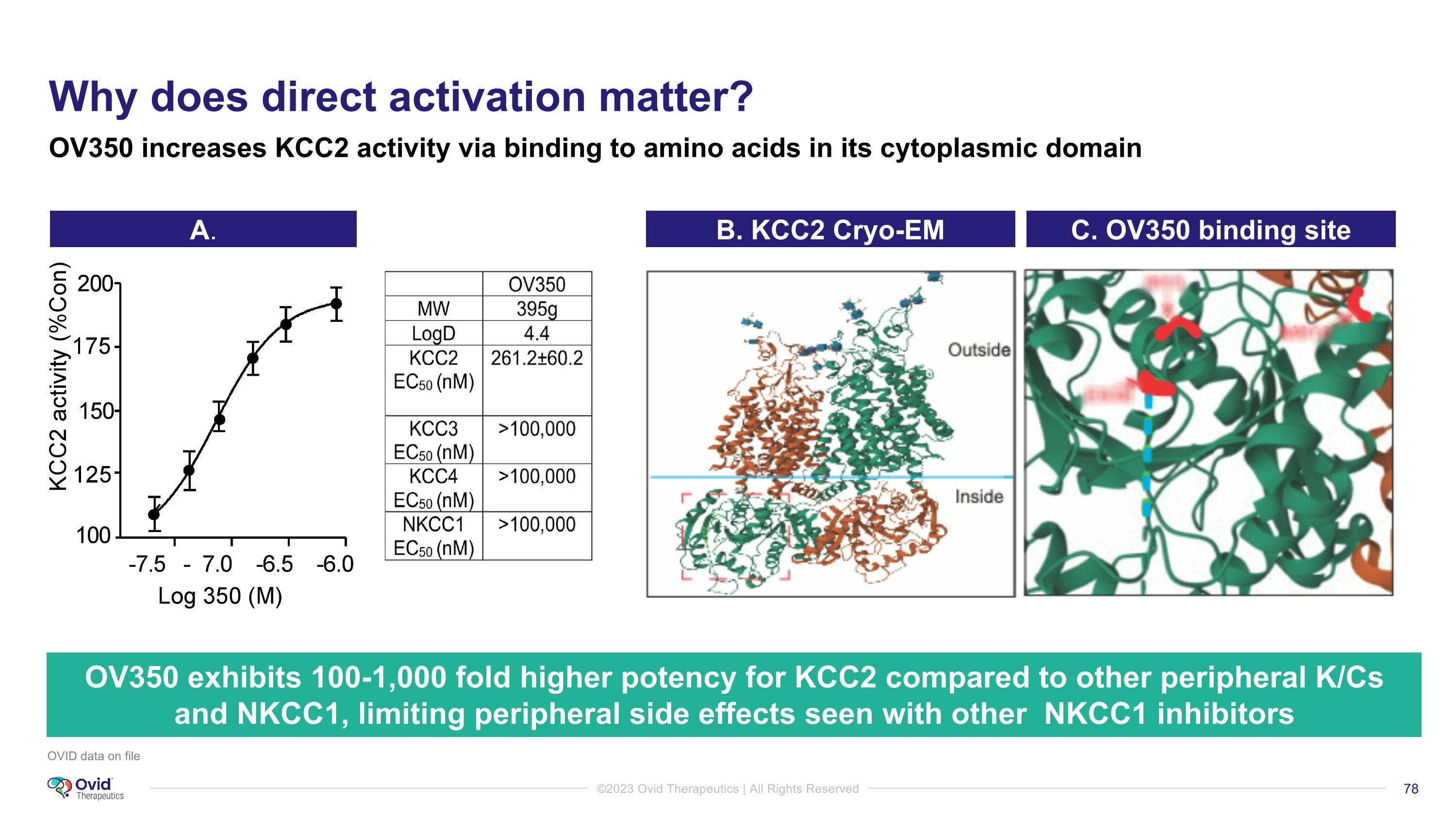
OVID data on file OV350 exhibits 100-1,000 fold higher potency for KCC2 compared to other peripheral K/Cs and NKCC1, limiting peripheral side effects seen with other NKCC1 inhibitors OV350 increases KCC2 activity via binding to amino acids in its cytoplasmic domain A. Why does direct activation matter? B. KCC2 Cryo-EM C. OV350 binding site
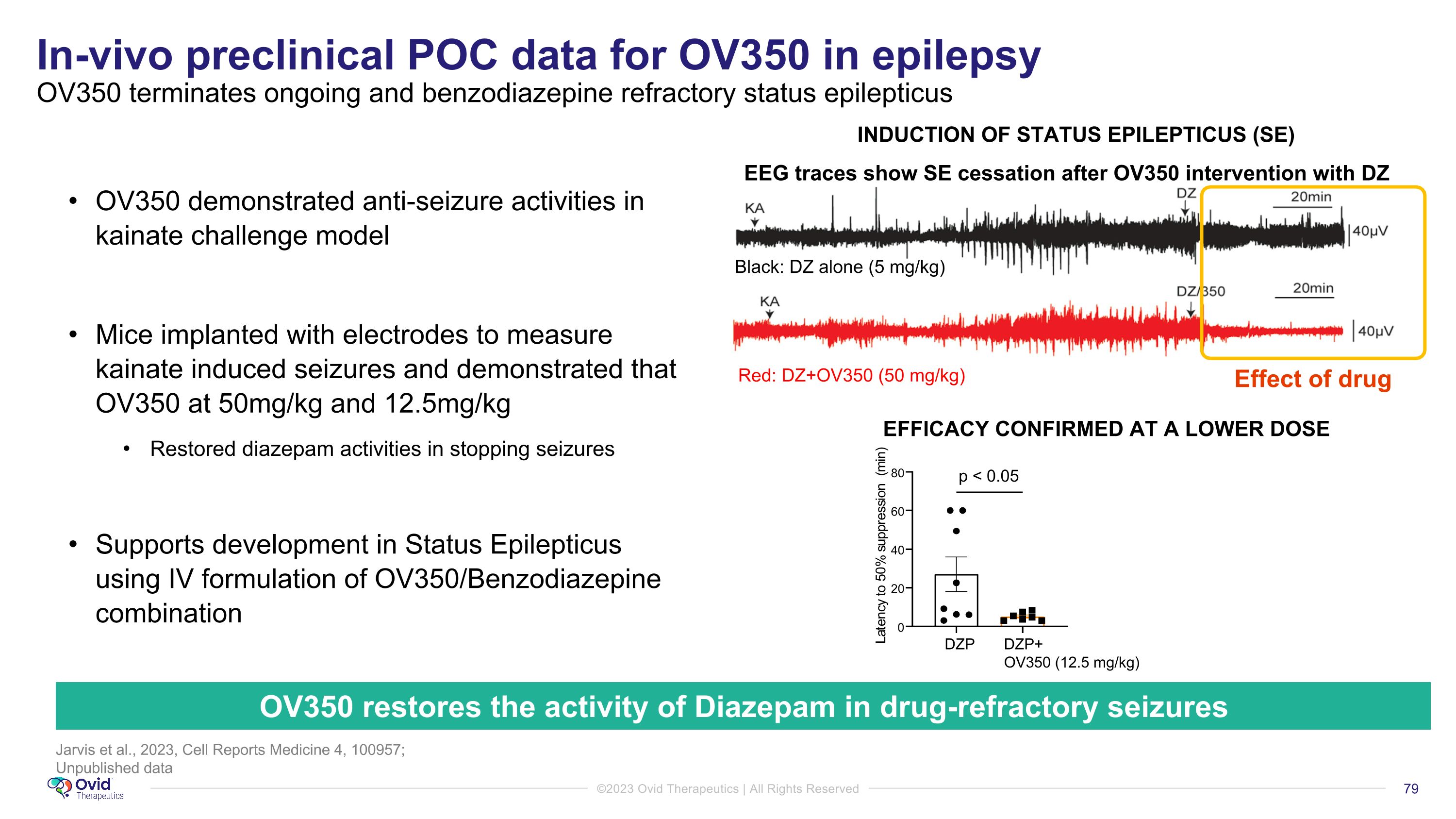
Jarvis et al., 2023, Cell Reports Medicine 4, 100957; Unpublished data In-vivo preclinical POC data for OV350 in epilepsy �OV350 terminates ongoing and benzodiazepine refractory status epilepticus 3 2 OV350 restores the activity of Diazepam in drug-refractory seizures EEG traces show SE cessation after OV350 intervention with DZ EFFICACY CONFIRMED AT A LOWER DOSE p < 0.05 INDUCTION OF STATUS EPILEPTICUS (SE) Effect of drug DZP DZP+ OV350 (12.5 mg/kg) 0 20 40 60 80 L a t e n c y t o 5 0 % s u p p r e s s i o n ( m i n ) Black: DZ alone (5 mg/kg) Red: DZ+OV350 (50 mg/kg) OV350 demonstrated anti-seizure activities in kainate challenge model Mice implanted with electrodes to measure kainate induced seizures and demonstrated that OV350 at 50mg/kg and 12.5mg/kg Restored diazepam activities in stopping seizures Supports development in Status Epilepticus using IV formulation of OV350/Benzodiazepine combination
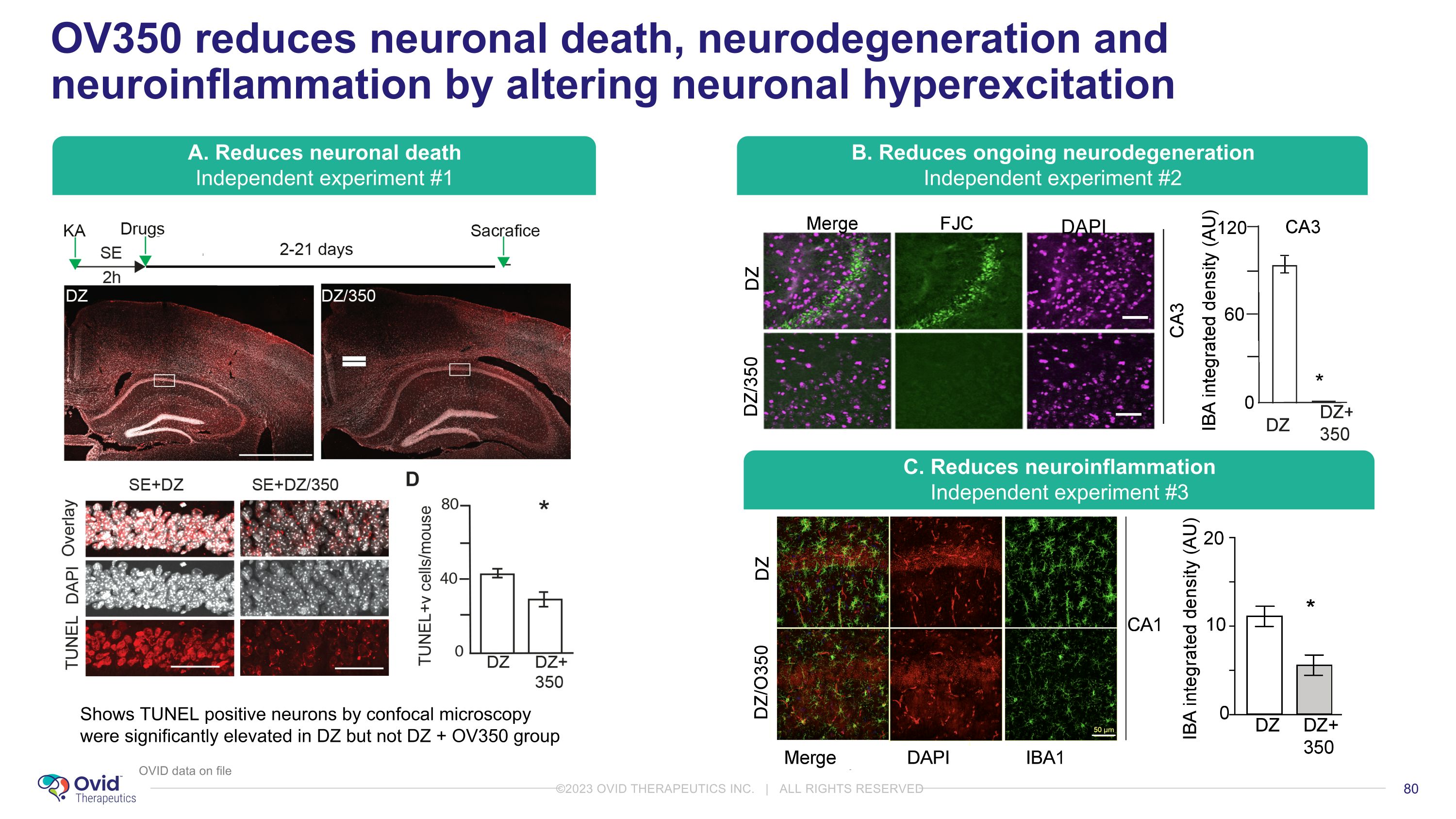
OV350 reduces neuronal death, neurodegeneration and neuroinflammation by altering neuronal hyperexcitation Shows TUNEL positive neurons by confocal microscopy were significantly elevated in DZ but not DZ + OV350 group A. Reduces neuronal death Independent experiment #1 B. Reduces ongoing neurodegeneration Independent experiment #2 C. Reduces neuroinflammation Independent experiment #3 OVID data on file
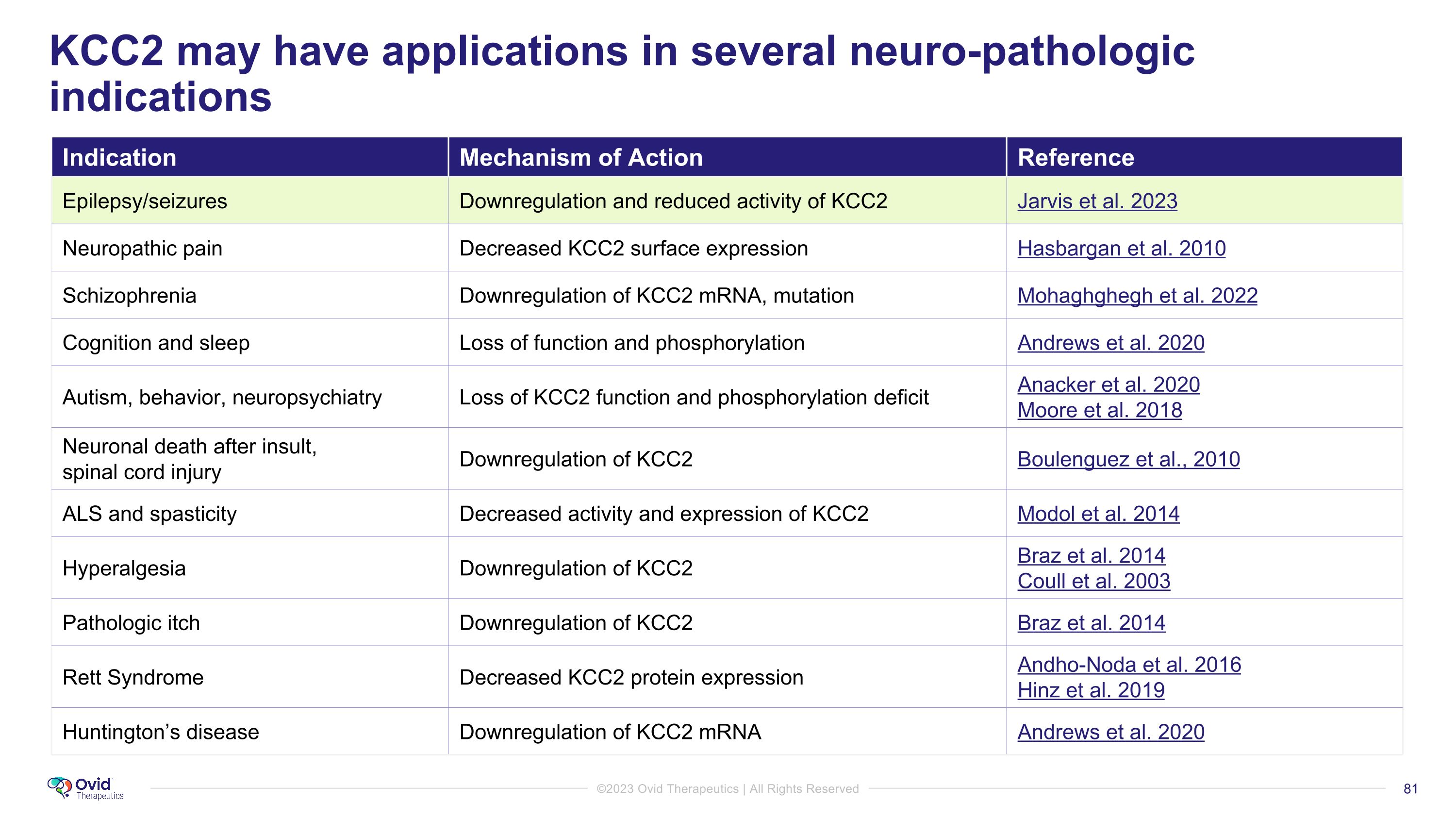
KCC2 may have applications in several neuro-pathologic indications Indication Mechanism of Action Reference Epilepsy/seizures Downregulation and reduced activity of KCC2 Jarvis et al. 2023 Neuropathic pain Decreased KCC2 surface expression Hasbargan et al. 2010 Schizophrenia Downregulation of KCC2 mRNA, mutation Mohaghghegh et al. 2022 Cognition and sleep Loss of function and phosphorylation Andrews et al. 2020 Autism, behavior, neuropsychiatry Loss of KCC2 function and phosphorylation deficit Anacker et al. 2020 Moore et al. 2018 Neuronal death after insult, �spinal cord injury Downregulation of KCC2 Boulenguez et al., 2010 ALS and spasticity Decreased activity and expression of KCC2 Modol et al. 2014 Hyperalgesia Downregulation of KCC2 Braz et al. 2014 Coull et al. 2003 Pathologic itch Downregulation of KCC2 Braz et al. 2014 Rett Syndrome Decreased KCC2 protein expression Andho-Noda et al. 2016 Hinz et al. 2019 Huntington’s disease Downregulation of KCC2 mRNA Andrews et al. 2020
