false000185083800018508382023-12-312023-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________________________
FORM 8-K
___________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): December 31, 2023
__________________________________
Omega Therapeutics, Inc.
(Exact Name of Registrant as specified in its charter)
__________________________________
|
|
|
|
|
Delaware |
|
001-40657 |
|
81-3247585 |
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
140 First Street, Suite 501
Cambridge, Massachusetts 02141
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (617) 949-4360
N/A
(Former Name or Former Address, if Changed Since Last Report)
____________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading Symbols |
|
Name of each exchange on which registered |
Common Stock, $0.001 par value per share |
|
OMGA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
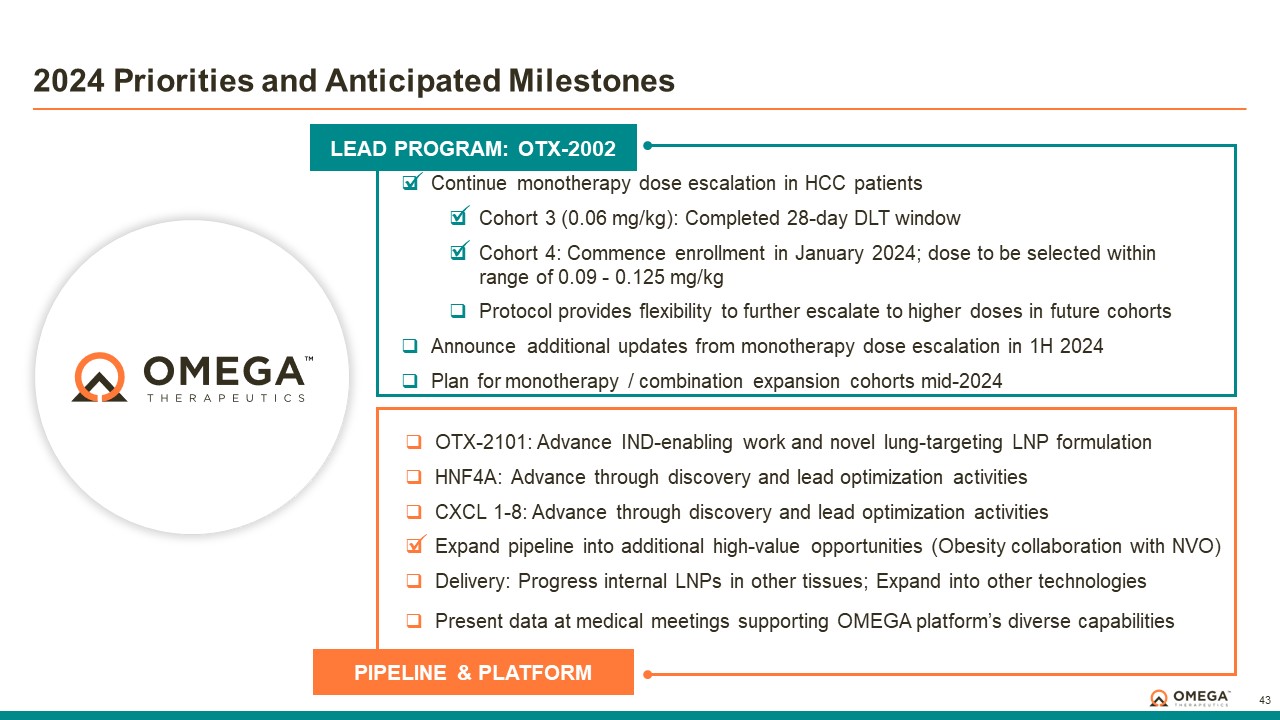
Item 1.01. Entry into a Material Definitive Agreement.
On December 31, 2023, Omega Therapeutics, Inc. (the “Company”) entered into a Research Collaboration Agreement (the “Agreement”) with Novo Nordisk A/S (“Novo Nordisk”), Pioneering Medicines 08, Inc. (“PM SpinCo”), and, with respect to certain provisions set forth in the Agreement, Pioneering Medicines (NN), LLC (“Shareholder”) and PM (NN) Explorations, Inc. (“PMCo” and together with PM SpinCo and Shareholder, the “PM Entities”). PM SpinCo, Shareholder and PMCo are entities affiliated with Flagship Pioneering, a significant stockholder of the Company. Christian Schade, Michelle C. Werner, and John Mendlien, Ph.D., JD. are each a member of the Company’s Board of Directors and Growth Partner, CEO-Partner, and Executive Partner, respectively, of Flagship Pioneering.
Under the terms of the Agreement, the Company granted to Novo Nordisk an exclusive, royalty-bearing, transferable license, with the right to grant sublicenses through multiple tiers, for certain of the Company’s intellectual property to conduct research and development activities under an agreed-upon research and development plan, together with the PM Entities, relating to a product candidate, or program target, for the prevention, treatment or control of a cardiometabolic disease, including diabetes, in humans throughout the world (the “Territory”).
In connection with the Agreement, Novo Nordisk will have the right to exercise its irrevocable option to purchase from Shareholder all of the outstanding equity securities of PM SpinCo (the “Option”) pursuant to the terms of a separate option agreement (the “Option Agreement”) and a separate share purchase agreement (the “Share Purchase Agreement”). Certain provisions in the Agreement are triggered in connection with the exercise of the Option pursuant to the Option Agreement (the “Program Handoff Date”) and the closing of the Option pursuant to the Share Purchase Agreement (the “Closing Date”), but the Company is not a party to the Option Agreement or Share Purchase Agreement. Following the Program Handoff Date, subject to certain exceptions set forth in the Agreement, Novo Nordisk will take over responsibilities for development, manufacturing and commercialization of the product candidate in accordance with the terms of the Agreement.
During the term of the Agreement (and for a period of time thereafter if the Agreement is terminated prior to the Closing Date), and subject to certain exceptions set forth in the Agreement, the Company is prohibited from, alone or with any third party, directly or indirectly, exploiting any product that (a) uses the same (i) epigenomic controller included in the licensed product candidate in the Territory or (ii) custom lipid nanoparticle component included in the licensed product candidate in the Territory for the prevention, treatment or control of a cardiometabolic disease (including diabetes) in humans or (b) is directed to (i) with respect to the initial program target, the initial program target or (ii) if a backup target replaces the initial program target, the selected program target for the prevention, treatment or control of a cardiometabolic disease (including diabetes) in humans. The Company is also prohibited from exploiting the program target or any of the potential backup program targets during the period in which Novo Nordisk has a right to replace the initial program target with a backup target for the prevention, treatment or control of a cardiometabolic disease (including diabetes) in humans.
In connection with the execution of the Agreement, Novo Nordisk agreed to make an upfront cash payment of $10 million, up to $522 million in future development and sales milestone payments, and mid and high-single digit to low double digit percentage royalties on net sales of the licensed product. These payments will be shared approximately equally between the Company and Shareholder. Novo Nordisk’s obligations to pay royalties with respect to a licensed product and country will expire upon the latest of ten years following first commercial sale of a licensed product in such country, the expiration of the last-to-expire of certain valid patent claims applicable to such licensed product in such country, and the expiration of regulatory exclusivity for such licensed product in such country (the “Royalty Term”), subject to certain royalty reduction and step-down provisions set forth in the Agreement.
