false 0001422143 0001422143 2025-02-05 2025-02-05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): February 5, 2025
KURA ONCOLOGY, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-37620 |
|
61-1547851 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 12730 High Bluff Drive, Suite 400, San Diego, CA |
|
92130 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (858) 500-8800
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
KURA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On February 5, 2025, Kura Oncology, Inc. (“Kura” or the “Company”) and Kyowa Kirin Co., Ltd. (“Kyowa Kirin”) announced positive topline results from KOMET-001, the Phase 2 registration-directed trial of ziftomenib, a highly selective, once-daily, oral investigational menin inhibitor, in patients with relapsed/refractory (“R/R”) NPM1-mutant (“NPM1-m”) acute myeloid leukemia (“AML”). Topline data for KOMET-001 has been submitted for presentation at an upcoming medical conference in the second quarter of 2025, and Kura is on track to submit a New Drug Application (“NDA”) to the U.S. Food and Drug Administration (“FDA”) for ziftomenib in the second quarter of 2025.
Kura and Kyowa Kirin, which announced their joint collaboration to commercialize ziftomenib in 2024, also announced they plan to initiate a single protocol containing two independently powered, randomized, double-blind, placebo-controlled, registrational Phase 3 trials to evaluate ziftomenib in combination with both intensive and non-intensive combination regimens in patients with newly diagnosed NPM1-m and KMT2A-rearranged (“KMT2A-r”) AML, following successful interactions with the FDA. Each frontline trial design includes dual-primary endpoints to support potential U.S. accelerated approval and full approval. The companies plan to initiate the two frontline Phase 3 trials in the second half of 2025 and anticipate multiple clinical data presentations for the ziftomenib AML program as well as Kura’s pipeline programs in 2025.
Kura and Kyowa Kirin also announced positive topline results from KOMET-001, a Phase 2 registration-directed trial of ziftomenib, in patients with R/R NPM1-m AML. The KOMET-001 trial achieved its primary endpoint of complete remission (“CR”) plus CR with partial hematological recovery (“CRh”) and the primary endpoint was statistically significant. The benefit-risk profile for ziftomenib is highly encouraging, and safety and tolerability were consistent with previous reports.
The KOMET-001 registration-directed trial is designed to assess evidence of clinical activity, safety and tolerability of ziftomenib, the only investigational therapy to receive Breakthrough Therapy Designation (“BTD”) from the FDA for treatment of R/R NPM1-mutant AML. Full results from the KOMET-001 trial will be presented at a future medical meeting in the second quarter of 2025. After successful FDA interactions in part facilitated by BTD, Kura announced that it is on track to submit an NDA to the FDA for ziftomenib for the treatment of patients with R/R NPM1-mutant AML in the second quarter of 2025.
Kura and Kyowa Kirin recently announced plans for KOMET-017, a global protocol evaluating ziftomenib in combination with standards of care for adults with newly diagnosed NPM1-m or KMT2A-r AML. Following successful End-of-Phase 1 meetings with the FDA, the companies announced they will proceed with plans to initiate the KOMET-017 trial, comprising of two independent, global, randomized, double-blind, placebo-controlled Phase 3 trials to evaluate ziftomenib in combination with both intensive and non-intensive combination regimens in patients with newly diagnosed NPM1-m and/or KMT2A-r AML. The positive feedback from the FDA, along with data from the KOMET-007 trial presented at the 2024 American Society of Hematology Annual Meeting, reinforces Kura’s and Kyowa Kirin’s commitment to evaluating ziftomenib in patients across the continuum of frontline treatment options.
The registrational KOMET-017-IC (Intensive Combination) trial will evaluate the combination of ziftomenib with induction chemotherapy (“7+3”) in newly diagnosed NPM1-m and KMT2A-r AML patients. Patients will be randomized to receive ziftomenib or placebo, in combination with standard induction, consolidation chemotherapy and post consolidation maintenance. The KOMET-017-IC trial will assess minimum residual disease (“MRD”) negative complete response (“CR”) and event-free survival (“EFS”) as dual-primary endpoints to support potential U.S. accelerated approval and full approval, respectively and is anticipated to be initiated in the second half of 2025.
The registrational KOMET-017-NIC (Non-Intensive Combination) trial will evaluate the combination of ziftomenib with venetoclax plus azacitidine in newly diagnosed NPM1-m patients unfit to receive intensive chemotherapy. The KOMET-017-NIC trial will assess CR and overall survival (“OS”) as dual-primary endpoints to support potential U.S. accelerated approval and full approval, respectively. Patients will be randomized to receive ziftomenib or placebo, in combination with venetoclax and azacitidine. The KOMET-017-NIC trial is anticipated to be initiated in the second half of 2025.
MRD is a term describing small numbers of leukemic cells, which are still detectable during or after treatment, even when a patient has achieved CR by standard criteria. Remaining leukemia cells in the body can become active and start to multiply, resulting in a relapse of the disease, which may be fatal for patients. Achieving MRD negativity, which may be associated with longer remissions and improved survival, means that a treatment has reduced the number of leukemic cells to below the limit of detection by the most sensitive analytical methods.
Kura and Kyowa Kirin expect to present multiple clinical data updates from their ziftomenib AML program, and Kura expects to present updates from its KO-2806 and tipifarnib programs, in 2025 as follows:
| |
• |
|
Topline data from the KOMET-001 trial of ziftomenib monotherapy in R/R NPM1-m AML (2Q 2025) |
| |
• |
|
KOMET-007 Phase 1b data of ziftomenib in combination with 7+3 in newly diagnosed NPM1-m AML and KMT2A-r AML (2Q 2025) |
| |
• |
|
FIT-001 Phase 1 data of KO-2806 monotherapy in HRAS-mutant and KRAS-mutated solid tumors (2H 2025) |
| |
• |
|
FIT-001 Phase 1 data of KO-2806 in combination with cabozantinib in renal cell carcinoma (“RCC”) (2H 2025) |
| |
• |
|
KURRENT-HN Phase 1 data of tipifarnib in combination with alpelisib in PIK3CA-dependent head and neck squamous cell carcinoma (“HNSCC”) (2H 2025) |
| |
• |
|
KOMET-007 Phase 1b data of ziftomenib in combination with venetoclax / azacitidine in NPM1-m AML (2H 2025) |
On February 5, 2025, the Company will host a virtual investor event and present certain materials related to the Company (the “Presentation”). A copy of the Presentation is attached hereto as Exhibit 99.1 and incorporated herein by reference.
