UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-K
(Mark
One)
☒ ANNUAL
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For
the fiscal year ended June 30, 2023
OR
☐
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For
the transition period from to
Commission
file number: 001-39685
INMED
PHARMACEUTICALS INC.
(Exact
name of registrant as specified in its charter)
| British Columbia, Canada | | 98-1428279 |
(State or other jurisdiction of
incorporation or organization) | | (IRS employer
Identification number) |
| | | |
| Suite 310 – 815 W Hastings, Vancouver, B.C., Canada | | V6C 1B4 |
| (Address of principal executive office) | | (Zip Code) |
(604)
669-7207
(Registrant’s
telephone number, including area code)
Securities
registered pursuant to Section 12(b) of the Exchange Act:
| Title of Each Class | | Trading Symbol | | Name of Each Exchange On Which Registered |
| Common Stock, no par value | | INM | | The Nasdaq Capital Market |
Securities
registered pursuant to Section 12(g) of the Act: None
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐
No ☒
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate
by check mark whether the registrant has submitted electronically, every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☒ No ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer” and
“smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | | Smaller reporting company | ☒ |
| | | | Emerging growth company | ☒ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has fi led a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting fi rm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation
received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of December 31, 2022, the last business day of the Registrant’s
most recently completed second fiscal quarter, the aggregate market value of the Company’s voting and non-voting common equity held
by non-affiliates of the Registrant was $3,180,248.
On September 29, 2023, there were 3,328,191 shares of the registrant’s
common stock outstanding.
DOCUMENTS
INCORPORATED BY REFERENCE
Portions
of the registrant’s definitive proxy statement for the registrant’s 2023 annual meeting of stockholders to be filed pursuant
to Regulation 14A within 120 days of the registrant’s fiscal year ended June 30, 2023 are incorporated herein by reference into
Part III of this Annual Report on Form 10-K.
InMed
Pharmaceuticals Inc.
TABLE
OF CONTENTS
PART
I
Special
Note Regarding Forward-Looking Statements
This
Annual Report on Form 10-K, including the sections entitled “Business,” “Risk Factors,” and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations”, contains forward-looking statements that involve risks
and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995 and other federal securities laws. All statements, other than statements of historical facts contained herein, regarding
our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management
and expected market growth are forward-looking statements. We may, in some cases, use words such as “anticipate”, “believe”,
“could”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict”,
“project”, “will”, “would”, and similar expressions that convey uncertainty of future events or outcomes
to identify these forward-looking statements. Any statements contained herein that are not statements of historical facts may be deemed
to be forward-looking statements. Forward-looking statements in this Annual Report on Form 10-K include, but are not limited to, statements
about:
| |
● |
The
Company’s ability to stem operating losses and the Company’s ability to find further financing to fund operations. |
| |
● |
The
revenues of BayMedica, LLC (“BayMedica”) and the commercial viability of the products in its portfolio; |
| |
● |
Our researching, developing,
manufacturing and commercializing cannabinoid-based biopharmaceutical products will treat diseases with high unmet medical needs; |
| |
● |
The continued optimization
of cannabinoid manufacturing approaches; |
| |
● |
Our success in initiating
discussions with potential partners for licensing various aspects of our Product Candidates; |
| |
● |
Our ability to commercialize and, where required, register products
in the pharmaceutical R&D programs (“Product Candidates”) and those targeted to the health and wellness sector (“Products”)
in the United States and other jurisdictions; |
| |
● |
Our ability to
successfully access existing manufacturing capacity via leases with third-parties or to transfer our manufacturing processes to
contract manufacturing organizations; |
| |
● |
Our belief that our manufacturing
approaches that we are developing are robust and effective and will result in high yields of cannabinoids and will be a significant
improvement upon existing manufacturing platforms; |
| |
● |
The ability of the IntegraSyn
approach to introduce a revenue stream to us before the expected commercial approval of our therapeutic programs; |
| |
● |
Our ability to successfully scale up our IntegraSyn or other cost-effective
approaches so that it will be commercial-scale ready after Phase 2 clinical trials are completed, after which time we may no longer need
to source active pharmaceutical ingredients (“APIs”) from API manufacturers; |
| |
● |
The success of the key
next steps in our manufacturing approaches, including continuing efforts to diversify the number of cannabinoids produced, scaling-up
the processes to larger vessels and identifying external vendors to assist in the commercial scale-up of the process; |
| |
● |
Our ability to successfully
make determinations as to which research and development programs to continue based on several strategic factors; |
| |
● |
Our ability to monetize
our IntegraSyn manufacturing approach to the broader pharmaceutical industry; |
| |
● |
Our ability to continue
to outsource the majority of our research and development activities through scientific collaboration agreements and arrangements
with various scientific collaborators, academic institutions and their personnel; |
| |
● |
The success of work to
be conducted under the research and development collaboration between us and various contract development and manufacturing organizations
(“CDMOs”); |
| |
● |
Our ability to develop
our therapies through early human testing; |
| |
● |
Our ability to evaluate the financial returns on various commercialization
approaches for our Product Candidates, such as a ‘go-it-alone’ commercialization effort, out-licensing to third parties, or
co-promotion agreements with strategic collaborators; |
| |
● |
Our ability to find a partnership
early in the development process for our various programs; |
| |
● |
Our ability to explore
our manufacturing technologies as processes which may confer certain benefits, either cost, yield, speed, or all of the above, when
pursuing specific types of cannabinoids, and filing a provisional patent application for same; |
| |
● |
Plans regarding our next
steps, options, and targeted benefits of our manufacturing technologies; |
| |
● |
Our IntegraSyn or BayMedica
derived products being bio-identical to the naturally occurring cannabinoids, and offering superior ease, control and quality of
manufacturing when compared to alternative methods; |
| |
● |
Our ability to potentially
earn revenue from our IntegraSyn approach by (i) becoming a supplier of APIs to the pharmaceutical industry and/or (ii) providing
pharmaceutical-grade ingredients to the non-pharmaceutical market; |
| |
● |
U.S. Food and Drug Administration
(“FDA”) regulatory acceptance of synthesizing rare cannabinoids for potential use in the pharmaceutical industry; |
| |
● |
Our ability to successfully
prosecute patent applications; |
| |
● |
INM-088 being a once-a-day
or twice-a-day eye drop medication that will compete with treatment modalities in the medicines category, and with the potential
of INM-088 assisting in reducing the high rate of non-adherence with current glaucoma therapies; |
| |
● |
Our belief that with a novel delivery system, the reduction of interocular
pressure (“IOP”) and/or providing neuroprotection in glaucoma patients by topical (eye drop) application of cannabinoids will
hold significant promise as a new therapy; |
| |
● |
The potential for any of
our patent applications to provide intellectual property protection for us; |
| |
● |
Our ability to secure insurance
coverage for shipping and storage of Product Candidates, and clinical trial insurance; |
| |
● |
Our ability to expand our
insurance coverage to include the commercial sale of Products and Product Candidates; |
| |
● |
Developing patentable New
Chemical Entities (“NCE”) which, if issued, will confer market exclusivity to us for the potential development into pharmaceutical
Product Candidates, license, partner or sell to interested external parties; |
| |
● |
Our ability to initiate
discussions and conclude strategic partnerships to assist with development of certain programs; |
| |
● |
Our ability to position
ourselves to achieve value-driving, near term milestones for our Product Candidates with limited investment; |
| |
● |
Our ability to execute
our business strategy; |
| |
● |
Our
disclosure controls and procedures and internal control over financial reporting |
| |
● |
Critical accounting estimates; |
| |
● |
Management’s assessment
of future plans and operations; |
| |
● |
The outlook of our business
and the global economic and geopolitical conditions; and |
| |
● |
The competitive environment
in which we and our business units operate. |
Any
forward-looking statements in this Annual Report on Form 10-K reflect our management’s beliefs and views with respect to future
events and are based on estimates and assumptions as of the date of this 10-K and are subject to risks and uncertainties. We discuss
many of these risks in greater detail under “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing
environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact
of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially
from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on
these forward-looking statements.
You
should read this Annual Report on Form 10-K and the documents that we reference in this Form 10-K and have filed as exhibits, completely
and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking
statements in this Annual Report on Form 10-K by these cautionary statements. Except as required by law, each forward-looking statement
speaks only as of the date of the particular statement, and we undertake no obligation to publicly update any forward-looking statements,
whether as a result of new information, future events or otherwise.
As
used in this Annual Report on Form 10-K, unless otherwise stated or the context otherwise indicates, references to “InMed,”
the “Company,” “we,” “our,” “us” or similar terms refer to InMed Pharmaceuticals Inc.,
and our wholly owned subsidiaries.
Overview
We are a clinical stage pharmaceutical company developing a pipeline
of prescription-based products, including rare cannabinoids and novel cannabinoid analogs, targeting the treatment of diseases with high
unmet medical needs (“Product Candidates”). We are dedicated to delivering new therapeutic alternatives to patients and consumers
who may benefit from cannabinoid-based products. Our approach leverages on the several thousand years’ history of health benefits
attributed to the Cannabis plant and brings this anecdotal information into the 21st century by applying tried, tested and true
scientific approaches to establish non-plant-derived (synthetically manufactured), individual cannabinoid compounds in important market
segments. Such segments include clinically proven, FDA-approved pharmaceuticals, referred to herein as our “Product Candidates”,
and “Products”, synthesized cannabinoids that are provided to wholesalers and end-product manufacturers in the health and
wellness sector. Together with our subsidiary, BayMedica, we are developing multiple manufacturing approaches for synthesizing rare cannabinoids
for potential use in pharmaceutical Product Candidates as well as leveraging this significant manufacturing know-how as a business to
business (B2B) supplier to wholesalers and end-product manufacturers / marketers in the health and wellness sector. Our know-how includes
traditional approaches such as chemical synthesis and biosynthesis, as well as a proprietary, integrated manufacturing approach called
IntegraSyn. While our activities do not involve direct use of Cannabis nor extracts from the plant, we note that the US Food and
Drug Administration (“FDA”) has, to date, not approved any marketing application for Cannabis for the treatment of
any disease or condition and has approved only one Cannabis-derived and three Cannabis-related drug products as prescription-based
drugs. Our ingredients are synthetically made and, therefore, we have no interaction with the Cannabis plant. We do not grow nor
utilize Cannabis nor its extracts in any of our Products or Product Candidates and we do not utilize tetrahydrocannabinol (“THC”)
or cannabidiol (“CBD”), the most common cannabinoid compounds that are typically extracted from the Cannabis plant,
in any of our Products or Product Candidates. The API under development for our initial two lead drug candidates, INM-755 for Epidermolysis
bullosa (“EB”) and INM-088 for glaucoma, is cannabinol (“CBN”). Additional uses of both INM-755 and INM-088 are
being explored, as well as the application of novel cannabinoid analogs in our ocular program and for our INM-900 series program to treat
neurodegenerative diseases including but not limited to Alzheimer’s, Parkinson’s, and Huntington’s.

We
believe we are positioned to develop multiple pharmaceutical Product Candidates in diseases which may benefit from medicines based on
rare cannabinoid compounds. Most currently approved cannabinoid therapies are based specifically on CBD and/or THC and are often delivered
orally, which has limitations and drawbacks, such as side effects (including the intoxicating effects of THC). Currently, we intend to
deliver our rare cannabinoid pharmaceutical drug candidates through various topical formulations (cream for dermatology, eye drops for
ocular diseases) as a way of enabling treatment of the specific disease at the site of disease while seeking to minimize systemic exposure
and any related unwanted systemic side effects, including any drug-drug interactions and any metabolism of the active pharmaceutical
ingredient by the liver. The cannabinoids products sold through our B2B raw material supply business are integrated into various product
formats by companies who then further commercializes such products. We access rare cannabinoids via all non-extraction approaches, including
chemical synthesis, biosynthesis and our proprietary integrated IntegraSyn approach, thus negating any interaction with or exposure to
the Cannabis plant.

Corporate
Information
We
were originally incorporated in the Province of British Columbia, under the Business Corporations Act (British Columbia) (the
“BCBCA”), on May 19, 1981 and we have undergone a number of executive management, corporate name and business sector changes
since this incorporation, ultimately changing our name to “InMed Pharmaceuticals Inc.” on October 6, 2014 to signify our
intent to specialize in cannabinoid pharmaceutical product development. Our principal executive offices are located at Suite 310 –
815 W. Hastings Street, Vancouver, BC, Canada, V6C 1B4 and our telephone number is +1-604-669-7207. Our internet address is https://www.inmedpharma.com/.
Employees
and Human Capital
Our
management team is comprised of highly experienced pharmaceutical and biotechnology executives with successful track records in researching,
developing, gaining approval for and commercializing novel medicines to treat serious diseases. Each member of our management team has
over 20 to 30 years of industry experience, including our CEO, COO, General Manager, and (Sr.) Vice Presidents of Clinical and Regulatory
Affairs, of Preclinical Research and Development, of Discovery Research, of Chemistry, of Synthetic Biology, of Sales & Marketing
and of Commercial Operations. Together, this team has covered the spectrum of pharmaceutical drug discovery, preclinical research, formulation
development, manufacturing, human clinical trials, regulatory submissions and approval, and global commercialization. Additionally, the
team has significant experience in company formation, capital raises, mergers/acquisitions, business development, and sales and marketing
in the pharmaceutical industry. Our Board is constituted by individuals with significant experience in the pharmaceutical and biotechnology
industries. As of September 20, 2023, including our management team, we had 13 full time employees and we also utilize the services of
several consultants. None of our employees are represented by a collective bargaining agreement, nor have we experienced any work stoppage.
We believe that our relations with our employees are good.
We
are committed to growing our business over the long-term. As a result of the competitive nature of the industry in which we operate,
employees have significant career mobility and as a result, the competition for experienced employees is great. The existence of this
competition, and the need for talented and experienced employees to realize our business objectives, underlies the design and implementation
of our compensation programs. At the same time, we seek to keep our approach to compensation simple and streamlined to reflect the still
relatively moderate size of our company. We have implemented compensation, leave and benefits programs necessary to attract and retain
the talented and experienced employees necessary to develop our business including competitive salaries, stock options awards to permanent
employees (both upon initial hiring and annually thereafter), and pay annual bonuses to permanent employees contingent on the achievement
of corporate and/or personal objectives. We have developed an Employee Handbook that contains all corporate policies and guidelines for
professional behavior. Our policies and practices apply to all employees, regardless of title. These guidelines include, among others,
our Code of Business Conduct as well as our policies for corporate disclosure, insider trading and whistle blower, all of which are posted
on our website.
Rationale
for Use of CBN and Cannabinoid Analogs in Pharmaceutical Drug Development
CBN
is one of several non-intoxicating rare cannabinoids naturally produced in the Cannabis plant, albeit at significantly lower levels
relative to the more commonly known THC and CBD. Despite their common origin, different cannabinoids have been observed to have distinct
physiological properties. We are specifically exploring these unique effects of CBN, as well as other rare cannabinoids, and their therapeutic
potential to treat disease. Extensive preclinical testing undertaken by us has identified several unique properties of CBN that outperformed
both THC and CBD in various disease-related assays and models. CBN can act with higher potency when interacting with some receptor systems
in the body, while acting with lower potency for others.
Rare
vs. Major Cannabinoids: Types, Prevalence& Application

The
CBN molecular formula is C21H26O2 and the molecular weight is 310.43 g/mol. CBN has no chiral centers. See Figure 1 below.
Figure
1. Structural Formula of CBN

CBN
occurs naturally as a trace component of Cannabis, or as a degradation product of THC. However, our Product Candidates utilizing
CBN contain highly purified, chemically synthesized CBN, rather than a biological extract from the plant.
We
believe that we offer a differentiated approach to selecting and delivering rare cannabinoids vis-à-vis other current competitors,
many of whom are exclusively focused on THC and/or CBD as their therapeutic agents. We believe that rare cannabinoids in general, and
CBN in particular, represent significant opportunities to treat a wide spectrum of diseases with high unmet medical need. In our preclinical
testing, CBN has demonstrated therapeutic potential beyond CBD for several symptoms and disease-modifying effects for dermatological
conditions and has demonstrated benefits beyond CBD and THC for ocular diseases. We believe that a topical application of CBN may maximize
the clinical benefit at the disease site (skin, eye) while minimizing the systemic exposure and any corresponding adverse effects.
INM-755,
our lead product candidate, is being developed as a topical skin cream formulation containing CBN for the treatment of symptoms related
to EB, a rare genetic skin disease characterized by fragile skin that blisters easily from minimal friction that causes shearing of the
skin layers. In this patient population, it is common for the blisters to become open wounds that do not heal well.
In
addition to relief of symptoms such as itch, inflammation, pain, and others, we believe INM-755 may impact the underlying disease by
enhancing skin integrity in a subset of EB patients. We have completed more than 30 preclinical pharmacology and toxicology studies to
investigate the effects of CBN. Several of these non-clinical studies explored the effect on important symptoms such as pain and inflammation.
In in vitro pharmacology studies, CBN demonstrated activity in reducing markers of prolonged inflammation. CBN upregulated expression
of a type of keratin called keratin 15, or “K15”, which might lead to skin strengthening and reduced blister formation in
EB simplex, or “EBS”, patients with mutations in another keratin called keratin 14, or “K14”. The anti-inflammatory
activity of CBN may be beneficial in healing chronic wounds caused by prolonged inflammation.
Following a review of our toxicology studies, a regulatory application
to support our first Phase 1 clinical study in healthy volunteers with INM-755 (755-101-HV) was submitted November 4, 2019 and approved
December 6, 2019 in the Netherlands. The initial Phase 1 clinical study evaluated the safety, tolerability, and pharmacokinetics of INM-755
cream in healthy volunteers with normal, intact skin; the volunteers had cream applied once daily for a period of 14 days. All subjects
in this first clinical trial completed treatment and evaluations by March 27, 2020. A regulatory application was approved April 17, 2020,
for a second Phase 1 clinical study of healthy volunteers to test the local safety and tolerability of applying sterile INM-755 cream
to small wounds once daily for 14 days. As with the initial Phase 1 trial, the second trial (755-102-HV) was conducted with two different
drug concentrations and a vehicle control. Enrollment began in early July 2020 and the clinical trial completed treatment and evaluations
at the end of September 2020. The safety of INM-755 will continue to be assessed throughout its clinical development. INM-755 cream was
well tolerated in the two Phase 1 clinical studies in healthy volunteers and, based upon this outcome, we advanced the product candidate
into a Phase 2 clinical trial in patients with EB (Study 755-201-EB). Patient treatment in this study commenced in December 2021 and took
place across 13 sites in seven countries including Austria, Germany, Greece, France, Italy, Israel and Spain. The Study completed enrollment
in April 2023 and we released preliminary results in June 2023. INM-755 demonstrated sufficient anti-itch activity to warrant its further
development as an anti-itch therapy in patients with EB or other diseases.
The
purpose of the Phase 2 trial was to evaluate the safety of INM-755 CBN cream, which consists of the control cream plus the active pharmaceutical
ingredient CBN, and obtain preliminary evidence of efficacy in treating symptoms and healing wounds over a 28-day period in patients
with EB. All four subtypes of inherited EB, including EB Simplex, Dystrophic EB, Junctional EB, and Kindler Syndrome were accepted into
the Phase 2 trial. The Phase 2 trial used a within-patient, double-blind design whereby matched index areas were randomized to INM-755
CBN cream or control cream.
Results from the Phase 2 clinical
trial showed a positive indication of enhanced anti-itch activity for INM-755 cannabinol cream versus the control cream alone in an exploratory
clinical evaluation. The results for non-wound itch were not statistically significant in this small trial due, in part, to the clinically
important anti-itch effect of the underlying control cream. We are, nevertheless, encouraged by and satisfied with the INM-755 clinical
data for non-wound itch treatment. That the majority of the assessed patients in the trial showed important improvement in non-wound itch
from the application of INM-755, be it with similar outcomes to the control cream or better than the control cream, can be considered
impressive. For a discussion of the results, please refer to Summary of Completed and Contemplated Clinical Development Plans.
Full data from the study is expected to be published in the second quarter of fiscal year 2024.
CBN
is also the active pharmaceutical ingredient in our second pharmaceutical drug candidate, INM-088, which is in preclinical studies as
a potential treatment for glaucoma. Current treatments for glaucoma primarily focus on decreasing fluid build-up in the eye. We are conducting
preclinical studies to test INM-088’s ability to provide both neuroprotection and reduce intraocular pressure in the eye. We compared
several cannabinoids, including CBD and THC, to determine which cannabinoid was the best drug candidate for the treatment of glaucoma.
Of all the cannabinoids examined in preclinical studies, CBN demonstrated the most optimal neuroprotective effect. Notably, exposure
of retinal neurons, called retinal ganglion cells (“RGCs”) to increasing concentrations of several cannabinoids, including
THC and CBD, resulted in dose dependent cytotoxicity, or cell death, over time. Importantly, CBN-exposed RGCs demonstrated the lowest
level of toxicity among the cannabinoids used in these experiments. We also verified that CBN has an anti-apoptotic effect on differentiated
RGCs when subjected to elevated hydrostatic pressure.
Furthermore,
CBN also exhibited intraocular pressure reduction capability. We selected a final delivery technology (MiDROPS®, EyeCRO
LLC) based on the extensive data collected from these assessments that included solubility, drug delivery localization and sustained
effect.
The
Company continues to advance discovery work for the potential use of a rare cannabinoid to improve neuronal function and provide neuroprotection
for treating neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease.
To date, screening for this indication has yielded some meaningful analog candidates and we will continue to proceed with our plan to
find an appropriate compound for a pre-clinical development program.
For
all current and future pharmaceutical Product Candidates we intend to submit new drug applications (“NDAs”) (or their international
equivalents) in most major jurisdictions, including the U.S. either alone or with development/commercial partners.
We
are actively establishing a broad patent portfolio to protect our commercial interests in utilizing CBN, other rare cannabinoid and cannabinoid
analogs across these and other diseases. We have also filed multiple patent applications for our integrated, biosynthesis-based manufacturing
approach. If granted, these patents may confer meaningful protection to the commercial potential for these technologies.
Rare
Cannabinoid Products in the Health and Wellness Sector
Our
wholly-owned subsidiary, BayMedica, LLC, is a revenue-stage biotechnology company leveraging its significant expertise in synthetic biology
and pharmaceutical chemistry to develop efficient, scalable, and proprietary manufacturing approaches to produce high quality, regulatory-compliant
rare cannabinoids for consumer applications. BayMedica is currently commercializing rare cannabinoids as a B2B supplier to distributors
and manufacturers/ marketers in the health and wellness sector, including nutraceuticals, cosmetics, functional foods and beverages,
as well as animal health markets. BayMedica currently has a robust portfolio of four different non-psychoactive cannabinoids including:
cannabichromene (“CBC”), cannabidivarin (“CBDV”) tetrahydrocannabivarin (“THCV”) and cannabicitran
(“CBT”).
Following the acquisition of BayMedica in 2021, a key priority in 2022
was accelerating commercial activities and building out a robust product portfolio as a supplier of raw ingredients to the health and
wellness sector. While there was slower than expected revenue growth in 2022, we have seen increased demand through the first half of
calendar 2023 due to better research of rare cannabinoids, companies and brands looking for product innovation and effects-based outcomes,
and the ability of companies like ours to be able to reliably supply high quality rare cannabinoids with low batch-to-batch variations.
During the financial year ended June 30, 2023, BayMedica had sales of approximately $4.1 million.
Supply chain optimization has been prioritized over the last 12 months
to ensure there is an adequate inventory to meet demand for our products. BayMedica continues to optimize its manufacturing processes
and supply chain logistics to reduce the overall cost of goods, while also improving the already high quality and purity levels for all
products in its portfolio. BayMedica has also been working hard to augment sources of raw materials and to add to its downstream purification
partners.
Although
there are no assurances this growth will continue in future quarters, this recent trend is encouraging. The increase in sales results
from expanded marketing efforts and increased demand in certain cannabinoid Products. BayMedica will continue to evaluate opportunities
for potential structured supply arrangements and collaborations for the commercial business. Sales and marketing efforts will remain
focused on Products and Product Candidates that contribute highest margins, where BayMedica continues to hold a strong competitive position.
Our
Business Strategy
Our
goal is to develop a pipeline of cannabinoid and proprietary cannabinoid analog prescription-based Product Candidates targeting treatments
for diseases with high unmet medical needs as well as to develop proprietary manufacturing technologies to produce rare cannabinoid Products
as raw ingredients to end-product manufactures within the health and wellness sector.
| |
● |
Advance cannabinoid pharmaceutical drug Product Candidates through preclinical and clinical development, thereby establishing important human proof-of-concept in multiple therapeutic applications |
| |
● |
Develop and produce proprietary cannabinoid analogs for use in our drug development program |
These
activities are well underway, at various stages, for INM-755 for diseases of the skin, INM-088 for diseases of the eye and the INM-900
series for neurodegenerative diseases. Building upon preclinical data sets, we have the internal capabilities to design and execute,
together with multiple external vendors, the preclinical data sets and clinical studies required to advance pharmaceutical drug candidates
towards commercialization.
| ● | Actively
seek avenues to accelerate drug development via licensing, partnering or sale to external
companies |
We
do not currently have an internal organization for the sales, marketing and distribution of pharmaceutical products. With respect to
the commercialization of each Product Candidate, we may rely on either i) a “go-it-alone” commercialization effort; ii) out-licensing
to third parties; or, iii) co-promotion agreements with strategic collaborators for our Product Candidates. Any decision on a “go-it-alone”
commercialization effort versus out-licensing to third parties will depend on various factors including, but not limited to, the complexity,
the expertise required and related cost of building any such infrastructure for our Product Candidates. For INM-755 in EB, we are actively
seeking development and commercial partnerships. For INM-088 in glaucoma, because of the potentially large number of clinical trial participants
and the extensive sales effort required to reach a large number of prescribing physicians, we may consider exploring partnership opportunities
early in the development process. The commercial strategy for the INM-900 series of compounds will be evaluated in due course.
| ● | Expand
portfolio and revenues of rare cannabinoids into existing distribution network and to end-product
manufacturers of specialty health and wellness products |
| ● | Develop
multiple cost-efficient manufacturing processes for high quality rare cannabinoids as APIs
for our core internal drug candidate pipeline and for licensing opportunities of non-core
drug candidates. |
Leveraging
off our extensive chemical synthesis and biosynthesis know-how, we are developing an integrative cannabinoid synthesis approach designed
to produce bio-identical, economical, pharmaceutical-grade cannabinoids in a cost-efficient manner, called IntegraSyn. IntegraSyn is
designed to offer superior yield, control, consistency and quality of rare cannabinoids when compared to alternative methods.
At
the core of our activities, we are a pharmaceutical drug development company and a developer and supplier of rare, naturally occurring
cannabinoids and their analogs that is focused on commercializing important cannabinoid-based medicines to treat diseases with high unmet
medical needs.
Our
Strengths
We
are the only clinical-stage company with multiple cannabinoid drug candidates, in multiple therapeutic categories, that is also currently
supplying rare cannabinoids to manufacturers in the health and wellness sector and who has internal expertise in multiple manufacturing
approaches including chemical synthesis, biosynthesis and a proprietary, integrated biosynthesis-based manufacturing approach, called
IntegraSyn, to meet the needs of the rapidly evolving markets for rare cannabinoids. Key strengths include:
Experienced
executive team and board of directors with proven track records.
One
key critical success factor in the field of pharmaceutical drug development is the experience and skill set of the individuals leading
the company. We have been successful in attracting and retaining executive and directors with extensive (20+ years) experience in all
facets of the pharmaceutical industry, including fundamental research and development, multiple manufacturing techniques, drug formulation,
clinical trial execution, regulatory approvals, pharmaceutical commercialization, company and capital formation, business development,
legal, and corporate governance. Our leadership team is well-poised to lead us through all facets of drug development and product commercialization,
either internally or externally via partnerships. It is this group of individuals that will help optimize our chances for success.
Multiple
manufacturing approaches.
The
combined manufacturing technologies from InMed and BayMedica provide us with a competitive advantage to utilize the most cost-efficient
methodology (i.e. chemical synthesis, biosynthesis, IntegraSyn) for the development and commercialization of new Products and Product
Candidates and provision of rare bio-identical cannabinoids or their analogs to a wide spectrum of market opportunities.
Early
mover status as a B2B supplier of rare cannabinoids to the health and wellness sector.
As
demonstrated by the launch of several rare cannabinoids into the health and wellness sector, the team at BayMedica has substantial expertise
in the commercial manufacturing scale-up to produce rare cannabinoids at large scale as well as extensive cannabinoid sales and marketing
expertise. This know-how is important to establishing an early-mover status and to maintain cost leadership with regards to specific
rare cannabinoids.
Leading
experts in the therapeutic potential of the rare cannabinoid CBN.
We
have invested significant time and effort in understanding the characteristics and therapeutic potential of our first rare cannabinoid
drug candidate, CBN. As such, we are positioning ourselves to be a world leader in the pharmaceutical development of this rare cannabinoid.
We anticipate that CBN will be the first of several such drug candidates.
Targeting
medical applications of rare cannabinoids to treat diseases with high unmet medical needs.
Significant
investment in understanding the therapeutic potential of CBN has provided us with important insight as to how best to develop this class
of compounds for treating various diseases. We intend to apply this know-how across several diseases that may benefit from cannabinoid-based
medicines.
Diverse
portfolio of patent applications covering a spectrum of commercial opportunities.
Success
in pharmaceutical markets often rests with the strength of intellectual property, including patents, to protect our commercialization
interests. We have filed several patents on our novel findings and expect to continue to do so. The acquisition of BayMedica brought
several additional new patent families to enrichen our manufacturing as well as drug development opportunities.
Cannabinoid
Science Overview
Cannabinoids
are a class of compounds that exist throughout nature and can be found in significant numbers and varying quantities in the Cannabis
plant. The two predominant, or major, cannabinoids in the Cannabis plant are THC and CBD. These two exist in relatively large
quantities in the plant and can be easily extracted, which has led to significant research into these two compounds over the previous
several decades. Nevertheless, there are over 140 additional cannabinoid compounds found in the plant, referred to as minor or rare cannabinoids.
Each cannabinoid has one or more specific chemical differences that may confer unique physiological properties in humans.
Cannabinoid
receptors are found throughout the body and are involved in many different functions, such as pain perception, memory, immune function
and sleep. Cannabinoids act as messengers that bind to cannabinoid receptors, as well as other receptors, signaling the endocannabinoid
system into action. The relevance of the endocannabinoid system on many important physiological processes has made cannabinoids an important
target to potentially treat a number of diseases and symptoms.
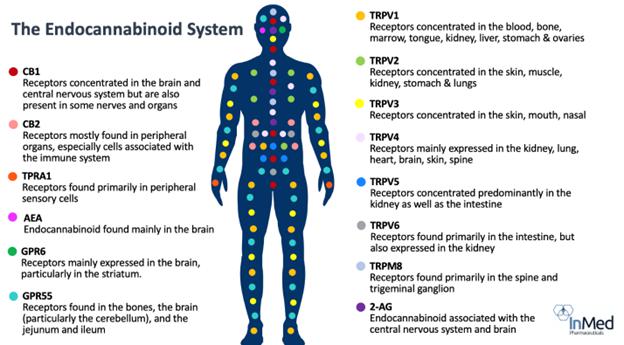
Two
cannabinoid receptors in the human body are the endocannabinoid receptor 1 (“CB1”), which is more significant to the central
nervous system, and endocannabinoid receptor 2 (“CB2”), which is more common with the immune system.
Significant
investigation is currently underway to determine the role of cannabinoids in affecting other receptor systems in the human body.
Our
Products, Product Candidates and Technologies
Development
of a Flexible Suite of Processes for the Manufacturing of Cannabinoids
Introduction
While there are over 140 different individual cannabinoids in the Cannabis
plant, the two most well-known and studied compounds are also the two that occur in the largest quantities: THC and CBD. Due to their
relative abundance in the Cannabis plant, it is also only THC and CBD that can currently be extracted economically; this also now
includes genetically modified plants designed to significantly increase quantities of cannabigerol (“CBG”) in harvested crops.
Among other challenges, the expense of extraction – or that of synthetic manufacturing – of the remaining minor or rare cannabinoids,
may be orders of magnitude greater than that of THC, CBD and CBG.
Nevertheless,
like the major cannabinoids THC, CBD and CBG, these rare cannabinoids may hold very important physiological benefits in humans. The challenge,
and opportunity, that we have identified, and seek to solve, is selecting and engineering the most appropriate manufacturing approach
to making a specific rare cannabinoid, at the desired quantity and requisite quality, which is cost-efficient and consistently yields
bio-identical cannabinoids as compared to the compounds found in nature, among several other benefits. We believe that providing this
solution will be a critical success factor not only for our own drug development strategy, but also for other pharmaceutical and health
and wellness companies.
Development
of InMed’s biosynthesis and IntegraSyn technologies:
In
2015, we commenced the development of a biosynthesis process for the manufacturing of cannabinoids through a research collaboration with
Dr. Vikramaditya Yadav from the Department of Biological and Chemical Engineering at the University of British Columbia. Utilizing the
basis of a specific vector created for us, Dr. Yadav initiated a Research and Development Project titled “The Metabolic Engineering
of yeast and bacteria for synthesis of cannabinoids and Cannabis-derived terpenoids” under a collaborative research agreement.
Subsequently, we signed a Technology Assignment Agreement with the University of British Columbia whereby we retain sole worldwide rights
to all patents emergent from the technology under development in exchange for a royalty of less than 1% on sales revenues from products
utilizing cannabinoids manufactured using the technology and a single digit royalty on any sub-licensing revenues. Other than the 1%
royalty, we do not have any ongoing financial commitments under these arrangements with the University of British Columbia.
Microorganisms
do not naturally produce cannabinoids nor the enzymes required for their assembly. However, utilizing genome engineering to modify their
metabolism, we have systematically introduced different aspects of the Cannabis plant’s metabolic pathways into a bacteria
(E. coli), referred to as a host, and have reported what we believe to be the first-of-its-kind production of fully differentiated
cannabinoids in this bacteria. This research served as the basis for the subsequent development of a new, integrated approach to cannabinoid
manufacturing that we refer to as IntegraSyn. IntegraSyn is a flexible, integrative cannabinoid synthesis approach utilizing novel enzyme(s)
to efficiently produce bio-identical, economical, pharmaceutical-grade cannabinoids without the risk and high-resource requirements of
an agriculture growing operation.
IntegraSyn integrates various
pharmaceutical manufacturing processes to maximize yield and minimize the cost of cannabinoid synthesis, in particular for pharmaceutical-grade
products. We utilize proprietary, high efficiency enzymes produced via the E. coli biofermentation portion of the IntegraSyn approach
for the production of a cannabinoid. Our enzymes are used in combination with cost-effective yet sophisticated substrates (or, starting
materials) to produce a cannabinoid in bulk via a biotransformation process, which is then further processed with downstream purification
steps including separation, purification and drying. This cannabinoid can be inventoried in bulk and used either as a finished API cannabinoid
product or as a starting material for other cannabinoids. This further differentiation can utilize any one of several well-established
manufacturing approaches – including enzymatic biotransformation and traditional chemical synthesis – to optimize yield, time
and cost.
IntegraSyn
BayMedica’s
Chemical Synthesis and Biosynthesis Technologies for the Development and Production of Cannabinoids, Their Variants and Analogs
BayMedica
continues to develop cannabinoid manufacturing techniques that are ‘method agnostic’, utilizing the most practicable, expeditious
and cost-effective means to produce any particular cannabinoid or novel cannabinoid compound.
Chemical
Synthesis for the Development and Production of Cannabinoids
Chemical
synthesis is a well-established, long-standing, robust and reliable method for the production of a myriad compounds for use in both
consumer and pharmaceutical products, including such commonly used medications as vitamin-D and acetaminophen. The production of
cannabinoids, in particular CBD and THC, by synthetic methods was first described in 1965 by Mechoulam, et. al. Although yields were
typically less than 10% and the scales were small, this work paved the way for methods to synthesize these common cannabinoids as
well as many rare and novel cannabinoid compounds. Today several companies such as Purisys, Biovectra, Kinetochem and Benuvia
reliably manufacture a wide variety of common and rare cannabinoids to pharmaceutical API standards. However, because price points
for these compounds have remained high, their utility in non-pharmaceutical applications has historically been limited.
Pharmaceutical
chemistry methods have also been of paramount utility in the creation of combinatorial libraries of new chemical entities for drug discovery.
We believe these pharmaceutical chemistry methods will also be of great value in the preparation of novel cannabinoid compounds with
enhanced pharmacological activities and have leveraged our expertise to this end. Using combinations of pharmaceutical chemistry and
biosynthesis, the BayMedica team has generated a wide variety of these compounds with the primary focus being on modifying the pentyl
side chain found on many naturally occurring cannabinoids. We believe that unlike a traditional drug discovery approach using combinatorial
libraries, our approach, because it does not change the core structure of each cannabinoid type, will result in a larger proportion of
novel cannabinoids showing pharmacological activity, thus increasing the probability of a successful drug candidate. Prior to the acquisition
by InMed, BayMedica delivered multiple novel cannabinoid analogs to InMed for evaluation. Since that time, we have developed additional
New Chemical Entities (“NCEs”) with the ability to expand through existing and novel methods currently under development.
Chemical
Synthesis-Derived Cannabinoids Commercialized by BayMedica
Cannabichromene
(CBC)
The
historically high cost of goods for cannabinoids manufactured by chemical synthesis has largely precluded their widespread adoption for
non-pharmaceutical applications. BayMedica has successfully manufactured and commercialized a rare cannabinoid, CBC, for sale to distributors
into the health and wellness industry. The development of a scalable process for the manufacturing of CBC began in 2018 using well established
chemical synthesis protocols.
In
calendar 1Q2019, a Material Services Agreement was completed with a multinational contract research, development and manufacturing organization
(“Chemistry CDMO”) to facilitate the optimization and scale-up of BayMedica’s proprietary CBC manufacturing process
using commercially available starting materials sourced from various manufacturers. We scaled to a batch size of greater than 1kg by
calendar 3Q2019 at which time we contracted a leading U.S. manufacturer to provide the final purification of CBC to greater than 95%
purity. This manufacturer also operates a North American (NA) based toll-processing facility with the capability to process from 10kg
to metric ton quantities of our crude CBC material under food-grade GMP conditions. By late 4Q2019 our Chemistry CDMO had scaled the
process to greater than 10kg, and by year end 2019 to almost 30kg with final purification at the NA contractor. We commenced commercial
sales of CBC in November 2019.
Large
scale manufacturing of crude CBC began at our Chemistry CDMO in 1Q2020 at >40kg. The emergence of the Covid-19 pandemic significantly
impacted sales beginning in calendar 1H2020. Large scale production continued with current batch sizes exceeding 100kg.
Cannabicitran
(CBT)
We
have developed a process for the efficient chemical synthesis of CBT through both in-house R&D efforts and via our CDMO. We began
scaling this process and conducted downstream processing and purification trials in late calendar 2H2021. We received initial purchase
orders and commenced commercial sales of CBT in calendar 1Q2022.
Cannabidivarin
(CBDV)
Beginning
in early 2021, BayMedica worked internally and with external parties to access and develop manufacturing technologies for the chemical
synthesis of the rare, non-intoxicating “varin” cannabinoid, CBDV. In calendar 4Q2021, via a Chemistry CDMO, we successfully
scaled CBDV synthesis to commercial quantities and initiated procurement of starting materials sufficient to meet expected customer demand.
In April 2022, we commenced B2B sales of CBDV to the health and wellness sector. We have since developed a novel and efficient
method allowing for the scalable preparation of CBDV at an improved costs of goods.
Tetrahydrocannabivarin
(THCV)
As
part of the R&D into manufacturing techniques to synthesize and produce CBDV, we also began researching and developing processes
to convert CBDV to the non-intoxicating rare cannabinoid THCV. In conjunction with our Chemistry CDMO and our in-house team, we developed
a robust pilot-scale process that produces THCV. We have now developed a purification process to produce the finished THCV material.
We began scale-up of our novel process with this CDMO in calendar 1Q2022 and commenced sales in calendar 2Q2022. As new manufacturing
approaches lead to reduced cost of goods for CBDV, these cost improvements benefit the THCV product as well.
Analogs
of Cannabinoids / New Chemical Entities
In
addition to the natural cannabinoids above, we have leveraged our expertise in pharmaceutical chemistry and biosynthesis to produce a
number of novel cannabinoid analogs and variants of pharmaceutical interest.
In
the field of pharmaceutical drug development, the term analog is used to describe structural and functional similarity between an original
(or parent) molecule and one that has been somewhat modified. While any company researching a naturally occurring compound, like cannabinoids,
cannot own a patent on the molecule itself for commercial exclusivity, a modified molecule, which has certain structural and pharmacological
similarities with the original compound, can be patented. As well, modifications of the original molecule (ie, the analog) can be designed
to confer certain improvement in activity of the parent, such as an elevation of the desired physiological effects, a decrease in unwanted
side effects, improvement in aspects related to drug delivery to targeted tissues, etc., or a combination of these targeted outcomes.
We have filed patents covering numerous structural additions and modification of the naturally occurring cannabinoids. Each individual
modification to each individual cannabinoid represents a New Chemical Entity (“NCE”) which can be patented. If issued, this
patent family will confer market exclusivity to us for the analogs that we intend to develop into pharmaceutical Product Candidates,
license, partner or sell to interested external parties.
Competitive
Conditions:
Other
companies deploy a diversified number of cannabinoid synthesis manufacturing techniques, including:
| |
● |
Biosynthesis (generation
of the final compound inside a single system) using yeast, non-E. coli bacteria, or other approaches (algae, etc.) as a host
organism; |
| |
● |
Synthetic chemistry; and |
| |
● |
Combinations of these above-listed technologies |
Several
companies (see chart below) are active in the cannabinoid manufacturing space including BioVectra, CB Therapeutics, Cellibre, Cronos,
Ginko Bioworks, Hyasynth, Intrexon, KinetoChem, Librede, and Purisys, among several others.
Key
Milestones:
On
May 21, 2015, we commenced the development of our biosynthesis process for the manufacturing of cannabinoids through a research collaboration
with Dr. Vikramaditya Yadav from the Department of Biological and Chemical Engineering at the University of British Columbia under a
project titled “The Metabolic Engineering of yeast and bacteria for synthesis of cannabinoids and Cannabis derived terpenoids”.
On May 31, 2017, we signed a Technology Assignment Agreement with the University of British Columbia whereby we retain sole worldwide
rights to all patents emergent from the technology under development in exchange for a royalty of less than 1% on sales revenues from
products utilizing cannabinoids manufactured using the technology and a single digit royalty on sub-licensing revenues. Royalties are
payable, on a country-by-country basis, until such time as there is no longer a patent pending, unexpired patent or issued patent derived
from the transfer technology, in any country. On May 15, 2018, we extended our Collaborative Research Agreement, which may be terminated
by either party upon 30 calendar days written notice, with the University of British Columbia for an additional three years, which expired
in 2021. Other than the 1% royalty, we do not have any ongoing financial commitments under these arrangements with the University of
British Columbia.
We,
in conjunction with our collaboration partners at the University of British Columbia, continue to advance the production platform for
the biofermentation of cannabinoids. Optimization of the vector continued in parallel with the identification of optimal fermentation
conditions and down-stream purification processes with third party contract manufacturing organizations. Optimization of the fermentation
conditions was a project conducted with the National Research Council Canada at their dedicated fermentation facility in Montreal, Quebec.
While we do not anticipate any new intellectual property arising from this venture, under the terms of this research agreement, the National
Research Council of Canada owns all new intellectual property (“IP”) and we have a sole, fully-paid-up license to all commercialization
rights of such IP. This project was initiated in October 2018 and concluded in the second half of 2019.
In
February 2019, we entered into a separate process development collaboration by way of a Master Service Agreement with the Almac Group
(UK), or “Almac”, a seasoned GMP pharmaceutical CDMO. Almac was initially tasked to develop a down-stream purification process
to support the fermentation optimization activities at the National Research Council of Canada. In addition, we also engaged Almac to
assist in the development of an “alternative” manufacturing process for cannabinoids which integrates the best available
technologies across the spectrum of pharmaceutical drug production. This process is now referred to as IntegraSyn. In May 2020, we announced
our working relationship with Almac on an integrated approach to augment current biosynthesis-based methods for cannabinoid production.
The companies have been engaged in developing a streamlined cannabinoid manufacturing process, specifically optimizing the upstream cannabinoid
assembly processes as well as downstream purification processes, to achieve cost-efficient, GMP-grade active pharmaceutical ingredients
for prescription-based cannabinoid medications. Almac is an international, privately-owned organization which has grown organically over
the past five decades now employing over 5,600 highly skilled personnel across 18 facilities including Europe, the US and Asia. We retain
all rights to this new process while Almac retains certain rights-of-first refusal on the production and supply of certain precursors,
or starting materials, for this alternative process.
