Filed Pursuant to Rule 424(b)(5)
Registration No. 333-274554
Prospectus Supplement No. 2, Dated November 17, 2023
(to Prospectus dated September 28, 2023)

HEART TEST LABORATORIES, INC.
Up to $11,036,310
Common Stock
________________________
This Prospectus Supplement No. 2 (this “prospectus supplement”), amends and supplements our at-the-market offering prospectus dated September 28, 2023, as amended and supplemented by the prospectus supplement dated November 9, 2023 (collectively, the “at-the-market offering prospectus”). This prospectus supplement should be read in conjunction with the at-the-market offering prospectus, which is to be delivered with this prospectus supplement. This prospectus supplement amends and supplements only those sections of the at-the-market offering prospectus listed in this prospectus supplement; all other sections of the at-the-market offering prospectus remain as is.
We previously entered into an Equity Distribution Agreement, dated September 18, 2023 (the “Original EDA”), with Maxim Group LLC (“Maxim”, or the “Sales Agent”), pursuant to which we may offer and sell, from time to time, an aggregate of up to $3,250,000 of shares of our common stock, $0.001 par value per share (“common stock”), in an “at the market” offering (as defined in Rule 415(a)(4) under the Securities Act of 1933, as amended (the “Securities Act”)). On November 9, 2023, we entered into Amendment No. 1 to the Original EDA with Maxim pursuant to which, among other things, we may issue and sell up to $10,000,000 of shares of common stock from time to time through the Sales Agent subject to certain selling limitations (the “First Amendment”). As of November 17, 2023, we have sold 25,345,416 shares of common stock with an aggregate offering price of approximately $6.0 million. On November 17, 2023, we entered into Amendment No. 2 to the Original EDA (the “Second Amendment”, and together with the Original EDA and the First Amendment, the “EDA”) with Maxim pursuant to which, among other things, we may issue and sell up to $15,000,000 of shares of common stock (“Shares”) from time to time through the Sales Agent; provided, however, that in no event will we issue or sell through the Sales Agent such number of shares of common stock that would cause us or the offering of our shares of common stock to not satisfy the eligibility and transaction requirements for use of Form S-3 (including General Instruction I.B.6 of Form S-3). Pursuant to the Second Amendment, we have agreed to reimburse Maxim Group’s legal fees and expenses up to $90,000.
The offering pursuant to this prospectus supplement will terminate upon the sale of all Shares subject to this prospectus supplement. We may file one or more additional prospectus supplements to offer and sell the remaining Shares subject to the EDA. Once all Shares covered by the EDA are issued and sold, the EDA will terminate. The EDA may be terminated by us or by the Sales Agent at any time.
Our common stock and our IPO Warrants are listed on the Nasdaq Capital Market under the symbols “HSCS” and “HSCSW,” respectively. On November 16, 2023, the last reported sale price of our common stock on Nasdaq Capital Market was $0.17 per share and the price of our warrants was $0.08. Sales of our common stock, if any, under this prospectus supplement, will be made by any method permitted that is deemed an “at the market offering” as defined in Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”). Maxim is not required to sell any specific amount, but will act as our sales agent using commercially reasonable efforts consistent with its normal trading and sales practices. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Maxim will be entitled to compensation at a commission rate equal to 4.0% of the gross sales price per share sold pursuant to this prospectus supplement and the EDA (excluding any shares of common stock sold under the Original EDA and the First Amendment, whereby Maxim was entitled to compensation at a commission rate equal to 3.0% of the gross sales price per share sold). See “Plan of Distribution” beginning on page S-32 of this prospectus supplement for additional information regarding the compensation to be paid to Maxim. In connection with the sale of the common stock on our behalf, the Sales Agent will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of the Sales Agent will be deemed to be underwriting commissions or discounts. We have also agreed in the EDA to provide indemnification and contribution to Maxim with respect to certain liabilities, including liabilities under the Securities Act and the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
As of November 17, 2023, the aggregate market value of our outstanding common stock held by non-affiliates was $33,108,931, which was calculated based on 47,707,393 outstanding shares of our common stock held by non-affiliates on November 17, 2023 and a price per share of $0.69, which was the closing price of our common stock on September 18, 2023 and is the highest closing sale price of our common stock on the Nasdaq Capital Market within the prior 60 days. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell the shelf securities in a public primary offering with a value exceeding more than one-third of the aggregate market value of our voting and non-voting ordinary shares held by non-affiliates in any 12-month period as long as the aggregate market value of our outstanding ordinary shares held by non-affiliates is less than $75 million. During the 12 calendar months prior to and including the date of this prospectus supplement, we have sold approximately $6.0 million worth of our securities pursuant to General Instruction I.B.6 of Form S-3.
Investing in our common stock involves a high degree of risk. Please read the information contained in and incorporated by reference under the heading “Risk Factors” beginning on page S-20 of this prospectus supplement, P-18 of the at-the-market offering prospectus, the section captioned “Item 1A — Risk Factors” in our most recently filed Annual Report on Form 10-K and in our most recently filed Quarterly Report on Form 10-Q, which we have incorporated by reference into this prospectus supplement, the at-the-market prospectus and under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this prospectus supplement.
Neither the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the at-the-market offering prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
________________________
Maxim Group LLC
The date of this Prospectus Supplement is November 17, 2023.
Table of Contents
S-i
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the at-the-market offering prospectus are part of a registration statement on Form S-3 (No. 333-274554) that we filed with the U.S. Securities and Exchange Commission (the “SEC”), using a “shelf” registration process. Under the at-the-market offering prospectus, as amended and supplemented by this prospectus supplement, we may offer shares of our common stock having an aggregate offering price of up to $11,036,310 from time to time at prices and on terms to be determined by market conditions at the time of the offering, subject to the General Instruction I.B.6 of Form S-3 as applicable.
In making your investment decision, you should rely only on the information contained or incorporated by reference in this prospectus supplement, the at-the-market offering prospectus and any free writing prospectus with respect to this offering filed by us with the SEC. You should also read and consider the information in the documents we have referred you to in the at-the-market offering prospectus under the headings “Where You Can Find More Information” and in this prospectus supplement under “Incorporation of Certain Information by Reference.” These documents contain important information that you should consider when making your investment decision. We have not, and Maxim has not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information you should not rely on it. You should assume that the information appearing in this prospectus supplement, the at-the-market offering prospectus, any free writing prospectus with respect to the offering filed by us with the SEC and the documents incorporated by reference herein and therein is accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed since those dates.
We are offering to sell shares of common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement, the at-the-market offering prospectus and the offering of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and at-the-market offering prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of the prospectus supplement and the at-the-market offering prospectus outside the United States. This prospectus supplement and the at-the-market offering prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the at-the-market offering prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in the at-the-market offering prospectus or in any document incorporated by reference that was filed with the SEC before the date of this prospectus supplement, on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having a later date — for example, a document incorporated by reference in the at-the-market offering prospectus — the statement in the document having the later date modifies or supersedes the earlier statement.
References in this prospectus supplement to “HeartSciences,” “Heart Test Laboratories,” “we,” “us,” “our” and the “Company” refer to Heart Test Laboratories, Inc. together with its subsidiaries. References to our “common stock” refer to the common stock of Heart Test Laboratories, Inc.
All references in this prospectus to our financial statements include, unless the context indicates otherwise, the related notes.
No action is being taken in any jurisdiction outside the United States to permit a public offering of the securities or possession or distribution of this prospectus supplement and the at-the-market offering prospectus in that jurisdiction. Persons who come into possession of this prospectus supplement or the at-the-market offering prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus supplement and the at-the-market offering prospectus applicable to that jurisdiction.
S-ii
Table of Contents
PROSPECTUS SUMMARY
The following is a summary of what we believe to be the most important aspects of our business and the offering of our securities under this prospectus supplement. We urge you to read this entire prospectus supplement, including the more detailed financial statements, notes to the financial statements and other information incorporated by reference from our other filings with the SEC or included in any applicable prospectus supplement. Investing in our securities involves risks. Therefore, carefully consider the risk factors set forth in any prospectus supplements and in our most recent annual and quarterly filings with the SEC, as well as other information in this prospectus and any prospectus supplements and the documents incorporated by reference herein or therein, before purchasing our securities. Each of the risk factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities.
Company Overview
We are a medical technology company focused on applying innovative AI-based technology to an ECG, also known as an “EKG,” to expand and improve an ECG’s clinical usefulness. Our objective is to make an ECG a far more valuable cardiac screening tool. HeartSciences’ first product candidate for FDA clearance, the MyoVista, is a resting 12-lead ECG that will incorporate HeartSciences’ first AI-based algorithm designed to provide diagnostic information related to cardiac dysfunction as well as conventional ECG information in the same test. In the future, we intend to incorporate additional AI-based algorithms in the MyoVista and develop a cloud-based platform to provide access to a range of AI-based ECG cardiovascular algorithms on an ECG hardware agnostic basis. The AI-based ECG algorithms provide diagnostic information related to structural heart disease which traditionally has only been available through the use of cardiac imaging. We believe, the MyoVista is particularly well suited for a frontline or point of care clinical setting and the initial revenue model, which involves the use of the MyoVista hardware, associated software and consumables for each test, is expected to be “razor-razorblade” as the cable connection to the electrodes used with the MyoVista are proprietary to HeartSciences, and new electrodes are used for every test performed. As further algorithms are made commercially available via the MyoVista or cloud-based platform we would expect to adopt revenue models based on algorithm usage and/or recurring subscriptions.
On September 20, 2023, we entered into multiple definitive license agreements (each a “License Agreement” and collectively, the “License Agreements”) with Icahn School of Medicine at Mount Sinai (“Mount Sinai”) to commercialize a range of AI-based cardiovascular algorithms developed by Mount Sinai as well as a memorandum of understanding for ongoing cooperation encompassing de-identified data access, on-going research, and the evaluation of the MyoVista. The License Agreements, of which there are eleven in total, cover rights to thirteen AI-based cardiovascular algorithms, two data science methods for use with ECG waveforms and three filed patents.
Neither the MyoVista hardware, nor any of the AI-based ECG algorithms, are cleared for marketing by the FDA and our future success is dependent upon receiving FDA clearances. Additional funding may be required as part of achieving FDA clearance and thereafter would be required to support the sales launch of the MyoVista into the U.S., provide working capital and support further research and development (“R&D”).
We believe that there is currently no low-cost, front-line, medical device that is effective at screening broadly for many types of heart disease. As a result, we believe that frontline physicians face a significant challenge in determining if a patient has heart disease. Although many think of the ECG as the frontline test for heart disease, in 2012, the United States Preventive Services Task Force conducted an evaluation of conventional ECG testing and stated: “There is no good evidence the test, called an ECG, helps doctors predict heart risks any better than traditional considerations such as smoking, blood pressure and cholesterol levels in people with no symptoms.”
ECG devices record the electrical signals of a patient’s heart. The ECG is a ubiquitous, relatively low-cost, simple and quick test; it is portable and can be performed in a wide range of clinical settings by a non-specialist clinician or clinical aide. There are three basic categories of heart disease: electrical (such as an arrhythmia), structural (such as valvular disease) and ischemic (such as coronary artery disease, or CAD). Conventional resting ECGs have limited sensitivity in detecting structural and ischemic disease and are typically used for diagnosing cardiac rhythm abnormalities, such as atrial fibrillation, or acute coronary syndrome, such as a myocardial infarction which is also known as a heart attack. However, traditional ECGs have a limited role in identifying cardiac dysfunction associated with structural and ischemic disease.
S-1
Table of Contents
HeartSciences has designed or licensed algorithms to help address these limitations and extend the clinical capability of an ECG to detect cardiac dysfunction or specific cardiovascular disease types.
The first AI algorithm to be incorporated into the MyoVista has been designed by the Company and applies AI-machine learning to the signal processed ECG signal to develop a proprietary algorithm designed to detect cardiac dysfunction caused by heart disease and/or age-related cardiac dysfunction. We recently proposed adjustment of the echocardiographic measurement thresholds to the FDA to reflect recent clinical findings in respect of ≥60 year old patients which we believe will further increase the clinical value of the algorithm. The FDA has now confirmed this approach and we are in the process of updating our algorithm to reflect these updated echo measurement thresholds. The MyoVista has not yet received FDA clearance.
The editorial comment associated with the study titled “Prediction of Abnormal Myocardial Relaxation from Signal Processed Surface ECG” presented below discusses recent applications of machine learning to data derived from surface 12-lead ECGs in relation to cardiac dysfunction:
“These represent some of the most significant advances in electrocardiography since its inception, which has historically had a limited, if any, role in the evaluation of cardiac dysfunction. In the past, our cardiovascular community was resigned to the fact that surface ECGs are poor indicators for cardiac dysfunction.”
Khurram Nasir, MD, MPH, MSC, Department of Cardiology, Houston Methodist DeBakey Heart & Vascular Center, Houston, Texas, et. al., Journal of American College of Cardiology Editorial Comment Volume 76 Number 8 2020.
Almost all forms of heart disease, including CAD and structural disease, affect heart muscle, or cardiac, function prior to symptoms. Impaired cardiac function is first observed as impaired cardiac relaxation which is an early indicator of diastolic dysfunction and usually continues to increase in severity as heart disease progresses. The diastolic phase of the cardiac cycle occurs when the heart muscle relaxes (following contraction). Diastolic dysfunction may also be related to age-related cardiac dysfunction.