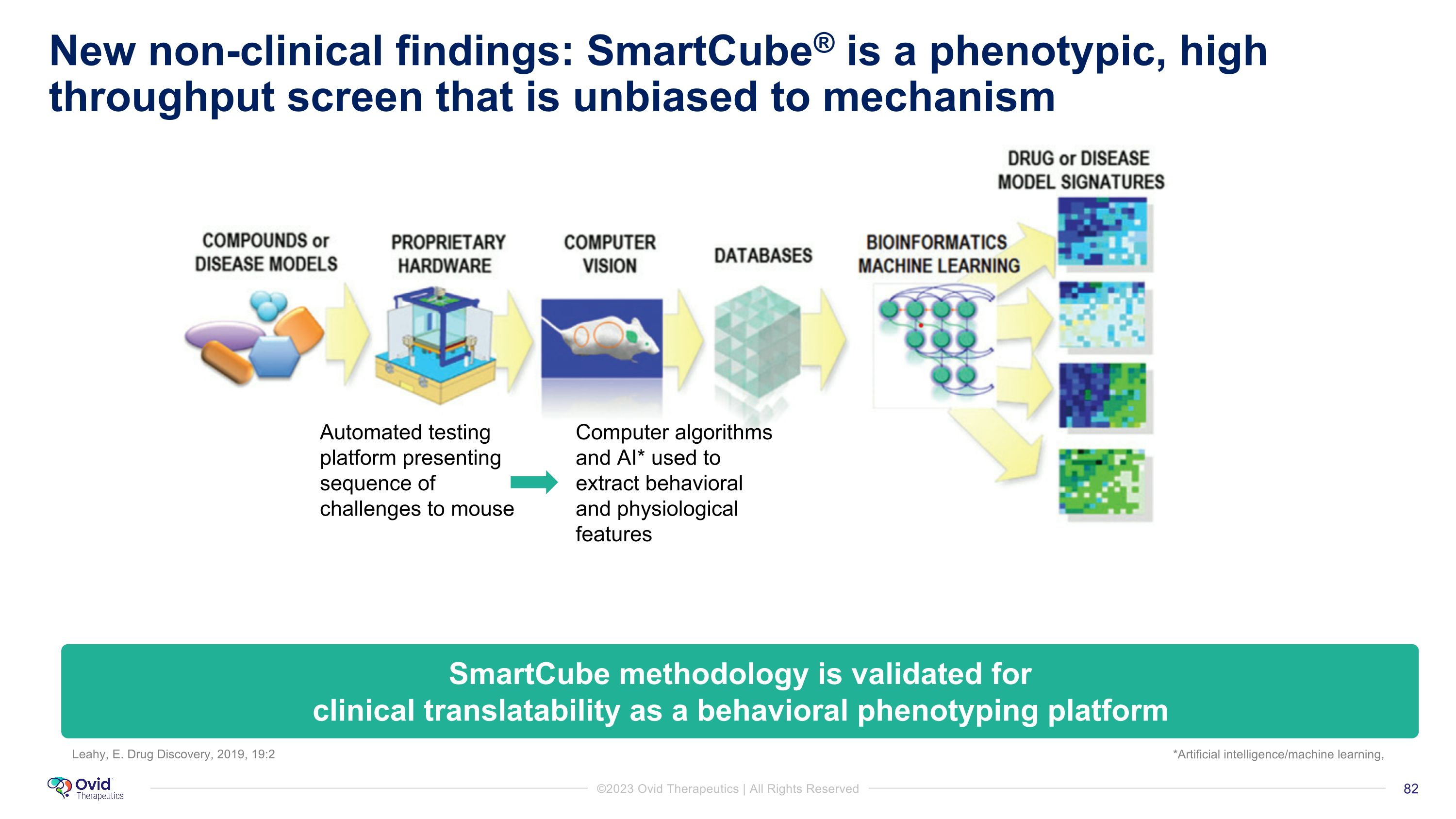
New non-clinical findings: SmartCube® is a phenotypic, high throughput screen that is unbiased to mechanism Automated testing platform presenting sequence of challenges to mouse Computer algorithms and AI* used to extract behavioral and physiological features Leahy, E. Drug Discovery, 2019, 19:2 SmartCube methodology is validated for �clinical translatability as a behavioral phenotyping platform *Artificial intelligence/machine learning,
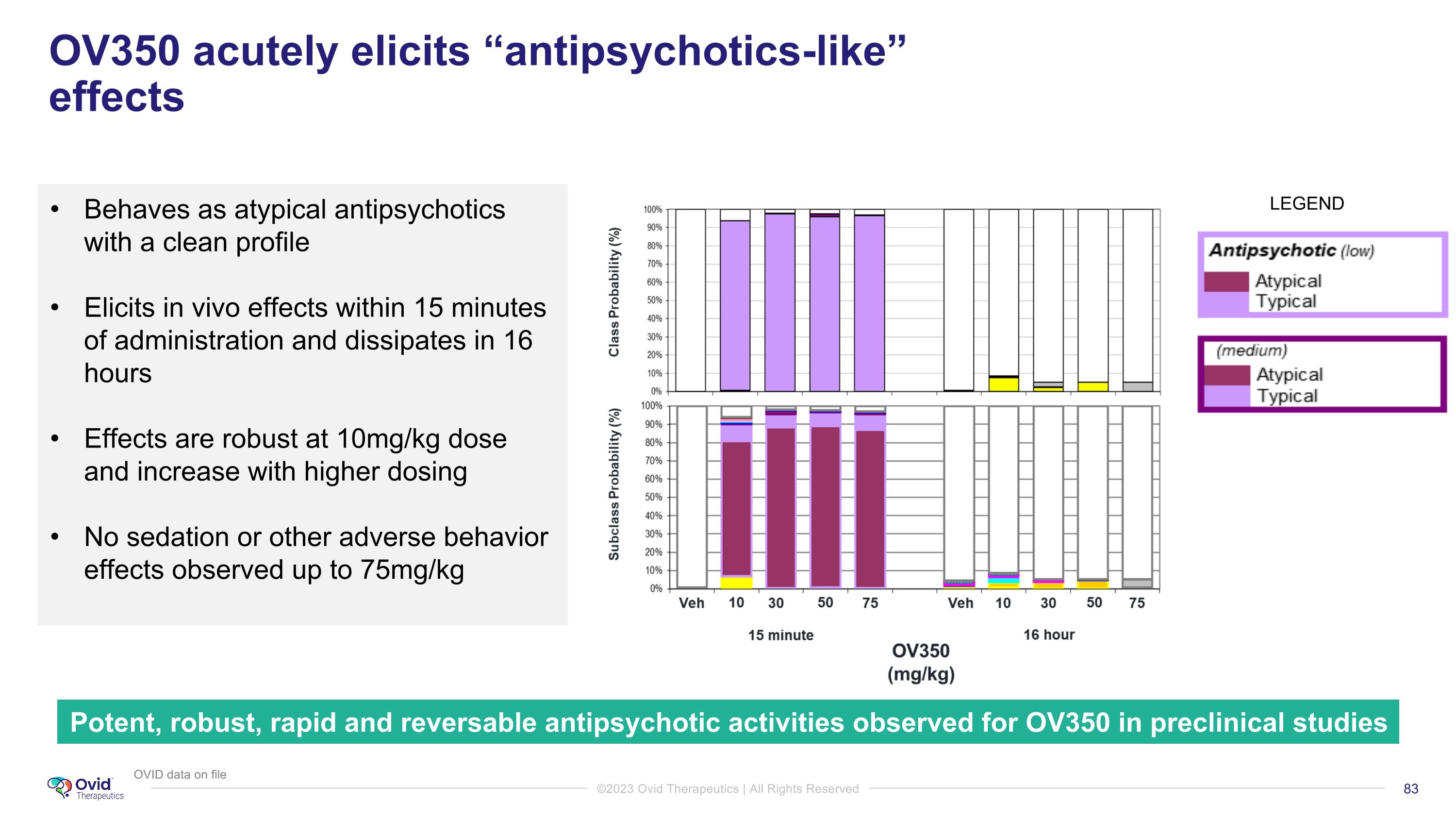
OV350 acutely elicits “antipsychotics-like” effects https://www.nature.com/articles/s41537-021-00190-z Behaves as atypical antipsychotics with a clean profile Elicits in vivo effects within 15 minutes of administration and dissipates in 16 hours Effects are robust at 10mg/kg dose and increase with higher dosing No sedation or other adverse behavior effects observed up to 75mg/kg Potent, robust, rapid and reversable antipsychotic activities observed for OV350 in preclinical studies LEGEND OVID data on file

OV350 anti-psychotic effects demonstrated in a schizophrenia model PCP Rx Phencyclidine-induced psychosis is characterized by: Confusion, excitation, aggression, paranoia, hallucinations, and can be experimentally measured by hyperlocomotion Phencyclidine challenge is a widely used model for schizophrenia OV350 inhibited PCP induced hyperlocomotion with clear dose dependent responses OV350 appears to have anxiolytic effects without causing sedation OVID data on file
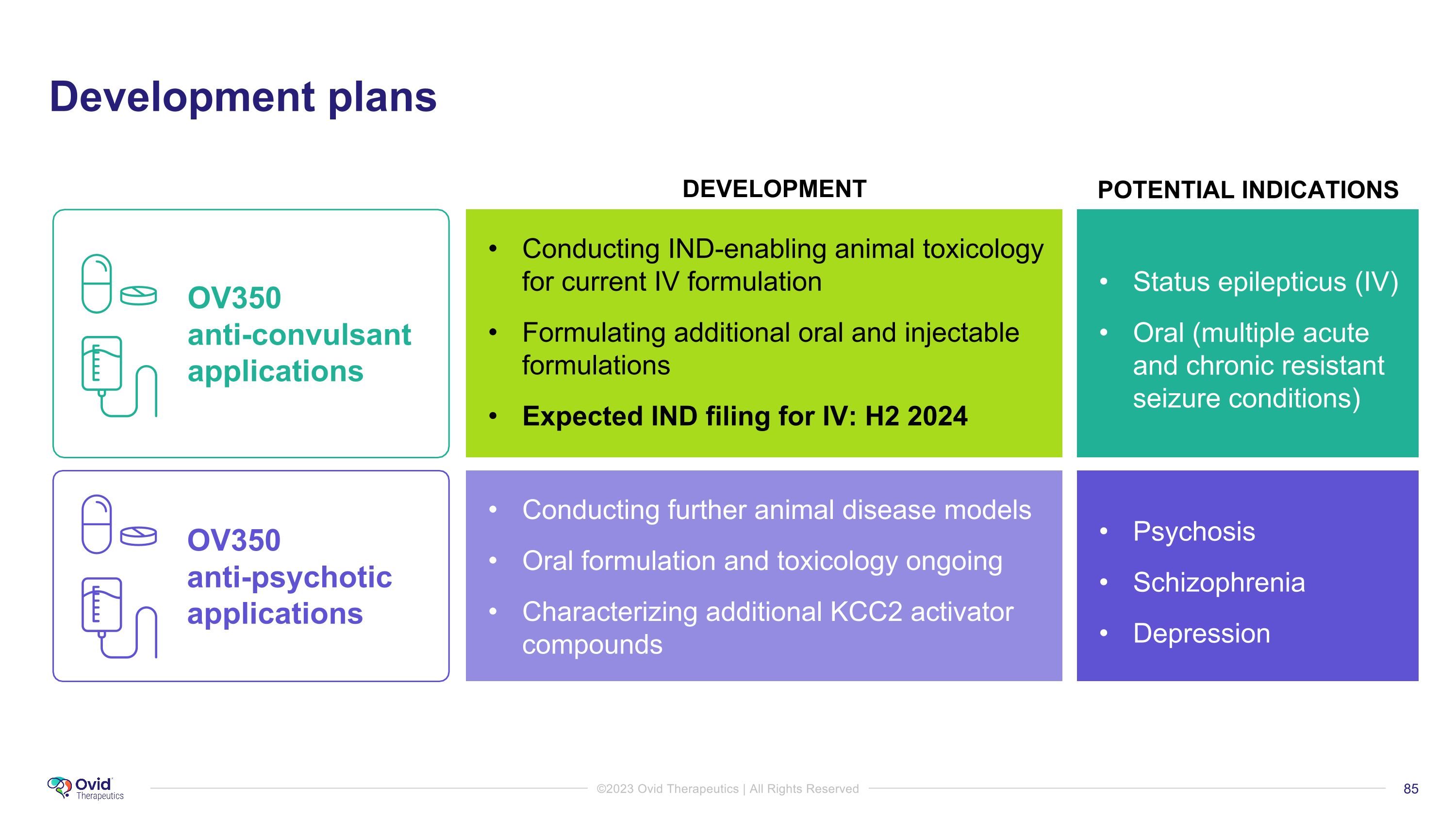
OV350 �anti-convulsant applications OV350 �anti-psychotic applications Conducting IND-enabling animal toxicology for current IV formulation Formulating additional oral and injectable formulations Expected IND filing for IV: H2 2024 Conducting further animal disease models Oral formulation and toxicology ongoing Characterizing additional KCC2 activator compounds Potential to partner or out-license Status epilepticus (IV) Oral (multiple acute and chronic resistant seizure conditions) DEVELOPMENT POTENTIAL INDICATIONS Psychosis Schizophrenia Depression Development plans

Soticlestat anticipated profile��Mr. Jason Tardio Chief Operating Officer

Soticlestat��Making a difference in Dravet & Lennox-Gastaut syndromes

