|
|
PROSPECTUS SUPPLEMENT NO. 9 (to Prospectus dated April 12, 2024) |
Filed Pursuant to Rule 424(b)(3) Registration No. 333-278566 |
6,974,248 Shares of Common Stock

This prospectus supplement is being filed to update and supplement the information contained in the prospectus dated April 12, 2024 (as supplemented from time to time, the “Prospectus”), which forms a part of our registration statement on Form S-1 (No. 333-278566), with the information contained in our Current Report on Form 8-K filed with the Securities and Exchange Commission on October 9, 2024 (the “Current Report”). Accordingly, we have attached the Current Report to this prospectus supplement. This prospectus relates to the offer and resale from time to time of up to 6,974,248 shares (the “Shares”) of common stock, par value $0.0001 per share (“Common Stock”), of Lexeo Therapeutics, Inc., a Delaware corporation (the “Company”), by the selling stockholders identified in this prospectus, including their transferees, pledgees or donees or their respective successors (the “Selling Stockholders”). The Shares consist of (i) 6,278,905 shares which were issued and sold to the Selling Stockholders on March 13, 2024 (the “Closing Date”) in a private placement (the “Private Placement”) pursuant to a common stock purchase agreement among us and such Selling Stockholders dated March 11, 2024 (the “Purchase Agreement”) and (ii) 695,343 shares of Common Stock held by the Selling Stockholders as of March 11, 2024. Concurrently with the Purchase Agreement, we entered into a registration rights agreement (the “Registration Rights Agreement”) with the Selling Stockholders, and we are registering the Shares being offered hereunder pursuant to such registration rights agreement on behalf of the Selling Stockholders, to be offered and sold by them from time to time.
This prospectus supplement updates and supplements the information in the Prospectus and is not complete without, and may not be delivered or utilized except in combination with, the Prospectus, including any amendments or supplements thereto. This prospectus supplement should be read in conjunction with the Prospectus and if there is any inconsistency between the information in the Prospectus and this prospectus supplement, you should rely on the information in this prospectus supplement.
Our Common Stock is listed on The Nasdaq Global Market (“Nasdaq”) under the symbol “LXEO”. On October 8, 2024, the last quoted sale price for our Common Stock as reported on Nasdaq was $8.75 per share.
We are an “emerging growth company” and a “smaller reporting company” as defined under the federal securities laws, and, as such, may elect to comply with certain reduced public company reporting requirements for this prospectus and for future filings.
Investing in our securities involves a high degree of risk. Before buying any securities, you should carefully read the discussion of the risks of investing in our securities in “Risk Factors” beginning on page 13 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Prospectus supplement dated October 9, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): October 09, 2024 |
Lexeo Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-41855 |
85-4012572 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
345 Park Avenue South, Floor 6 |
|
New York, New York |
|
10010 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 212 547-9879 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.0001 par value per share |
|
LXEO |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On October 9, 2024, Lexeo Therapeutics, Inc. (the “Company”) posted on its website an updated corporate presentation (the “Corporate Presentation”), noting the Company expects to share Cohort 1 data from LX2020 for the treatment of PKP2-ACM in the late first quarter or the early second quarter of 2025 and that the Company plans to present data from LX1001 for the treatment of APOE4-associated Alzheimer’s disease at the Clinical Trials on Alzheimer’s Disease conference in October 2024. The Corporate Presentation will be used from time to time in meetings with investors and analysts. A copy of the Corporate Presentation is attached hereto as Exhibit 99.1 and is incorporated by reference herein.
Cautionary Note Regarding Forward-Looking Statements
This report contains certain forward-looking statements regarding the business of the Company that are not a description of historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding the Company’s expected plans with respect to clinical trials of the Company’s gene therapy candidates and the timing for announcement of data from such trials. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, expectations regarding the initiation, progress, and expected results of the Company’s preclinical studies, clinical trials and research and development programs, the unpredictable relationship between preclinical study results and clinical study results, delays in submission of regulatory filings or failure to receive regulatory approval and liquidity and capital resources. Additional risks and uncertainties that could cause actual results to differ materially from those contemplated by the forward-looking statements are included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, Quarterly Report on Form 10-Q for the quarter ended June 30, 2024 and subsequent future filings the Company may make with the Securities and Exchange Commission from time to time that are available at www.sec.gov.
You are cautioned not to place undue reliance on forward-looking statements which are current only as of the date hereof. Except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Lexeo Therapeutics, Inc. |
|
|
|
|
Date: |
October 9, 2024 |
By: |
/s/ R. Nolan Townsend |
|
|
|
R. Nolan Townsend, Chief Executive Officer |

Lexeo Therapeutics�Corporate Overview���October 2024 Exhibit 99.1

Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, statements regarding Lexeo’s expectations and plans regarding its current product candidates and programs, including statements regarding the timing, progress and results of preclinical and clinical trials of Lexeo’s gene therapy product candidates, the anticipated benefits of its current product candidates and expected cash runway. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the U.S. Securities and Exchange Commission (SEC)), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward looking statements as a result of many factors, including but not limited to: risks and uncertainties related to expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 11, 2024, Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, filed with the SEC on August 12, 2024, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law.