Forward-Looking Statements
Statements contained in this Current Report on Form 8-K regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements, including, but not limited to, statements regarding, among other things, the therapeutic potential and potential success of ziftomenib, KO-2806 and tipifarnib; plans, trial designs and expected timing of clinical trials; the expected timing and presentation of data from clinical trials; the anticipated timing of the submission of a New Drug Application for ziftomenib; the potential for U.S. accelerated approval and full approval of product candidates; and the success and impact of interactions with the FDA.
Any forward-looking statements in this Current Report on Form 8-K are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: the risk that compounds that appeared promising in early research or clinical trials do not demonstrate safety and/or efficacy in later preclinical studies or clinical trials, the risk that the Company may not obtain approval to market its product candidates, uncertainties associated with performing clinical trials, regulatory filings, applications and other interactions with regulatory bodies, risks associated with reliance on third parties to successfully conduct clinical trials, the risks associated with reliance on outside financing to meet capital requirements, and other risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs, as well as those risks and uncertainties set forth more fully under the caption “Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2024 filed with the Securities and Exchange Commission (“SEC”) on November 7, 2024, as well as discussions of potential risks, uncertainties and other important factors in the Company’s other filings and reports with the SEC. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
KURA ONCOLOGY, INC. |
|
|
|
| Date: February 5, 2025 |
|
By: |
|
/s/ Teresa Bair |
|
|
|
|
Teresa Bair |
|
|
|
|
Chief Legal Officer |
February 5, 2025 ZIFTOMENIB PROGRAM
UPDATES Our goal is to develop transformative therapies to extend and improve the lives of patients with cancer Exhibit 99.1

FORWARD-LOOKING STATEMENTS This
presentation contains forward-looking statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for ziftomenib, KO-2806 and