Other
Milestones Include:
| |
● |
September 22, 2020 –
We announced the filing of a PCT patent application as part of a growing portfolio of intellectual property related to the IntegraSyn
manufacturing approach for producing low-cost, pharmaceutical-grade cannabinoids (refer to “Intellectual Property”, below). |
| |
● |
April 26, 2021 – We announced that the IntegraSyn cannabinoid
manufacturing approach has achieved a level of 2g/L cannabinoid yield, a milestone that signals commercial viability and supports advancement
to large-scale production in the coming months. Having achieved a 2g/L yield level, we will now focus on manufacturing scale-up to larger
batch sizes while continuing process and enzyme optimization, targeting increased cannabinoid yield and further reducing the overall cost
of goods. In parallel, we continue to prepare the manufacturing process to be Good Manufacturing Practice (“GMP”)-ready for
pharmaceutical quality production. |
Research
and Development Pipeline of Therapeutic Drug Candidates
INM-755
for the Treatment of Epidermolysis bullosa (“EB”)
Introduction
INM-755
(CBN) cream is being developed as a proprietary, topical, single-cannabinoid product candidate intended as a therapy in dermatological
diseases. The first clinical indication under development is EB. EB is a collective name for a group of genetic disorders of connective
tissues characterized by skin fragility leading to extensive blistering and wounding. It affects skin and mucous membranes, particularly
of the gastrointestinal tract, genitourinary and respiratory systems. EB is a debilitating disease affecting a small proportion of people
in the United States, thus earning it an orphan-disease status. The disease has no definitive cure and all current treatments are directed
towards symptom relief. There are, however, a number of products, mainly gene therapies, currently in clinical trials, in which a cure
is being explored, according to several recent scientific publications. Our preclinical research has identified a specific cannabinoid,
CBN, that may prove beneficial to patients: first, by addressing certain key disease hallmarks (which may include wound healing, infection,
pain, inflammation, and itch); and second, by regulating the expression of various proteins (keratins) that may compensate for reduced
expression of others.
The
active ingredient in INM-755, CBN, is an agonist for both cannabinoid (CB) 1 and CB2 receptors, with a higher affinity for CB2, which
means it should have a greater effect on the immune system than on the central nervous system. The distribution of CB1 and CB2 receptors
in sensory nerves and inflammatory cells in the skin make it an attractive pharmaceutical agent for dermal treatments in medical conditions
characterized by inflammation and pain.
In
preclinical pharmacology studies, CBN demonstrated activity as an anti-inflammatory, antipruritic and antinociceptive agent. CBN upregulated
expression of keratin 15 (K15), which might lead to skin strengthening and reduced blister formation in EBS patients with keratin 14
(K14) mutations. At the cream concentrations chosen for clinical development, it does not appear to impede wound healing of partial-thickness
wounds. Its anti-inflammatory activity may be beneficial in healing chronic wounds caused by prolonged inflammation.
We
have completed 20 safety pharmacology and toxicology studies to investigate the effects of CBN, with the longest treatment up to 28 days.
We have also completed three Phase 1 safety and tolerability studies in healthy volunteers, two studies of which were conducted with
varying concentrations of INM-755 cream and one study of which examined the non-CBN components of the cream base for INM-755. These foundational
preclinical and clinical studies would support the use of INM-755 cream in clinical trials for many skin diseases, with treatment up
to 28 days.
In
June 2023 we completed a Phase 2 clinical trial in patients with EB. INM-755 demonstrated sufficient anti-itch activity to warrant its
further development as an anti-itch therapy in patients with EB or other diseases.
The
Science Behind EB
At
the most basic level, the hallmark of EB is poor anchorage of the epidermis to the dermis such that the skin and mucous membranes of
the affected individuals tend to shear and blister on minimal friction. This is due to the genetically inherited defect in certain genes
(multiple genes have been shown to be associated with the different subtypes of EB) that code for some specific proteins that are concerned
with maintaining the integrity of skin and mucous membranes.
There are four main subtypes of the inherited condition. Each of these
subtypes can display a spectrum of phenotypic severity reflecting the types of mutations in different genes, together with modifying environmental
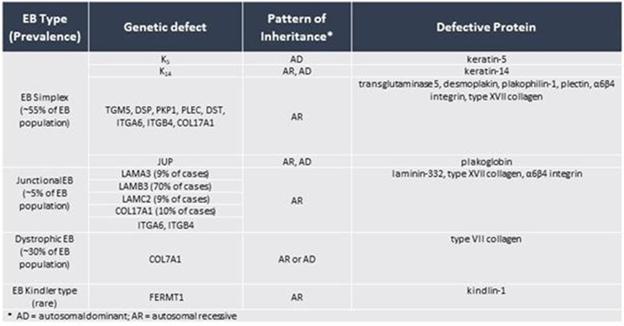
factors. The types of mutations also determine the mode of inheritance, either autosomal dominant or autosomal recessive. The following
table shows the pattern of inheritance and the defective genes and proteins in each:
Classification
of EB Types
(a)
EBS
This
is the most common form of EB and is characterized by a lack of adhesion of the skin directly above the basement membrane (the basal
layer). An estimated 55% of people with EB have EBS resulting from a genetic defect of the keratins K5 and K14, with the incidence between
the two defects estimated to be essentially equal. The most common form of EBS manifests itself as blistering confined to the hands and
feet while in others blistering can occur all over the body. Blistering generally appears during the neonatal period but it can also
manifest itself in later childhood (or even in adult life). Painful skin blisters are accentuated by friction, especially on the feet
where footwear causes increased irritation. Friction injuries tend to occur more commonly in warm weather and secondary infections are
common.
(b)
Junctional EB
Junctional
EB is characterized by a lack of adhesion of the skin through the basement membrane and affects some 5% of those with EB. The generalized
type of junctional disease (about half of cases of junctional EB) is usually fatal in infancy. This is often as a result of anemia and
malnutrition due to poor feeding caused by the serious blistering in the pharynx and esophagus. The milder form of the disease can cause
life-long pain and disability.
(c)
Dystrophic EB, or “DEB”
DEB
is characterized by a lack of adhesion of the skin under the basement membrane. Approximately 30% of people with EB have DEB. Patients
with DEB tend to develop blisters that heal with fibrosis, leading to joint contracture, fusion of the fingers, contractures of the mouth
membranes and narrowing of the esophagus. Often the dominant inherited type of DEB is the least severe type and the patient can lead
an almost normal life. However, the severity of the condition does increase with age due to scarring, syndactyly and generalized skin
atrophy. Those with recessive DEB have a high chance of developing a squamous cell carcinoma, often before the age of 35.
(d)
Kindler Syndrome
This
type of EB is rare and usually becomes apparent at birth or soon after. This condition is called mixed type because blisters appear across
the skin layers. The condition usually improves with time and can disappear. It is the only type that causes patchy discoloring (mottling)
of skin exposed to the sun. Kindler syndrome is recessive.
(e)
Epidermolysis bullosa acquisita
Epidermolysis
bullosa acquisita is a rare type that is not inherited. The blisters result from the immune system attacking healthy tissue by mistake.
It’s similar to another immune system disorder called bullous pemphigoid. It tends to cause blisters on the hands, feet and mucous
membranes.
Epidemiology,
Morbidity and Mortality
The
most reliable figures on prevalence and incidence of EB are derived from the National EB Registry, or “NEBR”, which collected
cross-sectional and longitudinal data on about 3,300 EB patients in the United States from 1986 through 2002. The prevalence of EB was
estimated to be approximately 11 per million and the incidence approximately 20 per million live births. In the United States, assuming
that mild cases of EBS are reported only 10% of the time, the affected population in the United States is approximately 12,500. Other
sources cite populations of up to 25,000 in the United States.
Generalized
blistering caused by any subtype may be complicated by infection, sepsis, and death especially in infancy. Severe forms of EB increase
the mortality risk during infancy. In patients with EB that survive childhood, the most common cause of death is metastatic squamous
cell carcinoma. This skin cancer occurs most frequently in patients with recessively inherited DEB who are aged 15-35 years. In contrast,
dominantly inherited EBS and DEB and milder forms of junctional EB may not affect a patient’s life expectancy adversely. Onset
of EB is at birth or shortly after. The exception occurs in mild cases of EBS, which may remain undetected until adulthood or remain
undiagnosed. The disease appears to have equal incidences in both sexes.
Current
Treatments
As
a genetic disease, EB has no cure and, as a designated orphan-disease, there are no approved products specifically to treat this indication.
Effective management of EB patients involves a collaborative approach between several specialists, including surgeons, dermatologists,
ophthalmologists, dentists, psychologists, podiatrists, physiotherapists and geneticists. The aim is to provide support to the patient
by alleviating symptoms and managing complications; in particular, the patient caregivers must assess and act daily to treat the wound
and enable wound healing, address the current level of pain and itch, provide adequate antimicrobial protection, reduce inflammation
(as a source of depressed wound healing abilities) and address the emotional state of the patient.
Current
medications are employed in control of pain (various types of analgesics including nonsteroidal anti-inflammatory drugs, or “NSAIDS”,
tricyclic antidepressants, gabapentin, and narcotics) and pruritus (antihistamines, etc.) and to address complications such as local
infection and septicemia (local and systemic antibiotics). Steroids and phenytoin are also used in managing dysphagia-associated pain.
Tetracycline is considered to be beneficial in improving the blistering and epithelial disadhesion. The complications of these classes
of medications are well known and the drugs are most likely to further complicate the patients’ conditions since they will be used
on long-term basis.
Competitive
Landscape
We
are developing INM-755, our proprietary, topical, single-cannabinoid product candidate, as a first-line therapy in all EB patients for
symptom relief and may explore its effects in EBS with a K14 genetic mutation as a therapy to potentially strengthen skin integrity via
up-regulation of the keratin K15.
There are only two therapies
approved specifically for the treatment of EB. Filsuvez® (Oleogel-S10) has been approved in Europe and Great Britain for the treatment
of skin wounds in patients with dystrophic or junctional EB and Krystal Biotech’s Vyjuvek® (beremagene gerperpavec, formerly
B-Vec), a topical gene therapy for dystrophic EB. For those products currently envisioned or in clinical trials as topical treatments,
wound healing and symptom relief are the primary endpoints.
According
to public information, several topical investigational drug formulations are currently at various stages of clinical development for
the treatment of EB, including:
| |
● |
Amryt Pharma’s Filsuvez® (formerly Oleogel-S10, or AP101),
which is a topical product incorporating a betulin-based active ingredient formulated with sunflower oil. It causes the keratinocytes
to migrate faster and to differentiate into mature epithelial skin cells. This product is currently approved in some jurisdictions for
the treatment of partial-thickness wounds in adults and was recently approved for patients with dystrophic and junctional EB in Europe.
The Phase 3 double blind study is complete and met its primary endpoint on time to first target wound closure (p=0.013). The 24-month
open label extension study is ongoing (NCT03068780). The product was also submitted to the FDA for approval, where it had been granted
orphan drug, rare pediatric disease and fast track designations. However, in February 2022 after several review extensions, the FDA formally
rejected the application and asked for additional evidence. Amryt plans to work with FDA to address their concerns. Amyrt was acquired
by the Italian company Chiesi Farmaceutici SpA in April, 2023. |
| |
● |
Krystal
Biotech’s Vyjuvek® (beremagene gerperpavec, formerly B-Vec) is a topical gene therapy for dystrophic EB. Vyjuvek® uses
a modified herpes simplex virus to deliver instructions to generate type VII collagen (that is mutated in dystrophic EB) to skin cells.
In May 2023, the FDA approved Vyjuvek®, for the treatment of wounds in patients 6 months of age and older with dystrophic epidermolysis
bullosa with mutation(s) in the collagen type VII alpha 1 chain (COL7A1) gene. Krystal had previously completed a Phase 3 study
in 31 children and adults with dystrophic EB, where a significantly greater proportion of Vyjuvek®-treated wounds were completely
healed at 3 months (71% vs 20%) and 6 months (67% vs 22%) compared with those given placebo gel. An 18-month open-label extension study
is ongoing and expected to conclude this year. |
| |
|
|
| |
● |
Abeona EB-101 is an autologous, engineered cell therapy for RDEB, a
rare connective tissue disorder without an approved treatment. Treatment with EB-101 involves using gene transfer to deliver COL7A1 genes
into an RDEB patient’s own skin cells (keratinocytes) and transplanting them back to the patient to enable normal Type VII collagen
expression and skin function. The VIITAL™ study is a Phase 3 randomized clinical trial investigating EB-101, an engineered cell
therapy for the treatment of RDEB. Large chronic wounds treated in VIITAL™ measured greater than 20 cm2 of surface area and had
remained open for more than six months. The VIITAL™ study met its two co-primary efficacy endpoints demonstrating statistically
significant, clinically meaningful improvements in wound healing and pain reduction in large chronic RDEB wounds. EB-101 was shown to
be well-tolerated with no serious treatment-related adverse events observed, consistent with past clinical experience. |
| |
● |
Castle Creek Biosciences has a Phase 3 clinical trial that is active,
but not recruiting. The purpose of this study is to determine whether administration of FCX-007 in addition to standard of care improves
wound healing as compared to standard of care alone (control) in children, adolescents, and adults with Recessive Dystrophic Epidermolysis
Bullosa. DEFI-RDEB is a multi-center, intra-patient randomized, controlled, open-label, Phase 3 study of FCX-007 for the treatment of
persistent non-healing and recurrent RDEB wounds in approximately 24 subjects. |
Other
approaches have shown promise and are under investigation for the treatment of EB.
Regulatory
Perspectives
According
to the National Epidermolysis Bullosa Registry, the overall incidence is about 20 per million live births and prevalence is 11 per million
in the United States. EB is designated as an “orphan disease”, and we plan to seek regulatory designation of INM-755 as such
in the U.S. and similar designations in various jurisdictions should clinical trials progress. The FDA defines orphan products as “those
intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people
in the United States, or that affect more than 200,000 persons but are not expected to recover the costs of developing and marketing
a treatment drug”. The EMA has its own definition of orphan disease and, under the European definition, EB is also an orphan disease.
The
mission of the FDA Office of Orphan Products Development, or “OOPD”, is to advance the evaluation and development of products
(drugs, biologics, devices, or medical foods) that demonstrate promise for the diagnosis and/or treatment of rare diseases or conditions.
This arm of the agency evaluates scientific and clinical data to identify and designate products as promising for rare diseases and to
further advance scientific development of such promising medical products. The OOPD also works on rare disease issues with the medical
and research communities, professional organizations, academia, governmental agencies, industry, and rare disease patient groups. The
OOPD provides incentives for sponsors to develop products for rare diseases. The Orphan-Drug Designation program, which is administered
by the OOPD, provides orphan status to drugs and biologics which are defined using the FDA definition above. The Orphan Products Grants
Program, which is administered by the OOPD, provides funding for clinical research that tests the safety and efficacy of drugs, biologics,
medical devices and medical foods in rare diseases or conditions.
It
is worth noting that there is a common pathway for application of orphan status for a product to both the FDA and EMA, and applicants
to the FDA are advised to use the common application platform. With regards to the data to be used in the application, it is expected
that applicants demonstrate that there is “promise” that the drug will be effective in treating said disease. “Promise”
is interpreted to include either data from clinical trials, data from case studies/reports, data from appropriate animal models or, on
rare occasions where there is no appropriate animal, data from in vitro experiments in addition to supporting information.
Regulatory
Incentives for Orphan Product Development

Summary
of Completed and Contemplated Clinical Development Plans
Phase
1 Clinical Trials (Studies 755-101-HV and 755-102-HV)
A regulatory application to
support our first Phase 1 clinical trial in healthy volunteers with INM-755 (755-101-HV) was submitted November 4, 2019 and approved December
6, 2019 in the Netherlands. The initial Phase 1 clinical trial evaluated the safety, tolerability, and pharmacokinetics of INM-755 cream
in 22 healthy volunteers with normal, intact skin; the volunteers had cream applied once daily for a period of 14 days. All subjects in
this first clinical trial completed treatment and evaluations by March 27, 2020. Database completion and data analyses were delayed by
pandemic restrictions. Study results were reported November 25, 2020. A blinded interim safety review from the first 16 subjects in this
Phase 1 clinical trial were included in a regulatory application that was approved April 17, 2020, for a second Phase 1 clinical trial
of 8 healthy volunteers to test the local safety and tolerability of applying sterile INM-755 cream to small wounds once daily for 14
days. As with the initial Phase 1 trial, the second clinical trial (755-102-HV) was conducted with two different drug concentrations and
a vehicle control. Enrollment began in early July 2020 and the clinical trial completed treatment and evaluations at the end of September
2020. Study results were reported January 8, 2021.
Phase
2 Clinical Trial (Study 755-201-EB)
Regulatory
applications to support this global trial were filed for review by the National Competent Authorities and Ethics Committees in 8 countries
for 13 clinical sites. Approvals were obtained in all countries (Austria, France, Germany, Greece, Israel, Italy, Serbia, and Spain)
as of March 2022. Enrollment and patient treatment began in December 2021 and completed in April 2023.
The goal of the Phase 2 study was to obtain safety and preliminary
efficacy of INM-755 cream in treating symptoms and wound healing in patients with EB, using a within-patient design in which matched index
areas were randomized to INM-755 cream or vehicle (no drug) cream in a blinded manner. A target of up to 20 patients were to be enrolled
with treatment for 28 days, the longest period supported by nonclinical toxicology studies.
No
single primary endpoint was set for the trial to allow for possible variations in presenting symptoms in each patient. These include
the presence of open wounds, wound pain associated with dressing changes, background wound pain, wound itch, and itch in non-wound areas.
To this end, InMed’s goal was to harvest data from the trial to evaluate the ability of INM-755 to treat chronic non-wound itch
and to heal wounds and treat associated pain and itch.
The
Phase 2 Trial enrolled a total of 19 patients. Data from one patient were excluded from efficacy analyses due to a significant protocol
deviation. Of the 18 remaining patients whose data were considered reliable for clinical review, 17 were treated for chronic non-wound
itch and one patient was treated for wound-related itch. The remaining endpoints (pain, wound healing) could not be analyzed due to too
few enrollees with such symptoms.
Of
the 18 participants assessed, chronic itch improved by a clinically meaningful amount in 12 patients (66.7%), of whom:
| ● | 6
patients (33.3%) had the same level of itch improvement with INM-755 cream as with control cream; |
| ● | 5
patients (27.8%) treated with INM-755 showed meaningful anti-itch activity beyond that of the control cream; and |
| ● | 1
patient (5.6%) showed better itch reduction with the control cream. |
In
summary, results from the Phase 2 clinical trial showed a positive indication of enhanced anti-itch activity for INM-755 cannabinol cream
versus the control cream alone in an exploratory clinical evaluation. The results for non-wound itch were not statistically significant
in this small trial due, in part, to the clinically important anti-itch effect of the underlying control cream. We are, nevertheless,
encouraged by and satisfied with the INM-755 clinical data for non-wound itch treatment. That the majority of the assessed patients in
the trial showed clinically meaningful improvement in non-wound itch from the application of INM-755, be it with similar outcomes to
the control cream or better than the control cream, can be considered impressive.
There are a number of challenges
associated with conducting an international multi-site clinical trial in an orphan disease, the largest being patient recruitment. For
INM-755, the difficulties in patient enrollment were due to the low prevalence of EB (there are estimated to be only 50K people worldwide
with variations of this particular genetic condition) and further complicated by COVID-related disruptions during the enrollment period.
The protocol-specified statistical
analyses for non-wound itch were not statistically significant in favor of INM-755 due in part to the clinically important anti-itch effect
of the underlying control cream. Nevertheless, that the majority of the assessed patients in the trial showed clinically meaningful improvement
in non-wound itch from the application of INM-755, be it with similar outcomes to the control cream or better than the control cream,
can be considered impressive. Notwithstanding the fact that statistical significance allows researchers to hold a degree of confidence
that their findings are reliable, being not statistically significant but clinically important is a combination that typically occurs
in a study that does not have a large enough sample size to detect a difference between groups such as is the case in this INM-755 trial.
Under such circumstances, statistically significant differences between groups might fail to be detected.
The
Phase 2 Clinical trial also demonstrated that CBN is safe and well-tolerated. As expected based on a Phase 1 safety study (755-101-HV)
undertaken by the Company, systemic exposure of CBN was measured at very low concentrations (picograms/mL in plasma). There were no serious
drug-related adverse events (“AEs”) and there were no withdrawals from treatment. Moderate headaches in one study participant
were the only systemic AEs deemed ‘possibly related’ to study drug. Very few local AEs were reported in the treatment areas;
they were transient and resolved without cessation of treatment. The Phase 2 Trial indicated that INM-755 CBN cream was very well tolerated
on sensitive EB skin.
Based
on the safety and efficacy data for treating non-wound itch in this EB study, as well as previous safety data from Phase 1 trials, we
are now seeking R&D and commercial partnership opportunities for any continued development of INM-755 CBN cream. Continued development
of INM-755 CBN cream will likely move beyond EB into broader indications involving chronic itch, with potentially much larger target
populations and commercial opportunities than offered solely by the EB indication.
On
average, it takes at least ten years to complete the development of an investigational drug from its initial discovery to the marketplace,
with clinical trials alone taking six to seven years on average. It is not possible with any degree of certainty to estimate how long
it will take to complete clinical trials and potentially obtain marketing approval for INM-755. To the extent that INM-755 may potentially
be designated as either a Fast Track drug, a Breakthrough Therapy, or eligible for Priority/Accelerated Review, our timeline to any potential
marketing approval may be shorter than might otherwise be the case.
Key Milestones for the EB Program:
| |
● |
April 30, 2020 – We announced clinical trial application approval in the Netherlands for Study 755-102-HV, a randomized, double-blind, vehicle-controlled Phase 1 study designed to evaluate the safety and tolerability of INM-755 (two strengths) applied daily for 14 days on epidermal wounds in 8 healthy volunteers. |
| |
● |
November 25, 2020 – We announced the top-line results of Study 755-101-HV (“Study 101”). Study 101 was a randomized, vehicle-controlled, double-blind, Phase 1 trial, that examined the safety and tolerability of two strengths of INM-755 cream on intact skin in 22 healthy adult volunteers over a 14-day treatment period. The Study 101 results indicate that INM-755 was safe and well-tolerated on intact skin, caused no systemic or serious adverse effects. In addition, there were no subject withdrawals due to adverse events. Drug concentrations in the blood were very low, as expected. |
| |
● |
January 8, 2021 – We announced the top-line results of Study 755-102-HV (“Study 102”). Study 102 was a randomized, double-blind, vehicle controlled, single-center study, in 8 healthy adult volunteers to test the tolerability of 14 days of application of the INM-755 cream on epidermal wounds under treatment procedures designed to simulate wound care for Epidermolysis Bullosa (“EB”) patients with open wounds. Results of Study 102 indicate that INM-755 cream was safe and well-tolerated on induced open epidermal wounds, caused no systemic or serious adverse effects. In addition, there were no subject withdrawals due to adverse events. These data from Study 101 and Study 102 support moving forward into clinical trials in patients with EB. |
| |
● |
April 28, 2021 – We announced that we filed Clinical Trial Applications (“CTAs”) in Austria, Israel and Serbia as part of a Phase 2 clinical trial of INM-755 (cannabinol) cream in Epidermolysis Bullosa (“EB”). Additional CTAs for 755-201-EB (the ‘201 study) will be submitted to National Competent Authorities (“NCAs”) and Ethics Committees (“ECs”) in France, Germany, Greece, and Italy in the coming weeks. |
| |
● |
On September 30, 2021 - We announced commencement of a Phase 2 clinical
trial, the 755-201-EB study, of INM-755 (cannabinol) cream in the treatment of EB, marking the first time cannabinol has advanced to a
Phase 2 clinical trial to be studied as a therapeutic option to treat a disease. The 755-201-EB study is designed to enroll up to 20 patients.
InMed will evaluate the safety of INM-755 (cannabinol) cream and its preliminary efficacy in treating symptoms and wound healing over
a 28-day treatment period. All four subtypes of inherited EB; EB Simplex, Dystrophic EB, Junctional EB, and Kindler Syndrome are eligible
for this study. |
| |
|
|
| |
● |
On July 25, 2022 – We announced, based on the safety data of the first five adult patients who completed treatment with INM-755 CBN cream for the treatment of symptoms in the Phase 2 clinical trial, an independent Data Monitoring Committee agreed it was safe to allow the enrollment of adolescent patients, defined as persons aged twelve to seventeen. |
| |
● |
On March 28, 2023 - We announced we had concluded enrollment of our Phase 2 clinical trial using investigational drug INM-755 cannabinol (“CBN”) cream for the treatment of patients with EB. The Phase 2 study enrolled 19 of its targeted 20 patients. |
| |
● |
On June 22, 2023 – We announced safety and efficacy results from the Phase 2 clinical trial (755-201-EB) for the treatment of symptoms in patients with EB. |
Additional Indications for INM-755
Once a company has gone to the significant investments of bringing
a new chemical entity into human clinical trials, the traditional approach is to investigate as many therapeutic uses of that product
in different indications, or specific diseases. We intend to pursue this strategy with a co-development partner as a way to leverage our
knowledge of CBN and investment in the development of INM-755 as a topical skin cream. Under the assumption that we would use the same
formulation for other dermatological indications, there should be no need for further Phase 1 safety studies allowing us to proceed directly
to Phase 2 safety and preliminary efficacy studies in humans, since the toxicology and initial human safety studies have been completed;
however, the adequacy of the nonclinical and human safety data to support new dermatologic indications will be determined by the appropriate
health authority.
INM-088 for the Treatment of Glaucoma
Introduction
Glaucoma is a chronic optic neuropathy that is typically characterized
by high intraocular pressure (“IOP”). The cause of glaucoma is understood to be inadequate or obstructed drainage of the fluid
in the eye, or “aqueous humor”, through a drainage membrane called the trabecular meshwork (“TM”), increasing
the fluid pressure within the front part of the eye, or “anterior chamber”, and subsequently leading to pressure at the back
part of the eye, or “posterior chamber”. The increased intraocular pressure exacts a toll on the nerve cells, called neurons,
located at the back of the eye in the retina, thinning the mesh-like tissue in this region and resulting in damage to the neurons and
specifically to the optic nerve, which provides the impulses of sight to the brain. This damage leads to blindness. Glaucoma is currently
the second leading cause of blindness world-wide and is estimated to affect a population of about 76 million worldwide.
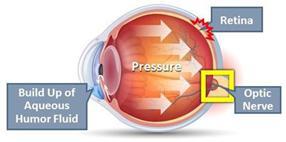
Current glaucoma therapies
generally act to lower intraocular pressure either by reducing the aqueous humor production by the cells around the eye, or the “ciliary
epithelial cells”, or by increasing fluid drainage through the TM. Nevertheless, we believe that there is considerable room for
improvement of existing drugs, most of which are formulated as eye drops, in terms of increasing the amount of drug that can be safely
delivered to increase its effect, improving the delivery of the drug into the eye, and reducing the common effect in currently used therapies
that, over time, their efficacy diminishes as the body becomes tolerant to these classes of drugs. Studies have shown that when drugs
are delivered as eye drops, less than 5% of the dose penetrates into the eye, indicating that 95% of the administered drug never reaches
its desired target as it is wiped away upon blinking. Thus, there is much room for improvement on the drug delivery as a means of increasing
clinical efficacy.
CBN is the key API in
our second drug candidate, INM-088, which is in preclinical studies as a potential treatment for glaucoma. We conducted studies to
test the ability of CBN to provide protection to the neurons at the back of the eye, referred to as “neuroprotection”,
and reduce the intraocular pressure in the eye. We compared several cannabinoids, including CBD and THC, to determine which
cannabinoid was the best drug candidate for the treatment of glaucoma. Of all of the cannabinoids we examined, CBN demonstrated the
most optimal effect of neuroprotection. Furthermore, CBN also exhibited intraocular pressure reduction capability.
Science behind Glaucoma
Glaucoma is a group of eye
diseases which results in degeneration of neurons, damage to the optic nerve and vision loss. The most common type is open-angle glaucoma,
or “OAG”, with less common types including closed-angle glaucoma, or “CAG”, and normal-tension (i.e., no increase
in intraocular pressure) glaucoma. OAG develops slowly over time and the patients normally don’t experience pain. If left untreated,
side vision may begin to decrease followed by central vision, resulting in blindness. CAG can present gradually or suddenly. The sudden
presentation may involve severe eye pain, blurred vision, mid-dilated pupil, redness of the eye and nausea. Vision loss from glaucoma,
once it has occurred, is permanent.
Risk factors for glaucoma
include increased pressure in the eye, the thinness of the cornea, a family history of the condition, age over 40 years in African Americans,
and age over 60 years for other ethnic groups (especially Mexican Americans). High intraocular pressure (those with a value of greater
than 21 mmHg or 2.8 kPa) is often associated with a greater risk of glaucoma. However, some people may have high eye pressure for years
and never develop damage. Conversely, neurodegeneration and optic nerve damage may occur with normal pressure, known as normal-tension
glaucoma. The mechanism of OAG is believed to be slow exit of aqueous humor through the trabecular meshwork while in CAG the iris blocks
the TM. Diagnosis is typically made by a dilated eye examination.
If treated early, it is possible
to slow or stop the progression of the disease with medication, laser treatment, or surgery. Currently, the goal of these treatments is
to decrease eye pressure. A number of different classes of glaucoma medication are available. Laser treatments may be effective in both
OAG and CAG. Several of types of glaucoma surgeries may be used in people who do not respond sufficiently to other measures. Treatment
of CAG is a medical emergency.
Epidemiology
The global prevalence of glaucoma
for population aged 40–80 years is 3.54%, of which 75% is OAG. As of 2010, there were 44.7 million people in the world with OAG
of which 2.8 million were in the United States. By 2020, the prevalence is projected to increase to 80 million worldwide and 3.4 million
the United States. It occurs more commonly among older people. CAG is more common in women. Both internationally and in the United States,
glaucoma is the second-leading cause of blindness.
Current Treatments in Glaucoma
Current treatments for glaucoma
include medication, laser treatment and surgery. The goals of glaucoma management are to avoid glaucomatous damage, nerve damage and preserve
visual field and total quality of life for patients, with minimal side effects. This requires appropriate diagnostic techniques and follow-up
examinations, and judicious selection of treatments for the individual patient. Although intraocular pressure is only one of the major
risk factors for glaucoma, lowering it via various pharmaceuticals and/or surgical techniques is currently the mainstay of glaucoma treatment.
Treatment Considerations based on Glaucoma Severity
Treatment considerations for
glaucoma span the therapeutic spectrum from drug intervention to surgery.

Pharmaceutical drug intervention
has the greatest potential to generate direct competition for INM-088.
Medicines for Glaucoma Treatment (Intraocular Pressure-Lowering
Drugs)
Current prescription eyedrop
medications targeting intraocular pressure reduction include:
| |
● |
Prostaglandins and prostaglandin analogs (“PGA”) such as latanoprost, bimatoprost and travoprost to increase the outflow of fluid from the eye and, thereby, reduce ocular pressure. The adverse effects of PGAs include conjunctival hyperemia, or eye redness, irreversible change in iris color, discoloration of the skin around the eyes, and droopiness of eyelids caused by the loss of orbital fat; |
| |
● |
Beta blockers, most commonly prescribed as drugs to treat hypertension, are also prescribed for glaucoma. With their MOA designed to inhibit aqueous production, they are one of the oldest approved drug classes for the reduction of IOP. The most commonly used drug in this class is timolol. Beta blockers are less effective than PGAs in terms of IOP reduction and are typically used twice daily. Beta blockers are the most commonly used non-PGA drug. They are used as an initially prescribed monotherapy and as an adjunctive therapy to PGAs when the efficacy of PGAs is insufficient. Beta blocker eye drops have contraindications in their label as a result of potential systemic exposures from the topical application of the eye drops, potentially leading to cardio-pulmonary events such as bronchospasm, arrhythmia and heart failure. Other possible side effects include wheezing or difficulty breathing, slowed heart rate, lower blood pressure, impotence and fatigue; |
| |
● |
Alpha agonists, with their MOA designed to inhibit aqueous production plus their effect on uveoscleral outflow, are less effective than PGAs and need to be dosed three times daily in order to obtain the desired IOP reduction. In clinical studies, the most frequently reported adverse reactions that occurred in individuals receiving brimonidine ophthalmic solution, a commonly prescribed alpha agonist, included allergic conjunctivitis, conjunctival hyperemia, eye pruritus, burning sensation, conjunctival folliculosis, hypertension, ocular allergic reaction, oral dryness and visual disturbance. |
| |
● |
Alpha-adrenergic agonists such as apraclonidine and brimonidine, both reduce the production of aqueous humor and increase the outflow of fluid from the eye. Side effects may include dry mouth, red eyes or eyelids, fatigue, low or high blood pressure, blurred vision and light sensitivity; and |
| |
● |
Carbonic anhydrase inhibitors such as dorzolamide and brinzolamide reduce the production of fluid in the eye, but they are associated with blurred vision, bitter metallic taste in the mouth, dry eyes, red/irritated eyes, headache, and upset stomach. |
Despite their modest efficacy,
safety and tolerability profiles, the requirement for two to three doses per day, and the fact that they do not target the diseased tissue
in glaucoma, beta blocker, carbonic anhydrase inhibitor and alpha agonist products account for a significant portion of the total prescription
volume for the treatment of glaucoma based on historical prescription patterns, with beta blocker timolol being the most widely prescribed
non-PGA drug. This is driven by the PGA products not being sufficiently effective as monotherapy for up to half of all glaucoma patients.
Often patients need to take a combination of different drugs and multiple eye drops throughout the day.
Fixed-dose combination glaucoma
products are also currently marketed in the United States, including Cosopt®, the combination of a beta blocker with a
carbonic anhydrase inhibitor, and Combigan®, the combination of a beta blocker with an alpha agonist. There are no fixed-dose
combinations of PGAs with other glaucoma drugs currently available in the United States.
Given side effect profiles,
many patients do not take their medications properly. Surgery and laser therapies are intended to physically improve the drainage of fluid
from the eyes and lowering of the intraocular pressure. Patients with OAG can have clogged channels in the TM opened with laser therapy,
filtering surgery (trabeculectomy) or electrocautery. In other cases, small drainage tubes may be implanted in the eye. Possible complications
include pain, redness, infection, inflammation, bleeding, abnormally high or low eye pressure and loss of vision. Some types of eye surgery
may accelerate the development of cataracts. Additional procedures may be needed if eye pressure continues to increase.
Emerging Competition for INM-088 in Glaucoma
Due to the large medical need
and potentially significant commercial opportunity, the competitive landscape of glaucoma is intense. As such, there are currently over
10 medications approved by the FDA for the treatment of glaucoma, which are summarized in the table below, according to drug class. In
addition to the currently approved medications, there are a multitude of other therapies being evaluated in clinical trials, and many
others at the preclinical stage. Finally, it should be noted that there are several laser surgeries, and other forms of surgical procedures
that are currently being performed to treat glaucoma, which also serve as a source of competition to the therapeutic alternatives.
In December 2017, the FDA approved RHOPRESSA® as the
first in a new class of glaucoma treatments known as Rho Kinase inhibitors. RHOPRESSA® is indicated for the reduction of
elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
INM-088 is envisioned as a once- or twice-a-day
eye drop medication to compete with treatment modalities in the medicines category if approved for commercialization.
In addition to INM-088, we
are aware of only one other pharmaceutical-grade cannabinoid-based therapy being evaluated for the treatment of glaucoma. Specifically,
Skye Biosciences Inc. (“Skye”, formerly Emerald Biosciences) is developing NB1111 (THC-Val-HS), later renamed SBI-100, for
the treatment of glaucoma. SBI-100 is a THC prodrug, which has demonstrated intraocular pressure-lowering efficacy in preclinical models.
On July 13, 2023, Skye announced positive safety review for the final cohort of their Phase 1 study of SBI-100 ophthalmic emulsion.
Investigational Glaucoma Treatments
Despite the treatments available
for lowering the intraocular pressure, there are some individuals for whom these treatments are either not tolerated due to side effects
or in whom the intraocular pressure is not sufficiently lowered. In these situations, both glaucoma patient and physician look for alternative
therapies.
While some experimental glaucoma
medications explore new ways of controlling intraocular pressure, other treatments are directed at protecting the optic nerve (neuroprotection)
to prevent eye damage, potential vision loss or even blindness. Many ongoing clinical studies are trying to find neuroprotective agents
that might benefit the optic nerve and certain retinal cells in glaucoma.
Some investigational treatments
are undergoing FDA clinical trials to prove safety and effectiveness. Other potential glaucoma treatments are strictly in experimental
stages and may be years away from the possibility of being available on the marketplace.
Key Preclinical Results for CBN as a Drug Candidate to
Treat Glaucoma
INM-088 is an eye-drop
CBN formulation being developed for the treatment of glaucoma. The preclinical development program for INM-088 has included a number of
studies comparing a number of cannabinoids, including CBN, THC and CBD, among others, to determine which cannabinoid holds the greatest
potential to treat glaucoma. This preclinical research to date is comprised of both in vitro and in vivo studies and led
to the selection of CBN as the lead drug candidate for further development.
The scope of the in vitro
studies to date include the following:
| 1) |
Evaluation of the neuroprotective effects of selected cannabinoids on the differentiated retinal ganglion cells, or “RGCs”, a thin layer of neurons responsible for relaying visual signals in the eye, under normal atmosphere pressure and elevated pressure conditions. |
Notably, exposure
of RGCs to increasing concentrations of several cannabinoids, including THC and CBD resulted in dose dependent cytotoxicity, or cell death,
over time. Importantly, CBN-exposed RGCs demonstrated the lowest level of toxicity among the cannabinoids used in these experiments.
Cytotoxicity and neuroprotective effects of
selected cannabinoids
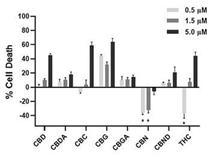
661W cells were
treated with selected cannabinoids (0.5, 1.5 and 5 µM) at normal atmospheric pressure for 72 hrs. Data normalized to vehicle under
normal pressure (NP-VC) was considered as 0% cell death. Note, CBN (0.5-5 µM) treatment under normal pressure did not result in
cytotoxicity. Other cannabinoids, including CBD, CBDA, CBC, CBG, CBGA, and CBND, displayed significant toxicity. Enhanced cell proliferation
in the presence of a low concentration of ∆9-THC (0.5 µM) was observed in contrast to increased cell death at higher concentrations
(1.5 and 5 µM). Experiments were performed in triplicates, two independent times. The data is presented as mean ± SEM. *p
< 0.05 against NP-VC (one-way ANOVA).
In addition, exposure
of the RGCs to elevated pressure in a cell-based model for glaucoma (without exposure to cannabinoids) for 72 hours resulted in high level
of cytotoxicity, whereas exposure of these cells to both an elevated pressure (20-40 mmHg) plus CBN, within the same time-period, resulted
in cell survival in a dose dependent fashion. A neuroprotective effect of CBN was also observed under elevated pressure conditions in
the pressurized chamber that is designed to mimic the clinical situation of increased intraocular pressure in glaucoma. CBN performed
better than both CBD and THC in this preclinical model under identical testing conditions.
Comparison of CBN versus THC-mediated inhibition
of elevated pressure-induced cell death
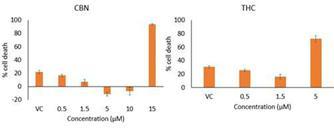
Significant neuroprotective
effect of CBN on differentiated 661W cells when cultured for 72 hours under a hydrostatic pressure of approximately 20 to 25 mmHg as compared
to ∆9-THC. Cell were treated with CBN at 0.5, 1.5, 5, 10 and 15 µM and ∆9-THC at 0.5, 1.5 and 5 µM respectively.
Vehicle Control (VC) contained 0.15% ethanol. Data presented as Cell Death (%) vs normal pressure Vehicle Control (taken as 0% cell death).
The data is presented as mean ± SEM.
CBN mediated inhibition of elevated pressure-induced
cell death
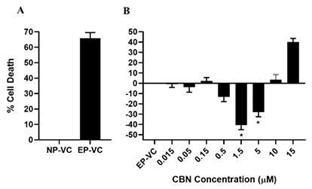
CBN increases cell
survival under elevated pressure. 661W cells were treated with CBN in a dose-dependent manner at increased atmospheric pressure (40 mmHg)
for 72 hours. A) Significant cytotoxicity was observed in untreated cells under elevated pressure in the pressure chamber (EP-VC) compared
to normal pressure vehicle control (NP-VC). Data normalized to normal pressure (NP-VC) was considered as 0% cell death. B) CBN treatment
under elevated pressure protected 661W cells from cell death at 0.5, 1.5 and 5 µM. Data normalized to elevated pressure vehicle
control (EP-VC) that was considered as 0% cell death. Protective effect was significantly different from pressure chamber vehicle control
(EP-VC) at 1.5 and 5 µM (p<0.0001 and p<0.001). CBN treatment of 661W cells at 15 µM under elevated pressure results
in cell death. Data presented as mean ± SEM of duplicate independent runs (n=6). Data analyzed statistically by one-way ANOVA.
| 2) |
Evaluation of anti-apoptotic effects of CBN on the differentiated RGCs when exposed to elevated pressure conditions. |
Using the same in vitro
model described above, we also looked at a specific, natural self-destruction process called programed cell death, or apoptosis. We verified
that CBN has an anti-apoptotic effect on differentiated RGCs when subjected to elevated hydrostatic pressure. Exposure of these cells
to high-pressure levels in the pressure chamber apparatus, without exposure to cannabinoids, for 6 hours resulted in an induction of apoptosis
ranging from 30-60% (n=3). Exposure of these cells under the same conditions concurrently with CBN prevented apoptosis and resulted in
a higher level of cell survival.
CBN mediated attenuation of elevated pressure-induced
apoptosis
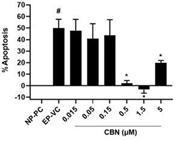
CBN mediated attenuation
of elevated pressure-induced apoptosis. 661W cells were treated in the presence of the indicated concentrations of CBN at increased atmospheric
pressure (~20–25 mm Hg) for 6 h. Significant apoptosis was observed under elevated pressure in the pressure chamber (EP-VC) when
compared to NP-VC. CBN at higher concentrations (0.5, 1.5 and 5 μM) but not at lower concentrations (0.015–0.15 μM) significantly
inhibited pressure-induced apoptosis of 661W cells. Experiments were performed in triplicates, two independent times. The data is presented
as mean ± SEM. #p < 0.05 against NP-VC analyzed by using an unpaired t-test; *p< 0.05 against EP-VC analyzed statistically
by one-way ANOVA.
| 3) |
Evaluation of CBN impact on the expression of specific extracellular matrix (ECM) markers on primary human trabecular meshwork (TM) cells under basal condition and following stress-induction with Transforming Growth Factor Beta 2 (TGF-ß2), a cytokine used to alter extracellular matrix metabolism. |
A key risk factor
for the development and progression of glaucoma is elevated IOP, the result of increased resistance to aqueous humor outflow through the
TM. Increased outflow resistance has been strongly correlated with aberrantly elevated levels of TGF-ß2, a cytokine used to alter
extracellular matrix metabolism of the TM of glaucoma patients compared to healthy individual. Therefore, evaluation of CBN effects on
the TM observed under elevated TGF-ß2conditions mimics the clinical presentation of IOP in glaucoma and is relevant in the clinical
context of the disease. Using human primary TM cells derived from various donors and propagated in vitro at different cell passages,
we were able to demonstrate that several extra-cellular matrix proteins, or “ECM” markers, were upregulated by TGF-ß2
induced condition. Furthermore, CBN treated TM cells under basal condition or TGF-ß2 induced conditions for a duration of 72 hours
resulted in reduction in the expression of several of these ECM protein markers.
CBN attenuated TGF-β2 induced changes on
TM markers Fibronectin (FN)
and Collagen 1A (COL1A) in hTM cells
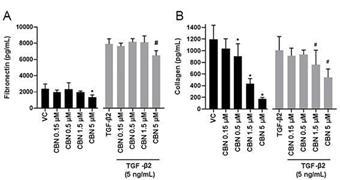
CBN attenuated TGF-β2
induced changes in TM markers Fibronectin (FN) and Collagen 1A (COL1A) in hTM cells. hTM cells were treated with CBN (0.15, 0.5, 1.5 and
5 μM) for 72 h with or without TGF-β2 (5 ng/mL). Post-treatment, cell lysates were collected, total protein was quantified by
Bradford assay, and ELISA was performed according to the manufacturer’s instructions. (A) TGF-β2 induced significant upregulation
of FN expression. Note, a significant reduction in basal or TGF-β2 induced FN levels was observed in the presence of CBN (5 μM).
(B) TGF-β2 did not induce upregulation of COL1A level in hTM cells, whereas CBN in a dose dependent manner (0.5–5 μM) attenuated
COL1A levels in the presence or absence of TGF-β2. Experiments were performed in triplicates, two independent times. The data is
presented as mean ± SEM. Changes in the treatment groups were compared to the vehicle control (VC) group (*) or TGF-β2 induced
group (#), and data were analyzed by using one-way ANOVA (p < 0.05).
Role of CBN in the inhibition of TGF-β2
induced changes in expression of α-SMA and pERK1/2

Figure 6: Role of CBN
in the inhibition of TGF-β2 induced changes in expression of α-SMA and pERK1/2.
Representative immunoblots
showing CBN mediated changes in expression of α-SMA and phospho-ERK1/2 in the presence or absence of TGF-β2. TGF-β2 stimulated
α-SMA expression and increased the level of ERK1/2 phosphorylation when compared to vehicle control. Note the inhibition of α-SMA
expression and ERK1/2 phosphorylation in the presence of CBN in comparison to vehicle control and/or TGF-β2 treatment. GAPDH was
used as the loading control. Histograms in the lower panel represent densitometry analysis of the Western blots (n=3–4). Changes
in the treatment groups were compared to the vehicle control (VC) group (*) or TGF-β2 group (#), and data were analyzed statistically
by unpaired t-test (p < 0.05).
| 4) |
Evaluation of CBN pharmacokinetic profile in the eye and plasma of a preclinical model (rat) by direct intravitreal (IVT) injection into the eye. |
Our first in vivo study
was designed to determine the pharmacokinetic profile of CBN in preclinical models, specifically measuring CBN levels in the eye and plasma
following direct bilateral IVT injection. This means that individual injections were made directly into the vitreous humor (fluid of the
central cavity of the eye). Following IVT delivery, CBN levels from the plasma (n=3 per time point) and the whole eye (n=6 per time point)
were measured at several timepoints using a qualified LC-MS/MS method. CBN levels in the plasma samples were below the detection limit
of the assay (0.05 ng/mL). Furthermore, CBN levels in the rat whole eye were shown to be slowly cleared from the eye with a projected
half-life (t1⁄2) of approximately 33 hrs.