Following the Closing Date and upon Novo Nordisk’s request, the Company and Novo Nordisk have agreed to negotiate in good faith and on commercially reasonable terms, for Novo Nordisk to buyout its future payment obligations under the Agreement (but neither the Company nor Novo Nordisk is obligated to enter into any such buyout arrangement).
The Agreement includes customary terms and conditions, including mutual representations and warranties and covenants, and indemnification, insurance, intellectual property, dispute resolution and confidentiality and publication provisions. Unless earlier terminated, the Agreement will terminate on a country-by-country and licensed
product-by-licensed product basis upon the expiration of the last Royalty Term in the Territory for such licensed product. Upon expiration of the Royalty Term for a given licensed product in a given country in the Territory, the licenses granted to Novo Nordisk pursuant to the Agreement under the Company’s licensed intellectual property survive and become perpetual, irrevocable, fully paid-up and royalty free with respect to such licensed product in such country. Subject to certain limited exceptions, the Agreement will automatically terminate in the event that (a) the Option Agreement expires in accordance with its terms without Novo Nordisk having exercised its option to acquire the outstanding equity interests of PM SpinCo or (b) the Option Agreement and the Share Purchase Agreement otherwise terminate in accordance therewith. Novo Nordisk may also terminate the Agreement in its entirety for convenience upon prior written notice as set forth in the Agreement. Both the Company and Novo Nordisk may terminate the Agreement upon written notice in the event of a material breach by the other party that has not been cured within a specified cure period. Both the Company and Novo Nordisk may also terminate the Agreement upon written notice if the other party undertakes certain bankruptcy, reorganization, liquidation or receivership proceedings or the assignment of a substantial portion of its assets for the benefit of creditors. The Company and PM SpinCo may terminate the Agreement upon certain patent challenges by Novo Nordisk with respect to each such party’s intellectual property, subject to certain exceptions and limitations set forth in the Agreement.
Item 7.01. Regulation FD Disclosure.
On January 4, 2024, the Company issued a press release announcing the entry into the Agreement. A copy of the press release is being furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The Company plans to present a corporate update on January 8, 2024 at the 2024 J.P. Morgan Healthcare Conference. A copy of the presentation that will be used is furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information contained in this Item 7.01 (including Exhibit 99.1 and Exhibit 99.2) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
Omega Therapeutics, Inc. |
|
|
|
|
Date: January 5, 2024 |
By: |
/s/ Mahesh Karande |
|
|
Mahesh Karande |
|
|
President and Chief Executive Officer |
Omega Therapeutics Announces Research Collaboration with Novo Nordisk to Develop a Novel Therapeutic for Obesity Management
-Collaboration will leverage Omega’s proprietary platform to develop an epigenomic controller designed to increase metabolic activity and support obesity management –
CAMBRIDGE, Mass., January 4, 2023 -- Omega Therapeutics, Inc. (Nasdaq: OMGA) (“Omega”), a clinical-stage biotechnology company pioneering the development of a new class of programmable epigenomic mRNA medicines, today announced a research collaboration with Novo Nordisk, a leading global healthcare company, to develop a novel therapeutic for obesity management. The collaboration will leverage Novo Nordisk’s expertise in research and development within cardiometabolic diseases and Omega’s proprietary platform technology to develop an epigenomic controller designed to enhance metabolic activity as a part of a potential new treatment approach for obesity management.
“By harnessing the body’s innate mechanisms to control cellular identity and gene expression, we believe that our epigenomic controllers offer an opportunity to therapeutically modulate genes linked to metabolism in a precise way,” said Mahesh Karande, President and Chief Executive Officer of Omega Therapeutics. “We look forward to leveraging our OMEGA platform and pursuing this ambitious strategy with Novo Nordisk to advance transformative developments for people living with obesity.”
Uli Stilz, Head of Novo Nordisk’s Bio Innovation Hub, added, “As the population of people living with obesity grows, it is vitally important that we seek out next generation therapeutic solutions to address the unmet need. Much of the scientific advancement in this area has been focused on appetite regulation, but by looking at new ways to increase energy expenditure, including through controlled epigenomic modulation, there is an opportunity to unlock a new path for intervention. We look forward to working together with Omega and leveraging our complementary capabilities to advance much needed new treatments.”
Globally, there are more than 800 million adults living with obesity1. Many of the existing therapeutic interventions for weight management have focused on appetite regulation. Thermogenesis, the production of heat within tissues to raise body temperature, is a natural metabolic function that critically regulates overall energy balance. By seeking to harness this naturally occurring metabolic function, Omega’s proprietary platform has the potential to create an epigenomic controller that can intervene in a unique way to possibly affect energy expenditure. This may ultimately lead to an alternative, and potentially more durable, approach to weight management.
This agreement was signed under the existing framework collaboration between Flagship Pioneering and Novo Nordisk to develop a portfolio of transformational medicines. Omega, Flagship’s Pioneering Medicines initiative, and Novo Nordisk will jointly advance this obesity management program through preclinical development and conduct foundational activities, after which point Novo Nordisk could further advance the program including through human proof-of-concept studies.
Under the terms of the agreement, Novo Nordisk will reimburse R&D costs and has the right to select one target to advance for clinical development. Omega and Pioneering Medicines are
____________________________
1 World_Obesity_Atlas_2023_Report.pdf (worldobesityday.org)
eligible to receive up to $532 million in upfront, development and commercial milestone payments, as well as tiered royalties on annual net sales of a licensed product.