If we receive FDA clearance for our first product candidates, the MyoVista hardware and its associated cardiac dysfunction algorithm, our main target markets would be frontline healthcare environments in the U.S., such as primary care, to assist physician decision making in the cardiology referral process. Currently, cardiology referral decisions are often based on a patient’s risk factors and/or a conventional ECG test. Accordingly, many patients with heart disease are left undetected while no current treatment or intervention is required for most patients referred for cardiac imaging. We believe that adding the capability to detect cardiac dysfunction to a standard 12-lead resting ECG could help improve cardiac referral pathways and be valuable for patients, physicians, health systems and third-party payors.
New Class II devices, such as the MyoVista, require FDA premarket review. The MyoVista along with its proprietary software and hardware is classified as a Class II medical device by the FDA. Premarket review and clearance by the FDA for these devices is generally accomplished through the 510(k) premarket notification process or De Novo classification request, or petition process. We previously submitted an FDA De Novo classification request in December 2019 and, following feedback and communications with the FDA during and since that submission, we have been making modifications to our device, including our proprietary algorithm. We are part-way through a new, pivotal clinical validation study and have been undertaking device and algorithm development testing for a revised FDA submission. Our discussions to date with the FDA have been in relation to a revised submission under the De Novo pathway, however, in August 2023, the FDA granted an industry first De Novo clearance and created a new Class II product code for cardiovascular machine learning-based notification software in respect of a hypertrophic cardiomyopathy algorithm. In late September 2023, the FDA cleared an algorithm for low ejection fraction (less than 40%) under the 510(k) pathway using the new product code. Accordingly, we now believe it is probable that we could submit the MyoVista algorithm for clearance under the 510(k) pathway. The 510(k) pathway is more common than De Novo and, on average, has a much quicker decision process than De Novo by the FDA. Accordingly, in October 2023, we submitted a pre-submission request to the FDA to, among other things, seek to confirm the appropriateness of a 510(k) submission. As a result, we now expect a revised submission to be filed during the first half of calendar year 2024. Assuming a submission under 510(k) in the first half of calendar year 2024, we would anticipate a determination by the FDA in 2024 which, if successful, would provide the ability to market and sell the MyoVista in the U.S. If successful, additional funding would be required to support the sales launch of the MyoVista in the U.S., provide working capital and support further R&D.
S-2
Table of Contents
Heart Disease Facts and Current ECG Testing Limitations
Heart disease refers to a variety of conditions that affect the heart — including heart rhythm problems, heart valve problems, genetic defects and blood-vessel diseases such as CAD. It is often referred to as the “silent killer.” According to the American Heart Association, one in three patients are not properly diagnosed until after a heart attack occurs and 50% of men and 64% of women who died suddenly of coronary heart disease showed no previous symptoms. Statistics published by the U.S. Centers for Disease Control and Prevention (the “CDC”), show that in the United States heart disease is the leading cause of death for both men and women, across most racial and ethnic groups. According to the CDC, in the United States, one person dies from cardiovascular disease every 34 seconds. In 2020, about 20.1 million adults aged 20 and older in the United States have CAD (about 7.2%), with approximately one in five heart attacks being a silent heart attack therefore the person is not even aware of it, but the damage is done. Approximately 697,000 people in the U.S. died from heart disease in 2020, that’s one in every five deaths. The scale of the problem is similar worldwide. In 2020, the World Health Organization confirmed that heart disease has remained the leading cause of death at the global level for the last 20 years. Cardiovascular diseases are the leading cause of death globally. An estimated 17.9 million people died from cardiovascular diseases in 2019, representing 32% of all global deaths.
The 2019 National Ambulatory Medical Care Survey showed there were approximately 1 billion ambulatory care visits in the U.S. with a high incidence of patients with risk factors for heart disease (33% had hypertension, 15% had diabetes and 7% had a history of CAD, ischemic heart disease or myocardial infarction).
As heart disease progresses to more acute stages, the cost to treat patients increases significantly. Cardiovascular disease is the leading cost to the healthcare system and is estimated to be responsible for one in every six healthcare dollars spent in the United States. Heart disease cost the United States about $229 billion in each of 2017 and 2018, including the cost of health care services, medicines, and lost productivity due to death. Governments, healthcare providers and payors are motivated to shift the diagnosis and management of these conditions to earlier stages where better patient outcomes can be delivered at lower costs.
We believe that there is currently no low-cost, front-line, medical device that is effective at screening for heart disease. As a result, frontline physicians face a significant challenge in determining if a patient has heart disease. The conventional ECG is thought of by many to be the front-line tool in cardiac testing, but it has poor sensitivity in detecting CAD or structural heart disease.
Overuse of Expensive Cardiology-Based Diagnostic Testing
We believe that the absence of cost-effective front-line or primary-care-based testing has resulted in the over-use of costly cardiology-based diagnostic tests. Noninvasive cardiac tests are significant contributors to healthcare costs, accounting for greater than 40% of Medicare Part B spending on medical imaging, or over $17 billion annually according to the U.S. Centers for Medicare & Medicaid Services (“CMS”). There are a variety of effective, though expensive, diagnostic tests used for patients to detect heart disease. These diagnostic tests are typically performed in a specialist cardiology or hospital setting and may include:
• Stress ECG testing, a non-invasive diagnostic test with a cost of approximately $200 with, according to the American College of Cardiology, a sensitivity of 68% in the detection of CAD;
• Echocardiogram, or echo, a non-invasive diagnostic imaging test, similar to an ultrasound, which is effective in the detection of heart disease; however, the Medicare cost of an echo in a hospital is approximately $600 and can be as much as $3,000 if performed privately;
• Cardiac imaging tests, such as nuclear stress tests and coronary computerized tomography angiograms alternatively can be conducted noninvasively, but typically cost $1,000 or more; or
• Coronary angiogram, an invasive test in which dye that is visible by X-ray is injected into the blood vessels of the heart. A coronary angiogram can cost in excess of $5,000.
S-3
Table of Contents
Diastolic Dysfunction, an Early Indicator of Heart Disease
The symptoms and causes of cardiac dysfunction have been researched for many years. The causes of cardiac dysfunction during the contraction (systolic) phase, also called reduced left ventricular ejection fraction, have been well understood for many years. However, according to the American Heart Association Statistics Committee report in 2013, approximately 50% of patients with heart failure (“HF”) symptoms have ejection fraction measures that are not markedly abnormal. In addition, multiple articles published by the National Institutes of Health (“NIH”), state that approximately 50% of HF cases are due to severe diastolic dysfunction, also called heart failure with preserved ejection fraction. HF with preserved ejection fraction (“HFpEF”) is a clinical syndrome in which patients have symptoms and signs of HF with normal or near-normal left ventricular ejection fraction (“LVEF”) (LVEF ≥50%). Roughly half of all patients with HF worldwide have an LVEF ≥50% and nearly half have an LVEF <50%. Thanks to the increased scientific attention about the condition and improved characterization and diagnostic tools, the incidence of HF with reduced ejection fraction (“HFrEF”) dropped while that of HFpEF has increased by 45%. As a result, understanding the causes and progression of diastolic dysfunction has become a key area of scientific and clinical interest. This research has led to the understanding that almost all patients with systolic dysfunction also have diastolic dysfunction and almost all types of heart disease including CAD, valvular disease, cardiomyopathy, hypertension, congenital heart disease, and pericardial disease induce diastolic dysfunction.
According to an article by Dr. Dalane W. Kitzman, MD and Dr. William C. Little, MD published in the February 14, 2012 issue of the Journal of the American Heart Association, diastolic performance is sensitive to nearly all of the common disease processes that affect cardiovascular function. The article indicates that left ventricular, or LV, diastolic function is impaired by all of the common disease processes that affect LV function or produce LV hypertrophy or fibrosis, including hypertension, diabetes, ischemia, myocarditis, toxins and infiltrative cardiomyopathies. LV diastolic dysfunction (“LVDD”) begins early in the heart disease process and continues to increase in severity as heart disease progresses. LVDD is now recognized as one of the earliest signs of heart disease and typical onset occurs when a patient is still asymptomatic. We believe that the early detection of diastolic dysfunction can be a clinically valuable marker for almost all forms of heart disease and age-related cardiac abnormalities that may otherwise be missed by current conventional ECG devices.
Product and Technology
The HeartSciences-developed cardiac dysfunction algorithm has been developed in response to the relatively recent understanding in cardiology that most forms of heart disease are associated with LV relaxation abnormalities and diastolic dysfunction. The MyoVista is a 12-lead resting ECG device featuring our proprietary algorithm designed to detect cardiac dysfunction in the diastolic phase, which is specifically slower than normal left ventricular relaxation rates, and incorporates echocardiographic measurement threshold adjustments for ≥60 year old patients in accordance with recent clinical findings and the American Society of Echocardiology Guidelines.
The MyoVista also includes the capabilities of a full-featured conventional 12-lead resting ECG including analysis using the Glasgow Algorithm, also known as the Glasgow ECG Interpretation Algorithm. Developed by the University of Glasgow in the United Kingdom, the 12-lead ECG Analysis Algorithm has been relied upon for more than 35 years and is a widely used resting ECG interpretive algorithm. The Glasgow Algorithm has been improved over the years and is licensed to us pursuant to a licensing agreement with The University Court of the University of Glasgow. Under this licensing agreement, we obtained a non-exclusive, worldwide license with automatic renewal provisions and the right to license: (i) software modules for an Android-based platform for the analysis of resting 12-lead electrocardiograms and (ii) all intellectual property rights (including patents, copyright, trademarks, trade secrets and know-how) relating to the software modules to be used in the MyoVista (the “Glasgow Licensing Agreement”).
In the MyoVista, the conventional ECG (including the Glasgow Algorithm) and our proprietary algorithm, designed to detect impaired left ventricular cardiac relaxation abnormalities, are combined as a single test with results presented separately. The MyoVista has a high-resolution touchscreen display and incorporates many intuitive features commonly associated with a tablet device.
S-4
Table of Contents
MyoVista device with 1 lead view of signal processed waveform
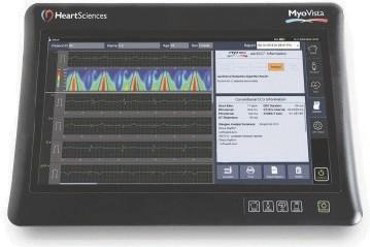
Market Opportunity
Diagnostic Gap
We believe that the significant diagnostic gap in heart disease is early identification. Heart disease often remains asymptomatic for many years until it reaches an acute stage, at which point many patients have a heart attack or die without prior diagnosis of disease. For this reason, heart disease is often referred to as the “silent killer.” In 2012, the United States Preventative Services Task Force stated that there is no good evidence that an ECG helps physicians predict heart risks in people with no symptoms any better than traditional considerations such as smoking, blood pressure and cholesterol levels, acknowledging the diagnostic gap that currently exists.
According to the CDC, cardiovascular disease remains the largest cost for the U.S. healthcare system at approximately $219 billion per year. The cost of treating acute cardiac events and heart failure is especially high in comparison to preventative treatment. Governments, healthcare providers and third-party payors are focused on shifting the diagnosis and management of heart disease to earlier stages where better patient outcomes can be delivered at lower cost; however, to make substantial progress the existing diagnostic gap needs to be closed.
We believe that the scale of cardiac disease as well as changing demographics, growing ECG market, impetus to identify risks earlier through low-cost testing which is better able to detect heart disease at an early stage, along with the increasing number and type of health care settings creates a significant opportunity for a device such as the MyoVista.
Changing Demographics
Heart disease is most commonly found in individuals aged 65 and older with incidences of heart disease increasing at 65 years for men and 71.8 years for women. According to the Organization for Economic Co-operation and Development, advances in the field of medicine have led to an increase in life expectancy which, as of 2020, was estimated to average 77.3. years for a person in the U.S., up from 75.4 years in 1990. As life expectancy increases, the average age of the population is expected to increase. According to the U.S. Health and Human Services — Office of the Inspector General (the “HHS”), the population age 65 and older increased from 38.8 million in 2008 to 52.4 million in 2018 (a 35% increase) and is projected to reach 94.7 million by 2060. By 2030, more than 20 percent of U.S. residents are projected to be age 65 and over. Since heart disease is most commonly found in individuals aged 65 years and older, and that population pool is increasing, we believe there is a significant opportunity for a device such as the MyoVista as well as our AI-based algorithms that are designed to assist frontline physicians in the early detection of heart disease.
S-5
Table of Contents
Growing ECG Market
The demand for electrocardiograph devices and related supplies known as electrodes is on the rise worldwide. Despite the limitations of the conventional ECG and healthcare guidance around the world that recommends against its use for screening, in the absence of a better alternative, the ECG remains a ubiquitous and widely used test throughout healthcare including non-cardiology settings. It is estimated that 1.5 million to 3.0 million ECGs are performed worldwide every day, making it one of the most commonly used cardiovascular diagnostic tests in healthcare and a fundamental tool in clinical practice. It is estimated that more than 100 million ECGs are performed each year in the United States. The 2019 National Ambulatory Medical Care Survey indicated that office-based patient care physicians, excluding anesthesiologists, radiologists and pathologists, ordered or provided 47 million ECG tests during office visits, and the 2020 National Hospital Ambulatory Medical Care Survey showed that during ambulatory care visits to hospital emergency departments, an additional 32 million ECG tests were ordered or performed by hospital emergency departments.