Percentage of CBN remaining in the whole eye
following single IVT injection at 10 µM initial dose
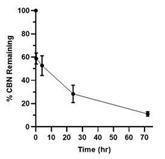
Percentage of CBN remaining
in the whole eye following single IVT injection at 10 μM initial dose. Each point represents the mean of left and right eye measurements.
The concentration-over-time elimination profile of CBN from IVT injection in the whole eye indicates a slow clearance rate of this cannabinoid,
with approximately 11% of the total CBN remaining at 72 h termination time-point (n = 6 eyes per time-point; values presented as mean±SEM).
Time dependent elimination of CBN from the whole
eye following
single IVT injection at 10 μM initial dose

Concentration-over-time elimination
profile of CBN from IVT injection in the whole eye indicates a slow clearance rate of this cannabinoid, with approximately 11% of the
total CBN remaining at 72 hrs termination time-point. Rats received a single bilateral IVT injection (5 μL) of CBN (50 μM) to target
a final concentration of 10 μM inside the eye. Values measured as ng/g ocular tissue or μM and presented as mean ± SEM. n
= 6 eyes per time-point. *596 ng/g represents an extrapolated initial dose intended for delivery inside the eye calculated based on the
actual whole eye tissue weight.
Pharmacokinetic parameters of CBN in the whole
eye following
single IVT injection at 10 μM initial dose
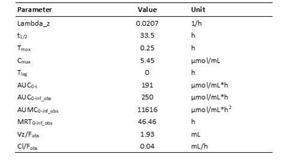
CBN was quantified by a qualified
LC-MS/MS method in the whole eye homogenates. The analysis of pharmacokinetic parameters was performed using Pharmacokinetic Solver 2.0
Software. To produce an output, a Non-Compartmental Analysis using the linear trapezoid rule after extravascular input was performed.
CBN was cleared at a slow rate from the ocular tissue with CLivt = ~0.04 mL/h, favorable AUC0-inf ocular exposure at 250 μmol/mL *
h, and relatively long ocular t1/2 =~33 h.
| 5) |
Evaluation of CBN neuroprotective and IOP-lowering effects in a rat preclinical glaucoma model (Rats) by IVT injection. |
We conducted a preclinical
efficacy study to evaluate neuroprotective and IOP lowering effects of CBN following IVT injection in a rat episcleral vein laser photocoagulation
model of glaucoma. The laser photocoagulation of episcleral veins was done unilaterally on the oculus dexter (OD) eye of the animals on
day 0 and day 7. The contralateral oculus sinister (OS) eye served as control and was not subjected to lasering. The rats were randomized
into 4 treatment groups based on their baseline pERG amplitudes measured at day - 4: Group 1: vehicle control (0.5% DMSO-PBS);
n=11. Group 2: CBN low dose at 5 µM final concentration inside the eye; n=13. Group 3: CBN high dose at 50 µM
final concentration inside the eye; n=11. Group 4: brimonidine tartrate (Alphagan®, 0.5% ophthalmic eye drops, a
registered trademark of Allergan PLC) a selective α2-adrenoceptor agonist clinical reference drug; n=14. Animals in Groups 1, 2
and 3 were administered either vehicle or CBN by IVT injection at 5 µl volume on day 0, 7 and 15. Animals in Group 4 were topically
instilled with brimonidine twice daily at 5 µl volume per eye 4 days before laser photocoagulation and throughout the study duration.
The study was terminated on day 21. High IOP was induced unilaterally by laser photocoagulation of episcleral veins (to approximately
19 mmHg). CBN was delivered by IVT injection after episcleral laser photocoagulation on three occasions on day 0, immediately after the
lasering, and on days 7 and 15 post-lasering. IOP and pERG were monitored at specific time points throughout the study. Significant reduction
in IOP (to approximately 13 mmHg) was observed for the CBN high dose treated group on Days 7 and 17 and significant improvement of pERG
amplitudes for CBN low dose treated group was observed on Day 21 (-39.8% from baseline for vehicle control group, -23.1% from baseline
for the active control (brimonidine tartrate) group, -16.6% from baseline for the CBN low dose group and -41.2% from baseline for the
CBN high dose group). These were the measured outcomes that are useful in evaluating candidates for a potential glaucoma treatment. In
summary, data from this study demonstrated a reduction of IOP and improvement of pERG function following IVT injection of CBN in the Rat
episcleral vein laser photocoagulation model of glaucoma.
CBN mediated effect on IOP in the rat episcleral
vein laser photocoagulation model of glaucoma

CBN mediated effect on IOP
in the rat episcleral vein laser photocoagulation model of glaucoma. The IOP lowering effect of CBN was analyzed in the lasered eyes of
different treatment groups versus the vehicle control group. At a low dose (5 μM), CBN treatment did not reduce IOP. At a high dose
(50 μM), CBN significantly reduced IOP on days 7 and 17. On day 21, IOP values in all groups normalized to the baseline levels. Data
analyses are presented for the time points with a statistically significant difference. Brimonidine (Alphagan®) was used
as a reference control. Data were analyzed statistically by unpaired t-test and presented as mean ± SEM. *p < 0.05 against vehicle
control (n =11–14 rats per treatment group).
CBN mediated changes in the pattern electroretinogram
(pERG) in the rat episcleral vein laser
photocoagulation model of glaucoma
A.

B.
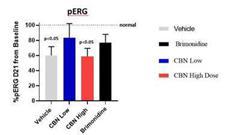
The pERG amplitudes (µV)
decreased in all treatment groups after the IOP elevation induced by episcleral vein laser photocoagulation. (A) pERG baseline-corrected
-Amplitude values (μV) (mean ± SEM) of animals in each treatment group were recorded at baseline before lasering and on days
7, 14 and 21 post-lasering. (B) %pERG reduction from 100% normal baseline values at Day 21. Significant RGC functional impairment was
observed in the vehicle group on day 21 and in the CBN high dose (50 µM) group on days 14 and 21 when compared to baseline. The
pERG amplitudes in the low dose CBN (5 μM) and brimonidine (Alphagan®) groups did not differ significantly from the
baseline on both follow-up days 14 and 21, indicating that CBN confers neuroprotective effect on RGCs. Data is presented as mean ±
SEM of baseline-corrected values and analyzed statistically by two-way ANOVA followed by Tukey’s multiple comparison test (*p <
0.05 against the baseline values; n=11-14 rats per treatment group).
Ocular Formulation Development for INM-088
There are a wide variety of
topically effective anti-glaucoma drugs that are available today and others in the developmental stage that represent significant advancements
for ocular therapeutics. Ophthalmologists typically prescribe drugs individually and then switch to different classes of drugs on a regular
basis in order to prevent the habituation phenomenon (reduction in effect of the drug over time) and negative side effects. There is an
opportunity for new therapies with low systemic toxicity and those which may not exhibit habituation.
Until very recently, studies
on novel topical ophthalmic formulations of cannabinoids have been largely non-existent. Designing an ideal delivery system for any ocular
disease depends on the molecular properties of the drug substance and incorporating it into the formulation while taking into consideration
parameters such as size, charge, and affinity towards various ocular tissues and pigments.
For all delivery technologies
under examination as candidates for INM-088, key design criteria include, among others:
| |
● |
Biocompatibility and biodegradability of the formulation; |
| |
● |
Viscous fluid behavior while inside the container (to facilitate ease of manufacturing, handling and dosing); |
| |
● |
Characterized and defined drug release, absorption and subsequent carrier degradation; |
| |
● |
Optimized particle size and surface charge to avoid irritation upon application to the eye and to facilitate ocular penetration; and |
| |
● |
Stable final drug product to ensure drug product quality storage over time. |
One of the delivery technologies
under development as a potential delivery vehicle for CBN in ocular disease is our proprietary, stimulus-responsive, nanoparticle-laden
hydrogel vehicle for spatiotemporal and dosage-controlled release of cannabinoids into the aqueous humor of the eye. This hydrogel is
envisioned to be packaged as a liquid and is intended for application as an eye drop. We investigated the compatibility and effectiveness
of our hydrogel formulation with CBN as compared to other third-party ocular drug delivery technologies such as EyeCRO’s MiDROPs®
microemulsion. We conducted an in vivo study that compared both the hydrogel and MiDROPs® formulated with CBN and
showed that a similar level of CBN was measured in the retina and retinal pigmented epithelium tissues following topical administration
of each formulation. In early December 2020, we selected a final delivery technology based on the extensive data collected from these
assessments that included solubility, drug delivery localization and sustained effect. This selection resulted in a licensing agreement
with EyeCRO LLC for its proprietary MiDROPs® technology. Through this agreement, InMed has secured an exclusive, global
commercial rights for the utilization of MiDROPs® for all cannabinoids, cannabinoid analogs and their variants. One key
benefit for our INM-088 program by working with EyeCRO is that their product development and testing with MiDROPs® is already
well advanced, having been previously reviewed by the US FDA during a pre-IND meeting.
Distribution of INM-088 formulation in Ocular
Tissue

Ocular pharmacokinetic study
of INM-088 following 5 days of bilateral ocular topical administration in New Zealand White Rabbits. INM-088 was formulated as 0.1% CBN-MiDROPS™
and dosed BID at 50 µl/dose for 5 days via bilateral topical ocular instillation in 3 naïve NZW rabbits. CBN level was measured
1 hour following the final dose administration on Day 5, Administration of INM-088 formulation resulted in measurable CBN concentrations
in each of the ocular tissues analyzed in this study. The study was performed independently by EyeCRO LLC.
Key Milestones:
| |
● |
December 20, 2021 – We announced that a peer-reviewed scientific article entitled “Cannabinol Modulates Neuroprotection and Intraocular Pressure: A Potential Multi-Target Therapeutic Intervention for Glaucoma”, has been published in Biochimica et Biophysical Acta (BBA - Molecular Basis of Disease), a leading international journal focused on biochemistry and molecular genetics of disease processes and models of human disease in the area of aging, cancer, metabolic-, neurological-, and immunological-based diseases. The peer-reviewed article highlights research evaluating the use of cannabinol, or CBN, as a potential treatment option for glaucoma. Several studies were conducted to evaluate the survival of retinal ganglion cells, modulation of intraocular pressure and its effects on extracellular matrix proteins using in vitro and in vivo glaucoma models. |
| |
● |
May 13, 2022 – We announced the Company recently completed a pre-Investigational New Drug (“pIND”) application discussion with the U.S. Food and Drug Administration (“FDA”) regarding manufacturing, preclinical studies and early clinical development plans for INM-088, a cannabinol (“CBN”) formulation in development for glaucoma. The Company has gained alignment with FDA on the design of the initial Phase 1-2 clinical trial to gather preliminary data on the safety and efficacy of INM-088 treatment. The FDA has provided guidance for the development program based on a summary of the available preclinical data, clinical safety data for CBN from the INM-755 program, study designs for additional IND-enabling preclinical studies, and Chemistry Manufacturing and Controls (“CMC”) information. |
Additional indications in ocular disease
Similar to the strategy being
pursued with INM-755, we intend to fully investigate the potential for CBN in INM-088 to treat a wide array of ocular diseases, in particular,
the potential for CBN to provide neuroprotection across several diseases where blindness is the ultimate outcome. We are currently pursuing
preclinical models to more closely study this effect and will leverage the toxicology and Phase 1 safety studies across these new indications,
if deemed applicable. In addition, we are actively working with our collaborators to test selected potential cannabinoid analogs, produced
by BayMedica, to demonstrate neuroprotection in other ocular indications such as Age-Related Macular Degeneration (AMD).
INM-900 series for the Treatment of Neurodegenerative diseases
Introduction
Our research demonstrating
the neuroprotective capabilities of cannabinoids in the eye led us to investigate how cannabinoids might play a role in protecting other
neurons in the human body, potentially, impacting different diseases. To this end, we initiated research on the neurons that are associated
with the brain and how rare cannabinoids and cannabinoid analogs could affect neurodegenerative diseases such as Alzheimer’s, Parkinson’s,
and Huntington’s.
Alzheimer’s disease (“AD”)
is a progressive neurodegenerative condition that predominantly afflicts the elderly, resulting in severe cognitive impairments. With
the expected increase in life expectancy, there is a projected surge in AD prevalence, estimating that as many as 13.8 million Americans
could be affected by the year 2050 (Diagnosis and Management of Dementia). This ailment is defined by the buildup of amyloid β (Aβ)
plaques and neurofibrillary tangles within the brain, making it a central focus of neurological research for many years.
Several published in
vitro and in vivo studies have been conducted to understand the effects of different cannabinoids in neuronal
disorders.
To date, InMed has advanced
discovery work for the potential use of proprietary cannabinoid analogs to improve neuronal function and provide neuroprotection for treating
neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease. Screening for
these indications has yielded interesting analog candidates and we will continue to conduct further research to find an appropriate compound
to support the preclinical development program.
Thus far, we have identified
two cannabinoid analogs (INM-900 series of compounds) demonstrating promising effects related to the treatment of neurodegenerative diseases
and we are currently conducting studies using in vivo models in neurodegenerative disease to select the most appropriate
candidate for clinical studies. Candidate selection and further efficacy readout is expected in Fall 2023.
Current treatments in Neurodegenerative Diseases
| Brand |
|
Company |
|
Mechanism of Action |
|
Status |
| Aducanumab (Aduhelm™) |
|
Biogen |
|
Anti-amyloid beta target both insoluble and soluble aggregates |
|
Approved June 2021 |
| Lecanemab (Leqembi™) |
|
Biogen/ Eisai |
|
Anti-amyloid beta, electively binds to large, soluble Aβ protofibrils |
|
Approved January 2023 |
| Gantenerumab |
|
Roche |
|
Anti-amyloid beta, target aggregated forms of AB including oligomers and plaques |
|
Phase 3 failed November 2022 |
| Donanemab |
|
Eli Lilly |
|
Anti-amyloid beta, target pyroglutamated AB in plaques |
|
Data Expected in 2023 |
| Semorinemab |
|
Genentech |
|
Anti-tau |
|
Phase 2 Failed |
| HMTM |
|
TauRx Therapeutics |
|
Anti-Tau, tau aggregation inhibitor |
|
Data expected 2023 |
Current medicines for Alzheimer’s treatments
Currently approved medications
for Alzheimer’s disease fall into two main categories. The first category comprises drugs designed to address symptoms related to memory
and cognitive function. While these medications cannot halt the damage that Alzheimer’s inflicts on brain cells, they can help alleviate
or stabilize symptoms for a limited duration by influencing specific chemicals responsible for transmitting messages between nerve cells
in the brain. Essentially, these medications aimed at preserving neurotransmitters. However, they do not replace the deteriorating ones
and thus do not impede the disease’s progression. Over the past three decades, only four drugs have received approval for Alzheimer’s
treatment, and while they can manage certain symptoms, they do not address the prevention or progression of the disease. These drugs,
known as cholinesterase inhibitors and glutamate regulators, primarily target cognitive symptoms.
In recent years, there has
been a growing emphasis on developing disease-modifying treatments that target the underlying biology of Alzheimer’s. One major focus
of these research and development endeavors has centered on addressing the accumulation of amyloid plaques and the removal of both these
plaques and tau proteins. This approach aligns with the long-standing amyloid hypothesis, which posits that Alzheimer’s is triggered by
the buildup of amyloid beta protein (Aβ) in the brain. This accumulation leads to neuronal toxicity within the central nervous system,
disrupting neuronal and synaptic function, ultimately culminating in neuronal degeneration and cell death.
Despite numerous challenges
and a history of limited success, the approach to Alzheimer’s disease treatment has seen recent developments. In the past two years, the
FDA has granted accelerated approvals to drugs developed by Biogen, namely aducanumab (approved in June 2021) and lecanemab (approved
in January 2023), both are biologics. These medications mark the first two disease-modifying therapeutic interventions for Alzheimer’s
disease.
Aducanumab is designed to
target specific forms of beta-amyloid that accumulate and form plaques in the brain, with the goal of removing them. On the other hand,
lecanemab works by inhibiting the formation of amyloid plaques within the brain. Although these drugs have shown promise in their ability
to remove plaques or reduce their production, their effectiveness in enhancing cognitive function is limited.
Role of Cannabinoids in Alzheimer’s disease:
Numerous studies have indicated
dysregulation of the Endocannabinoid System (ECS), which encompasses receptors, endocannabinoids, and synthesizing/metabolizing enzymes,
in various neurodegenerative conditions, notably Alzheimer’s disease. These investigations have unveiled the potential of cannabinoids,
both endogenous and synthetic, in mitigating the harmful effects of Alzheimer’s pathology. These cannabinoids have been suggested to diminish
amyloid beta (Aβ) toxicity, reduce tau hyper-phosphorylation, and suppress neuroinflammatory responses while curbing the production
of reactive oxygen species (ROS). As a result, they may enhance the survival of neurons in the aftermath of Aβ aggregation.
Cannabinoids exert their biological
effects through two primary membrane receptors, Cannabinoid receptors 1 and 2 (CB1 and CB2), which are widely distributed in the central
nervous system (CNS) and peripheral tissues. Activation of CB1 has demonstrated its ability to alleviate neurotoxicity in various Alzheimer’s
disease models. Conversely, CB2 agonism and increased expression have been associated with the removal of Aβ by macrophages.
The precise molecular mechanisms
responsible for safeguarding specific neuronal populations remain elusive. However, several observations support this concept: a) cannabinoids
possess a capacity to exert broad effects on multiple molecular targets, including critical brain structures and behaviour; b) cannabinoids
act not only through ECS receptors but also interact with other non-ECS receptors such as transient receptor potential vanilloid 1 (TRPV1),
peroxisome proliferator-activated receptors (PPARs), and transcription factors like Nuclear factor kappa B; and c) cannabinoids exhibit
anti-inflammatory properties, modulate neurotransmitter release, and limit oxidative stress, collectively contributing to the enhancement
of neuronal viability.
Alzheimer’s disease (AD) is
a progressive neurodegenerative condition primarily driven by the toxicity and disruption of proteostasis caused by misfolded Aβ
protein. Cannabinoids have emerged as promising agents capable of preserving neuronal integrity and functionality, offering a potential
strategy to slow down disease progression and enhance the quality of life for affected individuals. Furthermore, cannabinoids exhibit
the capacity to mitigate neuroinflammation, shield against beta-amyloid-induced neurotoxicity, and mitigate neurodegeneration in animal
models of AD. Additionally, research has unveiled dysregulation of the ECS in the brains of AD patients, which could contribute to the
cognitive and behavioural symptoms associated with the disease.
Cannabinoids are highly lipophilic
(dissolve in fats, oils and lipids) and can easily cross the blood-brain barrier, making them potential pharmaceutical targets for neurodegenerative
disease. The capability to traverse the blood-brain barrier renders cannabinoid analogs promising candidates for pharmaceutical use in
the treatment of neurological disorders.
The use of cannabinoids in
AD treatment holds great promise; however, further research is needed to fully understand the mechanisms to develop safe and effective
cannabinoid-based therapeutics. In addition to our INM-900 series of compounds, we are aware of no other pharmaceutical-grade cannabinoid-based
therapy being evaluated for the treatment of glaucoma
Key Preclinical Results to Treat Neurodegenerative Diseases
Figure 1. Neuroprotection of human neuronal cells
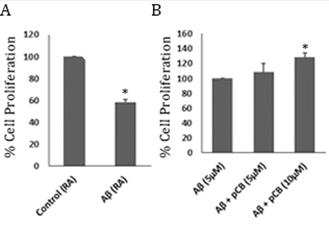
Phyto-cannabinoids (pCBx) promote neuroprotection.
(A) Amyloid peptide (Aβ, 5µM) induces cytotoxicity in SHSY5Y cells. Aβ1-42 insult induced approximately
~45% cytotoxicity of the SH-SY5Y cells. (B) Concurrent exposure of Aβ with pCBx at 5 µM and 10 µM concentrations
protected cells from Aβ induced toxicity in a dose-dependent manner. Cell viability was determined by MTT assay.
Figure 2. Neurogenesis of human neuronal cells

Cannabinoid (pCBx) promotes neuritogenesis.
Tuj1 Tubulins are building blocks of microtubules. As such, Tuj1 expression can reveal the fine details of axonal structures
and dendrites. Therefore, changes in Tuj1 expression can be directly correlated with neuronal health and communication. (A) Photomicrographs
illustrating Tuj1 expression in control and pCBx (5 and 10µM) treated cells. The formation of extended neurites and arborization
is evident upon pCBx treatment.
Key Results:
In our investigations related to neurodegeneration
and cannabinoids, we have demonstrated the following:
| ● | phyto(p)CBx significantly increases cell survival
and attenuated increased Bax/Cas-3 expression in the presence of Aβ (5µM) in SH-SY5Y neuroblastoma cells; |
| ● | pCBx treatment also improves neuritogenesis in
human neuroblastoma cells, enhanced expression of neurite marker MAP2 and Tuj1; and |
| ● | For behavioural studies, we have now established
and validated the control and transgenic models for the study. 4-5 months old 5XFAD tg mice exhibited motor impairments in the open field,
reduced anxiety behaviour in the elevated maze plus, and reduced exploratory behaviour in the novel object recognition test, indicating
disease progression and impaired cognitive function. Experiments using cannabinoids in 5XFAD mice are in progress. |
Next Steps for the INM-900 series in Neurodegenerative Diseases
Program:
| ● | Complete ongoing proof of concept studies using in
vivo models in neurodegenerative disease to select the most appropriate candidate for clinical studies |
| ● | Commence additional preclinical studies starting
in 2024 |
Key Milestones
| ● | November
3, 2021 - We announced the filing of an international patent application demonstrating neuroprotection and enhanced neuronal function
using a rare cannabinoid for the potential treatment of neurodegenerative diseases such as Alzheimer’s Disease, Parkinson’s
Disease, Huntington’s Disease and others. This Patent Cooperation Treaty (PCT) application, entitled “Compositions and Methods
for Treating Neuronal Disorders with Cannabinoids”, specifies a rare cannabinoid that may inhibit or slow the progression of neurodegenerative
diseases by providing neuroprotection in a population of affected neurons. Furthermore, the PCT application also demonstrates the subject
cannabinoid compound can also be used to promote neurite outgrowth, signifying the potential to enhance neuronal function. The rare cannabinoid
included in the PCT application is new to InMed’s portfolio. |
| ● | April
28, 2022 – We announced initiated a research collaboration with the Department of Biotechnological and Applied Clinical Sciences,
University of L’Aquila (Italy) in the laboratory of Dr. Mauro Maccarrone, an international expert in cannabinoid research and founding
member of the European Cannabinoid Research Alliance. Dr. Maccarrone’s lab will be screening the Company’s novel cannabinoid
analogs to investigate pharmacological properties and potential therapeutic uses. |
| ● | November
16, 2022- We announced announces the launch of its neurodegenerative disease program (INM-900 series), investigating the effects of cannabinoid
analogs in diseases such as Alzheimer’s, Huntington’s and Parkinson’s. In addition, Dr. Ujendra Kumar of the Faculty
of Pharmaceuticals Sciences at UBC has been awarded an Alliance grant from NSERC, with InMed as the named industry partner. The funding
will support the research and development studies of InMed’s cannabinoid pharmaceutical candidates, investigating their potential
therapeutic effects in neurodegenerative diseases. The collaboration project is entitled “Pharmacological Characterization of Phytocannabinoids
and the Endocannabinoid System.” |
| ● | June 1, 2023 – We announced that
results from a neurodegenerative disease study was presented in a scientific poster at the Canadian Neuroscience Meeting in
Montreal from May 28-31, 2023. The InMed sponsored research, entitled “Cannabinoids modulate cytotoxicity and
neuritogenesis in Amyloid-beta-treated neuronal cells”, demonstrated the ability of a specific rare cannabinoid
(“pCBx”) in our INM-900 series of potential candidates that reduces amyloid toxicity and tau protein expression while
enhancing neuronal cell growth and neuritogenesis markers in vitro, all considered to be important targets in the potential
treatment of neurodegenerative diseases such as Alzheimer’s. |
Manufacturing of Our Active Pharmaceutical Ingredients (API)
The CBN used in INM-755 and
INM-088 and cannabinoid analogs used in the INM-900 series is currently either made in-house at BayMedica, or sourced from contract manufacturers
or, for smaller quantities, from research material suppliers, that typically utilize synthetic chemistry. Changes in contract manufacturers
or suppliers may require additional verification of the vendor’s quality systems, compliance, manufacturing process, testing and
equivalency to the currently supplied CBN prior to use. This is intended to be an interim step to enable us to proceed with developing
its formulations, execute preclinical toxicology studies and progress through Phase 1 and 2 clinical trials.
Bridging studies consisting
of chemical analysis and, possibly, animal bioavailability studies may be required in order to switch our API from the current external
manufacturing sources to our internal IntegraSyn based APIs.
We expect that the final formulations
(API + excipients + packaging) of INM-755 topical cream and the INM-088 eye drop formulation will be manufactured by contract manufacturers
and sub-component fabricators. The contract manufacturers and sub-component fabricators will be selected based on their specific competencies
in manufacturing, quality standards, and materials. FDA regulations require that products be produced under current cGMP.
Intellectual Property
A patent is a monopoly granted
by a government for a period of up to 20 years. A patent provides an enforceable legal right to prevent others from exploiting an invention
being a product, device, system, substance, process or method in the country of grant. For an invention to be patentable, it must be novel,
involve an inventive step and useful at the time of filing the initial patent application for that invention. At 18 months from the initial
patent application, the detailed description of the invention is published. In order to secure patent protection, a patent application
is filed with the patent office in each country of interest, the application is considered under the patent laws of that country, and
a patent will issue if the application meets the patentability criteria of that country. After a patent expires or lapses, anyone can
then use the invention.
The grant of a patent does
not guarantee validity and a patent may be challenged by third parties at a patent office by re-examination in some countries or through
the courts by revocation proceedings. The grant of a valid patent does not mean that the invention may be exploited in a given country
without infringing third party intellectual property rights in that country.
The owner of a patent has
the exclusive right to prevent others from making, selling, importing or otherwise using the patented invention for the life of the patent.
Patent infringement occurs when someone makes, hires, uses, imports or sells the patented invention, or a product made by a patented method,
or offers to do these things, within the country covered by the patent without the permission of the owner of the patent.
Adequate protection of intellectual
property is a means to ensure that we can commercialize our intellectual property and reduce the likelihood of imitation by competitors.
We intend to utilize patents available to protect our IP wherever commercially realizable. In addition, we also rely on trade-secrets
and process know-how to protect our intellectual property. While we cannot patent the naturally occurring individual cannabinoids used
in our Products and Product Candidates, there are a number of other approaches to protect our inventions. These include:
| |
● |
patents on individual or combinations of cannabinoids that provide novel methods for treating diseases; |
| |
● |
cannabinoid delivery technology, formulations designed specifically to increase the safety and efficacy of drug treatments; and |
| |
● |
manufacturing processes for cannabinoids. |
The patent methodologies listed
above will be designed with the intention to maximize the protection of our multi-faceted approach to developing novel cannabinoid medicines.
We typically file patent applications in US, Canada, EU and other selected commercially significant foreign jurisdictions.
InMed Patent Portfolio, August 2023
| Subject Matter |
|
Scope |
|
Ownership/
Origin |
|
Filing Status /
Filing Date |
|
Patent Reference
Number |
|
Earliest
Potential/
Patent
Expiry2 |
|
Jurisdictions |
| Metabolic engineering of E. coli for the biosynthesis of cannabinoid products |
|
Manufacturing Process |
|
InMed, UBC1 |
|
PCT Application
filed 09/05/2018 |
|
WO2019/046941 |
|
2038 |
|
Pending: AU, CA, EP, IL, IN, JP, KR, US |
| Compositions and methods for biosynthesis of terpenoids or cannabinoids in a heterologous system |
|
Manufacturing Process |
|
InMed, UBC1 |
|
PCT Application
filed 3/6/2020 |
|
WO2020/176998 |
|
2040 |
|
Pending: AU, CA, CN, EP, JP, SG, US |
|
Ocular drug delivery formulation (Hydrogel) |
|
Formulation, Use |
|
InMed |
|
PCT Application
filed 05/08/2018 |
|
WO2018/205022
|
|
2038 |
|
Granted: AU, EP, IN, JP
Pending: CA, JP, MX, SG, US |
| Compositions and methods for use of cannabinoids for neuroprotection |
|
Use |
|
InMed |
|
PCT Application
filed 04/24/2020 |
|
WO2020/215164 |
|
2040 |
|
Pending: AU, CA, CN, EP, IL, IN, JP, KR, MX, SG, US, ZA |
| Topical formulations of cannabinoids and use thereof in the treatment of pain |
|
Formulation, Use |
|
InMed |
|
PCT Application
filed 09/21/2018 |
|
WO2019/056123 |
|
2038 |
|
Pending: EP, US |
| Use of topical formulations of cannabinoids in the treatment of epidermolysis bullosa and related connective tissue disorders |
|
Use |
|
InMed |
|
PCT Application
filed 05/04/2017 |
|
WO2017/190249 |
|
2037 |
|
Granted: IL, JP
Pending: AU, CA, EP, JP, SG, US |
| Compositions and methods for treating neuronal disorders with cannabinoids |
|
Use |
|
InMed |
|
PCT Application
filed 10/21/2022 |
|
WO 2022/082313
|
|
2041 |
|
Pending: AU, CA, CN, EP, IL, JP, MX, US |
| BayMedica Patent Portfolio, September 2023 |
|
|
| |
|
|
| Subject Matter |
|
Scope |
|
Ownership/
Origin |
|
Filing Status /
Filing Date |
|
Patent Reference
Number |
|
Earliest
Potential/
Patent
Expiry2 |
|
Jurisdictions |
| Recombinant production systems for prenylated polyketides of the cannabinoid family |
|
Manufacturing Process |
|
BayMedica |
|
PCT Application
filed 05/10/2018 |
|
WO2018/209143 |
|
2038 |
|
Granted: US, US
Pending: AU, CA, CN, EP, IN, MX |
| Cannabinoid analogs and methods for their preparation |
|
New Chemical Entity; Manufacturing Process |
|
BayMedica |
|
PCT Application
filed 10/31/2019 |
|
WO2020/092823 |
|
2039 |
|
Pending: AU, CA, CN, EP, IL, IN, JP, MX, US |
| Use of Type I and Type II polyketide synthases for the production of cannabinoids and cannabinoid analogs |
|
Manufacturing Process |
|
BayMedica |
|
PCT Application
filed 11/13/2019 |
|
WO2020/102430
|
|
2039 |
|
Pending: US |
| Preparation of cannabichromene and related cannabinoids |
|
Manufacturing Process |
|
BayMedica |
|
PCT Application
filed 12/23/2020 |
|
WO2021/133989 |
|
2040 |
|
Pending: CA, CN, EP, IN, JP, US |
| Genetically modified yeast for the production of cannabigerolic acid, cannabichromenic acid and related cannabinoids |
|
Manufacturing Process |
|
BayMedica |
|
PCT Application
filed 01/20/2021 |
|
WO2021/150636 |
|
2041 |
|
Pending: CA, CN, EP, IN, JP, US |
| Acyl activating enzymes for preparation of cannabinoids |
|
Manufacturing Process |
|
BayMedica |
|
PCT Application
filed 01/20/2022 |
|
WO2022/159589 |
|
2042 |
|
Pending: CA, EP, IN, JP, US |
| 1 |
UBC is a co-inventor and has assigned all commercial rights to InMed in exchange for a royalty of less than 1% on sales revenues from products utilizing cannabinoids manufactured using the technology and a single digit royalty on any sub-licensing revenues. |
| 2 |
Patents typically expire 20 years from their filing dates, if granted, the patent expiry may be extended by patent agencies and/or health regulatory authorities. |
PCT = Patent Cooperation Treaty. Members in this treaty includes over
150 countries including USA, Canada, Europe and others.
As of August 31, 2023, we
have thirteen patent families covering manufacturing, Product Candidates, and novel methods for treating diseases including two for our
INM-755 program (WO/2017/190249 and WO/2019/056123), one for our INM-088 program (WO/2020/215164) and one for treating neurodegenerative
diseases (WO/2022/082313). If these patent applications are granted and all maintenance fees or annuities are paid, these patents are
expected to expire in 2037-2042. In some situations, the patent may be eligible for adjustment or extension of the patent terms due to
delay in the patent office during the prosecution phase. The expiration date above does not include the adjustments or extensions.
As of August 31, 2023, we
have one patent family covering cannabinoid delivery technology for the ocular program (WO/2018/205022). If these patent applications
are granted and all maintenance fees or annuities are paid, these patents are expected to expire in 2038. In some situations, the patent
may be eligible for adjustment or extension of the patent terms due to delay in the patent office during the prosecution phase. The expiration
date above does not include the adjustments or extensions.
As of August 31, 2023, we
have eight patent families covering manufacturing process for cannabinoids of interest (WO2019/046941, WO2020/176998, WO2018/209143, WO2020/092823,
WO2020/102430, WO2021/133989, WO2021/150636 and WO2022/159589). If these patent applications are granted and all maintenance fees or annuities
are paid, these patents are expected to expire in 2038-2042. In some situations, the patent may be eligible for adjustment or extension
of the patent terms due to delay in the patent office during the prosecution phase. The expiration date above does not include the adjustments
or extensions.
The Patent Cooperation Treaty,
or “PCT”, is an international patent law treaty, which provides a unified procedure for filing patent applications to protect
inventions in each of its member states. There are 151 member countries within the PCT, enabling near-global patent coverage through successful
patent prosecution in the U.S., Japan, Europe, Canada, Australia, New Zealand, China, Brazil, Russia, India and many other countries.
We have several filed patent applications currently either in the provisional stage or PCT stage of review as shown above. None have been
granted to date. We retain the full commercial rights to all of these patents with any exceptions noted in the above table.
ITEM 1A. RISK FACTORS
Summary of Risk Factors
The following is a summary
of material risks that could affect the Company. This summary may not contain all of our material risks, and it is qualified in its entirety
by the more detailed risk factors set forth below.
| |
● |
Our IntegraSyn manufacturing approach may prove unsuccessful in achieving
yields and/or cost levels required to be economically competitive with alternative methods of manufacturing. |
| |
● |
Our prospects depend on the success of our Product Candidates which are at early-stages of development with a statistically high probability of failure and are subject to lengthy, time-consuming and inherently unpredictable regulatory processes. |
| |
|
|
| |
● |
Even if our Product Candidates advance through preclinical studies and clinical trials, we may experience difficulties in managing our growth and expanding our operations. |
| |
|
|
| |
● |
If we have difficulty enrolling patients in clinical trials, the completion of the trials may be delayed or cancelled. |
| |
● |
If clinical trials of our Product Candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we would incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our Product Candidates. |
| |
|
|
| |
● |
If we experience delays in clinical testing, we will be delayed in commercializing our Product Candidates, and our business may be substantially harmed. |
| |
|
|
| |
● |
Negative results from clinical trials or studies of others and adverse safety events involving the targets of our products may have an adverse impact on our future commercialization efforts. |
| |
|
|
| |
● |
We intend to expend our limited resources to pursue our Product Candidates for certain indications and may fail to capitalize on other Product Candidates or other indications for our Product Candidates that may be more profitable or for which there is a greater likelihood of success. |
| |
|
|
| |
● |
The regulatory approval processes of the FDA, HC, the EMA and other comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our Product Candidates, our business will be substantially harmed. |
| |
|
|
| |
● |
We intend to conduct clinical trials for our Product Candidates in several international jurisdictions, and acceptance by all regulatory authorities for such “international” data is not certain. |
| |
● |
Our Product Candidates contain compounds that may be classified as “controlled substances”, the use of which may generate public controversy and restrict their development or commercialization. |
| |
● |
Research restrictions, product shipment delays or prohibitions could have a material adverse effect on our business, results of operations and financial condition. |
| |
● |
Healthcare legislation, including potentially unfavorable pricing regulations or other healthcare reform initiatives, may increase the difficulty and cost for us to obtain marketing approval of and commercialize our Product Candidates. |
| |
|
|
| |
● |
Increased scrutiny on drug pricing or changes in pricing regulations could restrict the amount that we are able to charge for our Product Candidates, which could adversely affect our revenue and results of operations. |
| |
|
|
| |
● |
Even if we are able to commercialize our Product Candidates, they may not receive coverage and adequate reimbursement from third-party payors, which could harm our business. |
| |
|
|
| |
● |
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse, federal exclusion or debarment, and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings. |
| |
|
|
| |
● |
Failure to comply with the U.S. Foreign Corrupt Practices Act, or “FCPA”, the Canadian Corruption of Foreign Public Officials Act, or “CFPOA”, and other global anti-corruption and anti-bribery laws could subject us to penalties and other adverse consequences. |
| |
|
|
| |
● |
Recent federal legislation and actions by state and local governments may permit reimportation of drugs from/to foreign countries where the drugs are sold at lower prices than in the country of origination, which could materially adversely affect our business and financial condition. |
| |
|
|
| |
● |
We are dependent upon our key personnel to achieve our business objectives. |
| |
● |
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could subject us to significant liability and harm our reputation. |
| |
● |
Our insurance may be insufficient to cover losses that may occur as a result of our operations. |
| |
|
|
| |
● |
There may be changes in laws, regulations and guidelines which are detrimental to our business. |
| |
|
|
| |
● |
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected. |
| |
|
|
| |
● |
Our proprietary information, or that of our customers, suppliers and business partners, may be lost or we may suffer security breaches. |
| |
|
|
| |
● |
We expect to face intense competition, often from companies with greater resources and experience than we have. |
| |
|
|
| |
● |
If we receive regulatory approvals, we intend to market our Product Candidates in multiple jurisdictions where we have limited or no operating experience and may be subject to increased business and economic risks that could affect our financial results. |
| |
|
|
| |
● |
Controlled substance legislation may differ in other jurisdictions and could restrict our ability to market our products internationally, which would result in increased business and economic risks that could affect our financial results. |
| |
● |
Product liability lawsuits against us could cause us to incur substantial liabilities. |
| |
|
|
| |
● |
Failure to protect our information technology infrastructure against cyber-based attacks, network security breaches, service interruptions, or data corruption could significantly disrupt our operations and adversely affect our business and operating results. |
| |
|
|
| |
● |
Our failure to comply with data protection laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results. |
| |
|
|
| |
● |
The COVID-19 coronavirus could adversely impact our business, including several key activities that are critical to our success. |
| |
● |
The market prices for our common shares are volatile and will fluctuate. |
| |
● |
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or Product Candidates. |
| |
● |
Future offerings of debt or equity securities may rank senior to common shares. |
| |
|
|
| |
● |
Future sales of common shares by officers and directors may negatively impact the market price for our common shares. |
| |
|
|
| |
● |
We do not currently pay dividends on our common shares and have no intention to pay dividends on our common shares for the foreseeable future. |
| |
|
|
| |
● |
We are exposed to risks related
to currency exchange rates.
|
| |
● |
For as long as we are an “emerging growth company” we intend to take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common shares being less attractive to investors and could make it more difficult for us to raise capital as and when we need it. |
| |
|
|
| |
● |
If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common shares. |
| |
|
|
| |
● |
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud. |
| |
● |
Deficiencies in disclosure controls and procedures and internal control over financial reporting could result in a material misstatement in our financial statements. |
| |
● |
In connection with the audit of our financial statements as of and for the years ended June 30, 2023 and 2022, a significant deficiency and a material weakness, respectively in our internal control over financial reporting were identified and we may identify additional material weaknesses in the future. |
| |
● |
We have incurred, and will continue to incur, increased costs as a result of operating as a public company, and our management has been required, and will continue to be required, to devote substantial time to new compliance initiatives. |
| |
|
|
| |
● |
Future sales and issuances of our common shares or rights to purchase common shares pursuant to our equity incentive plan could result in additional dilution of the percentage ownership of our shareholders and may cause our share price to fall. |
| |
● |
Provisions in our corporate charter documents and certain Canadian laws could delay or deter a change of control. |
| |
|
|
| |
● |
If securities or industry analysts publish inaccurate or unfavorable research about our business, our share price and trading volume may decline. |
| |
|
|
| |
● |
We are incorporated in Canada, with our assets and officers primarily located in Canada, with the result that it may be difficult for investors to enforce judgments obtained against us or some of our officers. |
| |
|
|
| |
● |
Our operating losses have raised substantial doubt regarding our ability to continue as a going concern. |
| |
|
|
| |
● |
We have incurred significant losses since our inception, we anticipate that we will continue to incur losses in the future. |
| |
|
|
| |
● |
We will require additional capital to fund our operations and if we fail to obtain necessary financing, we will not be able to complete the development and commercialization of our Product Candidates. |
| |
● |
We currently have limited commercial revenue and may never become profitable. |
| |
|
|
| |
● |
Changes in tax laws and unanticipated tax liabilities could adversely affect our effective income tax rate and ability to achieve profitability. |
| |
|
|
| |
● |
Our ability to use our net operating loss carryforwards and other tax attributes may be limited. |
| |
|
|
| |
● |
Changes to accounting standards may adversely impact the manner in which we report our financial position and operating results. |
| |
|
|
| |
● |
There is currently general economic uncertainty in the global markets. |
| |
|
|
| |
● |
Our success is largely dependent upon our patents, proprietary technology, and other intellectual property. |
| |
|
|
| |
● |
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements. |
| |
|
|
| |
● |
We may become subject to claims or become involved in lawsuits related to intellectual property. |
| |
● |
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful and have a material adverse effect on the success of our business. |
| |
● |
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of our technology and products could be significantly diminished. |
| |
|
|
| |
● |
We may not be able to protect our intellectual property rights throughout the world. |
| |
|
|
| |
● |
Patent terms may be inadequate to protect our competitive position on our Product Candidates for an adequate amount of time. |
| |
|
|
| |
● |
Intellectual property rights do not necessarily address all potential threats to our competitive advantage. |
| |
|
|
| |
● |
We rely heavily on contract manufacturers over whom we have limited control and our existing collaboration agreements and any that we may enter into in the future may not be successful. |
| |
|
|
| |
● |
Our existing collaboration agreements and any that we may enter into in the future may not be successful. |
Risk Factors
Investing in our common
shares involves a high degree of risk. You should carefully consider each of the following risks, together with all other information
set forth in this Annual Form on 10-K, including the consolidated financial statements and the related notes, before making a decision
to buy our common shares. If any of the following risks actually occurs, our business could be harmed. In that case, the trading price
of our common shares could decline, and you may lose all or part of your investment.
Risks Related to our Business
and Industry
Our IntegraSyn manufacturing approach may prove
unsuccessful in achieving yields and/or cost levels required to be economically competitive with alternative methods of manufacturing.
Given the early-stage of development
of the IntegraSyn program and the risks inherent in research and development, it is too early to project the commercial viability of cannabinoids
produced via this process. Potential negative outcomes from this program include but are not limited to:
| |
● |
the technology fails to produce sufficient quantities of cannabinoids or ones for which we or others have a need; or |
| |
● |
the cost structure of the technology is such that it is not commercially competitive with alternate methods of cannabinoid manufacturing leading to the technology having no value proposition nor incremental value to the Company. |
Our prospects depend on
the success of our Product Candidates which are at early-stages of development with a statistically high probability of failure.
Given the early-stage of
development, we can make no assurance that our research and development programs will result in regulatory approval or commercially viable
products. To achieve profitable operations, we, alone or with others, must successfully develop, gain regulatory approval, and market
our future products. We currently have no products that have been approved by the FDA, HC, or any similar regulatory authority. To obtain
regulatory approvals for our Product Candidates being developed and to achieve commercial success, clinical trials must demonstrate that
the Product Candidates are safe for human use and that they demonstrate efficacy. We have no products or technologies which are currently
in human clinical trials. Additionally, we have no products for commercial sale or licensed for commercial sale, nor do we expect to
have any such products for the next several years.
Many potential pharmaceuticals products never reach the stage of clinical testing and
even those that do have only a small chance of successfully completing clinical development and gaining regulatory approval. Our Product
Candidates may fail for a number of reasons, including, but not limited to, being unsafe for human use or due to the failure to provide
therapeutic benefits equal to or better than the standard of treatment at the time of testing. Positive results of early preclinical
research may not be indicative of the results that will be obtained in later stages of preclinical or clinical research. Similarly, positive
results from early-stage clinical trials may not be indicative of favorable outcomes in later-stage clinical trials. We can make no assurance
that any future studies, if undertaken, will yield favorable results.
The early-stage of our product
development makes it particularly uncertain whether any of our product development efforts will prove to be successful and meet applicable
regulatory requirements, and whether any of our Product Candidates will receive the requisite regulatory approvals, be capable of being
manufactured at a reasonable cost or be successfully marketed. If we are successful in developing our current and future Product Candidates
into approved products, we will still experience many potential obstacles, such as the need to develop or obtain manufacturing, marketing
and distribution capabilities. If we are unable to successfully commercialize any of our products, our financial condition and results
of operations may be materially and adversely affected.