tipifarnib, plans regarding regulatory filings, our expectations regarding the relative benefits of our product candidates versus competitive therapies, and our expectations regarding the therapeutic and commercial potential of our product
candidates. The words “believe,” “may,” “should,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,”
“expect,” “potential” and similar expressions (including the negative thereof) are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ
materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food
and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials;
we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our product candidates, or in the reporting of data from such clinical testing, or significant issues regarding the adequacy of our
clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our product candidates may not receive regulatory approval or be
successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; and we may not be able to obtain additional financing.
Additional risks and uncertainties may emerge from time to time, and it is not possible for Kura’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of
the date on which they were made. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or
circumstances that exist after the date on which they were made. This presentation also contains statistical data obtained from and prepared by third parties. The recipient is cautioned not to give undue weight to such disclosures. Neither the
Company nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation.
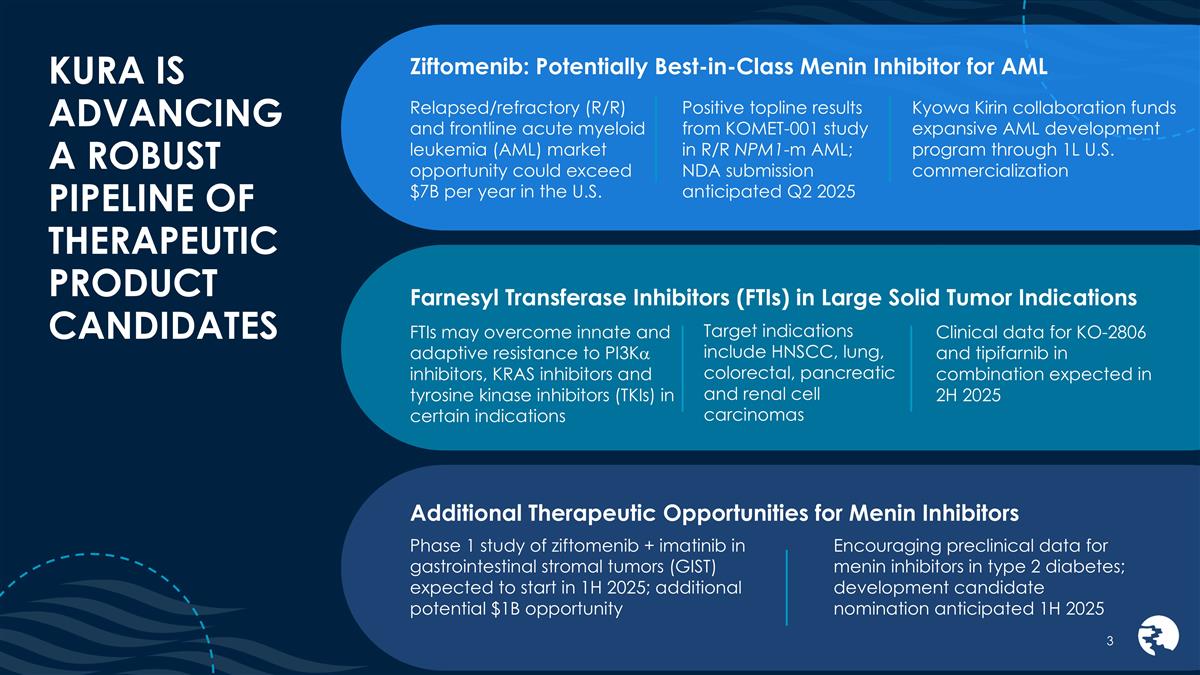
KURA IS ADVANCING A ROBUST PIPELINE OF
THERAPEUTIC PRODUCT CANDIDATES Ziftomenib: Potentially Best-in-Class Menin Inhibitor for AML Relapsed/refractory (R/R) and frontline acute myeloid leukemia (AML) market opportunity could exceed $7B per year in the U.S. Positive topline results from
KOMET-001 study in R/R NPM1-m AML; NDA submission anticipated Q2 2025 Kyowa Kirin collaboration funds expansive AML development program through 1L U.S. commercialization Target indications include HNSCC, lung, colorectal, pancreatic and renal cell
carcinomas Farnesyl Transferase Inhibitors (FTIs) in Large Solid Tumor Indications FTIs may overcome innate and adaptive resistance to PI3Ka inhibitors, KRAS inhibitors and tyrosine kinase inhibitors (TKIs) in certain indications Clinical data