… The most distinctive feature of this drug is that it has a new mechanism of action. There has never been such a drug before. This product has a good efficacy rates compared to others, but it seems to have a better safety profile, which is a strong suit. It also doesn’t interact with other medications, so it’s probably going to be something I’d want to include as an adjunct treatment …My likelihood to prescribe is high” -Epileptologist, Children’s Hospital “ Source: Interviews from Ovid 2022 Global market research. ”
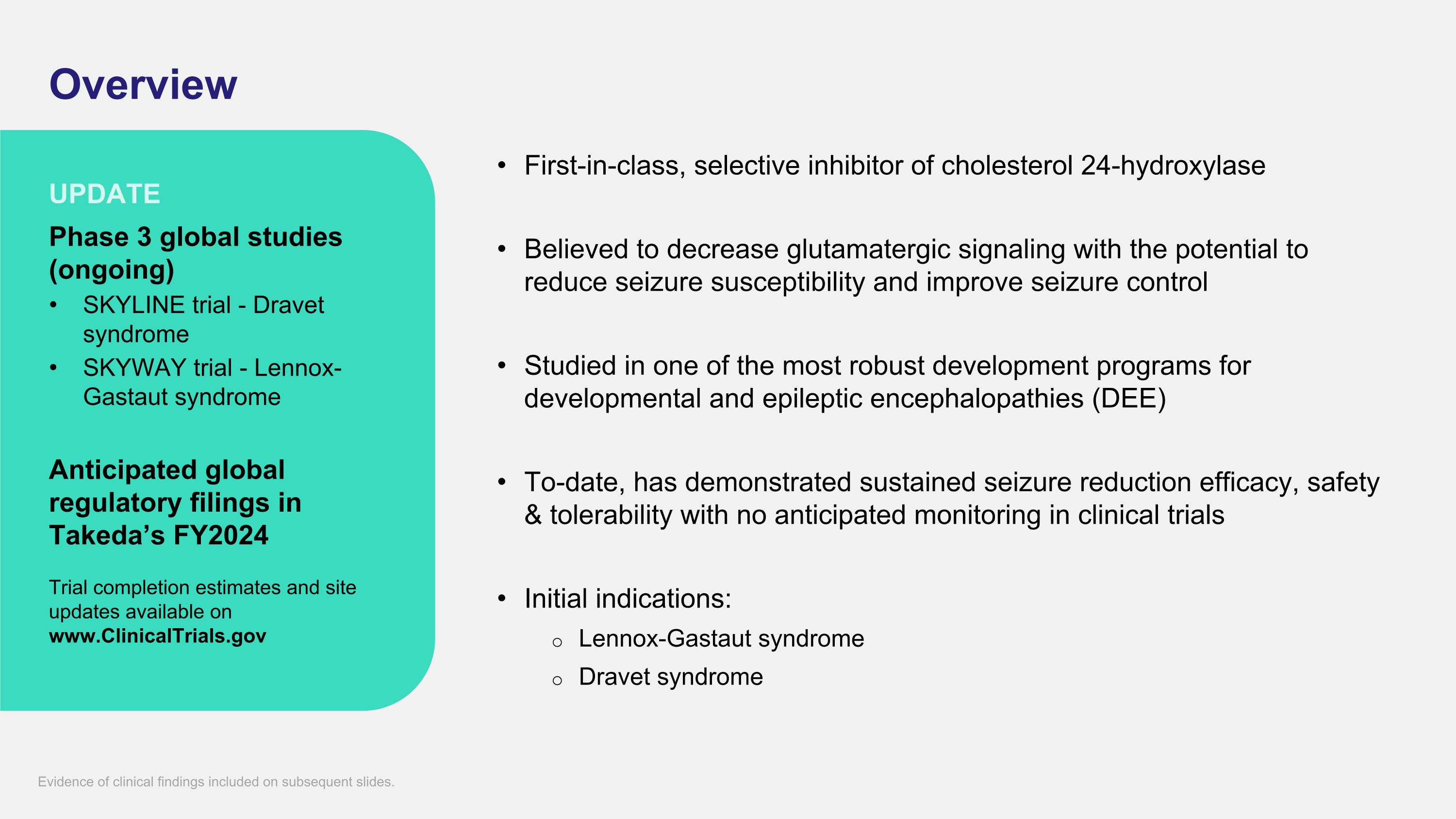
Overview First-in-class, selective inhibitor of cholesterol 24-hydroxylase Believed to decrease glutamatergic signaling with the potential to reduce seizure susceptibility and improve seizure control Studied in one of the most robust development programs for developmental and epileptic encephalopathies (DEE) To-date, has demonstrated sustained seizure reduction efficacy, safety & tolerability with no anticipated monitoring in clinical trials Initial indications: Lennox-Gastaut syndrome Dravet syndrome Phase 3 global studies (ongoing) SKYLINE trial - Dravet syndrome SKYWAY trial - Lennox-Gastaut syndrome Anticipated global regulatory filings in Takeda’s FY2024 Trial completion estimates and site updates available on www.ClinicalTrials.gov UPDATE Evidence of clinical findings included on subsequent slides.

Role of cholesterol 24 hydroxylase in excitability To maintain the steady-state level, excess brain cholesterol is converted into the metabolite 24-S-hydroxycholesterol (24HC) by the enzyme cholesterol 24-hydroxylase (CH24H) 24HC is a positive allosteric modulator of the NMDA receptor and modulates glutamatergic signaling CH24H is involved in over-activation of the glutamatergic pathway Modulation of the NMDA channel Increases expression of CH24H Disrupts the reuptake of glutamate by astrocytes, resulting in hyperexcitability It is believed inhibition of CH24H lowers levels of 24HC and thus reduces glutamate hyperexcitability 1) Gamba P, Giannelli S, Staurenghi E, Testa G, Sottero B, Biasi F, Poli G, Leonarduzzi G. The Controversial Role of 24-S-Hydroxycholesterol in Alzheimer's Disease. Antioxidants (Basel). 2021 May 7;10(5):740. 2)Salamone A, et al. Cholesterol 24-hydroxylase is a novel pharmacological target for antiictogenic and disease modification effects in epilepsy. Neurobiology of Disease. 173, 2022.
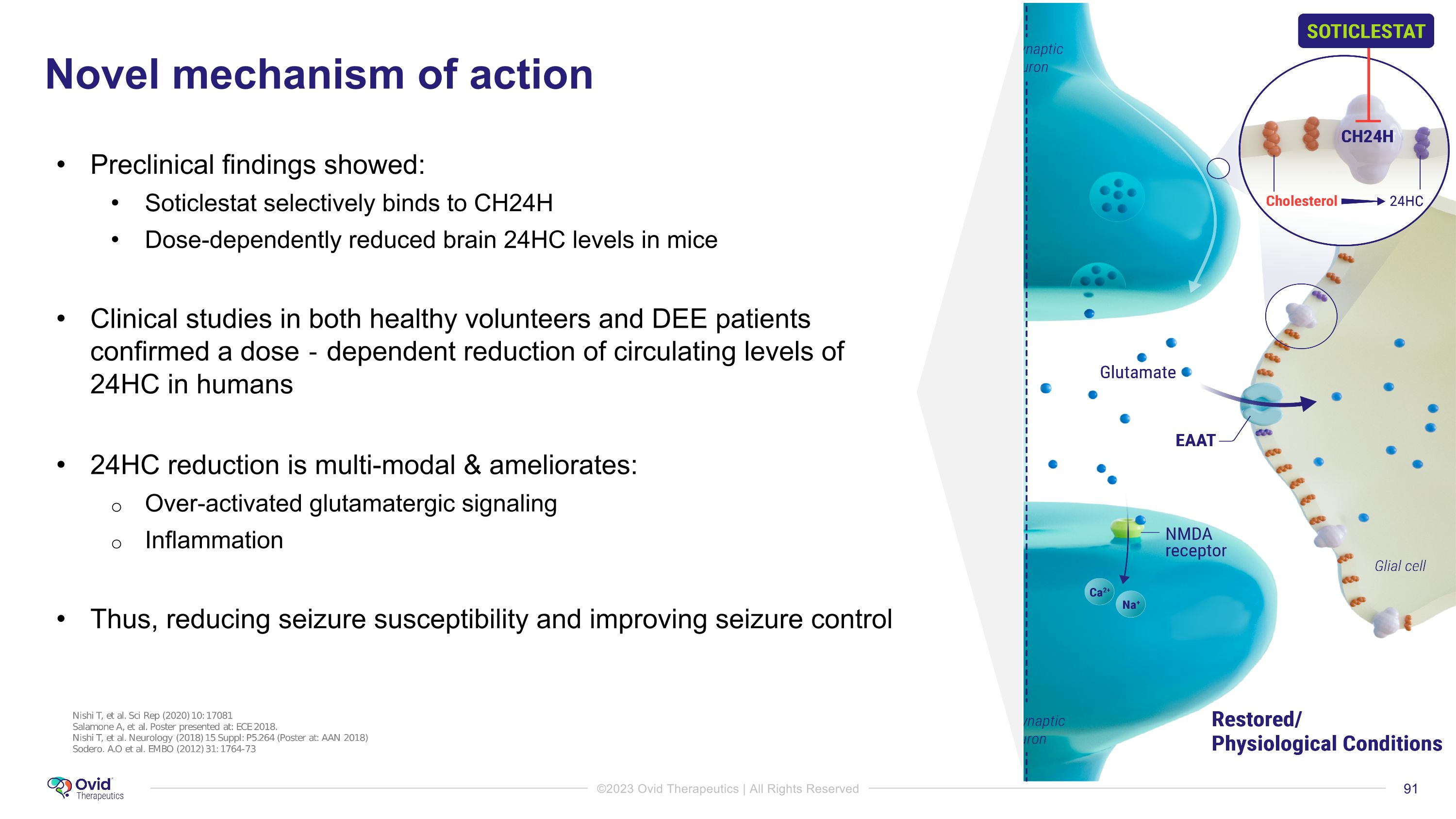
Preclinical findings showed: Soticlestat selectively binds to CH24H Dose-dependently reduced brain 24HC levels in mice Clinical studies in both healthy volunteers and DEE patients confirmed a dose‐dependent reduction of circulating levels of �24HC in humans 24HC reduction is multi-modal & ameliorates: Over-activated glutamatergic signaling Inflammation Thus, reducing seizure susceptibility and improving seizure control Novel mechanism of action