Even if our Product Candidates
advance through preclinical studies and clinical trials, we may experience difficulties in managing our growth and expanding our operations.
We have limited resources
to carry out objectives for our current and future preclinical studies and clinical trials. Since our inception as a pharmaceutical company
in October 2014, we have conducted numerous preclinical experiments and are currently conducting early-stage clinical trials, which is
a time-consuming, expensive and uncertain process. In addition, while we have experienced management and expect to contract out many of
the activities related to conducting these programs, we are a small company with less than 15 employees and, therefore, have limited internal
resources both to conduct preclinical studies and clinical trials and to monitor third-party providers. As our Product Candidates advance
through preclinical studies and clinical trials, we will need to expand our development, regulatory and manufacturing operations, either
by expanding our internal capabilities or contracting with other organizations to provide these capabilities for us. In the future, we
expect to have to manage additional relationships with collaborators or partners, suppliers and other organizations. Our ability to manage
our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting
systems and procedures.
If we have difficulty
enrolling patients in clinical trials, the completion of the trials may be delayed or cancelled.
As our Product Candidates
advance from preclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will
need to enroll an increasing number of patients that meet the eligibility criteria for those trials. The factors that affect our ability
to enroll patients are largely uncontrollable and include, but are not limited to, the following:
| |
● |
size and nature of the patient population; |
| |
● |
inclusion and exclusion criteria for the trial; |
| |
● |
design of the study protocol; |
| |
● |
competition with other companies for clinical sites or patients; |
| |
● |
the perceived risks and benefits of the product candidate under study; |
| |
● |
the patient referral practices of physicians; and |
| |
● |
the number, availability, location and accessibility of clinical trial sites. |
As a result of the foregoing
factors, we may have difficulty enrolling or maintaining the enrollment of patients in any clinical trials conducted for our products,
which may result in the delay or cancellation of such trials. The delay or cancellation of any clinical trials could shorten any periods
during which we may have the exclusive right to commercialize our Product Candidates or allow our competitors to bring products to market
before us, which would impair our ability to successfully commercialize our Product Candidates and may harm our financial condition, results
of operations and prospects.
If clinical trials of our Product Candidates
fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we
would incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization
of our Product Candidates.
Before obtaining marketing
approval from regulatory authorities for the sale of our Product Candidates, we must conduct preclinical studies in animals and extensive
clinical trials in humans to demonstrate the safety and efficacy of the Product Candidates. Clinical testing is expensive and difficult
to design and implement, can take many years to complete and has uncertain outcomes. The outcome of preclinical studies and early clinical
trials may not predict the success of later clinical trials and interim results of a clinical trial do not necessarily predict final results.
A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials
due to lack of efficacy or unacceptable safety profiles, notwithstanding promising results in earlier trials. We do not know whether the
clinical trials we may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market any of our Product
Candidates in any jurisdiction. A product candidate may fail for safety or efficacy reasons at any stage of the testing process. A major
risk we face is the possibility that none of our Product Candidates under development will successfully gain market approval from the
FDA or other regulatory authorities, resulting in us being unable to derive any commercial revenue from them after investing significant
amounts of capital in multiple stages of preclinical and clinical testing.
If we experience delays
in clinical testing, we will be delayed in commercializing our Product Candidates, and our business may be substantially harmed.
We cannot predict whether
any clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, or at all. Our product development
costs will increase if we experience delays in clinical testing. Significant clinical trial delays could shorten any periods during which
we may have the exclusive right to commercialize our Product Candidates or allow our competitors to bring products to market before us,
which would impair our ability to successfully commercialize our Product Candidates and may harm our financial condition, results of operations
and prospects. The commencement and completion of clinical trials for our products may be delayed for a number of reasons, including delays
related, but not limited, to:
| |
● |
failure by regulatory authorities to grant permission to proceed or placing the clinical trial on hold; |
| |
● |
import/export and research restrictions for cannabinoid-based pharmaceuticals may delay or prevent clinical trials in various geographical jurisdictions; |
| |
● |
patients failing to enroll or remain in our trials at the rate we expect; |
| |
● |
suspension or termination of clinical trials by regulators for many reasons, including concerns about patient safety or failure of our contract manufacturers to comply with current good manufacturing practice, or “cGMP”, requirements; |
| |
● |
any changes to our manufacturing process that may be necessary or desired; |
| |
● |
delays or failure to obtain clinical supply from contract manufacturers of our products necessary to conduct clinical trials; |
| |
● |
Product Candidates demonstrating a lack of safety or efficacy during clinical trials; |
| |
● |
patients choosing an alternative treatment for the indications for which we are developing any of our Product Candidates or participating in competing clinical trials and/or scheduling conflicts with participating clinicians; |
| |
● |
patients failing to complete clinical trials due to dissatisfaction with the treatment, side effects or other reasons; |
| |
● |
reports of clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
| |
● |
clinical investigators not performing our clinical trials on their anticipated schedule, dropping out of a trial, or employing methods not consistent with the clinical trial protocol, regulatory requirements or other third parties not performing data collection and analysis in a timely or accurate manner; |
| |
● |
failure of our CROs, to satisfy their contractual duties or meet expected deadlines; |
| |
● |
inspections of clinical trial sites by regulatory authorities or Institutional Review Boards, or “IRBs”, or ethics committees finding regulatory violations that require us to undertake corrective action, resulting in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study; |
| |
● |
one or more IRBs or ethics committees rejecting, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; or |
| |
● |
failure to reach agreement on acceptable terms with prospective clinical trial sites. |
Our product development costs
will increase if we experience delays in testing or approval or if we need to perform more or larger clinical trials than planned. Additionally,
changes in regulatory requirements and policies may occur, and we may need to amend study protocols to reflect these changes. Amendments
may require us to resubmit our study protocols to regulatory authorities or IRBs or ethics committees for re-examination, which may impact
the cost, timing or successful completion of that trial. Delays or increased product development costs may have a material adverse effect
on our business, financial condition and prospects.
Negative results from clinical trials or studies
of others and adverse safety events involving the targets of our products may have an adverse impact on our future commercialization efforts.
From time to time, studies
or clinical trials on various aspects of pharmaceutical products are conducted by academic researchers, competitors or others. The results
of these studies or trials, when published, may have a significant effect on the market for the pharmaceutical product that is the subject
of the study. The publication of negative results of studies or clinical trials or adverse safety events related to our Product Candidates,
or the therapeutic areas in which our Product Candidates compete, could adversely affect the price of our common shares and our ability
to finance future development of our Product Candidates, and our business and financial results could be materially and adversely affected.
We intend to expend our limited resources to
pursue our Product Candidates for certain indications and may fail to capitalize on other Product Candidates or other indications for
our Product Candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial
and managerial resources, we are focusing on research programs relating to our Product Candidates for certain indications, primarily for
the treatment of EB, which concentrates the risk of product failure in the event our Product Candidates prove to be unsafe or ineffective
or inadequate for clinical development or commercialization. As a result, we may forego or delay pursuit of opportunities with other Product
Candidates or for other indications that could later prove to have greater commercial potential. We may also deem it advisable to refocus
our clinical development programs based on clinical trial results.
The regulatory approval processes of the FDA,
HC, the EMA and other comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable, and if we are
ultimately unable to obtain regulatory approval for our Product Candidates, our business will be substantially harmed.
We are not permitted to market
our Product Candidates in any jurisdiction until we receive formal approval from the appropriate regulatory authorities. For example,
prior to submitting an NDA to the FDA or an MAA to the EMA for approval of our Product Candidates, we will need to complete our preclinical
studies and clinical trials. Successfully completing our clinical program and obtaining approval of an application seeking commercialization
approval is a complex, lengthy, expensive and uncertain process, and the regulatory authorities may delay, limit or deny approval of our
Product Candidates for many reasons, including, among others, because:
| |
● |
we may not be able to demonstrate that our Product Candidates are safe and effective in treating patients to the satisfaction of the regulatory authorities such as the FDA, HC or EMA; |
| |
● |
the results of our clinical trials may not meet the level of statistical or clinical significance required by the regulatory authorities for marketing approval; |
| |
● |
the regulatory authorities may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| |
● |
the regulatory authorities may require that we conduct additional clinical trials; |
| |
● |
the regulatory authorities or other applicable foreign regulatory authorities may not approve the formulation, labeling or specifications of our Product Candidates; |
| |
● |
the contract manufacturing organizations and other contractors that we may retain to conduct our clinical trials may take actions outside of our control that materially adversely impact our clinical trials; |
| |
● |
the regulatory authorities may find the data from clinical studies and clinical trials insufficient to demonstrate that our Product Candidates are safe and effective for their proposed indications; |
| |
● |
the regulatory authorities may disagree with our interpretation of data from our preclinical studies and clinical trials; |
| |
● |
the regulatory authorities may not accept data generated at our clinical trial sites or may disagree with us over whether to accept efficacy results from clinical trial sites outside the United States, Canada or outside the European Union, as applicable, where the standard of care is potentially different from that in the United States, Canada or in the European Union, as applicable; |
| |
● |
if our applications are submitted to the regulatory authorities, the regulatory authorities may have difficulties scheduling the necessary review meetings in a timely manner, may recommend against approval of our application or may recommend or require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| |
● |
the FDA may require development of a Risk Evaluation and Mitigation Strategy which would use risk minimization strategies to ensure that the benefits of certain prescription drugs outweigh their risks, as a condition of approval or post-approval, and the EMA may grant only conditional marketing authorization or impose specific obligations as a condition for marketing authorization, or may require us to conduct post-authorization safety studies; |
| |
● |
the FDA, DEA, HC, EMA or other applicable foreign regulatory agencies may not approve the manufacturing processes or facilities of third-party manufacturers with which we contract or DEA or other applicable foreign regulatory agency quotas may limit the quantities of controlled substances available to our manufacturers; or |
| |
● |
the FDA, HC, EMA or other applicable foreign regulatory agencies may change their approval policies or adopt new regulations. |
In the United States, our
activities are potentially subject to additional regulation by various federal, state and local authorities in addition to the FDA, including,
among others, the Centers for Medicare and Medicaid Services, other divisions of the United States Department of Health and Human Services,
or “HHS”, (for example, the Office of Inspector General), the Department of Justice, or “DOJ”, and individual
United States Attorney offices within the DOJ, and state and local governments. Because of the breadth of these laws and the narrowness
of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under
one or more of such laws. If our operations are found to be in violation of any of the federal and state laws described above or any other
governmental regulations that apply to us, we may be subject to penalties, including criminal and significant civil monetary penalties,
damages, fines, imprisonment, exclusion from participation in government programs, injunctions, recall or seizure of products, total or
partial suspension of production, denial or withdrawal of pre marketing product approvals, private “qui tam” actions brought
by individual whistleblowers in the name of the government or refusal to allow us to enter into supply contracts, including government
contracts, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business
and our results of operations. To the extent that any of our products are sold in a foreign country, we may be subject to similar foreign
laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud
and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
Any of these factors, many
of which are beyond our control, could increase development costs, jeopardize our ability to obtain regulatory approval for and successfully
market our Product Candidates and generate product revenue.
We intend to conduct clinical trials for our
Product Candidates in several international jurisdictions, and acceptance by all regulatory authorities for such “international”
data is not certain.
We intend to conduct clinical
trials for our Product Candidates both inside and outside the United States. To date, all of our clinical development has been conducted
outside of the United States. Ultimately, we plan to submit NDAs for our Product Candidates to the FDA and other regulatory authorities
upon completion of all requisite clinical trials. As an example, although the FDA may accept data from clinical trials conducted outside
the United States, acceptance of such study data by the FDA is subject to certain conditions. For example, the clinical trial must be
conducted in accordance with FDA regulations relating governing human subject protection and the conduct of clinical trials, which are
referred to as “Good Clinical Practice”, or “GCP” requirements and the FDA must be able to validate the data from
the clinical trial through an onsite inspection if it deems such inspection necessary. Where data from foreign clinical trials are intended
to serve as the sole basis for marketing approval in the United States, the FDA will not approve the application on the basis of foreign
data alone unless those data are considered applicable to the U.S. patient population and U.S. medical practice, the clinical trials were
performed by clinical investigators of recognized competence, and the data is considered valid without the need for an on-site inspection
by the FDA or, if the FDA considers such an inspection to be necessary, the FDA is able to validate the data through an on-site inspection
or other appropriate means. In addition, such clinical trials would be subject to the applicable local laws of the foreign jurisdictions
where the clinical trials are conducted. There can be no assurance the FDA or any other regulatory authorities will accept data from clinical
trials conducted outside of the United States or other international jurisdictions. If the FDA or any other regulatory authorities does
not accept any such data, it would likely result in the need for additional clinical trials, which would be costly and time-consuming
and delay aspects of our development plan.
In addition, the conduct of
clinical trials outside the United States could have a significant impact on us. Risks inherent in conducting international clinical trials
include:
| |
● |
foreign regulatory requirements that could burden or limit our ability to conduct our clinical trials; |
| |
● |
administrative burdens of conducting clinical trials under multiple foreign regulatory schema; |
| |
● |
foreign currency fluctuations which could negatively impact our financial condition since certain payments are paid in local currencies; |
| |
● |
manufacturing, customs, shipment and storage requirements; |
| |
● |
cultural differences in medical practice and clinical research; and |
| |
● |
diminished protection of intellectual property in some countries. |
Our Product Candidates contain compounds that
may be classified as “controlled substances”, the use of which may generate public controversy and restrict their development
or commercialization.
If a drug has a potential
for abuse, the NDA or other regulatory submission must include a description and analysis of studies or information related to abuse of
the drug, including a proposal for scheduling (for example, in the U.S. under the federal Controlled Substances Act, or “CSA”).
A description of any studies related to overdosage is also required, including information on dialysis, antidotes, or other treatments,
if known. While we believe there would be relatively minimal abuse potential with our Product Candidates given the low drug concentration
and topical route of administration, we could be incorrect or they may be perceived as having the potential for substance abuse. In either
case, there may be a negative effect on our ability to successfully develop or commercialize our Product Candidates. Since our Product
Candidates contain purified substances that are chemically identical to those occurring in nature, they may, therefore, be classified
as “controlled substances”, and their regulatory approval may generate public controversy. Political and social pressures
and adverse publicity could lead to delays in approval of, and increased expenses for, our Product Candidates. These pressures could also
limit or restrict the introduction and marketing of our Product Candidates. Despite that fact that our APIs, which are the ingredients
that give medicines their effects, are synthetically made and, therefore, we have no interaction with the Cannabis plant, adverse publicity
from Cannabis misuse or adverse side effects from Cannabis or other cannabinoid products may adversely affect the commercial success or
market penetration achievable for our Product Candidates. The nature of our business attracts a high level of public and media interest,
and in the event of any resultant adverse publicity, our reputation may be harmed. Furthermore, if our Product Candidates are classified
as “controlled substances”, they may be subject to import/export and research restrictions that could delay or prevent the
development of our products in various geographical jurisdictions. The successful commercialization of our Product Candidates may require
permits or approvals from regulatory bodies, such as the DEA, that regulate controlled substances.
Research restrictions, product shipment delays
or prohibitions could have a material adverse effect on our business, results of operations and financial condition.
Research on and the
shipment, import and export of our Product Candidates and the API used in our Product Candidates will require research permits, import
and export licenses by many different authorities. For instance, in the United States, the FDA, U.S. Customs and Border Protection, and
the DEA; in Canada, the Canada Border Services Agency, and HC; in Europe, the EMA and the European Commission; in Australia and New Zealand,
the Australian Customs and Border Protection Service, the Therapeutic Goods Administration, the New Zealand Medicines and Medical Device
Safety Authority and the New Zealand Customs Service; and in other countries, similar regulatory authorities, regulate the research on
and import and export of pharmaceutical products that contain controlled substances. Specifically, the import and export process requires
the issuance of import and export licenses by the relevant controlled substance authority in both the importing and exporting country.
We may not be granted, or if granted, maintain, such licenses from the authorities in certain countries. Even if we obtain the relevant
licenses, shipments of API and our Product Candidates may be held up in transit, which could cause significant delays and may lead to
product batches being stored outside required temperature ranges. Inappropriate storage may damage the product shipment resulting in delays
in clinical trials or, upon commercialization, a partial or total loss of revenue from one or more shipments of API or our Product Candidates.
Once shipment is complete, we or the research contractors we are working with may also suffer further delays or restrictions as a result
of regulations governing research on cannabinoids. A delay in a clinical trial or, upon commercialization, a partial or total loss of
revenue from one or more shipments of API or our Product Candidates could have a material adverse effect on our business, results of operations
and financial condition. The aforementioned examples and lists of various authorities that may currently, or in the future, affect our
ability to conduct research on or import or export our Product Candidates and/or API, should not be construed as exhaustive or comprehensive
in any way.
Healthcare legislation, including potentially
unfavorable pricing regulations or other healthcare reform initiatives, may increase the difficulty and cost for us to obtain marketing
approval of and commercialize our Product Candidates.
Particularly in the United States but also in other jurisdictions,
there have been a number of legislative and regulatory changes and proposed changes in recent years regarding the healthcare system that
could prevent or delay marketing approval of our Product Candidates, restrict or regulate post-approval activities or affect our ability
to profitably sell any Product Candidates for which we obtain marketing approval. Healthcare reform measures that have been and may be
adopted in the future may result in more rigorous coverage criteria, new payment methodologies and in additional downward pressure on
the price that we receive for any approved product, and could seriously harm our future revenue. Any reduction in reimbursement from Medicare
or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment
measures or other healthcare reforms may compromise our ability to generate revenue, attain profitability or commercialize our products.
Increased scrutiny on drug pricing or changes
in pricing regulations could restrict the amount that we are able to charge for our Product Candidates, which could adversely affect our
revenue and results of operations.
Drug pricing by pharmaceutical
companies is currently under increased scrutiny and is expected to continue to be the subject of intense political and public debate in
the United States and other jurisdictions. Specifically, there have been several recent U.S. Congressional inquiries and hearings with
respect to pharmaceutical drug pricing practices, including in connection with the investigation of specific price increases by several
pharmaceutical companies. Additionally, several states have recently passed laws designed to, among other things, bring more transparency
to drug pricing, and other states may pursue similar initiatives in the future. We cannot predict the extent to which our business may
be affected by these or other potential future legislative or regulatory developments. However, increased scrutiny on drug pricing, negative
publicity related to the pricing of pharmaceutical drugs generally, or changes in pricing regulations could restrict the amount that we
are able to charge for our Product Candidates, which could have a material adverse effect on our revenue and results of operations.
Even if we are able to commercialize our Product
Candidates, they may not receive coverage and adequate reimbursement from third-party payors, which could harm our business.
The availability of reimbursement
by governmental and private payors is essential for most patients to be able to afford their treatments. Sales of our Product Candidates,
if approved, will depend substantially on the extent to which the costs of these Product Candidates will be paid by health maintenance,
managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities,
private health coverage insurers and other third-party payors. If reimbursement is not available, or is available only to limited levels,
we may not be able to successfully commercialize our Product Candidates. Even if coverage is provided, the approved reimbursement amount
may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.
In the United States, the
Medicare Modernization Act, established the Medicare Part D program and provided authority for limiting the number of drugs that will
be covered in any therapeutic class thereunder. The Medicare Modernization Act, including its cost reduction initiatives, could decrease
the coverage available for any of our approved products. Furthermore, private payors often follow Medicare in setting their own coverage
policies. Therefore, any reduction in coverage that results from the Medicare Modernization Act may result in a similar reduction from
private payors.
There is significant uncertainty
related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement
for new medicines are typically made by the Centers for Medicare & Medicaid Services, or “CMS”, an agency within the HHS,
as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors tend to follow
CMS to a substantial degree.
The intended use of a drug
product by a physician can also affect pricing. For example, CMS could initiate a National Coverage Determination administrative procedure,
by which the agency determines which uses of a therapeutic product would and would not be reimbursable under Medicare. This determination
process can be lengthy, thereby creating a long period during which the future reimbursement for a particular product may be uncertain.
Outside the United States,
particularly in EU Member States, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations
or the successful completion of Health Technology Assessment, or “HTA”, procedures with governmental authorities can take
considerable time after receipt of marketing authorization for a product. In addition, there can be considerable pressure by governments
and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Certain countries allow companies
to fix their own prices for medicines but monitor and control company profits. Political, economic and regulatory developments may further
complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by
various EU Member States and parallel distribution, or arbitrage between low-priced and high-priced EU member states, can further reduce
net realized prices. In some countries, we or our collaborators may be required to conduct a clinical trial or other studies that compare
the cost-effectiveness of our Product Candidates to other available therapies in order to obtain or maintain reimbursement or pricing
approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels
within the country of publication and other countries. If reimbursement of any product candidate approved for marketing is unavailable
or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business, financial condition, results of operations
or prospects could be adversely affected.
Our relationships with customers and third-party
payors will be subject to applicable anti-kickback, fraud and abuse, federal exclusion or debarment, and other healthcare laws and regulations,
which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future
earnings.
Healthcare providers, physicians
and third-party payors play a primary role in the recommendation and prescription of any Product Candidates for which we obtain marketing
approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other
healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell
and distribute our products for which we obtain marketing approval. As a pharmaceutical company, even though we do not and will not control
referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state healthcare
laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. Restrictions
under applicable federal and state healthcare laws and regulations that may affect our ability to operate include the following:
| |
● |
the U.S. federal healthcare Anti-Kickback Statute impacts our marketing practices, educational programs, pricing policies and relationships with healthcare providers or other entities, by prohibiting, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid; |
| |
● |
federal civil and criminal false claims laws and civil monetary penalty laws impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, false or fraudulent claims for payment of government funds (including through reimbursement by Medicare or Medicaid or other federal health care programs), which has been applied to impermissible promotion of pharmaceutical products for off-label uses, or making a false statement or record to avoid, decrease or conceal an obligation to pay money to the federal government; |
| |
● |
the U.S. Health Insurance Portability and Accountability Act, or “HIPPA”, as amended by the Health Information Technology for Economic and Clinical Health Act, or “HITECH Act”, among other things, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services; |
| |
● |
the U.S. federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires applicable manufacturers of covered drugs, devices, biologics and medical supplies to report annually to HHS information related to payments and other transfers of value to physicians and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members; |
| |
● |
analogous state laws and regulations, such as state anti-kickback laws, false claims laws and privacy and security of health information laws, may apply to sales or marketing arrangements, claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, or health information; and |
| |
● |
certain state laws require pharmaceutical companies to adopt codes of conduct consistent with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; restrict certain marketing-related activities including the provision of gifts, meals, or other items to certain health care providers; and/or require drug manufacturers to report information related to payments and other transfers of value to physicians and certain other healthcare providers or marketing expenditures. |
Comparable laws and regulations
exist in the countries within the European Economic Area, or “EEA”. Although such laws are partially based upon European Union,
or “EU”, law, they may vary from country to country. Healthcare specific, as well as general EU and national laws, regulations
and industry codes constrain, for example, our interactions with government officials and healthcare professionals, and the collection
and processing of personal health data. Non-compliance with any of these laws or regulations could lead to criminal or civil liability.
Efforts to ensure that our
business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It
is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations
or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation
of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and
administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid,
and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect
to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions,
including exclusions from government funded healthcare programs.
Failure to comply with the U.S. Foreign Corrupt
Practices Act, or “FCPA”, the Canadian Corruption of Foreign Public Officials Act, or “CFPOA”, and other global
anti-corruption and anti-bribery laws could subject us to penalties and other adverse consequences.
The FCPA and the CFPOA, as
well as any other applicable domestic or foreign anti-corruption or anti-bribery laws to which we are or may become subject generally
prohibit corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working
in an official capacity and requires companies to maintain accurate books and records and internal controls, including at foreign-controlled
subsidiaries. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government
staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working
in an official capacity.
Compliance with these anti-corruption
laws and anti-bribery laws may be expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition,
these laws present particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government,
and physicians and other hospital employees are considered to be foreign officials. Certain payments by other companies to hospitals in
connection with clinical trials and other work have been deemed to be improper payments to governmental officials and have led to FCPA
enforcement actions.
Our internal control policies
and procedures may not protect us from reckless or negligent acts committed by our employees, future distributors, licensees or agents.
We are currently working to get policies and processes in place to monitor compliance with the FCPA and CFPOA. We can make no assurance
that they will not engage in prohibited conduct, and we may be held liable for their acts under applicable anti-corruption and anti-bribery
laws. Noncompliance with these laws could subject us to investigations, sanctions, settlements, prosecution, other enforcement actions,
disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions, suspension or debarment from contracting
with certain persons, the loss of export privileges, whistleblower complaints, reputational harm, adverse media coverage, and other collateral
consequences. Any investigations, actions or sanctions or other previously mentioned harm could have a material negative effect on our
business, operating results and financial condition.
Recent federal legislation and actions by state
and local governments may permit reimportation of drugs from/to foreign countries where the drugs are sold at lower prices than in the
country of origination, which could materially adversely affect our business and financial condition.
We may face competition for
our Product Candidates, if approved, from cheaper generics and/or cannabinoid therapies sourced from foreign countries that have placed
price controls on pharmaceutical products. This is referred to as parallel importation. For instance, the Medicare Modernization Act contains
provisions that may change U.S. importation laws and expand pharmacists’ and wholesalers’ ability to import cheaper versions
of an approved drug and competing products from Canada, where there are government price controls. These changes to U.S. importation laws
will not take effect unless and until the Secretary of HHS certifies that the changes will pose no additional risk to the public’s
health and safety and will result in a significant reduction in the cost of products to consumers. The Secretary of HHS has so far declined
to approve a reimportation plan. Proponents of drug reimportation, including certain state legislatures, may attempt to pass legislation
that would directly allow reimportation under certain circumstances. Legislation or regulations allowing the reimportation of drugs, if
enacted, could decrease the price we receive for any products that we may develop, including our Product Candidates, and adversely affect
our future revenues and prospects for profitability.
We are dependent upon
our key personnel to achieve our business objectives.
We depend on key personnel,
the loss of any of whom could harm our business. Our future performance and development will depend to a significant extent on the efforts
and abilities of its executive officers, key employees, and consultants. The loss of the services of one or more of these individuals
could harm our business. Our success will depend largely on our continuing ability to attract, develop and retain skilled employees and
consultants in our business. Because of the specialized scientific and managerial nature of our business, we rely heavily on our ability
to attract and retain qualified scientific, technical and managerial personnel. The competition for qualified personnel in our field is
intense. Due to this intense competition, we may be unable to continue to attract and retain qualified personnel necessary for the development
of our business or to recruit suitable replacement personnel. Any delay in replacing such persons, or an inability to replace them with
persons of similar expertise, would have a material adverse effect on our business, financial condition and results of operations.
Our employees may engage in misconduct or other
improper activities, including noncompliance with regulatory standards and requirements, which could subject us to significant liability
and harm our reputation.
We are exposed to the risk
of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with regulations of domestic
or foreign regulatory authorities. In addition, misconduct by employees could include intentional failures to comply with certain development
standards, to report financial information or data accurately, or to disclose unauthorized activities to us. Employee misconduct could
also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and
serious harm to our reputation. While prohibited, it is not always possible to identify and deter employee misconduct, and the precautions
we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting
us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations.
If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions
could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Our insurance may be insufficient
to cover losses that may occur as a result of our operations.
We currently maintain directors’
and officers’ liability insurance, clinical trial insurance and property and general liability insurance and intend in the future
to obtain shipping and storage insurance for Product Candidates. This insurance may not remain available to us or be obtainable by us
at commercially reasonable rates, and the amount of our coverage may not be adequate to cover any liability we incur. Future increases
in insurance costs, coupled with the increase in deductibles, will result in higher operating costs and increased risk. If we were to
incur substantial liability and such damages were not covered by insurance or were in excess of policy limits, or if we were to incur
such liability at a time when we were not able to obtain liability insurance, our business, results of operations and financial condition
could be materially adversely affected.
There may be changes in
laws, regulations and guidelines which are detrimental to our business.
Our operations are subject
to a variety of laws, regulations and guidelines relating to pharmacology, cannabinoids and drug delivery, as well as laws and regulations
relating to health and safety, the conduct of operations, and the protection of the environment. While, to the knowledge of our management,
we are currently in compliance with all such laws, changes to such laws, regulations and guidelines due to matters beyond our control
may cause adverse effects to our operations and financial condition. These changes may require us to incur substantial costs associated
with legal and compliance fees and ultimately require us to alter our business plan. In addition, if the governments of Canada or the
United States were to enact or amend laws relating to our industry, it may decrease the size of, or eliminate entirely, the market for
our Product Candidates, may introduce significant new competition into the market and may otherwise potentially materially and adversely
affect our business, results of operations and financial condition.
If we do not comply with
laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
The research and development
that we carry out either directly or through third-parties involves, and may in the future involve, the use of potentially hazardous materials
and chemicals. Our operations may produce hazardous waste products. Although we believe that our safety procedures for handling and disposing
of these materials comply with the standards mandated by local, state and federal laws and regulations, the risk of accidental contamination
or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could
be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations and fire and building
codes. Although we maintain workers’ compensation insurance as prescribed by the Province of British Columbia to cover us for costs
and expenses we may incur due to injuries to our employees, this insurance may not provide adequate coverage against potential liabilities.
We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us. Additional federal, state
and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and
substantial fines or penalties if we violate, any of these laws or regulations.
Our proprietary information,
or that of our customers, suppliers and business partners, may be lost or we may suffer security breaches.
In the ordinary course of
our business, we may collect and store sensitive data, including intellectual property, data from preclinical studies, clinical trial
data, our proprietary business information and that of our customers, suppliers and business partners, and personally identifiable information
of our customers, clinical trial subjects and employees, in our data centers and on our networks. The secure processing, maintenance and
transmission of this information is critical to our operations. Despite our security measures, our information technology and infrastructure
may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Although to our knowledge
we have not experienced any such material security breach to date, any such breach could compromise our networks and the information stored
there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in
legal claims or proceedings, liability under laws that protect the privacy of personal information, regulatory penalties, disrupt our
operations, damage to our ability to obtain patent protection for our Product Candidates, damage to our reputation, and cause a loss of
confidence in our products and our ability to conduct clinical trials, which could adversely affect our business and reputation and lead
to delays in gaining regulatory approvals.
We expect to face intense
competition, often from companies with greater resources and experience than we have.
The pharmaceutical industry
is highly competitive and subject to rapid change. The industry continues to expand and evolve as an increasing number of competitors
and potential competitors enter the market. Many of these competitors and potential competitors have substantially greater financial,
technological, managerial and research and development resources and experience than we have. Some of these competitors and potential
competitors have more experience than we have in the development of pharmaceutical products, including validation procedures and regulatory
matters. Other companies researching in the same disease areas may develop products that are competitive or superior to our Product Candidates.
Other companies working in cannabinoid research may develop products targeting the same diseases that we are focused on that are competitive
or superior to our Product Candidates. In addition, there are non-FDA approved Cannabis / cannabinoid preparations being made available
from companies in the so-called “medical marijuana” industry, which may be competitive to our products. If we are unable to
compete successfully, our commercial opportunities will be reduced and our business, results of operations and financial conditions may
be materially harmed.
If we receive regulatory approvals, we intend
to market our Product Candidates in multiple jurisdictions where we have limited or no operating experience and may be subject to increased
business and economic risks that could affect our financial results.
If we receive regulatory approvals,
we may plan to market our Product Candidates in jurisdictions where we have limited or no experience in marketing, developing and distributing
our products. Certain markets have substantial legal and regulatory complexities that we may not have experience navigating. We are subject
to a variety of risks inherent in doing business internationally, including risks related to the legal and regulatory environment in non-U.S.
jurisdictions, including with respect to privacy and data security, trade control laws and unexpected changes in laws, regulatory requirements
and enforcement, as well as risks related to fluctuations in currency exchange rates and political, social and economic instability in
foreign countries. If we are unable to manage our international operations successfully, our financial results could be adversely affected.
Controlled substance legislation
may differ in other jurisdictions and could restrict our ability to market our products internationally, which would result in increased
business and economic risks that could affect our financial results.
Controlled substance legislation
may differ in other jurisdictions and could restrict our ability to market our products internationally. Most countries are parties to
the Single Convention on Narcotic Drugs 1961, which governs international trade and domestic control of narcotic substances, including
Cannabis extracts. Countries may interpret and implement their treaty obligations in a way that creates a legal obstacle to our obtaining
marketing approval for Product Candidates in those countries. These countries may not be willing or able to amend or otherwise modify
their laws and regulations to permit our Product Candidates to be marketed or achieving such amendments to the laws and regulations may
take a prolonged period of time. We would be unable to market our Product Candidates in countries with such obstacles in the near future
or perhaps at all without modification to laws and regulations.
Product liability lawsuits
against us could cause us to incur substantial liabilities.
Our use of our Product Candidates
in clinical trials and the sale of our Product Candidates, if approved, exposes us to the risk of product liability claims. Product liability
claims might be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with our Product
Candidates. For example, we may be sued if any product we develop allegedly causes injury or is alleged to be otherwise unsuitable during
product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing,
defects in design, a failure to warn of dangers inherent in the product, including as a result of interactions with alcohol or other drugs,
negligence, strict liability, and a breach of warranties. Claims could also be asserted under local jurisdiction consumer protection acts.
If we become subject to product liability claims and cannot successfully defend ourselves against them, we could incur substantial liabilities.
In addition, regardless of merit or eventual outcome, product liability claims may result in, among other things:
| |
● |
withdrawal of patients from our clinical trials; |
| |
● |
substantial monetary awards to patients or other claimants; |
| |
● |
decreased demand for our Product Candidates following marketing approval, if obtained; |
| |
● |
damage to our reputation and exposure to adverse publicity; |
| |
● |
increased FDA warnings on product labels or increased warnings imposed by the EMA or other regulatory authorities; |
| |
● |
distraction of management’s attention from our primary business; |
| |
● |
the inability to successfully commercialize our Product Candidates, if approved. |
Our current clinical trial
liability insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage
is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient
amounts to protect us against losses due to liability. If we obtain marketing approval for our Product Candidates, we intend to expand
our insurance coverage to include the sale of commercial products; however, we may be unable to obtain product liability insurance on
commercially reasonable terms or in adequate amounts. Large judgments have been awarded in class action lawsuits based on drugs that had
unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be
substantial, particularly in light of the size of our business and financial resources. A product liability claim or series of claims
brought against us could cause our share price to decline and, if we are unsuccessful in defending such a claim or claims and the resulting
judgments exceed our insurance coverage, our financial condition, results of operations, business and prospects could be materially adversely
affected.
Failure to protect our information technology
infrastructure against cyber-based attacks, network security breaches, service interruptions, or data corruption could significantly disrupt
our operations and adversely affect our business and operating results.
We rely on information technology,
telephone networks and systems, including the internet, to process and transmit sensitive electronic information and to manage or support
a variety of business processes and activities. We use enterprise information technology systems to record, process and summarize financial
information and results of operations for internal reporting purposes and to comply with regulatory, financial reporting, legal and tax
requirements. Despite the implementation of security measures, our information technology systems, and those of our third-party contractors
and consultants, are vulnerable to a cyber-attack, malicious intrusion, breakdown, destruction, loss of data privacy or other significant
disruption. Any such successful attacks could result in the theft of intellectual property or other misappropriation of assets, or otherwise
compromise our confidential or proprietary information and disrupt our operations. Cyber-attacks are becoming more sophisticated and frequent,
and our systems could be the target of malware and other cyber-attacks. We have invested in our systems and the protection of our data
to reduce the risk of an intrusion or interruption, and we monitor our systems on an ongoing basis for any current or potential threats.
Nonetheless, our computer systems are subject to penetration and our data protection measures may not prevent unauthorized access. We
can give no assurances that these measures and efforts will prevent interruptions or breakdowns. If we are unable to detect or prevent
a security breach or cyber-attack or other disruption from occurring, then we could incur losses or damage to our data, or inappropriate
disclosure of our confidential information or that of others; and we could sustain damage to our reputation, suffer disruptions to our
research and development and incur increased operating costs including increased cybersecurity and other insurance premiums, costs to
mitigate any damage caused and protect against future damage, and be exposed to additional regulatory scrutiny or penalties and to civil
litigation and possible financial liability. For instance, the loss of preclinical or clinical data could result in delays in our development
and regulatory filing efforts and significantly increase our costs.
Our failure to comply with data protection
laws and regulations could lead to government enforcement actions and significant penalties against us, and adversely impact our operating
results.
We are subject to various
domestic and international data protection laws and regulations (i.e., laws and regulations that address privacy and data security). The
legislative and regulatory landscape for data protection continues to evolve, and in recent years there has been an increasing focus on
privacy and data security issues. Numerous laws, including data breach notification laws, health information privacy laws and consumer
protection laws, govern the collection, use and disclosure of health-related and other personal information. In addition, we may obtain
health information from third parties (e.g., healthcare providers who prescribe our products) that are subject to privacy and security
requirements under HIPAA regulations.
EU Member States, Australia
and other countries have also adopted data protection laws and regulations, which impose significant compliance obligations. For example,
the collection and use of personal data in the EU is governed by the provisions of the General Data Protection Regulation, or “GDPR”.
The GDPR and the national implementing legislation of the EU Member States impose strict obligations and restrictions on the ability to
collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting. In particular, these
obligations and restrictions concern the consent of the individuals to whom the personal data relates, the information provided to the
individuals, the rights of individuals to control personal data and the security and confidentiality of the personal data. In addition,
the Australian Privacy Act 1988 (Cth), and other laws in the states and territories in Australia where we conduct certain of our clinical
trials, apply similar restrictions on our ability to collect, analyze and transfer medical records and other patient data.
A claim or series of claims
brought against us alleging a failure to comply with these laws, or changes in the way in which these laws are implemented, could lead
to government enforcement actions and significant penalties against us, and adversely impact our operating results and could cause our
share price to decline and, if we are unsuccessful in defending such a claim or claims and the resulting judgments exceed our insurance
coverage, our financial condition, results of operations, business and prospects could be materially adversely affected.
The COVID-19 coronavirus
could adversely impact our business, including several key activities that are critical to our success.
The global outbreak of COVID-19
continues to rapidly evolve. As a result, businesses have closed and limits have been placed on travel. The extent to which COVID-19 may
impact our business will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the
ultimate impact of the disease on specific geographies, the duration of the outbreak, travel restrictions and social distancing in the
United States, Canada and other countries, business closures or business disruptions and the effectiveness of actions taken in the United
States, Canada and other countries to contain and treat the disease.
The spread of COVID-19 throughout
the world has also created global economic uncertainty, which may cause partners, suppliers and potential customers to closely monitor
their costs and reduce their spending budget. Any of the foregoing could materially adversely affect our research and development activities,
clinical trials, supply chain, financial condition and cash flows.
If the COVID-19 outbreak continues
to spread, we may need to limit operations or implement other limitations on our activities. There is a risk that countries or regions
outside the United States and Canada may be less effective at vaccinations and containing COVID-19, in which case the risks described
herein could be elevated significantly.
Risks Related to our Securities
The market prices for
our common shares are volatile and will fluctuate.
The market price for our common
shares may be volatile and subject to wide fluctuations in response to numerous factors, many of which are beyond our control, including
the following: (i) actual or anticipated fluctuations in our quarterly financial results; (ii) recommendations by securities research
analysts; (iii) changes in the economic performance or market valuations of other issuers that investors deem comparable to ours; (iv)
addition or departure of our executive officers or members of our Board and other key personnel; (v) release or expiration of lock-up
or other transfer restrictions on outstanding common shares; (vi) sales or perceived sales of additional common shares; (vii) liquidity
of the common shares; (viii) significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments
by or involving us or our competitors; and (ix) news reports relating to trends, concerns, technological or competitive developments,
regulatory changes and other related issues in our industry or target markets. Financial markets often experience significant price and
volume fluctuations that affect the market prices of equity securities of public entities and that are, in many cases, unrelated to the
operating performance, underlying asset values or prospects of such entities. Accordingly, the market price of our common shares may decline
even if our operating results, underlying asset values or prospects have not changed. Additionally, these factors, as well as other related
factors, may cause decreases in asset values that are deemed to be other than temporary, which may result in impairment losses. As well,
certain institutional investors may base their investment decisions on consideration of our environmental, governance and social practices
and performance against such institutions’ respective investment guidelines and criteria, and failure to meet such criteria may
result in limited or no investment in our common shares by those institutions, which could materially adversely affect the trading price
of our common shares. There can be no assurance that continuing fluctuations in price and volume will not occur. If such increased levels
of volatility and market turmoil continue for a protracted period of time, our operations could be materially adversely impacted and the
trading price of our common shares may be materially adversely affected.
Raising additional capital
may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or Product
Candidates.
We will seek additional capital
through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing ownership interests
will be diluted and the terms of such financings may include liquidation or other preferences that adversely affect the rights of existing
shareholders. Debt financings may be coupled with an equity component, such as warrants to purchase shares, which could also result in
dilution of our existing shareholders’ ownership. The incurrence of indebtedness would result in increased fixed payment obligations
and could also result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our
ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to
conduct our business and may result in liens being placed on our assets and intellectual property. If we were to default on such indebtedness,
we could lose such assets and intellectual property. If we raise additional funds through strategic partnerships and alliances and licensing
arrangements with third parties, we may have to relinquish valuable rights to our Product Candidates or grant licenses on terms that are
not favorable to us.
Future offerings of debt
or equity securities may rank senior to common shares.
If we decide to issue debt
or equity securities in the future ranking senior to our common shares or otherwise incur additional indebtedness, it is possible that
these securities or indebtedness will be governed by an indenture or other instrument containing covenants restricting our operating flexibility
and limiting our ability to pay dividends to shareholders. Additionally, any convertible or exchangeable securities that we issue in the
future may have rights, preferences and privileges, including with respect to dividends, more favorable than those of common shares and
may result in dilution to shareholders. Because our decision to issue debt or equity securities in any future offering or otherwise incur
indebtedness will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or
nature of our future offerings or financings, any of which could reduce the market price of our common shares and dilute their value.
Future sales of common
shares by officers and directors may negatively impact the market price for our common shares.
Subject to compliance with
applicable securities laws, our directors and officers and their affiliates may sell some or all of their common shares in the future.
No prediction can be made as to the effect, if any, such future sales of common shares may have on the market price of the common shares
prevailing from time to time. However, the future sale of a substantial number of common shares by our directors and officers and their
affiliates, or the perception that such sales could occur, could adversely affect prevailing market prices for our common shares.
We do not currently pay
dividends on our common shares and have no intention to pay dividends on our common shares for the foreseeable future.
No dividends on our common
shares have been paid by us to date. We do not intend to declare or pay any cash dividends in the foreseeable future. Payment of any future
dividends will be at the discretion of our Board, after taking into account a multitude of factors appropriate in the circumstances, including
our operating results, financial condition and current and anticipated cash needs. In addition, the terms of any future debt or credit
facility may preclude us from paying any dividends unless certain consents are obtained and certain conditions are met.
We are exposed to risks
related to currency exchange rates.
We currently hold the majority
of our cash, cash equivalents and short-term investments in U.S. dollars which is our functional currency. A portion of our current operations
is conducted in Canadian dollars. Exchange rate fluctuations between other currencies and the U.S. dollar create risk in several ways,
including the following:
| |
● |
weakening of the Canadian dollar may decrease the value of our Canadian dollar cash, cash equivalents and short-term investments; |
| |
|
|
| |
● |
weakening of the U.S. dollar may increase the cost of operations and products/services sourced in Canada; |
| |
|
|
| |
● |
the exchange rates on non-U.S. dollar transactions and cash deposits can distort our financial results; and |
| |
|
|
| |
● |
commercial product pricing and profit margins are affected by currency fluctuations. |
For as long as we are
an “emerging growth company” we intend to take advantage of reduced disclosure and governance requirements applicable to emerging
growth companies, which could result in our common shares being less attractive to investors and could make it more difficult for us to
raise capital as and when we need it.
We are an “emerging
growth company,” as defined in the JOBS Act, and we have taken advantage, and intend to continue to take advantage, of certain exemptions
from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but
not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced
disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements
of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously
approved.
Investors may find our common
shares less attractive because we rely on these exemptions, which could contribute to a less active trading market for our common shares
or volatility in our share price. In addition, we may be less attractive to investors and it may be difficult for us to raise additional
capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that
our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and
when we need it, our financial condition and results of operations may be materially and adversely affected.
We may take advantage of these
reporting exemptions until we are no longer an emerging growth company.
If we fail to maintain
an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial
condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our
common shares.
We will be required, under
Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control
over financial reporting. This assessment includes disclosure of any material weaknesses identified by our management in our internal
control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial
reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will
not be prevented or detected on a timely basis. Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our
independent registered public accounting firm on the effectiveness of our internal control over financial reporting. However, for as long
as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage of the exemption permitting us not to
comply with the independent registered public accounting firm attestation requirement.
Our compliance with Section 404 will require that we incur substantial
accounting expense and expend significant management efforts. We may not be able to complete our evaluation, testing and any required
remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal
control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot
assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in
the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report
our financial condition, results of operations or cash flows. This may expose us, including individual executives, to potential liability
which could significantly affect our business. If we are unable to conclude that our internal control over financial reporting is effective,
or if our independent registered public accounting firm determines we have a material weakness in our internal control over financial
reporting once that firm begins its audits of internal control over financial reporting, we could lose investor confidence in the accuracy
and completeness of our financial reports, the market price of our common shares could decline, and we could be subject to sanctions or
investigations by Nasdaq, the SEC, or other regulatory authorities. Failure to remedy any material weakness in our internal control over
financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our
future access to the capital markets.