for KO-2806 and tipifarnib in combination expected in 2H 2025 Additional Therapeutic Opportunities for Menin Inhibitors Encouraging preclinical data for menin inhibitors in type 2 diabetes; development candidate nomination anticipated 1H 2025 Phase
1 study of ziftomenib + imatinib in gastrointestinal stromal tumors (GIST) expected to start in 1H 2025; additional potential $1B opportunity

AGENDA Positive FDA Feedback for
Upcoming Frontline Combination Trial Designs Positive KOMET-001 Ziftomenib Monotherapy Trial in R/R NPM1-m AML 2025 Anticipated Milestones for Ziftomenib and Pipeline Programs
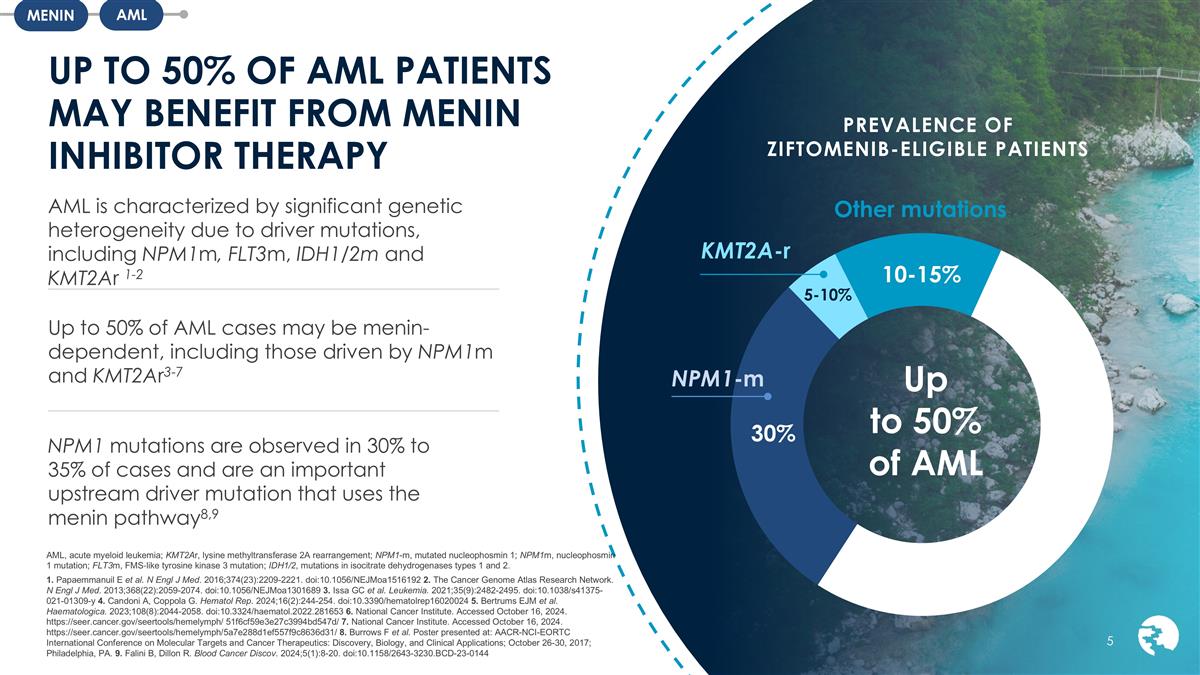
PREVALENCE OF ZIFTOMENIB-ELIGIBLE
PATIENTS UP TO 50% OF AML PATIENTS MAY BENEFIT FROM MENIN INHIBITOR THERAPY AML, acute myeloid leukemia; KMT2Ar, lysine methyltransferase 2A rearrangement; NPM1-m, mutated nucleophosmin 1; NPM1m, nucleophosmin 1 mutation; FLT3m, FMS‐like
tyrosine kinase 3 mutation; IDH1/2, mutations in isocitrate dehydrogenases types 1 and 2. 1. Papaemmanuil E et al. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192 2. The Cancer Genome Atlas Research Network. N Engl J Med.
2013;368(22):2059-2074. doi:10.1056/NEJMoa1301689 3. Issa GC et al. Leukemia. 2021;35(9):2482-2495. doi:10.1038/s41375-021-01309-y 4. Candoni A, Coppola G. Hematol Rep. 2024;16(2):244-254. doi:10.3390/hematolrep16020024 5. Bertrums EJM et al.
Haematologica. 2023;108(8):2044-2058. doi:10.3324/haematol.2022.281653 6. National Cancer Institute. Accessed October 16, 2024. https://seer.cancer.gov/seertools/hemelymph/ 51f6cf59e3e27c3994bd547d/ 7. National Cancer Institute. Accessed October 16,
2024. https://seer.cancer.gov/seertools/hemelymph/5a7e288d1ef557f9c8636d31/ 8. Burrows F et al. Poster presented at: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics: Discovery, Biology, and Clinical Applications;
October 26-30, 2017; Philadelphia, PA. 9. Falini B, Dillon R. Blood Cancer Discov. 2024;5(1):8-20. doi:10.1158/2643-3230.BCD-23-0144 AML is characterized by significant genetic heterogeneity due to driver mutations, including NPM1m, FLT3m, IDH1/2m
and KMT2Ar 1-2 Up to 50% of AML cases may be menin-dependent, including those driven by NPM1m and KMT2Ar3-7 NPM1 mutations are observed in 30% to 35% of cases and are an important upstream driver mutation that uses the menin pathway8,9 10-15% 5-10%
Up to 50% of AML 30% NPM1-m KMT2A-r Other mutations AML MENIN

AGENDA Positive FDA Feedback for