Anticonvulsive properties of soticlestat, a novel cholesterol 24-hydroxylase inhibitor. Nishi T, Metcalf CS, Fujimoto S, Hasegawa S, Miyamoto M, Sunahara E, Watanabe S, Kondo S, White HS. Epilepsia. 2022 Mar 22. doi: 10.1111/epi.17232 Demonstrated efficacy & reduced 24HC levels in several animal models of epilepsy Reduced total seizure burden Delayed acquisition of kindling seizures Prevented progression of spontaneous recurrent seizures Increased seizure threshold against environmental stimuli Improved survival Protection from seizure-related mortality Demonstrated Neuroprotection Protection against glutamate excitotoxicity Preserved cognitive functions Selectively binds to CH24H and dose-dependently reduced brain 24HC levels Highly consistent profile across robust development program Preclinical Phase 1 Phase 1b/2a Phase 2 Open Label Extension Phase 3 FINDINGS
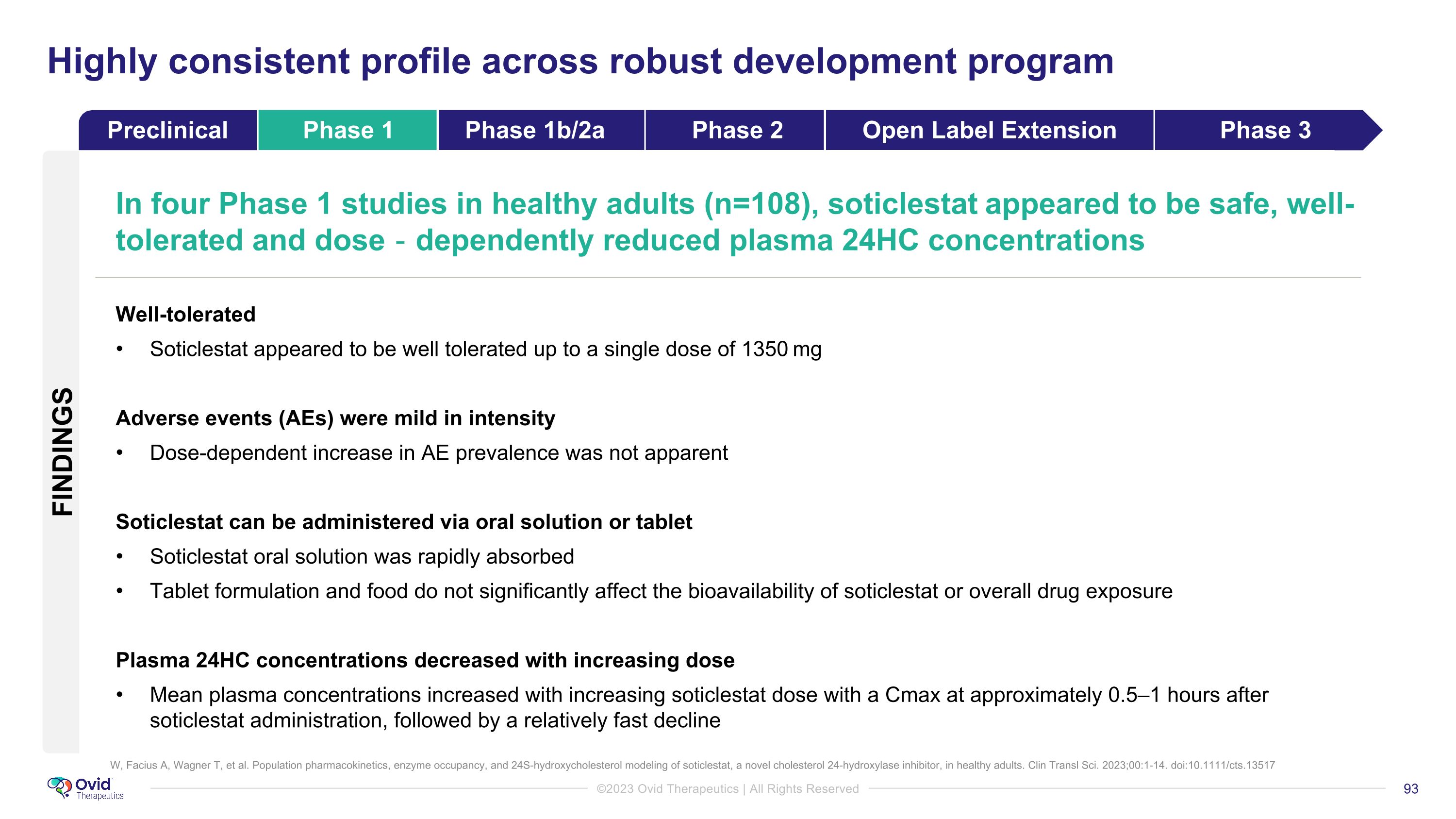
W, Facius A, Wagner T, et al. Population pharmacokinetics, enzyme occupancy, and 24S-hydroxycholesterol modeling of soticlestat, a novel cholesterol 24-hydroxylase inhibitor, in healthy adults. Clin Transl Sci. 2023;00:1-14. doi:10.1111/cts.13517 Highly consistent profile across robust development program In four Phase 1 studies in healthy adults (n=108), soticlestat appeared to be safe, well-tolerated and dose‐dependently reduced plasma 24HC concentrations Well-tolerated Soticlestat appeared to be well tolerated up to a single dose of 1350 mg Adverse events (AEs) were mild in intensity Dose-dependent increase in AE prevalence was not apparent Soticlestat can be administered via oral solution or tablet Soticlestat oral solution was rapidly absorbed Tablet formulation and food do not significantly affect the bioavailability of soticlestat or overall drug exposure Plasma 24HC concentrations decreased with increasing dose Mean plasma concentrations increased with increasing soticlestat dose with a Cmax at approximately 0.5–1 hours after soticlestat administration, followed by a relatively fast decline Preclinical Phase 1 Phase 1b/2a Phase 2 Open Label Extension Phase 3 FINDINGS
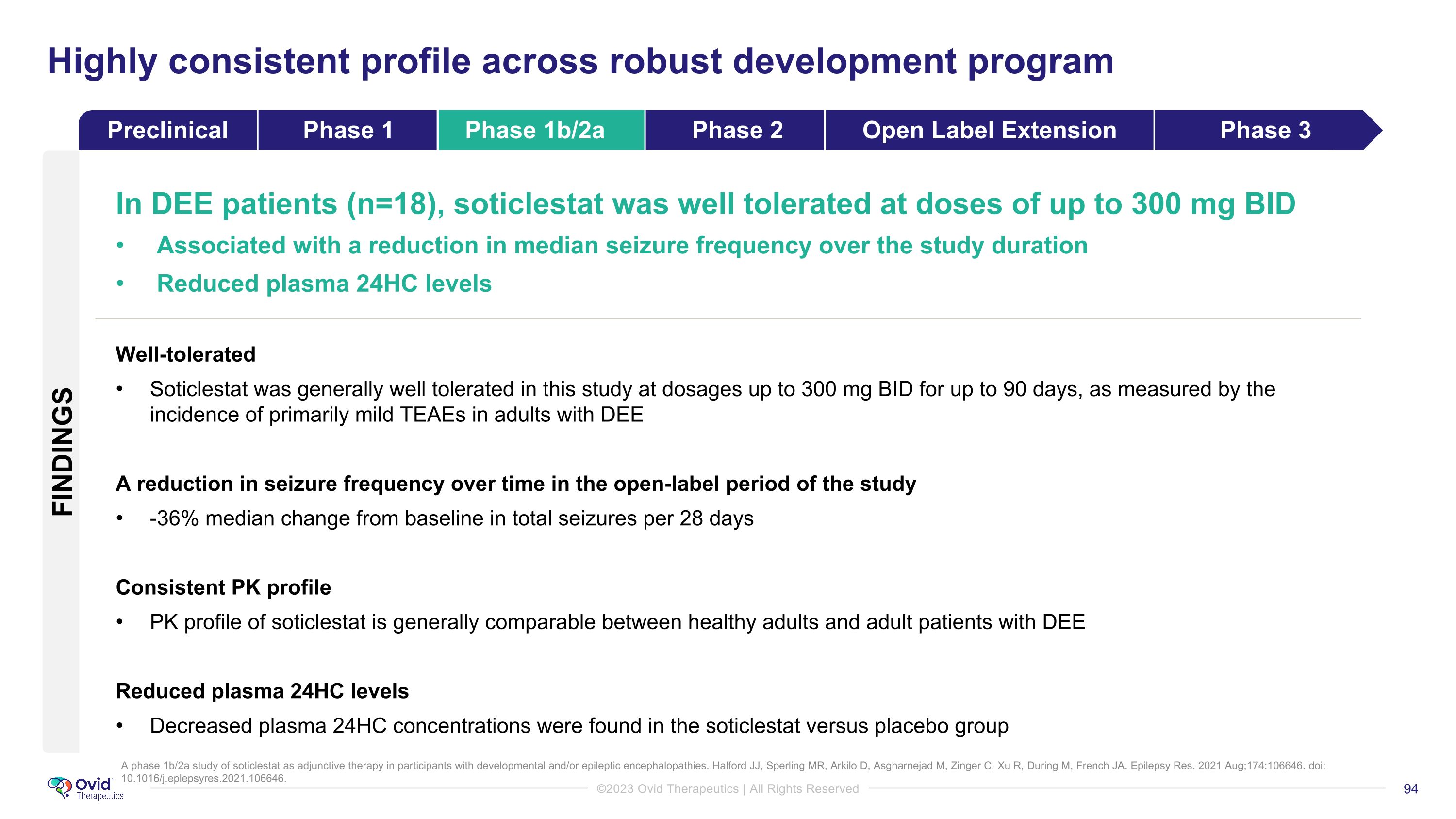
A phase 1b/2a study of soticlestat as adjunctive therapy in participants with developmental and/or epileptic encephalopathies. Halford JJ, Sperling MR, Arkilo D, Asgharnejad M, Zinger C, Xu R, During M, French JA. Epilepsy Res. 2021 Aug;174:106646. doi: 10.1016/j.eplepsyres.2021.106646. Highly consistent profile across robust development program FINDINGS In DEE patients (n=18), soticlestat was well tolerated at doses of up to 300 mg BID Associated