With the advent of advanced technology, ECG testing market research reports demonstrate that market growth in ECG devices and use is increasing. Precedence Research, a Canada/India based market research company recently released market research on the global electrocardiograph market for 2023, the market size is expected grow significantly from $10.93 billion in 2023 to $25.56 billion by 2032.
Impetus to Identify Risks Earlier for More Effective Low-Cost Testing
A key goal of the HHS is reducing healthcare costs. This places pressure on physicians and healthcare institutions to contain healthcare costs. Additionally, one of the key objectives of HHS’s Healthy People 2030, is to increase preventive care for people of all ages. We believe that efforts towards preventive care and maintenance will lead to more testing for high-risk individuals and patients who have existing cardiac conditions. This trend, we believe, in tandem with the push to shorten hospital stays, has created an impetus to identify pre-symptomatic patients at risk more effectively at the front-line physician or clinic level and to treat recovering cardiac patients through outpatient care and rehabilitation.
It is our belief that the MyoVista and our AI-based algorithms are positioned to respond to the global need for more effective, low-cost ECG testing that screens for heart disease.
Changing Nature of Healthcare Providers
The delivery of healthcare in the U.S. is evolving. Alternative treatment sites, such as retail clinics, concierge medicine, urgent care clinics and ambulatory surgical centers, deliver care from qualified providers in settings outside of emergency departments, hospitals or traditional physician offices. We expect this trend to accelerate the drive to provide more effective preventative care and represents a significant opportunity for the introduction of our AI-based algorithms that offer an enhanced ability to screen for heart disease.
Capitation Provides an Incentive to Identify Medicare Advantage Patients
Healthcare providers are paid either through fee-for-service or capitation. Fee-for-service is a payment model where services are unbundled and paid for separately. In health care, the fee-for-service payment model incentivizes physicians to provide more treatments because payment is dependent on the quantity, rather than quality, of care. Capitation is a payment arrangement that pays a physician or group of physicians a set amount for each enrolled person assigned to them, per period of time, whether or not that person seeks care. Under capitation, the amount of remuneration is based on the average expected healthcare utilization of that patient, with greater payment for patients with a significant history of medical problems.
Approximately 48% (approximately 28 million people) of those covered by Medicare according to CMS are enrolled in a Medicare Advantage plan. With respect to these patients, CMS pays capitation to healthcare providers. CMS uses risk adjustment to adjust capitation payments to health plans, either higher or lower, to account for the differences in the health costs of individuals with ailments such as heart failure, CAD, angina and valvular heart disease. Accordingly, under CMS guidelines, risk factor adjustments per patient will provide payment that is higher for sicker patients who have conditions where diagnosis codes are documented in the medical record as a result of a face-to-face visit. Therefore, there is a financial incentive to identify those Medicare Advantage patients who are
S-6
Table of Contents
sicker, including those who have undiagnosed ailments such as heart disease. We believe that undiagnosed heart disease represents a significant problem, and we believe insurance plans that have a high number of Medicare Advantage patients could be a target market for the MyoVista.
Market Strategy
General
Our objective is to make our AI-based ECG algorithms widely available to significantly improve front-line testing for heart disease. Our business model involves the capital sale of the MyoVista device, including its proprietary supplies (electrodes) for each test, as well as the revenue from the use of our AI-based algorithms. The algorithms are intended to be delivered either through the MyoVista hardware or through a cloud platform which will process electronic ECG files sent from non-MyoVista ECG devices to the cloud platform that contains the algorithms where the ECG records will be processed by HeartSciences algorithms and results returned to the physicians that ordered the tests. The electrode connection system of the MyoVista is patented which, together with our proprietary high-quality electrodes, facilitates high quality, stable ECG signal capture. New electrodes are needed for each test. Our proprietary electrodes, when purchased, would provide recurring per-test revenue for each MyoVista sold. In short, we do not expect to primarily rely on high initial device pricing and instead will seek to encourage the adoption of the MyoVista when possible and intend to focus on recurring revenue from electrode sales as well as use of HeartSciences’ AI-based algorithms.
Territories
Our initial sales focus will primarily be within the U.S. We intend to market our products in the U.S. using a direct sales force following FDA clearance. Outside of the U.S., for markets such as Europe and Latin America, we intend to utilize medical device distributors that have existing healthcare provider relationships and experience selling ECG devices, which will be supported by a small number of local field personnel.
Potential Markets
We believe that there is a large variety of potential markets for AI-based ECG algorithms with new diagnostic capabilities that are not currently available for ECG devices. Conventional ECGs are used throughout healthcare in almost every clinical setting including clinics, doctor’s offices, urgent care centers, and hospitals. We believe that, in many of those settings, the additional information provided by the AI-based ECG algorithms such as cardiac dysfunction which the MyoVista is designed to provide, in addition to the conventional ECG information provided, could be extremely valuable.
Our AI-based algorithms range of applications and potential uses are vast, and include providing:
• Primary care — front-line cardiac testing/referral tool, heart disease screening.
• Retail Healthcare — access to ECG testing at retail sites such as CVS, Walmart and Walgreens.
• Emergency Departments — enhanced ECG testing for emergency room patients.
• Cardiologists — prescreening cardiology patients.
• Hospitals — in-patient testing or testing prior to discharge, particularly cardiac wards.
• Surgery — pre-anesthesia testing, pre/post intervention.
• Life Insurance testing — ECGs when required in connection with the issuance of life insurance policies.
• Specialty Environments — screening for conditions such as, cardiomyopathy, cardiac oncology, drug trials, heart failure, and diabetes.
• Athlete testing — cardiac screening programs for athletes.
S-7
Table of Contents
Early Target Markets
Initially, our focus markets will include: cardiology; primary care providers that serve upper to middle income regions including concierge medicine providers; retail clinics; and insurers with high levels of Medicare Advantage patients. As additional algorithms obtain FDA clearance, HeartSciences will extend its sales efforts to clinics and physicians that will benefit the most from the specific AI-based algorithm.
Reimbursement
In addition to targeting the health care settings described above, a key element of our strategy is to ensure each algorithm qualifies for reimbursement from third-party payors such as CMS (Medicare payor). CPT codes are numbers assigned to each task or service provided by a healthcare provider including medical, surgical and diagnostic services. Insurers use the numbers to determine the procedure and the amount to pay a provider. The American Medical Association has already issued a temporary Current Procedural Terminology (CPT) Category III code for novel AI assistive algorithmic ECG risk assessment for cardiac dysfunction. These codes are designed to facilitate the use, adoption, and potential reimbursement of emerging technologies. This provides physicians and clinical institutions the ability to bill for HeartSciences algorithms that detect different types of heart dysfunction such as systolic and diastolic dysfunction. While we cannot be certain that these new codes will ultimately lead to the issuance of permanent CPT Category I codes, or that insurance coverage or payment can be obtained, if successful, this could potentially provide total reimbursement that is larger than reimbursement for conventional ECG devices, which, in turn, could provide MyoVista with a competitive advantage as compared to conventional ECG devices. The MyoVista device also includes conventional ECG testing capabilities and is expected to also qualify for Medicare reimbursement for existing ECG testing procedures with interpretation and report ranges from approximately $17 to $55 depending on the type of healthcare facility. These charges would go directly to the healthcare facility/physician.
Competition
The medical device industry is characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. There are many medical device companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to HeartSciences AI-based algorithms and MyoVista hardware. Competitors could include traditional ECG manufacturers such as GE Healthcare Technologies, Inc., (“GE Healthcare”), Koninklijke Philips N.V. (“Phillips”), Baxter International, Inc. (“Baxter”), and Nihon Kohden Corporation that may seek to innovate, and new commercial entrants to the AI ECG market, such as Anumana, Inc. or companies involved in AI healthcare, such as Tempus Labs, Inc. or VIZ.ai that also see the opportunity to bring innovation in a market that, we believe, has significant need for improved products and technology change.
Intellectual Property
Our technology is protected by a patent portfolio as well as trade secrets, which together comprise an important part of the intellectual property protection for our existing and licensed proprietary algorithms (especially when developing proprietary algorithms). We believe that the combination of patents and trade secrets creates valuable competitive barriers in favor of HeartSciences.
The USPTO has issued eight utility patents and one design patent to us. The patent expiration dates range from March 2031 to August 2040. We also have fourteen international design registrations and eighteen international utility patents granted (with expiration dates ranging from September 2036 to March 2037) in jurisdictions such as China, Japan, South Korea, the United Kingdom, France, Germany, Mexico, the United Arab Emirates, Brazil, and Australia. We currently have two patent allowances in Europe and Canada, and also have additional pending patent applications in various jurisdictions.
In addition, we have entered into two agreements that are material to our rights to the intellectual property utilized in the MyoVista:
• In January 2014, we entered into an invention assignment agreement under which certain specified MyoVista technology and proprietary and intellectual property rights thereto (including patents, copyright, trademarks, trade secrets and know-how) were transferred and assigned to us by the inventor; and
S-8
Table of Contents
• In December 2015, we entered the Glasgow Licensing Agreement with The University Court of the University of Glasgow under which we obtained a non-exclusive, worldwide license to software modules for an Android platform for analysis of resting 12-lead electrocardiograms and all intellectual property rights (including patents, copyright, trademarks, trade secrets and know-how) relating to the software modules to be used in the MyoVista.
Research and Development
The Company’s R&D staff designs our hardware, software and internally developed AI-based algorithms. Hardware development assistance is provided by outside consulting firms. The Company internally develops the signal processing software elements along with outside assistance. The user interface software of the MyoVista is designed by the Company along with the assistance of outside consultants. The data science work necessary to build the AI-based algorithms is performed both internally and externally using outside consultants.
Incorporation of all software elements into the MyoVista hardware is performed internally. We currently employ four full-time R&D staff.
We believe, based on our research and other published research, that further algorithms could be developed for a range of additional clinical indications. To accelerate HeartSciences’ route to market with additional algorithms we entered into multiple license agreements with Mount Sinai on September 20, 2023. Please see the section, “Agreements with Mount Sinai related to Commercialization of Multiple AI-based Cardiovascular ECG Algorithms developed by Mount Sinai” for additional information regarding these license agreements. Studies involving the use of the MyoVista and proof of concept algorithms for alternative clinical indications have already been published and there is a growing body of third-party published research in this field.
On November 29, 2022, we entered into a multi-year collaboration agreement with Rutgers, The State University of New Jersey, to develop additional AI-based ECG algorithms with our intention being to augment our product development pipeline for additional new ECG algorithms in the medium term. We believe that in the future the ECG will have significantly greater clinical value and will facilitate far more effective heart disease screening and referral as these AI-based ECG algorithms obtain regulatory approval.
Implications of Being an “Emerging Growth Company” and a “Smaller Reporting Company”
We qualify as an “emerging growth company” under the Jumpstart our Business Startups Act of 2012, or the JOBS Act. For so long as we remain an emerging growth company, we may take advantage of relief from certain reporting requirements and other burdens generally applicable to public companies. In particular, as an emerging growth company we:
• are not required to obtain an attestation and report from our auditors on our management’s assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act;
• are not required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives (commonly referred to as “compensation discussion and analysis”);
• are not required to obtain a non-binding advisory vote from our shareholders on executive compensation or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on-frequency” and “say-on-golden-parachute” votes);
• are exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure;
• may present only two years of audited financial statements and only two years of related Management’s Discussion & Analysis of Financial Condition and Results of Operations (“MD&A”); and
• are eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act.
S-9
Table of Contents
We intend to take advantage of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under §107 of the JOBS Act. Please see “Risk Factors — We are an ‘emerging growth company,’ and any decision on our part to comply with certain reduced disclosure requirements applicable to emerging growth companies could make the common stock less attractive to investors.”
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions for up to five years after our initial sale of common equity pursuant to a registration statement declared effective under the Securities Act of 1933, as amended (the “Securities Act”), or such earlier time that we no longer meet the definition of an emerging growth company. In this regard, the JOBS Act provides that we would cease to be an “emerging growth company” if we have more than $1.235 billion in annual revenue, have more than $700 million in market value of our common stock held by non-affiliates (and are not otherwise eligible to be a smaller reporting company), or issue more than $1 billion in principal amount of non-convertible debt over a three-year period. Further, under current SEC rules we will continue to qualify as a “smaller reporting company” for so long as we have a public float (i.e., the market value of common equity held by non-affiliates) of less than $250 million as of the last business day of our most recently completed second fiscal quarter.
Certain of the reduced reporting requirements and exemptions available to us as an “emerging growth company” are also available to us due to the fact that we also qualify as a “smaller reporting company” under the SEC rules. For instance, smaller reporting companies are not required to obtain an auditor attestation and report regarding internal control over financial reporting; are not required to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure; and may present only two years of audited financial statements and related MD&A disclosure.
If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. We will continue to be a smaller reporting company so long as (i) the market value of our stock held by non-affiliates is less than $250 million as of the last business day of our second fiscal quarter or (ii) our annual revenue was less than $100 million during our most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700 million as of the last business day of our second fiscal quarter. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Reports on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Recent Developments
Going Concern
On July 18, 2023, our independent registered public accounting firm issued an opinion on our audited financial statements, included in our Annual Report on Form 10-K for the year ended April 30, 2023, that contained an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern because we have experienced recurring losses, negative cash flows from operations, and limited capital resources. These events and conditions raise substantial doubt about our ability to continue as a going concern.