Our disclosure controls
and procedures may not prevent or detect all errors or acts of fraud.
Our disclosure controls and
procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Securities
Exchange Act of 1934 is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods
specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures,
no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system
are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur
because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of
two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system,
misstatements or insufficient disclosures due to error or fraud may occur and not be detected.
Deficiencies in disclosure
controls and procedures and internal control over financial reporting could result in a material misstatement in our financial statements.
We could be adversely affected
if there are deficiencies in our disclosure controls and procedures or in our internal controls over financial reporting. The design and
effectiveness of our disclosure controls and procedures and our internal controls over financial reporting may not prevent all errors,
misstatements or misrepresentations. Consistent with other entities in similar stages of development, we have a limited number of employees
currently in the accounting group, limiting our ability to provide for segregation of duties and secondary review. A lack of resources
in the accounting group could lead to material misstatements resulting from undetected errors occurring from an individual performing
primarily all areas of accounting with limited secondary review. Deficiencies in internal controls over financial reporting which may
occur could result in material misstatements of our results of operations, restatements of financial statements, other required remediations,
a decline in the price of our common shares, or otherwise materially adversely affect our business, reputation, results of operations,
financial condition or liquidity.
In connection with the
audit of our financial statements as of and for the years ended June 30, 2023 and 2022, material weaknesses in our internal control over
financial reporting were identified and we may identify additional material weaknesses in the future.
In connection with the preparation
and audits of our financial statements as of and for the years ended June 30, 2023 and 2022, a material weakness, (as defined under the
Exchange Act and by the auditing standards of the U.S. Public Company Accounting Oversight Board, or “PCAOB”), was identified
in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control
over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual financial statements
will not be prevented or detected on a timely basis. The identified control deficiencies arose from a lack of resources in our finance
function, which rose to a material weakness due to segregation of duty issues.
In light of the identified
material weakness, it is possible that, had we performed a formal assessment of our internal control over financial reporting or had our
independent registered public accounting firm performed an audit of our internal control over financial reporting in accordance with PCAOB
standards, additional control deficiencies may have been identified.
We have begun taking measures, and plan to continue to take measures,
to remediate this material weakness. However, the implementation of these measures may not fully address this material weakness in our
internal control over financial reporting, and, if so, we would not be able to conclude that they have been fully remedied. Our failure
to correct this material weakness or our failure to discover and address any other control deficiencies could result in inaccuracies in
our financial statements and could also impair our ability to comply with applicable financial reporting requirements and make related
regulatory filings on a timely basis. As a result, our business, financial condition, results of operations and prospects, as well as
the trading price of our common shares, may be materially and adversely affected.
We have incurred, and will continue to incur,
increased costs as a result of operating as a public company, and our management has been required, and will continue to be required,
to devote substantial time to new compliance initiatives.
As a public company, we have
incurred and are continuing to incur significant legal, accounting and other expenses and these expenses may increase even more after
we are no longer an “emerging growth company.” We are subject to the reporting requirements of the Exchange Act and the rules
adopted, and to be adopted, by the SEC. Our management and other personnel devote a substantial amount of time to these compliance initiatives.
Moreover, these rules and
regulations have substantially increased our legal and financial compliance costs and made some activities more time-consuming and costly.
The increased costs have increased our net loss. These rules and regulations may make it more difficult and more expensive for us to maintain
sufficient director’s and officer’s liability insurance coverage. We cannot predict or estimate the amount or timing of additional
costs we may continue to incur to respond to these requirements. The ongoing impact of these requirements could also make it more difficult
for us to attract and retain qualified persons to serve on our Board, our Board committees or as executive officers.
Future sales and issuances of our common shares
or rights to purchase common shares pursuant to our equity incentive plan could result in additional dilution of the percentage ownership
of our shareholders and may cause our share price to fall.
We expect that significant additional capital will be needed in the
future to continue our planned operations. To raise capital, we may sell substantial amounts of common shares or securities convertible
into or exchangeable for common shares. These future issuances of common shares or common share-related securities to purchase common
shares, together with the exercise of outstanding options and any additional shares issued in connection with acquisitions, if any, may
result in material dilution to our investors. Such sales may also result in material dilution to our existing shareholders, and new investors
could gain rights, preferences and privileges senior to those of holders of our common shares.
Pursuant to our 2017 Amended and Restated Stock Option Plan, and as
amended at our Annual General Meeting in November 2020, our compensation committee is authorized to grant equity-based incentive awards
in the form of options to purchase common shares to our directors, executive officers and other employees and service providers. As of
September 20, 2023, there were 51,633 options available for future allocation pursuant to the 20% of the issued and outstanding shares
allowed to be issued according to the terms of the Plan. Future equity incentive grants under our stock option plan may result in material
dilution to our shareholders and may have an adverse effect on the market price of our common shares.
Provisions in our corporate
charter documents and certain Canadian laws could delay or deter a change of control.
Provisions in our articles
and our by-laws, as well as certain provisions under the BCBCA and applicable Canadian securities laws, may discourage, delay or prevent
a merger, acquisition, tender offer or other change in control of us that some shareholders may consider favorable. In addition, because
our Board is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by
our shareholders to replace or remove our current management by making it more difficult for shareholders to replace members of our Board.
As well, our preferred shares are available for issuance from time to time at the discretion of our Board, without shareholder approval.
Our articles allow our Board, without shareholder approval, to determine the special rights to be attached to our preferred shares, and
such rights may be superior to those of our common shares.
In addition, limitations on
the ability to acquire and hold our common shares may be imposed by the Competition Act in Canada. This legislation permits the Commissioner
of Competition of Canada, or “Commissioner”, to review any acquisition of a significant interest in us. This legislation grants
the Commissioner jurisdiction to challenge such an acquisition before the Canadian Competition Tribunal if the Commissioner believes that
it would, or would be likely to, result in a substantial lessening or prevention of competition in any market in Canada. The Investment
Canada Act subjects an acquisition of control of a company by a non-Canadian to government review if the value of our assets, as calculated
pursuant to the legislation, exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied
that the investment is likely to result in a net benefit to Canada. Any of the foregoing could prevent or delay a change of control and
may deprive or limit strategic opportunities for our shareholders to sell their shares.
If securities or industry
analysts publish inaccurate or unfavorable research about our business, our share price and trading volume may decline.
The trading market for our
common shares depends in part on the research and reports that securities or industry analysts publish about us or our business. If one
or more of the analysts who cover us downgrade our shares or publish inaccurate or unfavorable research about our business, our shares
price may decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for
our shares may decrease, which may cause our shares price and trading volume to decline.
We are incorporated in Canada, with our assets
and officers primarily located in Canada, with the result that it may be difficult for investors to enforce judgments obtained against
us or some of our officers.
We are a company organized
and existing under the laws of British Columbia, Canada. Many of our directors and officers and the experts named in this Annual Form
on 10-K are residents of Canada or otherwise reside outside the United States, and all or a substantial portion of their assets, and a
substantial portion of our assets, are located outside the United States. It may be difficult for holders of common shares who reside
in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the
United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon
judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts
under the U.S. federal securities laws. Our Canadian counsel has advised us that there is doubt as to the enforceability in Canada against
us or against our directors, officers and experts who are not residents of the United States, in original actions or in actions for enforcement
of judgments of courts of the United States, of liabilities predicated solely upon U.S. federal or state securities laws.
Conversely, some of our directors
and officers reside outside Canada and some of our assets are also located outside Canada. Therefore, it may not be possible for you to
enforce in Canada against our assets or those directors and officers residing outside Canada, judgments obtained in Canadian courts based
upon the civil liability provisions of the Canadian securities laws or other laws of Canada.
Risks Related to our Financial Position and
Capital Needs
Our operating losses have raised substantial
doubt regarding our ability to continue as a going concern.
Our operating losses raise substantial doubt about
our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph
in its report on our financial statements as of and for the years ended June 30, 2023 and June 30, 2022 with respect to this uncertainty.
The perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation
of our operations and could result in the loss of confidence by investors, suppliers and employees.
We have incurred significant
losses since our inception and anticipate that we will continue to incur losses in the future.
Since our inception as a pharmaceutical
company in October 2014, we have devoted substantially all of our resources to the development of our proprietary Product Candidates.
We have generated significant operating losses since our inception with an accumulated deficit to June 30, 2023 of approximately $101.4
million. Our comprehensive losses for the fiscal years ended June 30, 2023 and 2022 were approximately $8.0 million and $18.6 million,
respectively. Substantially all of our losses have resulted from expenses incurred in connection with our research and development programs
and from general and administrative costs associated with our operations.
We expect to continue to incur
significant expenses and operating losses for the foreseeable future. We anticipate these losses will increase as we continue the research
and development of, and clinical trials for, our Product Candidates. In addition to budgeted expenses, we may encounter unforeseen expenses,
difficulties, complications, delays and other unknown factors that may adversely affect our business. If our Product Candidates fail in
preclinical or clinical trials, or do not gain regulatory approval, or even if approved, fail to achieve market acceptance, we may never
become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
Due to our limited operating
history and history of losses, any predictions about our future success, performance or viability may not be accurate.
We will require additional
capital to fund our operations and if we fail to obtain necessary financing, we will not be able to complete the development and commercialization
of our Product Candidates.
Our operations have consumed
substantial amounts of cash since inception. We expect to continue to spend substantial and increasing amounts to conduct further research
and development, preclinical testing and clinical trials of our Product Candidates, to seek regulatory approvals and reimbursement for
our Product Candidates and to launch and commercialize any Product Candidates for which we receive regulatory approval.
As of June 30, 2023, we had
approximately $9.0 million in cash, cash equivalents and short-term investments, which, we currently estimate funds our operations into
the second half of fiscal 2024, and possibly into the third quarter of fiscal 2024 (being the second calendar quarter of 2024), depending
on the level and timing of realizing revenues from the sale of BayMedica inventory as well as the level and timing of the Company’s
operating expenses. Our ability to develop our research and development programs is subject to accessing additional capital, including
through the sale of equity, partnership revenues, and out-licensing activities. There is no assurance that we will be successful in these
efforts.
The progress of our Product
Candidates for both current and prospective target indication(s) is uncertain because it is difficult to predict our spending for our
Product Candidates up to the time that we seek FDA approval due to numerous factors, including, without limitation, the rate of progress
of clinical trials, the results of preclinical studies and clinical trials for such indication, the costs and timing of seeking and obtaining
FDA and other regulatory approvals for clinical trials and FDA guidance regarding clinical trials for such indication. Moreover, changing
circumstances may cause us to expend cash significantly faster than we currently anticipate, and we may need to spend more cash than currently
expected because of circumstances beyond our control. For these reasons, we are unable to state unequivocally the actual funds we will
require for development and any approved marketing and commercialization activities. Our future funding requirements, both near and long-term,
will depend on many factors, including, but not limited to:
| |
● |
the initiation, progress, timing, costs and results of preclinical studies and clinical trials for our Product Candidates; |
| |
● |
any change in the clinical development plans or target indications for these Product Candidates; |
| |
● |
the number and characteristics of Product Candidates that we develop or may in-license; |
| |
● |
the terms of any collaboration agreements we may choose to execute; |
| |
● |
the outcome, timing and cost of meeting regulatory requirements established by the Drug Enforcement Administration, or “DEA”, the FDA, the European Medicines Agency, or “EMA”, Health Canada, or “HC”, or other comparable foreign regulatory authorities; |
| |
● |
The cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights; |
| |
● |
the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us; |
| |
● |
the effect of competing product and market developments; |
| |
● |
the costs and timing of the implementation of commercial scale manufacturing activities; and |
| |
● |
the cost of establishing, or outsourcing, sales, marketing and distribution capabilities for any Product Candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own. |
We cannot be certain that
additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts
or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one
or more of our Product Candidates or one or more of our other research and development initiatives.
Any doubt about our ability
to continue as a going concern may materially and adversely affect the price of our common shares, and it may be more difficult for us
to obtain financing. Any doubt about our ability to continue as a going concern may also adversely affect our relationships with current
and future collaborators, contract manufacturers and investors, who may become concerned about our ability to meet our ongoing financial
obligations. If potential collaborators decline to do business with us or potential investors decline to participate in any future financings
due to such concerns, our ability to increase our financial resources may be limited. We have prepared our financial statements on a going
concern basis, which assumes that we will be able to meet our commitments, realize our assets and discharge our liabilities in the normal
course of business. Our consolidated financial statements do not include any adjustment to reflect the possible future effects on the
recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this
uncertainty.
We currently have limited commercial revenue
and may never become profitable.
In addition to the limited
revenues from our BayMedica Products, our ability to generate revenue and become profitable depends upon our ability to obtain regulatory
approval for, and successfully commercialize, our Product Candidates that we may develop, in-license or acquire in the future.
Even if we are able to successfully
achieve regulatory approval for these Product Candidates, we do not know what the reimbursement status of our Product Candidates will
be or when any of these products will generate revenue for us, if at all. We have not generated, and do not expect to generate, any revenue
from Product Candidates for the foreseeable future, and we expect to continue to incur significant operating losses for the foreseeable
future due to the cost of research and development, preclinical studies and clinical trials and the regulatory approval process for our
Product Candidates. The amount of future losses is uncertain and will depend, in part, on the rate of growth of our expenses.
Our ability to generate revenue
and become profitable depends upon a number of additional factors, including our ability to:
| |
● |
successfully complete development activities, including the remaining preclinical studies and ongoing and planned clinical trials for our Product Candidates; |
| |
● |
in-license or acquire in the future, Product Candidates and other potential lines of business that we may develop; |
| |
● |
complete and submit NDAs to the FDA and Marketing Authorization Applications, or “MAAs”, to the EMA, and obtain regulatory approval for indications for which there is a commercial market; |
| |
● |
complete and submit applications to, and obtain regulatory approval from, other foreign regulatory authorities; |
| |
● |
manufacture any approved products in commercial quantities and on commercially reasonable terms; |
| |
● |
develop a commercial organization, or find suitable partners, to market, sell and distribute approved products in the markets in which we have retained commercialization rights; |
| |
● |
achieve acceptance among patients, clinicians and advocacy groups for any products we develop; |
| |
● |
obtain coverage and adequate reimbursement from third parties, including government payors; and |
| |
● |
set a commercially viable price for any products for which we may receive approval. |
We are unable to predict the
timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. Even if we are able to complete
the processes described above, we anticipate incurring significant costs associated with commercializing our Product Candidates.
Changes in tax laws and
unanticipated tax liabilities could adversely affect our effective income tax rate and ability to achieve profitability.
We are subject to income taxes
in the United States and Canada. As our operations expand, we may become subject to income tax in jurisdictions outside of the United
States and Canada. Our effective income tax rate in the future could be adversely affected by a number of factors including changes in
the mix of earnings (losses) in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities
and changes in tax laws. We regularly assess all of these matters to determine the adequacy of our tax provision which is subject to discretion.
If our assessments are incorrect, it could have an adverse effect on our business and financial condition. There can be no assurance that
income tax laws and administrative policies with respect to the income tax consequences generally applicable to us or to our subsidiaries
will not be changed in a manner which adversely affects our shareholders.
Our ability to use our
net operating loss carryforwards and other tax attributes may be limited.
As of our last fiscal year
end, we had non-capital loss, or “NOL”, carry-forwards of approximately $71.6 million available to offset future taxable income
in Canada and the United States. These NOL carry-forwards begin to expire in 2026.
Our NOL carryforwards could
expire unused and be unavailable to offset future income tax liabilities. Under provisions in the Canadian Income Tax Act, and corresponding
provisions of Canadian provincial law, if a corporation undergoes an “ownership change,” generally defined as a greater than
50% change, by value, the corporation’s ability to use its pre-change Canadian NOLs and other pre-change tax attributes, such as
research and development tax credits, to offset its post-change income may be limited. Specifically, NOLs from a business before the change
of control may be carried forward to taxation years after the change of control, but only if the same business is carried forward on after
the change in control with a reasonable expectation of profit, and only to offset income from that business or a similar business. We
have not performed any analyses under the applicable provisions in the Canadian Income Tax Act and cannot forecast or otherwise determine
our ability to derive benefit from our various federal or provincial tax attribute carryforwards. As a result, if we earn net taxable
income, our ability to use our pre-change NOL carryforwards to offset Canadian federal taxable income may be subject to limitations, which
could potentially result in increased future tax liability to us. In addition, at the provincial level, there may be periods during which
the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase provincial taxes owed.
In addition, we may experience
ownership changes in the future as a result of subsequent shifts in our share ownership, including in any future offerings, some of which
may be outside of our control. If we determine that an ownership change has occurred and our ability to use our NOL carryforwards is materially
limited, it would harm our future operating results by effectively increasing our future tax obligations.
Changes to accounting
standards may adversely impact the manner in which we report our financial position and operating results.
There are ongoing projects
conducted by the Financial Accounting Standards Board in the United States that are expected to result in new pronouncements that continue
to evolve, which could adversely impact the manner in which we report our financial position and operating results.
Global Economic Uncertainty
Changes in the global economic
environment have created market uncertainty and volatility in recent years. The market and demand for metal commodities and related products
has in recent years been adversely affected by global economic uncertainty, reduced confidence in financial markets, the COVID-19 pandemic,
bank failures and credit availability concerns. These macro-economic events negatively affected the mining and minerals sectors in general.
Global financial conditions remain subject to sudden and rapid destabilizations in response to economic shocks. A slowdown in the financial
markets or other economic conditions, including but not limited to reduced consumer spending, decreased employment rates, adverse business
conditions, high inflation, high fuel and energy costs, high consumer debt levels, a lack of available credit, the state of turmoil in
the financial markets, high interest rates and/or tax rates, may adversely affect the Company’s growth and profitability. Future
economic shocks may be precipitated by a number of causes, including the slowdown in the Chinese economy, a rise in the price of oil and
other commodities, climate change disasters, geopolitical instability, further wars or acts of terrorism, the devaluation and volatility
of global stock markets and natural disasters. Any sudden or rapid destabilization of global economic conditions could impact the Company’s
ability to obtain equity or debt financing in the future on terms favorable to the Company or at all. In such an event, the Company’s
operations and financial condition could be adversely impacted.
The Company assesses on a
quarterly basis the carrying values of its assets. Should market conditions and commodity prices worsen and persist in a worsened state
for a prolonged period of time, an assessment of the Company’s assets for impairment may be required.
Risks Related to our Intellectual Property
Our success is largely
dependent upon our patents, proprietary technology, and other intellectual property.
Our success will depend, in
part, on our ability to obtain patents, protect our trade secrets and operate without infringing on the proprietary rights of others.
Patents and other proprietary rights are essential to our business. We rely on trade secret, patent, copyright and trademark laws, and
confidentiality and other agreements with employees and third parties, all of which offer only limited protection. Our general policy
has been to file patent applications to protect our inventions and improvements to our inventions that are considered important to the
development of our business. In certain cases, we have chosen to protect our intellectual property by treating it as confidential internal
know-how. Our success will depend in part on our ability to obtain patents, defend patents, maintain internal know-how/trade secret protection
and operate without infringing on the proprietary rights of others. Interpretation and evaluation of pharmaceutical patent claims present
complex legal and factual questions. Further, patent protection may not be available for some of the products or technology we are developing.
If we are placed in a position where we must spend significant time and money defending or enforcing our patents, designing around patents
held by others or licensing patents or other proprietary rights held by others, our business, results of operations and financial condition
may be harmed. In seeking to protect our inventions using patents it is important to note that we have no assurance that:
| |
● |
patent applications will result in the issuance of patents; |
| |
● |
additional proprietary products developed will be patentable; |
| |
● |
patents issued will provide adequate protection or any competitive advantages; |
| |
● |
patents issued will not be successfully challenged by third parties; |
| |
● |
commercial exploitation of our inventions does not infringe the patents or intellectual property of others; or |
| |
● |
we will be able to obtain any extensions of the patent term. |
A number of pharmaceutical,
biotechnology and medical device companies and research and academic institutions have developed technologies, filed patent applications
or received patents on various technologies that may be related to our business. Some of these technologies, applications or patents could
limit the scope of the patents, if any, that we may be able to obtain. It is also possible that these technologies, applications or patents
may preclude us from obtaining patent protection for our inventions. Further, there may be uncertainty as to whether we may be able to
successfully defend any challenge to our patent portfolio. Moreover, we may have to participate in derivation proceedings, inter partes
review proceedings, post-grant review proceedings, or opposition proceedings in the various jurisdictions around the world. An unfavorable
outcome in a derivation proceeding, an inter partes review proceeding, a post-grant review proceeding, or an opposition proceeding
could preclude us or our collaborators or licensees from making, using or selling products using the technology, or require us to obtain
license rights from third parties. It is not known whether any prevailing party would offer a license on commercially acceptable terms,
if at all. Further, any such license could require the expenditure of substantial time and resources and could harm our business. If such
licenses are not available, we could encounter delays or prohibition of the development or introduction of our product. In the case of
intellectual property where we have chosen to protect it by treating it as internal knowhow, there can be no assurance that others with
greater expertise or access to greater resources do not develop similar or superior technology that impairs the competitive value of our
internal know-how.
Obtaining and maintaining our patent protection
depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent
agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The U.S. Patent and Trademark
Office, or “PTO”, and various foreign national or international patent agencies require compliance with a number of procedural,
documentary, fee payment and other similar provisions during the patent application process. Periodic maintenance fees on any issued patent
are due to be paid to the PTO and various foreign national or international patent agencies in several stages over the lifetime of the
patent. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable
rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in
partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse
of patent rights include, but are not limited to, failure to timely file national and regional stage patent applications based on our
international patent application, failure to respond to official actions within prescribed time limits, non-payment of fees and failure
to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our Product Candidates,
our competitors might be able to enter the market, which would have a material adverse effect on our business.
We may become subject to claims by third parties
asserting that we or our employees have misappropriated their intellectual property or claiming ownership of what we regard as our own
intellectual property.
Our commercial success depends
upon our ability to develop, manufacture, market and sell our Product Candidates, and to use our related proprietary technologies without
violating the intellectual property rights of others. We may become party to, or threatened with, future adversarial proceedings or litigation
regarding intellectual property rights with respect to our Product Candidates, including interference or derivation proceedings before
the PTO or other international patent offices. Third parties may assert infringement claims against us based on existing patents or patents
that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required
to obtain a license from such third party to continue commercializing our Product Candidates. However, we may not be able to obtain any
required license on commercially reasonable terms or at all. Under certain circumstances, we could be forced, including by court order,
to cease commercializing the applicable product candidate. In addition, in any such proceeding or litigation, we could be found liable
for monetary damages. A finding of infringement could prevent us from commercializing our Product Candidates or force us to cease some
of our business operations, which could materially harm our business. Any claims by third parties that we have misappropriated their confidential
information or trade secrets could have a similar negative impact on our business.
While our preclinical studies
are ongoing, we believe that the use of our Product Candidates in these preclinical studies fall within the scope of the exemptions provided
by 35 U.S.C. Section 271(e) in the United States, which exempts from patent infringement liability activities reasonably related to the
development and submission of information to the FDA. As our Product Candidates progress toward clinical trials and, ultimately, commercialization,
the possibility of a patent infringement claim against us increases. We attempt to ensure that our Product Candidates and the methods
we employ to manufacture them, as well as the methods for their uses we intend to promote, do not infringe other parties’ patents
and other proprietary rights. There can be no assurance they do not, however, and competitors or other parties may assert that we infringe
their proprietary rights in any event.
We may become involved in lawsuits to protect
or enforce our intellectual property, which could be expensive, time consuming and unsuccessful and have a material adverse effect on
the success of our business.
Competitors may infringe our
patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, litigation
may be necessary in the future to enforce or defend our intellectual property rights, to protect our trade secrets or to determine the
validity and scope of our own intellectual property rights or the proprietary rights of others. Also, third parties may initiate legal
proceedings against us to challenge the validity or scope of intellectual property rights we own. These proceedings can be expensive and
time consuming. Many of our current and potential competitors have the ability to dedicate substantially greater resources to defend their
intellectual property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing
upon or misappropriating our intellectual property. Litigation could result in substantial costs and diversion of management resources,
which could harm our business and financial results. In addition, in an infringement proceeding, a court may decide that a patent owned
by us is invalid or unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents
do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of
being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in
connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure
during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings
or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on
the price of our common shares.
If we are not able to adequately prevent disclosure
of trade secrets and other proprietary information, the value of our technology and products could be significantly diminished.
We rely on trade secrets to
protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade
secrets are difficult to protect. We rely in part on confidentiality agreements with our current and former employees, consultants, outside
scientific collaborators, sponsored researchers, contract manufacturers, vendors and other advisors to protect our trade secrets and other
proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate
remedy in the event of unauthorized disclosure of confidential information. In addition, we cannot guarantee that we have executed these
agreements with each party that may have or have had access to our trade secrets. Any party with whom we or they have executed such an
agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain
adequate remedies for such breaches.
Enforcing a claim that a party
illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In
addition, some courts are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained
or independently developed by a competitor, we would have no right to prevent them, or those to whom they disclose such trade secrets,
from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed
by a competitor or other third-party, our competitive position would be harmed.
We may not be able to protect our intellectual
property rights throughout the world.
Filing, prosecuting and defending
patents on all of our Product Candidates throughout the world would be prohibitively expensive. Therefore, we have filed applications
and/or obtained patents only in key markets such as the United States, Canada, Japan and Europe. Competitors may use our technologies
in jurisdictions where we have not obtained patent protection to develop their own products and, further, may be able to export otherwise
infringing products to territories where we have patent protection but where enforcement is not as strong as that in the United States.
These products may compete with our products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual
property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered
significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of certain
countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection,
particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing
of competing products in violation of our proprietary rights generally. For example, an April 2016 report from the Office of the United
States Trade Representative identified a number of countries, including India and China, where challenges to the procurement and enforcement
of patent rights have been reported. Several countries, including India and China, have been listed in the report every year since 1989.
As a result, proceedings to enforce our patent rights in certain foreign jurisdictions could result in substantial cost and divert our
efforts and attention from other aspects of our business and could be unsuccessful.
Patent terms may be inadequate to protect our
competitive position on our Product Candidates for an adequate amount of time.
Given the amount of time required
for the development, testing and regulatory review of new Product Candidates, patents protecting such candidates might expire before or
shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the United States and, if available,
in other countries where we are prosecuting patents. In the United States, the Drug Price Competition and Patent Term Restoration Act
of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent, which is limited to the approved
indication (or any additional indications approved during the period of extension). However, the applicable authorities, including the
FDA and the PTO, and any equivalent regulatory authorities in other countries, may not agree with our assessment of whether such extensions
are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs,
our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical
data and launch their product earlier than might otherwise be the case.
Intellectual property
rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection
afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately
protect our business, or permit us to maintain our competitive advantage. For example:
| |
● |
others may be able to make compounds that are the same as or similar to our Product Candidates but that are not covered by the claims of the patents that we own; |
| |
● |
we might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own; |
| |
● |
we might not have been the first to file patent applications covering certain of our inventions; |
| |
● |
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| |
● |
it is possible that our pending patent applications will not lead to issued patents; |
| |
● |
issued patents that we own may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges; |
| |
● |
our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; or |
| |
● |
the patents of others may have an adverse effect on our business. |
Risks Related to our Third Parties
We rely heavily on contract manufacturers over
whom we have limited control. If we are subject to quality, cost or delivery issues with the preclinical and clinical grade materials
supplied by contract manufacturers, our business operations could suffer significant harm.
We currently have no manufacturing
capabilities and rely on contract development and manufacturing organizations, or “CDMOs”, to manufacture our Product Candidates
for preclinical studies and clinical trials. We rely on CDMOs for manufacturing, filling, packaging, testing, storing and shipping of
drug products in compliance with cGMP, regulations applicable to our products. The FDA and other regulatory agencies ensure the quality
of drug products by carefully monitoring drug manufacturers’ compliance with cGMP regulations. The cGMP regulations for drugs contain
minimum requirements for the methods, facilities and controls used in manufacturing, processing and packaging of a drug product. If our
CDMOs increase their prices or fail to meet our quality standards, or those of regulatory agencies such as the FDA, and cannot be replaced
by other acceptable CDMOs, our ability to obtain regulatory approval for and commercialize our Product Candidates may be materially adversely
affected.
The APIs used in all of our
Product Candidates are currently sourced from either contract manufacturers or, for smaller quantities, from research material suppliers,
that typically utilize synthetic chemistry as their manufacturing method. This is intended to be an interim step to enable us to proceed
with developing our formulation, execute preclinical toxicology studies and progress through Phase 1 and 2 clinical trials, after which
time we anticipate that we will have been able to successfully scale-up our IntegraSyn manufacturing approach so that it will be GMP-
ready at pharmaceutical grade. Bridging studies consisting of chemical analysis and, possibly, animal studies may be required in order
to switch our APIs from the current external manufacturing sources to our internally manufactured products. There is no guarantee that
we will be successful in scaling up our IntegraSyn manufacturing process for cannabinoids, or successfully complete any required bridging
studies, or be able to successfully transfer our IntegraSyn manufacturing process to a CDMO. The key risks and challenges associated with
the development of the IntegraSyn process include: failure to continue optimization and development of the process manufacturing steps
from the current scale while maintaining the same or greater output of the selected cannabinoid; equipment and techniques may not be able
to be scaled up using existing commercial processing equipment; supply of the key starting materials for the process may not be secured
to ensure stability and security of commercial supply; and, failure of the large scale process to consistently produce the selected cannabinoid
within set specifications and meeting the process parameters and in process controls to enable the manufacturing process to be validated
for GMP commercial production of an API, among others. Failing to accomplish these or other criteria for the IntegraSyn manufacturing
process with a CDMO may mean that we are not able to produce certain cannabinoids in a cost-effective manner. This could result in us
not being able to successfully commercialize or utilize our APIs in our Product Candidates, if any, that may obtain regulatory approval.
Our existing collaboration
agreements and any that we may enter into in the future may not be successful.
We also have relationships
with scientific collaborators at academic and other institutions, some of whom conduct research at our request or assist us in formulating
our research and development strategies. These scientific collaborators are not our employees and may have commitments to, or consulting
or advisory contracts with, companies that conflict in interests with and pose a competitive threat to us. Moreover, to the extent that
we decide to enter into collaboration agreements, we will face significant competition in seeking appropriate collaborators. Collaboration
arrangements are complex and time consuming to negotiate, document and implement. We may not be successful in our efforts to establish,
implement and maintain collaborations or other alternative arrangements if we choose to enter into such arrangements and our selected
partners may be given, and may exercise, a right to terminate their agreement with us without cause. Our Collaborative Research Agreement
with the University of British Columbia may be terminated by either party upon 30 calendar days written notice. The terms of any collaboration
or other arrangements that we may establish may not be favorable to us.
For all of the aforesaid
reasons and others set forth in this Annual Form on 10-K, an investment in our common shares and any other securities that we may offer
from time to time involves a certain degree of risk. Any person considering an investment in our common shares or any other of our securities
should be aware of these and other factors set forth in this 10-K and should consult with his or her legal, tax and financial advisors
prior to making an investment in our common shares or any other of our securities that may be offered from time to time. Our common shares
and any other securities that we may offer from time to time should only be purchased by persons who can afford to lose all of their investment.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our corporate headquarters
are located at Suite 310 - 815 W. Hastings Street, Vancouver, British Columbia V6C 1B4, Canada. This office occupies approximately 4,477
square feet with a monthly basic rental rate and operating charges of an estimated C$17,402 for the first two years, C$17,775 for the
third and fourth years, and C$18,521 for the fifth year. This lease expires on August 31, 2024.
In July 2019, InMed entered
into a facility lease agreement for approximately 4,000 square feet of office space in Vancouver, BC, which serves as our corporate headquarters.
The lease was set to expire in August 2024. The lease has an option to renew for an additional three-year period at our discretion.
In conjunction with the acquisition
of BayMedica, the Company acquired a facility lease agreement for approximately 7,000 square feet of office space in South San Francisco,
California. The lease is set to expire in April 2024.
We believe substantially all
of our property and equipment is in good condition and that InMed has sufficient capacity to meet its current operational needs. We further
believe that, should it be needed, suitable additional space is available to accommodate any expansion of our operations, but such space
may not be available in the same building, if and when such space is needed.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we are
subject to various legal proceedings, claims and administrative proceedings that arise in the ordinary course of our business activities.
Although the results of the litigation and claims cannot be predicted with certainty, as of the date of this report, we do not believe
we are party to any claim, proceeding or litigation the outcome of which, if determined adversely to us, would individually or in the
aggregate be reasonably expected to have a material adverse effect on our business. Regardless of the outcome, litigation can have an
adverse impact on us because of defense and settlement costs, diversion of management resources and other factors. However, as of the
date of this Annual Form on 10-K, we are not involved in any material pending legal or governmental proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S
COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
The Company’s shares
are listed on the on the Nasdaq Capital Market (“Nasdaq”) under the trading symbol “INM”).
There were approximately 770
holders of record of our common stock as of September 20, 2023. On September 20, 2022, the last reported sales price per share of our
common stock was $0.81 per share.
Unregistered Sales of Equity Securities
None.
Repurchases of Equity Securities
None.
ITEM 6. [RESERVED]
We are a smaller reporting
company as defined by Rule 12b-2 of the Exchange Act and have therefore omitted the information required by this Item 6.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This
discussion and analysis contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”),
and is subject to the safe harbor created by those sections. For more information, see “Cautionary Note Regarding Forward-Looking
Statements.” When reviewing the discussion below, you should keep in mind the substantial risks and uncertainties that impact our
business. In particular, we encourage you to review the risks and uncertainties described in “Risk Factors” in this Annual
Report on Form 10-K. These risks and uncertainties could cause actual results to differ materially from those projected or implied by
our forward-looking statements contained in this report. These forward-looking statements are made as of the date of this report, and
we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law.
The
following discussion and analysis should be read in conjunction with our audited consolidated financial statements for the year ended
June 30, 2023, and the related notes thereto, which have been prepared in accordance with U.S. GAAP, included in our Form 10-K filing.
Throughout this discussion, unless the context specifies or implies otherwise the terms “InMed,” “Company,” “we,”
“us,” and “our” refer to InMed Pharmaceuticals Inc.
All dollar amounts stated herein are in
U.S. dollars unless specified otherwise.
Overview
We are a clinical stage pharmaceutical
company developing a pipeline of prescription-based products, including rare cannabinoids and novel cannabinoid analogs, targeting the
treatment of diseases with high unmet medical needs. Together with our subsidiary, BayMedica, we also have significant know-how in developing
proprietary manufacturing approaches to produce cannabinoids for various market sectors. Our know-how includes traditional approaches
such as chemical synthesis and biosynthesis, as well as a proprietary, integrated manufacturing approach called IntegraSyn. We are dedicated
to delivering new therapeutic alternatives to patients and consumers who may benefit from cannabinoid-based products. Our approach leverages
on the several thousand years’ history of health benefits attributed to the Cannabis plant and brings this anecdotal information
into the 21st century by applying tried, tested and true scientific approaches to establish non-plant-derived (synthetically manufactured),
individual cannabinoid compounds as Product Candidates for InMed’s pharmaceutical product development pipeline or specific rare
cannabinoid Products sold to end-product manufacturers by BayMedica. While our activities do not involve direct use of Cannabis
nor extracts from the plant, we note that the FDA has, to date, not approved any marketing application for Cannabis for the treatment
of any disease or condition and has approved only one Cannabis-derived and three Cannabis-related drug products. Our ingredients
are synthetically made and, therefore, we have no interaction with the Cannabis plant. We do not grow nor utilize Cannabis
nor its extracts in any of our Products or Product Candidates; our current pharmaceutical drug Product Candidates are applied topically
(not inhaled nor ingested); and, we do not utilize THC or CBD, the most common cannabinoid compounds that are typically extracted from
the Cannabis plant, in any of our Products or Product Candidates. The API under development for our initial two drug candidates,
INM-755 for EB and INM-088 for glaucoma, is CBN. Additional uses of both INM-755 and INM-088 are being explored, as well as the application
of novel cannabinoid analogs to treat diseases including but not limited to neurodegenerative diseases such as Alzheimer’s, Parkinson’s,
and Huntington’s.
We believe we are positioned
to develop multiple pharmaceutical Product Candidates in diseases which may benefit from medicines based on rare cannabinoid compounds.
Most currently approved cannabinoid therapies are based specifically on CBD and/or THC and are often delivered orally, which has limitations
and drawbacks, such as side effects (including the intoxicating effects of THC). Currently, we intend to deliver our rare cannabinoid
pharmaceutical Product Candidates through various topical formulations (cream for dermatology, eye drops for ocular diseases) as a way
of enabling treatment of the specific disease at the site of disease while seeking to minimize systemic exposure and any related unwanted
systemic side effects, including any drug-drug interactions and any metabolism of the active pharmaceutical ingredient by the liver. The
cannabinoid Products sold through our B2B raw material supply business are integrated into various product formats by the companies who
then further commercializes such products. We access rare cannabinoids via all non-extraction approaches, including chemical synthesis,
biosynthesis and our proprietary integrated IntegraSyn approach, thus negating any interaction with or exposure to the Cannabis
plant.
Since our acquisition of Biogen
Sciences Inc., a privately held British Columbia pharmaceutical company focused on drug discovery and development of cannabinoids in 2014,
our operations have focused on conducting research and development for our Product Candidates and for our integrated, biosynthesis-based
manufacturing technology, establishing our intellectual property, organizing and staffing our Company, business planning and capital raising.
On October 13, 2021, we acquired BayMedica, Inc., now named BayMedica, LLC. Upon closing of the transaction, BayMedica became a wholly-owned
subsidiary of InMed. To date, we have funded our operations primarily through the issuance of common shares.
We have incurred significant operating losses since our inception and
since the acquisition of Biogen Science Inc. and we expect to continue to incur significant operating losses for the foreseeable future.
Our ability to generate product revenue that is sufficient to achieve profitability will depend heavily on the revenues generated from
our products in the Health and Wellness sector, on the successful development and eventual commercialization of one or more of our Product
Candidates and/or the success of our manufacturing technologies. Our net loss was $8.0 million and $18.6 million for the year ended June
30, 2023 and 2022, respectively. As of June 30, 2023, we had an accumulated deficit of $101.4 million, which includes all losses since
our inception in 1981. We expect our expenses will remain steady as we:
| |
● |
seek partnerships to advance the INM-755 program, our lead drug candidate for the treatment of EB; |
| |
● |
continue to further advance research into the role of cannabinoids in treating ocular diseases; |
| |
● |
continue to advance research in the INM-900 series program, using
cannabinoid analogs in treating neurodegenerative diseases such as Alzheimer’s, Huntington’s and Parkinson’s; |
| |
● |
investigate our Product Candidates for additional uses beyond their initial target indications; |
| |
● |
pursue the discovery of drug targets based on proprietary cannabinoid analogs for other diseases with high unmet medical needs and the subsequent development of any resulting new Product Candidates; |
| |
● |
seek regulatory approvals for any Product Candidates that successfully complete clinical trials; |
| |
● |
scale-up our manufacturing processes and
capabilities, or arrange for a third party to do so on our behalf; |
| |
● |
continue to support our commercial operations
and revenue-generating Products at BayMedica; |
| |
● |
execute on business development activities, including but not limited to company mergers/acquisitions and acquisition or in-licensing of externally developed products and/or technologies; |
| |
● |
maintain, expand, enforce, defend and protect
our intellectual property; |
| |
● |
continue to further advance the research
and development of various manufacturing technologies; |
| |
● |
build internal infrastructure, including personnel, to meet our milestones; and |
| |
● |
add operational, financial and management information
systems and personnel, including personnel to support product development and potential future commercialization efforts and our operations
as a public company.
|
As a result of these activities
as well as our working capital requirements, we will need substantial additional funding to support our continuing operations and pursue
our growth strategy. We expect to finance our operations through product sales, the sale of equity, debt financings or other capital sources,
including collaborations with other companies or other strategic transactions. We may be unable to raise additional funds or enter into
such other agreements or arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such agreements
as and when needed, we may have to significantly delay, scale back or discontinue the development and commercialization of one or more
of our Products and Product Candidates or grant rights to external entities to develop and market our Product Candidates, even if we would
otherwise prefer to develop and market such Products and Product Candidates ourselves.
Because of the numerous risks
and uncertainties associated with drug development and commercial growth, we are unable to predict the timing or amount of increased expenses
and working capital requirements or the timing of when or if we will be able to achieve or maintain profitability. If we fail to become
profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels
and be forced to reduce or terminate our operations.
Recent Developments
We completed our Phase 2 trial
on INM-755 in April of 2023, and we have released preliminary results in June 2023. We anticipate publishing the full data from the study
in the second quarter of fiscal 2024.
Components of Results of Operations
Revenue
Our revenue consists of manufacturing
and distribution sales of bulk rare cannabinoid Products, which are generally recognized at a point in time. The Company recognizes revenue
when control over the products have been transferred to the customer and the Company has a present right to payment.
Cost of Sales
Cost
of sales consist primarily of the purchase price of goods and cost of services rendered, freight costs, warehousing costs, and purchasing
costs. Cost of sales also includes production and labor costs for our manufacturing business.
Operating Expenses
Research and Development and Patent Expenses
Research and development and
patent expenses represent costs incurred by us for the discovery, development, and manufacture of our Products and Product Candidates
and include:
| |
● |
external research and development expenses incurred under agreements with contract research organizations, or “CROs”, contract development and manufacturing organization, or “CDMOs”, and consultants; |
| |
|
|
| |
● |
salaries, payroll taxes, employee benefits expenses for individuals involved in research and development efforts; |
| |
|
|
| |
● |
research supplies; and |
| |
|
|
| |
● |
legal and patent office fees related to patent and intellectual property matters. |
We expense research and development costs
as incurred. We recognize expenses for certain development activities, such as preclinical studies and manufacturing, based on an evaluation
of the progress to completion of specific tasks using data or other information provided to us by our vendors. Payments for these activities
are based on the terms of the individual agreements, which may differ from the pattern of expenses incurred. Non-refundable advance payments
for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. These
amounts are recognized as an expense as the goods are delivered or the related services are performed, or until it is no longer expected
that the goods will be delivered, or the services rendered.
External costs represent a
significant portion of our research and development expenses, which we track on a program-by-program basis following the nomination of
a development candidate. Our internal research and development expenses consist primarily of personnel-related expenses, including salaries,
benefits and stock-based compensation expense. We do not track our internal research and development expenses on a program-by-program
basis as the resources are deployed across multiple projects.
The successful development
of our Products and Product Candidates is highly uncertain. At this time, we cannot reasonably estimate or know the nature, timing, and
estimated costs of the efforts that will be necessary to complete the remainder of the development of our Product Candidates or to develop
and commercialize additional Products. We are also unable to predict when, if ever, material net cash inflows will commence from our Product
Candidates, if approved. This is due to the numerous risks and uncertainties associated with development, including the uncertainty related
to:
| |
● |
the timing and progress of preclinical and clinical development activities; |
| |
● |
the number and scope of preclinical and clinical
programs we decide to pursue; |
| |
● |
our ability to raise additional funds necessary to complete preclinical and clinical development and commercialization of our Product Candidates, to further advance the development of our manufacturing technologies, and to develop and commercialize additional Products, if any; |
| |
● |
our ability to maintain our current research and development programs and to establish new ones; |
| |
● |
our ability to establish sales, licensing
or collaboration arrangements; |
| |
● |
the progress of the development efforts of parties with whom we may enter into collaboration arrangements; |
| |
● |
the successful initiation and completion of clinical trials with safety, tolerability and efficacy profiles that are satisfactory to the FDA or any comparable foreign regulatory authority; |
| |
● |
the receipt and related terms of regulatory approvals from applicable regulatory authorities; |
| |
● |
the availability of materials for use in production of our Products and Product Candidates; |
| ● | our ability to secure manufacturing supply through relationships
with third parties or establish and operate a manufacturing facility; |
| ● | our ability to consistently manufacture our Product Candidates
in quantities sufficient for use in clinical trials; |
| ● | our ability to obtain and maintain intellectual property protection
and regulatory exclusivity, both in the United States and internationally; |
| ● | our ability to maintain, enforce, defend and protect our rights
in our intellectual property portfolio; |
| ● | the commercialization of our Product Candidates, if and when
approved, and of new Products; |
| ● | our ability to obtain and maintain third-party payor coverage
and adequate reimbursement for our Product Candidates, if approved; |
| ● | the acceptance of our Product Candidates, if approved, by
patients, the medical community and third-party payors; |
| ● | competition with other products; and |
| ● | a continued acceptable safety profile of our Product Candidates
following receipt of any regulatory approvals. |
A change in the outcome of
any of these variables with respect to the development of any of our Products or Product Candidates would significantly change the costs
and timing associated with the development of those Products or Product Candidates.
Research and development activities account for a significant portion
of our operating expenses. Research and development expenses decreased in fiscal 2023 as compared to fiscal 2022, largely due to high
start-up costs associated with the multicenter Phase 2 clinical trial in our INM-755 program during fiscal 2022. However, we expect our
research and development expenses to increase significantly in future periods as we continue to implement our business strategy, which
includes advancing our drug candidates and our manufacturing technologies into and through clinical development, expanding our research
and development efforts, including hiring additional personnel to support our research and development efforts, ultimately seeking regulatory
approvals for our drug candidates that successfully complete clinical trials, and further developing selected R&D and commercial BayMedica
activities. In addition, drug candidates in later stages of clinical development generally incur higher development costs than those in
earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. Accordingly,
although we expect our research and development expenses to increase as our drug candidates advance into later stages of clinical development,
we do not believe that it is possible, at this time, to accurately project total program-specific expenses through to commercialization.