with a reduction in median seizure frequency over the study duration Reduced plasma 24HC levels Well-tolerated Soticlestat was generally well tolerated in this study at dosages up to 300 mg BID for up to 90 days, as measured by the incidence of primarily mild TEAEs in adults with DEE A reduction in seizure frequency over time in the open-label period of the study -36% median change from baseline in total seizures per 28 days Consistent PK profile PK profile of soticlestat is generally comparable between healthy adults and adult patients with DEE Reduced plasma 24HC levels Decreased plasma 24HC concentrations were found in the soticlestat versus placebo group Preclinical Phase 1 Phase 1b/2a Phase 2 Open Label Extension Phase 3
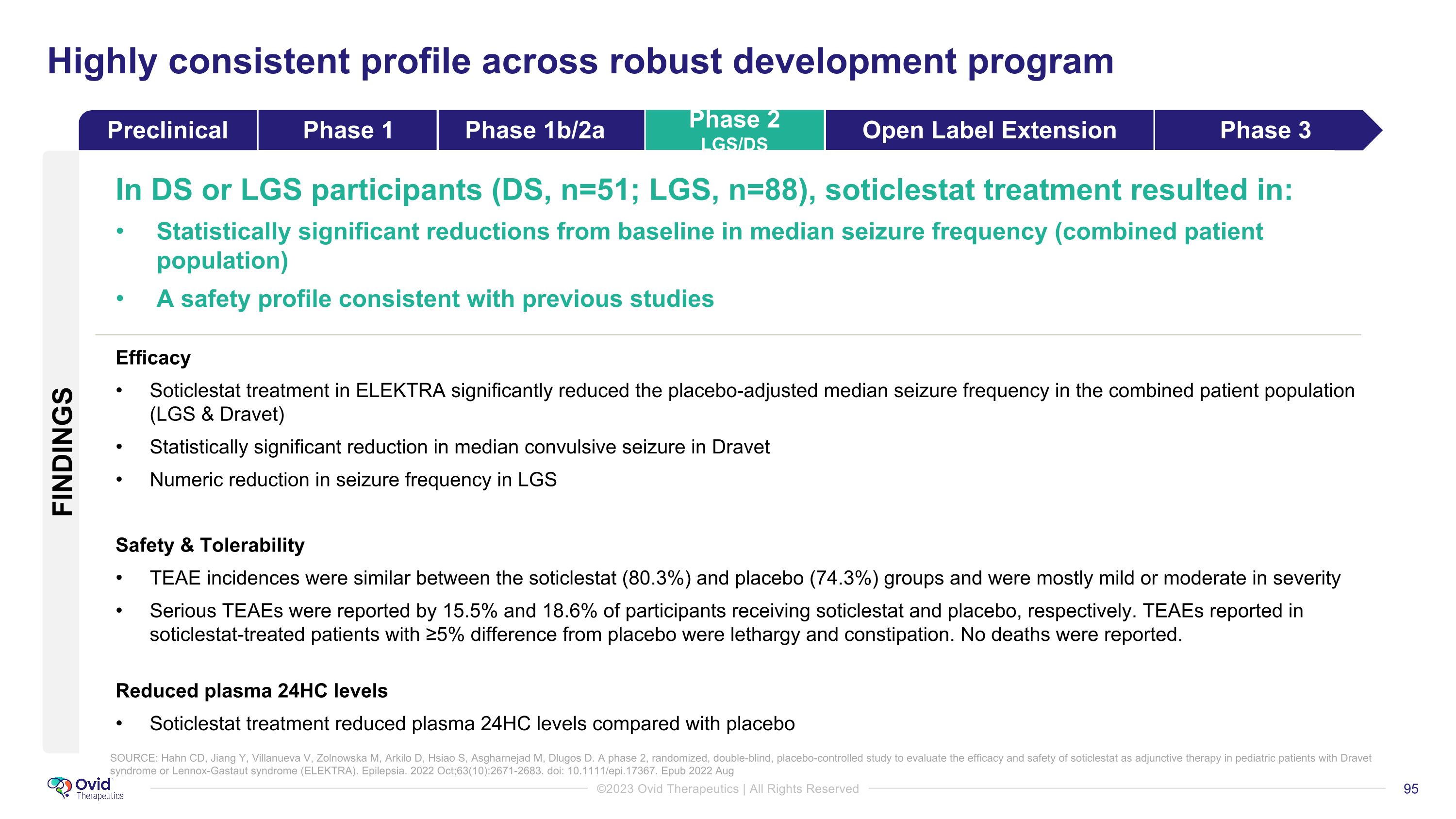
SOURCE: Hahn CD, Jiang Y, Villanueva V, Zolnowska M, Arkilo D, Hsiao S, Asgharnejad M, Dlugos D. A phase 2, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of soticlestat as adjunctive therapy in pediatric patients with Dravet syndrome or Lennox-Gastaut syndrome (ELEKTRA). Epilepsia. 2022 Oct;63(10):2671-2683. doi: 10.1111/epi.17367. Epub 2022 Aug Highly consistent profile across robust development program In DS or LGS participants (DS, n=51; LGS, n=88), soticlestat treatment resulted in: Statistically significant reductions from baseline in median seizure frequency (combined patient population) A safety profile consistent with previous studies Efficacy Soticlestat treatment in ELEKTRA significantly reduced the placebo-adjusted median seizure frequency in the combined patient population (LGS & Dravet) Statistically significant reduction in median convulsive seizure in Dravet Numeric reduction in seizure frequency in LGS Safety & Tolerability TEAE incidences were similar between the soticlestat (80.3%) and placebo (74.3%) groups and were mostly mild or moderate in severity Serious TEAEs were reported by 15.5% and 18.6% of participants receiving soticlestat and placebo, respectively. TEAEs reported in soticlestat-treated patients with ≥5% difference from placebo were lethargy and constipation. No deaths were reported. Reduced plasma 24HC levels Soticlestat treatment reduced plasma 24HC levels compared with placebo FINDINGS Preclinical Phase 1 Phase 1b/2a Phase 2 LGS/DS Open Label Extension Phase 3
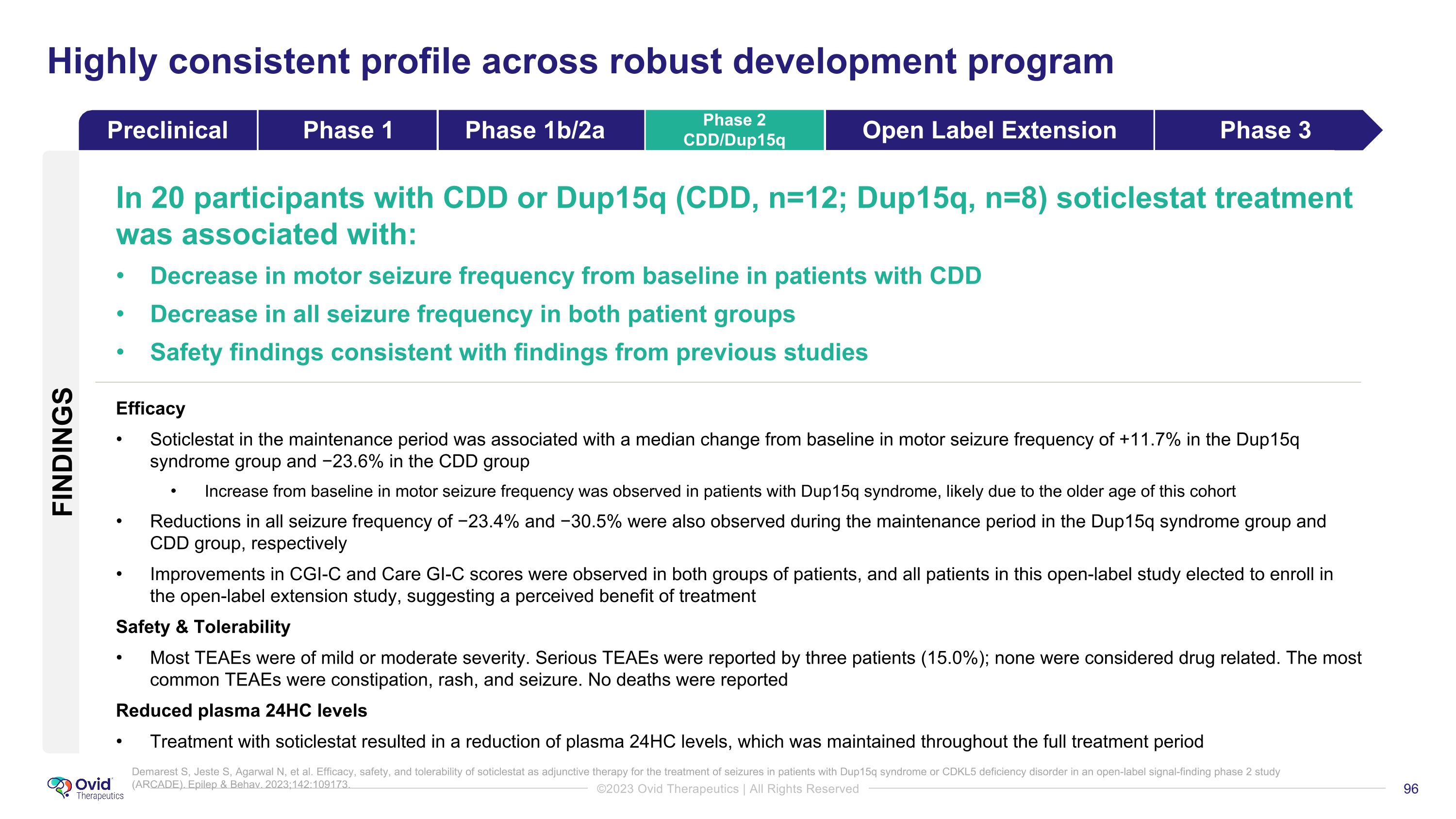