Lexeo Therapeutics Team R. Nolan Townsend Chief Executive Officer Ronald Crystal, M.D. Founder & Chief Scientific Adviser Professor and Chairman, Weill Cornell Medicine Director, Belfer Gene Therapy Core Facility Steven Altschuler, M.D. Chairman Management team with broad leadership experience in gene therapy and rare disease Eric Adler, M.D. Chief Medical Officer and Head of Research Jenny R. Robertson Chief Business and Legal Officer Former Chief,�Pulmonary Branch Founder / Co-founder José Manuel Otero, Ph.D. Chief Technical Officer Management Team & Key Advisors Chair and Scientific Founder Sandi See Tai, M.D. Chief Development Officer Rajiv Patni, M.D. Senior Advisor to the CEO and Board of Directors
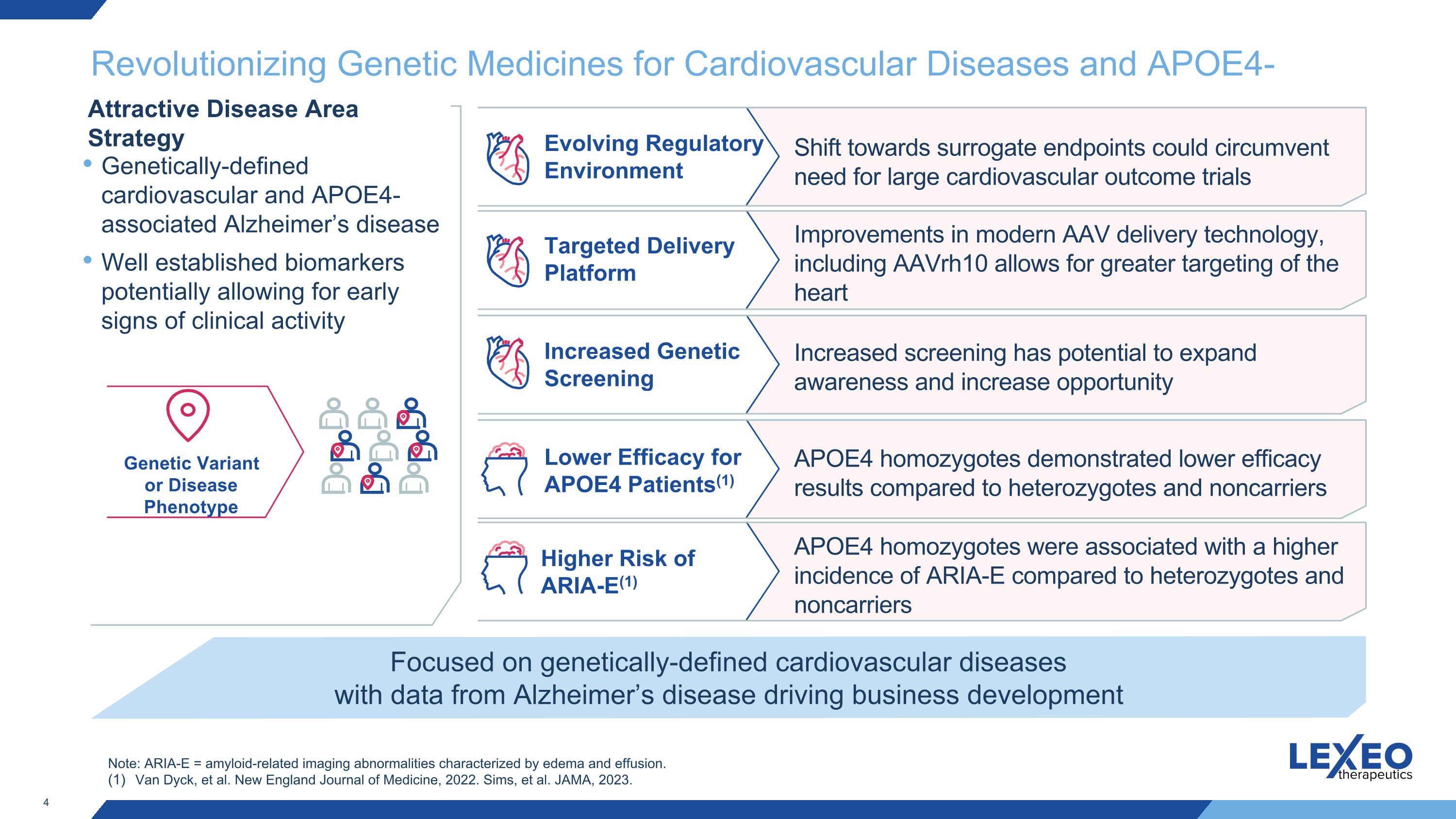
Revolutionizing Genetic Medicines for Cardiovascular Diseases and APOE4-Alzheimer’s Genetic Variant or Disease Phenotype Targeted Delivery Platform Shift towards surrogate endpoints could circumvent need for large cardiovascular outcome trials Improvements in modern AAV delivery technology, including AAVrh10 allows for greater targeting of the heart Increased Genetic Screening Increased screening has potential to expand awareness and increase opportunity Lower Efficacy for APOE4 Patients(1) APOE4 homozygotes demonstrated lower efficacy results compared to heterozygotes and noncarriers Focused on genetically-defined cardiovascular diseases with data from Alzheimer’s disease driving business development Attractive Disease Area Strategy Evolving Regulatory Environment Genetically-defined cardiovascular and APOE4-associated Alzheimer’s disease Well established biomarkers potentially allowing for early signs of clinical activity Higher Risk of ARIA-E(1) APOE4 homozygotes were associated with a higher incidence of ARIA-E compared to heterozygotes and noncarriers Note: ARIA-E = amyloid-related imaging abnormalities characterized by edema and effusion. Van Dyck, et al. New England Journal of Medicine, 2022. Sims, et al. JAMA, 2023.
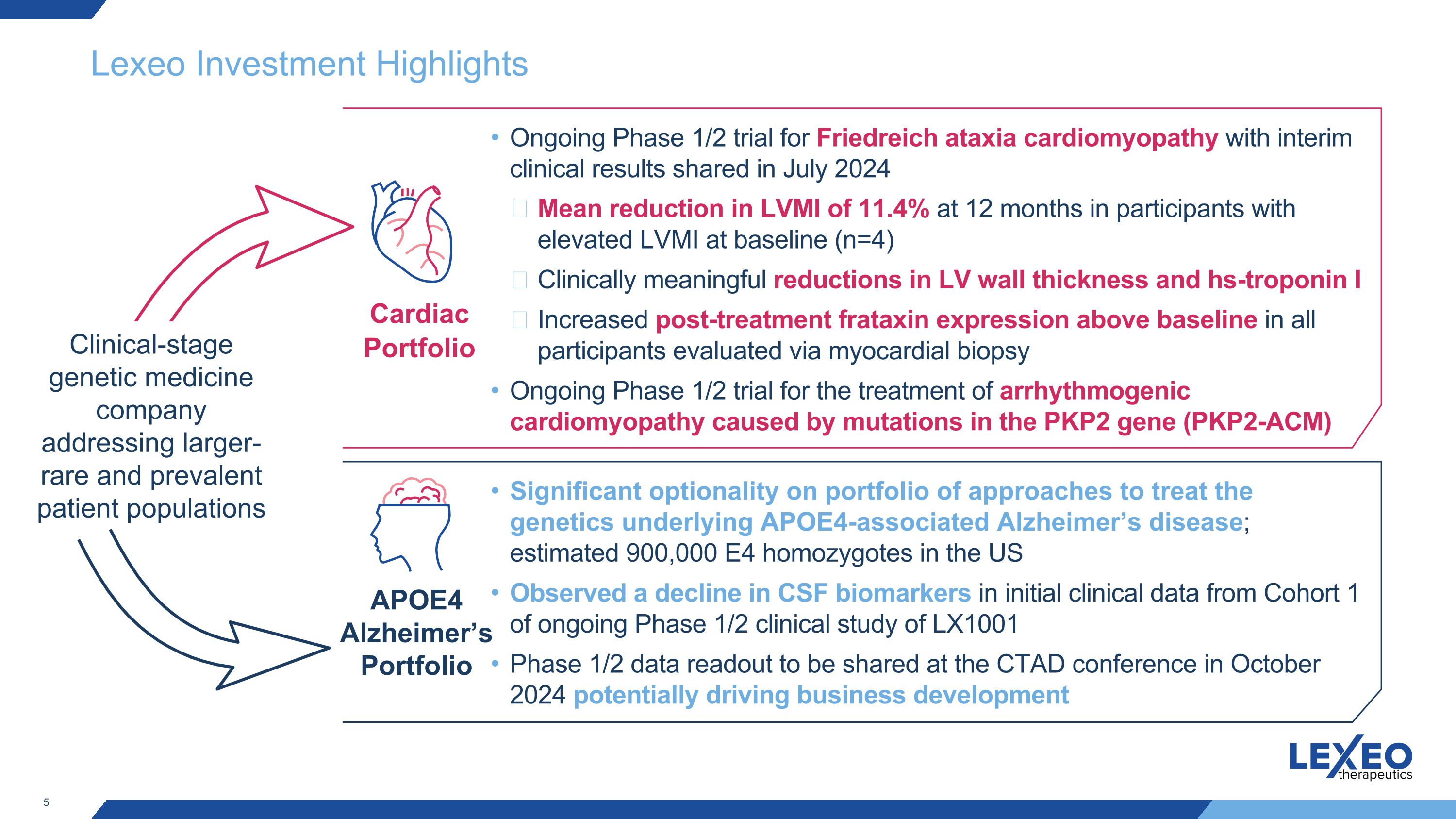
Lexeo Investment Highlights Clinical-stage genetic medicine company addressing larger-rare and prevalent patient populations Cardiac Portfolio APOE4 Alzheimer’s Portfolio Ongoing Phase 1/2 trial for Friedreich ataxia cardiomyopathy with interim clinical results shared in July 2024 Mean reduction in LVMI of 11.4% at 12 months in participants with elevated LVMI at baseline (n=4) Clinically meaningful reductions in LV wall thickness and hs-troponin I Increased post-treatment frataxin expression above baseline in all participants evaluated via myocardial biopsy Ongoing Phase 1/2 trial for the treatment of arrhythmogenic cardiomyopathy caused by mutations in the PKP2 gene (PKP2-ACM) Significant optionality on portfolio of approaches to treat the genetics underlying APOE4-associated Alzheimer’s disease; estimated 900,000 E4 homozygotes in the US Observed a decline in CSF biomarkers in initial clinical data from Cohort 1 of ongoing Phase 1/2 clinical study of LX1001 Phase 1/2 data readout to be shared at the CTAD conference in October 2024 potentially driving business development
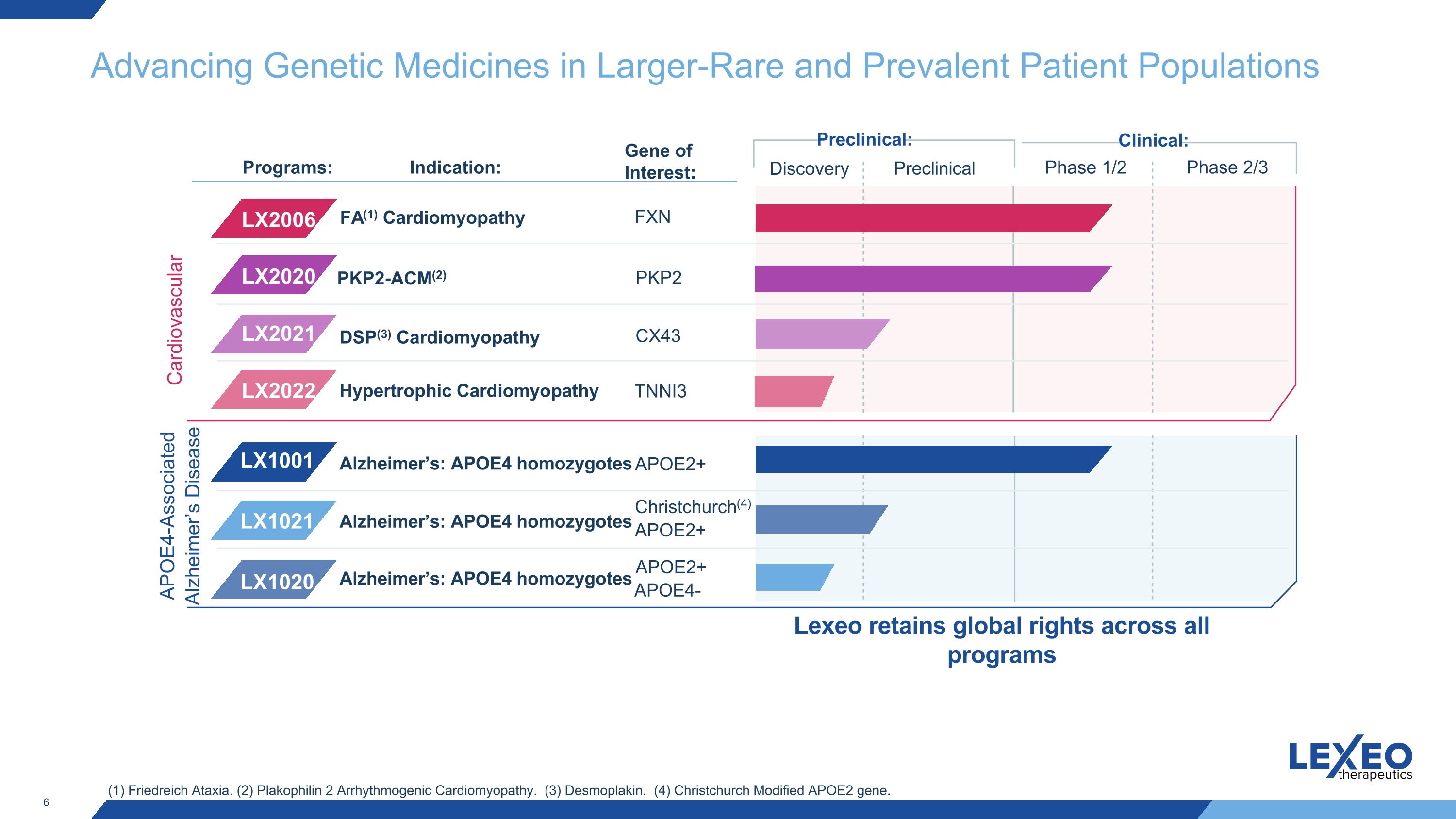
Advancing Genetic Medicines in Larger-Rare and Prevalent Patient Populations Clinical: Discovery Preclinical Phase 1/2 Phase 2/3 Preclinical: Cardiovascular Indication: LX1001 LX1020 LX1021 Programs: Alzheimer’s: APOE4 homozygotes APOE4-Associated Alzheimer’s Disease FA(1) Cardiomyopathy PKP2-ACM(2) FXN PKP2 APOE2+ Gene of Interest: LX2006 LX2020 APOE2+ Christchurch(4) APOE2+ Alzheimer’s: APOE4 homozygotes Alzheimer’s: APOE4 homozygotes APOE4- (1) Friedreich Ataxia. (2) Plakophilin 2 Arrhythmogenic Cardiomyopathy. (3) Desmoplakin. (4) Christchurch Modified APOE2 gene. CX43 LX2021 DSP(3) Cardiomyopathy Hypertrophic Cardiomyopathy TNNI3 LX2022 Lexeo retains global rights across all programs
Cardiovascular diseases 7
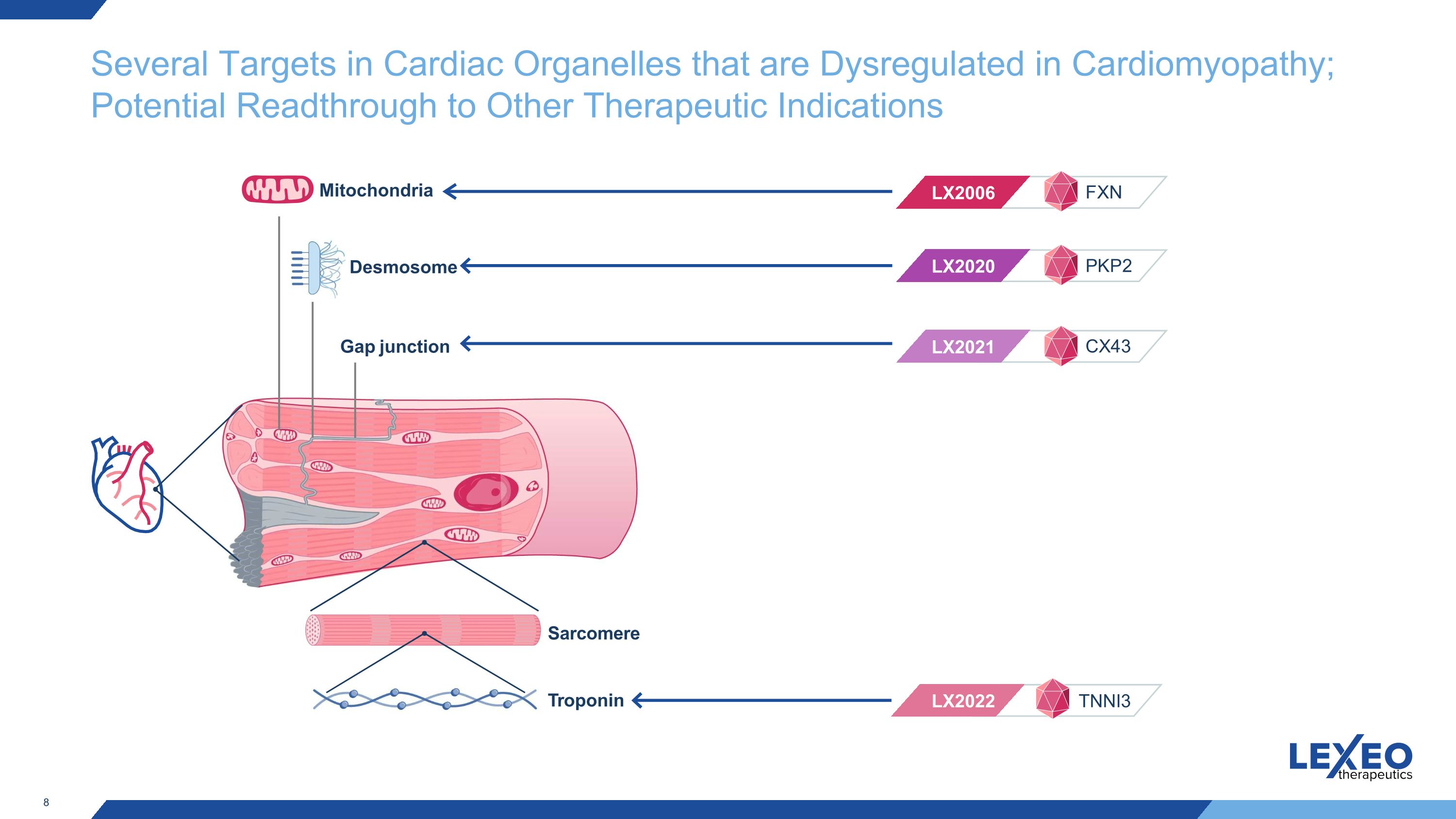
Several Targets in Cardiac Organelles that are Dysregulated in Cardiomyopathy; Potential Readthrough to Other Therapeutic Indications Troponin LX2020 LX2021 LX2006 LX2022 Desmosome Mitochondria Gap junction PKP2 CX43 FXN TNNI3 Sarcomere
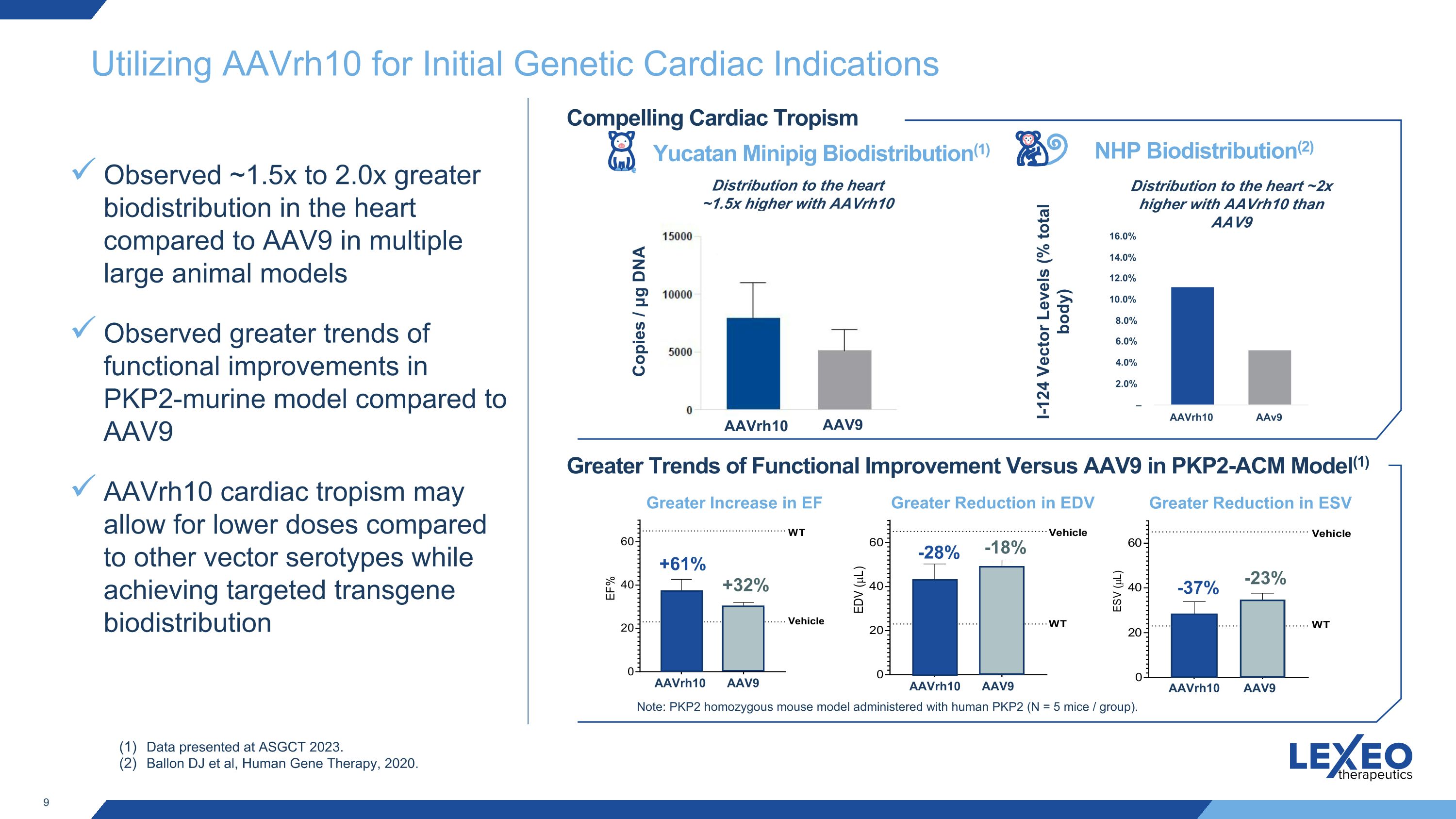
Utilizing AAVrh10 for Initial Genetic Cardiac Indications Observed ~1.5x to 2.0x greater biodistribution in the heart compared to AAV9 in multiple large animal models Observed greater trends of functional improvements in PKP2-murine model compared to AAV9 AAVrh10 cardiac tropism may allow for lower doses compared to other vector serotypes while achieving targeted transgene biodistribution Yucatan Minipig Biodistribution(1) NHP Biodistribution(2) I-124 Vector Levels (% total body) Data presented at ASGCT 2023. Ballon DJ et al, Human Gene Therapy, 2020. Compelling Cardiac Tropism Greater Trends of Functional Improvement Versus AAV9 in PKP2-ACM Model(1) Distribution to the heart ~1.5x higher with AAVrh10 than AAV9 Greater Increase in EF Greater Reduction in EDV Greater Reduction in ESV +61% +32% -28% -18% -37% -23% Note: PKP2 homozygous mouse model administered with human PKP2 (N = 5 mice / group). Copies / μg DNA Distribution to the heart ~2x higher with AAVrh10 than AAV9 AAVrh10 AAV9 AAVrh10 AAV9 AAVrh10 AAV9 AAVrh10 AAV9

LX2006 (FA Cardiomyopathy) 10
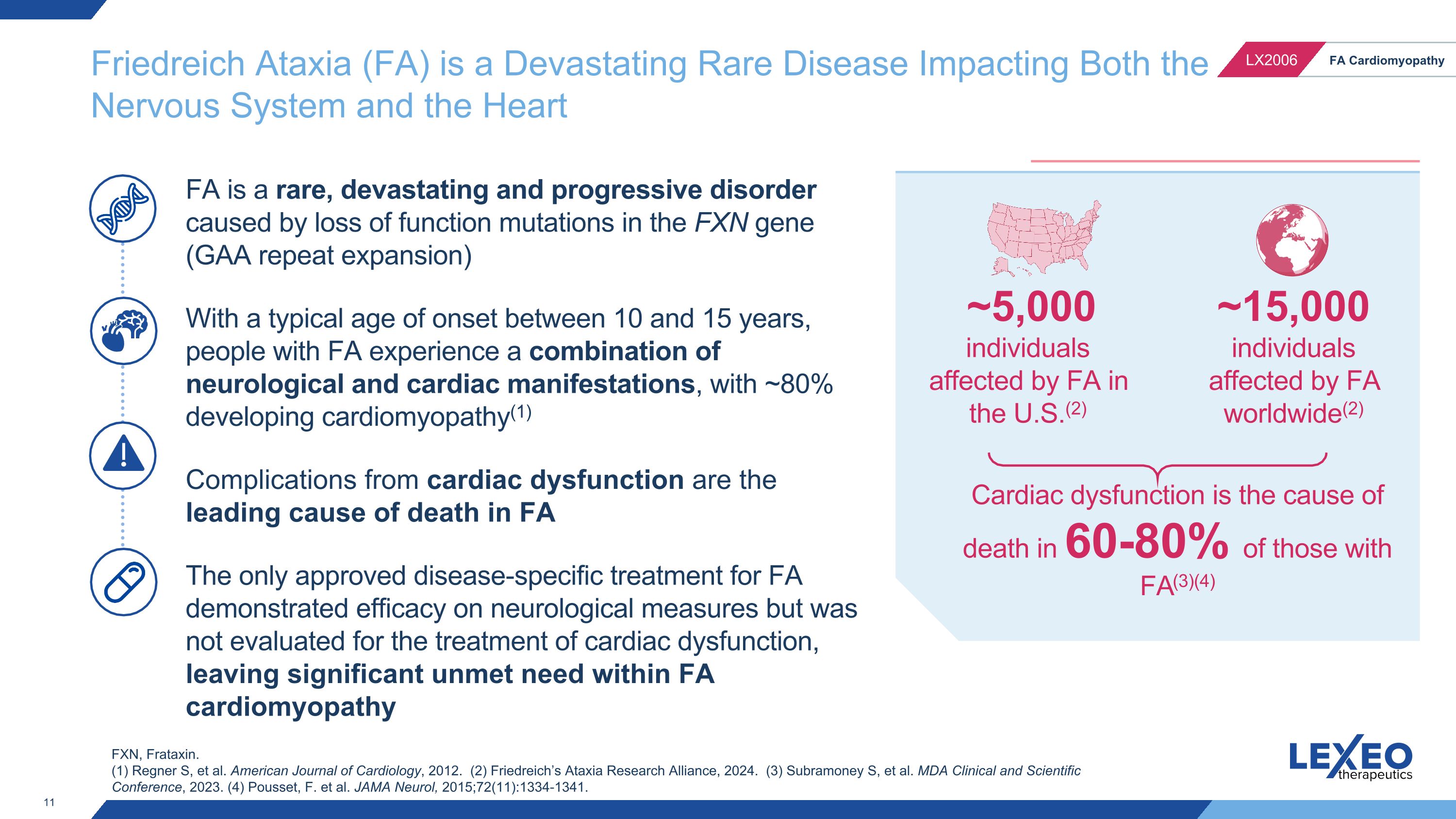
FA is a rare, devastating and progressive disorder caused by loss of function mutations in the FXN gene (GAA repeat expansion) With a typical age of onset between 10 and 15 years, people with FA experience a combination of neurological and cardiac manifestations, with ~80% developing cardiomyopathy(1) Complications from cardiac dysfunction are the leading cause of death in FA The only approved disease-specific treatment for FA demonstrated efficacy on neurological measures but was not evaluated for the treatment of cardiac dysfunction, leaving significant unmet need within FA cardiomyopathy Cardiac dysfunction is the cause of death in 60-80% of those with FA(3)(4) Friedreich Ataxia (FA) is a Devastating Rare Disease Impacting Both the �Nervous System and the Heart ~15,000 individuals affected by FA worldwide(2) ~5,000 individuals affected by FA in the U.S.(2) FXN, Frataxin. (1) Regner S, et al. American Journal of Cardiology, 2012. (2) Friedreich’s Ataxia Research Alliance, 2024. (3) Subramoney S, et al. MDA Clinical and Scientific Conference, 2023. (4) Pousset, F. et al. JAMA Neurol, 2015;72(11):1334-1341.