There are numerous factors associated with the successful commercialization of any of our Product Candidates, including future trial design
and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development.
General and Administrative Expenses
General and administrative
expenses consist of personnel-related costs, including salaries, benefits and stock-based compensation expense, for our personnel in executive,
finance and accounting, human resources, business operations and other administrative functions, investor relations activities, legal
fees related to corporate matters, fees paid for accounting and tax services, consulting fees and facility-related costs.
Amortization and Depreciation
Intangible assets are comprised
of intellectual property that we acquired in 2014 and 2015 and trade secrets, product formulation knowledge, patents that we acquired
in October 2021. The acquired intellectual property and patents are amortized on a straight-line basis based on their estimated useful
lives. Equipment and leasehold improvements are depreciated using the straight-line method based on their estimated useful lives.
Impairment of Long-Lived Assets
We assess the recoverability
of our long-lived assets whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Recoverability of the long-lived asset is measured by a comparison of the carrying amount of the asset to future undiscounted net cash
flows expected to be generated by the asset or assets. If carrying value exceeds the sum of undiscounted cash flows, we then determine
the fair value of the underlying asset. Any impairment to be recognized is measured as the amount by which the carrying amount of the
asset group exceeds the estimated fair value of the asset group. Assets classified as held for sale are reported at the lower of the carrying
amount or fair value, less costs to sell.
Share-based Payments
Share-based payments is the
stock-based compensation expense related to our granting of stock options to employees and others. The fair value, at the grant date,
of equity-settled share awards is charged to our loss over the period for which the benefits of employees and others providing similar
services are expected to be received. The vesting components of graded vesting employee awards are measured separately and expensed over
the related tranche’s vesting period. The amount recognized as an expense is adjusted to reflect the number of share options expected
to vest. The fair value of awards is calculated using the Black-Scholes option pricing model, which considers the exercise price, current
market price of the underlying shares, expected life of the award, risk-free interest rate, expected volatility and the dividend yield.
Other Income
Other income consists primarily
of interest income earned on our cash, cash equivalents and short-term investments.
Results of Operations
As of the closing of the BayMedica
acquisition, the Company aligned into two operating and reportable segments, InMed Pharmaceuticals (the “InMed” segment) and
BayMedica (the “BayMedica” segment).
Comparison of the year ended June 30, 2023
and 2022 for InMed Segment
| | |
Year
Ended
June 30, | | |
| | |
| |
| | |
2023 | | |
2022 | | |
Change | | |
% Change | |
| | |
(in thousands) | | |
| | |
| |
| Operating expenses: | |
| | |
| | |
| | |
| |
| Research and development and patents | |
| 2,864 | | |
| 5,986 | | |
| (3,122 | ) | |
| (52 | )% |
| General and administrative | |
| 4,022 | | |
| 5,906 | | |
| (1,884 | ) | |
| (32 | )% |
| Amortization and depreciation | |
| 105 | | |
| 107 | | |
| (2 | ) | |
| (2 | )% |
| Total operating expenses | |
| 6,991 | | |
| 11,999 | | |
| (5,008 | ) | |
| (42 | )% |
| Interest and other income | |
| 303 | | |
| 20 | | |
| 283 | | |
| 1,415 | % |
| Warrant modification expense | |
| - | | |
| (1,314 | ) | |
| 1,314 | | |
| (100 | )% |
| Foreign exchange (loss) gain | |
| (48 | ) | |
| (118 | ) | |
| 70 | | |
| (59 | )% |
| Net loss | |
$ | (6,736 | ) | |
$ | (13,411 | ) | |
$ | 6,675 | | |
| (50 | )% |
Research and Development and Patents Expenses
Research and development and patents expenses decreased by $3.1 million
in our InMed segment, or 52%, for the year ended June 30, 2023 compared to the year ended June 30, 2022. The decrease in research and
development and patents expenses was due to a combination of lower personnel expenses, legal fees and decreased expenses related to the
INM-755 program as a result of high start-up costs associated with the multicenter Phase 2 clinical trial during fiscal 2022.
General and administrative expenses
General and administrative
expenses decreased by $1.9 million in our InMed segment, or 32%, for the year ended June 30, 2023 compared to the year ended June 30,
2022. The decrease results primarily from a combination of changes including lower office and admin fees, investor relation expenses,
stock-based compensation expenses, personnel expenses, accounting, and legal fees.
Foreign exchange loss
The Company’s functional currency is US dollar and our foreign
exchange loss is predominantly due to transactions with foreign currency. Foreign exchange loss increased
by less than $0.1 million in our InMed segment, or 59%, for the year ended June 30, 2023, compared to the year ended June 30, 2022, as
a consequence of holding non-US denominated assets and liabilities combined with fluctuations in foreign exchange rates.
Comparison of the year ended June 30, 2023
and 2022 for BayMedica Segment
| | |
Year Ended
June 30, | | |
| | |
| |
| | |
2023 | | |
2022 | | |
Change | | |
% Change | |
| | |
| | |
| | |
| | |
| |
| | |
(in thousands) | | |
| | |
| |
| Sales | |
$ | 4,136 | | |
$ | 1,089 | | |
$ | 3,047 | | |
| 280 | % |
| Cost of sales | |
| 2,424 | | |
| 546 | | |
| 1,878 | | |
| 344 | % |
| Loss on decline in NRV | |
| 309 | | |
| - | | |
| 309 | | |
| 100 | % |
| Gross profit | |
| 1,403 | | |
| 544 | | |
| 860 | | |
| 158 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Research and development and patents | |
| 868 | | |
| 1,296 | | |
| (429 | ) | |
| (33 | )% |
| General and administrative | |
| 1,825 | | |
| 961 | | |
| 864 | | |
| 90 | % |
| Amortization and depreciation | |
| 98 | | |
| 79 | | |
| 19 | | |
| 24 | % |
| Impairment of intangible assets and goodwill | |
| - | | |
| 3,473 | | |
| (3,473 | ) | |
| (100 | )% |
| Total operating expenses | |
| 2,791 | | |
| 5,809 | | |
| (3,018 | ) | |
| (52 | )% |
| Interest and other income | |
| 189 | | |
| 76 | | |
| 111 | | |
| 142 | % |
| Tax expense | |
| (13 | ) | |
| - | | |
| (13 | ) | |
| nm | |
| Net loss | |
$ | (1,212 | ) | |
$ | (5,189 | ) | |
$ | 3,977 | | |
| (77 | )% |
Sales
Sales increased by $3.0 million
in our BayMedica segment, or 280%, for the year ended June 30, 2023 compared to the year ended June 30, 2022. BayMedica has now realized
three consecutive quarters of revenue growth, with increases of 46%, 100%, and 123% in Q2, Q3, and Q4 of fiscal year 2023, respectively.
While we expect revenue fluctuations based on distributor order patterns, there are no assurances that this growth will continue in future
quarters. However, the recent trend of increased sales is encouraging. The increase in distribution sales results from expanded marketing
efforts and increased demand in certain cannabinoid products. BayMedica will continue to evaluate
opportunities for potential structured supply arrangements and collaborations for the commercial business. Sales and marketing efforts
will remain focused on products that contribute highest margins, where BayMedica continues to hold a strong competitive position.
Cost of Sales
Cost of goods sold
increased by $1.9 million in our BayMedica segment, or 344%, for the year ended June 30, 2023 compared to the year ended June 30,
2022. The increase in cost of goods sold is a result from the increase in sales mentioned above Our cost of sales percentage
fluctuates based on the Products mix sold.
Inventory Write-Down
The
write-down of inventories to net realizable value was $0.3 million in our BayMedica segment for the year ended June 30, 2023, with no
comparable expenses in 2022. Contributing factors to the decrease in net realizable value included lower demand and downward pricing pressure
in the first quarter of fiscal 2023. BayMedica continues to evaluate new manufacturing approaches for certain products to increase competitive
position in the marketplace.
Research and Development and Patents Expenses
Research and development and
patents expenses decreased by $0.4 million in our BayMedica segment, or 33%, for the year ended June 30, 2023 compared to the year ended
June 30, 2022. The decrease in research and development and patents expenses was primarily due to lower personnel expenses and external
consultants. This was offset by an increase in research supplies.
General and administrative expenses
General and administrative
expenses increased by $0.9 million in our BayMedica segment, or 90%, for the year ended June 30, 2023 compared to the year ended June
30, 2022. The increase results primarily from a combination of changes including higher personnel expenses, accounting fees, legal fees
and sales and marketing expenses.
Liquidity and Capital Resources
Since our inception, we have
only generated limited revenue from product sales, no sales from any other sources and have incurred significant operating losses and
negative cash flows from our operations. We have only commenced commercial sales with the acquisition of BayMedica and not yet commercialized
any of our Product Candidates and we do not expect to generate revenue from sales of any Product Candidates for several years, if at all.
We have funded our operations to date primarily with proceeds from the sale of common shares.
As of June 30, 2023, we had cash, cash equivalents
and short-term investments of $9.0 million.
The following table summarizes our cash flows
for each of the periods presented:
| (in thousands) | |
Year Ended
June 30,
2023 | | |
Year Ended
June 30,
2022 | |
| Net cash (used in) operating activities | |
$ | (7,283 | ) | |
$ | (15,584 | ) |
| Net cash (used in) investing activities | |
| (662 | ) | |
| (673 | ) |
| Net cash provided by financing activities | |
| 10,680 | | |
| 15,071 | |
| Net increase (decrease) in cash and cash equivalents | |
$ | 2,735 | | |
$ | (1,186 | ) |
Operating Activities
During the year ended June
30, 2023, we used cash in operating activities of $7.3 million, primarily resulting from our net loss of $7.9 million combined with $0.6 million used in changes in our non-cash working capital, partially offset by non-cash share-based compensation expenses and inventory
write-down.
During the year ended June
30, 2022, we used cash in operating activities of $15.9 million, primarily resulting from our net loss of $18.6 million combined with
$2.7 million used in changes in our non-cash working capital, partially offset by non-cash share-based compensation expenses, impairment
of intangible assets and goodwill and warrant modification expense related to the change in fair value of warrants that were re-priced
during the year.
Investing Activities
During the year ended June
30, 2023, cash used in investing activities of $0.7 million resulted from escrow payments made to BayMedica’s historical equity
and convertible debt holders and purchase of property and equipment.
During the year ended June
30, 2022, cash used in investing activities of $0.7 million resulted from escrow payments made to BayMedica’s historical equity
and convertible debt holders, settlement of loan receivable from BayMedica and purchases of property and equipment, partially offset by
cash acquired from the acquisition of BayMedica.
Financing Activities
During the year ended June
30, 2023, cash provided by financing activities of $10.7 million consisted of $12.0 million of gross proceeds from private placements
of our common shares, offset by total transaction costs of $1.3 million.
During the year ended June
30, 2022, cash provided by financing activities of $15.1 million consisted of $12.0 million of gross proceeds from a private placement
of our common shares and $5.0 million of gross proceeds from a registered direct offering and concurrent private placement of our common
shares, offset by total transaction costs of $1.8 million and $0.3 million for the repayment of debt assumed in the BayMedica acquisition.
Funding Requirements
We expect our expenses to
increase substantially in connection with our ongoing research and development activities, particularly as we continue the research and
development of and the clinical trials for our Product Candidates. In addition, we expect to incur additional costs associated with operating
as a US-listed public company and associated with any required investment into BayMedica’s R&D efforts targeting cannabinoid
analogs. As a result, we expect to incur substantial operating losses and negative operating cash flows for the foreseeable future.
In accordance with the Financial
Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) 2014-15, Disclosure of Uncertainties about
an Entity’s Ability to Continue as a Going Concern (Subtopic 205-40), we have evaluated whether there are conditions and events,
considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one
year after the date that the consolidated financial statements are issued.
Through June 30, 2023, we
have funded our operations primarily with proceeds from the sale of common stock. We have incurred recurring losses and negative cash
flows from operations since its inception, including net losses of $7.9 million and $18.6 million for the year ended June 30, 2023 and
2022, respectively. In addition, we have an accumulated deficit of $101.4 million as of June 30, 2023.
As
of the issuance date of the consolidated interim financial statements, we expect our cash and cash, cash equivalents and short-term investments
of $9.0 million as of June 30, 2023 will be sufficient to fund our operating expenses and capital expenditure requirements into the first
quarter of calendar year 2024. depending on the level and timing of realizing BayMedica revenues from the sale of Products in the Health
& Wellness sector as well as the level and timing of the Company operating expenses. Our future viability is dependent on our ability
to raise additional capital to finance our operations. In addition, there are a number of uncertainties in estimating our operating expenses
and capital expenditure requirements including the impact of potential acquisitions.
As a result, we have concluded that there is substantial doubt about
our ability to continue as a going concern within one year after the date that the consolidated financial statements are issued.
We expect to continue to seek
additional funding through equity financings, debt financings or other capital sources, including collaborations with other companies,
government contracts or other strategic transactions. We may not be able to obtain financing on acceptable terms, or at all. The terms
of any financing may adversely affect the holdings or the rights of our existing stockholders.
Our funding requirements and timing and amount
of our operating expenditures will depend largely on:
| ● | the
scope, progress, results and costs of discovery research, preclinical development, laboratory testing and clinical trials for our Product
Candidates; |
| |
● |
the scope, progress, results and costs of
development of our manufacturing technologies; |
| |
● |
the number of and development requirements for other Products and Product Candidates that we pursue; |
| |
● |
the costs, timing and outcome of regulatory review of our Product Candidates; |
| |
● |
our ability to enter into contract manufacturing
arrangements for supply of materials and manufacture of our Products and Product Candidates and the terms of such arrangements; |
| |
● |
the impact of any acquired, or in-licensed, externally developed product(s) and/or technologies; |
| |
● |
our ability to establish and maintain strategic collaborations, licensing or other arrangements, including sales arrangements, and the financial terms of such arrangements; |
| |
● |
the sales, costs and timing of future commercialization activities, including product manufacturing, sales, marketing and distribution, for any of our Products and for Product Candidates for which we may receive marketing approval; |
| |
● |
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property and proprietary rights and defending any intellectual property- related claims; |
| |
● |
expansion costs of our operational, financial and management systems and increases to our personnel, including personnel to support our clinical development, manufacturing and commercialization efforts and our operations as a dual listed company; |
| |
● |
the costs to obtain, maintain, expand and protect our intellectual property portfolio; and |
| |
● |
the level and timing of realizing revenues from the BayMedica commercial operations. |
A change in the outcome of
any of these, or other variables with respect to the development of any of our Products and Product Candidates, could significantly change
the costs and timing associated with their development. We will need to continue to rely on additional financing to achieve our business
objectives.
In addition to the variables
described above, if and when any of our Product Candidates successfully complete development, we will incur substantial additional costs
associated with regulatory filings, marketing approval, post-marketing requirements, maintaining our intellectual property rights, and
regulatory protection, in addition to other commercial costs. We cannot reasonably estimate these costs at this time.
Until such time, if ever,
as we can generate substantial revenues from either our Products or Product Candidates, we expect to finance our cash needs through a
combination of equity or debt financings and collaboration arrangements. We currently have no credit facility or committed sources of
capital. To the extent that we raise additional capital through the future sale of equity securities, the ownership interests of our shareholders
will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our
existing common shareholders. If we raise additional funds through the issuance of debt securities, these securities could contain covenants
that would restrict our operations. We may require additional capital beyond our currently anticipated amounts, and additional capital
may not be available on reasonable terms, or at all. If we raise additional funds through collaboration arrangements or other strategic
transactions in the future, we may have to relinquish valuable rights to our technologies, future revenue streams, Products or Product
Candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt
financings when needed, we may be required to delay, limit, reduce or terminate development or future commercialization efforts or grant
rights to develop and market Products or Product Candidates that we would otherwise prefer to develop and market ourselves.
Off-Balance Sheet Arrangements
During the periods presented
we did not have, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Critical Accounting Policies and Significant
Judgments and Estimates
We periodically review our
financial reporting and disclosure practices and accounting policies to ensure that they provide accurate and transparent information
relative to the current economic and business environment. As part of this process, we have reviewed our selection, application and communication
of critical accounting policies and financial disclosures. Management has discussed the development and selection of the critical accounting
policies with the Audit Committee of the Board of Directors and the Audit Committee has reviewed the disclosure relating to critical accounting
policies in this Management’s Discussion and Analysis.
This discussion and analysis
of our financial condition and results of operations is based on our consolidated financial statements included as part of this report,
which have been prepared in accordance with U.S. GAAP. The preparation of our consolidated financial statements requires us to make estimates
and assumptions that affect the reported amounts of assets and liabilities and the revenue and expenses incurred during the reported periods.
We base estimates on our historical experience, known trends and various other factors that we believe are reasonable under the circumstances,
the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from
other sources. Actual results may differ from these estimates under different assumptions or conditions.
The full details of our
accounting policies are presented in Note 2 of our audited consolidated financial statements for the year ended June 30, 2023. These
policies are considered by management to be essential to understanding the processes and reasoning that go into the preparation of
our consolidated financial statements and the uncertainties that could have a bearing on its financial results. The significant
accounting policies that we believe to be most critical in fully understanding and evaluating our financial results are research and
development costs and share based payments.
Research &
Development and Patents costs:
Research
and development and patents costs is a critical accounting estimate due to the magnitude and nature of the assumptions that are required
to calculate third-party accrued and prepaid research and development expenses. Research and development costs are charged to expense
as incurred and include, but are not limited to, personnel compensation, including salaries and benefits, services provided by CROs that
conduct preclinical and clinical studies, costs of filing and prosecuting patent applications, and lab supplies.
The
amount of expenses recognized in a period related to service agreements is based on estimates of the work performed using an accrual basis
of accounting. These estimates are based on services provided and goods delivered, contractual terms and experience with similar contracts.
We monitor these factors and adjust our estimates accordingly.
Share-based payments:
The
fair value, at the grant date, of equity share awards is charged to income or loss over the period for which the benefits of employees
and others providing similar services are expected to be received, generally the vesting period. The corresponding accrued entitlement
is recorded in contributed surplus. The amount recognized as an expense is adjusted to reflect the number of share options expected to
vest. The fair value of awards is calculated using the Black-Scholes option pricing model which considers the following factors:
| |
● |
Current market price of the underlying shares |
| |
● |
Expected life of the award |
| |
● |
Risk-free interest rate |
Management
determines costs for share-based payments using market-based valuation techniques. The fair value of the market-based and performance-based
share awards are determined at the date of grant using generally accepted valuation techniques. Assumptions are made and judgment used
in applying valuation techniques. These assumptions and judgments include estimating the future volatility of the stock price, expected
dividend yield, forfeiture rates and corporate performance. For employee awards, we use the “simplified method” to determine
the expected term of options. Under this method, the expected term represents the average of the vesting period and the contractual term.
Such judgments and assumptions are inherently uncertain. Changes in these assumptions affect the fair value estimates. If we had made
different judgments and assumptions than those described previously, the amount of our share-based payments expense, net loss and net
loss per common shares amounts could have been materially different.
Impairment of Intangible
Assets:
We
assess the recoverability of our long-lived assets whenever events or changes in circumstances indicate that the carrying amount of an
asset may not be recoverable. Recoverability of the long-lived asset is measured by a comparison of the carrying amount of the asset to
future undiscounted net cash flows expected to be generated by the asset or assets. If carrying value exceeds the sum of undiscounted
cash flows, we then determine the fair value of the underlying asset. Any impairment to be recognized is measured as the amount by which
the carrying amount of the asset group exceeds the estimated fair value of the asset group.
Due
to the impairment indicators discussed in Note 5 of our consolidated financial statements, as of June 30, 2022, the Company determined
that intangibles assets of BayMedica that were associated with manufacturing and commercialization of our health and wellness products
were impaired during the year ended June 30, 2022. Refer to Note 5 of our consolidated financial statements.
Business Combination
Business
combinations are accounted for using the acquisition method. The fair value of total purchase consideration is allocated to the fair values
of identifiable tangible and intangible assets acquired and liabilities assumed, with the remaining amount being classified as goodwill.
All assets and liabilities acquired or assumed in a business combination are recorded at their fair values at the date of acquisition.
If the Company’s interest in the fair value of the acquiree’s net identifiable assets exceeds the cost of the acquisition,
the excess is recognized in earnings or loss immediately. Transaction costs that are incurred in connection with a business combination,
other than costs associated with the issuance of debt or equity securities, are expensed as incurred.
As
part of our acquisition of BayMedica Inc, on October 13, 2021, goodwill, trade secrets, product formulation knowledge, patents, trademarks,
Technology and In-Process Research and Development Intangible (“IPR&D”) intangible assets were recognized. The fair
value of the aggregate intangible assets was determined to be $2.7 million and goodwill was $2.0 million at the acquisition date. IPR&D
was classified as indefinite-lived and was not amortized. The multi-period excess earnings method was used to determine the fair value
of these assets as at the date of acquisition. All research and development costs incurred subsequent to the acquisition of IPR&D
are expensed as incurred. Patents are expected to have a finite life and are being amortized on a straight-line basis over their estimated
useful lives. Amortization begins when intangible assets with finite lives are put into use.
Going Concern
Through June 30, 2023, we
have funded our operations primarily with proceeds from the sale of common shares. We have incurred recurring losses and negative cash
flows from operations since our inception, including net losses of $7.9 million and $18.6 million for the years ended June 30, 2023 and
2022, respectively. In addition, we have an accumulated deficit of $101.4 million as of June 30, 2023.
As of the issuance date of
the consolidated financial statements, we expect our cash and cash equivalents and short-term investments of $9.0 million as of June
30, 2023 will be sufficient to fund our operating expenses and capital expenditure requirements into the first quarter of calendar 2024,
and possibly into the second quarter of calendar year 2024, depending on the level and timing of realizing revenues from the BayMedica
commercial operations as well as the level and timing of the Company operating expense. Our future viability is dependent on our ability
to raise additional capital to finance our operations. In addition, there are a number of uncertainties in estimating our operating expenses
and capital expenditure requirements including the impact of potential acquisitions.
As a result, we have concluded
that there is substantial doubt about our ability to continue as a going concern within one year after the date that the consolidated financial statements are issued.
We expect to seek additional
funding through equity financings, debt financings or other capital sources, including collaborations with other companies, government
contracts or other strategic transactions. We may not be able to obtain financing on acceptable terms, or at all. The terms of any financing
may adversely affect the holdings or the rights of our existing shareholders.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS
We are a smaller reporting
company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA

Consolidated Financial Statements of
InMed Pharmaceuticals Inc.
For the Year Ended June 30, 2023 and 2022

InMed Pharmaceuticals Inc.
(Expressed in U.S. Dollars)
June 30, 2023
Report of Independent
Registered Public Accounting Firm
To the Shareholders and Board of Directors of
InMed Pharmaceuticals Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheet of InMed Pharmaceuticals Inc. (the “Company”) as of June 30, 2023, the related consolidated statements of operations,
changes in shareholders’ equity and cash flows for the year ended June 30, 2023 and the related notes (collectively referred to
as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial
position of the Company as of June 30, 2023, and the results of its operations and its cash flows for the year ended June 30, 2023, in
conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been
prepared assuming that the Company will continue as a going concern. As more fully described in Note 1, the Company has incurred significant
losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt
about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1.
The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We
are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are
required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were
we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal
control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 2023
New York, NY
September 29, 2023
Report of Independent
Registered Public Accounting Firm
To the Shareholders and Board of Directors
InMed Pharmaceuticals Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of InMed
Pharmaceuticals Inc. (the Company) as of June 30, 2022, the related consolidated statement of operations, shareholders’ equity,
and cash flows for the year ended June 30, 2022, and the related notes (collectively, the consolidated financial statements). In
our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as
of June 30, 2022, and the results of its operations and its cash flows for the year ended June 30, 2022, in conformity with U.S.
generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared
assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company
has incurred recurring losses and negative cash flows and has an accumulated deficit that raise substantial doubt about its ability to
continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial
statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required
to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations
of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB.
Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements
are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform,
an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal
control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal
control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material
misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those
risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial
statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as
evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for
our opinion.
/s/ KPMG LLP
Chartered Professional Accountants
We have served as the Company’s auditor since 2017.
Vancouver, Canada
September 23, 2022
InMed Pharmaceuticals Inc.
CONSOLIDATED BALANCE SHEETS
Expressed in U.S. Dollars
| | |
| | |
June 30, | | |
June 30, | |
| | |
Note | | |
2023 | | |
2022 | |
| ASSETS | |
| | |
$ | | |
$ | |
| Current | |
| | |
| | |
| |
| Cash
and cash equivalents | |
| | |
| 8,912,517 | | |
| 6,176,866 | |
| Short-term
investments | |
| | |
| 44,422 | | |
| 44,804 | |
| Accounts
receivable, net | |
| | |
| 260,399 | | |
| 88,027 | |
| Inventories | |
3 | | |
| 1,616,356 | | |
| 2,490,854 | |
| Prepaids
and other current assets | |
| | |
| 498,033 | | |
| 797,225 | |
| Total
current assets | |
| | |
| 11,331,727 | | |
| 9,597,776 | |
| | |
| | |
| | | |
| | |
| Non-Current | |
| | |
| | | |
| | |
| Property,
equipment and ROU assets, net | |
4 | | |
| 723,426 | | |
| 904,252 | |
| Intangible
assets, net | |
6 | | |
| 1,946,279 | | |
| 2,108,915 | |
| Other
assets | |
| | |
| 104,908 | | |
| 176,637 | |
| Total
Assets | |
| | |
| 14,106,340 | | |
| 12,787,580 | |
| | |
| | |
| | | |
| | |
| LIABILITIES
AND SHAREHOLDERS’ EQUITY | |
| | |
| | | |
| | |
| Current | |
| | |
| | | |
| | |
| Accounts
payable and accrued liabilities | |
8 | | |
| 1,608,735 | | |
| 2,415,265 | |
| Current
portion of lease obligations | |
11 | | |
| 375,713 | | |
| 404,276 | |
| Deferred
rent | |
| | |
| 16,171 | | |
| - | |
| Acquisition
consideration payable | |
| | |
| - | | |
| 500,000 | |
| Total
current liabilities | |
| | |
| 2,000,619 | | |
| 3,319,541 | |
| | |
| | |
| | | |
| | |
| Non-current | |
| | |
| | | |
| | |
| Lease
obligations, net of current portion | |
11 | | |
| 15,994 | | |
| 389,498 | |
| Total
Liabilities | |
| | |
| 2,016,613 | | |
| 3,709,039 | |
| Commitments
and Contingencies (Note 14) | |
| | |
| | | |
| | |
| | |
| | |
| | | |
| | |
| Shareholders’ Equity | |
| | |
| | | |
| | |
Common shares, no par value, unlimited authorized shares: 3,328,191 (June 30, 2022 - 650,667) issued and outstanding | |
9 | | |
| 77,620,252 | | |
| 70,718,461 | |
| Additional
paid-in capital | |
9,
10 | | |
| 35,741,115 | | |
| 31,684,098 | |
| Accumulated
deficit | |
| | |
| (101,400,209 | ) | |
| (93,452,587 | ) |
| Accumulated
other comprehensive income | |
| | |
| 128,569 | | |
| 128,569 | |
| Total
Shareholders’ Equity | |
| | |
| 12,089,727 | | |
| 9,078,541 | |
| Total
Liabilities and Shareholders’ Equity | |
| | |
| 14,106,340 | | |
| 12,787,580 | |
| Related
Party Transactions (Note 15) | |
| | |
| | | |
| | |
| Subsequent
Events (Note 16) | |
| | |
| | | |
| | |
The accompanying notes form an integral part of these consolidated
financial statements.
InMed Pharmaceuticals Inc.
CONSOLIDATED STATEMENTS OF OPERATIONS
Expressed in U.S.
Dollars
| |
|
|
|
|
For the Years
Ended |
|
| |
|
|
|
|
June 30 |
|
| |
|
Note |
|
|
2023 |
|
|
2022 |
|
| |
|
|
|
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
| Sales |
|
|
|
|
|
4,135,561 |
|
|
|
1,089,435 |
|
| Cost of sales |
|
|
|
|
|
2,423,588 |
|
|
|
545,889 |
|
| Inventory write-down |
|
3 |
|
|
|
308,937 |
|
|
|
- |
|
| Gross profit |
|
|
|
|
|
1,403,036 |
|
|
|
543,546 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| Operating Expenses |
|
|
|
|
|
|
|
|
|
|
|
| Research and development and patents |
|
|
|
|
|
3,732,056 |
|
|
|
7,282,615 |
|
| General and administrative |
|
|
|
|
|
5,847,518 |
|
|
|
6,867,030 |
|
| Amortization and depreciation |
|
4, 6 |
|
|
|
202,249 |
|
|
|
185,657 |
|
| Impairment of intangible assets and goodwill |
|
5 |
|
|
|
- |
|
|
|
3,472,593 |
|
| Total operating expenses |
|
|
|
|
|
9,781,823 |
|
|
|
17,807,895 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| Other Income (Expense) |
|
|
|
|
|
|
|
|
|
|
|
| Interest and other income |
|
|
|
|
|
492,440 |
|
|
|
96,090 |
|
| Warrant modification expense |
|
|
|
|
|
- |
|
|
|
(1,314,307 |
) |
| Foreign
exchange loss |
|
|
|
|
|
(48,175 |
) |
|
|
(117,551 |
) |
| Loss before income tax expense |
|
|
|
|
|
(7,934,522 |
) |
|
|
(18,600,117 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
| Income tax expense |
|
|
|
|
|
(13,100 |
) |
|
|
- |
|
| Net loss for the year |
|
|
|
|
|
(7,947,622 |
) |
|
|
(18,600,117 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share for the year |
|
|
|
|
|
|
|
|
|
|
|
| Basic and diluted |
|
|
|
|
|
(3.25 |
) |
|
|
(33.17 |
) |
| Weighted average outstanding common shares |
|
|
|
|
|
|
|
|
|
|
|
| Basic and diluted |
|
|
|
|
|
2,448,458 |
|
|
|
560,829 |
|
The accompanying notes form an integral part of these consolidated
financial statements.
InMed Pharmaceuticals Inc.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
For the years ended June 30, 2023 and 2022
Expressed in U.S.
Dollars
| | |
| |
| | |
| | |
Additional | | |
| | |
Accumulated
Other | | |
| |
| | |
Note | |
Common Shares | | |
Paid-in
Capital | | |
Accumulated
Deficit | | |
Comprehensive
Income | | |
Total | |
| | |
| |
# | | |
$ | | |
$ | | |
$ | | |
$ | | |
$ | |
| Balance June 30, 2022 | |
| |
| 650,667 | | |
| 70,718,461 | | |
| 31,684,098 | | |
| (93,452,587 | ) | |
| 128,569 | | |
| 9,078,541 | |
| Private placement | |
9 | |
| 240,000 | | |
| 673,748 | | |
| 11,326,042 | | |
| - | | |
| - | | |
| 11,999,790 | |
| Share issuance costs | |
9 | |
| - | | |
| (115,955 | ) | |
| (1,895,311 | ) | |
| - | | |
| - | | |
| (2,011,266 | ) |
| Agents’ investment options | |
| |
| - | | |
| - | | |
| 691,483 | | |
| - | | |
| - | | |
| 691,483 | |
| Exercise of pre-funded warrants | |
9 | |
| 2,437,524 | | |
| 6,343,998 | | |
| (6,343,352 | ) | |
| - | | |
| - | | |
| 646 | |
| Loss for the period | |
| |
| - | | |
| - | | |
| - | | |
| (7,947,622 | ) | |
| - | | |
| (7,947,622 | ) |
| Share-based compensation | |
10 | |
| - | | |
| - | | |
| 278,155 | | |
| - | | |
| - | | |
| 278,155 | |
| Balance June 30, 2023 | |
| |
| 3,328,191 | | |
| 77,620,252 | | |
| 35,741,115 | | |
| (101,400,209 | ) | |
| 128,569 | | |
| 12,089,727 | |
| | |
| |
| | |
| | |
Additional | | |
| | |
Accumulated
Other | | |
| |
| | |
Note | |
Common Shares | | |
Paid-in
Capital | | |
Accumulated
Deficit | | |
Comprehensive
Income | | |
Total | |
| | |
| |
# | | |
$ | | |
$ | | |
$ | | |
$ | | |
$ | |
| Balance June 30, 2021 | |
| |
| 322,028 | | |
| 60,587,417 | | |
| 21,513,051 | | |
| (74,852,470 | ) | |
| 128,569 | | |
| 7,376,567 | |
| Private placement | |
9 | |
| 35,600 | | |
| 1,459,051 | | |
| 10,540,635 | | |
| - | | |
| - | | |
| 11,999,686 | |
| ATM offering, net of issuance costs | |
9 | |
| 10,759 | | |
| 146,533 | | |
| - | | |
| - | | |
| - | | |
| 146,533 | |
| Registered direct and private placement | |
| |
| 65,002 | | |
| 754,072 | | |
| 4,245,508 | | |
| - | | |
| - | | |
| 4,999,580 | |
| Share issuance costs | |
9 | |
| - | | |
| (375,220 | ) | |
| (2,506,795 | ) | |
| - | | |
| - | | |
| (2,882,015 | ) |
| Agents’ warrants | |
| |
| - | | |
| - | | |
| 739,920 | | |
| - | | |
| - | | |
| 739,920 | |
| Agents’ investment options | |
| |
| - | | |
| - | | |
| 192,492 | | |
| - | | |
| - | | |
| 192,492 | |
| Exercise of pre-funded warrants | |
9 | |
| 125,853 | | |
| 4,283,969 | | |
| (4,283,654 | ) | |
| - | | |
| - | | |
| 315 | |
| Exercise of warrants | |
9 | |
| 6,293 | | |
| 769,260 | | |
| (769,260 | ) | |
| - | | |
| - | | |
| - | |
| Acquisition of BayMedica | |
7 | |
| 82,000 | | |
| 3,013,500 | | |
| - | | |
| - | | |
| - | | |
| 3,013,500 | |
| Shares issued for consulting services | |
| |
| 3,132 | | |
| 79,879 | | |
| - | | |
| - | | |
| - | | |
| 79,879 | |
| Warrant modification expense | |
9 | |
| - | | |
| - | | |
| 1,314,307 | | |
| - | | |
| - | | |
| 1,314,307 | |
| Loss for the period | |
| |
| - | | |
| - | | |
| - | | |
| (18,600,117 | ) | |
| - | | |
| (18,600,117 | ) |
| Share-based compensation | |
10 | |
| - | | |
| - | | |
| 697,894 | | |
| - | | |
| - | | |
| 697,894 | |
| Balance June 30, 2022 | |
| |
| 650,667 | | |
| 70,718,461 | | |
| 31,684,098 | | |
| (93,452,587 | ) | |
| 128,569 | | |
| 9,078,541 | |
The accompanying notes form
an integral part of these consolidated financial statements.
InMed Pharmaceuticals Inc.
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the years ended June 30, 2023 and 2022
Expressed in U.S.
Dollars
| | |
Note | |
2023 | | |
2022 | |
| | |
| |
$ | | |
$ | |
| Cash provided by (used in): | |
| |
| | |
| |
| | |
| |
| | |
| |
| Operating Activities | |
| |
| | |
| |
| Net loss | |
| |
| (7,947,622 | ) | |
| (18,600,117 | ) |
| Items not requiring cash: | |
| |
| | | |
| | |
| Amortization and depreciation | |
4, 6 | |
| 202,249 | | |
| 185,657 | |
| Share-based compensation | |
10 | |
| 278,155 | | |
| 697,894 | |
| Shares issued for services | |
| |
| - | | |
| 79,879 | |
| Amortization of right-of-use assets | |
| |
| 393,748 | | |
| 326,133 | |
| Loss on disposal of assets | |
| |
| - | | |
| 11,355 | |
| Interest income received on short-term investments | |
| |
| (803 | ) | |
| (115 | ) |
| Unrealized foreign exchange loss | |
| |
| 1,183 | | |
| 1,770 | |
| Impairment of intangible assets and goodwill | |
| |
| - | | |
| 3,472,593 | |
| Inventory write-down | |
3 | |
| 308,937 | | |
| - | |
| Bad debts | |
| |
| 46,775 | | |
| - | |
| Warrant modification expense | |
| |
| - | | |
| 1,314,307 | |
| Changes in operating assets and liabilities: | |
| |
| | | |
| | |
| Inventories | |
| |
| 565,561 | | |
| (2,003,732 | ) |
| Prepaids and other currents assets | |
| |
| 299,192 | | |
| 190,661 | |
| Other non-current assets | |
| |
| 5,507 | | |
| (61,432 | ) |
| Accounts receivable | |
| |
| (219,147 | ) | |
| (40,008 | ) |
| Accounts payable and accrued liabilities | |
| |
| (806,530 | ) | |
| (811,599 | ) |
| Deferred rent | |
| |
| 16,171 | | |
| (5,142 | ) |
| Lease obligations | |
| |
| (426,575 | ) | |
| (341,862 | ) |
| Total cash used in operating activities | |
| |
| (7,283,199 | ) | |
| (15,583,758 | ) |
| | |
| |
| | | |
| | |
| Investing Activities | |
| |
| | | |
| | |
| Cash acquired from acquisition of BayMedica | |
| |
| - | | |
| 91,566 | |
| Payment of acquisition consideration payable | |
| |
| (500,000 | ) | |
| (300,457 | ) |
| Payment of deposit on equipment | |
| |
| (1,790 | ) | |
| - | |
| Purchase of property and equipment | |
| |
| (160,014 | ) | |
| (39,108 | ) |
| Sale of short-term investments | |
| |
| (42,268 | ) | |
| - | |
| Purchase of short-term investments | |
| |
| 42,268 | | |
| - | |
| Loan receivable | |
| |
| - | | |
| (425,000 | ) |
| Total cash (used in) provided by investing activities | |
| |
| (661,804 | ) | |
| (672,999 | ) |
| | |
| |
| | | |
| | |
| Financing Activities | |
| |
| | | |
| | |
| Shares issued for cash | |
9 | |
| 12,000,436 | | |
| 17,146,114 | |
| Share issuance costs | |
9 | |
| (1,319,782 | ) | |
| (1,784,791 | ) |
| Repayment of debt | |
| |
| - | | |
| (290,826 | ) |
| Total cash provided by financing activities | |
| |
| 10,680,654 | | |
| 15,070,497 | |
| Increase (decrease) in cash during the period | |
| |
| 2,735,651 | | |
| (1,186,260 | ) |
| Cash and cash equivalents beginning of the period | |
| |
| 6,176,866 | | |
| 7,363,126 | |
| Cash and cash equivalents end of the period | |
| |
| 8,912,517 | | |
| 6,176,866 | |
| | |
| |
| | | |
| | |
| SUPPLEMENTARY CASH FLOW INFORMATION: | |
| |
| | | |
| | |
| Cash Paid During the Year for: | |
| |
| | | |
| | |
| Income taxes | |
| |
$ | - | | |
$ | - | |
| Interest | |
| |
$ | - | | |
$ | - | |
| | |
| |
| | | |
| | |
| SUPPLEMENTARY DISCLOSURE OF NON-CASH INVESTING AND FINANCING ACTIVITIES: | |
| |
| | | |
| | |
| Preferred investment options to its placement agent | |
| |
$ | 691,484 | | |
$ | 192,491 | |
| Warrants to its placement agent | |
| |
$ | - | | |
$ | 739,920 | |
| Shares issued for acquisition | |
| |
$ | - | | |
$ | 3,013,500 | |
The accompanying notes form
an integral part of these consolidated financial statements.
| 1. | CORPORATE INFORMATION AND CONTINUING OPERATIONS |
Business
InMed Pharmaceuticals
Inc. (“InMed” or the “Company”) was incorporated in the Province of British Columbia on May 19, 1981 under the
Business Corporations Act of British Columbia. InMed is a clinical stage pharmaceutical company developing a pipeline of prescription-based
products, including rare cannabinoids and novel cannabinoid analogs, targeting the treatment of diseases with high unmet medical needs
as well as developing proprietary manufacturing technologies to produce rare cannabinoids for sale in the health and wellness industry.
The Company’s
shares are listed on the Nasdaq Capital Market (“Nasdaq”) under the trading symbol “INM”. InMed’s office
and principal place of business is located at #310 – 815 West Hastings Street, Vancouver, B.C., Canada, V6C 1B4.
Going Concern
In accordance
with the Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) 2014-15, Disclosure
of Uncertainties about an Entity’s Ability to Continue as a Going Concern (Subtopic 205-40), the Company has evaluated whether there
are conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as
a going concern within one year after the date that the consolidated financial statements are issued.
Through June 30,
2023, the Company has funded its operations primarily with proceeds from the sale of common stock. The Company has incurred recurring
losses and negative cash flows from operations since its inception, including net losses of approximately $7.9 million and $18.6 million
for the years ended June 30, 2023 and 2022, respectively. In addition, the Company had an accumulated deficit of approximately $101.4
million at June 30, 2023. The Company expects to continue to generate operating losses for the foreseeable future.
As of the issuance
date of these consolidated annual financial statements, the Company expects its cash, cash equivalents and short-term investments of $9.0
million as of June 30, 2023 will be sufficient to fund its operating expenses and capital expenditure requirements into the first quarter
of calendar 2024, depending on the level and timing of realizing BayMedica revenues from the sale of bulk rare cannabinoids in the health
& wellness sector as well as the level and timing of the Company operating expenses. The future viability of the Company is dependent
on its ability to raise additional capital to finance its operations. The Company has concluded that there is substantial doubt about
its ability to continue as a going concern within one year after the date that the consolidated financial statements are issued.
The Company expects
to continue to seek additional funding through equity financings, debt financings or other capital sources, including collaborations with
other companies, government contracts or other strategic transactions. The Company may not be able to obtain financing on acceptable terms,
or at all. The terms of any financing may adversely affect the holdings or the rights of the Company’s existing shareholders.
These consolidated
financial statements have been prepared on a going concern basis, which assumes that the Company will be able to meet its commitments,
realize its assets and discharge its liabilities in the normal course. These consolidated financial statements do not reflect adjustments
to the carrying values of assets and liabilities that would be necessary if the Company was unable to continue as a going concern and
such adjustments could be material.
COVID-19 Impacts
The full extent
to which the COVID-19 pandemic may directly or indirectly impact the Company’s business, results of operations and financial condition,
including expenses, research and development costs and employee-related amounts, will depend on future developments that are evolving
and highly uncertain, such as the duration and severity of outbreaks, including potential future waves or cycles, and the effectiveness
of actions taken to contain and treat COVID-19. The Company considered the potential impact of COVID-19 when making certain estimates
and judgments relating to the preparation of these consolidated financial statements. While there was no material impact to the Company’s
consolidated financial statements as of and for the and for the year ended June 30, 2023, the Company’s future assessment of the
magnitude and duration of COVID-19, as well as other factors, could result in a material impact to the Company’s consolidated financial
statements in future reporting periods.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
Basis
of Presentation
These consolidated
financial statements have been prepared in accordance with generally accepted accounting principles as applied in the United States (“US
GAAP”) and pursuant to the rules and regulations of the United States Securities and Exchange Commission (“SEC”) for
financial information.
Reclassifications
Certain prior year amounts in the consolidated financial statements
and the notes thereto have been reclassified where necessary to conform to the current year’s presentation. These reclassifications
did not affect the prior period’s total assets, total liabilities, stockholders’ deficit, net loss or net cash used in operating
activities.
Use of Estimates
The preparation
of financial statements in compliance with US GAAP requires management to make estimates and assumptions that affect the reported amount
of assets and liabilities as of the balance sheet date, and the corresponding revenues and expenses for the periods reported. It also
requires management to exercise judgment in applying the Company’s accounting policies. In the future, actual experience may differ
from these estimates and assumptions. The areas involving a higher degree of judgment or complexity, or areas where assumptions and estimates
are significant to these consolidated financial statements are the estimate of useful life of intangible assets, the application of the
going concern assumption, and determining the fair value of share-based payments, income tax provisions, write-down of inventories to
net realizable value, and warrant valuations.
Actual results
could differ from those estimates.
Basis
of Consolidation
These
consolidated financial statements include the accounts of the Company and its subsidiaries, including subsidiaries: InMed Pharmaceutical
Ltd., BayMedica, LLC, Biogen Sciences Inc., and Sweetnam Consulting Inc. A subsidiary is an entity that the Company controls, either directly
or indirectly, where control is defined as the power to govern the financial and operating policies of an entity so as to obtain benefits
from its activities. All inter-company transactions and balances including unrealized income and expenses arising from intercompany transactions
are eliminated in preparing these consolidated financial statements.
Foreign
Currency
The
functional currency of the Company and its subsidiaries is the U.S. Dollar. These consolidated financial statements are presented in U.S.
Dollars. References to “$” and “US$” are to United States (“U.S.”) dollars and references to “C$”
are to Canadian dollars.
Business
Combinations
Business
combinations are accounted for using the acquisition method. The fair value of total purchase consideration is allocated to the fair values
of identifiable tangible and intangible assets acquired and liabilities assumed, with the remaining amount being classified as goodwill.
All assets, liabilities and contingent liabilities acquired or assumed in a business combination are recorded at their fair values at
the date of acquisition. If the Company’s interest in the fair value of the acquiree’s net identifiable assets exceeds the
cost of the acquisition, the excess is recognized in earnings. Transaction costs that are incurred in connection with a business combination,
other than costs associated with the issuance of debt or equity securities, are expensed as incurred.