Highly consistent profile across robust development program In 20 participants with CDD or Dup15q (CDD, n=12; Dup15q, n=8) soticlestat treatment was associated with: Decrease in motor seizure frequency from baseline in patients with CDD Decrease in all seizure frequency in both patient groups Safety findings consistent with findings from previous studies Efficacy Soticlestat in the maintenance period was associated with a median change from baseline in motor seizure frequency of +11.7% in the Dup15q syndrome group and −23.6% in the CDD group Increase from baseline in motor seizure frequency was observed in patients with Dup15q syndrome, likely due to the older age of this cohort Reductions in all seizure frequency of −23.4% and −30.5% were also observed during the maintenance period in the Dup15q syndrome group and CDD group, respectively Improvements in CGI-C and Care GI-C scores were observed in both groups of patients, and all patients in this open-label study elected to enroll in the open-label extension study, suggesting a perceived benefit of treatment Safety & Tolerability Most TEAEs were of mild or moderate severity. Serious TEAEs were reported by three patients (15.0%); none were considered drug related. The most common TEAEs were constipation, rash, and seizure. No deaths were reported Reduced plasma 24HC levels Treatment with soticlestat resulted in a reduction of plasma 24HC levels, which was maintained throughout the full treatment period Demarest S, Jeste S, Agarwal N, et al. Efficacy, safety, and tolerability of soticlestat as adjunctive therapy for the treatment of seizures in patients with Dup15q syndrome or CDKL5 deficiency disorder in an open-label signal-finding phase 2 study (ARCADE). Epilep & Behav. 2023;142:109173. FINDINGS Preclinical Phase 1 Phase 1b/2a Phase 2 CDD/Dup15q Open Label Extension Phase 3
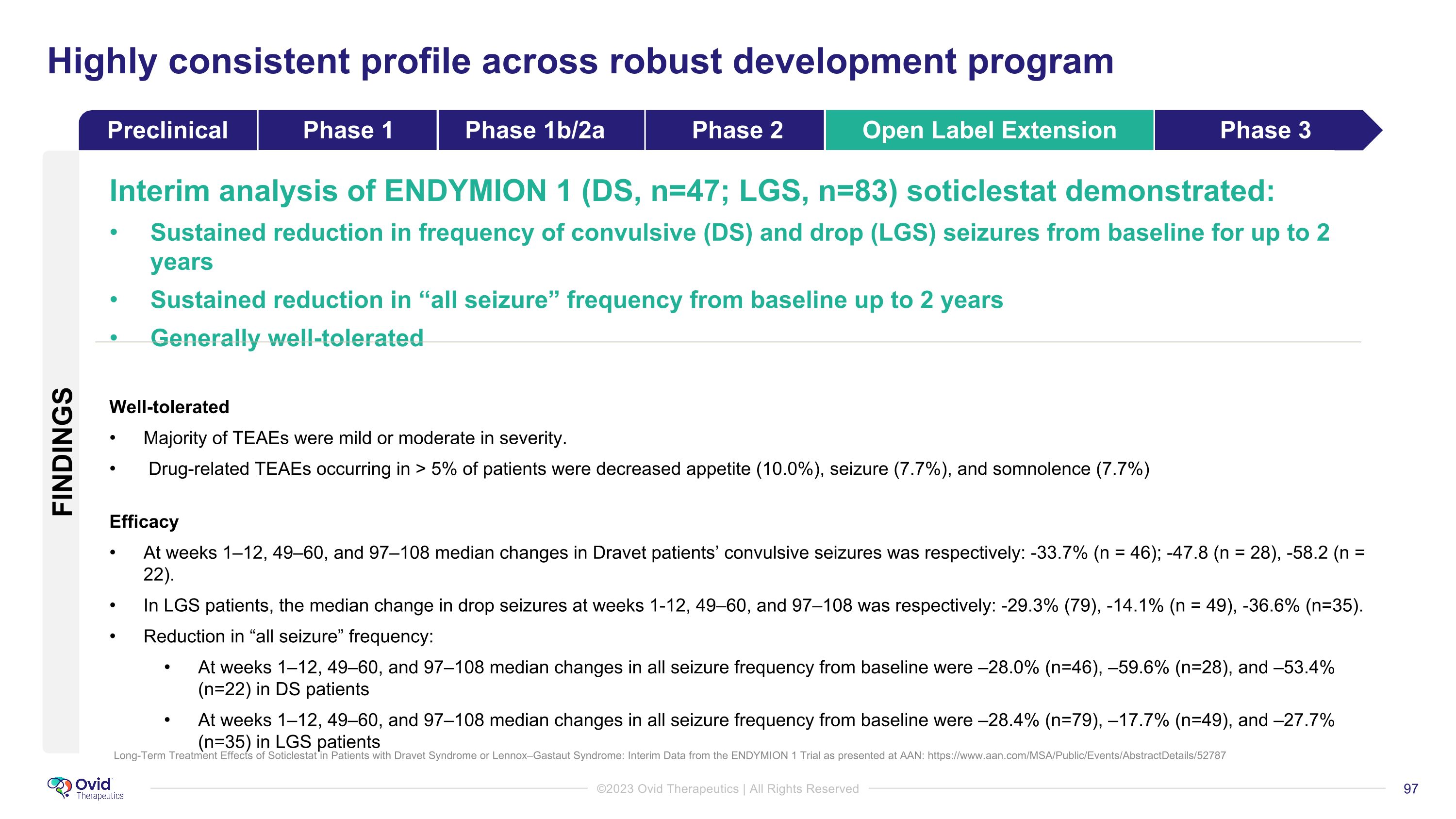
Long-Term Treatment Effects of Soticlestat in Patients with Dravet Syndrome or Lennox–Gastaut Syndrome: Interim Data from the ENDYMION 1 Trial as presented at AAN: https://www.aan.com/MSA/Public/Events/AbstractDetails/52787 Highly consistent profile across robust development program Interim analysis of ENDYMION 1 (DS, n=47; LGS, n=83) soticlestat demonstrated: Sustained reduction in frequency of convulsive (DS) and drop (LGS) seizures from baseline for up to 2 years Sustained reduction in “all seizure” frequency from baseline up to 2 years Generally well-tolerated Well-tolerated Majority of TEAEs were mild or moderate in severity. Drug-related TEAEs occurring in > 5% of patients were decreased appetite (10.0%), seizure (7.7%), and somnolence (7.7%) Efficacy At weeks 1–12, 49–60, and 97–108 median changes in Dravet patients’ convulsive seizures was respectively: -33.7% (n = 46); -47.8 (n = 28), -58.2 (n = 22). In LGS patients, the median change in drop seizures at weeks 1-12, 49–60, and 97–108 was respectively: -29.3% (79), -14.1% (n = 