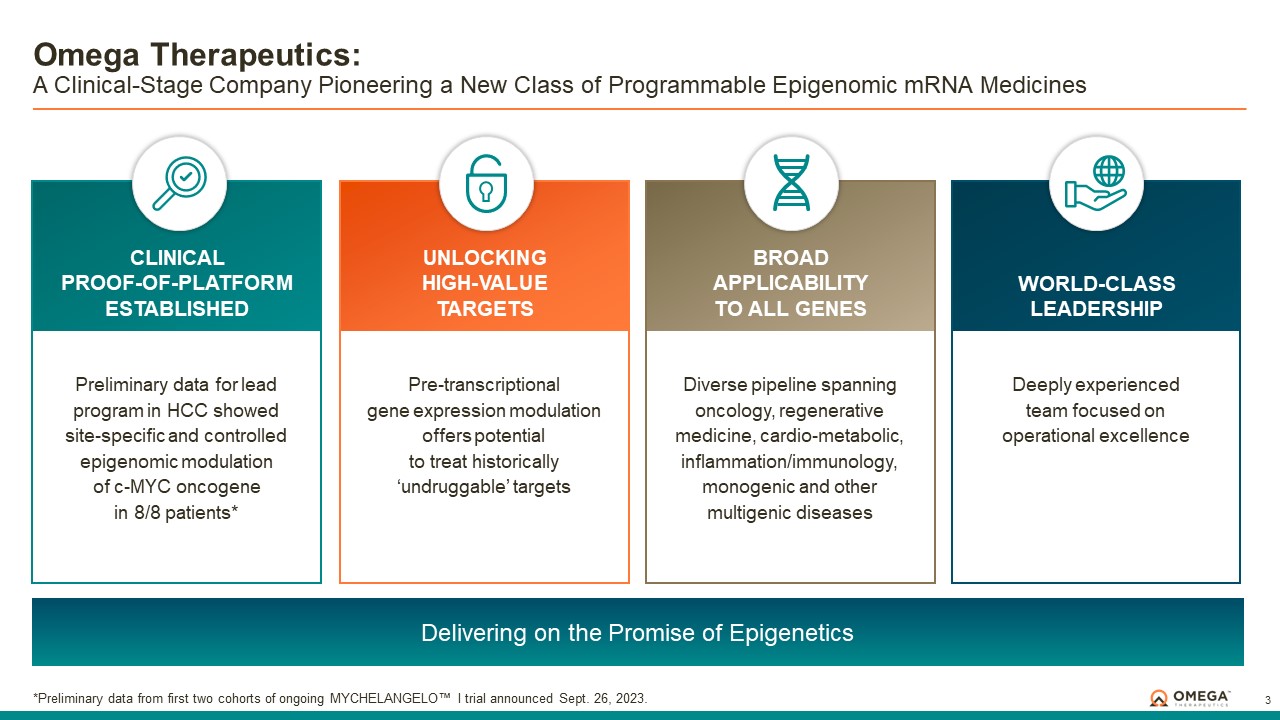
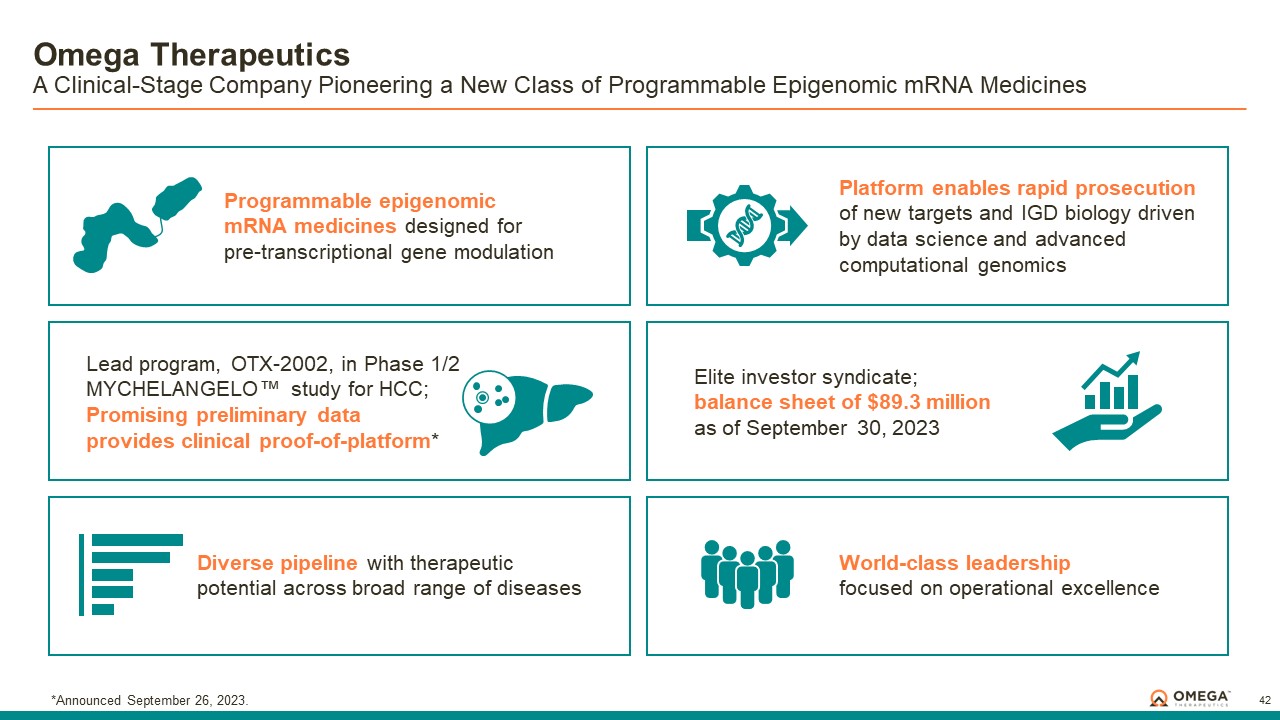
About Omega Therapeutics
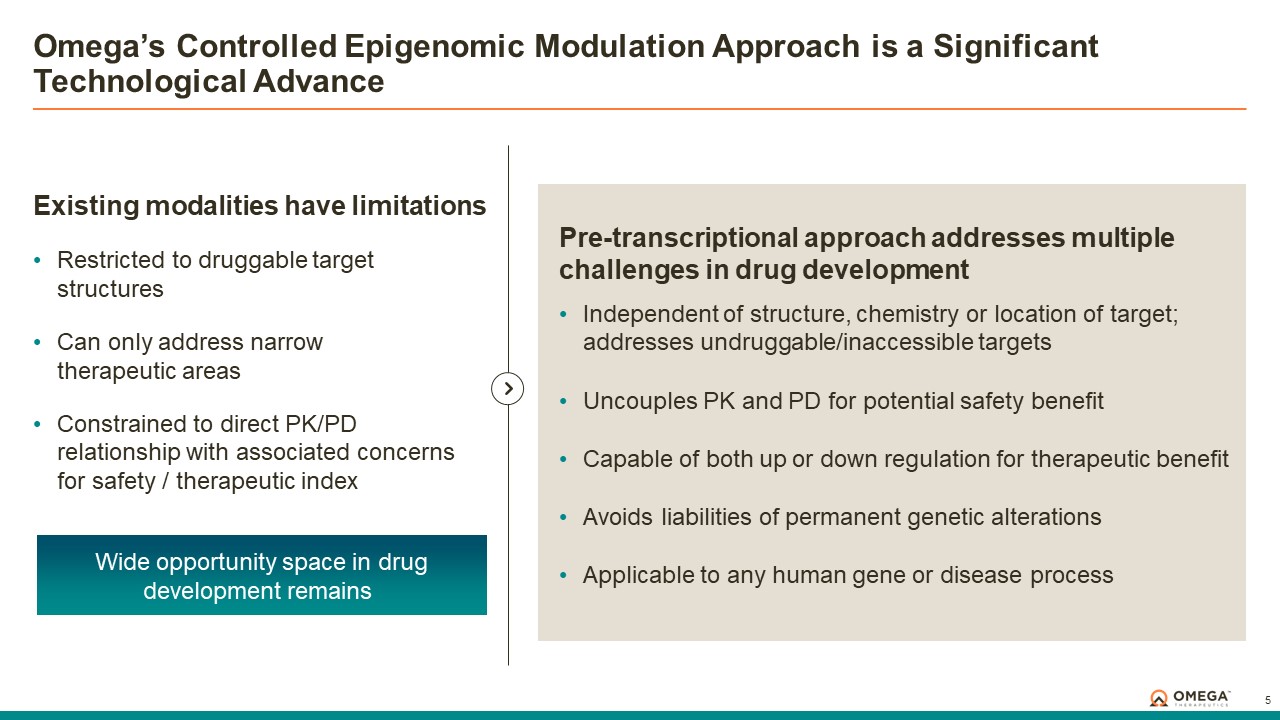
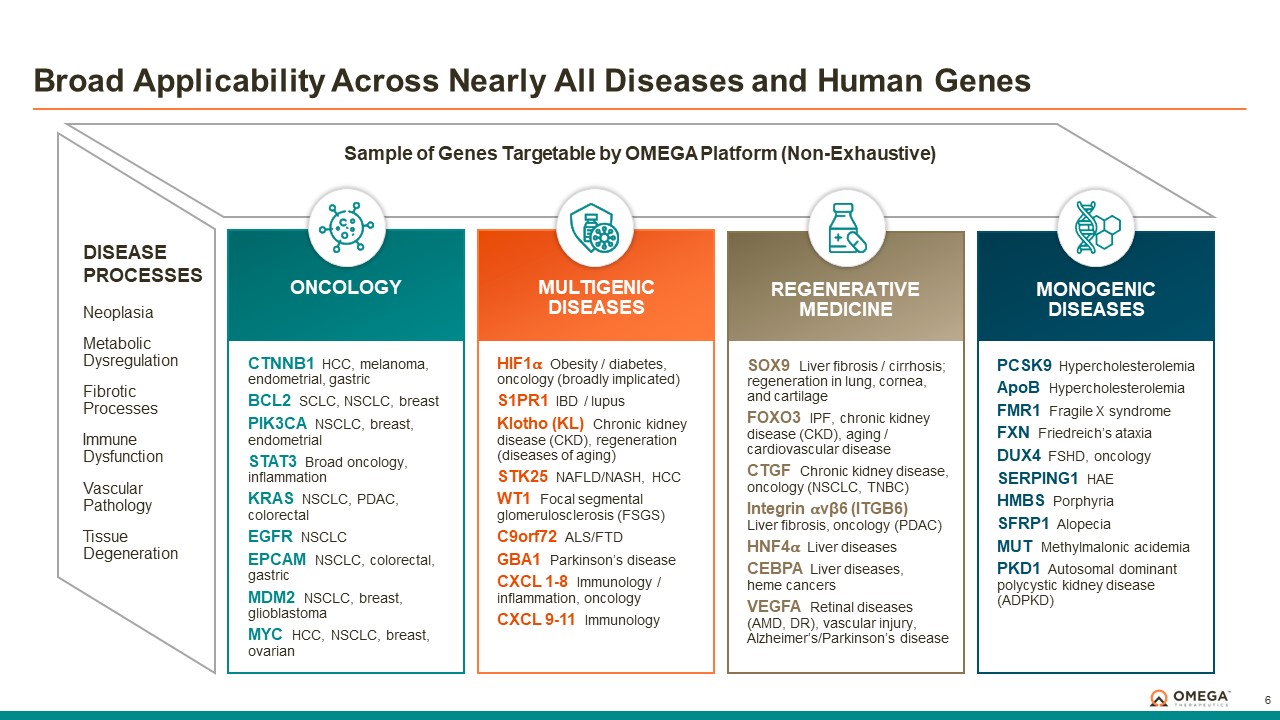
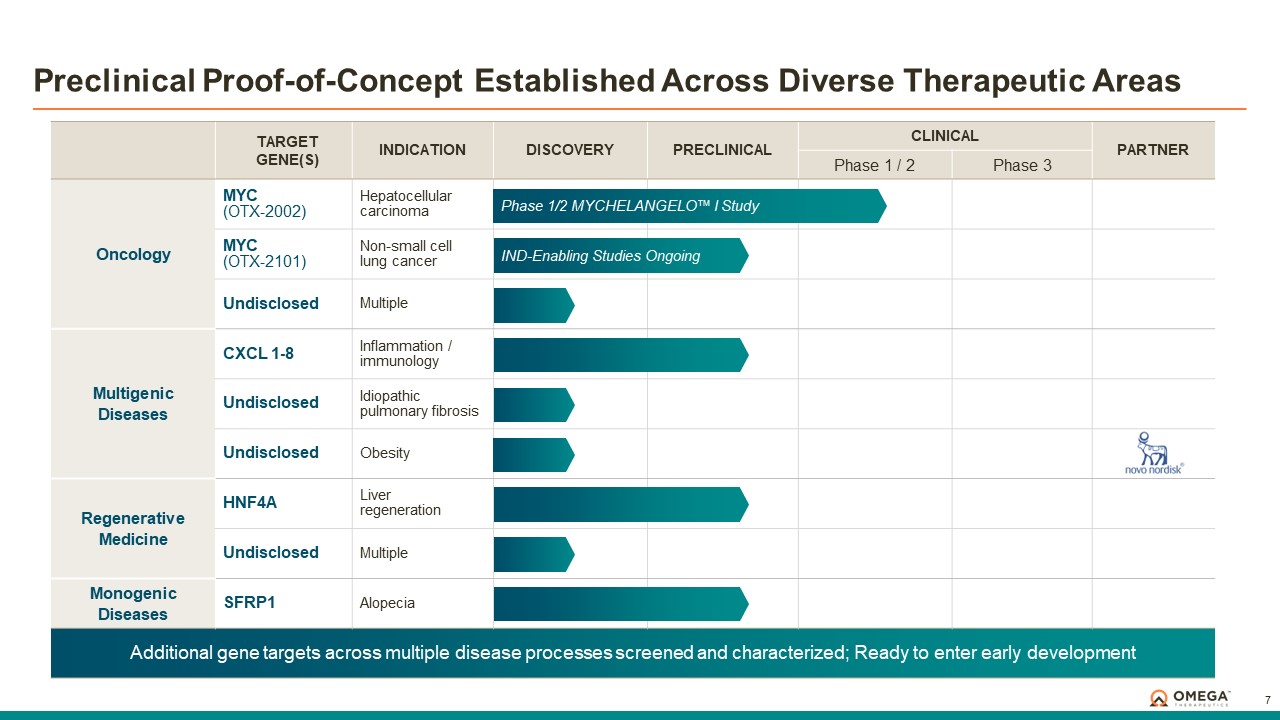
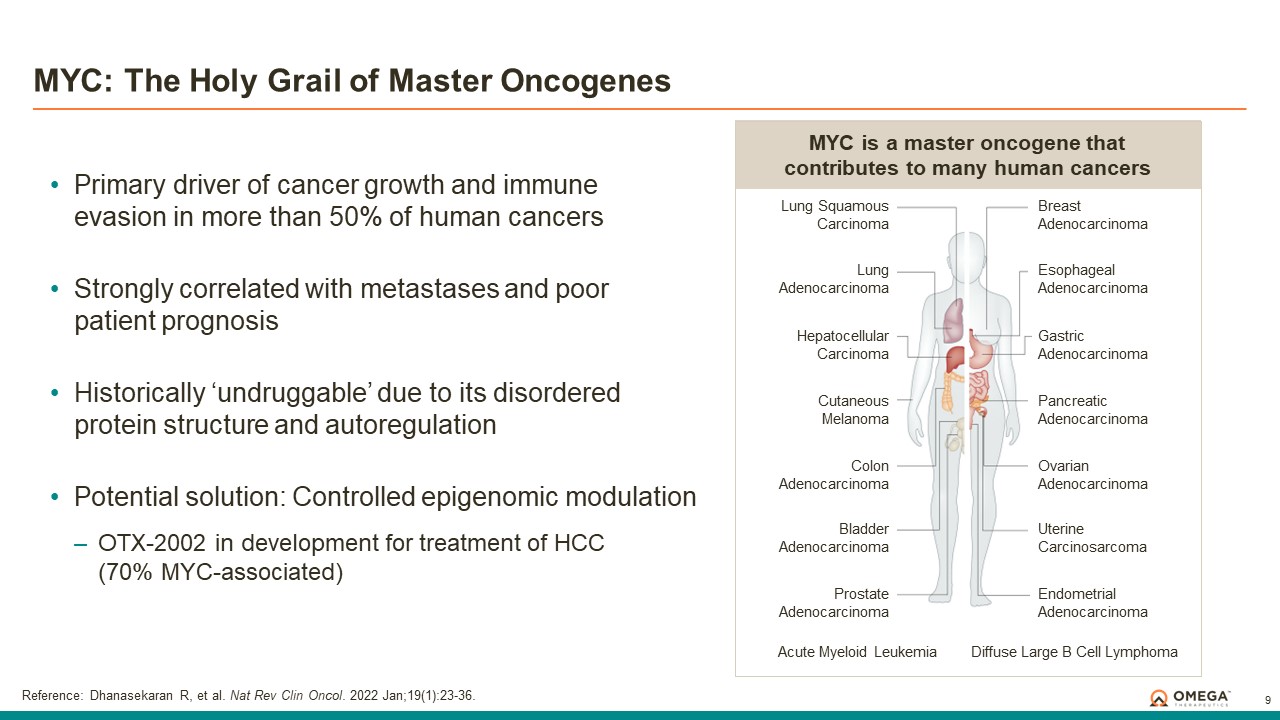
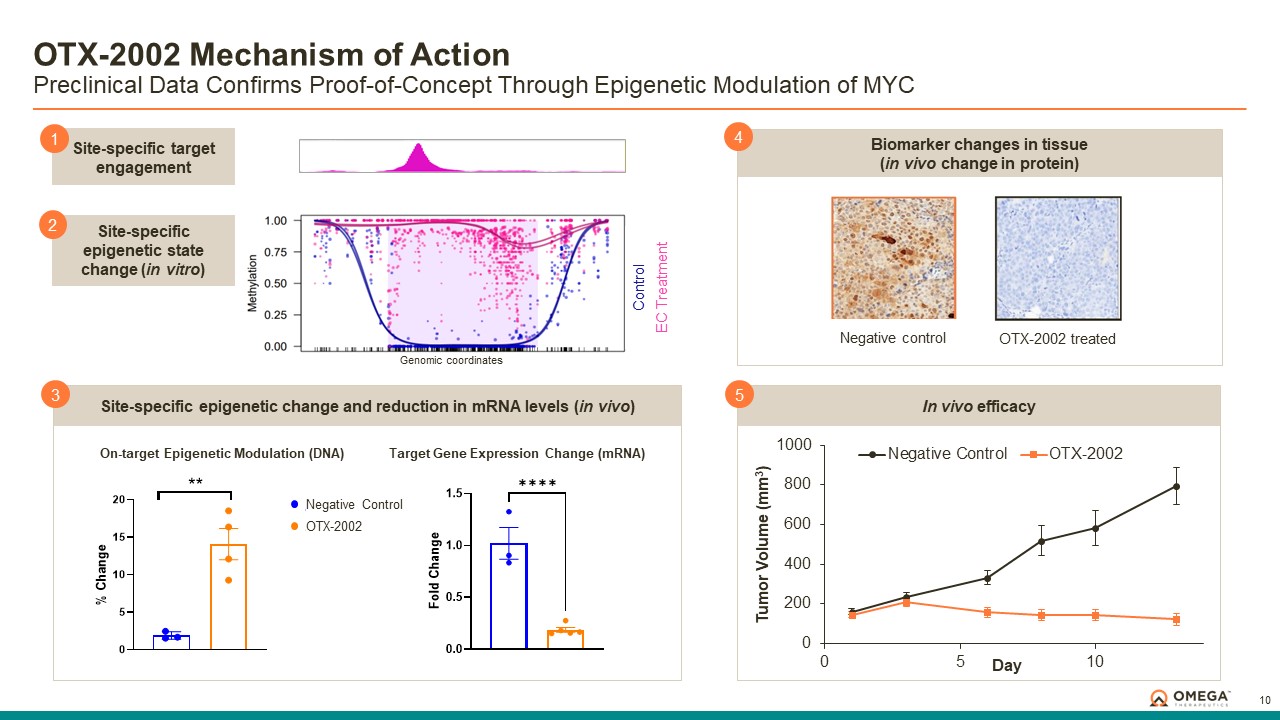
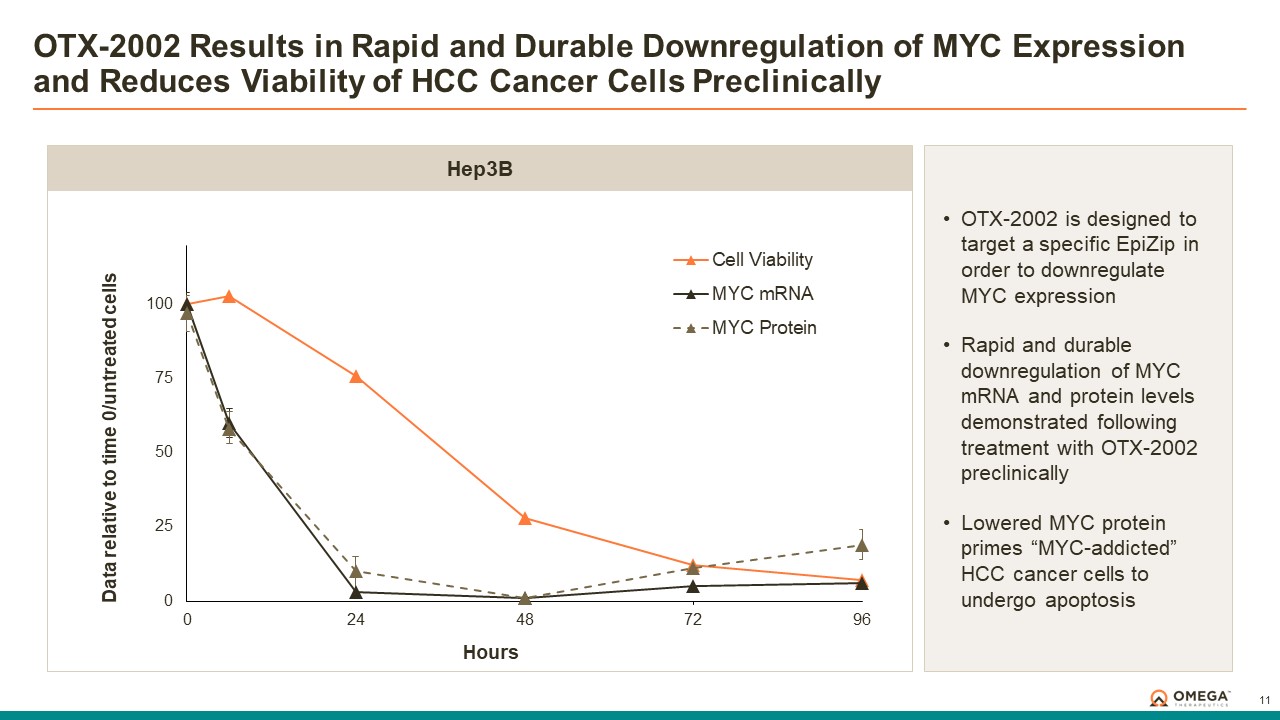