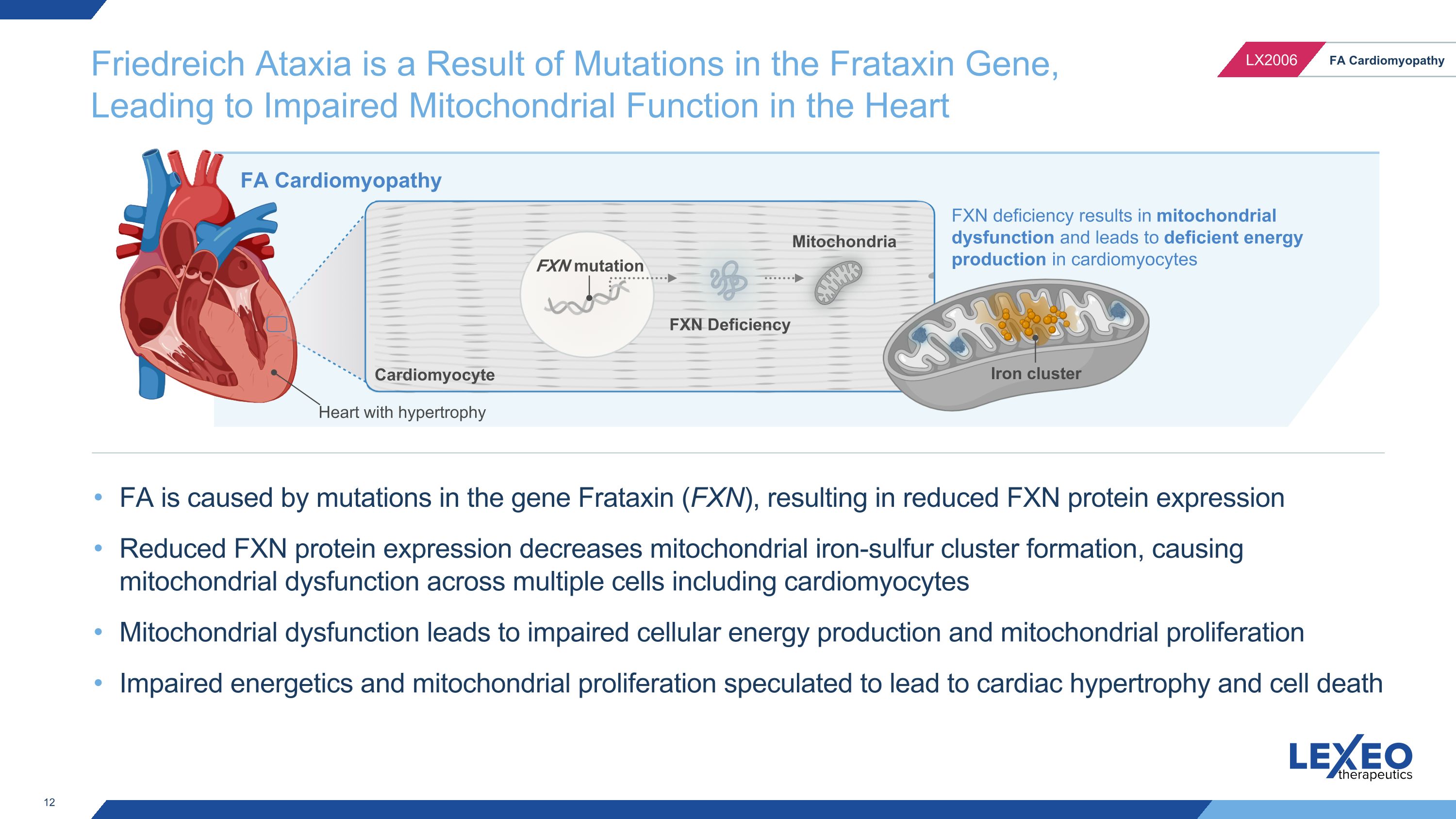
Friedreich Ataxia is a Result of Mutations in the Frataxin Gene, Leading to Impaired Mitochondrial Function in the Heart FXN mutation FXN Deficiency Mitochondria FA Cardiomyopathy Cardiomyocyte FXN deficiency results in mitochondrial dysfunction and leads to deficient energy production in cardiomyocytes Iron cluster Heart with hypertrophy FA is caused by mutations in the gene Frataxin (FXN), resulting in reduced FXN protein expression Reduced FXN protein expression decreases mitochondrial iron-sulfur cluster formation, causing mitochondrial dysfunction across multiple cells including cardiomyocytes Mitochondrial dysfunction leads to impaired cellular energy production and mitochondrial proliferation Impaired energetics and mitochondrial proliferation speculated to lead to cardiac hypertrophy and cell death
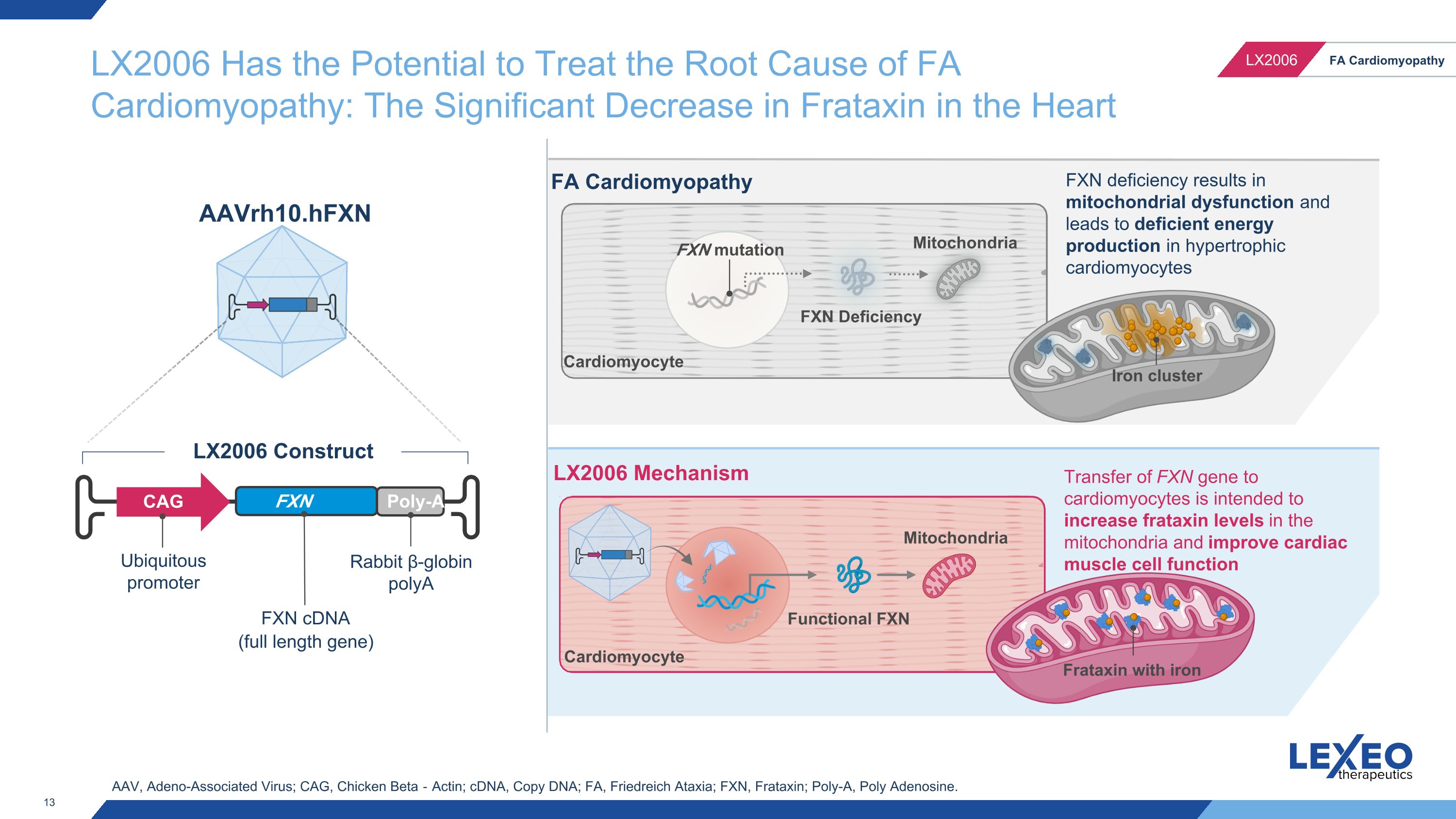
FXN mutation FXN Deficiency Mitochondria Functional FXN FA Cardiomyopathy LX2006 Mechanism Cardiomyocyte Transfer of FXN gene to cardiomyocytes is intended to increase frataxin levels in the mitochondria and improve cardiac muscle cell function FXN deficiency results in mitochondrial dysfunction and leads to deficient energy production in hypertrophic cardiomyocytes Mitochondria Cardiomyocyte AAV, Adeno-Associated Virus; CAG, Chicken Beta‐Actin; cDNA, Copy DNA; FA, Friedreich Ataxia; FXN, Frataxin; Poly-A, Poly Adenosine. LX2006 Has the Potential to Treat the Root Cause of FA Cardiomyopathy: The Significant Decrease in Frataxin in the Heart Ubiquitous promoter FXN cDNA (full length gene) CAG FXN gene Poly-A Rabbit β-globin polyA LX2006 Construct AAVrh10.hFXN Iron cluster Frataxin with iron
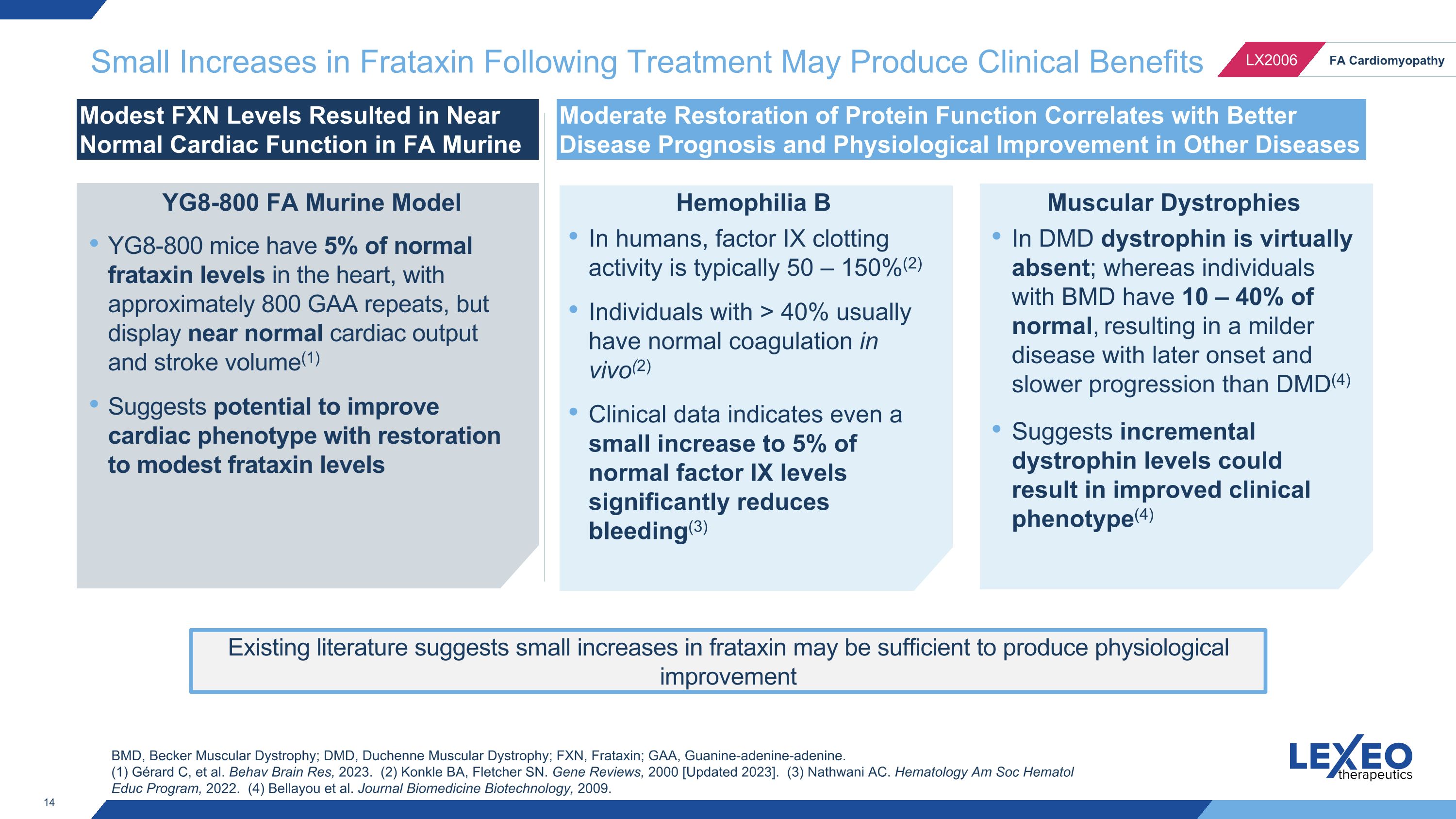
Small Increases in Frataxin Following Treatment May Produce Clinical Benefits Modest FXN Levels Resulted in Near Normal Cardiac Function in FA Murine Model Moderate Restoration of Protein Function Correlates with Better Disease Prognosis and Physiological Improvement in Other Diseases Hemophilia B Muscular Dystrophies BMD, Becker Muscular Dystrophy; DMD, Duchenne Muscular Dystrophy; FXN, Frataxin; GAA, Guanine-adenine-adenine. (1) Gérard C, et al. Behav Brain Res, 2023. (2) Konkle BA, Fletcher SN. Gene Reviews, 2000 [Updated 2023]. (3) Nathwani AC. Hematology Am Soc Hematol Educ Program, 2022. (4) Bellayou et al. Journal Biomedicine Biotechnology, 2009. Existing literature suggests small increases in frataxin may be sufficient to produce physiological improvement YG8-800 mice have 5% of normal frataxin levels in the heart, with approximately 800 GAA repeats, but display near normal cardiac output and stroke volume(1) Suggests potential to improve cardiac phenotype with restoration to modest frataxin levels YG8-800 FA Murine Model In humans, factor IX clotting activity is typically 50 – 150%(2) Individuals with > 40% usually have normal coagulation in vivo(2) Clinical data indicates even a small increase to 5% of normal factor IX levels significantly reduces bleeding(3) In DMD dystrophin is virtually absent; whereas individuals with BMD have 10 – 40% of normal, resulting in a milder disease with later onset and slower progression than DMD(4) Suggests incremental dystrophin levels could result in improved clinical phenotype(4)
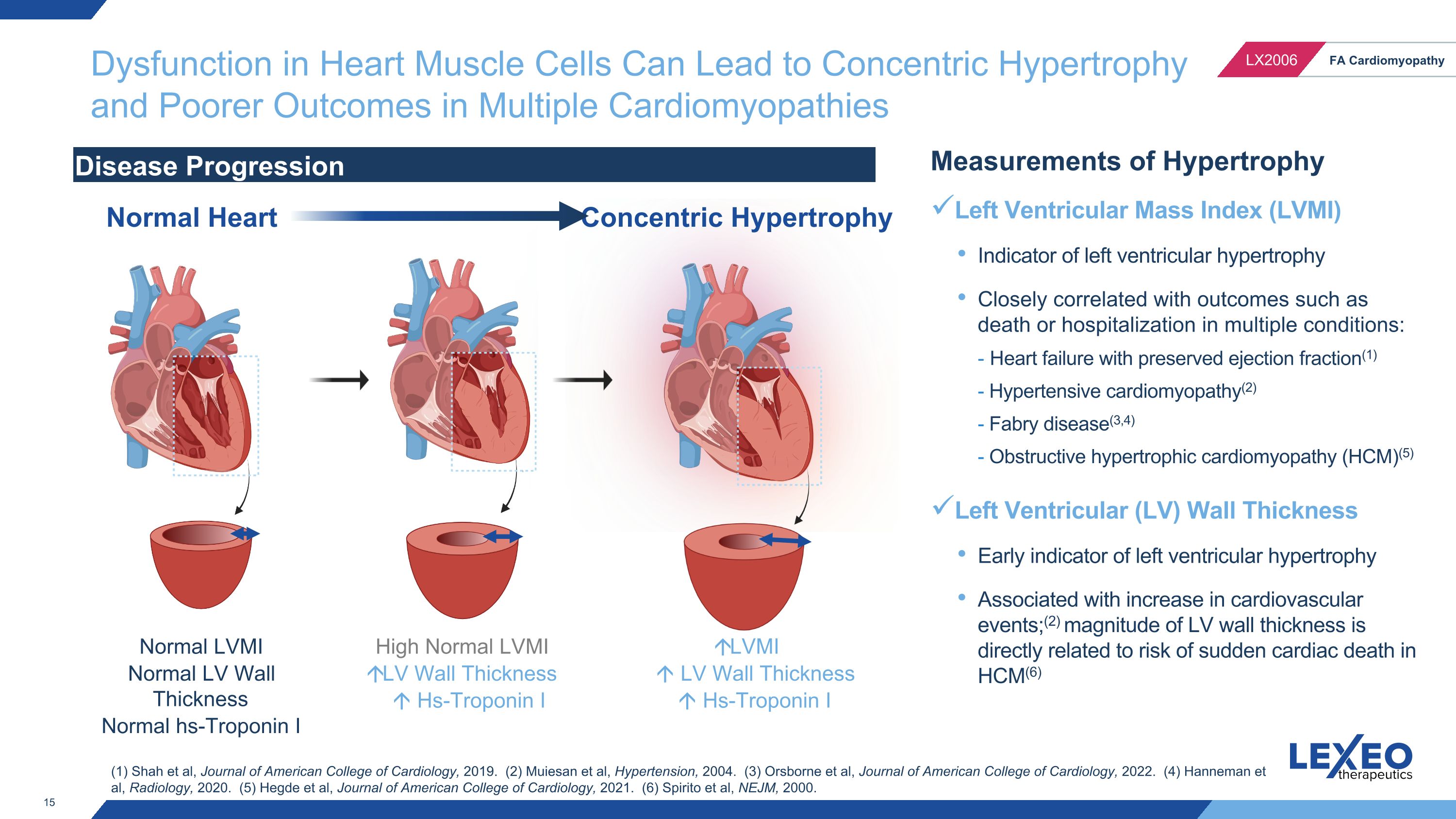
Dysfunction in Heart Muscle Cells Can Lead to Concentric Hypertrophy and Poorer Outcomes in Multiple Cardiomyopathies Normal LVMI Normal LV Wall Thickness Normal hs-Troponin I LVMI LV Wall Thickness Hs-Troponin I Disease Progression Normal Heart Concentric Hypertrophy Measurements of Hypertrophy Left Ventricular Mass Index (LVMI) Indicator of left ventricular hypertrophy Closely correlated with outcomes such as death or hospitalization in multiple conditions: - Heart failure with preserved ejection fraction(1) - Hypertensive cardiomyopathy(2) - Fabry disease(3,4) - Obstructive hypertrophic cardiomyopathy (HCM)(5) Left Ventricular (LV) Wall Thickness Early indicator of left ventricular hypertrophy Associated with increase in cardiovascular events;(2) magnitude of LV wall thickness is directly related to risk of sudden cardiac death in HCM(6) High Normal LVMI LV Wall Thickness Hs-Troponin I (1) Shah et al, Journal of American College of Cardiology, 2019. (2) Muiesan et al, Hypertension, 2004. (3) Orsborne et al, Journal of American College of Cardiology, 2022. (4) Hanneman et al, Radiology, 2020. (5) Hegde et al, Journal of American College of Cardiology, 2021. (6) Spirito et al, NEJM, 2000.