Cash and Cash Equivalents
Cash and cash
equivalents include cash-on-hand, demand deposits with financial institutions and other short-term, highly liquid investments with original
maturities of three months or less when acquired that are readily convertible to known amounts of cash and subject to an insignificant
risk of change in value.
Short-term Investments
Short-term investments
include fixed and variable rate guaranteed investment certificates, with terms greater than three months and less than twelve months.
Due to the short term nature of these investments the fair value of the investments approximates the current value. Guaranteed investment
certificates are convertible to known amounts of cash and are subject to an insignificant risk of change in value.
Accounts Receivable
Accounts receivable
are recorded at invoiced amounts, net of any allowance for doubtful accounts. The allowance for doubtful accounts is the Company’s
best estimate of the amount of probable credit losses in existing accounts receivable.
The Company evaluates
the collectability of accounts receivable on a regular basis based upon various factors including the financial condition and payment
history of customers, an overall review of collections experience on other accounts and economic factors or events expected to affect
future collections experience. Expected credit losses on our accounts receivable were $66,775 and $20,000 as at June 30, 2023 and 2022
respectively.
Concentration of Credit Risk
and Other Risks and Uncertainties
At times, cash balances may exceed the Federal Deposit Insurance Corporation
(“FDIC”) or Canadian Deposit Insurance Corporation (CDIC) insurable limits. The Company has not experienced any losses related
to these balances. The uninsured cash balance as of June 30, 2023, was $3.8 million. The Company does not believe it is exposed to significant
credit risk on cash and cash equivalents.
The Company’s
customers are primarily concentrated in the United States.
As of June 30,
2023, we had three customers with an accounts receivable balance representing 41%, 30% and 15% of total accounts receivable.
For the year ended
June 30, 2023, the Company had four customers that accounted for 22%, 17%, 16% and 11% of revenue. For the year ended June 30, 2022, the
Company had three customer that accounted for 21%, 20%, and 11% of revenue.
Inventories
Inventories are
initially valued at weighted average cost and subsequently valued at the lower of weighted average cost and net realizable value. Costs
included in inventories are the purchase price of goods and cost of services rendered, freight costs, warehousing costs, purchasing costs
and production and labor costs related to manufacturing.
In determining
any valuation allowances, the Company reviews inventory for obsolete, redundant, and slow-moving goods. As of June 30, 2023, the Company
has $93,820 as a valuation allowance to reduce weighted average cost to net realizable value. As of June 30, 2022, no amounts had been
charged to the valuation allowance. During the year ended June 30, 2023 and 2022 the Company record an inventory write-down of $308,937
and $Nil respectively.
Property, Equipment and ROU Assets,
Net
Computer equipment,
lab equipment and furnishings are recorded at cost, less accumulated depreciation and accumulated impairment losses. The initial cost
of computer equipment, lab equipment and furnishings comprises their purchase price. The computer equipment, lab equipment and furnishings
are reviewed at least once per year for impairment. Equipment and furniture are depreciated using the straight-line method based on their
estimated useful lives as follows:
| | ● | Computer equipment – 5 years |
| | | |
| | ● | Lab equipment – 6 - 10 years
|
Computer equipment, lab equipment and furnishings, acquired or disposed
of during the year, are depreciated proportionately for the period they are in use.
The right-of-use
assets are initially measured based on the initial amount of the lease liability adjusted for any lease payments made at or before the
commencement date, less any lease incentives received. The assets are amortized to the earlier of the end of the useful life of the right-of-use
asset or the lease term using the straight-line method as this most closely reflects the expected pattern of consumption of the future
economic benefits. The lease term includes periods covered by an option to extend if the Company is reasonably certain to exercise that
option. In addition, the right-of-use assets are periodically reduced by impairment losses, if any, and adjusted for certain re-measurements
of the lease liability (see Note 2 Lease (i)).
Intangible Assets, Net
Intangible assets
are comprised of acquired intellectual property, which consists of certain patents and technical know-how. The intellectual property is
recorded at cost and is amortized on a straight-line basis over an estimated useful life of 18 years net of any accumulated
impairment losses.
In-Process R&D
In-process R&D
(“IPR&D”) is classified as an indefinite-lived intangible asset and is not amortized. IPR&D becomes definite-lived
upon the completion or abandonment of the associated research and development efforts. All research and development costs incurred subsequent
to the acquisition of IPR&D are expensed as incurred. Indefinite-lived intangible assets are evaluated for impairment on an annual
basis or more frequently if an indicator of impairment is present.
Impairment of Long-Lived Assets
The Company assesses
the recoverability of its long-lived assets whenever events or changes in circumstances indicate that the carrying amount of an asset
may not be recoverable. Recoverability of the long-lived asset is measured by a comparison of the carrying amount of the asset to future
undiscounted net cash flows expected to be generated by the asset or assets. If carrying value exceeds the sum of undiscounted cash flows,
the Company then determines the fair value of the underlying asset. Any impairment to be recognized is measured as the amount by which
the carrying amount of the asset group exceeds the estimated fair value of the asset group. Assets classified as held for sale are reported
at the lower of the carrying amount or fair value, less costs to sell. Based on the completion of the impairment test, the Company recorded
an impairment charge of $Nil and $1,449,554 for Long-Lived Assets for the years ended June 30, 2023, and 2022, respectively. (See Note
5)
Goodwill
The Company tests
goodwill for potential impairment annually on June 30, or more frequently if an event or other circumstance indicates that the Company
may not be able to recover the carrying amount of the net assets of the reporting unit. The Company’s operations consist of two operating
and reportable segments, InMed Pharmaceuticals (the “InMed” segment) and BayMedica (the “BayMedica” segment).
In evaluating goodwill for impairment, the Company may assess qualitative factors to determine whether it is more likely than not that
the fair value of a reporting unit is less than its carrying amount. If a Company bypasses the qualitative assessment, or if the Company
concludes that it is more likely than not that the fair value of a reporting unit is less than its carrying value, then the Company performs
a quantitative impairment test by comparing the fair value of a reporting unit with its carrying amount and records an impairment charge
if the carrying value exceeds the fair value. Based on the completion of the annual impairment test, the Company recorded an impairment
charge of $2,023,039 for Goodwill for the years ended June 30, 2022, respectively. (See Note 5)
Financial Assets and Liabilities
Financial Assets
Financial assets
are initially recognized at fair value, plus transaction costs that are directly attributable to their acquisition or issue and subsequently
carried at amortized cost, using the effective interest rate method, less any impairment losses. No financial assets are or elected to
be carried at fair value through profit or loss or where changes in fair value are recognized in the consolidated statements of operations
and comprehensive loss in other comprehensive loss.
Short-term investments
are subsequently recorded at cost plus accrued interest, which approximates fair value due to short term nature. Accounts receivable are
reported at outstanding amounts, net of provisions for uncollectable amounts.
Financial Liabilities
To determine the
fair value of financial instruments, the Company uses the fair value hierarchy for inputs used to measure fair value of financial assets
and liabilities. This hierarchy prioritizes the inputs to valuation techniques used to measure fair value into three levels: Level 1 (highest
priority), Level 2, and Level 3 (lowest priority).
| |
Level 1 – |
Unadjusted quoted prices in active markets for identical instruments. |
| |
Level 2 – |
Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (i.e., interest rates, yield curves, etc.), and inputs that are derived principally from or corroborated by observable market data by correlation or other means (market corroborated inputs). |
| |
Level 3 – |
Inputs are unobservable and reflect the Company’s assumptions as to what market participants would use in pricing the asset or liability. The Company develops these inputs based on the best information available. Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Changes in the observability of valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy. |
The carrying value
of cash and cash equivalents, short-term investments, accounts receivable, and accounts payable and accrued liabilities, approximate their
carrying values as at June 30, 2023 and 2022 due to their immediate or short-term maturities.
Income Taxes
Deferred tax assets
and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts
of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred tax assets
and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences
are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income
in the period that includes the enactment date. At June 30, 2023, and June 30, 2022, the Company had a full valuation allowance against
its deferred tax assets.
Per FASB ASC 740-10,
disclosure is not required of an uncertain tax position unless it is considered probable that a claim will be asserted and there is a
more-likely-than-not possibility that the outcome will be unfavorable. Using this guidance, as of June 30, 2023, and 2022, the Company
has no uncertain tax positions that qualify for either recognition or disclosure in the financial statements. The Company’s 2023,
2022, 2021, and 2020 United States and Canadian tax returns remain subject to examination by their respective taxing authorities. Neither
of the Company’s tax returns are currently under examination.
Revenue Recognition
The Company recognizes
revenue when the Company satisfies the performance obligations under the terms of a contract and control of its products and services
is transferred to its customers in an amount that reflects the consideration the Company expects to receive from its customers in exchange
for those products and services. ASC 606, Revenue from Contracts with Customers defines a five-step process to recognize
revenue that requires judgment and estimates, including identifying the contract with the customer, identifying the performance obligations
in the contract, determining the transaction price, allocating the transaction price to the performance obligations in the contract, and
recognizing revenue when or as the performance obligation is satisfied.
Revenue consists
of manufacturing and distribution sales of bulk rare cannabinoids, which are generally recognized at a point in time. The Company recognizes
revenue when control over the products have been transferred to the customer and the Company has a present right to payment. Sales and
other taxes that are required to be remitted to regulatory authorities are recorded as liabilities and excluded from sales. Limited rights
of return, for claims of damaged or non-compliant products, exist with the Company’s customers.
The Company has
elected the practical expedient that allows it to recognize the incremental costs of obtaining a contract as an expense, when incurred,
if the amortization period of the asset that the Company otherwise would have recognized is one year or less.
Revenues within
the scope of ASC 606 do not include material amounts of variable consideration. Customer payments are generally due in advance of when
control is transferred to the customer. Some of our larger customers with which we have history with are eligible for payment terms up
to net 30.
Cost of Sales
Cost of sales
consists primarily of the purchase price of goods and cost of services rendered, freight costs, warehousing costs, and purchasing costs.
Cost of sales also includes production and labor costs for the Company’s manufacturing business.
Shipping and Handling
The Company records
freight billed to customers within Net sales. Shipping and handling costs associated with inbound freight and goods shipped to customers
are recorded in cost of sales. Other shipping and handling costs, such as for quality assurance, are recorded in operating expenses.
Earnings (Loss) Per Share
Basic earnings
(loss) per common share (“EPS”) is computed by dividing the net income or loss applicable to common shares of the Company
by the weighted average number of common shares outstanding for the relevant period. Diluted earnings (loss) per common share (“Diluted
EPS”) is computed by dividing the net income or loss applicable to common shares by the sum of the weighted average number of common
shares issued and outstanding and all additional common shares that would have been outstanding, if potentially dilutive instruments were
converted. If the conversion of outstanding stock options and warrants into common share is anti-dilutive, then diluted EPS is not presented
separately from EPS.
The
following table sets forth the number of potential shares of common stock that have been excluded from diluted net income (loss) per because
their effect was anti-dilutive:
| | |
Year ended June 30, | |
| | |
2023 | | |
2022 | |
| Options | |
| 102,642 | | |
| 55,603 | |
| Warrants | |
| 3,516,529 | | |
| 505,128 | |
| | |
| 3,619,171 | | |
| 560,731 | |
Share-based Payments
The Company follows
the requirements of FASB ASC 718-10-10, Share-Based Payments with regards to stock-based compensation issued to employees and non-employees.
The Company has agreements and arrangements that call for stock to be awarded to the employees and consultants at various times as compensation
and periodic bonuses. The expense for this stock-based compensation is equal to the fair value of the stock price on the day the stock
was awarded multiplied by the number of shares awarded. The Company has a relatively low forfeiture rate of stock-based compensation and
forfeitures are recognized as they occur.
The valuation
methodology used to determine the fair value of the options issued during the period is the Black-Scholes option-pricing model. The Black-Scholes
model requires the use of a number of assumptions including the volatility of the stock price, the average risk-free interest rate, and
the weighted average expected life of the options. Risk-free interest rates are calculated based on continuously compounded risk-free
rates for the appropriate term. The dividend yield is assumed to be zero as the Company has never paid or declared any cash dividends
on its Common Stock and does not intend to pay dividends on its Common Stock in the foreseeable future. The expected forfeiture rate is
estimated based on management’s best assessment.
Estimated volatility
is a measure of the amount by which InMed’s stock price is expected to fluctuate each year during the expected life of the award.
The Company’s calculation of estimated volatility is based on historical stock prices over a period equal to the expected life of
the awards.
Research and Development Costs
The Company conducts
research and development programs and incurs costs related to these activities, including research and development personnel compensation,
services provided by contract research organizations and lab supplies. Research and development costs are expensed in the periods in which
they are incurred.
Patents and Intellectual Property
Costs
The costs of filing
for patents and of prosecuting and maintaining intellectual property rights are expensed as incurred due to the uncertainty surrounding
the drug development process and the uncertainty of future benefits. Patents and intellectual property acquired from third parties for
approved products or where there are alternative future uses are capitalized and amortized over the remaining life of the patent.
Segment reporting
The Company’s
operations consist of two operating and reportable segments, the InMed segment and the BayMedica segment.
The InMed segment
is largely organized around the research and development of cannabinoid-based pharmaceuticals products and the BayMedica segment is largely
organized around developing proprietary manufacturing technologies to produce rare cannabinoids for sale in the health and wellness industry
(See Note 13).
Leases
At inception of
a contract, the Company assesses whether a contract is, or contains, a lease based on whether the contract conveys the right to control
the use of an identified asset for a period of time in exchange for consideration.
The lease liability
is initially measured as the present value of future lease payments excluding payments made at the commencement date, discounted using
the interest rate implicit in the lease or, if that rate cannot be readily determined, the Company’s incremental borrowing rate.
Generally, the Company uses its incremental borrowing rate as the discount rate. The lease liability is measured at amortized cost using
the effective interest method. It is re-measured when there is a change in future lease payments arising from a change in an index or
rate, if there is a change in the Company’s estimate of the amount expected to be payable under a residual value guarantee, or if
the Company changes its assessment of whether it will exercise a purchase, extension, or termination option. When the lease liability
is re-measured in this way, a corresponding adjustment is made to the carrying amount of the right-of-use asset or is recorded in profit
or loss if the carrying amount of the right-of-use asset has been reduced to zero.
The Company has
lease arrangements that include both lease and non-lease components. The Company accounts for each separate lease component and its associated
non-lease components as a single lease component for all of its asset classes.
The
Company has elected to apply the practical expedient to exclude initial direct costs such as annual operating costs from the measurement
of the right-of-use asset at the date of initial application. The Company has elected to apply the practical expedient not to recognize
right-of-use assets and lease liabilities for short-term leases that have a lease term of 12 months or less. The lease payments
associated with these leases is recognized as an expense on a straight- line basis over the lease term.
Recent Accounting Pronouncements
The Company has
reviewed recent accounting pronouncements and concluded that they are either not applicable to the Company or that there was no material
impact or no material impact is expected in the consolidated financial statements as a result of future adoption.
Inventories consisted of the following:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| | |
| | |
| |
| Raw materials | |
| 208,737 | | |
| 292,577 | |
| Work in process | |
| 514,113 | | |
| 1,724,851 | |
| Finished goods | |
| 893,506 | | |
| 473,426 | |
| Inventories | |
| 1,616,356 | | |
| 2,490,854 | |
During the year
ended June 30, 2023 and 2022, the write-down of inventories to net realizable value was $308,937 and $Nil respectively. Contributing factors
to the decrease in net realizable value included lower demand and downward pricing pressure for certain products. As of June 30, 2023
and 2022, the Company has $93,820 and $Nil respectively as a valuation allowance to reduce weighted average cost to new basis.
| 4. | PROPERTY, EQUIPMENT AND ROU ASSETS, NET |
Property, equipment and ROU assets consisted of
the following:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| | |
| | |
| |
| Right-of-Use Assets (leases) | |
| 1,167,436 | | |
| 1,167,436 | |
| Equipment | |
| 440,902 | | |
| 212,877 | |
Furnishing | |
| 40,409 | | |
| 40,409 | |
| Property and equipment | |
| 1,648,747 | | |
| 1,420,722 | |
| Less: accumulated depreciation and amortization | |
| (925,321 | ) | |
| (516,470 | ) |
| Property, equipment and ROU assets, net | |
| 723,426 | | |
| 904,252 | |
Depreciation expense
on computer equipment, lab equipment and furnishing for the year ended June 30, 2023 and 2022, was $39,613 and $26,426 respectively and
was recorded in general and administrative expenses. Amortization expense related to the right-of-use assets for the year ended June 30,
2023 and 2022, was $369,239 and $289,594 respectively and was recorded in general and administrative expenses.
| 5. | IMPAIRMENT OF INTANGIBLE
ASSETS AND GOODWILL |
During
the year ended June 30, 2022, the Company recorded goodwill of $2,023,039, definite lived intangible assets of $216,000, IPR&D of
$1,249,000 and patents of $1,191,000 in connection with the acquisition of BayMedica, as described in Note 7.
The
Company performs an annual impairment test at the reporting unit level as of June 30 of each fiscal year.
As
of June 30, 2022, the Company qualitatively assessed whether it is more likely than not that the respective fair value of the Company’s
BayMedica reporting unit was less than its carrying amount, including goodwill. For a variety of reasons, performance of the BayMedica
segment has not materialized as expected. Contributing factors include but are not limited to the following:
| |
- |
market demand for launched compounds has not materialized as quickly as the Company anticipated; |
| |
- |
recent overarching recessionary pressures have contributed to hesitation within the health and wellness (H&W) sector to invest in, and launch, new rare cannabinoid products; |
| |
- |
in this nascent market, BayMedica’s perceived competitive advantages of certified, high purity and reliability and consistency of supply have not resonated with the industry’s current product manufacturers; and |
| |
- |
additional downward pricing pressure for cannabinoids in the H&W sector. |
As
a result of this sustained decline in performance compared to expectations and continuing market uncertainties, the Company determined
that as of June 30, 2022, it was more likely than not that the carrying value of these acquired intangibles exceeded their estimated fair
value. Accordingly, the Company performed an impairment analysis as of that date using the income method, the relieve from royalty method
and the multi-period excess earnings method. This analysis required significant judgments, including the estimation of future revenues,
royalties, licensing fees, costs, the probability of success in various phases of its development programs, potential post launch cash
flows and discount rates. The Company recorded a goodwill and intangible asset impairment charge for the excess of the reporting unit’s
carrying value over its fair value.
As
of June 30, 2023, the Company did not identify any impairment indicators and no impairment was recorded on our remaining intangible assets.
The
following table provides the Company’s goodwill, indefinite and definite lived intangible assets as of June 30, 2023 and 2022. There
was no impairment of InMed long lived intangible assets as of June 30, 2023 and 2022.
| | |
$ | |
| | |
| |
| Goodwill | |
| |
| Balance at July 1, 2021 | |
| - | |
| Acquired at October 13, 2021 | |
| 2,023,039 | |
| Impairment losses | |
| (2,023,039 | ) |
| Balance at June 30, 2022 and 2023 | |
| - | |
| | |
| | |
| Indefinite lived intangible assets | |
| | |
| IPR&D | |
| | |
| Balance at July 1, 2021 | |
| - | |
| Acquired at October 13, 2021 | |
| 1,249,000 | |
| Impairment losses | |
| (1,249,000 | ) |
| Balance at June 30, 2022 and 2023 | |
| - | |
| | |
| | |
| Definite lived intangible assets | |
| | |
| Trademark and Intellectual Property | |
| | |
| Balance at July 1, 2021 | |
| 1,736,420 | |
| Acquired at October 13, 2021 | |
| 216,000 | |
| Amortization | |
| (786,637 | ) |
| Impairment losses | |
| (200,554 | ) |
| Balance at June 30, 2022 | |
| 965,229 | |
| Amortization | |
| (96,468 | ) |
| Impairment losses | |
| - | |
| Balance at June 30, 2023 | |
| 868,761 | |
| | |
| | |
| Definite lived intangible assets | |
| | |
| Patents | |
| | |
| Balance at July 1, 2021 | |
| - | |
| Acquired at October 13, 2021 | |
| 1,191,000 | |
| Amortization | |
| (47,314 | ) |
| Impairment losses | |
| - | |
| Balance at June 30, 2022 | |
| 1,143,686 | |
| Amortization | |
| (66,168 | ) |
| Impairment losses | |
| - | |
| Balance at June 30, 2023 | |
| 1,077,518 | |
| | |
| | |
| Intangible assets, net as of June 30, 2022 | |
| 2,108,915 | |
| | |
| | |
| Intangible assets, net as of June 30, 2023 | |
| 1,946,279 | |
During
the year ended June 30, 2022, the Company recognized a goodwill impairment charge of $2 million which is a non recuring level 3
measurement. For the identified indefinite lived assets, the Company recognized an impairment charge of $Nil and
$1.2 million during the years ended June 30, 2023 and 2022, respectively. For identified definite lived intangible assets,
the Company recognized an impairment charge of $Nil and $0.2 million during the years ended June 30, 2023 and 2022,
respectively.
The following table summarizes the Companies
intangible assets:
| | |
| | |
| |
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| | |
| | |
| |
| Intellectual property | |
| 1,736,420 | | |
| 1,736,420 | |
| Patents | |
| 1,191,000 | | |
| 1,191,000 | |
| Intangible assets | |
| 2,927,420 | | |
| 2,927,420 | |
Less: accumulated amortization | |
| (981,141 | ) | |
| (818,505 | ) |
| Intangible assets, net | |
| 1,946,279 | | |
| 2,108,915 | |
Acquired intellectual
property is recorded at cost and is amortized on a straight-line basis over 18 years. Acquired patents consist of patents related to the
development of cannabinoid analogs. This intangible asset is being amortized over an estimated useful life of 18 years. As at June 30,
2023, the definite-lived intangible assets had a weighted average estimated remaining useful life of approximately 12 years.
Amortization expense
on intangible assets for the year ended June 30, 2023 and 2022 was $162,636 and $159,228 respectively. The Company expects amortization
expense to be incurred over the next five years as follows:
| Twelve months ending June 30, | |
$ | |
| | |
| |
| 2024 | |
| 158,935 | |
| 2025 | |
| 158,935 | |
| 2026 | |
| 158,935 | |
| 2027 | |
| 158,935 | |
| 2028 | |
| 158,935 | |
| Thereafter | |
| 1,151,604 | |
| Total | |
| 1,946,279 | |
On
October 13, 2021, the Company completed the acquisition of BayMedica, a private company based in the U.S. that specializes in the manufacturing
and commercialization of rare cannabinoids. The Company acquired 100% of BayMedica in exchange for i) 82,000 common shares issued
to BayMedica’s equity and convertible debt holders, subject to a six-month contractual hold period and ii) $1 million to be held
in escrow, subject to reduction for certain post-closing adjustments or satisfaction of indemnification claims under the definitive agreement
(the “BayMedica Agreement”) in the six- and twelve-month periods following the closing.
Total
consideration for the acquisition of BayMedica is summarized as follows:
| | |
Purchase
Price
Consideration
($) | |
| Estimated fair value of common shares issued | |
| 3,013,500 | |
| Cash | |
| 1,000,000 | |
| Less: Post-closing adjustments | |
| (199,543 | ) |
| Estimated fair value of consideration transferred | |
| 3,813,957 | |
The 82,000 common
shares were valued at $36.75 per share, being the closing price of the Company’s common shares on Nasdaq on October 13, 2021. The
cash component is subject to reduction for certain post-closing adjustments or satisfaction of indemnification claims and therefore is
subject to further changes.
Prior
to the acquisition, the Company has a $425,000 loan receivable from BayMedica and BayMedica has an equal loan payable to the Company.
As a result of the acquisition of BayMedica, the loan receivable and payable is effectively settled between the parties.
In
accordance with the acquisition method of accounting, the purchase price of BayMedica has been allocated to the acquired assets and assumed
liabilities based on their estimated acquisition date fair values. The fair value estimates were based on income, estimates and other
analyses. The excess of the total consideration over the estimated fair value of the amounts initially assigned to the identifiable assets
acquired and liabilities assumed has been recorded as goodwill, which is not deductible for income taxes purposes. The goodwill balance
represents the assembled workforce acquired, the combined Company’s expectations of the strategic opportunities available as a result
of the acquisition, and other synergies that will be derived from the acquisition.
The
following table summarizes the final fair value of assets acquired and liabilities assumed as of the acquisition date:
| | |
Purchase Price | |
| | |
Allocation | |
| | |
($) | |
| Assets acquired: | |
| |
| Cash and cash equivalents | |
| 91,566 | |
| Accounts receivable | |
| 36,100 | |
| Inventories | |
| 487,122 | |
| Prepaid expenses and deposits | |
| 131,674 | |
| Property and equipment | |
| 133,911 | |
| IPR&D | |
| 1,249,000 | |
| Patents | |
| 1,191,000 | |
| Trademark | |
| 216,000 | |
| Goodwill | |
| 2,023,039 | |
| Total assets acquired | |
| 5,559,412 | |
| | |
| | |
| Liabilities assumed: | |
| | |
| Accounts payable and accrued liabilities | |
| 1,024,487 | |
| Other short-term liabilities | |
| 598,245 | |
| Long-term debt | |
| 122,723 | |
| Total liabilities acquired | |
| 1,745,455 | |
| Estimated fair value of net assets acquired | |
| 3,813,957 | |
Tangible
assets and liabilities were valued at their respective carrying amounts as management believes that these amounts approximated their acquisition-date
fair values.
The
Purchase Price allocation includes certain identifiable intangible assets with an estimated fair value of approximately $2,656,000. These
intangible assets include trade secrets, product formulation knowledge, patents and trademarks.
Acquired
IPR&D are related identifiable intangible assets associated with cannabinoid manufacturing processes and includes knowhow and trade
secrets. The multi-period excess earnings method was used to determine the fair value of these assets as at the date of acquisition.
The
acquired trademark represents the trade name ProDiol®. The fair value of the trademark was determined using the relief from royalty
method.
Acquired
patents consist of patents related to the development of cannabinoid analogs, the fair value of which was determined using the income
approach. This intangible asset is being amortized over an estimated useful life of 18 years.
Following
the acquisition date, the operating results of BayMedica have been included in the consolidated financial statements. For the period from
the October 13, 2021 acquisition date through June 30, 2022, sales attributable to BayMedica were $1.1 million and operating losses
attributable to BayMedica were $5.2 million. Acquisition-related expenses, which were comprised primarily of regulatory, financial
advisory and legal fees, totaled $0.2 million for the year ended June 30, 2022 and were included in general and administrative expenses
in the consolidated statements of operations and comprehensive loss.
The
following table presents the pro forma consolidated results of the Company assuming the BayMedica acquisition had been completed on July
1, 2021:
| | |
Year Ended
June 30,
2022 | |
| | |
| |
| | |
| |
| Sales | |
$ | 1,365,755 | |
| Net loss | |
$ | (19,260,014 | ) |
| Net loss per share | |
$ | (34.34 | ) |
| Weighted average number of shares outstanding | |
| 560,829 | |
| 8. | ACCOUNTS PAYABLE AND ACCRUED LIABILITIES |
Accounts payable and
accrued liabilities consist of the following:
| | |
June 30,
2023 | | |
June 30,
2022 | |
| | |
$ | | |
$ | |
| | |
| | | |
| | |
| Trade payables | |
| 544,179 | | |
| 1,166,068 | |
| Accrued research and development expenses | |
| 164,587 | | |
| 839,638 | |
| Employee compensation, benefits and related accruals | |
| 542,305 | | |
| 139,120 | |
| Accrued general and administrative expenses | |
| 357,664 | | |
| 270,439 | |
| Accounts payable and accrued liabilities | |
| 1,608,735 | | |
| 2,415,265 | |
| 9. | SHARE CAPITAL AND RESERVES |
On September 7,
2022, the Company effected a one-for-25 reverse stock split of its issued and outstanding common shares. Accordingly, all common share,
stock option, per common share and warrant amounts for all periods presented in the consolidated financial statements and notes thereto
have been adjusted retrospectively to reflect this reverse stock split.
As of June 30, 2023, the Company’s
authorized share structure consisted of: (i) an unlimited number of common shares without par value; and (ii) an unlimited number of preferred
shares without par value. No preferred shares were issued and outstanding as of June 30, 2023 and 2022.
The Company may issue preferred shares
and may, at the time of issuance, determine the rights, preference and limitations pertaining to these shares. Holders of preferred shares
may be entitled to receive a preference payment in the event of any liquidation, dissolution or winding up of the Company before any payment
is made to the holders of common shares.
During the year ended June 30, 2023,
the Company completed the following:
September 2022 Private Placement Offering:
| Transaction Description | |
Number | | |
Issue Price | | |
Total | |
| Shares Issued | |
| 90,000 | | |
$ | 8.680 | | |
$ | 781,200 | |
| Pre-funded Warrants Issued | |
| 601,245 | | |
$ | 8.6799 | | |
| 5,218,746 | |
| Gross Proceeds | |
| | | |
| | | |
$ | 5,999,946 | |
| Allocated to Additional Paid-in Capital | |
| | | |
| | | |
| (5,589,570 | ) |
| | |
| | | |
| | | |
$ | 410,376 | |
| Share Issuance Costs | |
| | | |
| | | |
$ | (77,242 | ) |
On September
13, 2022, the Company closed a private placement of its common shares and issued an aggregate of 90,000 common shares and 601,245 pre-funded
warrants, for gross proceeds of $5,999,946. The pre-funded warrants were determined to be common stock equivalents. Each common share
and each pre-funded warrant were sold in the offering with an investment option to purchase a common share. Transaction costs were allocated
proportionally between common shares and investment options with $77,242 allocated to common shares and the balance of $1,052,101 allocated
to additional paid-in capital and recorded as a component of shareholders’ equity in the consolidated balance sheet. As of June
30, 2023, there were no pre-funded warrants outstanding.
November 2022
Private Placement Offering:
| Transaction Description | |
Number | | |
Issue Price | | |
Total | |
| Shares Issued | |
| 150,000 | | |
$ | 3.300 | | |
$ | 495,000 | |
| Pre-funded Warrants Issued | |
| 1,668,185 | | |
$ | 3.2999 | | |
| 5,504,844 | |
| Gross Proceeds | |
| | | |
| | | |
$ | 5,999,844 | |
| Allocated to Additional Paid-in Capital | |
| | | |
| | | |
| (5,736,472 | ) |
| | |
| | | |
| | | |
$ | 263,372 | |
| Share Issuance Costs | |
| | | |
| | | |
$ | (38,713 | ) |
On November 21,
2022, the Company closed a private placement of its common shares and issued an aggregate of 150,000 common shares and 1,668,185 pre-funded
warrants, for gross proceeds of $5,999,844. The pre-funded warrants were determined to be common stock equivalents. Each common share
and each pre-funded warrant were sold in the offering with an investment option to purchase a common share. Transaction costs were allocated
proportionally between common shares and investment options with $38,713 allocated to common shares and the balance of $831,292 allocated
to additional paid-in capital and recorded as a component of shareholders’ equity in the consolidated balance sheet. As of June
30, 2023, there were no pre-funded warrants outstanding.
During
the year ended June 30, 2022, the Company completed the following:
July
2021 Private Placement Offering:
Transaction Description | |
Number | | |
Issue Price | | |
Total | |
| Shares Issued | |
| 35,600 | | |
$ | 74.325 | | |
$ | 2,645,970 | |
| Pre-funded Warrants Issued | |
| 125,853 | | |
$ | 74.3226 | | |
| 9,353,716 | |
| Gross Proceeds | |
| | | |
| | | |
$ | 11,999,686 | |
| Allocated to Additional Paid-in Capital | |
| | | |
| | | |
| (10,540,635 | ) |
| | |
| | | |
| | | |
$ | 1,459,051 | |
| Share Issuance Costs | |
| | | |
| | | |
$ | (247,336 | ) |
On
July 2, 2021, the Company closed a private placement of its common shares and issued an aggregate of 35,600 common shares and 125,853 pre-funded
warrants, for gross proceeds of $11,999,686. The pre-funded warrants were determined to be common stock equivalents. Each common share
and each pre-funded warrant were sold in the offering with a warrant to purchase a common share. Transaction costs were allocated proportionally
between common shares and warrants with $247,336 allocated to common shares and the balance of $1,786,831 allocated to additional
paid-in capital and recorded as a component of shareholders’ equity in the consolidated balance sheet. The 125,853 pre-funded
warrants were fully exercised for 125,853 common shares during the year ended June 30, 2022, resulting in a $4,283,654 reclassification
from additional paid-in capital to common shares.
June
2022 Registered Direct and Private Placement Offerings:
| Transaction Description | |
Number | | |
Issue Price | | |
Total | |
| Shares Issued | |
| 65,002 | | |
$ | 21.450 | | |
$ | 1,394,286 | |
| Pre-funded Warrants Issued | |
| 168,099 | | |
$ | 21.4474 | | |
| 3,605,294 | |
| Gross Proceeds | |
| | | |
| | | |
$ | 4,999,580 | |
| Allocated to Additional Paid-in Capital | |
| | | |
| | | |
| (4,245,508 | ) |
| | |
| | | |
| | | |
$ | 754,072 | |
| Share Issuance Costs | |
| | | |
| | | |
$ | (127,884 | ) |
On
April 22, 2022, the Company issued 10,759 common shares under an at-the-market offering (“ATM”) for proceeds of
$146,533, net of issuance costs.
On
June 6, 2022, the Company closed a registered direct offering and concurrent private placement of its common shares. In the registered
direct offering, the Company issued an aggregate of 65,002 common shares and 98,169 pre-funded warrants, for gross
proceeds of $3,500,002. In the concurrent private placement, the Company issued an aggregate of 69,930 pre-funded warrants for
gross proceeds of $1,499,999. The pre-funded warrants were determined to be common stock equivalents. Each common stock and each pre-funded
warrant were sold in the offerings with a preferred investment option to purchase a common share. Transaction costs were allocated proportionally
between common shares and warrants with $127,884 allocated to common shares and the balance of $719,964 allocated to additional
paid-in capital and recorded as a component of shareholders’ equity in the consolidated balance sheet.
During
the year ended June 30, 2022, in accordance with the BayMedica Agreement, the Company issued 82,000 common shares to BayMedica’s
historical equity and convertible debt holders (See Note 7). In addition, the Company issued 78,312 common shares for consulting
services.
As part of the Company’s
financing in the year ended June 30, 2023 and 2022, the units included pre-funded warrants of 2,269,430 and 293,952 respectively. These
warrants contained an exercise price of $.0001 and were exercised in the year issued.
| c) | Share Purchase Warrants |
On July 2, 2021,
161,453 warrants were issued with an exercise price of $71.20 per share, were immediately exercisable upon issuance, and expire 5 years
following the date of issuance. The pre-funded and common warrants did not meet the criteria to be classified as a liability award and
therefore were treated as an equity award and recorded as a component of shareholders’ equity in the consolidated balance sheet.
On June 6, 2022, the Company amended the warrants to re-price them to $18.50 per share with an expiry date of June 6, 2029. Accordingly,
the Company has calculated the incremental fair value from the modification to be $1,194,752 and is recognized as a warrant modification
expense in the statement of operations.
The following is a summary of changes in share purchase
warrants from July 1, 2021 to June 30, 2023:
| | |
Number | | |
Weighted
Average Share Price | | |
Aggregate
Intrinsic
Value | |
| Balance as at July 1, 2021 | |
| 98,920 | | |
$ | 75,47 | | |
$ | - | |
| Granted | |
| 161,453 | | |
| 18.50 | | |
| - | |
| Exercised | |
| (15,606 | ) | |
| 11.25 | | |
| 125,611 | |
| Balance as at June 30, 2022 | |
| 244,767 | | |
| 41.99 | | |
| - | |
| Granted | |
| - | | |
| - | | |
| - | |
| Expired / Cancelled | |
| (191,345 | ) | |
| 18.04 | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Balance as at June 30, 2023 | |
| 53,422 | | |
$ | 92.91 | | |
$ | - | |
The intrinsic value
of warrants as of June 30, 2023 and 2022 was $Nil.
On July 2, 2021, 12,109 warrants were
issued for services with an exercise price of $92.9075 per share, were immediately exercisable upon issuance, and expire 5 years following
the date of issuance. The agents’ warrants did not meet the criteria to be classified as a liability award and therefore were treated
as an equity award and recorded as a component of shareholders’ equity in the consolidated balance sheet.
The following is a summary of changes in agents’
warrants from July 1, 2021 to June 30, 2023:
| | |
Number | | |
Weighted
Average Share Price | | |
Aggregate
Intrinsic
Value | |
| Balance as at July 1, 2021 | |
| - | | |
$ | - | | |
$ | - | |
| Granted | |
| 12,109 | | |
| 92.91 | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Balance as at June 30, 2022 | |
| 12,109 | | |
| 92.91 | | |
| - | |
| Granted | |
| - | | |
| - | | |
| - | |
| Expired / Cancelled | |
| - | | |
| - | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Balance as at June 30, 2023 | |
| 12,109 | | |
$ | 92.91 | | |
$ | - | |
| e) | Preferred Investment Options |
On September 13, 2022, the Company
closed a private placement of its common shares and 1,382,490 preferred investment options were issued with an exercise price of $8.44
per share, were immediately exercisable upon issuance, and expire 7 years following the date of issuance. The fair value of preferred
investment options was calculated using the Black-Scholes option pricing model and was determined to be $10.91 per option. Assumptions
used included a weighted average risk-free interest rate of 3.12%, expected term of 7 years, weighted average volatility factor of 114.42%
and a weighted average dividend yield of 0%. The allocated value of the investment options was recorded in additional paid-in capital.
On November 21, 2022, these preferred investment options were surrendered to the Company for cancellation.
On November 21, 2022, the Company closed a private placement of its
common shares and 3,272,733 preferred investment options were issued with an exercise price of $3.044 per share, were immediately exercisable
upon issuance, and expire 7 years following the date of issuance. The fair value of preferred investment options was calculated using
the Black-Scholes option pricing model and was determined to be $2.278 per option. Assumptions used included a weighted average risk-free
interest rate of 2.92%, expected term of 7 years, weighted average volatility factor of 116.52% and a weighted average dividend yield
of 0%. The allocated value of these investment options was recorded in additional paid-in capital.
On June 6, 2022, 233,100 preferred
investment options were issued with an exercise price of $19.75 per share, were immediately exercisable upon issuance, and expire 6.5 years
following the date of issuance.
The following is a summary of changes
in preferred investment options from July 1, 2021 to June 30, 2023:
| | |
Number | | |
Weighted
Average Share Price | | |
Aggregate
Intrinsic Value | |
| Balance as at July 1, 2021 | |
| - | | |
$ | - | | |
$ | - | |
| Granted | |
| 233,100 | | |
| 19.75 | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Balance as at June 30, 2022 | |
| 233,100 | | |
| 19.75 | | |
| - | |
| Granted | |
| 4,655,223 | | |
| 4.65 | | |
| - | |
| Expired / Cancelled | |
| (1,615,590 | ) | |
| 9.89 | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Balance as at June 30, 2023 | |
| 3,272,733 | | |
$ | 3.04 | | |
$ | - | |
| f) | Agents’ Investment Options |
On September 13, 2022, the Company
closed a private placement of its common shares and 44,931 preferred investment options were issued for services with an exercise price
of $10.85 per share, were immediately exercisable upon issuance, and expire approximately 7 years following the date of issuance. The
fair value of agents’ investment options was calculated using the Black-Scholes option pricing model and was determined to be $10.06
per option. Assumptions used included a weighted average risk-free interest rate of 3.24%, expected term of 5 years, weighted average
volatility factor of 116.88% and a weighted average dividend yield of 0%. The allocated value of these agents’ investment options
was recorded in additional paid-in capital.
On November 21, 2022, the Company
closed a private placement of its common shares and 118,182 preferred investment options were issued for services with an exercise price
of $4.125 per share, were immediately exercisable upon issuance, and expire approximately 7 years following the date of issuance. The
fair value of agents’ investment options was calculated using the Black-Scholes option pricing model and was determined to be $2.03
per option. Assumptions used included a weighted average risk-free interest rate of 3.18%, expected term of 5 years, weighted average
volatility factor of 117.97% and a weighted average dividend yield of 0%. The allocated value of these agents’ investment options
was recorded in additional paid-in capital.
On June 6, 2022, 15,152 preferred
investment options were issued for services with an exercise price of $26.8125 per share, were exercisable 4 months upon
issuance, and expire 5 years following the date of issuance.
The following is a summary of changes
in Agents’ Investment Options from July 1, 2021 to June 30, 2023:
| | |
Number | | |
Weighted
Average Share Price | | |
Aggregate
Intrinsic
Value | |
| Balance as at July 1, 2021 | |
| - | | |
$ | - | | |
$ | - | |
| Granted | |
| 15,152 | | |
| 26.81 | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Balance as at June 30, 2022 | |
| 15,152 | | |
| 26.81 | | |
| - | |
| Granted | |
| 163,113 | | |
| 5.98 | | |
| - | |
| Expired / Cancelled | |
| - | | |
| | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | |
| Balance as at June 30, 2023 | |
| 178,265 | | |
$ | 7.75 | | |
$ | - | |
On March 24,
2017, and as amended on November 20, 2020, the Company’s shareholders approved: (i) the adoption of a new stock option plan (the
“Plan”) pursuant to which the Board of Directors may, from time to time, in its discretion and in accordance with regulatory
requirements, grant to directors, officers, employees and consultants of the Company, non-transferable options to purchase common shares,
provided that the number of common shares reserved for issuance will not exceed twenty percent (20%) of the issued and outstanding common
shares at the date the options are granted (on a non-diluted and rolling basis); and (ii) the application of the new stock option plan
to all outstanding stock options of the Company that were granted prior to March 24, 2017 under the terms of the Company’s previous
stock option plan.
As of June 30,
2023 and 2022, there were 51,633 and 18,163, respectively, options available for future allocation pursuant to SEC rules and 20% of the
issued and outstanding shares according to the terms of the Plan. The option price under each option shall not be less than the closing
price on the day prior to the date of grant. All options vest upon terms as set by the Board of Directors, either over time, up to 36
months, or upon the achievement of certain corporate milestones.
Stock options
granted prior to May 2021 were granted with Canadian dollar exercise prices (United States dollar amounts for weighted average exercise
prices and aggregate intrinsic value are calculated using prevailing rates as at June 30, 2022). Commencing in May 2021, stock options
are granted with United States dollar exercise prices.
The following is a summary of changes
in outstanding options from July 1, 2021 to June 30, 2023:
| | |
Number | | |
Weighted
Average Exercise
Price | |
| | |
| | |
| |
| Balance as at June 30, 2021 | |
| 36,472 | | |
$ | 215.35 | |
| Granted | |
| 31,160 | | |
| 34.20 | |
| Expired/Forfeited | |
| (12,029 | ) | |
| 122.38 | |
| Balance as at June 30, 2022 | |
| 55,603 | | |
| 128.59 | |
| Granted | |
| 61,720 | | |
| 1.85 | |
| Expired/Forfeited | |
| (14,681 | ) | |
| 267.13 | |
| Balance as at June 30, 2023 | |
| 102,642 | | |
$ | 31.28 | |
| June 30, 2022: | |
| | |
| |
| Vested and exercisable | |
| 26,182 | | |
| 228.87 | |
| Unvested | |
| 29,421 | | |
$ | 39.35 | |
June 30, 2023: | |
| | |
| |
| Vested and exercisable | |
| 51,067 | | |
| 57.44 | |
| Unvested | |
| 51,575 | | |
$ | 5.38 | |
| b) | Fair Value of Options Issued During the Period |
| i) | Weighted Average Fair Value at Grant Date of Options Granted: |
The weighted average
fair value at grant date of options granted during the year ended June 30, 2023 and 2022, was $1.85 and $21.04 respectively, per option.
Assumptions used for options granted during the year ended June 30, 2023 and 2022 included a weighted average risk-free interest rate
of 3.74% and 1.17% respectively, weighted average expected life of 3.3 years calculated using the Simplified Method for directors, officers
and employees, weighted average volatility factor of 122.98% and 97.15% respectively, weighted average dividend yield of 0% and 0% respectively
and a 5% and 5% respectively.
| ii) | Expenses Arising from Share-based Payment Transactions: |
Total expenses
arising from share-based payment transactions recognized during the year months ended June 30, 2023 and 2022, were $278,154 and $697,894
respectively. $162,200 and $419,075 respectively, was allocated to general and administrative expenses and the remaining $115,954 and
$278,819 respectively, was allocated to research and development expenses. Unrecognized compensation cost at June 30, 2023 related to
unvested options was $51,575 which will be recognized over a weighted-average vesting period of 3.6 years.