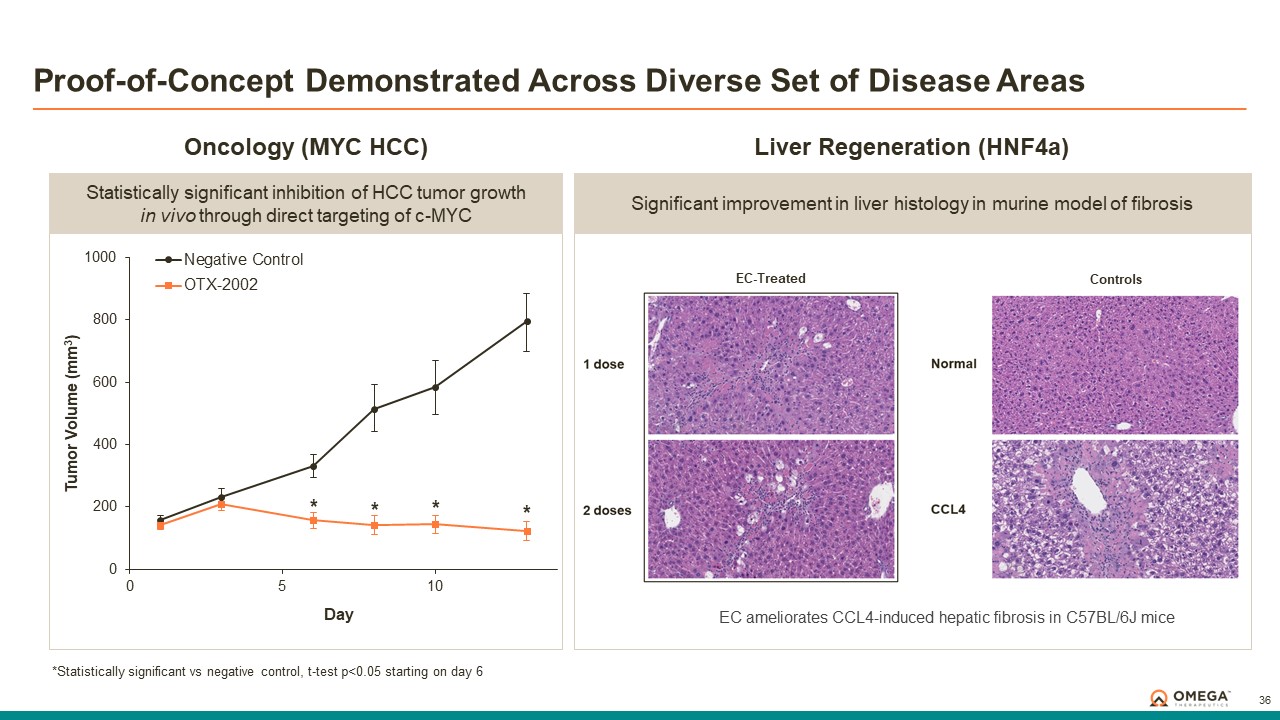
Omega Therapeutics is a clinical-stage biotechnology company pioneering the development of a new class of programmable epigenomic mRNA medicines to treat or cure a broad range of diseases. By pre-transcriptionally modulating gene expression, Omega’s approach enables precision epigenomic control of nearly all human genes, including historically undruggable and difficult-to-treat targets, without altering native nucleic acid sequences. Founded in 2017 by Flagship Pioneering following breakthrough research by world-renowned experts in the field of epigenetics, Omega is led by a seasoned and accomplished leadership team with a track record of innovation and operational excellence. The Company is committed to revolutionizing genomic medicine and has a diverse pipeline of therapeutic candidates derived from its OMEGA platform spanning oncology, regenerative medicine, multigenic diseases including immunology, and select monogenic diseases.
For more information, visit omegatherapeutics.com, or follow us on X and LinkedIn.