Compliance with Nasdaq Listing Requirements
On December 21, 2022, we received notice from the Listing Qualifications Staff (“Staff”) of The Nasdaq Stock Market LLC (“Nasdaq”), indicating that we were not in compliance with the minimum stockholders’ equity requirement for continued listing on the Nasdaq Capital Market, under Listing Rule 5550(b)(1) (the “Minimum Stockholders’ Equity Requirement”), because our stockholders’ equity of $1,082,676 as reported in our Quarterly Report on Form 10-Q for the period ended October 31, 2022 was below the required minimum of $2.5 million, and because, as of October 31, 2022, we did not meet the alternative compliance standards, relating to the market value of listed securities of $35 million or net income from continuing operations of $500,000 in the most recently completed fiscal year or in two of the last three most recently completed fiscal years.
S-10
Table of Contents
On February 3, 2023, we submitted a plan to Nasdaq to regain compliance with the Minimum Stockholders’ Equity Requirement. On February 8, 2023, Nasdaq notified us that they have granted us an extension of up to 180 calendar days from December 21, 2022, i.e., through June 19, 2023, to regain compliance. On June 20, 2023, we received a delist determination letter from Nasdaq advising us that Nasdaq determined that we did not meet the terms of the extension by the June 19, 2023 deadline.
On June 27, 2023, we submitted a hearing request to the Nasdaq Hearing Panel (the “Panel”) to appeal the delisting determination. In response to our request for a hearing, on June 27, 2023, we received a letter from Nasdaq stating that its delisting action has been stayed, pending a final decision by the Panel and a hearing will be held on August 17, 2023.
On August 2, 2023, we received a letter from the Staff indicating that, based upon the closing bid price of our common stock for the last 30 consecutive business days, we no longer met the requirement to maintain a minimum bid price of $1 per share (the “Minimum Bid Price Requirement”). In accordance with Nasdaq listing rules, we have until January 29, 2024 to regain compliance with the Minimum Bid Price Requirement. In the event we do not regain compliance during this period, we may be eligible to seek an additional 180 calendar day compliance period if we meet the Nasdaq continued listing requirement for market value of publicly held shares and all other initial listing standards, with the exception of the Minimum Bid Price Requirement, and provide written notice to Nasdaq of our intent to cure the deficiency during this second compliance period.
We attended an August 17, 2023 hearing before the Panel, and requested the continued listing of our securities on the Nasdaq Capital Market pending our return to compliance with the Minimum Stockholder’s Equity Requirement and Minimum Bid Price Requirement.
On August 28, 2023, we received a decision from the Panel granting our request for continued listing on the Nasdaq Capital Market, subject to us demonstrating compliance with the Minimum Stockholders’ Equity Requirement on or before November 21, 2023, and certain other conditions. In addition, we have until January 29, 2024, to demonstrate compliance with the Minimum Bid Price Requirement.
Patents
In September 2023, we were issued a notice of patent allowance from the Brazilian Patent and Trademark Office and the United Arab Emirates Ministry of Economy covering MyoVista wavelet technology utilizing AI for early detection of heart disease.
Bridge Warrant Amendment No. 2
On February 3, 2023, we entered into a second amendment to the Bridge Warrants (as defined in the Glossary of Terms), which we refer to as the Bridge Warrant Amendment No. 2. The Bridge Warrant Amendment No. 2 amended the Bridge Warrants (as previously amended) by (i) lowering the exercise price of $4.25 for a period of ten (10) business days beginning February 3, 2023 and ending February 16, 2023 (the “Limited Period”), during which period the exercise price was set at $1.00, subject to adjustments set forth in the Bridge Warrant; (ii) providing that during the Limited Period, the holder was able, in its sole discretion, to elect a cashless exercise of the Bridge Warrant in whole or in part, pursuant to which the holder received a net number of shares of common stock equal to one-third of the total number of shares into which the Bridge Warrant could otherwise have been exercised; and (iii) removing the exercise price adjustment provisions of the Bridge Warrants with limited exceptions for transactions such as stock dividends, stock splits, stock combinations and reverse stock splits. Additionally, the Bridge Warrant Amendment No. 2 provided that in the event that the aggregate number of shares of common stock to be received by a holder upon an exercise of its Bridge Warrant during the Limited Period would result in such holder’s receiving shares of common stock in excess of its applicable Bridge Maximum Percentage (as defined in the Glossary of Terms), in lieu of delivery of shares of common stock in excess of the Bridge Maximum Percentage, the holder would receive such excess shares as pre-funded warrants substantially in the form of the Pre-Funded Bridge Warrants (as defined in the Glossary of Terms), with certain exercise price adjustment provisions removed.
Further, the Bridge Warrant Amendment No. 2 included a waiver of Section 4(w) of the Bridge SPA (as defined in the Glossary of Terms), which placed certain restrictions on the Company’s ability to issue securities for a specified period of time.
S-11
Table of Contents
During the Limited Period, we issued 1,172,304 shares of common stock and a pre-funded warrant to purchase 150,000 shares of common stock (the “Remaining Pre-Funded Bridge Warrant”) pursuant to exercises of the Bridge Warrants and received approximately $1.3 million in proceeds from these exercises. At the end of the Limited Period, Bridge Warrants to purchase 298,667 shares of common stock (the “Remaining Bridge Warrants”) remained outstanding, with an exercise price of $4.25 per share, subject to adjustments as set forth in the Bridge Warrants.
Lincoln Park Purchase Agreement
On March 10, 2023, we entered into a Purchase Agreement (the “Lincoln Park Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln Park”) pursuant to which we have the right, but not the obligation, to sell to Lincoln Park up to $15,000,000 of Purchase Shares from time to time over a 36-month term beginning only after certain conditions set forth in the Lincoln Park Purchase Agreement have been satisfied, including that the registration statement registering the Purchase Shares for resale (the “Lincoln Park Registration Statement”) shall have been declared effective under the Securities Act, which we refer to as the Commencement Date. In accordance with the Lincoln Park Purchase Agreement, on March 13, 2023, we issued 100,000 shares of our common stock (the “Initial Commitment Shares”) to Lincoln Park as consideration for its commitment to purchase the Purchase Shares under the Lincoln Park Purchase Agreement. At the time Lincoln Park’s purchases cumulatively reach an aggregate amount of $2,000,000 of Purchase Shares, in accordance with the Lincoln Park Purchase Agreement, we will issue an additional 62,500 shares of our common stock (the “Additional Commitment Shares”, and, together with the Initial Commitment Shares, the “Commitment Shares”) to Lincoln Park as consideration for such purchases.
Under applicable rules of Nasdaq, in no event may we issue or sell to Lincoln Park under the Lincoln Park Purchase Agreement shares of our common stock, including the Commitment Shares, in excess of 1,927,022 shares, which is equal to 19.99% of the shares of our common stock outstanding immediately prior to the execution of the Lincoln Park Purchase Agreement (the “Exchange Cap”) unless (i) we obtain shareholder approval to issue shares of our common stock in excess of the Exchange Cap or (ii) the average price of all shares of common stock issued to Lincoln Park under the Lincoln Park Purchase Agreement equals or exceeds $1.16 per share (which represents the official closing price of our common stock on The Nasdaq Capital Market the day of signing of the Lincoln Park Purchase Agreement), such that the transactions contemplated by the Lincoln Park Purchase Agreement are exempt from the Exchange Cap limitation under applicable Nasdaq rules. In any event, the Lincoln Park Purchase Agreement specifically provides that we may not issue or sell any shares of our common stock under the Lincoln Park Purchase Agreement if such issuance or sale would breach any applicable rules or regulations of the Nasdaq. The Lincoln Park Purchase Agreement also prohibits us from directing Lincoln Park to purchase any shares of our common stock if those shares, when aggregated with all other shares of our common stock then beneficially owned by Lincoln Park (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Rule 13d-3 thereunder), would result in Lincoln Park and its affiliates beneficially owning more than 9.99% of the then total outstanding shares of common stock, which we refer to herein as the Beneficial Ownership Limitation.
Lincoln Park Registration Rights Agreement
Concurrently with entering into the Lincoln Park Purchase Agreement, we entered into a registration rights agreement with Lincoln Park (the “Registration Rights Agreement”) pursuant to which we agreed to register the resale of the Purchase Shares and Commitment Shares that have been and may be issued to Lincoln Park under the Lincoln Park Purchase Agreement pursuant to the Lincoln Park Registration Statement. On March 29, 2023, we filed with the SEC the Lincoln Park Registration Statement registering the resale of the Purchase Shares and Commitment Shares that have been and may be issued to Lincoln Park under the Lincoln Park Purchase Agreement, and the SEC declared the Lincoln Park Registration Statement effective on April 10, 2023.
As of November 17, 2023, we have issued 1,864,522 shares to Lincoln Park, including the Initial Commitment Shares, receiving gross proceeds of approximately $1.1 million.
Senior Unsecured Promissory Drawdown Loan Note
On September 6, 2023, we entered into a Senior Unsecured Promissory Drawdown Loan Note (the “MSW Note”) with Matthews Southwest Holdings, Inc. (the “Lender”). The MSW Note provided for an unsecured drawdown loan of up to $1,000,000, drawn in installments consisting of (i) $250,000 on or prior to September 8, 2023, (ii) $250,000 on or prior to September 20, 2023, and (iii) further drawdowns of up to $500,000 in such amounts and such times to be mutually agreed upon between us and the Lender.
S-12
Table of Contents
In consideration of the MSW Note, we agreed to pay a facility fee to the Lender as follows:
• warrants to acquire 500,000 shares of common stock, exercisable at $1.00 per share, which shall be issued to the Lender upon the completion of the first drawdown;
• warrants to acquire 500,000 shares of common stock, exercisable at $1.25 per share, which shall be issued to the Lender upon the completion of the first drawdown and 250,000 of such Warrants shall be issued to the Lender pro-rata based on further drawdowns up to $500,000; and
• warrants to acquire up to 500,000 shares of common stock, exercisable at $1.50 per share, of which 250,000 of such Warrants shall be issued to the Lender upon the completion of the first drawdown and 250,000 of such Warrants to be issued to the Lender pro-rata based on further drawdowns up to $500,000.
As of November 14, 2023, we had drawn $500,000 under the MSW Note and issued 1,000,000 Warrants (the “Existing MSW Warrants”) to purchase shares of common stock in lieu of a facility fee.
On November 16, 2023, we entered into a note conversion letter agreement with the Lender (the “MSW Note Conversion Letter Agreement”). Pursuant to the MSW Note Conversion Letter Agreement, in consideration for the conversion of the aggregate principal and interest amount due under the MSW Note, on November 16, 2023, we (i) issued to the Lender 3,125,000 shares of common stock at a conversion price of $0.16 per share; and (ii) entered into a Warrant Amendment Agreement with the Lender, amending the Existing MSW Warrants to reduce the exercise price of an aggregate of 1,000,000 Existing MSW Warrants to $0.16 per share (the “MSW Warrant Amendment”). Except as expressly set forth in the MSW Warrant Amendment, the terms and provisions of the warrants held by the Lender shall remain in full force and effect.
Agreements with Mount Sinai related to Commercialization of Multiple AI-based Cardiovascular ECG Algorithms developed by Mount Sinai
On September 20, 2023, we entered into the License Agreements, each of which are subject to certain financing requirement conditions (as further described below), for the purpose of acquiring certain rights related to intellectual property developed by Mount Sinai. We will use these rights to focus on commercialization efforts of the licensed cardiovascular AI-based ECG algorithms developed by Mount Sinai. Commercialization will include efforts related to pursuing FDA clearance and European regulatory approval as well as ongoing sales efforts once an algorithm achieves regulatory clearance. The License Agreements include a total of eleven (11) license agreements for Mount Sinai-developed intellectual property, a memorandum of understanding between HeartSciences and Mount Sinai for on-going cooperation, collaboration, and data access as well as a Securities Purchase Agreement (as defined below) required to secure the rights related to the License Agreements.
Intellectual Property Description
Mount Sinai has a clinical database with millions of patients’ ECG records for the use of building AI-based electrocardiography (ECG) algorithms. The database includes clinical, as well as diagnostic imaging information related to many types of cardiovascular diseases. This has enabled its researchers to develop a range of AI-based ECG algorithms using state-of-the art data science development methods including the use of cutting-edge vision transformers (ViT).
We have entered into the License Agreements which covers certain usage rights to Mount Sinai’s AI-based ECG algorithms, technology and patents in the field of screening for, or diagnosis of, certain cardiovascular diseases using electrocardiogram ECG data. The following License Agreements provide for worldwide rights to the ECG algorithms and all but two of the ECG algorithms provide for exclusive rights to the algorithms:
• Deep learning ECG algorithms to derive Left Ventricular Ejection measures and detect Right Ventricular dysfunction;
• Deep Learning ECG algorithm to detect Pulmonary Embolism;
• Deep Learning ECG algorithms to predict right ventricle size and right ventricle systolic function;
S-13
Table of Contents
• Deep Learning ECG algorithm to Predict Premature Ventricular Contraction (PVC) related cardiomyopathy;
• Deep learning ECG algorithm to identify left heart valvular dysfunction — mitral regurgitation- (nonexclusive);
• Deep learning ECG algorithm to identify left heart valvular dysfunction — aortic stenosis (nonexclusive);
• Electrocardiogram deep learning interpretability toolbox;
• HeartBEiT Vision Transformer development platform;
• Vision Transformer based ECG algorithm to derive Left Ventricular Ejection Fraction;
• Vision Transformer based ECG algorithm to detection elevated ST segment; and
• Vision Transformer based ECG algorithm to detect Hypertrophic Cardiomyopathy.