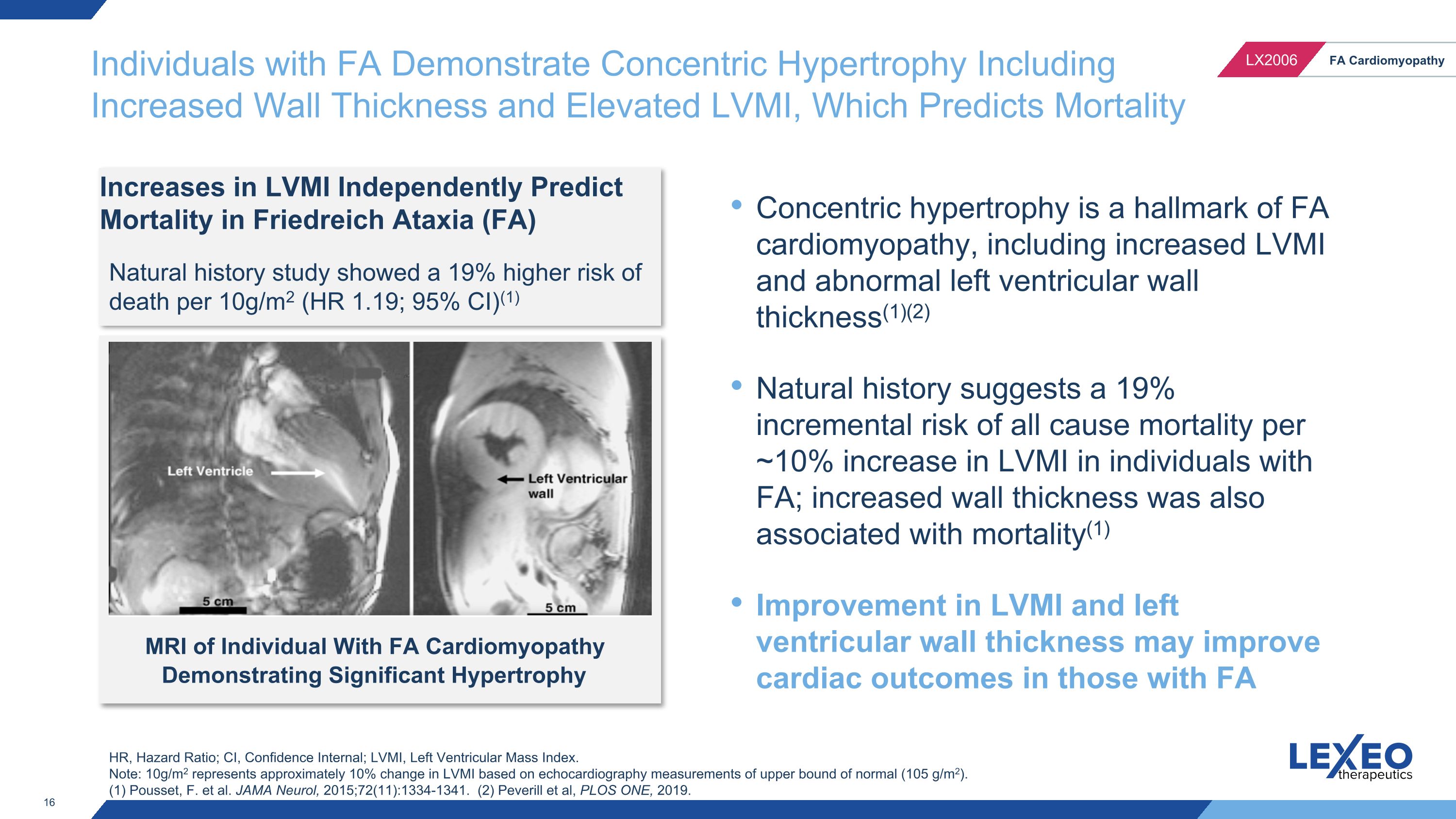
Individuals with FA Demonstrate Concentric Hypertrophy Including Increased Wall Thickness and Elevated LVMI, Which Predicts Mortality Increases in LVMI Independently Predict Mortality in Friedreich Ataxia (FA) Natural history study showed a 19% higher risk of death per 10g/m2 (HR 1.19; 95% CI)(1) Concentric hypertrophy is a hallmark of FA cardiomyopathy, including increased LVMI and abnormal left ventricular wall thickness(1)(2) Natural history suggests a 19% incremental risk of all cause mortality per ~10% increase in LVMI in individuals with FA; increased wall thickness was also associated with mortality(1) Improvement in LVMI and left ventricular wall thickness may improve cardiac outcomes in those with FA MRI of Individual With FA Cardiomyopathy Demonstrating Significant Hypertrophy HR, Hazard Ratio; CI, Confidence Internal; LVMI, Left Ventricular Mass Index. Note: 10g/m2 represents approximately 10% change in LVMI based on echocardiography measurements of upper bound of normal (105 g/m2). (1) Pousset, F. et al. JAMA Neurol, 2015;72(11):1334-1341. (2) Peverill et al, PLOS ONE, 2019.
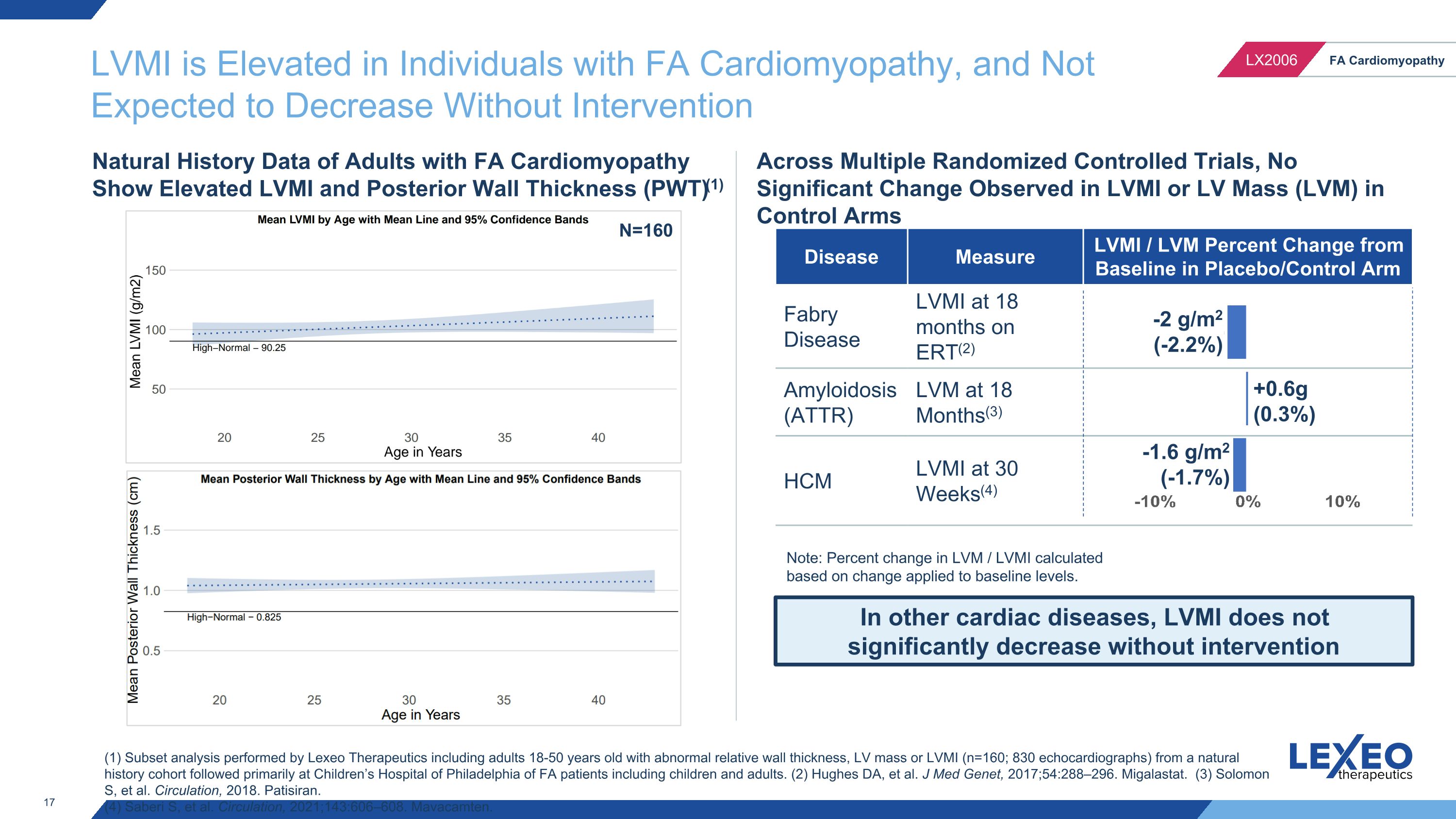
LVMI is Elevated in Individuals with FA Cardiomyopathy, and Not Expected to Decrease Without Intervention In other cardiac diseases, LVMI does not significantly decrease without intervention Across Multiple Randomized Controlled Trials, No Significant Change Observed in LVMI or LV Mass (LVM) in Control Arms Disease Measure LVMI / LVM Percent Change from Baseline in Placebo/Control Arm Fabry Disease LVMI at 18 months on ERT(2) Amyloidosis (ATTR) LVM at 18 Months(3) HCM LVMI at 30 Weeks(4) (1) Subset analysis performed by Lexeo Therapeutics including adults 18-50 years old with abnormal relative wall thickness, LV mass or LVMI (n=160; 830 echocardiographs) from a natural history cohort followed primarily at Children’s Hospital of Philadelphia of FA patients including children and adults. (2) Hughes DA, et al. J Med Genet, 2017;54:288–296. Migalastat. (3) Solomon S, et al. Circulation, 2018. Patisiran. (4) Saberi S, et al. Circulation, 2021;143:606–608. Mavacamten. Natural History Data of Adults with FA Cardiomyopathy Show Elevated LVMI and Posterior Wall Thickness (PWT)(1) -2 g/m2 (-2.2%) +0.6g (0.3%) -1.6 g/m2 (-1.7%) N=160 Note: Percent change in LVM / LVMI calculated based on change applied to baseline levels.

Recent LX2006 Program Updates In April 2024, Lexeo announced a license agreement with Cornell University for intellectual property rights including current and future clinical data from the ongoing Weill Cornell Medicine investigator-initiated trial of AAVrh10.hFXN (LX2006) Lexeo-sponsored SUNRISE-FA trial and Weill Cornell Medicine investigator-initiated trial utilize identical drug product manufactured at Weill Cornell for these ongoing studies Both studies share similar inclusion and exclusion criteria, however the Weill Cornell trial does not conduct cardiac biopsies
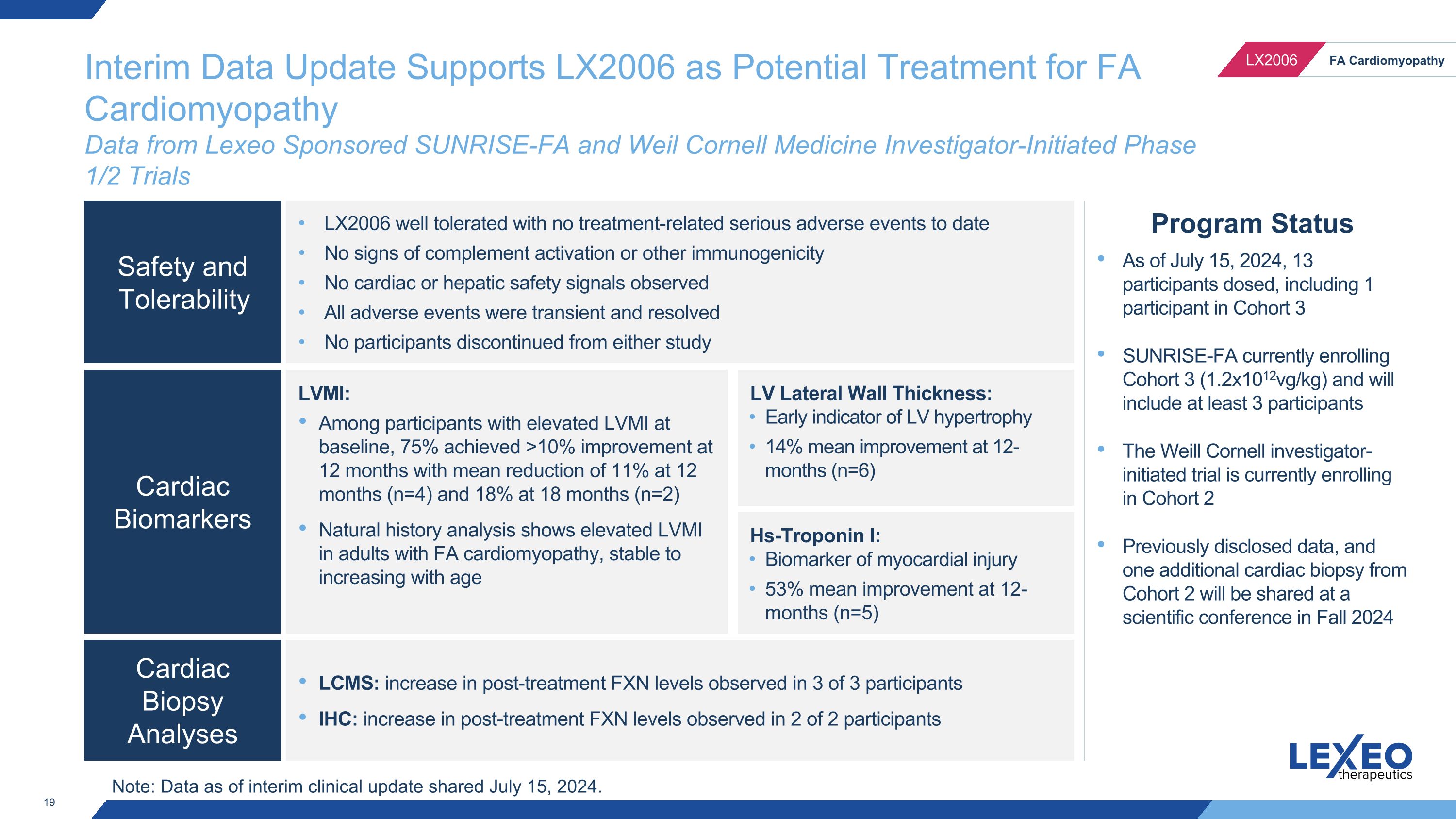
Interim Data Update Supports LX2006 as Potential Treatment for FA Cardiomyopathy�Data from Lexeo Sponsored SUNRISE-FA and Weil Cornell Medicine Investigator-Initiated Phase 1/2 Trials Program Status As of July 15, 2024, 13 participants dosed, including 1 participant in Cohort 3 SUNRISE-FA currently enrolling Cohort 3 (1.2x1012vg/kg) and will include at least 3 participants The Weill Cornell investigator-initiated trial is currently enrolling in Cohort 2 Previously disclosed data, and one additional cardiac biopsy from Cohort 2 will be shared at a scientific conference in Fall 2024 Safety and Tolerability Cardiac Biomarkers Cardiac Biopsy Analyses LX2006 well tolerated with no treatment-related serious adverse events to date No signs of complement activation or other immunogenicity No cardiac or hepatic safety signals observed All adverse events were transient and resolved No participants discontinued from either study LVMI: Among participants with elevated LVMI at baseline, 75% achieved >10% improvement at 12 months with mean reduction of 11% at 12 months (n=4) and 18% at 18 months (n=2) Natural history analysis shows elevated LVMI in adults with FA cardiomyopathy, stable to increasing with age LCMS: increase in post-treatment FXN levels observed in 3 of 3 participants IHC: increase in post-treatment FXN levels observed in 2 of 2 participants LV Lateral Wall Thickness: Early indicator of LV hypertrophy 14% mean improvement at 12-months (n=6) Hs-Troponin I: Biomarker of myocardial injury 53% mean improvement at 12-months (n=5) Note: Data as of interim clinical update shared July 15, 2024.