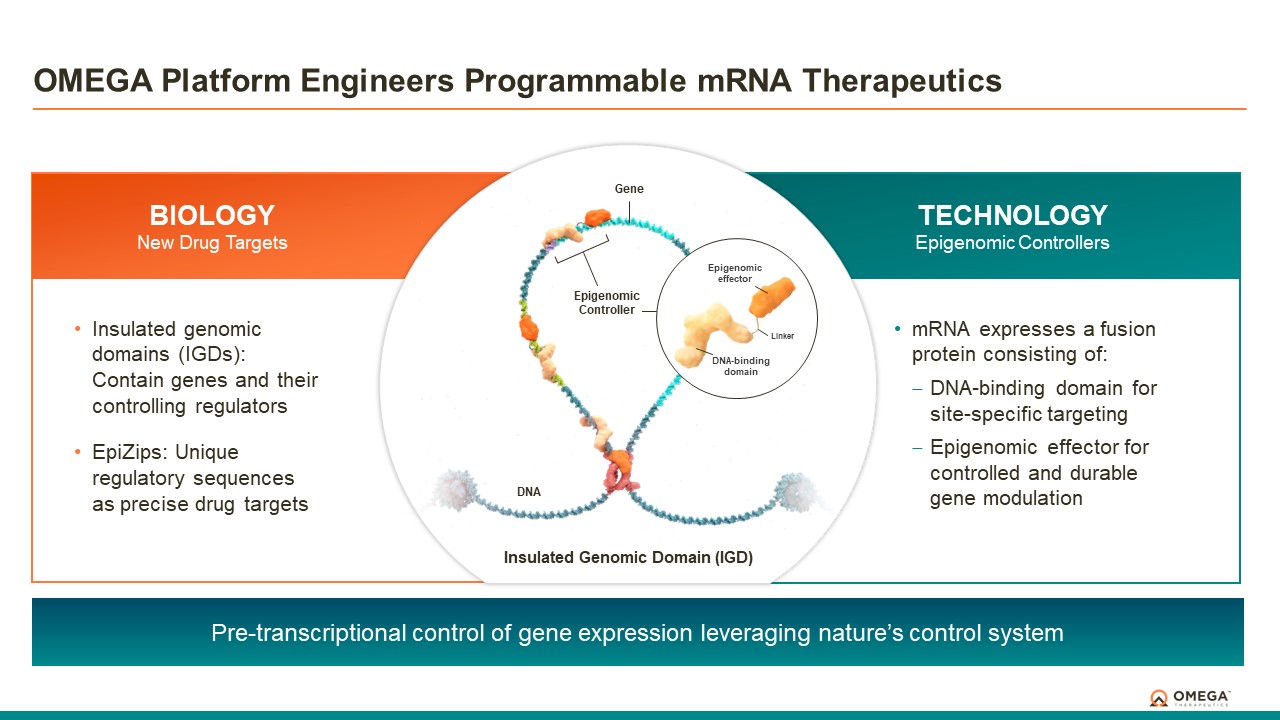
About the OMEGA Platform
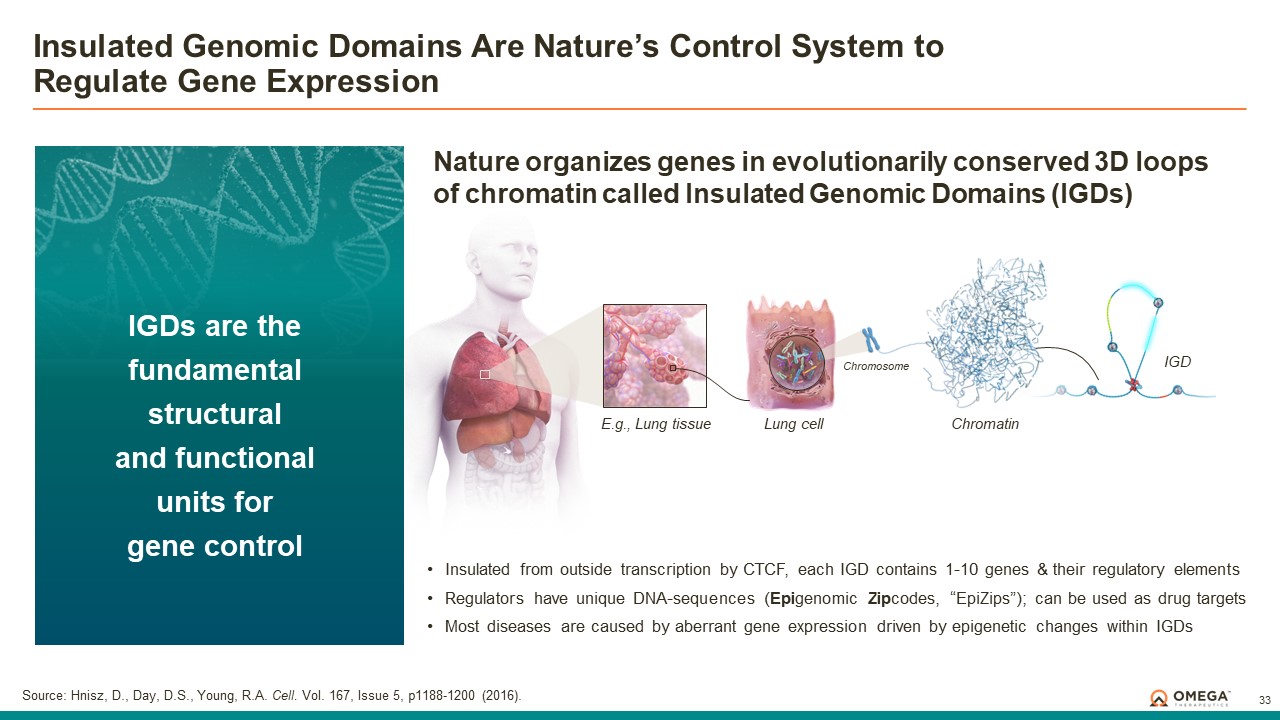
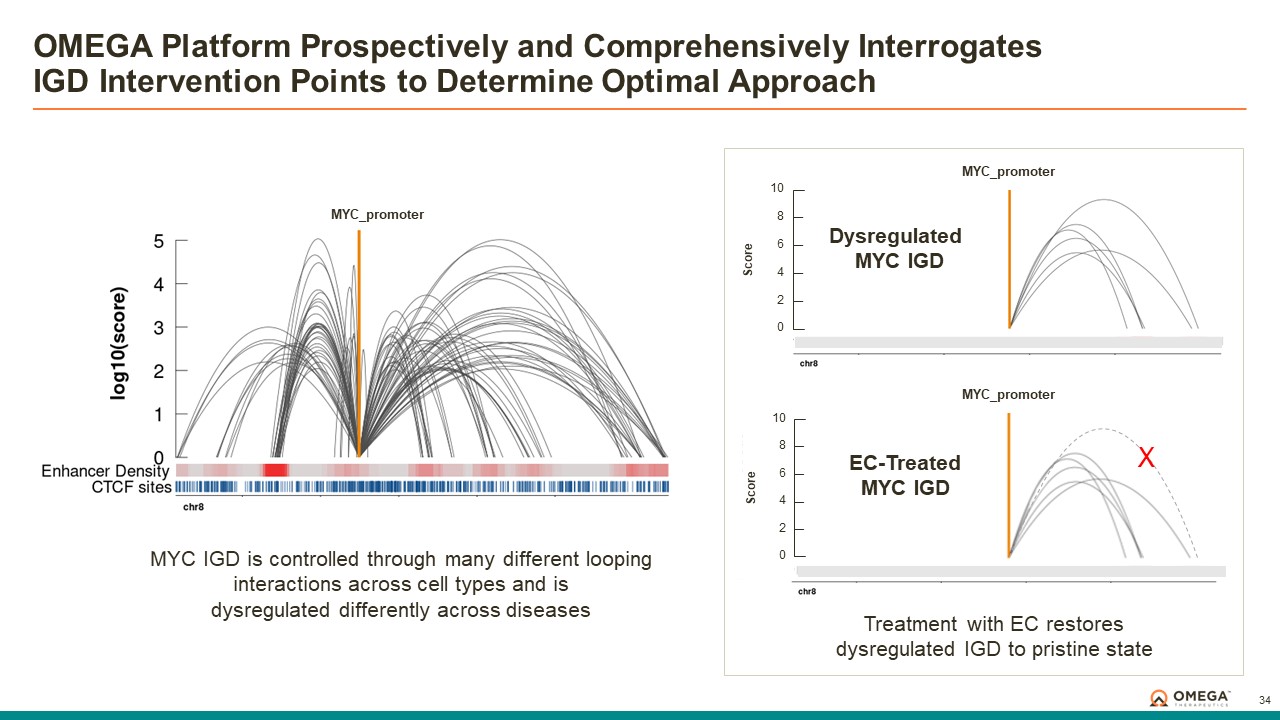
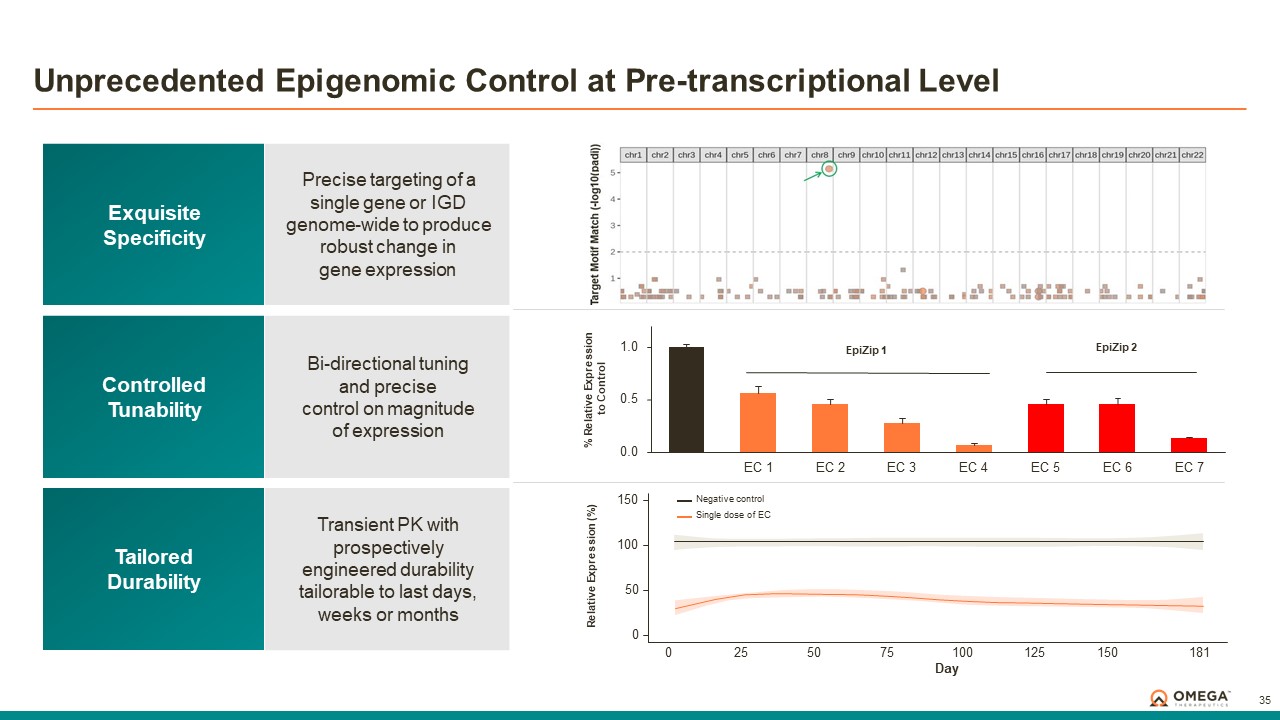
The OMEGA platform leverages the Company’s deep understanding of gene regulation, genomic architecture and epigenetic mechanisms to design programmable epigenomic mRNA medicines that precisely target and modulate gene expression at the pre-transcriptional level. Combining a biology-first approach and world-class data science capabilities with rational drug design and customized delivery, the OMEGA platform enables control of fundamental epigenetic processes to correct the root cause of disease by returning aberrant gene expression to a normal range. Omega’s modular and programmable mRNA medicines, called epigenomic controllers, target specific genomic loci within insulated genomic domains with high specificity to durably tune single or multiple genes to treat and cure diseases through unprecedented precision epigenomic control.
Omega Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding the research collaboration with Novo Nordisk and the development of a programmable epigenomic mRNA candidate designed to increase metabolic activity and support weight management; the potential of the OMEGA platform to engineer programmable epigenomic mRNA therapeutics that successfully regulate gene expression by targeting insulated genomic domains; and expectations surrounding the potential of our product candidates. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the novel technology on which our product candidates are based makes it difficult to predict the time and cost of preclinical and clinical development and subsequently obtaining regulatory approval, if at all; the substantial development and regulatory risks associated with epigenomic controllers due to the novel and unprecedented nature of this new category of medicines; our limited operating history; the incurrence of
2
significant losses and the fact that we expect to continue to incur significant additional losses for the foreseeable future; our need for substantial additional financing; our investments in research and development efforts that further enhance the OMEGA platform, and their impact on our results; uncertainty regarding preclinical development, especially for a new class of medicines such as