The Company is committed to minimum
lease payments as follows:
| Maturity Analysis | |
June 30,
2023 | |
| | |
$ | |
| | |
| |
| Less than one year | |
| 384,713 | |
| One to five years | |
| 9,017 | |
| More than five years | |
| - | |
| Total undiscounted lease liabilities(1) | |
| 393,730 | |
| Less: imputed interest | |
| (2,023 | ) |
| Present value of lease liabilities | |
| 391,707 | |
| | |
| | |
| Less: Current portion of lease liabilities | |
| (375,713 | ) |
| Non-current portion of lease liabilities | |
| (15,994 | ) |
The
following is a reconciliation of income taxes calculated at the combined Canadian federal and provincial income statutory corporate tax
rate of 27.0% to the tax expense:
| | |
2023 | | |
2022 | |
| | |
$ | | |
$ | |
| US net loss before taxes | |
| (1,212,372 | ) | |
| (5,189,364 | ) |
Canada net loss before tax | |
| (6,735,250 | ) | |
| (13,410,749 | ) |
| Net loss before taxes | |
| (7,947,622 | ) | |
| (18,600,117 | ) |
| | |
| | | |
| | |
| Income tax expense (recovery) at the statutory rate | |
| (2,073,116 | ) | |
| (4,710,669 | ) |
| Increase (reduction) in income taxes resulting from: | |
| | | |
| | |
| Change in valuation allowance | |
| 2,532,867 | | |
| 4,112,045 | |
| State taxes | |
| 9,638 | | |
| (220,491 | ) |
| Permanent differences | |
| 76,605 | | |
| 613,269 | |
| Foreign exchange differences | |
| 417,194 | | |
| 591,263 | |
| Share issuance cost capitalized in equity | |
| (553,877 | ) | |
| (582,548 | ) |
| Other | |
| (396,211 | ) | |
| 197,131 | |
| Income tax expense (recovery) | |
| 13,100 | | |
| - | |
As
of June 30, 2023, the Company has non-capital loss carry-forwards of approximately $67,875,659 (June 30, 2022 - $62,921,785) available
to offset future taxable income in Canada. These non-capital loss carryforwards begin to expire in 2026. As of June 30, 2023, the Company
has US Federal net operating losses of $4,295,287 and state net operating losses of $3,328,922. The US Federal NOLs have an indefinite
carryforward period, and the state NOLs begin to expire in 2042.
Deferred
tax assets and liabilities are as follows:
| | |
2023 | | |
2022 | |
| | |
$ | | |
$ | |
| Non-capital losses | |
| 19,490,270 | | |
| 17,003,766 | |
| Financing costs | |
| 1,265,542 | | |
| 702,479 | |
| Accrued expenses | |
| 11,638 | | |
| 193,549 | |
| Intangible assets, net | |
| 131,714 | | |
| 553,392 | |
| Tax credits | |
| 241,270 | | |
| 248,254 | |
| Lease liability | |
| 98,827 | | |
| 51,994 | |
| | |
| 21,239,261 | | |
| 18,753,434 | |
| Property and equipment, net | |
| (119,889 | ) | |
| (16,546 | ) |
| Lease obligations | |
| (91,377 | ) | |
| (55,260 | ) |
| | |
| (211,266 | ) | |
| (71,806 | ) |
| Net deferred tax asset | |
| 21,027,995 | | |
| 18,681,628 | |
| Valuation allowance | |
| (21,027,995 | ) | |
| (18,681,628 | ) |
| | |
| - | | |
| - | |
A
full valuation allowance has been applied against the net deferred tax assets because it is more likely than not that future taxable income
will be available against which the Company can utilize the benefits therefrom.
The
Company recognizes tax benefits from an uncertain tax position only if it is more likely than not that the tax position will be sustained
on examination by taxing authorities, based on the technical merits of the position. The tax benefits recognized in the consolidated financial
statements from any such position would be measured based on the largest benefit that has a greater than fifty percent likelihood
of being realized upon ultimate settlement. It is the Company’s policy to recognize interest and penalties accrued on any uncertain
tax benefits as a component of income tax expense. As of June 30, 2023, the Company has one uncertain tax position relating to IRS code
280E. The effect of this uncertainty relates to the deductions available to the Company regarding cost of goods sold.
The
Company files income tax returns in the U.S. federal jurisdiction, various state jurisdictions, and Canada. With few exceptions, the Company
is no longer subject to U.S. federal and state tax examinations for fiscal years prior to 2019.
The
Company is subject to taxation at the federal, state, and local levels in the United States and Canada.
As of the closing
of the BayMedica acquisition, the Company aligned into two operating and reportable segments, the InMed segment and the BayMedica segment.
The Company reports segment information based on the management approach which designates the internal reporting used by the Chief Operating
Decision Maker (“CODM”), which is the Company’s Chief Executive Officer, for making decisions and assessing performance
as the source of the Company’s reportable segments. The CODM allocates resources and assesses the performance of each operating
segment based on potential licensing opportunities, historical and potential future product sales, operating expenses, and operating income
(loss) before interest and taxes. The Company has determined its reportable segments to be InMed and BayMedica based on the information
used by the CODM. Other than cash, cash equivalents and short-term investments (“Unrestricted cash”) balances, the CODM does
not regularly review asset information by reportable segment and therefore, the Company does not report asset information by reportable
segment.
The InMed segment
is largely organized around the research and development of cannabinoid-based pharmaceuticals products and the BayMedica segment is largely
organized around developing proprietary manufacturing technologies to produce rare cannabinoids for sale in the health and wellness industry.
Total assets as of June 30, 2023 and 2022, held in the InMed segment are $9,498,752 and $8,010,832 respectively. Total assets as of June
30, 2023 and 2022, held in the BayMedica segment are $4,607,588 and $4,776,746 respectively.
The following table presents information
about the Company’s reportable segments for the year ended June 30, 2023 and 2022:
| | |
Year Ended June 30, | |
| | |
2023 | | |
2022 | |
| | |
InMed | | |
BayMedica | | |
Total | | |
InMed | | |
BayMedica | | |
Total | |
| | |
$ | | |
$ | | |
$ | | |
$ | | |
$ | | |
$ | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Sales | |
| - | | |
| 4,135,561 | | |
| 4,135,561 | | |
| - | | |
| 1,089,435 | | |
| 1,089,435 | |
| Cost of sales | |
| - | | |
| (2,423,588 | ) | |
| (2,423,588 | ) | |
| - | | |
| (545,889 | ) | |
| (545,889 | ) |
| Inventory write-down | |
| - | | |
| (308,937 | ) | |
| (308,937 | ) | |
| - | | |
| - | | |
| - | |
| Operating expenses | |
| (6,990,477 | ) | |
| (2,791,346 | ) | |
| (9,781,823 | ) | |
| (11,998,435 | ) | |
| (5,809,460 | ) | |
| (17,807,895 | ) |
| Other income (expense) | |
| 255,227 | | |
| 175,938 | | |
| 431,165 | | |
| (1,412,318 | ) | |
| 76,550 | | |
| (1,335,768 | ) |
| Net loss | |
| (6,735,250 | ) | |
| (1,212,372 | ) | |
| (7,947,622 | ) | |
| (13,410,753 | ) | |
| (5,189,364 | ) | |
| (18,600,117 | ) |
| Unrestricted cash | |
| 8,036,714 | | |
| 875,803 | | |
| 8,912,517 | | |
| 5,984,622 | | |
| 192,244 | | |
| 6,176,866 | |
| 14. | COMMITMENTS AND CONTINGENCIES |
Pursuant to the
terms of agreements with various contract research organizations, as of June 30, 2023, the Company is committed for contract research
services and materials at a cost of approximately $2.0 million, expected to occur in the twelve months following period.
Pursuant to the
terms of agreements with various vendors, as of June 30, 2023, the Company is committed for contract materials and equipment at a cost
of approximately $0.7 million, expected to occur in the twelve months following June 30, 2023.
Pursuant to the terms of a May 31,
2017 Technology Assignment Agreement between the Company and the University of British Columbia (“UBC”), the Company is committed
to pay royalties to UBC on certain licensing and royalty revenues received by the Company for biosynthesis of certain drug products that
are covered by the agreement. To date, no payments have been required to be made.
Pursuant to the terms of a December
13, 2018 Collaborative Research Agreement with UBC in which the Company owns all rights, title and interests in and to any intellectual
property, in addition to funding research at UBC, the Company is committed to make a one-time payment upon filing of any PCT patent application
arising from the research. To date, one such payment has been made to UBC.
Pursuant to the
terms of a November 1, 2018 Contribution Agreement with National Research Council Canada, as represented by its Industrial Research Assistance
Program (NRC-IRAP), under certain circumstances contributions received, including the disposition of the underlying intellectual property
developed in part with NRC-IRAP contributions, may become repayable.
Short-term investments include guaranteed
investment certificates, with one year terms, of $44,422 and 44,676 as of June 30, 2023 and 2022 respectively, that are pledged as security
for a corporate credit card.
The Company has entered into certain
agreements in the ordinary course of operations that may include indemnification provisions, which are common in such agreements. In some
cases, the maximum amount of potential future indemnification is unlimited; however, the Company currently holds commercial general liability
insurance. This insurance limits the Company’s liability and may enable the Company to recover a portion of any future amounts paid.
Historically, the Company has not made any indemnification payments under such agreements and it believes that the fair value of these
indemnification obligations is minimal. Accordingly, the Company has not recognized any liabilities relating to these obligations for
any period presented.
Pursuant to a technology licensing
agreement, the Company is committed to issue, subject to regulatory approval, up to 700 warrants to purchase 700 common shares upon the
achievement of certain milestones. The exercise price of the warrants will be equal to the five-day VWAP of the common shares prior to
each milestone achievement and the warrants will be exercisable for a period of three years for issuance date.
BayMedica LLC, a wholly-owned subsidiary
of the Company, entered into a patent license agreement (“Agreement”) with a third party (the “Licensor”) in an
agreement dated February 15, 2021. The Company is required to make future royalty payments to the Licensor based on net sales of licensed
products, with minimum payments required starting in 2021 to maintain an exclusive license. In December 2021, the Company amended the
License Agreement including the deferral of the 2021 minimum payments to 2022. As of June 30, 2023, the Company has paid $300,000 for
the minimum payments under the agreement. On February 10, 2023, BayMedica received a letter from the Licensor alleging a breach of the
Agreement and asserting a right to monies thereunder. On April 6, 2023, BayMedica sent a letter to the Licensor disputing the Licensor’s
interpretation of the Agreement and considering the counterparty’s only remedy under the Agreement to be either (a) the conversion
of an exclusive technology license into a non-exclusive one or (b) to terminate the Agreement. The interpretation of a contract under
Ontario law requires consideration of the surrounding circumstances at the time the contract was negotiated, and BayMedica is of the view
that the text of the Agreement and the surrounding circumstances show that the remedy discussed above reflects the intention of the parties.
To date, the Licensor has not initiated a lawsuit. If a lawsuit is brought alleging a breach of the Agreement, the proceeding will be
subject to final, binding and non-appealable arbitration under the Arbitration Act, 1991 (Ontario) and determined pursuant to Ontario
law. BayMedica intends to vigorously defend its position. At this time, it is not possible to reasonably estimate a potential loss due
to the terms of the Agreement, the nature of the legal theory advanced by the counterparty, and the requirement under Ontario law that
a contract must be interpreted in light of the “surrounding circumstances” at the time the contract was formed. Management
will be better positioned to determine whether it is possible to estimate any potential loss following documentary and oral discovery,
if any.
From time to time, the Company may
be subject to various legal proceedings and claims related to matters arising in the ordinary course of business. The Company does not
believe it is currently subject to any material matters where there is at least a reasonable possibility that a material loss may be incurred.
| 15. | RELATED PARTY TRANSACTIONS |
On February 11, 2022, the Board of Directors appointed Janet Grove
as a director of the Company. Ms. Grove is a Partner of Norton Rose Fulbright Canada LLP (“NRF”). From February 11, 2022 to
June 30, 2022, NRF rendered legal services in the amount of $345,935 to the Company. During the year ended June 30, 2023, NRF rendered
legal services in the amount of $634,208 to the Company. These transactions were in the normal course of operations and were measured
at the exchange amount which represented the amount of consideration established and agreed to by NRF. No legal services rendered by NRF
were rendered by Ms. Grove directly.
The Company has
evaluated subsequent events through the date of the filing of this Annual Report on Form 10-K and determined that there have been no events
that have occurred that would require adjustments to our disclosures in the consolidated financial statements except for the transactions
described below.
On
September 19, 2023, the Company received written notice from the listing qualifications department staff of The Nasdaq Capital Market
(“Nasdaq”) notifying it that the average closing bid price of the Company’s common shares over a period of 30 consecutive
trading days was below the minimum $1.00 per share requirement for continued listing on the Nasdaq under Nasdaq Listing Rule 5550(a)(2).
In
accordance with applicable Nasdaq procedures, the Company has a period of 180 calendar days following the receipt of the written notice
mentioned above to cure the deficiency and regain compliance. The notice has no immediate impact on the listing of the Company’s common
shares, which will continue to trade on the Nasdaq subject to the Company’s continued compliance with the other listing requirements
of the Nasdaq. The common shares of the Company will continue to trade under the symbol “INM”. The Company intends to monitor
the closing share price for its common shares and explore available options to regain compliance.
In
the event the Company does not evidence compliance with the minimum bid price requirement during the 180-day grace period, it is expected
that Nasdaq would notify the Company that its common shares are subject to delisting. At such time, the Company may appeal such determination
to a Nasdaq Hearings Panel (the “Panel”) and it is expected that the Company’s securities would continue to be listed
and available to trade on Nasdaq at least pending the completion of the appeal process. There can be no assurance that any such appeal
would be successful or that the Company would be able to evidence compliance with the terms of any extension that may be granted by the
Panel.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation
of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures
as of June 30, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the
Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by
a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time
periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures
designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act
is accumulated and communicated to its management, including its principal executive and principal financial officers, as appropriate
to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed
and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating
the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as
of June 30, 2023, our principal executive officer and principal financial officer concluded that, as of such date, our disclosure controls
and procedures were not effective.
Management’s Report on Internal Control over Financial
Reporting
Our management is responsible
for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined
in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision
of, the Company’s principal executive and principal financial officers and effected by the company’s board of directors, management
and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures
that:
| |
● |
Pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| |
● |
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and directors of the Company; and |
| |
● |
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations,
internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective
can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation
of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over
financial reporting as of June 30, 2023. In making this assessment, management used the criteria set forth by the Committee of Sponsoring
Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework (2013). Based on this evaluation,
management has concluded our internal control over financial reporting as of June 30, 2023 was not effective due to the material weakness
in the Company’s internal control over financial reporting as disclosed below.
Notwithstanding the identified
material weakness, our Chief Executive Officer and our Chief Financial Officer believe the consolidated financial statements included
in this Annual Report on Form 10-K fairly represent in all material respects our financial condition, results of operations and cash flows
at and for the periods presented in accordance with U.S. GAAP.
Ongoing Remediation of Previously Reported Material Weakness
In connection with the audits of our consolidated financial statements
as of and for the years ended June 30, 2023 and 2022, our management previously identified a material weakness in the Company’s
internal control over financial reporting, primarily the result of inadequate resources required to respond to financial reporting matters
other than in the normal course of business. In connection with the preparation of the consolidated financial statements as of June 30,
2022, we identified that we did not maintain effective processes and controls over financial reporting matters other than in the normal
course of business. Because of this weakness, which is pervasive in nature, there is a reasonable possibility that a material misstatement
of our consolidated financial statements will not be prevented or detected on a timely basis. In the year ended June 30, 2022, this deficiency
resulted in certain material audit adjustments related to the presentation of pre-funded warrants associated with a financing transaction,
and the fair value on the purchase consideration for the acquisition of BayMedica Inc. The presence of these adjustments is indicative
of failures in design and effectiveness of internal controls. The identified material weakness from June 30, 2022 has been remediated
as of June 30, 2023 with the hiring of an outside consulting team with experience in our industry. Since the resignation of our VP of
Accounting and Controller in July of 2023, the new material weakness is around lack of personnel and inadequate controls around segregation
of duties in the accounting department from employee turnover.
Management has implemented a remediation plan to address the root causes
that contributed to the material weakness and is committed to a strong Internal Control over Financial Reporting (ICFR) environment. Management
has begun looking for a replacement for our VP of Accounting and Controller role within the accounting department, who has the requisite
technical accounting knowledge, which management believes addresses the material weakness. The identified material weakness will only
be considered remediated once the applicable remedial controls operate for a sufficient period of time and management has concluded, through
testing, that these controls are operating effectively. The Company has engaged Brio Financial Group to fulfill the VP of Accounting and
Controller position. The engagement is expected to commence by mid-October.
Changes in Internal Control over Financial Reporting
Other than the actions we
have taken and are continuing to take in order to remediate the material weakness described above for the financial year ended June 30,
2023 there were no changes in our internal control over financial reporting identified in connection with the evaluation required by Rule
13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the year ended June 30, 2023 that have materially affected, or are reasonably
likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
Not applicable
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENTS
INSPECTIONS
Not applicable
PART III
The information required by
Part III is omitted from this report because we will file a definitive proxy statement within 120 days after the end of our 2023 fiscal
year pursuant to Regulation 14A for our 2023 Annual Meeting of Stockholders, or the 2023 Proxy Statement, will be filed pursuant to Regulation
14A of the Securities Exchange Act of 1934, as amended. If the 2023 Proxy Statement is not filed within 120 days after the end of the
fiscal year covered by this Annual Report on Form 10-K, the omitted information will be included in an amendment to this Annual Report
on Form 10-K filed not later than the end of such 120-day period.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The response to this item
is incorporated by reference from the discussion responsive thereto under the captions “Management and Corporate Governance,”
“Section 16(a) Beneficial Ownership Reporting Compliance,” and “Code of Business Conduct and Ethics” in the Company’s
Proxy Statement for the 2023 Annual Meeting of Stockholders.
ITEM 11. EXECUTIVE COMPENSATION
The response to this item
is incorporated by reference from the discussion responsive thereto under the caption “Executive Officer and Director Compensation”
in the Company’s Proxy Statement for the 2023 Annual Meeting of Stockholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL
OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The response to this item
is incorporated by reference from the discussion responsive thereto under the captions “Security Ownership of Certain Beneficial
Owners and Management,” and “Equity Compensation Plan Information” in the Company’s Proxy Statement for the 2023
Annual Meeting of Stockholders.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
The response to this item
is incorporated by reference from the discussion responsive thereto under the captions “Certain Relationships and Related Person
Transactions” and “Management and Corporate Governance” in the Company’s Proxy Statement for the 2023 Annual Meeting
of Stockholders.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Our independent registered public accounting firm is Marcum LLP PCAOB
Auditor ID 688. The information required by this item will be included in our definitive proxy statement with respect to our 2023 Annual
Meeting of Shareholders to be filed with the SEC, and is incorporated herein by reference.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
The following documents are
being filed as part of this report:
| |
(1) |
The following financial statements of the Company and the report of
KPMG LLP and Marcum LLP are included in Part II, Item 8: |
| |
(2) |
All financial statement supporting schedules are omitted because the information is inapplicable or presented in the Notes to Consolidated Financial Statements. |
| |
(3) |
A list of exhibits filed with this report or incorporated herein by reference is found in the Exhibit Index immediately following the signature page of this Annual Report. |
| EXHIBIT |
|
|
| NUMBER |
|
DESCRIPTION |
| |
|
|
| 2.1 |
|
Amended and Restated Agreement and Plan of Reorganization, dated as of October 13, 2021, by and among InMed Pharmaceuticals Inc., InMed LLC, BayMedica, Inc., BM REP, LLC, as the stockholder representative, and certain stockholders thereto (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the SEC on October 13, 2021). |
| 3.1 |
|
Amended and Restated Articles of InMed Pharmaceuticals Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form S-1 filed with the SEC on June 19, 2020). |
| 4.1 |
|
Form of Specific Common Share Certificate (incorporated by reference to Exhibit 4.3 to the Company’s Registration Statement on Form S-1 filed with the SEC on July 13, 2021). |
| 4.2 |
|
Form of Common Shares Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on November 12, 2020). |
| 4.3 |
|
Form of Common Shares Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on February 5, 2021). |
| 4.4 |
|
Form of Series A Warrant (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on June 30, 2021). |
| 4.5 |
|
Form of Pre-Funded Warrants (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on June 30, 2021). |
| 4.6 |
|
Form of Preferred Investment Option (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2022). |
| 4.7 |
|
Form of Pre-Funded Warrant (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2022). |
| 4.8 |
|
Form of Pre-Funded Warrant (incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2022). |
| 4.9 |
|
Warrant Amendment Agreement (incorporated by reference to Exhibit 4.4 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2022). |
| 4.1 |
|
Form of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on September 14, 2022). |
| 4.11 |
|
Form of Preferred Investment Option (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on September 14, 2022). |
| 4.12 |
|
Form of Placement Agent Preferred Investment Option (incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form 8-K filed with the SEC on September 14, 2022). |
| 4.13 |
|
Description of Securities of InMed Pharmaceuticals Inc. |
| 10.1† |
|
InMed Pharmaceuticals Inc. 2017 Amended and Restated Stock Option Plan, as amended (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form S-8 filed with the SEC on March 5, 2021). |
| 10.2† |
|
Form of Stock Option Agreement pursuant to the InMed Pharmaceuticals Inc. 2017 Amended and Restated Stock Option Plan (incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form S-8 filed with the SEC on March 5, 2021). |
| 10.3 |
|
Registration Rights Agreement, dated February 5, 2021, between InMed Pharmaceuticals Inc. and several purchasers thereto (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on February 5, 2021). |
| 10.4 |
|
Registration Rights Agreement, dated June 28, 2021, between InMed Pharmaceuticals Inc. and several purchasers thereto (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on June 30, 2021). |
| 10.5 |
|
Registration Rights Agreement, dated June 1, 2022, between InMed Pharmaceuticals Inc. and the purchasers thereto (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2022). |
| 10.6 |
|
Registration Rights Agreement, dated September 9, 2022, between InMed Pharmaceuticals Inc. and the purchasers thereto (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on September 14, 2022). |
| 10.7† |
|
Amended and Restated Executive Employment Agreement, dated March 1, 2021, between Eric A. Adams and InMed Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.3 to the Company’s Registration Statement on Form S-1 filed with the SEC on July 13, 2021). |
| 10.8† |
|
Amendment dated July 11, 2022 to Eric Adams’ Employment Agreement dated 1 March 2021 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 18, 2022). |
| 10.9† |
|
Amended and Restated Executive Employment Agreement, dated March 1, 2021, between Eric Hsu and InMed Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.4 to the Company’s Registration Statement on Form S-1 filed with the SEC on July 13, 2021). |
| 10.10† |
|
Amended and Restated Executive Employment Agreement, dated March 1, 2021, between Alexandra Mancini and InMed Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement on Form S-1 filed with the SEC on July 13, 2021). |
| 10.11† |
|
Amended and Restated Executive Employment Agreement, dated March 1, 2021, between Bruce S. Colwill and InMed Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.7 to the Company’s Registration Statement on Form S-1 filed with the SEC on July 13, 2021). |
| 10.12† |
|
Employment Agreement dated July 15, 2022, between InMed Pharmaceuticals Inc. and Michael Woudenberg (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 20, 2022) |
| 10.13† |
|
Consulting Agreement dated as of April 1, 2022, between InMed Pharmaceuticals Inc. and Brenda Edwards. |
| 10.14† |
|
Form of InMed Pharmaceuticals Inc. Indemnification Agreement entered into with each member of the board of directors and Chief Financial Officer (incorporated by reference to Exhibit 10.10 to the Company’s Annual Report on Form 10-K filed with the SEC on September 24, 2021) |
| 10.15 |
|
Office Premises Lease, dated January 14, 2019, between InMed Pharmaceuticals Inc. and 815 West Hastings Ltd. (incorporated by reference to Exhibit 10.8 to the Company’s Registration Statement on Form S-1 filed with the SEC on June 19, 2020). |
| 10.16 |
|
Form of Amendment of Purchase Agreement and Common Stock Purchase Warrant, dated March 21, 2022 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on March 22, 2022). |
| 10.17 |
|
At the Market Offering Agreement dated April 7, 2021 by and between InMed Pharmaceuticals Inc., and H.C. Wainwright & Co., LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on April 7, 2022). |
| 21.1* |
|
Subsidiaries of the Company. |
| 23.1* |
|
Consent of Marcum LLP. |
| 23.2* |
|
Consent of KPMG LLP. |
| 31.1* |
|
Certification of Principal Executive Officer Pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities and Exchange Act of 1934, as amended, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 31.2* |
|
Certification of Principal Financial Officer Pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities and Exchange Act of 1934, as amended, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 32.1* |
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| 32.2* |
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| 101.INS* |
|
Inline XBRL Instance Document. |
| 101.SCH* |
|
Inline XBRL Taxonomy Extension Schema Document. |
| 101.CAL* |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF* |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB* |
|
Inline XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE* |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| 104* |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
ITEM 16. 10-K SUMMARY
Not applicable.
SIGNATURES
Pursuant to the requirements
of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
INMED PHARMACEUTICALS INC. |
| |
(Registrant) |
| |
|
|
| September 29, 2023 |
By: |
/s/ Jonathan Tegge |
| |
|
Jonathan Tegge |
| |
|
Interim Chief Financial Officer
and Principal Accounting Officer |
Pursuant to the requirements
of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and
in the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Eric A. Adams |
|
President, Chief Executive Officer and Director |
|
September 29, 2023 |
| Eric A. Adams |
|
(Principal Executive Officer) |
|
|
| |
|
|
|
|
| /s/ Jonathan Tegge |
|
Interim Chief Financial Officer |
|
September 29, 2023 |
| Jonathan Tegge |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/ Andrew Hull |
|
Director (Chairman to the Board of Directors) |
|
September 29, 2023 |
| Andrew Hull |
|
|
|
|
| |
|
|
|
|
| /s/ Janet Grove |
|
Director |
|
September 29, 2023 |
| Janet Grove |
|
|
|
|
| |
|
|
|
|
| /s/ Bryan Baldasare |
|
Director |
|
September 29, 2023 |
| Bryan Baldasare |
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
| /s/ Nicole Lemerond |
|
Director |
|
September 29, 2023 |
| Nicole Lemerond |
|
|
|
|
96
Unlimited
Unlimited
33.17
3.25
2448458
560829
7547
false
FY
0001728328
0001728328
2022-07-01
2023-06-30
0001728328
2022-12-31
0001728328
2023-09-29
0001728328
2023-06-30
0001728328
2022-06-30
0001728328
2021-07-01
2022-06-30
0001728328
us-gaap:CommonStockMember
2022-06-30
0001728328
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001728328
us-gaap:RetainedEarningsMember
2022-06-30
0001728328
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001728328
us-gaap:CommonStockMember
2022-07-01
2023-06-30
0001728328
us-gaap:AdditionalPaidInCapitalMember
2022-07-01
2023-06-30
0001728328
us-gaap:RetainedEarningsMember
2022-07-01
2023-06-30
0001728328
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-07-01
2023-06-30
0001728328
us-gaap:CommonStockMember
2023-06-30
0001728328
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001728328
us-gaap:RetainedEarningsMember
2023-06-30
0001728328
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001728328
us-gaap:CommonStockMember
2021-06-30
0001728328
us-gaap:AdditionalPaidInCapitalMember
2021-06-30
0001728328
us-gaap:RetainedEarningsMember
2021-06-30
0001728328
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-06-30
0001728328
2021-06-30
0001728328
us-gaap:CommonStockMember
2021-07-01
2022-06-30
0001728328
us-gaap:AdditionalPaidInCapitalMember
2021-07-01
2022-06-30
0001728328
us-gaap:RetainedEarningsMember
2021-07-01
2022-06-30
0001728328
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-07-01
2022-06-30
0001728328
inm:OneCustomersMember
us-gaap:AccountsReceivableMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001728328
inm:TwoCustomersMember
us-gaap:AccountsReceivableMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001728328
inm:ThreeCustomersMember
us-gaap:AccountsReceivableMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001728328
inm:OneCustomersMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001728328
inm:TwoCustomersMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001728328
inm:ThreeCustomersMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001728328
inm:FourCustomersMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2022-07-01
2023-06-30
0001728328
inm:OneCustomersMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2021-07-01
2022-06-30
0001728328
inm:TwoCustomersMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2021-07-01
2022-06-30
0001728328
inm:ThreeCustomersMember
us-gaap:SalesRevenueNetMember
us-gaap:CustomerConcentrationRiskMember
2021-07-01
2022-06-30
0001728328
us-gaap:ComputerEquipmentMember
2023-06-30
0001728328
srt:MinimumMember
us-gaap:EquipmentMember
2023-06-30
0001728328
srt:MaximumMember
us-gaap:EquipmentMember
2023-06-30
0001728328
us-gaap:FurnitureAndFixturesMember
2023-06-30
0001728328
us-gaap:EmployeeStockOptionMember
2022-07-01
2023-06-30
0001728328
us-gaap:EmployeeStockOptionMember
2021-07-01
2022-06-30
0001728328
us-gaap:WarrantMember
2022-07-01
2023-06-30
0001728328
us-gaap:WarrantMember
2021-07-01
2022-06-30
0001728328
2022-01-01
2022-06-30
0001728328
inm:RightOfUseAssetMember
2023-06-30
0001728328
inm:RightOfUseAssetMember
2022-06-30
0001728328
us-gaap:EquipmentMember
2023-06-30
0001728328
us-gaap:EquipmentMember
2022-06-30
0001728328
us-gaap:FurnitureAndFixturesMember
2022-06-30
0001728328
inm:IPRDMember
2022-06-30
0001728328
inm:BayMedicaMember
2022-06-30
0001728328
us-gaap:GoodwillMember
2021-06-30
0001728328
us-gaap:GoodwillMember
2021-07-01
2022-06-30
0001728328
us-gaap:GoodwillMember
2022-06-30
0001728328
inm:IPRDMember
2021-06-30
0001728328
inm:IPRDMember
2021-07-01
2022-06-30
0001728328
inm:IPRDMember
2022-06-30
0001728328
us-gaap:TrademarksMember
2021-06-30
0001728328
us-gaap:TrademarksMember
2021-07-01
2022-06-30
0001728328
us-gaap:TrademarksMember
2022-06-30
0001728328
us-gaap:TrademarksMember
2022-07-01
2023-06-30
0001728328
us-gaap:TrademarksMember
2023-06-30
0001728328
us-gaap:PatentsMember
2021-06-30
0001728328
us-gaap:PatentsMember
2021-07-01
2022-06-30
0001728328
us-gaap:PatentsMember
2022-06-30
0001728328
us-gaap:PatentsMember
2022-07-01
2023-06-30
0001728328
us-gaap:PatentsMember
2023-06-30
0001728328
us-gaap:FiniteLivedIntangibleAssetsMember
2022-07-01
2023-06-30
0001728328
inm:BayMedicaMember
2021-10-13
0001728328
2021-10-13
2021-10-13
0001728328
us-gaap:CommonStockMember
2021-10-13
2021-10-13
0001728328
inm:BayMedicaMember
us-gaap:CommonStockMember
2021-10-13
0001728328
2021-10-13
0001728328
inm:BayMedicaMember
2021-10-13
2022-06-30
0001728328
inm:BayMedicaMember
2022-07-01
2023-06-30
0001728328
inm:BayMedicaMember
2023-06-30
0001728328
inm:September2022PrivatePlacementOfferingMember
2022-09-01
2022-09-13
0001728328
inm:November2022PrivatePlacementOfferingMember
2022-11-01
2022-11-21
0001728328
inm:November2022PrivatePlacementOfferingMember
2022-11-21
0001728328
inm:July2021PrivatePlacementOfferingMember
2021-07-02
2021-07-02
0001728328
inm:July2021PrivatePlacementOfferingMember
2021-07-02
0001728328
inm:July2021PrivatePlacementOfferingMember
2022-06-30
0001728328
inm:July2021PrivatePlacementOfferingMember
2021-07-01
2022-06-30
0001728328
inm:ATMMember
2022-04-01
2022-04-22
0001728328
inm:June2022RegisteredDirectAndPrivatePlacementOfferingsMember
2022-06-06
2022-06-06
0001728328
inm:June2022RegisteredDirectAndPrivatePlacementOfferingsMember
2022-06-06
0001728328
2022-06-06
2022-06-06
0001728328
inm:BayMedicaAgreementMember
2022-06-30
0001728328
inm:BayMedicaAgreementMember
2021-07-01
2022-06-30
0001728328
2021-07-02
2021-07-02
0001728328
2021-07-02
0001728328
2022-06-06
0001728328
us-gaap:WarrantMember
2021-07-02
0001728328
us-gaap:WarrantMember
2021-07-02
2021-07-02
0001728328
inm:AgentsInvestmentOptionsMember
2022-09-01
2022-09-13
0001728328
inm:AgentsInvestmentOptionsMember
2022-09-13
0001728328
inm:AgentsInvestmentOptionsMember
2022-11-21
0001728328
inm:AgentsInvestmentOptionsMember
2022-11-01
2022-11-21
0001728328
inm:PreferredInvestmentOptionsMember
2022-06-06
0001728328
inm:PreferredInvestmentOptionsMember
2022-06-06
2022-06-06
0001728328
inm:PreferredInvestmentOptionsMember
2022-09-01
2022-09-13
0001728328
inm:PreferredInvestmentOptionsMember
2022-09-13
0001728328
inm:PreferredInvestmentOptionsMember
2022-11-01
2022-11-21
0001728328
inm:PreferredInvestmentOptionsMember
2022-11-21
0001728328
inm:AgentsInvestmentOptionsMember
2022-06-06
0001728328
inm:AgentsInvestmentOptionsMember
2022-06-06
2022-06-06
0001728328
inm:SharesIssuedMember
2022-09-30
0001728328
inm:SharesIssuedMember
2022-09-01
2022-09-30
0001728328
inm:PrefundedWarrantsIssuedMember
2022-09-30
0001728328
inm:PrefundedWarrantsIssuedMember
2022-09-01
2022-09-30
0001728328
inm:GrossProceedsMember
2022-09-01
2022-09-30
0001728328
inm:AllocatedToAdditionalPaidinCapitalMember
2022-09-01
2022-09-30
0001728328
2022-09-01
2022-09-30
0001728328
inm:ShareIssuanceCostsMember
2022-09-01
2022-09-30
0001728328
inm:SharesIssuedMember
2022-11-30
0001728328
inm:SharesIssuedMember
2022-11-01
2022-11-30
0001728328
inm:PrefundedWarrantsIssuedMember
2022-11-30
0001728328
inm:PrefundedWarrantsIssuedMember
2022-11-01
2022-11-30
0001728328
inm:GrossProceedsMember
2022-11-01
2022-11-30
0001728328
inm:AllocatedToAdditionalPaidinCapitalMember
2022-11-01
2022-11-30
0001728328
2022-11-01
2022-11-30
0001728328
inm:ShareIssuanceCostsMember
2022-11-01
2022-11-30
0001728328
inm:SharesIssuedMember
2020-07-31
0001728328
inm:SharesIssuedMember
2020-07-01
2020-07-31
0001728328
inm:PrefundedWarrantsIssuedMember
2020-07-31
0001728328
inm:PrefundedWarrantsIssuedMember
2020-07-01
2020-07-31
0001728328
inm:GrossProceedsMember
2020-07-01
2020-07-31
0001728328
inm:AllocatedToAdditionalPaidinCapitalMember
2020-07-01
2020-07-31
0001728328
2020-07-01
2020-07-31
0001728328
inm:ShareIssuanceCostsMember
2020-07-01
2020-07-31
0001728328
inm:SharesIssuedMember
2022-06-30
0001728328
inm:SharesIssuedMember
2022-06-01
2022-06-30
0001728328
inm:PrefundedWarrantsIssuedMember
2022-06-30
0001728328
inm:PrefundedWarrantsIssuedMember
2022-06-01
2022-06-30
0001728328
inm:GrossProceedsMember
2022-06-01
2022-06-30
0001728328
inm:AllocatedToAdditionalPaidinCapitalMember
2022-06-01
2022-06-30
0001728328
2022-06-01
2022-06-30
0001728328
inm:ShareIssuanceCostsMember
2022-06-01
2022-06-30
0001728328
inm:SharePurchaseWarrantsMember
2021-06-30
0001728328
inm:SharePurchaseWarrantsMember
2021-07-01
2022-06-30
0001728328
inm:SharePurchaseWarrantsMember
2022-06-30
0001728328
inm:SharePurchaseWarrantsMember
2022-07-01
2023-06-30
0001728328
inm:SharePurchaseWarrantsMember
2023-06-30
0001728328
inm:AgentsWarrantsMember
2021-06-30
0001728328
inm:AgentsWarrantsMember
2021-07-01
2022-06-30
0001728328
inm:AgentsWarrantsMember
2022-06-30
0001728328
inm:AgentsWarrantsMember
2022-07-01
2023-06-30
0001728328
inm:AgentsWarrantsMember
2023-06-30
0001728328
inm:PreferredInvestmentOptionsMember
2021-06-30
0001728328
inm:PreferredInvestmentOptionsMember
2021-07-01
2022-06-30
0001728328
inm:PreferredInvestmentOptionsMember
2022-06-30
0001728328
inm:PreferredInvestmentOptionsMember
2022-07-01
2023-06-30
0001728328
inm:PreferredInvestmentOptionsMember
2023-06-30
0001728328
inm:AgentsInvestmentOptionsMember
2021-06-30
0001728328
inm:AgentsInvestmentOptionsMember
2021-07-01
2022-06-30
0001728328
inm:AgentsInvestmentOptionsMember
2022-06-30
0001728328
inm:AgentsInvestmentOptionsMember
2022-07-01
2023-06-30
0001728328
inm:AgentsInvestmentOptionsMember
2023-06-30
0001728328
2017-03-01
2017-03-24
0001728328
us-gaap:EmployeeStockOptionMember
2023-06-30
0001728328
us-gaap:EmployeeStockOptionMember
2022-06-30
0001728328
us-gaap:GeneralAndAdministrativeExpenseMember
2022-07-01
2023-06-30
0001728328
us-gaap:GeneralAndAdministrativeExpenseMember
2021-07-01
2022-06-30
0001728328
us-gaap:ResearchAndDevelopmentExpenseMember
2022-07-01
2023-06-30
0001728328
us-gaap:ResearchAndDevelopmentExpenseMember
2021-07-01
2022-06-30
0001728328
srt:ScenarioForecastMember
2024-04-01
2024-04-30
0001728328
srt:ScenarioForecastMember
2024-08-01
2024-08-31
0001728328
country:US
2022-07-01
2023-06-30
0001728328
country:US
2021-07-01
2022-06-30
0001728328
country:CA
2022-07-01
2023-06-30
0001728328
country:CA
2021-07-01
2022-06-30
0001728328
inm:InMedMember
2023-06-30
0001728328
inm:InMedMember
2022-06-30
0001728328
inm:BayMedicaMember
2023-06-30
0001728328
inm:InMedMember
2022-07-01
2023-06-30
0001728328
inm:BayMedicaMember
2022-07-01
2023-06-30
0001728328
inm:InMedMember
2021-07-01
2022-06-30
0001728328
inm:BayMedicaMember
2021-07-01
2022-06-30
0001728328
inm:InMedMember
2023-06-30
0001728328
inm:BayMedicaMember
2023-06-30
0001728328
inm:InMedMember
2022-06-30
0001728328
inm:BayMedicaMember
2022-06-30
0001728328
us-gaap:WarrantMember
2022-07-01
2023-06-30
0001728328
2022-02-11
2022-06-30
0001728328
2023-09-19
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
The following description (this “Description”)
of our common shares is a summary and does not purport to be complete. It is subject to, and qualified in its entirety by reference to,
our Amended and Restated Articles (our “Articles”), which has been filed with the Securities and Exchange Commission. This
Description also summarizes relevant provisions of the British Columbia Business Corporations Act (the “BCBCA”)
and securities laws in the provinces and territories of Canada. We encourage you to read our Articles, the applicable provisions of the
BCBCA and the applicable provisions of securities laws in the provinces and territories of Canada for additional information.
Our authorized share capital consists of an unlimited
number of common shares without par value and an unlimited number of preferred shares without par value. As of the date of the Annual
Report on Form 10-K of which this Description forms a part, no preferred shares were issued and outstanding.
Each common share entitles the holder thereof
to one vote at all meetings of shareholders.
There are no limitations on the rights of non-Canadian owners to hold or vote common shares.
In the event of our liquidation, dissolution or
winding-up, whether voluntary or involuntary, or other distribution of our assets among shareholders for the purpose of winding up our
affairs, subject to the rights, privileges and restrictions attaching to any preferred shares that may then be outstanding, the shareholders
shall be entitled to receive our remaining property.
The shareholders are entitled to receive dividends,
as and when declared by our board of directors, subject to the rights, privileges and restrictions attaching to our securities, which
may be paid in money, property or by the issue of fully paid shares in our capital.
Unless such offer constitutes an exempt transaction,
an offer made by a person to acquire outstanding shares of a Canadian entity that, when aggregated with the offeror’s holdings (and
those of persons or companies acting jointly with the offeror), would constitute 20% or more of the outstanding shares, would be subject
to the take-over provisions of Canadian securities laws. The foregoing is a limited and general summary of certain aspects of applicable
securities law in the provinces and territories of Canada, all in effect as of the date of the Annual Report of which this Description
forms a part.
In addition to the take-over bid requirements
noted above, the acquisition of shares may trigger the application of additional statutory regimes including amongst others, the Investment
Canada Act and the Competition Act.
This summary is not a comprehensive description
of relevant or applicable considerations regarding such requirements and, accordingly, is not intended to be, and should not be interpreted
as, legal advice to any existing or prospective investor and no representation with respect to such requirements to any existing or prospective
investor is made. Existing and prospective investors should consult their own Canadian legal advisors with respect to any questions regarding
securities law in the provinces and territories of Canada.
Under the BCBCA, unless otherwise stated in our
Articles, certain corporate actions require the approval of a special majority of shareholders, meaning holders of shares representing
66 2/3% of those votes cast in respect of a shareholder vote addressing such matter. Those items requiring the approval
of a special majority generally relate to fundamental changes with respect to our business, and include amongst others, resolutions: (i)
removing a director prior to the expiry of his or her term; (ii) altering our Articles, (iii) approving an amalgamation; (iv) approving
a plan of arrangement; and (v) providing for a sale of all or substantially all of our assets.
A. The Company is a global leader in the research,
development, manufacturing and commercialization of rare cannabinoids and wishes to retain the consulting services of the Consultant;
B. The Consultant has relevant experience as financial
consultant;
C. Under an assignment agreement between Robert
Half Canada Inc. and the Company dated March 14, 2022 (the “Assignment Agreement”), the Consultant was seconded to the Company
as a Senior Financial Consultant from March 16, 2022 to March 31, 2022;
D. The Consultant executed a Confidentiality and
Assignment of Inventions Agreement dated March 13, 2022;
E. On March 31, 2022, Robert Half Canada Inc.
and the Company terminated the Assignment Agreement March 31, 2022 and executed and Executive Placement Agreement which provides for,
among other matters, the direct engagement of the Consultant by the Company;
F. The Company wishes to retain the Consultant
and the Consultant wishes to be retained to provide consulting advice to the Company with respect to the advancement of its Business and
other related matters on the terms and conditions set out in this Agreement; and
G. From April 1, 2022 until the Company determines
otherwise, the Consultant will hold the title of Interim Chief Financial Officer.
NOW THEREFORE THIS AGREEMENT WITNESSES that for
and in consideration of the premises and mutual covenants and agreements hereinafter contained, the Company and the Consultant agree as
follows:
If the Consultant is required to travel
long distance on behalf of the Company, the travel time (e.g. on airplane) will be paid at the full rate for time spent providing services
and at a reduced rate of CDN $77.50 per hour otherwise. The Consultant will submit an invoice to the Company, reflecting the services
provided twice per month. Invoices will be payable monthly in arrears, within five business days after receipt of the invoice.
We consent to the incorporation by reference in
this Registration Statement of InMed Pharmaceuticals Inc. on Forms S-1 [File Nos. 333-253925, 333-257858, 333-265731, 333-267831 and 333-268700],
on Forms S-3 [File Nos. 333-262533 and 333-264187], and on Forms S-8 [File Nos. 333-253912 and 333-260323] of our report dated September
29, 2023, which includes an explanatory paragraph as to the Company’s ability to continue as a going concern with respect to our
audit of the consolidated financial statements of InMed Pharmaceuticals Inc. as of June 30, 2023 and for the year then ended, appearing
in the Annual Report on Form 10-K of InMed Pharmaceuticals Inc. for the year ended June 30, 2023.
InMed Pharmaceuticals Inc.
We consent to the use of our report dated September 23, 2022 on the
consolidated financial statements of InMed Pharmaceuticals Inc. (the “Entity”) which comprise the consolidated balance sheet
as of June 30, 2022, the consolidated statement of operations, changes in shareholder’s equity and cash flow for the year ended
June 30, 2022, and notes to the consolidated financial statements, including a summary of significant accounting policies (collectively
the “consolidated financial statements”) which is included in the Annual Report on Form 10-K of the Entity for the fiscal
year ended June 30, 2023.
We also consent to incorporation by reference of such report in the
registration statements (Nos. 333-253925, 333-257858, 333-265731, 333-267831 and 333-268700) on Form S-1, (Nos. 333-262533 and 333-264187)
on Form S-3 and (Nos. 333-253912 and 333-260323) on Form S-8 of the Entity.
I, Eric A. Adams, certify that:
Pursuant to 18 U.S.C. Section
1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, I, Eric A. Adams, the President and Chief Executive Officer
of InMed Pharmaceuticals Inc. (the “Company”), hereby certify that, to my knowledge:
1. The Annual Report on Form 10-K for the year ended June 30, 2023 (the “Report”) of the Company fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934; and
2. The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Pursuant to 18 U.S.C. Section
1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, I, Jonathan Tegge, the Chief Financial Officer of InMed Pharmaceuticals
Inc. (the “Company”), hereby certify that, to my knowledge:
1. The Annual Report on Form 10-K for the year ended June 30, 2023 (the “Report”) of the Company fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934; and
2. The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.