Financing Requirement Conditions
The closing of the transactions contemplated under the Securities Purchase Agreement (the “MTS Transaction”), dated as of September 20, 2023 (the “Securities Purchase Agreement”), by and between us and Mount Sinai, and the effectiveness of the licenses under the License Agreements, are subject to the satisfaction or waiver of certain conditions, including a condition that we must complete one or more financings in which we receive aggregate gross proceeds of at least $5,000,000 (the “Financing Requirement”) prior to December 31, 2023 (the “Closing Date”).
On November 15, 2023, we satisfied the Financing Requirement and, accordingly, the licenses under the License Agreements were deemed effective.
Pursuant to the Securities Purchase Agreement, and on November 16, 2023, we issued to Mount Sinai the following:
• 4,854,853 shares of common stock;
• pre-funded warrants to purchase up to 710,605 shares of common stock, with an exercise price per share of $0.00001, which warrants were issued in lieu of shares of common stock issuable to Mount Sinai to ensure that the number of shares of common stock held by Mount Sinai does not exceed the Beneficial Ownership Limitation (the “MTS Pre-Funded Warrants”); and
• Common stock warrants to purchase up to 914,148 shares of common stock, having an exercise price per share equal to $0.5060, which warrants shall be exercisable immediately upon (x) completion of any financing of at least $10,000,000 raised by the Company (the “Additional Financing”) from the period commencing August 1, 2023 and ending on or prior to June 30, 2024 or (y) waiver by Mount Sinai of the Company’s Additional Financing Requirement (as defined below) (the “MTS Warrants” and collectively with the Consideration Shares and the MTS Pre-Funded Warrants, the “MTS Securities”).
The License Agreements may be terminated by Mount Sinai if we have not received aggregate gross total proceeds of at least $10,000,000 in qualified financing transactions by June 30, 2024 (the “Additional Financing Requirement”).
Registration rights related to the MTS Securities provide that on or prior to the date of one hundred and fifty days (150) days after the closing date, we shall prepare and file with the SEC a Registration Statement on Form S-1 (or such other form as applicable) covering the resale under the Securities Act of the MTS Securities issued to Mount Sinai, subject to any limitations imposed by the Nasdaq Rules.
Mount Sinai Licensed Algorithms Development Methods
The application of AI to the ECG, is playing an increasingly important role in patient screening, diagnosis, and management.
S-14
Table of Contents
As larger rich clinical data sets become available, more sophisticated AI-based algorithms are being developed and delivering improved diagnostic performance. Improved data sciences methods such as convolutional neural networking (“CNNs”) and recently emerged vision transformer (“ViT”) methods allow for developing algorithms to diagnose heart disease conditions that were not possible in the past. Some examples include detection of left ventricle systolic dysfunction, right ventricle dysfunction, hypertrophic cardiomyopathy and valvular heart disease through low-cost ECG testing.
Mount Sinai is one of few nationally recognized cardiology institutions available that has created clinical records data sets greater than five million. They have used these extremely large ECG clinical data sets to develop innovative AI-based ECG algorithms using state-of-the art data science methods. The Mount Sinai algorithms were developed using methods such as CNNs as well as state-of-the-art ViT methods. HeartSciences has secured license agreements for 13 of Mount Sinai-developed AI-based ECG algorithms. Below are examples of algorithms HeartSciences believes to have important clinical value and significant commercial potential:
• Left Ventricle Ejection Fraction <40%
• Left Ventricle Ejection Fraction >50%
• Right Ventricle Dysfunction
• Pulmonary Embolism
• LV Mitral Valve Regurgitation
• LV Aortic Valve Stenosis
• Hypertrophic Cardiomyopathy
Upon the completion of the offering, HeartSciences intends to conduct an expedited in-depth analysis to determine which of the ECG algorithms provide the best combination of clinical performance along with strong commercialization opportunity. HeartSciences also expects to increase internal data science staff to ensure the ability to respond to any data science needs related to fine tuning some of the existing algorithms if needed for optimal sensitivity/specificity performance.
Regulatory Approval
HeartSciences intends to work quickly to obtain regulatory approval on a number of key algorithms. This will require increased regulatory staff to support expedited regulatory submissions to the FDA for clearance and European regulatory approval. HeartSciences will use recruiting services that are specialized in finding and recruiting qualified candidates. Upon satisfying the Financing Requirement contemplated by the License Agreements, Mount Sinai will become a significant shareholder in the Company. Assuming at least $5,000,000 is raised in this offering, our anticipated commercialization efforts will initially focus on achieving FDA clearance and European CE Mark regulatory approval.
Algorithm Delivery Platforms
HeartSciences intends to make the algorithms available worldwide on an ECG hardware agnostic basis through the use of Health Insurance Portability and Accountability Act (HIPAA) and European General Data Protection Regulation (GDPR) compliant cybersecure cloud-based environment. Clinical institutions will be able to upload ECGs from anywhere and have the algorithm diagnostic results returned to them electronically. HeartSciences has identified software contractors that have developed ECG cloud environments in the past and are available to assist with design and development of a cloud environment that will meet these requirements. HeartSciences also intends to make multiple of these algorithms available on its MyoVista hardware platform. As the licensed algorithms have been developed with advanced data science methods using extremely large datasets, many of the advanced algorithms will require significant computing power. HeartSciences will need to test each algorithm to determine which one may be made available on the MyoVista hardware platform and may also need to upgrade the hardware platform of the MyoVista to increase hardware performance.
S-15
Table of Contents
U.S. Commercialization Strategy
Sales staffing requirements due to the additional Mount Sinai algorithms will not initially change as these algorithms require regulatory approvals and approvals will most likely be staggered over time. Sales efficiencies should actually improve since sales access to existing customers that have already adopted the use of one algorithm is easier and the technical ECG upload processes of using the cloud-based algorithms is the same for all cloud-based algorithms. The algorithms will also be used in many of the same front-line clinical environments. The algorithms will be used in front-line clinical pathways as ECGs are used today but provide improved ECG testing for a more efficient patient referral process by detecting patients earlier that need to have image-based cardiology testing. The improved referral process can reduce healthcare costs while improving patient care by improving early detection of heart disease while reducing patient referrals for testing not needed on patients that could not effectively be tested through older conventional ECG testing.
HeartSciences believes there is a significant opportunity to partner with both pharmaceutical and medical device companies that would benefit from improved ECG testing for heart diseases related to their drug treatments or medical devices, for example partnering with a heart valve replacement provider to accelerate sales efforts by increasing the use of a new ECG algorithm to detect valvular disease. This would lower the cost of initial sales efforts by partially funding HeartSciences sales efforts.
Revenue Model related to Reimbursement and Algorithm Use
Insurers and payors such as CMS (Medicare) use payment codes to determine the procedure and the amount to pay providers (physicians and clinical institutions). The American Medical Association (AMA) has already issued a temporary Current Procedural Terminology (CPT) Category III code for AI assistive algorithmic ECG risk assessment for cardiac dysfunction. These codes are designed to facilitate the use, adoption, and potential reimbursement of emerging technologies. This provides physicians and clinical institutions with the ability to bill for multiple licensed algorithms which would allow clinical institutions to bill payors for use of many of the algorithms as they obtain regulatory approval. While we cannot be certain that these new codes will ultimately lead to the issuance of permanent CPT Category I codes, or that insurance coverage or payment can be obtained, if either are successful this will assist with adoption of the AI-based algorithms. As further algorithms are made commercially available via the MyoVista or cloud-based platform we would expect to adopt revenue models based on algorithm usage and/or recurring subscriptions.
Significant Early European Opportunity
HeartSciences sees significant additional commercial opportunities in Europe based on HeartSciences ongoing engagement with European based key opinion leaders (KOLs). Due to the pandemic and a chronic lack of funding, many European national health systems are suffering from cardiology diagnostic backlogs that are up to one year for most types of diagnostic imaging. Today there is not an effective low-cost method to prioritize these patient backlogs. This has led to an increase in adverse cardiovascular events for patients suffering these increased waiting periods.
Below are excerpts from recent articles related to chronic backlog issues in multiple European national health systems.
Telegraph.co.uk August 2023 Titled “It’s not just the NHS: health services are imploding all over Europe” UK NHS (UK National Health Service) waiting list in the UK stood at 2.3 million in 2009 but by early 2020 had increased to 4.3 million. Today, the figure is about 7 million — equivalent to more than a tenth of the population. Overall, survey company Eurofound reported that more than one in five people in EU countries had foregone medical care, including examinations and treatments, during the first year of the pandemic — with a similar number reporting they still had unmet needs in spring 2022.
“We know that we’ve almost certainly missed a lot of serious illness during the pandemic,” says Anita Charlesworth, director of research at the Health Foundation and a former top civil servant. “And being able to have a timely diagnosis is really important. Early diagnosis tends to be associated with better outcomes and it tends to mean that you need less complex healthcare intervention, which is more costly in the end. “So early detection and diagnosis is in the patient’s interest and it’s also in the taxpayer’s interests.”
S-16
Table of Contents
WHO March 2023 titled “The health workforce crisis in Europe is no longer a looming threat — it is here and now.”
European Region, …national health systems are struggling to keep up with the rising demand for health care, exacerbated by service backlogs caused by the COVID-19 pandemic, rising expectations from patients and the health risks posed by climate change and emergencies.
New York Times July 16, 2023 “National Treasure, Tarnished: Can Britain Fix Its Health Service?”
Cardiovascular-related fatalities, which can be linked to delays in treatment, were up particularly sharply, according to Stuart McDonald, an expert on mortality data at LCP, a London-based pension and investment advisory firm.
These chronic issues have many national health systems in Europe seeking more effective low-cost methods to assist with prioritizing and reducing the backlog through improved low-cost testing. Due to typically shorter regulatory review periods for European regulatory submissions as compared to FDA review periods HeartSciences feels there will be significant commercial opportunities early-on in Europe.
Debt Conversion
As previously disclosed in our Current Report on Form 8-K filed with the SEC on January 24, 2023, we entered into Amendment No. 4 to the Loan and Security Agreement dated April 24, 2020 (the “Loan and Security Agreement”) with Front Range Ventures LLC (“FRV”) and John Q. Adams (“Adams”). Pursuant to the Loan and Security Agreement, a secured promissory note in the original principal amount of $500,000 was issued to FRV (the “FRV Note”) and a secured promissory note in the original principal amount of $500,000 was issued to Adams (the “Adams Note”). The Loan and Security Agreement was further amended on September 29, 2023 to amend the dates on which principal and accrued interest is due under the Adams Note. As consideration for such extension, we issued FRV and Adams warrants (the “$1M Lender Warrants”) to purchase an aggregate of 200,000 shares of common stock at an exercise price of $0.44 per share.
On November 16, 2023, we entered into a note conversion letter agreement with Adams (the “Adams Note Conversion Letter Agreements”). Pursuant to the Adams Note Conversion Letter Agreement, in consideration for the conversion of the principal and interest amounts due under the Adams Note, on November 16, 2023, we: (1) issued 3,656,288 shares of common stock to Adams; and (2) entered into a Warrant Amendment Agreement with Adams, amending the $1M Lender Warrants owned by Adams to reduce the exercise price of an aggregate of 107,575 $1M Lender Warrants to $0.16 per share (the “Adams Warrant Amendment”). Except as expressly set forth in the Adams Warrant Amendment, the terms and provisions of the warrants held by Adams shall remain in full force and effect.
Common Stock Warrants
On November 16, 2023, we issued warrants to purchase up to 240,000 shares of common stock, at an exercise price of $0.17 per share, to a consultant of the Company (the “Consultant Warrants”) as consideration for services rendered to the Company.
Corporate Information
We are a Texas corporation based in Southlake, Texas and were incorporated in Texas in August 2007. Our principal executive offices are located at 550 Reserve Street, Suite 360, Southlake TX 76092. Our telephone number is 682-237-7781. We are doing business under an assumed name, HeartSciences. Our website address is www.heartsciences.com. The information contained on, or that can be accessed through, our website is not part of this prospectus or the registration statement of which it forms a part. We have included our website address in this prospectus solely as an inactive textual reference.