epigenomic controllers; potential delays in and unforeseen costs arising from our clinical trials; the fact that our product candidates may be associated with serious adverse events, undesirable side effects or have other properties that could halt their regulatory development, prevent their regulatory approval, limit their commercial potential, or result in significant negative consequences; the impact of increased demand for the manufacture of mRNA and LNP based vaccines to treat COVID-19 on our development plans; difficulties manufacturing the novel technology on which our epigenomic controller candidates are based; our ability to adapt to rapid and significant technological change; our reliance on third parties for the manufacture of materials; our ability to successfully acquire and establish our own manufacturing facilities and infrastructure; our reliance on a limited number of suppliers for lipid excipients used in our product candidates; our ability to advance our product candidates to clinical development; and our ability to obtain, maintain, enforce and adequately protect our intellectual property rights. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, and our other filings with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change.
CONTACT
Investors:
Eva Stroynowski
617.949.4370
estroynowski@omegatx.com
Media:
Jason Braco, LifeSci Communications
646.751.4361
jbraco@lifescicomms.com
3















v3.23.4
Document and Entity Information
|
Dec. 31, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Dec. 31, 2023
|
| Entity Registrant Name |
Omega Therapeutics, Inc.
|
| Entity Central Index Key |
0001850838
|
| Entity Emerging Growth Company |
true
|
| Securities Act File Number |
001-40657
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
81-3247585
|
| Entity Address, Address Line One |
140 First Street
|
| Entity Address, Address Line Two |
Suite 501
|
| Entity Address, City or Town |
Cambridge
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02141
|
| City Area Code |
617
|
| Local Phone Number |
949-4360
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 par value per share
|
| Trading Symbol |
OMGA
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Omega Therapeutics (NASDAQ:OMGA)
과거 데이터 주식 차트
부터 10월(10) 2024 으로 11월(11) 2024

Omega Therapeutics (NASDAQ:OMGA)
과거 데이터 주식 차트
부터 11월(11) 2023 으로 11월(11) 2024