49), -36.6% (n=35). Reduction in “all seizure” frequency: At weeks 1–12, 49–60, and 97–108 median changes in all seizure frequency from baseline were –28.0% (n=46), –59.6% (n=28), and –53.4% (n=22) in DS patients At weeks 1–12, 49–60, and 97–108 median changes in all seizure frequency from baseline were –28.4% (n=79), –17.7% (n=49), and –27.7% (n=35) in LGS patients Preclinical Phase 1 Phase 1b/2a Phase 2 Open Label Extension Phase 3 FINDINGS

Phase 3 pivotal trials (Conducted by Takeda) Takeda anticipates global regulatory filing during its FY 2024 (April 1, 2024 – March 31, 2025) 1100 mg BID, 200 mg BID, 300 mg BID (mg/kg dosing) 2Clinical Trials.gov Accessed October 26, 2021. DS N = 142 LGS N = 234 Soticlestat BID (Add-on to SoC) Placebo BID (Add-on to SoC) Titration (4 weeks) 1 Maintenance (12 weeks) Full treatment period 16 weeks Entry into OLE TRIAL DESIGN Trial design based on feedback from FDA, EMA & PMDA Ages ≥ 2 years Adjunctive to ASMs Active seizures at baseline2 OUTCOME MEASURES Primary Frequency change in convulsive seizures (DS study) during full treatment period DS: ≥4 convulsive seizures at baseline LGS study uses Major Motor Drop seizure endpoint (frequency change in major motor drop seizures) during full treatment period LGS: ≥8 Major Motor Drop (MMD) seizures at baseline. For LGS, countable drop seizures reliably recognized by the caregivers and consistently implemented by the investigators Preclinical Phase 1 Phase 1b/2a Phase 2 Open Label Extension Phase 3

The soticlestat opportunity Market research supports potential use as an early line option for patients not well-controlled on other antiseizure medications: Anticipated profile:1 Efficacy on top of standard of care therapies Fewer side effects No clinically relevant drug-to-drug interactions No expected monitoring requirements Promising safety profile 1) Hahn et al. A phase 2, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of soticlestat as adjunctive therapy in pediatric patients with Dravet syndrome or Lennox–Gastaut syndrome (ELEKTRA). Epilepsia. 2022; 00: 1– 13. https://doi.org/10.1111/epi.17367 2) Ovid Global Market Reseach 2022 LARGE GLOBAL MARKET OPPORTUNITY2 If soticlestat is approved and commercialized, subsequent milestones (up to $660M) and royalties (tiered up to 20%) create a stream of non-dilutive capital to Ovid to fuel our pipeline ~110-135K Lennox-Gastaut syndrome (7 major markets) ~30-45K Dravet syndrome (7 major markets)

Milestones & financial strategy��Mr. Jeff Rona Chief Business & Financial Officer
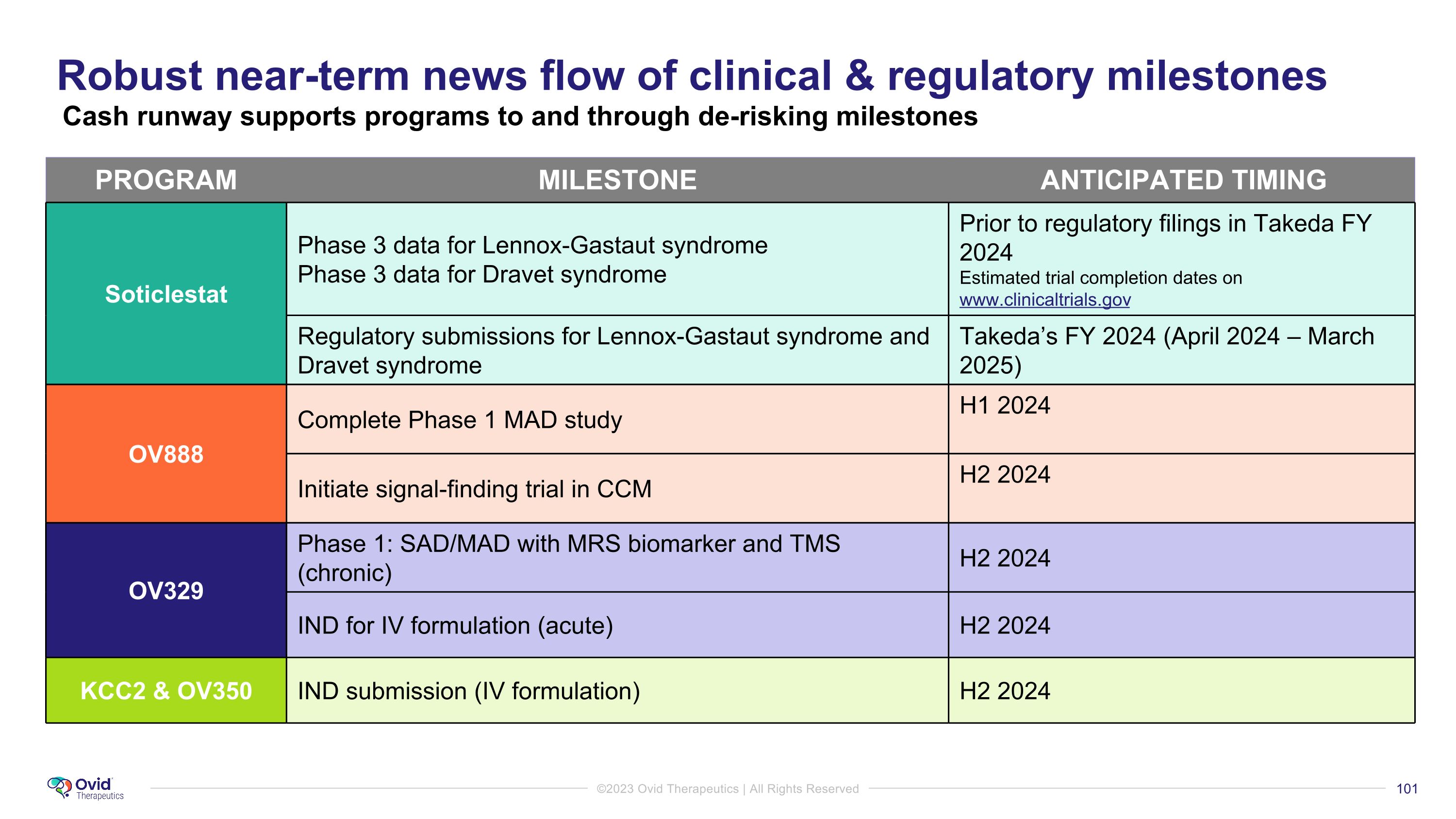