S-17
Table of Contents
THE OFFERING
|
Common stock offered by us
|
|
Shares of our common stock having an aggregate offering price of up to $11,036,310.
|
|
Common stock to be outstanding after this offering(1)
|
|
Up to 78,175,995 assuming sales at a price of $0.17 per share, which was the closing price on the Nasdaq Capital Market on November 16, 2023. Actual number of shares issued will vary depending on the sales price under this offering.
|
|
Plan of distribution
|
|
“At the market offering” that may be made from time to time through Maxim Group LLC, as agent or principal. See “Plan of Distribution” on page S-32 of this prospectus supplement.
|
|
Use of proceeds
|
|
We retain broad discretion over the use of the net proceeds from the sale of shares of common stock offered hereby. We intend to use the net proceeds from the sale of shares of our common stock for working capital and other general corporate purposes, which may include future acquisitions of businesses and content. See “Use of Proceeds” on page S-27 of this prospectus supplement.
|
|
Market for common stock:
|
|
Our common stock and our IPO Warrants are listed on the Nasdaq Capital Market under the symbols “HSCS” and “HSCSW,” respectively. On November 16, 2023, the last reported sale price of our common stock on Nasdaq Capital Market was $0.17 per share.
|
|
Risk factors
|
|
Investing in our common stock involves significant risk. Please see “Risk Factors” beginning on page S-20 of this prospectus supplement, the section captioned “Item 1A — Risk Factors” in our most recently filed Annual Report on Form 10-K and our Quarterly Report on Form 10-Q, which are incorporated by reference into this prospectus, and under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this prospectus.
|
• 5,289,143 shares of common stock issuable upon conversion of the 380,440 shares of issued and outstanding Series C Preferred Stock;
• 1,634,907 shares of common stock issuable upon the exercise of stock options issued to directors, employees and consultants of the Company, of which 398,925 have vested;
• 2,500,000 shares of common stock issuable pursuant to our 2023 Equity Incentive Plan (as amended, the “2023 Plan”), that are reserved for future issuance to our employees, directors and consultants, of which 931,500 shares of our common stock are underlying outstanding awards under the 2023 Plan as of November 17, 2023;
• 752,636 shares of common stock issuable upon exercise of outstanding warrants (the “Investor Warrants”), the $1M Lender Warrants and the $1.5M Lender Warrants;
• 1,000,000 shares of common stock issuable upon exercise of the warrants issued pursuant to the MSW Note;
• 298,667 shares of common stock issuable upon exercise of the Remaining Bridge Warrants;
• 1,725,000 shares of common stock issuable upon exercise of the IPO Warrants, which includes the IPO Warrants issued pursuant to the underwriter’s over-allotment option in the IPO;
• 105,000 shares of common stock issuable upon exercise of the IPO Underwriter Warrants;
• 1,624,753 shares of common stock issuable upon exercise of the MTS Warrants and MTS Pre-Funded Warrants;
S-18
Table of Contents
• 150,000 shares of common stock issuable upon exercise of the Remaining Pre-Funded Bridge Warrant; and
• 62,500 shares that may still be issued under the Lincoln Park Purchase Agreement based on 1,927,000 shares, which is the current maximum number of shares that may be issued and/or sold under the Lincoln Park Purchase Agreement based on the price of $1.16 per share (which represents the official closing price of our common stock on Nasdaq the day of signing of the Lincoln Park Purchase Agreement); and
• any additional shares that may be sold under the EDA beyond what is covered by this prospectus supplement.
Unless otherwise indicated, all information in this prospectus supplement assumes no exercise of any outstanding options or warrants to purchase our common stock and no vesting of restricted stock units.
S-19
Table of Contents
RISK FACTORS
Before you make a decision to invest in our securities, you should consider carefully the risks described below, together with other information in this prospectus and the information incorporated by reference herein, including any risk factors contained in our Annual Report on Form 10-K, filed with the SEC on July 19, 2023 and our Quarterly Report on Form 10-Q, filed with the SEC on September 14, 2023, and in our other reports filed with the SEC and in future reports that we will file periodically. If any of the following events actually occur, our business, operating results, prospects or financial condition could be materially and adversely affected. This could cause the trading price of our common stock to decline and you may lose part or all of your investment. The risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also significantly impair our business operations and could result in a complete loss of your investment.
Risks Related to This Offering
Management will have broad discretion as to the use of the proceeds from this offering and may not use the proceeds effectively.
Because we have not designated the amount of net proceeds from this offering to be used for any particular purpose, our management will have broad discretion as to the application of the net proceeds from this offering and could use them for purposes other than those contemplated at the time of the offering. Our management may use the net proceeds for corporate purposes that may not improve our financial condition or market value. The failure by management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the implementation of our corporate strategy. Pending their use to fund our operations, we may invest our cash, cash equivalents and short-term investments, including the net proceeds from this offering, in a manner that does not produce income or that loses value.
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase in the offering and may experience dilution as a result of future equity offerings.
The offering price per share in this offering may exceed the pro forma net tangible book value per share of our common stock outstanding as of July 31, 2023. Assuming that we sell an aggregate of 29,578,866 shares of our common stock at a price of $0.17 per share, the sale price of our common stock on the Nasdaq Capital Market on November 16, 2023, during the term of the EDA, for aggregate gross proceeds of approximately $5.0 million (excluding shares we have already sold pursuant to the EDA prior to the date of this prospectus supplement), and after deducting commissions and estimated aggregate offering expenses payable by us, you will experience immediate dilution of $0.01 per share, representing the difference between our pro forma as adjusted net tangible book value per share as of July 31, 2023 after giving effect to this offering and the assumed offering price. The exercise of outstanding stock options and warrants and the sale of additional shares of common stock pursuant to the EDA beyond those covered by this prospectus supplement may result in further dilution of your investment. Additionally, because the sales of shares of our common stock offered hereby will be made directly into the market, the prices at which we sell such securities will vary and these variations may be significant. As a result, you may suffer dilution if you purchase shares in this offering at a higher price than other shares offered hereby are sold. See the section titled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate in this offering.
We expect that significant additional capital will be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity and/or convertible securities, our stockholders may experience substantial dilution. We may sell or otherwise issue our common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell or issue our common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent issuances. These issuances may also result in material dilution to our existing stockholders, and new investors could gain rights superior to our existing stockholders. We may pay for future acquisitions with additional issuances of shares of our common stock as well, which would result in further dilution for existing stockholders.
S-20
Table of Contents
Pursuant to the 2023 Plan, there are 2,500,000 shares of our common stock plus (i) any shares of our common stock subject to options that expire or otherwise terminate without having been exercised in full, are tendered to or withheld by us for payment of an exercise price or for tax withholding obligations, or are forfeited to or repurchased by us due to failure to vest, with the maximum number of shares of our common stock to be added to the Equity Incentive Plan under this clause (ii) equal to 832,195 shares of our common stock, reserved for issuance to our employees, directors and consultants, of which 931,500 shares of our common stock are underlying outstanding awards under the 2023 Plan as of November 17, 2023. If our board of directors elects to issue stock, stock options and/or other equity-based awards under the 2023 Plan, our stockholders may experience additional dilution, which could cause our stock price to fall.
Market price of our common stock may be highly volatile, you may not be able to resell your shares at or above the public offering price and you could lose all or part of your investment.
The trading price of our common stock may be volatile. As an investor, you might never recoup all, or even part of, your investment and you may never realize any return on your investment. You must be prepared to lose all your investment. Our stock price could be subject to wide fluctuations in response to a variety of factors, including the following:
• actual or anticipated fluctuations in our revenue and other operating results;
• actions of securities analysts who initiate or maintain coverage of us, changes in financial estimates by any securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors;
• issuance of our equity or debt securities, or disclosure or announcements relating thereto;
• the lack of a meaningful, consistent and liquid trading market for our common stock;
• additional shares of our common stock being sold into the market by us or our stockholders or the anticipation of such sales;
• our convertible debt securities being converted into equity or the anticipation of such conversion;
• announcements by us or our competitors of significant events or features, technical innovations, acquisitions, strategic partnerships, joint ventures or capital commitments;
• changes in operating performance and stock market valuations of companies in our industry;
• price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole;
• lawsuits threatened or filed against us;
• changes in regulation or tax law;
• regulatory developments in the United States and foreign countries; and
• other events or factors, including those resulting from the impact of COVID-19 epidemic, war or incidents of terrorism, or responses to these events.
In addition, the stock market in general has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of certain companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
The actual number of shares we will issue under the EDA, at any one time or in total, is uncertain.
Subject to certain limitations in the EDA and compliance with applicable law, we have the discretion to deliver placement notices to Maxim at any time throughout the term of the EDA. The number of shares of common stock that are sold by Maxim after delivering a placement notice will fluctuate based on the market price of the common stock during the sales period and limits we set with Maxim.
S-21
Table of Contents
We do not intend to pay dividends on our common stock so any returns will be limited to the value of our stock.
We have never declared or paid any cash dividend on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Additionally, any credit and security agreement that we may enter into in the future will likely contain covenants that will restrict our ability to pay dividends. Any return to stockholders will therefore be limited to the appreciation of their stock.
Sales of a substantial number of shares of our common stock in the public market, or the perception that such sales could occur, could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
An active trading market for our common stock may not be maintained.
Our stock is currently traded on The Nasdaq Capital Market, but we can provide no assurance that we will be able to maintain an active trading market on this or any other exchange in the future. If an active market for our common stock is not maintained, it may be difficult for our stockholders to sell or purchase shares. An inactive market may also impair our ability to raise capital to continue to fund operations by selling shares and impair our ability to acquire other companies or technologies using our shares as consideration.
The common stock offered hereby will be sold in an “at the market offering”, and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares in this offering at different times will likely pay different prices, and so may experience different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold, and there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result of share sales made at prices lower than the prices they paid.
Risks Related to Our License Agreements with Mount Sinai
We may not ultimately retain the Licenses from Mount Sinai since the retention of the Licenses is subject to the Company raising at least $10,000,000 in gross proceeds from one or more financings raised prior to June 30, 2024.
The retention of the Licenses from Mount Sinai is subject to a condition which mandates that we raise an aggregate of $10,000,000 prior to June 30, 2024 (inclusive of the $5,000,000 we have already raised), otherwise Mount Sinai may terminate the License Agreements. Failure to meet such financing threshold within the stipulated time frame may result in the revocation of the Licenses issued and termination of our License Agreements with Mount Sinai. This condition presents a significant risk to our business, as our ability to meet the fundraising target may be influenced by various factors, including market conditions, investor sentiment, and economic uncertainties. If we are unable to secure the necessary capital within the specified timeframe, we may be unable to retain the Licenses, which could materially impact our ability to operate and generate revenue, potentially leading to a loss of business opportunities and financial harm to our company and shareholders.
As a result of the MTS Transaction, our shareholders have suffered and will suffer immediate and substantial dilution.
Because of the issuance of shares of common stock, common stock warrants and pre-funded warrants to Mount Sinai, our stockholders have experienced and may continue to experience significant dilution of their ownership interests. Furthermore, if we fail to meet the Additional Financing Requirement, Mount Sinai will have the right to retain the MTS Securities issued to them under the Securities Purchase Agreement at its own discretion. For a further description of the dilution that investors in this offering will experience, see “Dilution.”
S-22
Table of Contents
We are highly dependent on the Licenses, the termination of which may prevent us from commercializing our products, and which imposes significant obligations on us.
We are highly dependent on the intellectual property licensed from Mount Sinai, pursuant to which we aim to incorporate the licensed technology for use and development in our MyoVista products. Other products or services we may develop also may rely on the same technology. In the event that we fail to use no less than commercially reasonable efforts to develop and commercialize the licensed products in their respective field of use as soon as reasonably practicable, and we do not cure such breach within the applicable time period, Mount Sinai could terminate the Licenses. Any termination of the Licenses resulting in the loss of the licensed rights would prevent us from marketing and selling our anticipated MyoVista products and any other products or services we may develop based on such underlying licensed technology. Any termination of the exclusivity of the license, in those cases where applicable, could damage our competitive position within the marketplace.
Furthermore, the License Agreements impose significant obligations on us. We will be required to pay Mount Sinai royalties in low-single digit percentages of annual net sales of licensed products sold by the Company and a share of any sublicense revenue received by the Company from sublicensees of the licensed products as well as achieve the milestones prescribed by the License Agreements and Securities Purchase Agreement.
Our future financial performance will depend in part on the successful integration, improvement and software updates from the Mount Sinai algorithms.
Our future financial performance will depend in part on our ability to influence, anticipate, identify and respond to changing consumer preferences and needs and the technologies relating to the care and treatment of heart disease. We can provide no assurances that the Mount Sinai algorithms and products will be successfully integrated, achieve significant commercial success and gain meaningful market share. We may not correctly anticipate or identify trends in consumer preferences or needs or may identify them later than competitors do. In addition, difficulties in manufacturing or in obtaining regulatory approvals due to integrating the Mount Sinai algorithms or providing a cloud-based environment to host the algorithms may delay or prohibit our algorithm-based product offerings from completing the development process. Further, we may not be able to develop improvements and software updates to our “EKG” product at a cost that allows us to meet our goals for profitability. Service costs relating to our product may be greater than anticipated and we may be required to devote significant resources to address any quality issues associated with our product.
Failure to successfully introduce, improve or update our products on a cost-effective basis, or delays in customer decisions related to the evaluation of our products could cause us to lose market acceptance and could materially adversely affect our business, financial condition and results of operations.
Our future success depends on our ability to develop, receive regulatory clearance or approval for, and introduce the algorithms underlying the Mount Sinai Licenses to the market in a timely manner. If we do not obtain and maintain the regulatory registrations and clearances for the algorithms, we will be unable to market and sell the MyoVista utilizing the Licenses in the United States, Europe or other regions.
In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to, an existing product, we must first receive either approval of a Premarket Approval Application, or PMA, clearance under Section 510(k), or be granted a De Novo classification, in accordance with the Federal Food, Drug, and Cosmetic Act, or the FDCA. For additional information on the PMA or the De Novo classification processes, see “Company Overview” above.
The FDA can delay, limit or deny clearance or approval of a medical device for many reasons, including:
• we may not be able to demonstrate to the FDA’s satisfaction that the algorithms underlying the Mount Sinai Licenses are safe and effective for its intended use;
• the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and
• the manufacturing process or facilities we use or contract to use may not meet applicable requirements.
S-23
Table of Contents
Sales of our device outside of the United States and the EEA are also subject to foreign regulatory requirements that vary widely from country to country. Approval procedures vary among countries and can involve additional testing. Complying with foreign regulatory requirements, including obtaining registrations, clearances or approvals, can be expensive and time-consuming, and we may not receive regulatory clearances or approvals in each country in which we plan to market our device or we may be unable to do so on a timely basis. If we modify our device, we may need to apply for additional regulatory clearances or approvals before we are permitted to sell the modified device. In addition, we may not continue to meet the quality and safety standards required to maintain the authorizations that we have received. If we are unable to maintain our authorizations in a particular country, we will no longer be able to sell the applicable device in that country.