Upcoming Frontline Combination Trial Designs Positive KOMET-001 Ziftomenib Monotherapy Trial in R/R NPM1-m AML 2025 Anticipated Milestones for Ziftomenib and Pipeline Programs

Phase 2 registrational trial in
relapsed/refractory NPM1-m AML achieved its primary CR/CRh endpoint, and the primary endpoint was statistically significant The benefit-risk profile for ziftomenib is highly encouraging, and safety and tolerability were consistent with previous
reports Topline data submitted for presentation at an upcoming medical meeting Pre-NDA meeting with FDA completed; NDA submission for ziftomenib on track for 2Q 2025 POSITIVE TOPLINE RESULTS FROM KOMET-001 REGISTRATIONAL TRIAL IN R/R NPM1-m AML R/R
AML MENIN
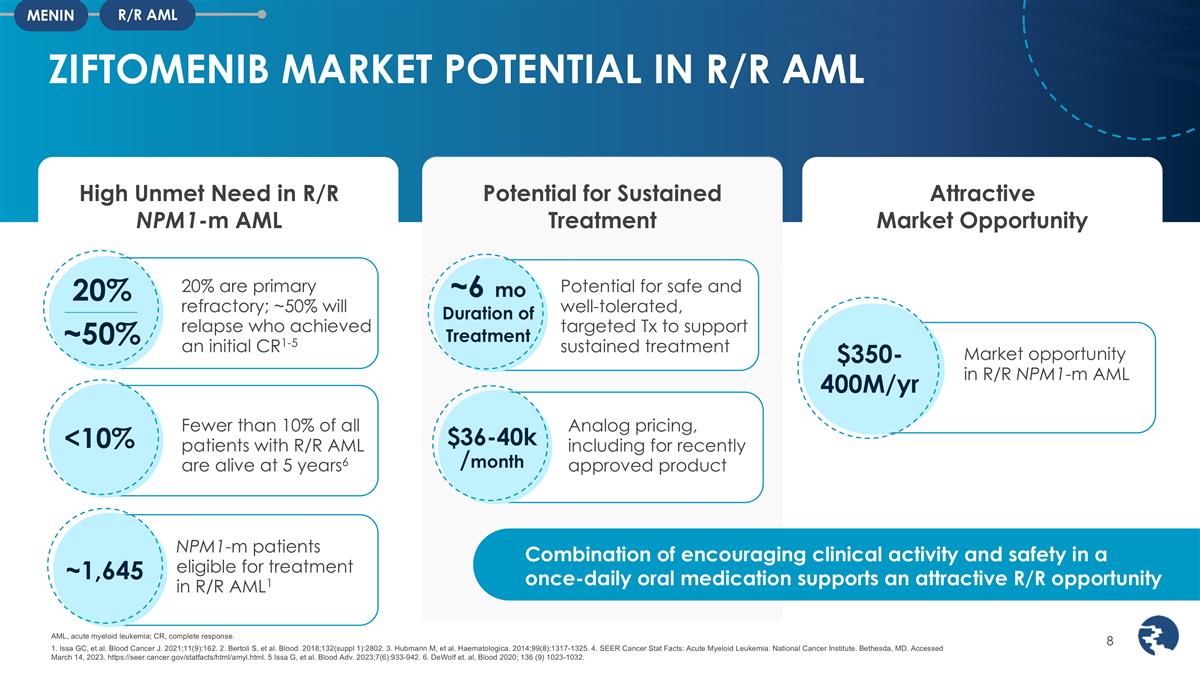
ZIFTOMENIB MARKET POTENTIAL IN R/R AML
R/R AML MENIN High Unmet Need in R/R NPM1-m AML Potential for Sustained Treatment Attractive Market Opportunity 20% are primary refractory; ~50% will relapse who achieved an initial CR1-5 20% ~50% Fewer than 10% of all patients with R/R AML are
alive at 5 years6 <10% Combination of encouraging clinical activity and safety in a once-daily oral medication supports an attractive R/R opportunity AML, acute myeloid leukemia; CR, complete response. 1. Issa GC, et al. Blood Cancer J.
2021;11(9):162. 2. Bertoli S, et al. Blood. 2018;132(suppl 1):2802. 3. Hubmann M, et al. Haematologica. 2014;99(8):1317-1325. 4. SEER Cancer Stat Facts: Acute Myeloid Leukemia. National Cancer Institute. Bethesda, MD. Accessed March 14, 2023.
https://seer.cancer.gov/statfacts/html/amyl.html. 5 Issa G, et al. Blood Adv. 2023;7(6):933-942. 6. DeWolf et. al, Blood 2020; 136 (9) 1023-1032. $36-40k /month Analog pricing, including for recently approved product $350-400M/yr Market opportunity
in R/R NPM1-m AML NPM1-m patients eligible for treatment in R/R AML1 ~1,645 Potential for safe and well-tolerated, targeted Tx to support sustained treatment ~6 mo Duration of Treatment

AGENDA Positive FDA Feedback for
Upcoming Frontline Combination Trial Designs Positive KOMET-001 Ziftomenib Monotherapy Trial in R/R NPM1-m AML 2025 Anticipated Milestones for Ziftomenib and Pipeline Programs

Single protocol encompassing two
independent, randomized, placebo-controlled Phase 3 trials Single protocol designed to facilitate study start-up and execution Single protocol is attractive to sites as it provides treatment options to the broadest potential patient pool FDA aligned