Robust near-term news flow of clinical & regulatory milestones Cash runway supports programs to and through de-risking milestones PROGRAM MILESTONE ANTICIPATED TIMING Soticlestat Phase 3 data for Lennox-Gastaut syndrome Phase 3 data for Dravet syndrome Prior to regulatory filings in Takeda FY 2024 Estimated trial completion dates on www.clinicaltrials.gov Regulatory submissions for Lennox-Gastaut syndrome and Dravet syndrome Takeda’s FY 2024 (April 2024 – March 2025) OV888 Complete Phase 1 MAD study H1 2024 Initiate signal-finding trial in CCM H2 2024 OV329 Phase 1: SAD/MAD with MRS biomarker and TMS (chronic) H2 2024 IND for IV formulation (acute) H2 2024 KCC2 & OV350 IND submission (IV formulation) H2 2024
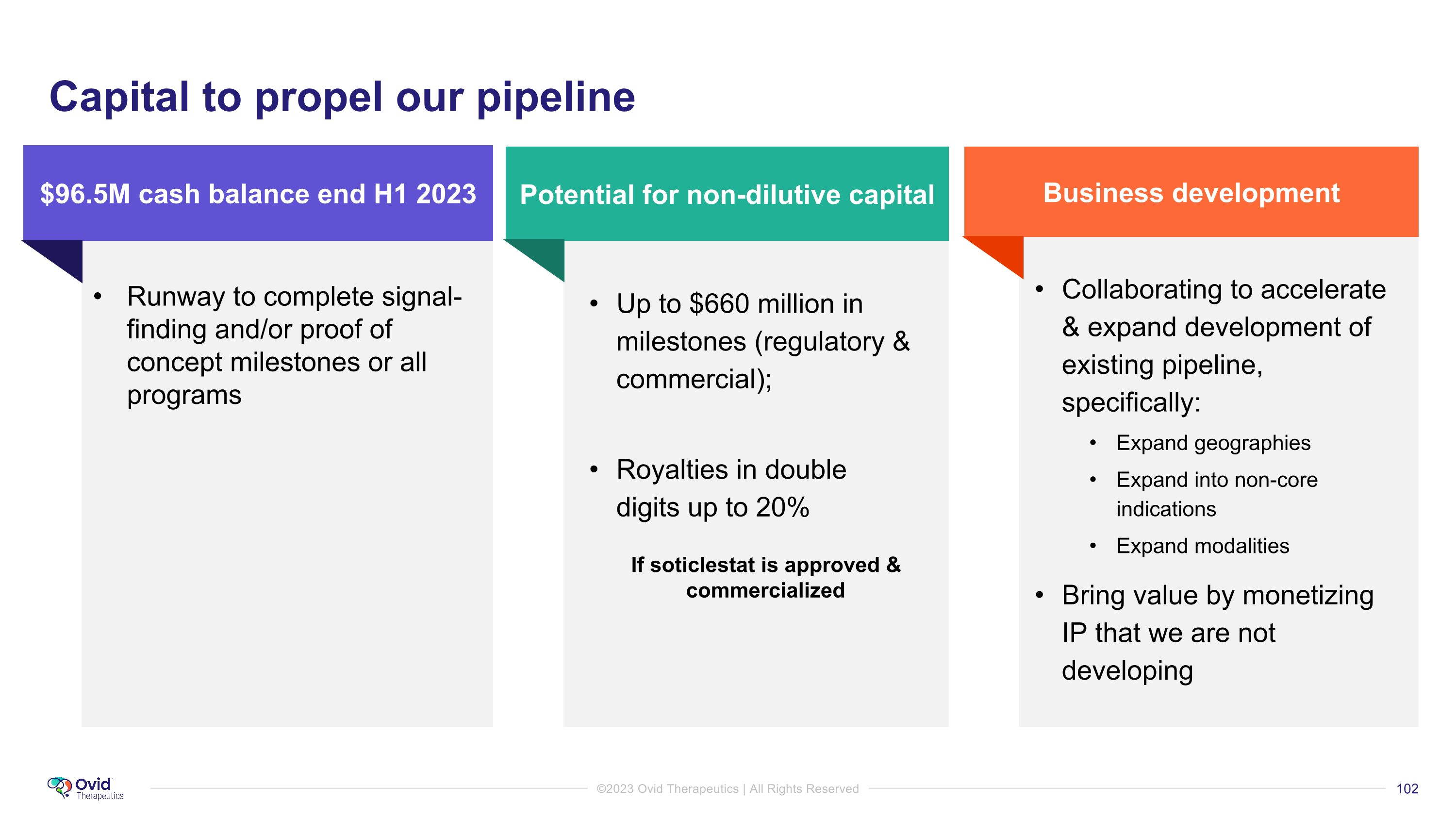
Runway to complete signal-finding and/or proof of concept milestones or all programs Business development Potential for non-dilutive capital $96.5M cash balance end H1 2023 Capital to propel our pipeline Up to $660 million in milestones (regulatory & commercial); Royalties in double digits up to 20% Collaborating to accelerate & expand development of existing pipeline, specifically: Expand geographies Expand into non-core indications Expand modalities Bring value by monetizing IP that we are not developing If soticlestat is approved & commercialized

Why Ovid? Differentiated and risk mitigated pipeline Programs with multiple value creating opportunities Financial interest in two imminent Phase 3 pivotal study readouts & regulatory filings 5 additional clinical milestones expected within the next 12-15 months Potential to partner non-seizure applications Strong capital base and financial strategy to fund anticipated milestones

Ovid is part of a revolution in targeted small molecules that can unlock epilepsy and an era of neurotherapeutics.
Open Q&A KCC2 direct activation and OV350 Soticlestat, a cholesterol 24 hydroxylase inhibitor Financial strategy
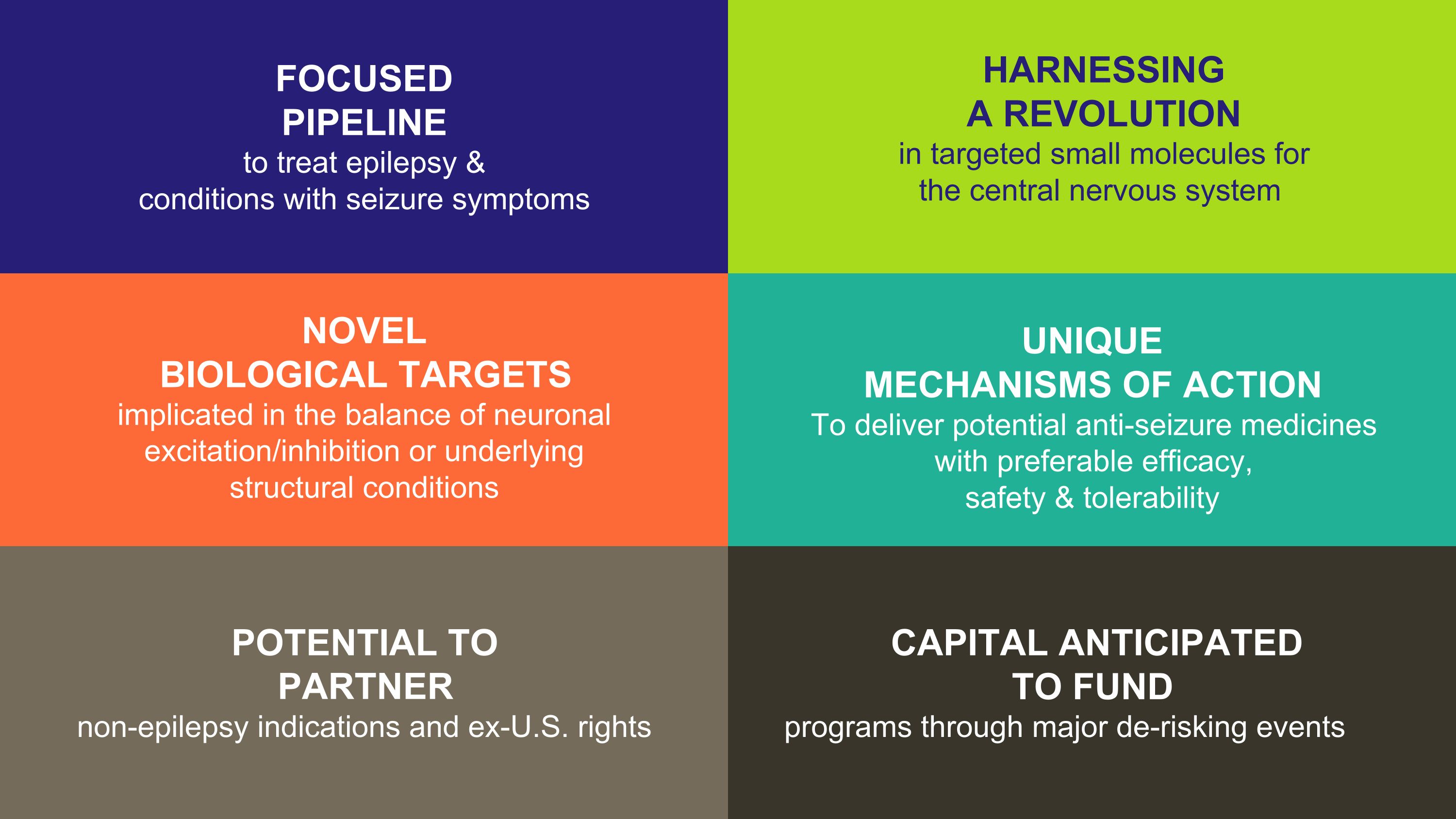
HARNESSING �A REVOLUTION�in targeted small molecules for �the central nervous system NOVEL �BIOLOGICAL TARGETS �implicated in the balance of neuronal excitation/inhibition or underlying �structural conditions UNIQUE �MECHANISMS OF ACTION To deliver potential anti-seizure medicines with preferable efficacy, �safety & tolerability FOCUSED �PIPELINE to treat epilepsy & �conditions with seizure symptoms POTENTIAL TO �PARTNER �non-epilepsy indications and ex-U.S. rights CAPITAL ANTICIPATED �TO FUND programs through major de-risking events
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Ovid Therapeutics (NASDAQ:OVID)
과거 데이터 주식 차트
부터 1월(1) 2025 으로 2월(2) 2025

Ovid Therapeutics (NASDAQ:OVID)
과거 데이터 주식 차트
부터 2월(2) 2024 으로 2월(2) 2025