Regulatory clearance or approval by the FDA does not ensure registration, clearance or approval by regulatory authorities in other countries, and registration, clearance or approval by one or more foreign regulatory authorities does not ensure registration, clearance or approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining registration or regulatory clearance or approval in one country may have a negative effect on the regulatory process in others.
S-24
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the at-the-market offering prospectus and the documents incorporated by reference herein or therein, contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or other comparable terms. All statements other than statements of historical facts included in this prospectus supplement, the at-the-market offering prospectus and the documents incorporated by reference herein or therein regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. These forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors that could cause our actual results of operations, financial condition, liquidity, performance, prospects, opportunities, achievements or industry results, as well as those of the markets we serve or intend to serve, to differ materially from those expressed in, or suggested by, these forward-looking statements. These forward-looking statements are based on assumptions regarding our present and future business strategies and the environment in which we expect to operate in the future. Important risks and factors that could cause those differences include, but are not limited to:
• our expectation regarding the sufficiency of our existing cash and cash equivalents to fund our current operations;
• our ability to receive regulatory clearance for the MyoVista wavECG (the “MyoVista”) from the U.S. Food and Drug Administration (the “FDA”), state regulators, if any, or other similar foreign regulatory agencies, including approval to conduct clinical trials, the timing and scope of those trials and the prospects for regulatory approval or clearance of, or other regulatory action with respect to the MyoVista or other future potential products;
• our ability to further advance the development of the MyoVista, our 12-lead electrocardiograph (“ECG”) device that also incorporates an additional proprietary artificial intelligence (“AI”) -based algorithm that we have been designing to detect cardiac dysfunction, and future potential products;
• our ability to launch sales of the MyoVista or any future potential products into the U.S.;
• our ability to retain the Licenses from Mount Sinai, which are subject to certain financing requirement conditions;
• our assessment of the potential of the MyoVista and any future potential products;
• our planned level of capital expenditures and liquidity;
• our plans to continue to invest in research and development to develop technology for new products;
• our failure to meet the continued listing requirements of The Nasdaq Stock Market LLC could result in a de-listing of our shares and penny stock trading;
• the regulatory environment and changes in the health policies and regimes in the countries in which we intend to operate, including the impact of any changes in regulation and legislation that could affect the medical device industry;
• our ability to meet our expectations regarding the commercial supply of the MyoVista and any future products;
• our ability to retain key executives;
• our ability to internally develop new inventions and intellectual property;
• the overall global economic environment;
S-25
Table of Contents
• the ultimate impact of the COVID-19 pandemic, or any other health epidemic, on our business, our clinical trials, our research programs, healthcare system or the global economy as a whole;
• the impact of competition and new technologies;
• general market, political and economic conditions in the countries in which we operate;
• our ability to develop new devices and intellectual property;
• changes in our strategy; and
• potential litigation.
Forward-looking statements are based only on our current beliefs, expectations and assumptions regarding the future of our business, strategies, projections, anticipated events and trends, and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in our forward-looking statements. Therefore, you should not rely on the occurrence of events described in any of these forward-looking statements. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of the information presented in this prospectus supplement, the at-the-market offering prospectus and any document incorporated herein or therein by reference, and particularly our forward-looking statements, by these cautionary statements.
S-26
Table of Contents
USE OF PROCEEDS
The amount of proceeds from this offering will depend upon the number of shares of our common stock sold and the market price at which they are sold. There can be no assurance that we will be able to sell any shares under or fully utilize the EDA with Maxim as a source of financing.
We intend to use the net proceeds pursuant to this prospectus supplement and the EDA for costs directly related to obtaining FDA clearance for the MyoVista, for R&D, working capital and general corporate purposes, including personnel costs, capital expenditures and the costs of operating as a public company. We have not allocated specific amounts of net proceeds for any of these purposes. Changing circumstances may cause us to consume capital significantly faster than we currently anticipate. The amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our global marketing and sales efforts, our development efforts and the overall economic environment.
Therefore, our management will retain broad discretion over the use of the proceeds from this offering. We may ultimately use the proceeds for different purposes than what we currently intend. Pending any ultimate use of any portion of the proceeds from this offering, if the anticipated proceeds will not be sufficient to fund all the proposed purposes, our management will determine the order of priority for using the proceeds, as well as the amount and sources of other funds needed. We believe that the funds raised in this offering will be sufficient to finance the purposes described above, and we do not think that material amounts of other funds will be necessary to finance such purposes.
S-27
Table of Contents
DILUTION
If you invest in our common stock in this offering, your interest will be diluted immediately to the extent of the difference between the price per share you pay in this offering and the as adjusted net tangible book deficit per share of our common stock after giving effect to this offering. Our historical net tangible book deficit as of July 31, 2023 was approximately negative $0.5 million, or negative $(0.05) per share of our common stock. Net tangible book deficit per share is calculated by subtracting our total liabilities from our total tangible assets, which is total assets less intangible assets (including goodwill), and dividing this amount by the number of shares of common stock outstanding.
Our pro forma historical net tangible book deficit as of July 31, 2023, was approximately $8.2 million, or $0.17 per share of our common stock. Pro forma historical net tangible book deficit per share represents the amount of our total tangible assets less our total liabilities, divided by the total number of shares of common stock outstanding at July 31, 2023, after giving effect to the (i) issuance of an aggregate of 944,592 shares of common stock pursuant to the Lincoln Park Purchase Agreement; (ii) the draw of $500,000 on the MSW Note; (iii) the issuance of warrants to purchase up to 200,000 shares of common stock as consideration for extension of the $1M Loan and Security agreement; (iv) the issuance of 1,000,000 shares of common stock in lieu of a facility fee; (v) issuance of warrants to purchase up to 240,000 shares of common stock to a consultant; (vi) the issuance of 25,345,416 shares in this at the market offering pursuant to the Original EDA and the First Amendment, (vii) the issuance of 6,781,288 shares of common stock for conversion of the Adams Note and MSW Note; and (viii) the issuance of 4,854,853 shares of common stock, pre-funded warrants to purchase 710,605 shares of common stock and warrants to purchase 914,148 shares of common stock to Mount Sinai.
After giving effect to the sale by us of approximately $5.03 million (which represents the remaining amount available for sale under this prospectus supplement after excluding shares we have already sold pursuant to the Original EDA and the First Amendment prior to the date of this prospectus supplement) worth of common stock that may be offered in this offering at an assumed offering price of $0.17 per share, which was the closing price of our common stock on The Nasdaq Capital Market on November 16, 2023, and after deducting estimated offering commissions and expenses payable by us, our as-adjusted net tangible book value as of July 31, 2023 would have been approximately $12.8 million, or $0.16 per share of common stock. This represents an immediate increase in the net tangible book value of $0.21 per share to our existing stockholders and an immediate and substantial dilution in net tangible book value of $0.01 per share to investors in this offering. The following table illustrates this hypothetical per share dilution:
|
Assumed public offering price per share
|
|
|
|
|
|
$
|
0.17
|
|
Net tangible book deficit per share as of July 31, 2023
|
|
$
|
(0.05
|
)
|
|
|
|
|
Change in net tangible book value per share as of July 31, 2023, after giving effect to the pro forma transactions
|
|
$
|
0.22
|
|
|
|
|
|
Change in net tangible book value per share as of July 31, 2023, attributable to this offering
|
|
$
|
(0.01
|
)
|
|
|
|
|
As adjusted net tangible book value per share as of July 31, 2023, after giving effect to this offering
|
|
|
|
|
|
$
|
0.16
|
|
Dilution per share to new investors purchasing shares in this offering
|
|
|
|
|
|
$
|
0.01
|
The table above assumes for illustrative purposes that an aggregate of 29,578,866 shares of our common stock are sold at a price of $0.17 per share, the last reported sale price of our common stock on The Nasdaq Capital Market on November 16, 2023, for aggregate gross proceeds of approximately $5.03 million (which represents the remaining amount available for sale under this prospectus supplement after excluding shares we have already sold pursuant to the Original EDA and the First Amendment prior to the date of this prospectus supplement). The shares sold in this offering, if any, will be sold from time to time at various prices. An increase of $0.05 per share in the price at which the shares are sold from the assumed offering price of $0.17 per share shown in the table above, would increase our pro forma as adjusted net tangible book value after giving effect to this offering by approximately $1.4 million, or approximately $0.02 per share, and the dilution per share to new investors in this offering by $0.03 per share, after deducting commissions and estimated aggregate offering expenses payable by us. A decrease of $0.05 per share in the price at which the shares are sold from the assumed offering price of $0.17 per share shown in the table above, would decrease our pro forma as adjusted net tangible book value after giving effect to this offering by approximately $1.4 million, or approximately $0.01 per share, and the dilution per share to new investors in this offering by $0.04 per share, after deducting commissions and estimated aggregate offering expenses payable by us. This information is supplied for illustrative purposes only.
S-28
Table of Contents
For purposes of calculating net tangible book value, the above table is based on 10,670,980 shares issued and outstanding as of July 31, 2023, and assumes the sale of up to 29,578,866 shares of our common stock by Maxim Group LLC, as Sales Agent, pursuant to the EDA, and does not include the following:
• 1,728,710 shares of common stock issuable upon conversion of the 380,440 shares of issued and outstanding Series C Preferred Stock;
• 1,634,907 shares of common stock issuable upon the exercise of stock options issued to directors, employees and consultants of the Company, of which 360,857 have vested;
• 2,500,000 shares of our common stock pursuant to our 2023 Equity Incentive Plan (as amended, the “2023 Plan”), that are reserved for future issuance to our employees, directors and consultants, of which 931,500 shares of our common stock are underlying outstanding awards under the 2023 Plan as of November 17, 2023;
• 303,969 shares of common stock issuable upon exercise of the Investor Warrants, the $1M Lender Warrants and the $1.5M Lender Warrants;
• 1,000,000 shares of common stock issuable upon exercise of the warrants issued pursuant to the MSW Note;
• 298,667 shares of common stock issuable upon exercise of the Remaining Bridge Warrants;
• 1,725,000 shares of common stock issuable upon exercise of the IPO Warrants, which includes the IPO Warrants issued pursuant to the underwriter’s over-allotment option in the IPO;
• 105,000 shares of common stock issuable upon exercise of the IPO Underwriter Warrants;
• 150,000 shares of common stock issuable upon exercise of the Remaining Pre-Funded Bridge Warrant; and
• 944,592 shares that may still be issued and/or sold under the Lincoln Park Purchase Agreement based on 1,927,000 shares. This reflects the current maximum number of shares that may be issued and/or sold under the Lincoln Park Purchase Agreement based on the price of $1.16 per share (which represents the official closing price of our common stock on Nasdaq the day of signing of the Lincoln Park Purchase Agreement).
S-29
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding beneficial ownership of our common stock and Series C Preferred Stock as of November 17, 2023 by:
• each person, or group of affiliated persons, known to us to be the beneficial owner of more than 5% of our outstanding common stock or Series C Preferred Stock;
• each of our directors and executive officers; and
• all of our directors and executive officers as a group.
Beneficial ownership is determined in accordance with the rules of the SEC, and includes voting or investment power with respect to the shares of capital stock indicated. Shares of capital stock that are issuable upon (i) the conversion of Series C Preferred Stock or (ii) exercise of options or warrants exercisable within 60 days after November 16, 2023, are deemed outstanding for the purpose of computing the percentage ownership of the person holding such Series C Preferred Stock, options or warrants, but are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
We are not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and there are no arrangements known to us which would result in a change in control of our Company at a subsequent date. Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such shareholders. Unless otherwise noted below, each beneficial owner’s address is c/o Heart Test Laboratories, Inc., 550 Reserve Street, Suite 360, Southlake, Texas 76092.