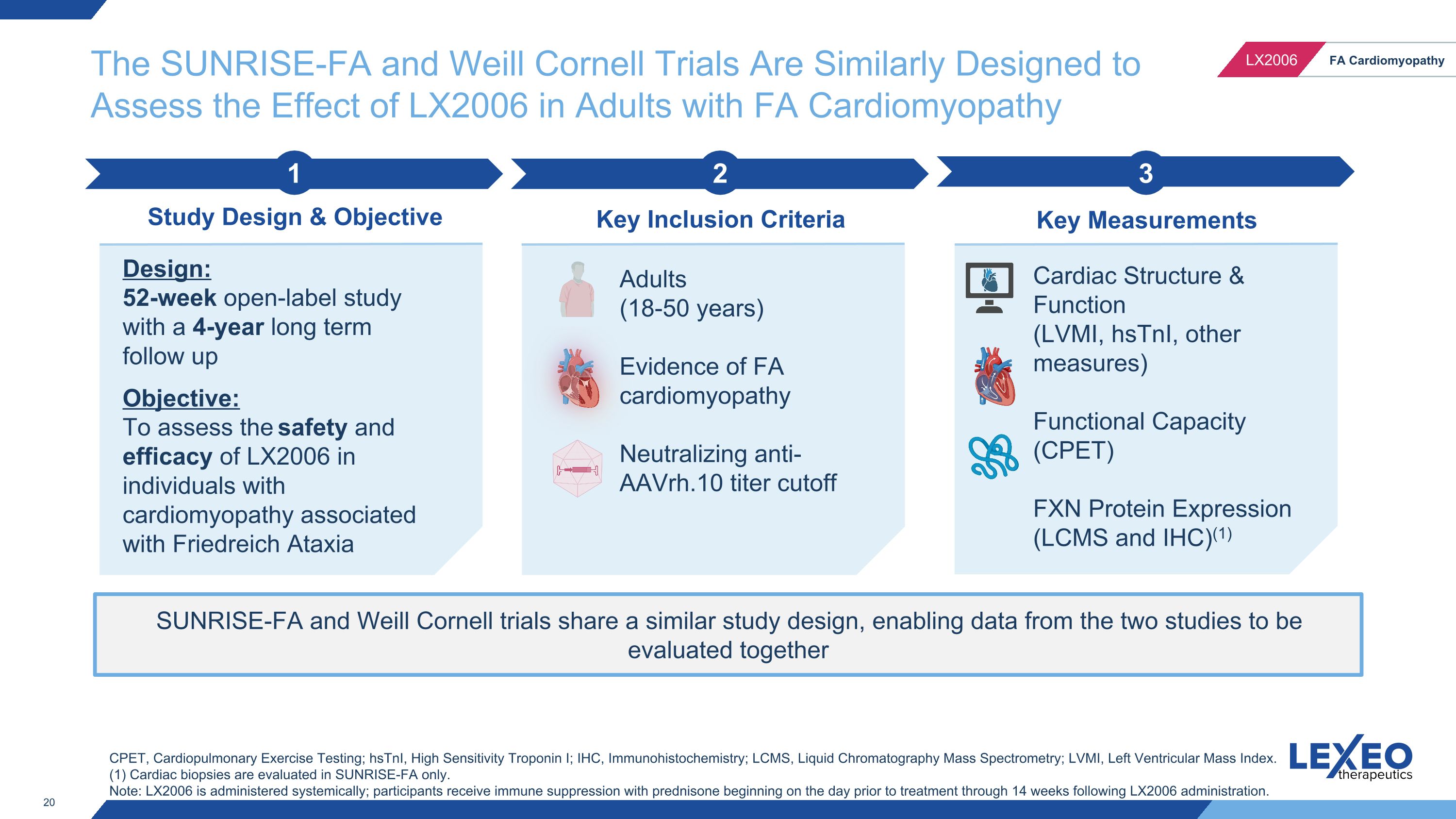
The SUNRISE-FA and Weill Cornell Trials Are Similarly Designed to Assess the Effect of LX2006 in Adults with FA Cardiomyopathy SUNRISE-FA and Weill Cornell trials share a similar study design, enabling data from the two studies to be evaluated together 2 Key Inclusion Criteria 3 Key Measurements 1 Study Design & Objective Design: 52-week open-label study with a 4-year long term follow up Objective: To assess the safety and efficacy of LX2006 in individuals with cardiomyopathy associated with Friedreich Ataxia Adults (18-50 years) Evidence of FA cardiomyopathy Neutralizing anti-AAVrh.10 titer cutoff Cardiac Structure & Function (LVMI, hsTnI, other measures) Functional Capacity (CPET) FXN Protein Expression (LCMS and IHC)(1) CPET, Cardiopulmonary Exercise Testing; hsTnI, High Sensitivity Troponin I; IHC, Immunohistochemistry; LCMS, Liquid Chromatography Mass Spectrometry; LVMI, Left Ventricular Mass Index. (1) Cardiac biopsies are evaluated in SUNRISE-FA only. Note: LX2006 is administered systemically; participants receive immune suppression with prednisone beginning on the day prior to treatment through 14 weeks following LX2006 administration.
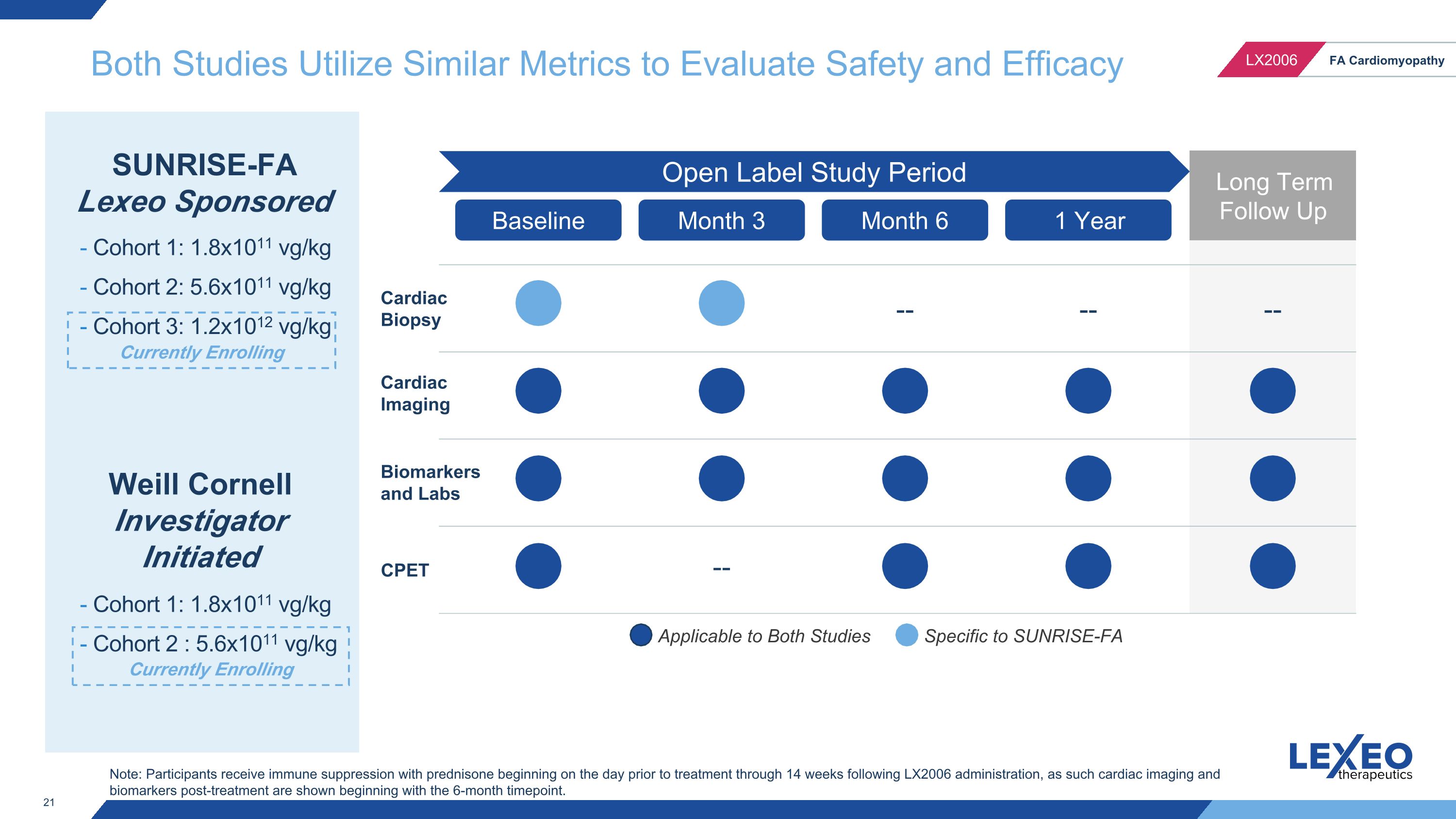
Both Studies Utilize Similar Metrics to Evaluate Safety and Efficacy Applicable to Both Studies Specific to SUNRISE-FA Baseline Month 6 1 Year Long Term Follow Up -- -- -- Cardiac Biopsy Cardiac Imaging Biomarkers and Labs CPET Open Label Study Period SUNRISE-FA Lexeo Sponsored - Cohort 1: 1.8x1011 vg/kg - Cohort 2: 5.6x1011 vg/kg - Cohort 3: 1.2x1012 vg/kg Weill Cornell Investigator Initiated - Cohort 1: 1.8x1011 vg/kg - Cohort 2 : 5.6x1011 vg/kg Month 3 -- Note: Participants receive immune suppression with prednisone beginning on the day prior to treatment through 14 weeks following LX2006 administration, as such cardiac imaging and biomarkers post-treatment are shown beginning with the 6-month timepoint. Currently Enrolling Currently Enrolling
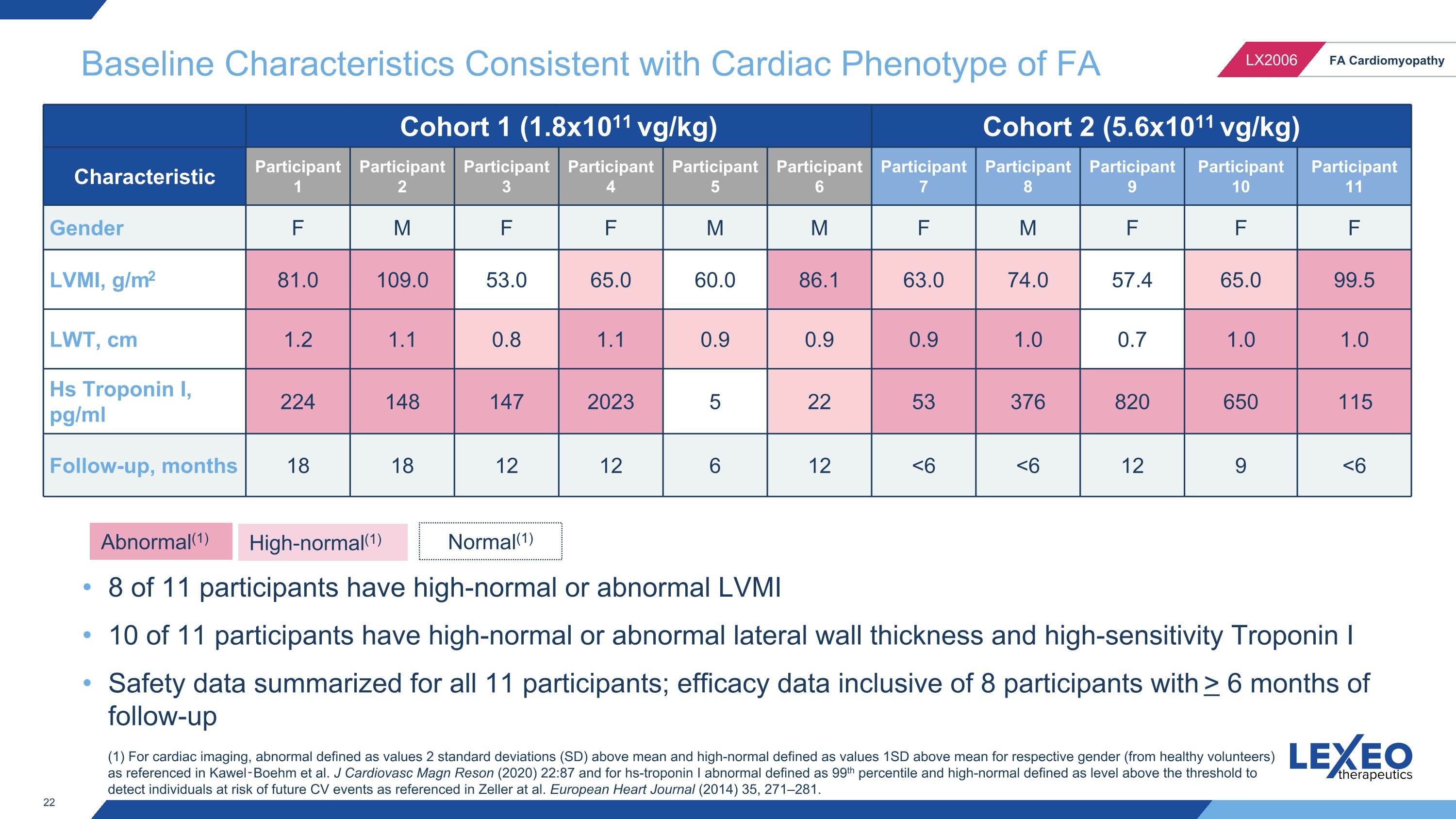
Baseline Characteristics Consistent with Cardiac Phenotype of FA Cohort 1 (1.8x1011 vg/kg) Cohort 2 (5.6x1011 vg/kg) Characteristic Participant 1 Participant 2 Participant 3 Participant 4 Participant 5 Participant 6 Participant 7 Participant 8 Participant 9 Participant 10 Participant 11 Gender F M F F M M F M F F F LVMI, g/m2 81.0 109.0 53.0 65.0 60.0 86.1 63.0 74.0 57.4 65.0 99.5 LWT, cm 1.2 1.1 0.8 1.1 0.9 0.9 0.9 1.0 0.7 1.0 1.0 Hs Troponin I, pg/ml 224 148 147 2023 5 22 53 376 820 650 115 Follow-up, months 18 18 12 12 6 12 <6 <6 12 9 <6 Abnormal(1) High-normal(1) (1) For cardiac imaging, abnormal defined as values 2 standard deviations (SD) above mean and high-normal defined as values 1SD above mean for respective gender (from healthy volunteers) as referenced in Kawel‑Boehm et al. J Cardiovasc Magn Reson (2020) 22:87 and for hs-troponin I abnormal defined as 99th percentile and high-normal defined as level above the threshold to detect individuals at risk of future CV events as referenced in Zeller at al. European Heart Journal (2014) 35, 271–281. 8 of 11 participants have high-normal or abnormal LVMI 10 of 11 participants have high-normal or abnormal lateral wall thickness and high-sensitivity Troponin I Safety data summarized for all 11 participants; efficacy data inclusive of 8 participants with > 6 months of follow-up Normal(1)

Treatment with LX2006 Has Been Well Tolerated to Date LX2006 has been well tolerated with no treatment-related serious adverse events No signs of complement activation or other immunogenicity No cardiac or hepatic safety signals All AEs were transient and resolved No participants discontinued from either study SUNRISE-FA independent Data and Safety Monitoring Board endorsed proceeding to Cohort 3 dose level (1.2x1012vg/kg) Note: Data as of interim clinical update shared July 15, 2024.