on endpoints, providing pathways for potential accelerated approval in both trials Continuing to work with global health authorities to gain alignment on protocol Both trials expected to initiate in 2H 2025 POSITIVE FDA FEEDBACK ON KOMET-017
REGISTRATIONAL TRIALS IN FRONTLINE INTENSIVE AND NON-INTENSIVE AML FRONTLINE AML MENIN MRD= Minimal Residual Disease
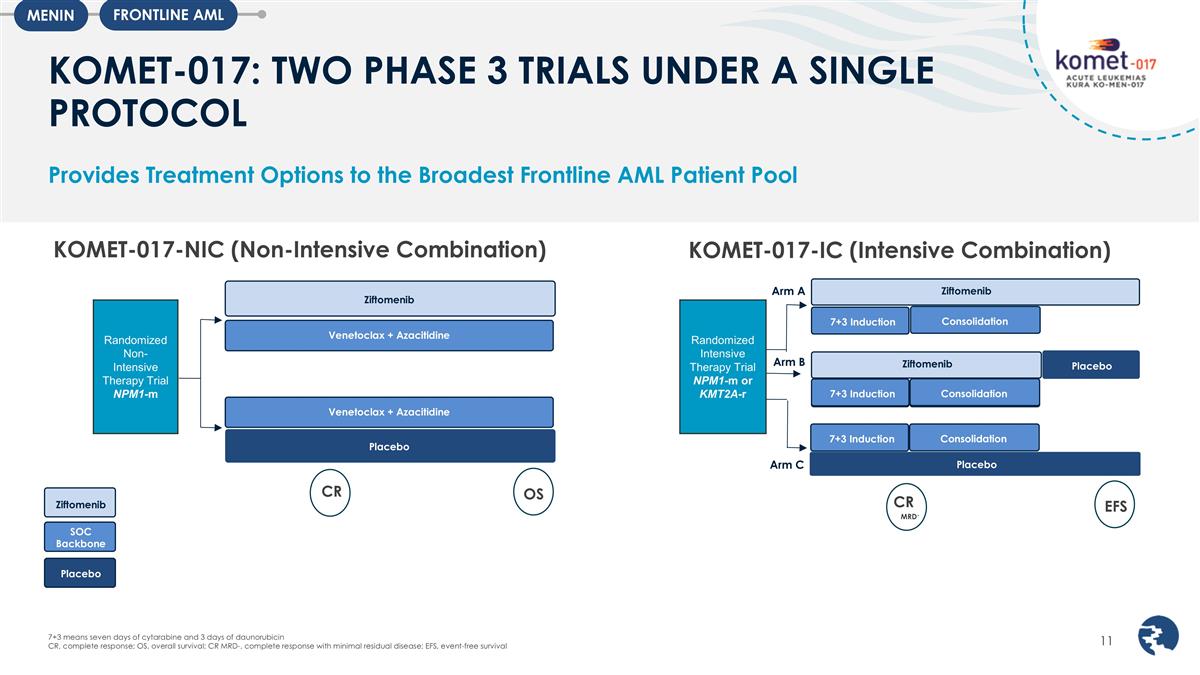
KOMET-017: TWO PHASE 3 TRIALS UNDER
A SINGLE PROTOCOL Provides Treatment Options to the Broadest Frontline AML Patient Pool Ziftomenib Venetoclax + Azacitidine Placebo Venetoclax + Azacitidine Randomized Non-Intensive Therapy Trial NPM1-m CR OS KOMET-017-NIC (Non-Intensive
Combination) 7+3 Induction Randomized Intensive Therapy Trial NPM1-m or KMT2A-r Arm C Arm A Arm B Ziftomenib Ziftomenib Consolidation 7+3 Induction Consolidation Placebo Placebo 7+3 Induction Consolidation CR 7+3 Induction Consolidation EFS MRD-
KOMET-017-IC (Intensive Combination) Ziftomenib SOC Backbone Placebo 7+3 means seven days of cytarabine and 3 days of daunorubicin CR, complete response; OS, overall survival; CR MRD-, complete response with minimal residual disease; EFS, event-free
survival FRONTLINE AML MENIN
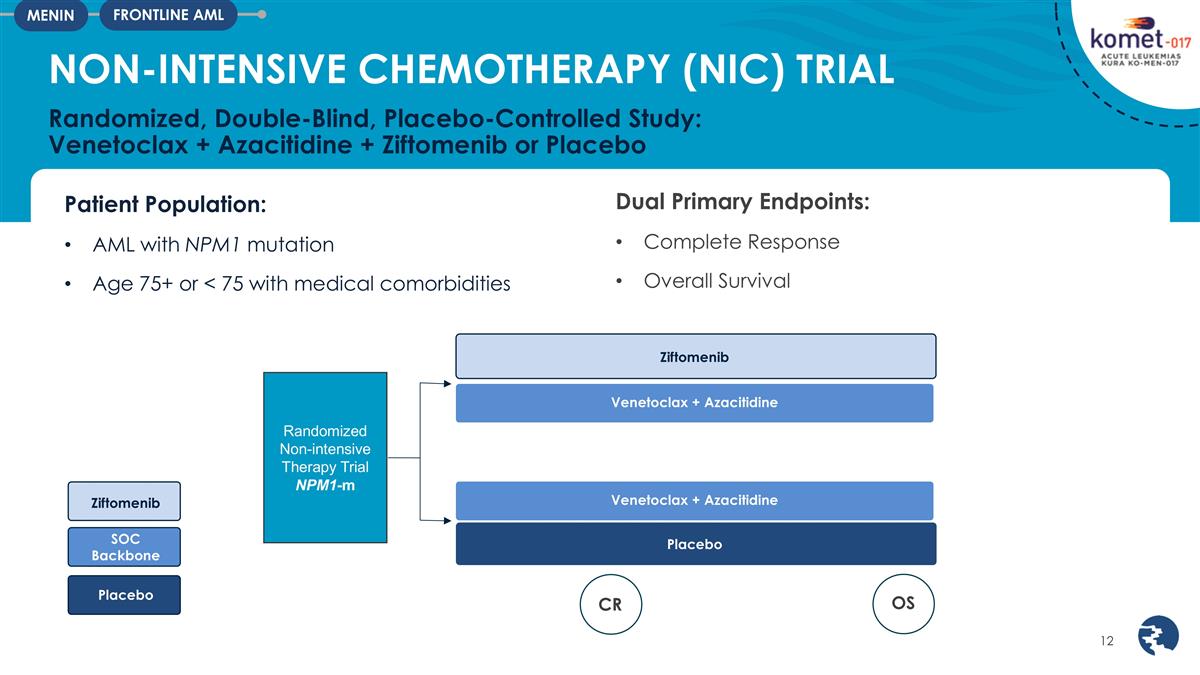
Patient Population: AML with NPM1
mutation Age 75+ or < 75 with medical comorbidities NON-INTENSIVE CHEMOTHERAPY (NIC) TRIAL Randomized, Double-Blind, Placebo-Controlled Study: Venetoclax + Azacitidine + Ziftomenib or Placebo FRONTLINE AML MENIN Dual Primary Endpoints: Complete
Response Overall Survival Ziftomenib Venetoclax + Azacitidine Placebo Ziftomenib SOC Backbone Placebo Venetoclax + Azacitidine Randomized Non-intensive Therapy Trial NPM1-m CR OS
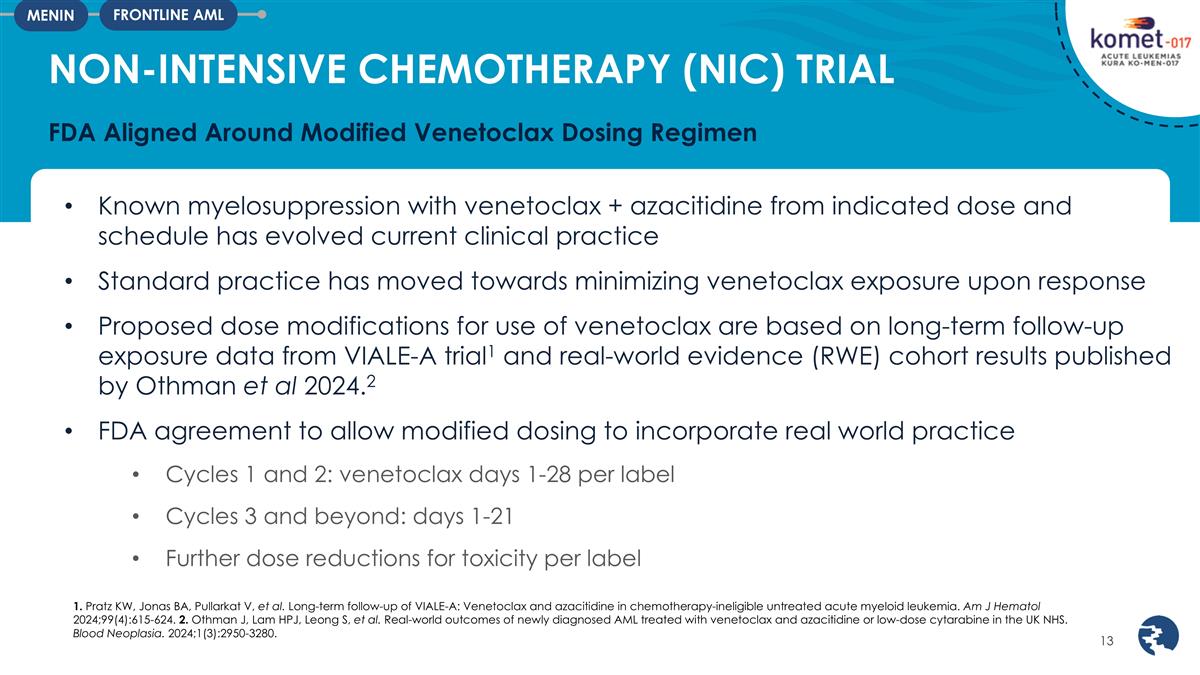
Known myelosuppression with
venetoclax + azacitidine from indicated dose and schedule has evolved current clinical practice Standard practice has moved towards minimizing venetoclax exposure upon response Proposed dose modifications for use of venetoclax are based on long-term
follow-up exposure data from VIALE-A trial1 and real-world evidence (RWE) cohort results published by Othman et al 2024.2 FDA agreement to allow modified dosing to incorporate real world practice Cycles 1 and 2: venetoclax days 1-28 per label
Cycles 3 and beyond: days 1-21 Further dose reductions for toxicity per label NON-INTENSIVE CHEMOTHERAPY (NIC) TRIAL FDA Aligned Around Modified Venetoclax Dosing Regimen FRONTLINE AML MENIN 1. Pratz KW, Jonas BA, Pullarkat V, et al. Long-term
follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am J Hematol 2024;99(4):615-624. 2. Othman J, Lam HPJ, Leong S, et al. Real-world outcomes of newly diagnosed AML treated with venetoclax
and azacitidine or low-dose cytarabine in the UK NHS. Blood Neoplasia. 2024;1(3):2950-3280.
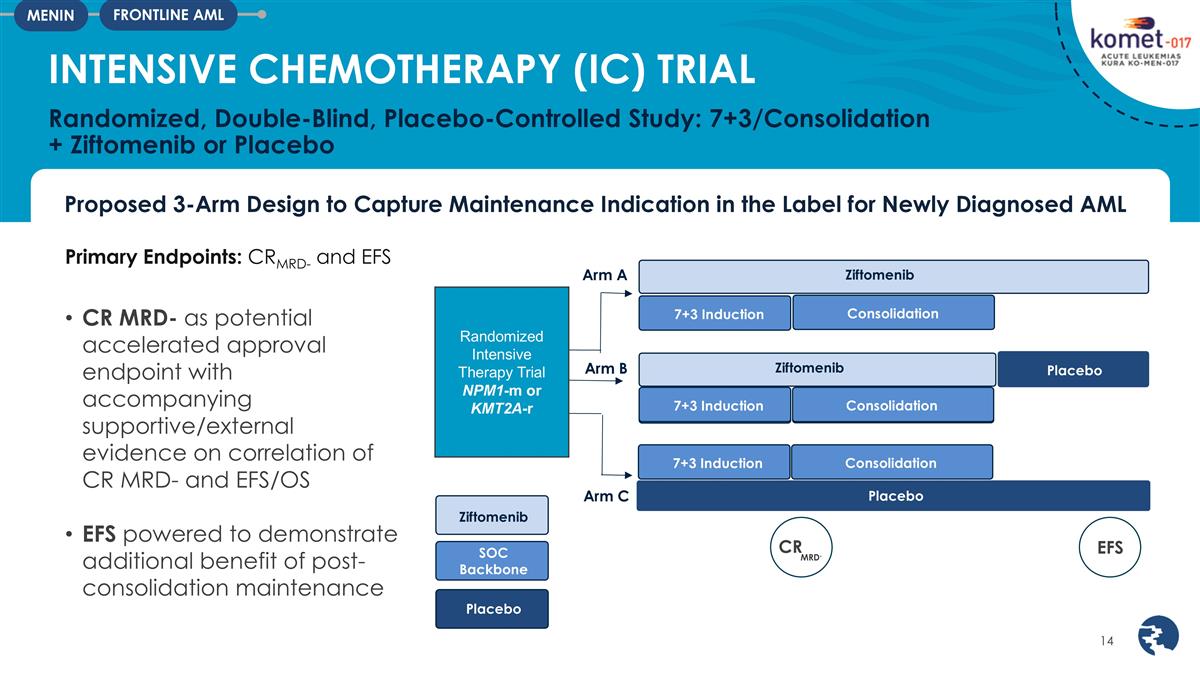
Proposed 3-Arm Design to Capture
Maintenance Indication in the Label for Newly Diagnosed AML INTENSIVE CHEMOTHERAPY (IC) TRIAL Randomized, Double-Blind, Placebo-Controlled Study: 7+3/Consolidation + Ziftomenib or Placebo FRONTLINE AML MENIN Primary Endpoints: CRMRD- and EFS 7+3
Induction Randomized Intensive Therapy Trial NPM1-m or KMT2A-r Ziftomenib SOC Backbone Placebo Arm C Arm A Arm B Ziftomenib Ziftomenib Consolidation 7+3 Induction Consolidation Placebo Placebo 7+3 Induction Consolidation CR 7+3 Induction
Consolidation EFS MRD- CR MRD- as potential accelerated approval endpoint with accompanying supportive/external evidence on correlation of CR MRD- and EFS/OS EFS powered to demonstrate additional benefit of post-consolidation maintenance
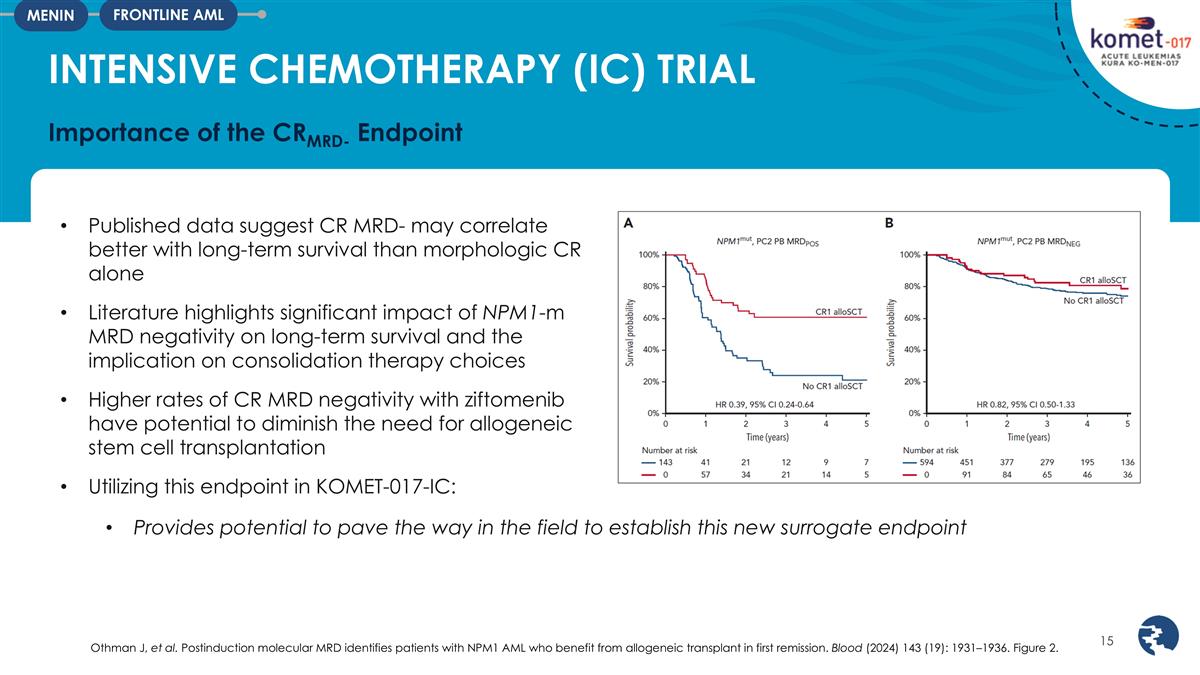
INTENSIVE CHEMOTHERAPY (IC) TRIAL
Importance of the CRMRD- Endpoint FRONTLINE AML MENIN Published data suggest CR MRD- may correlate better with long-term survival than morphologic CR alone Literature highlights significant impact of NPM1-m MRD negativity on long-term survival and
the implication on consolidation therapy choices Higher rates of CR MRD negativity with ziftomenib have potential to diminish the need for allogeneic stem cell transplantation Utilizing this endpoint in KOMET-017-IC: Provides potential to pave the
way in the field to establish this new surrogate endpoint Othman J, et al. Postinduction molecular MRD identifies patients with NPM1 AML who benefit from allogeneic transplant in first remission. Blood (2024) 143 (19): 1931–1936. Figure
2.
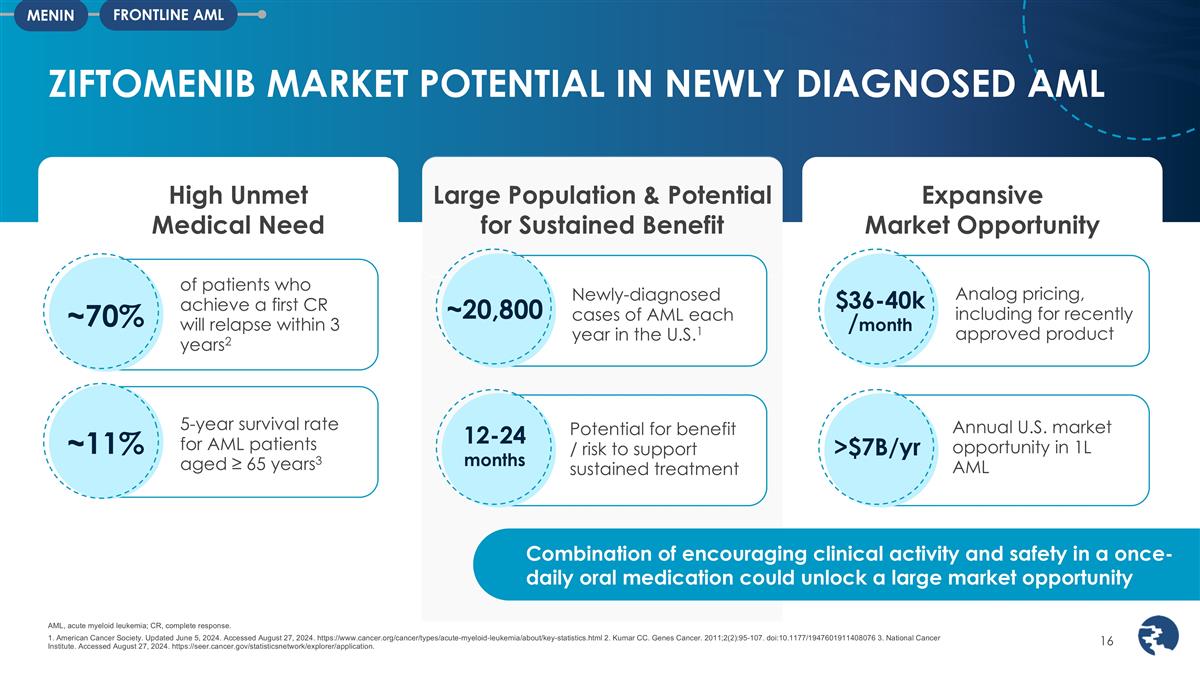
Expansive Market Opportunity
ZIFTOMENIB MARKET POTENTIAL IN NEWLY DIAGNOSED AML AML, acute myeloid leukemia; CR, complete response. 1. American Cancer Society. Updated June 5, 2024. Accessed August 27, 2024.
https://www.cancer.org/cancer/types/acute-myeloid-leukemia/about/key-statistics.html 2. Kumar CC. Genes Cancer. 2011;2(2):95-107. doi:10.1177/1947601911408076 3. National Cancer Institute. Accessed August 27, 2024.