With respect to the calculations set forth in the table below, the percentages of beneficial ownership prior to this offering are based on 48,597,129 shares of our common stock and 380,440 shares of Series C Preferred Stock outstanding as of November 17, 2023. The percentage of beneficial ownership in the table below is based on 78,175,995 shares of our common stock assumed to be outstanding if all 29,578,866 shares of common stock offered pursuant to this prospectus supplement are sold (based on a price per share of $0.17, which was the closing price of our common stock on the Nasdaq Capital Market on November 16, 2023. The calculations set forth in the table below include shares issuable pursuant to antidilution provisions set forth in the Series C Preferred Stock, which would be triggered as a result of this offering. See “— Antidilution Provisions” discussed in the at-the-market offering prospectus.
|
|
|
Beneficial Ownership
|
| |
|
Number of Shares(1)
|
|
Percentages(2)
|
|
Name of Beneficial Owner
|
|
Common Stock
Prior to
Offering
|
|
Common Stock
After
Offering
|
|
Series C
Preferred
Stock
|
|
Common Stock
Prior to
Offering
|
|
Common
Stock
After
Offering
|
|
Series C
Preferred
Stock
|
|
Combined
Voting
Power
Prior to
Offering(3)
|
|
Combined
Voting
Power
After
Offering(3)
|
|
Holder of 5% or more of each class of our securities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Front Range Ventures, LLC(4)
|
|
2,456,396
|
|
3,319,155
|
|
148,213
|
|
4.8
|
%
|
|
4.1
|
%
|
|
39.0
|
%
|
|
4.4
|
%
|
|
3.7
|
%
|
|
John H. Matthews(5)
|
|
4,952,778
|
|
5,241,758
|
|
—
|
|
9.9
|
%
|
|
6.6
|
%
|
|
—
|
|
|
9.5
|
%
|
|
6.0
|
%
|
|
Lary Snodgrass(6)
|
|
649,583
|
|
819,791
|
|
29,240
|
|
1.3
|
%
|
|
1.0
|
%
|
|
7.7
|
%
|
|
1.2
|
%
|
|
1.0
|
%
|
|
Paul Buchanan(7)
|
|
534,013
|
|
653,921
|
|
20,599
|
|
1.1
|
%
|
|
*
|
%
|
|
5.4
|
%
|
|
1.0
|
%
|
|
*
|
%
|
|
Mount Sinai(8)
|
|
4,854,853
|
|
6,479,606
|
|
—
|
|
9.9
|
%
|
|
8.1
|
%
|
|
__
|
|
|
9.0
|
%
|
|
7.4
|
%
|
|
Directors and executive officers:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Andrew Simpson, President, CEO and Chairman(9)
|
|
624,579
|
|
660,186
|
|
6,117
|
|
1.3
|
%
|
|
*
|
%
|
|
1.6
|
%
|
|
1.2
|
%
|
|
*
|
%
|
|
Mark Hilz, COO & Secretary(10)
|
|
577,425
|
|
589,533
|
|
2,080
|
|
1.2
|
%
|
|
*
|
%
|
|
*
|
|
|
1.1
|
%
|
|
*
|
%
|
|
Danielle Watson, CFO & Treasurer(11)
|
|
7,575
|
|
7,575
|
|
—
|
|
*
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
Bruce Bent, Director(12)
|
|
28,532
|
|
28,532
|
|
—
|
|
*
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
Brian Szymczak, Director(13)
|
|
57,863
|
|
60,191
|
|
400
|
|
*
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
David R. Wells, Director(14)
|
|
25,000
|
|
25,000
|
|
—
|
|
—
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
All directors and executive officers as a group (6 persons):
|
|
1,320,974
|
|
1,371,017
|
|
8,597
|
|
2.7
|
%
|
|
1.7
|
%
|
|
2.3
|
%
|
|
2.4
|
%
|
|
1.6
|
%
|
S-30
Table of Contents
S-31
Table of Contents
PLAN OF DISTRIBUTION
We entered into the Original EDA on September 18, 2023, with Maxim, pursuant to which we may offer and sell, from time to time, an aggregate of up to $3,250,000 of shares of our common stock, in an “at the market” offering (as defined in Rule 415(a)(4) under the Securities Act). On November 9, 2023, we entered into the First Amendment with Maxim pursuant to which, among other things, we may issue and sell up to $10,000,000 of common stock from time to time through the Sales Agent subject to certain selling limitations. As of November 17, 2023, we have sold 25,345,416 shares of common stock with an aggregate offering price of approximately $6.0 million. On November 17, 2023, we entered into the Second Amendment with Maxim pursuant to which, among other things, we may issue and sell up to $15,000,000 of our shares of common stock from time to time through the Sales Agent; provided, however, that in no event will we issue or sell through the Sales Agent such number of shares of common stock that would cause us or the offering of our shares of common stock to not satisfy the eligibility and transaction requirements for use of Form S-3 (including General Instruction I.B.6 of Form S-3). Pursuant to the Second Amendment, we have also agreed to reimburse Maxim Group’s legal fees and expenses up to $90,000. As of November 17, 2023, the aggregate market value of our outstanding shares of common stock held by non-affiliates was $33,108,931, which was calculated based on 47,707,393 outstanding shares of common stock held by non-affiliates on November 17, 2023 and a price per share of $0.69, which was the closing price of the common stock on September 18, 2023 and is the highest closing sale price of common stock on the Nasdaq Capital Market within the prior 60 days. Therefore, this prospectus supplement covers the sale of up to $11,036,310 shares of common stock. We may file one of more prospectus supplements from time to time to sell additional shares of common stock as more availability under General Instruction I.B.6 of Form S-3 becomes available.
The following summary of the material provisions of the EDA does not purport to be a complete statement of its terms and conditions. The Original EDA has been filed as an exhibit to our Current Report on Form 8-K dated September 22, 2023, the First Amendment has been filed as an exhibit to our Current Report on Form 8-K dated November 13, 2023 and the Second Amendment has been filed as an exhibit to our Current Report on Form 8-K dated November 17, 2023. In accordance with the terms of the EDA, we may sell shares of our common stock from time to time through or to the Sales Agent, acting as sales agent or principal, subject to certain limitations, including the number or dollar amount of shares registered under the registration statement to which the offering relates. The sales, if any, of shares made under the EDA will be made by any method that is deemed an “at the market offering” as defined in Rule 415 promulgated under the Securities Act. We may instruct the Sales Agent not to sell common stock if the sales cannot be effected at or above the price designated by us from time to time. We or the Sales Agent may suspend the offering of common stock upon notice and subject to other conditions. Each time we wish to issue and sell common stock under the EDA, we will notify the Sales Agent of the number or dollar value of shares to be issued, the dates on which such sales are anticipated to be made, any minimum price below which sales may not be made and other sales parameters as we deem appropriate. Once we have so instructed such designated Sales Agent, unless such Sales Agent declines to accept the terms of the notice, such Sales Agent has agreed to use its commercially reasonable efforts consistent with such agent’s normal trading and sales practices to sell such shares up to the amount specified on such terms. The obligations of the Sales Agent under the EDA to sell our common stock is subject to a number of conditions that we must meet.
We will pay the Sales Agent commissions for its services in acting as agent in the sale of our common stock. The Sales Agent will be entitled to a commission equal to 4.0% of the gross proceeds from the sale of common stock offered hereby (excluding any shares of common stock sold under the Original EDA and the First Amendment, whereby Maxim was entitled to compensation at a commission rate equal to 3.0% of the gross sales price per share sold). In addition, we have agreed to reimburse legal expenses of the Sales Agent in an amount not to exceed $90,000 in connection with the preparation and entering into the EDA and/or establishment of the “at the market offering” plus up to $2,500 per calendar quarter. In accordance with Financial Industry Regulatory Authority, Inc. Rule 5110, these fees and reimbursed expenses are deemed sales compensation in connection with this offering. We estimate that the total expenses for the offering, excluding compensation payable to the Sales Agent under the terms of the EDA, will be approximately $115,000.
S-32
Table of Contents
Settlement for sales of common stock will generally occur on the second business day following the date on which any sales are made, or on some other date that is agreed upon by us and the Sales Agent in connection with a particular transaction, in return for payment of the net proceeds to us. Sales of our common stock as contemplated in this prospectus supplement and the at-the-market offering prospectus will be settled through the facilities of The Depository Trust Company or by such other means as we and the Sales Agent may agree upon. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
The actual proceeds to us will vary depending on the number of shares sold and the prices of such sales. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions, and proceeds to us, if any, are not determinable at this time.
In connection with the sale of the common stock on our behalf, the Sales Agent may be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of the Sales Agent may be deemed to be underwriting commissions or discounts. We have agreed to provide indemnification and contribution to the Sales Agent against certain civil liabilities, including liabilities under the Securities Act. We have also agreed to reimburse the sales agent for certain other specified expenses. The offering of our common stock pursuant to this prospectus supplement will terminate upon the earlier of (i) the sale of all shares of our common stock provided for in this prospectus supplement, or (ii) termination of the EDA as provided therein.
The Sales Agent and its affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates, for which services they may in the future receive customary fees. To the extent required by Regulation M, the Sales Agent will not engage in any market making activities involving our common stock while the offering is ongoing under this prospectus supplement and the at-the-market offering prospectus.
This prospectus supplement and the at-the-market offering prospectus in electronic format may be made available on a website maintained by the Sales Agent, and the Sales Agent may distribute this prospectus supplement and the at-the-market offering prospectus electronically.
S-33
Table of Contents
LEGAL MATTERS
Certain legal matters relating to the validity of the securities offered by this prospectus supplement and the at-the-market offering prospectus will be passed upon for us by Foley Shechter Ablovatskiy LLP (“FSA”), New York, New York. FSA may receive shares of our common stock in connection with the satisfaction of outstanding legal fees payable to FSA. Although FSA is not under any obligation to accept shares of our common stock in payment for services, it may do so in the future. The Sales Agent is being represented in connection with this offering by Sullivan and Worcester LLP, New York, New York.
EXPERTS
The financial statements as of April 30, 2023 and 2022, and for the years then ended incorporated by reference in this prospectus supplement, the “at-the-market” prospectus and the Registration Statement have been so incorporated in reliance on the report of Haskell & White LLP, an independent registered public accounting firm, incorporated herein by reference, given on the authority of said firm as experts in auditing and accounting. The report on the financial statements contains an explanatory paragraph regarding our ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the reporting requirements of the Exchange Act, and file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains a web site that contains reports, proxy and information statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov. You may access these materials free of charge as soon as reasonably practicable after they are electronically filed with or furnished to the SEC.
Our web site address is https://heartsciences.com. The information on our web site, however, is not, and should not be deemed to be, a part of, or incorporated by reference into, this prospectus.
This prospectus supplement is only part of a registration statement on Form S-3 that we have filed with the SEC under the Securities Act and therefore omits certain information contained in the registration statement. We have also filed exhibits and schedules with the registration statement that are excluded from this prospectus supplement or the at-the-market offering prospectus, and you should refer to the applicable exhibit or schedule for a complete description of any statement referring to any contract or other document. You may inspect a copy of the registration statement, including the exhibits and schedules, without charge, at the public reference room or obtain a copy from the SEC upon payment of the fees prescribed by the SEC.
We also maintain a website at www.heartsciences.com, through which you can access our SEC filings. The website addresses referenced herein are not intended to function as hyperlinks, and the information contained in our website, the SEC’s website or any other website referenced herein is not incorporated by reference into this prospectus supplement and should not be considered to be part of this prospectus supplement.
S-34
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information that we file with them. Incorporation by reference allows us to disclose important information to you by referring you to those other documents. The information incorporated by reference is an important part of this prospectus supplement, and information that we file later with the SEC will automatically update and supersede this information. This prospectus supplement omits certain information contained in the registration statement, as permitted by the SEC. You should refer to the registration statement, including the exhibits, for further information about us and the securities we may offer pursuant to this prospectus supplement. Statements in this prospectus supplement regarding the provisions of certain documents filed with, or incorporated by reference in, the registration statement are not necessarily complete and each statement is qualified in all respects by that reference. Copies of all or any part of the registration statement, including the documents incorporated by reference or the exhibits, may be obtained upon payment of the prescribed rates at the offices of the SEC listed above in “Where You Can Find More Information.” The documents we are incorporating by reference are:
• our Annual Report on Form 10-K for the fiscal year ended April 30, 2023, filed with the SEC on July 19, 2023;
• our Quarterly Report on Form 10-Q for the quarter ended July 31, 2023, filed with the SEC on September 14, 2023;
• our Current Report on Form 8-K filed with the SEC on July 19, 2023;
• our Current Report on Form 8-K, filed with the SEC on August 4, 2023;
• our Current Report on Form 8-K filed with the SEC on August 17, 2023;
• our Current Report on Form 8-K filed with the SEC on August 30, 2023;
• our Current Report on Form 8-K filed with the SEC on September 7, 2023;
• our Current Report on Form 8-K filed with the SEC on September 21, 2023;
• our Current Report on Form 8-K filed with the SEC on September 22, 2023;
• our Current Report on Form 8-K filed with the SEC on November 13, 2023;
• our Current Report on Form 8-K filed with the SEC on November 17, 2023;
• the description of our common stock contained in our Registration Statement on Form 8-A, filed on June 14, 2022, pursuant to Section 12(b) of the Exchange Act, which incorporates by reference the description of the shares of our common stock contained in our Registration Statement on Form S-1 (Registration No. 333-265024) initially filed with the SEC on May 17, 2022, as amended, and declared effective by the SEC on June 14, 2022, and any amendment or report filed with the SEC for purposes of updating such description;
• all reports and other documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of this prospectus and prior to the termination or completion of the offering of securities under this prospectus supplement shall be deemed to be incorporated by reference in this prospectus supplement and to be a part hereof from the date of filing such reports and other documents.
Unless otherwise noted, the SEC file number for each of the documents listed above is 001-41422.
Unless expressly incorporated by reference, nothing in this prospectus supplement shall be deemed to incorporate by reference information furnished, but not filed, with the SEC.
S-35
Table of Contents
Any statement contained in this prospectus supplement or in a document incorporated or deemed to be incorporated by reference into this prospectus supplement will be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained in this prospectus supplement or any other subsequently filed document that is deemed to be incorporated by reference into this prospectus supplement modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement.
We will promptly provide, without charge to you, upon written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus supplement, other than exhibits to those documents, unless the exhibits are specifically incorporated by reference in those documents. Requests should be directed to:
Corporate Secretary
Heart Test Laboratories, Inc.
550 Reserve Street, Suite 360
Southlake, TX 76092
S-36
Table of Contents

HEART TEST LABORATORIES, INC.
_______________________________________
PROSPECTUS SUPPLEMENT
_______________________________________
Up to $11,036,310
Common Stock
Maxim Group LLC
The date of this prospectus supplement is November 17, 2023.