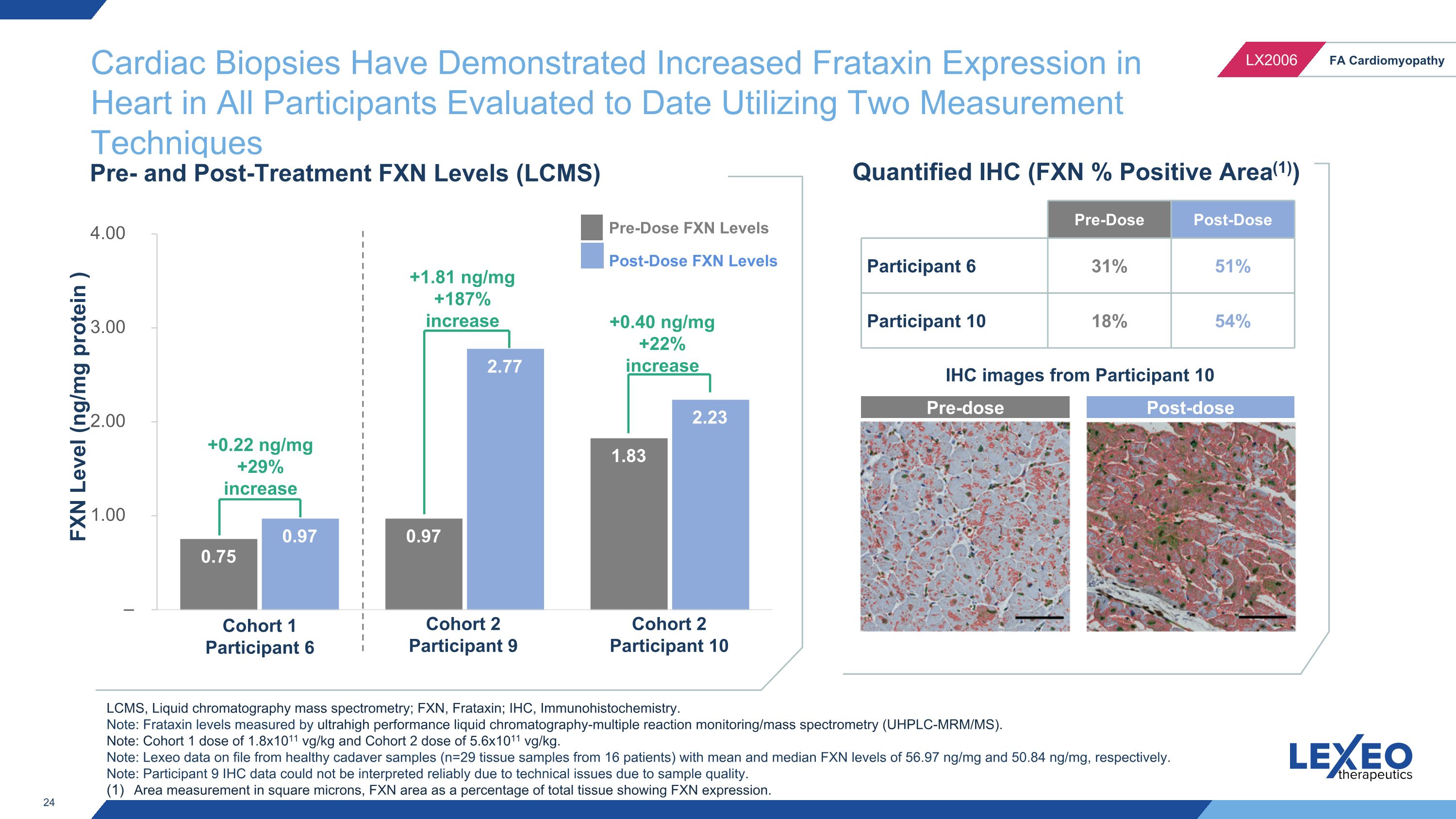
IHC images from Participant 10 Cardiac Biopsies Have Demonstrated Increased Frataxin Expression in Heart in All Participants Evaluated to Date Utilizing Two Measurement Techniques FXN Level (ng/mg protein ) Pre- and Post-Treatment FXN Levels (LCMS) Cohort 1 Participant 6 Cohort 2 Participant 9 Cohort 2 Participant 10 Pre-Dose FXN Levels Post-Dose FXN Levels Quantified IHC (FXN % Positive Area(1)) LCMS, Liquid chromatography mass spectrometry; FXN, Frataxin; IHC, Immunohistochemistry. Note: Frataxin levels measured by ultrahigh performance liquid chromatography-multiple reaction monitoring/mass spectrometry (UHPLC-MRM/MS). Note: Cohort 1 dose of 1.8x1011 vg/kg and Cohort 2 dose of 5.6x1011 vg/kg. Note: Lexeo data on file from healthy cadaver samples (n=29 tissue samples from 16 patients) with mean and median FXN levels of 56.97 ng/mg and 50.84 ng/mg, respectively. Note: Participant 9 IHC data could not be interpreted reliably due to technical issues due to sample quality. Area measurement in square microns, FXN area as a percentage of total tissue showing FXN expression. +0.22 ng/mg +29% increase +1.81 ng/mg +187% increase +0.40 ng/mg +22% increase Pre-dose Post-dose Pre-Dose Post-Dose Participant 6 31% 51% Participant 10 18% 54%
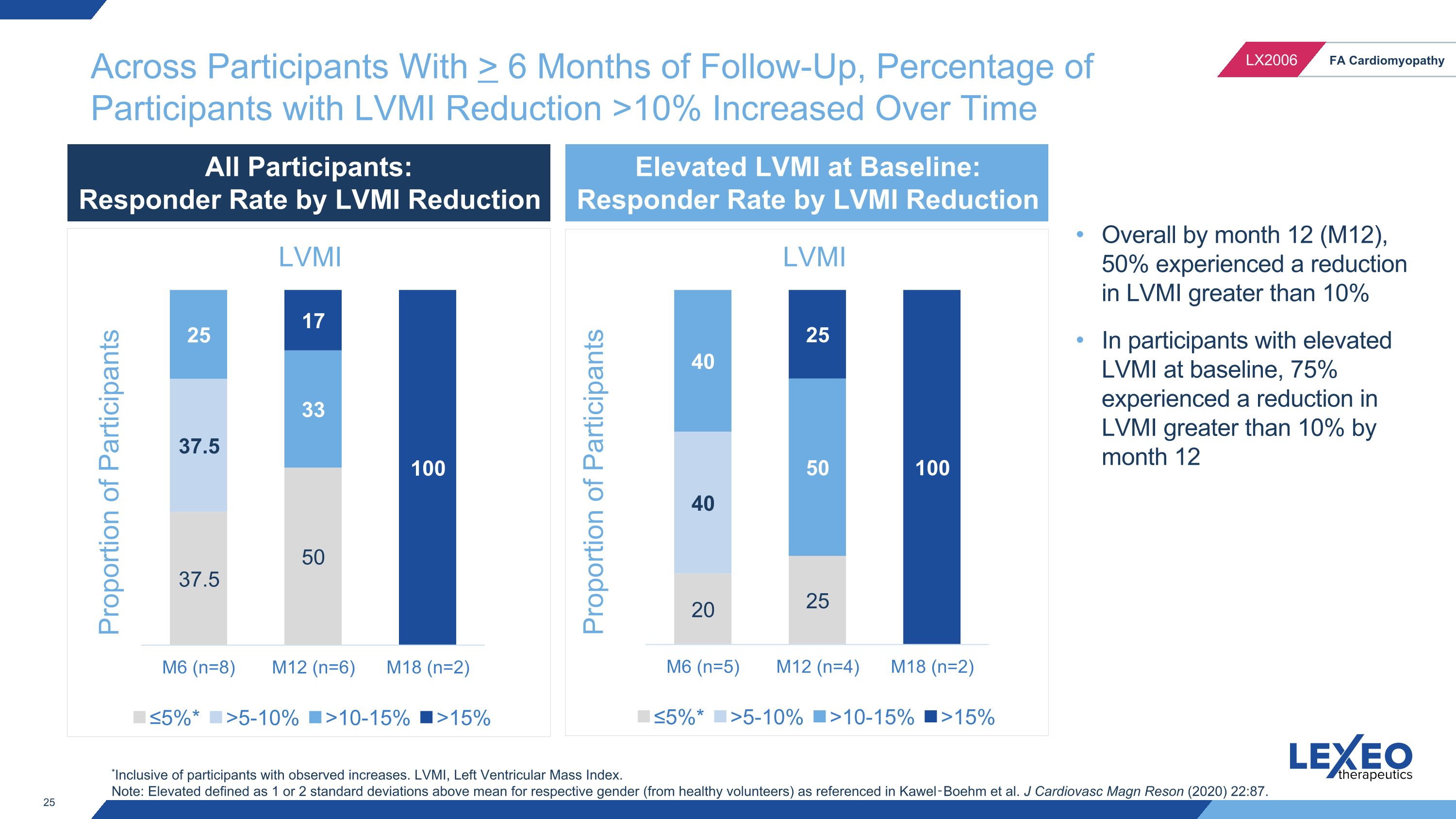
Across Participants With > 6 Months of Follow-Up, Percentage of Participants with LVMI Reduction >10% Increased Over Time Proportion of Participants *Inclusive of participants with observed increases. LVMI, Left Ventricular Mass Index. Note: Elevated defined as 1 or 2 standard deviations above mean for respective gender (from healthy volunteers) as referenced in Kawel‑Boehm et al. J Cardiovasc Magn Reson (2020) 22:87. Overall by month 12 (M12), 50% experienced a reduction in LVMI greater than 10% In participants with elevated LVMI at baseline, 75% experienced a reduction in LVMI greater than 10% by month 12 Proportion of Participants All Participants: Responder Rate by LVMI Reduction Elevated LVMI at Baseline: Responder Rate by LVMI Reduction
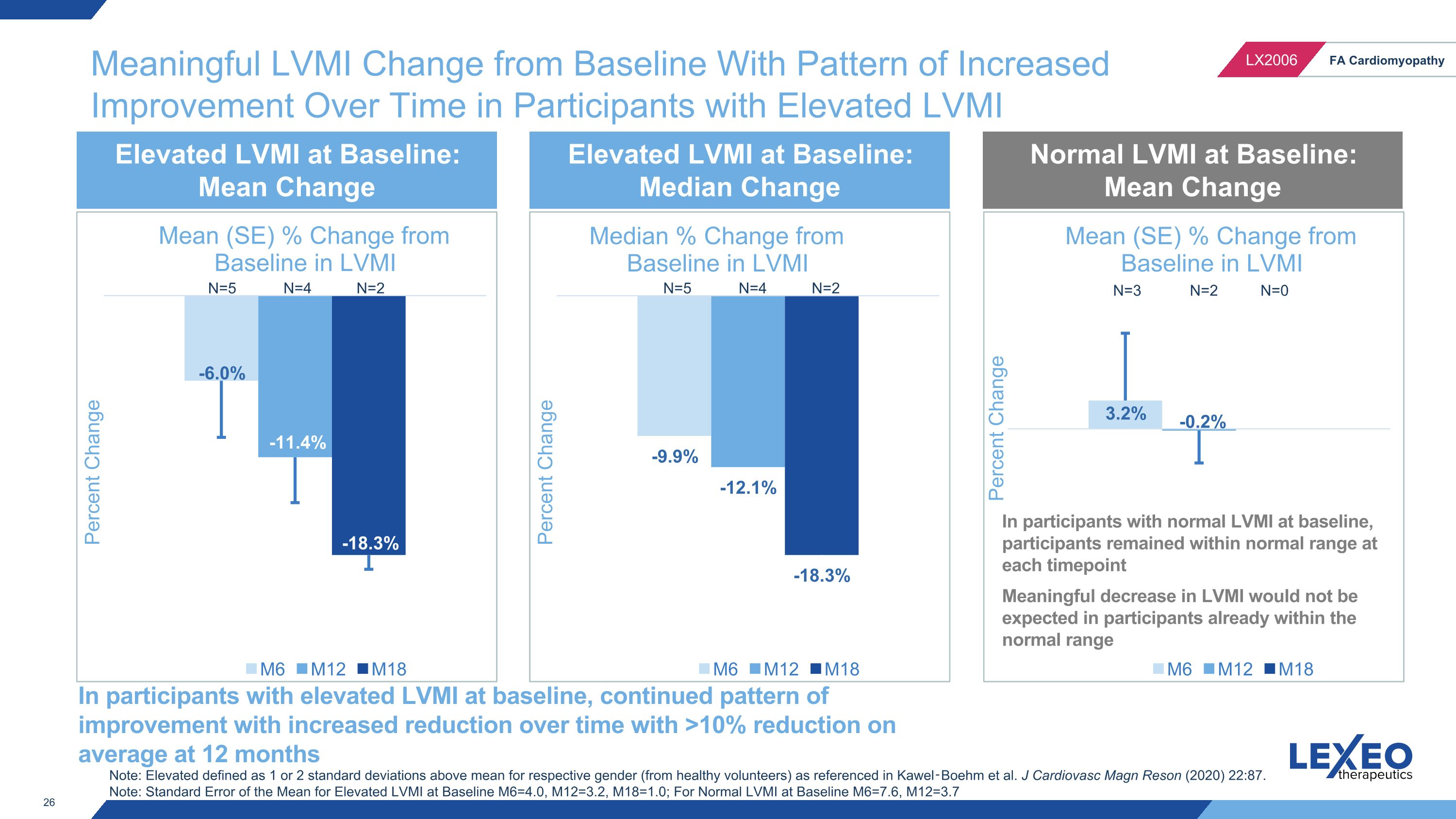
Meaningful LVMI Change from Baseline With Pattern of Increased Improvement Over Time in Participants with Elevated LVMI N=4 N=2 N=5 N=4 N=2 N=5 N=3 N=2 N=0 Elevated LVMI at Baseline: Mean Change Elevated LVMI at Baseline: Median Change Normal LVMI at Baseline: Mean Change In participants with elevated LVMI at baseline, continued pattern of improvement with increased reduction over time with >10% reduction on average at 12 months Note: Elevated defined as 1 or 2 standard deviations above mean for respective gender (from healthy volunteers) as referenced in Kawel‑Boehm et al. J Cardiovasc Magn Reson (2020) 22:87. Note: Standard Error of the Mean for Elevated LVMI at Baseline M6=4.0, M12=3.2, M18=1.0; For Normal LVMI at Baseline M6=7.6, M12=3.7 In participants with normal LVMI at baseline, participants remained within normal range at each timepoint Meaningful decrease in LVMI would not be expected in participants already within the normal range
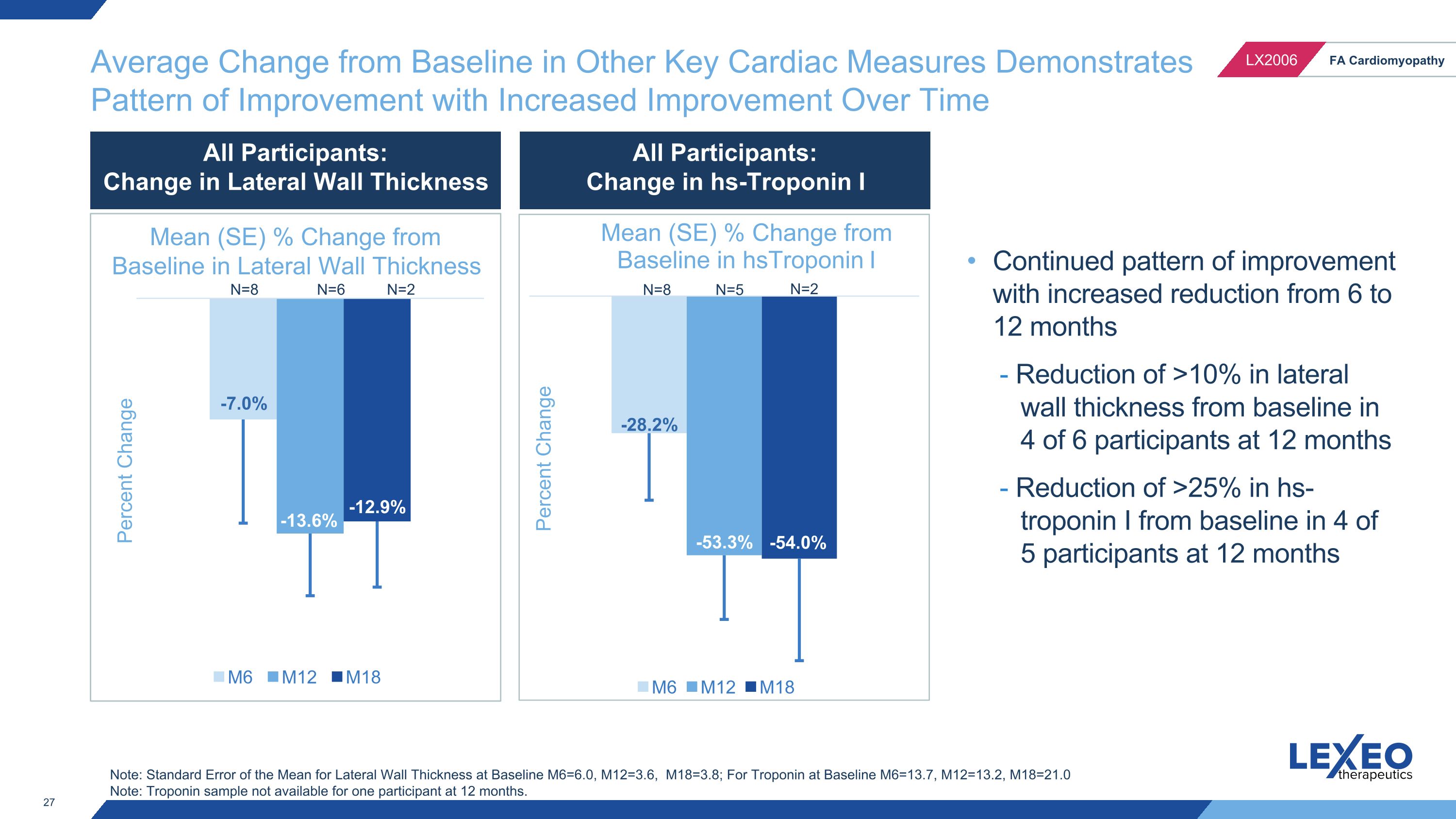
Average Change from Baseline in Other Key Cardiac Measures Demonstrates Pattern of Improvement with Increased Improvement Over Time N=8 N=5 N=2 Continued pattern of improvement with increased reduction from 6 to 12 months - Reduction of >10% in lateral wall thickness from baseline in 4 of 6 participants at 12 months - Reduction of >25% in hs-troponin I from baseline in 4 of 5 participants at 12 months Note: Standard Error of the Mean for Lateral Wall Thickness at Baseline M6=6.0, M12=3.6, M18=3.8; For Troponin at Baseline M6=13.7, M12=13.2, M18=21.0 Note: Troponin sample not available for one participant at 12 months. N=8 N=6 N=2 Percent Change All Participants: Change in Lateral Wall Thickness All Participants: Change in hs-Troponin I Mean (SE) % Change from Baseline in Lateral Wall Thickness