https://seer.cancer.gov/statisticsnetwork/explorer/application. Potential for benefit / risk to support sustained treatment 12-24 months Analog pricing, including for recently approved product $36-40k months Potential peak sales for menin inhibitors
in 1L AML >$7B/yr Combination of encouraging clinical activity and safety in a once-daily oral medication could unlock a large market opportunity Potential for benefit / risk to support sustained treatment 12-24 months Newly-diagnosed cases of
AML each year in the U.S.1 ~20,800 Analog pricing, including for recently approved product $36-40k /month Large Population & Potential for Sustained Benefit High Unmet Medical Need of patients who achieve a first CR will relapse within 3 years2
~70% 5-year survival rate for AML patients aged ≥ 65 years3 ~11% >$7B/yr Annual U.S. market opportunity in 1L AML FRONTLINE AML MENIN

AGENDA Positive FDA Feedback for
Upcoming Frontline Combination Trial Designs Positive KOMET-001 Ziftomenib Monotherapy Trial in R/R NPM1-m AML 2025 Anticipated Milestones for Ziftomenib and Pipeline Programs

Ziftomenib ANTICIPATED UPCOMING
MILESTONES: SEVERAL EXPECTED 2025 DATA READ-OUTS ACROSS MULTIPLE PROGRAMS Report topline results from KOMET-001 Phase 2 registration-directed trial in R/R NPM1-m AML ü FDA feedback on KOMET-017 registration-enabling protocol in 1L NPM1-m and
KMT2A-r intensive and non-intensive AML ü NDA submission for ziftomenib in R/R NPM1-m AML 2Q 2025 Present topline data from KOMET-001 Phase 2 registration-directed trial in R/R NPM1-m AML 2Q 2025 Present preliminary clinical data from KOMET-007
Phase 1b trial in 1L intensive AML 2Q 2025 Initiate KOMET-015 Phase 1 trial of ziftomenib in combination with imatinib in patients with advanced GIST 1H 2025 Initiate KOMET-017 Phase 3 registration-enabling trials in 1L NPM1-m and KMT2A-r intensive
and non-intensive AML 2H 2025 Present preliminary clinical data from Phase 1b expansion of KOMET-007 in 1L non-intensive AML 2H 2025 KO-2806 / tipifarnib Initiate one or more expansion cohorts in combination with cabozantinib in RCC 1H 2025 Present
preliminary clinical data from FIT-001 trial for KO-2806 as monotherapy and combo with cabozantinib in RCC 2H 2025 Present full clinical data from KURRENT-HN trial of tipifarnib in combination with alpelisib in PIK3CA-m HNSCC 2H 2025 Next-gen Menin
Nominate a development candidate for next-generation menin inhibitor program for diabetes 1H 2025
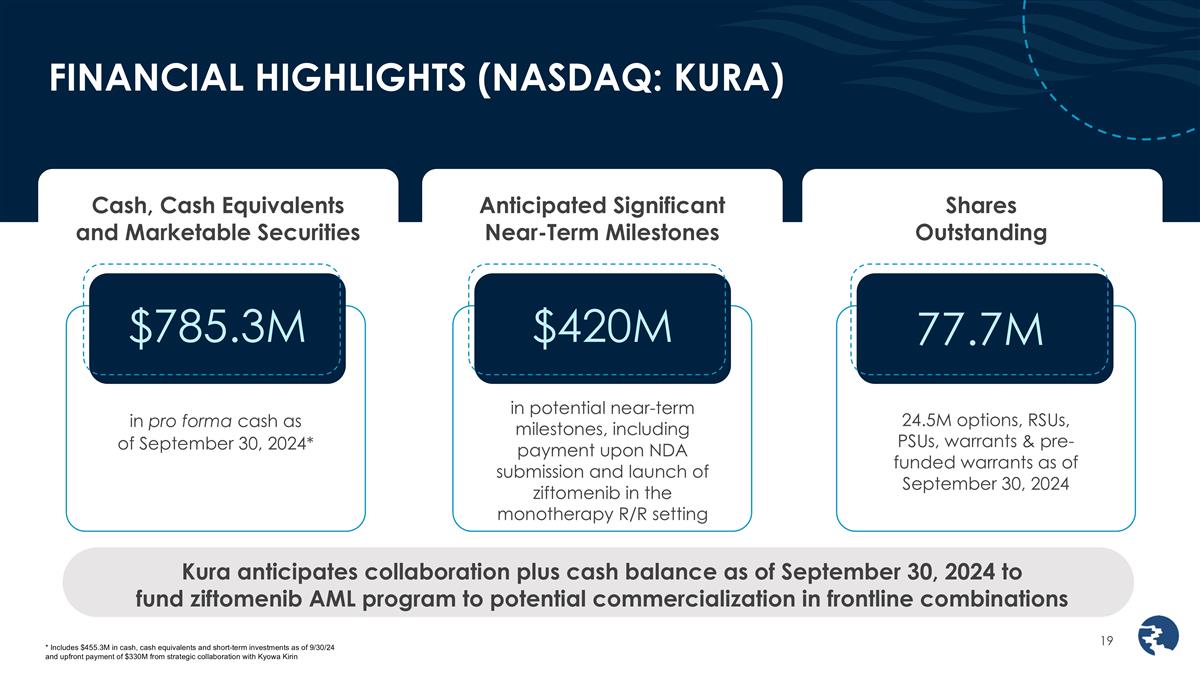
FINANCIAL HIGHLIGHTS (NASDAQ: KURA)
Cash, Cash Equivalents and Marketable Securities Anticipated Significant Near-Term Milestones Shares Outstanding in pro forma cash as of September 30, 2024* Kura anticipates collaboration plus cash balance as of September 30, 2024 to fund ziftomenib
AML program to potential commercialization in frontline combinations in potential near-term milestones, including payment upon NDA submission and launch of ziftomenib in the monotherapy R/R setting 24.5M options, RSUs, PSUs, warrants &
pre-funded warrants as of September 30, 2024 * Includes $455.3M in cash, cash equivalents and short-term investments as of 9/30/24 and upfront payment of $330M from strategic collaboration with Kyowa Kirin $785.3M $420M 77.7M
February 2025 ZIFTOMENIB PROGRAM
UPDATES Our goal is to develop transformative therapies to extend and improve the lives of patients with cancer
v3.25.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Kura Oncology (NASDAQ:KURA)
과거 데이터 주식 차트
부터 1월(1) 2025 으로 2월(2) 2025

Kura Oncology (NASDAQ:KURA)
과거 데이터 주식 차트
부터 2월(2) 2024 으로 2월(2) 2025