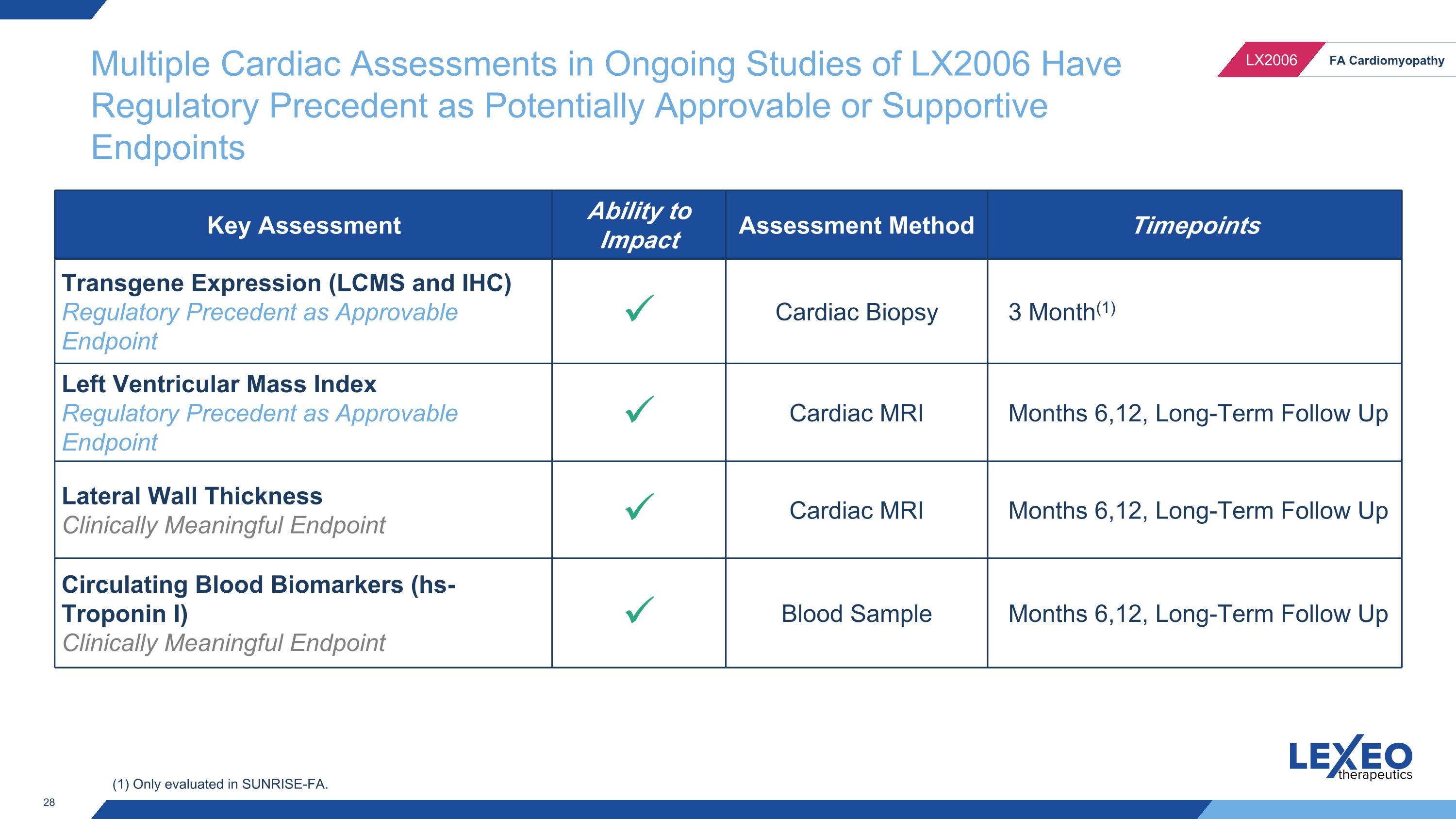
Multiple Cardiac Assessments in Ongoing Studies of LX2006 Have Regulatory Precedent as Potentially Approvable or Supportive Endpoints Key Assessment Ability to Impact Assessment Method Timepoints Transgene Expression (LCMS and IHC) Regulatory Precedent as Approvable Endpoint Cardiac Biopsy 3 Month(1) Left Ventricular Mass Index Regulatory Precedent as Approvable Endpoint Cardiac MRI Months 6,12, Long-Term Follow Up Lateral Wall Thickness Clinically Meaningful Endpoint Cardiac MRI Months 6,12, Long-Term Follow Up Circulating Blood Biomarkers (hs-Troponin I) Clinically Meaningful Endpoint Blood Sample Months 6,12, Long-Term Follow Up (1) Only evaluated in SUNRISE-FA.

Summary of Results and Next Steps for LX2006 LX2006 (AAVrh10.hFXN) has been well tolerated with no treatment-related serious adverse events to date Improvements in key clinical parameters observed at 12-months: - 75% of participants with elevated LVMI at baseline experienced >10% reduction in LVMI (n=4) - 14% mean reduction from baseline in lateral left ventricular wall thickness (n=6) - 53% mean reduction from baseline in hs-troponin I (n=5) As of July 15, 2024, 13 participants dosed, including 1 participant in Cohort 3 - SUNRISE-FA Data and Safety Monitoring Board endorsed proceeding to Cohort 3 dose level (1.2x1012vg/kg); this cohort has started enrollment with 1 participant dosed, and will include at least 3 participants - The Weill Cornell investigator-initiated trial is currently enrolling in Cohort 2 Previously disclosed data, and one additional cardiac biopsy from Cohort 2 will be shared at a scientific conference in Fall 2024 Note: Data as of interim clinical update shared July 15, 2024.

LX2020 (PKP2-ACM) 30
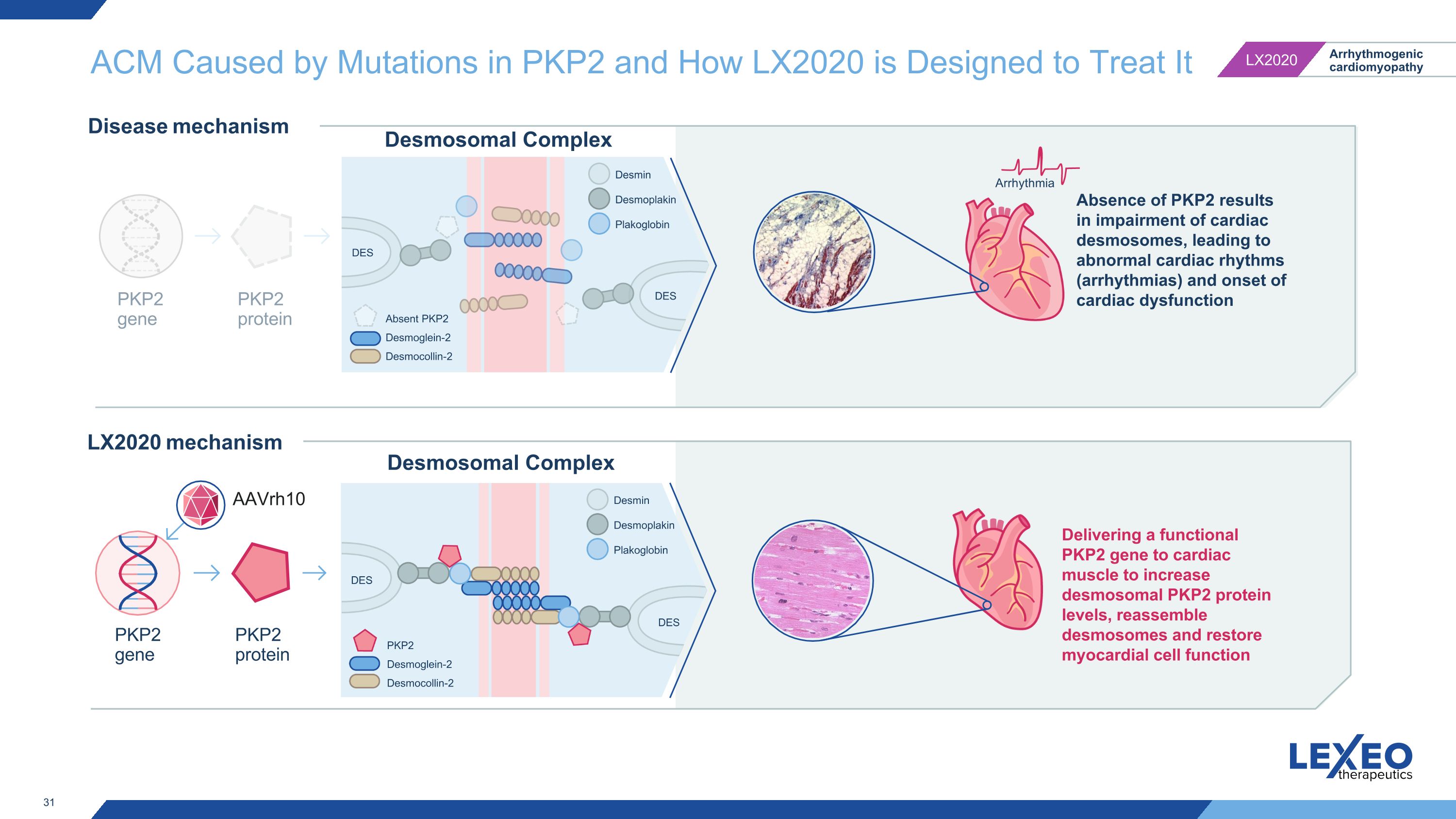
ACM Caused by Mutations in PKP2 and How LX2020 is Designed to Treat It Arrhythmia Desmosomal Complex PKP2 gene PKP2 protein PKP2 gene PKP2 protein AAVrh10 Desmosomal Complex Delivering a functional PKP2 gene to cardiac muscle to increase desmosomal PKP2 protein levels, reassemble desmosomes and restore myocardial cell function LX2020 mechanism Disease mechanism Absence of PKP2 results in impairment of cardiac desmosomes, leading to abnormal cardiac rhythms (arrhythmias) and onset of cardiac dysfunction DES DES Absent PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin DES DES PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin
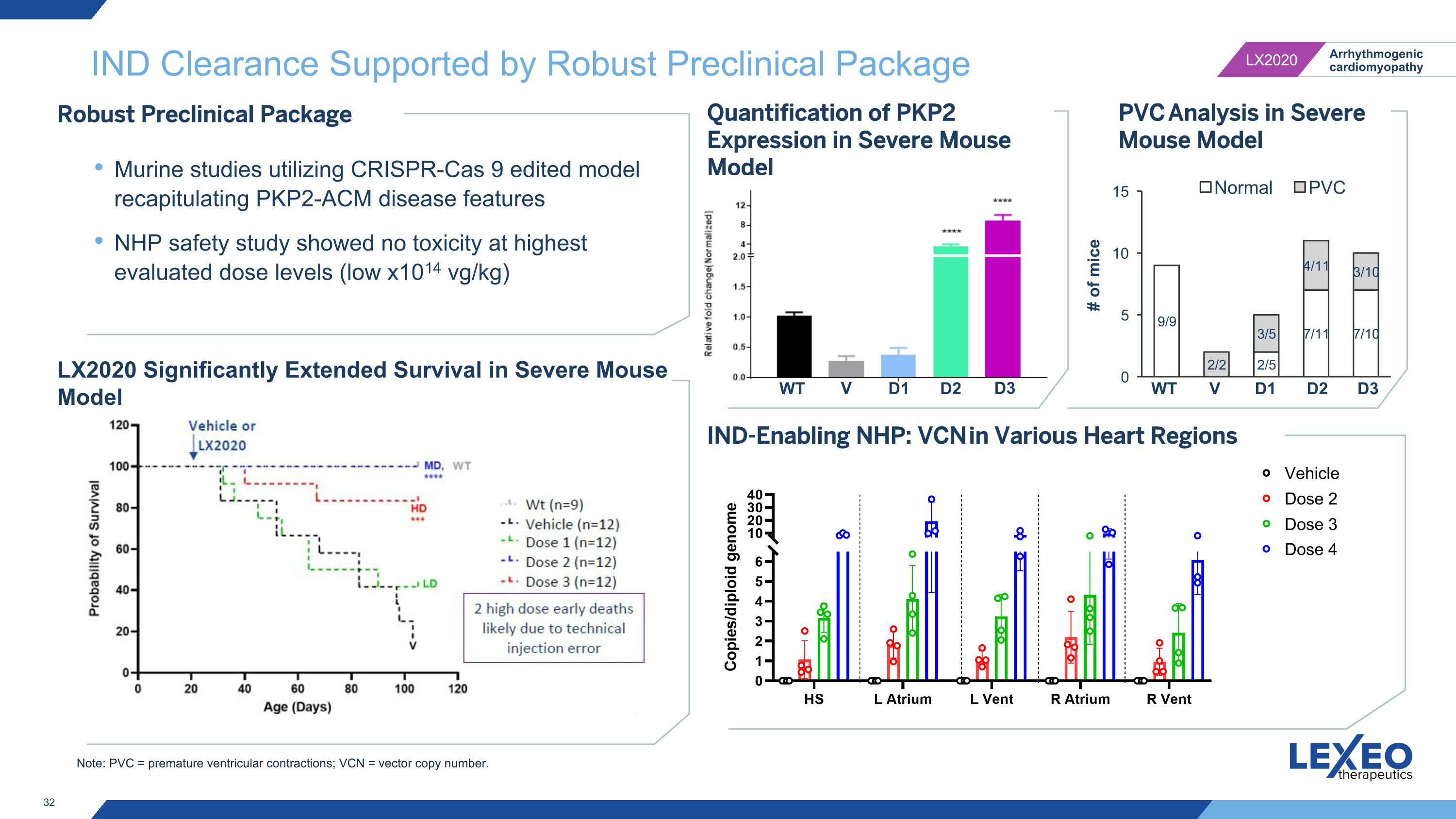
Note: PVC = premature ventricular contractions; VCN = vector copy number. IND Clearance Supported by Robust Preclinical Package IND-Enabling NHP: VCN in Various Heart Regions Robust Preclinical Package Murine studies utilizing CRISPR-Cas 9 edited model recapitulating PKP2-ACM disease features NHP safety study showed no toxicity at highest evaluated dose levels (low x1014 vg/kg) LX2020 Significantly Extended Survival in Severe Mouse Model Quantification of PKP2 Expression in Severe Mouse Model D1 D2 D3 V WT PVC Analysis in Severe Mouse Model V WT Vehicle Dose 2 Dose 3 Dose 4 D1 D2 D3
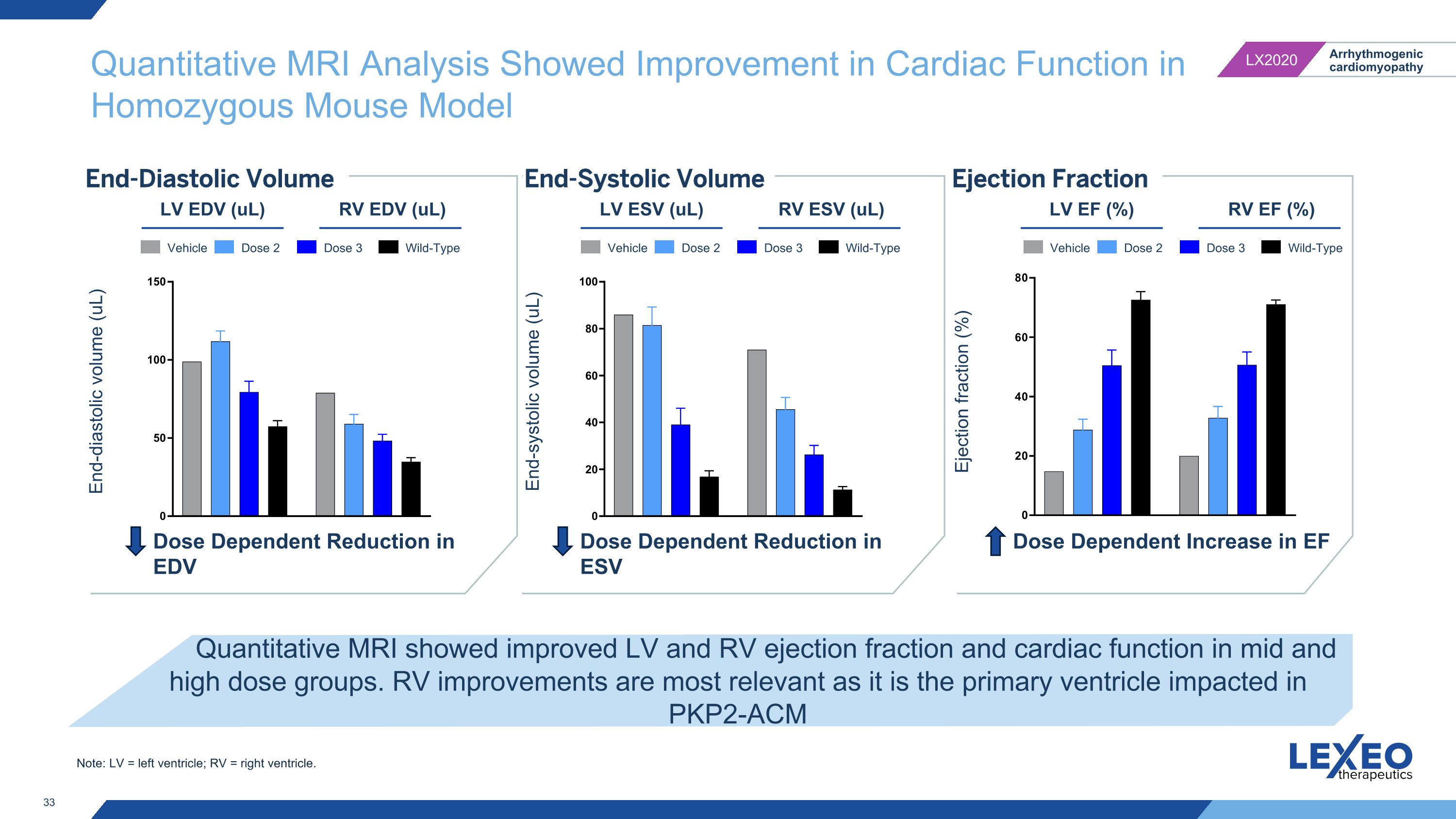
Quantitative MRI Analysis Showed Improvement in Cardiac Function in Homozygous Mouse Model End-Diastolic Volume Quantitative MRI showed improved LV and RV ejection fraction and cardiac function in mid and high dose groups. RV improvements are most relevant as it is the primary ventricle impacted in PKP2-ACM End-Systolic Volume Ejection Fraction LV EDV (uL) RV EDV (uL) End-diastolic volume (uL) End-systolic volume (uL) Ejection fraction (%) LV ESV (uL) RV ESV (uL) LV EF (%) RV EF (%) Dose Dependent Reduction in EDV Dose Dependent Reduction in ESV Dose Dependent Increase in EF Vehicle Dose 2 Dose 3 Wild-Type Vehicle Dose 2 Dose 3 Wild-Type Vehicle Dose 2 Dose 3 Wild-Type Note: LV = left ventricle; RV = right ventricle.
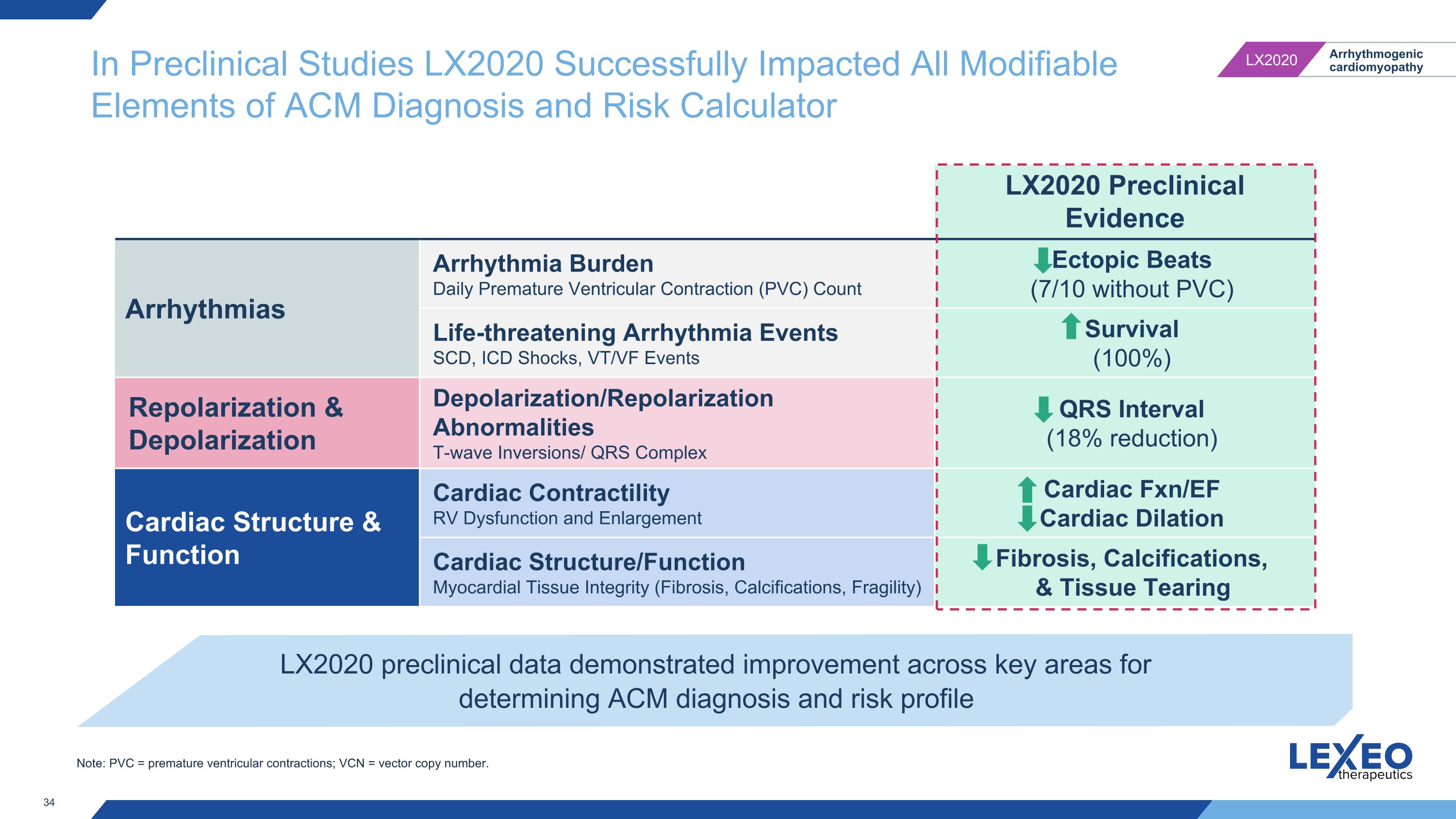
In Preclinical Studies LX2020 Successfully Impacted All Modifiable Elements of ACM Diagnosis and Risk Calculator LX2020 Preclinical Evidence Arrhythmias Arrhythmia Burden Daily Premature Ventricular Contraction (PVC) Count Ectopic Beats�(7/10 without PVC) Life-threatening Arrhythmia Events SCD, ICD Shocks, VT/VF Events Survival (100%) Repolarization & Depolarization Depolarization/Repolarization Abnormalities T-wave Inversions/ QRS Complex QRS Interval (18% reduction) Cardiac Structure & Function Cardiac Contractility RV Dysfunction and Enlargement Cardiac Fxn/EF Cardiac Dilation Cardiac Structure/Function Myocardial Tissue Integrity (Fibrosis, Calcifications, Fragility) Fibrosis, Calcifications, & Tissue Tearing LX2020 preclinical data demonstrated improvement across key areas for determining ACM diagnosis and risk profile Note: PVC = premature ventricular contractions; VCN = vector copy number.
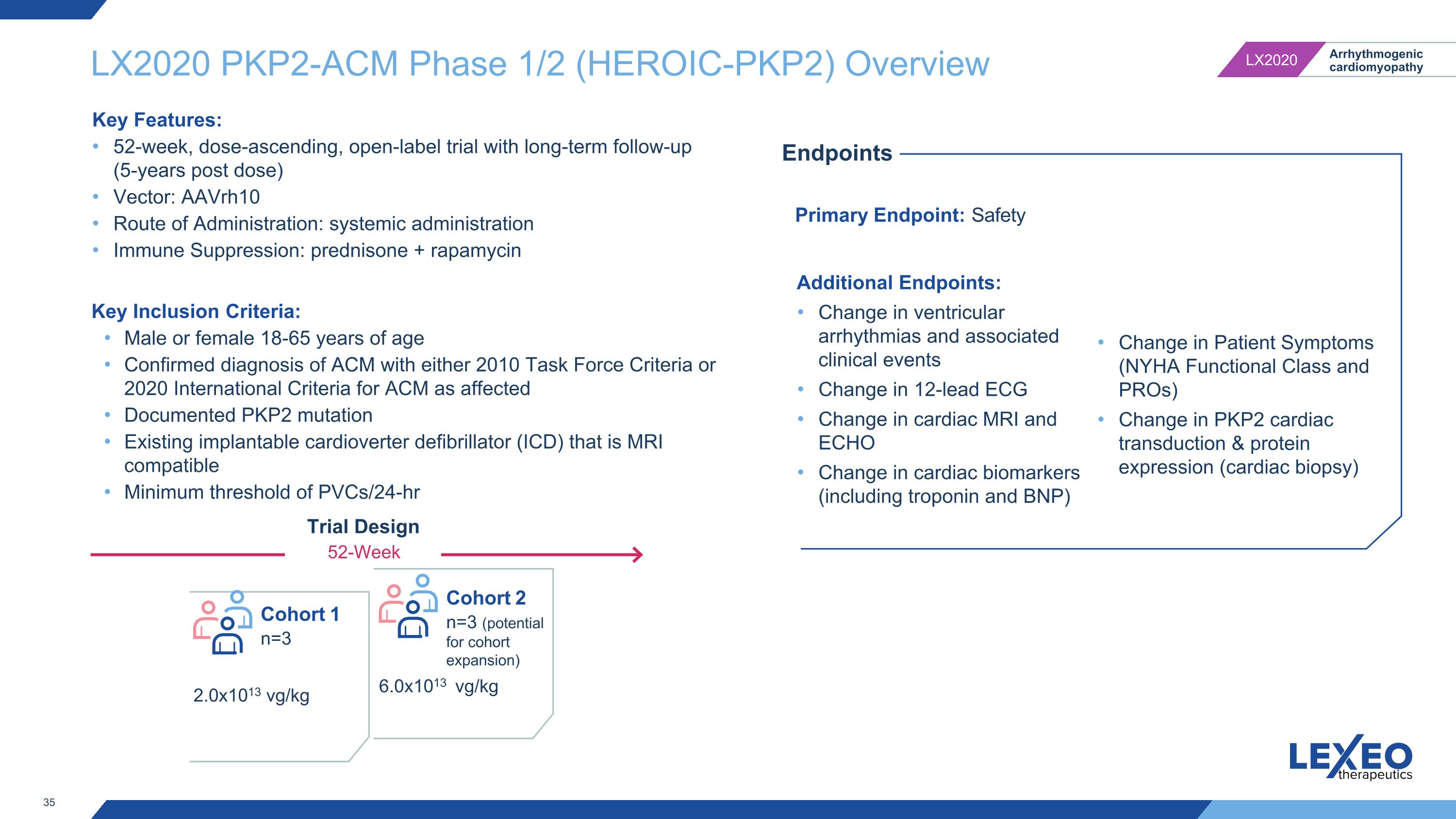
LX2020 PKP2-ACM Phase 1/2 (HEROIC-PKP2) Overview Primary Endpoint: Safety Endpoints Additional Endpoints: Change in ventricular arrhythmias and associated clinical events Change in 12-lead ECG Change in cardiac MRI and ECHO Change in cardiac biomarkers (including troponin and BNP) Change in Patient Symptoms (NYHA Functional Class and PROs) Change in PKP2 cardiac transduction & protein expression (cardiac biopsy) Key Inclusion Criteria: Male or female 18-65 years of age Confirmed diagnosis of ACM with either 2010 Task Force Criteria or 2020 International Criteria for ACM as affected Documented PKP2 mutation Existing implantable cardioverter defibrillator (ICD) that is MRI compatible Minimum threshold of PVCs/24-hr Trial Design 52-Week Cohort 1 n=3 2.0x1013 vg/kg 6.0x1013 vg/kg Cohort 2 n=3 (potential for cohort expansion) Key Features: 52-week, dose-ascending, open-label trial with long-term follow-up (5-years post dose) Vector: AAVrh10 Route of Administration: systemic administration Immune Suppression: prednisone + rapamycin

APOE4-Associated Alzheimer’s Disease 36
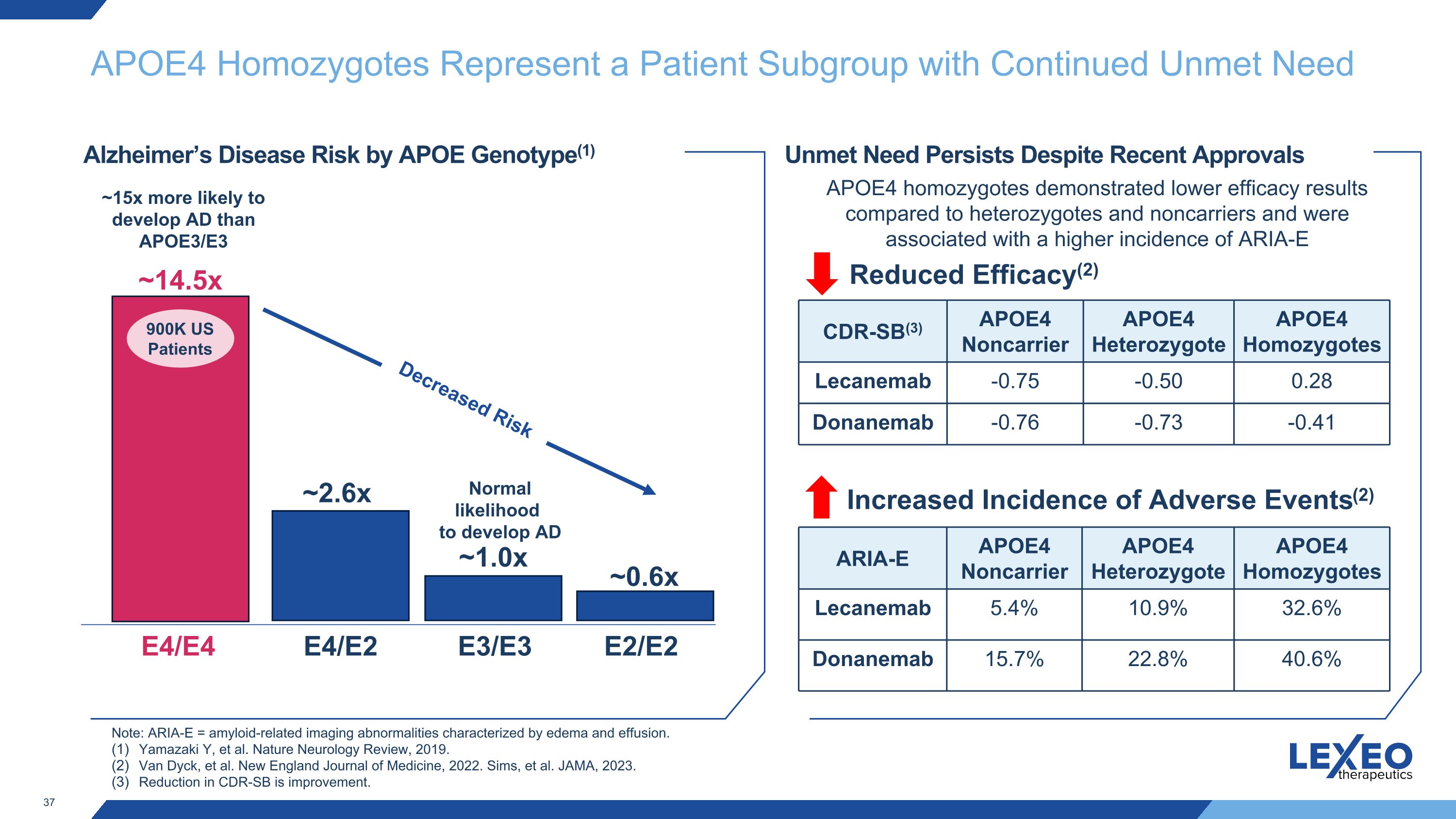
APOE4 Homozygotes Represent a Patient Subgroup with Continued Unmet Need E4/E4 E3/E3 ~14.5x E4/E2 ~2.6x ~1.0x Alzheimer’s Disease Risk by APOE Genotype(1) E2/E2 ~0.6x ~15x more likely to develop AD than APOE3/E3 Unmet Need Persists Despite Recent Approvals Increased Incidence of Adverse Events(2) Note: ARIA-E = amyloid-related imaging abnormalities characterized by edema and effusion. Yamazaki Y, et al. Nature Neurology Review, 2019. Van Dyck, et al. New England Journal of Medicine, 2022. Sims, et al. JAMA, 2023. Reduction in CDR-SB is improvement. APOE4 homozygotes demonstrated lower efficacy results compared to heterozygotes and noncarriers and were associated with a higher incidence of ARIA-E 900K US Patients Decreased Risk Normal likelihood to develop AD Reduced Efficacy(2) CDR-SB(3) APOE4 Noncarrier APOE4 Heterozygote APOE4 Homozygotes Lecanemab -0.75 -0.50 0.28 Donanemab -0.76 -0.73 -0.41 ARIA-E APOE4 Noncarrier APOE4 Heterozygote APOE4 Homozygotes Lecanemab 5.4% 10.9% 32.6% Donanemab 15.7% 22.8% 40.6%
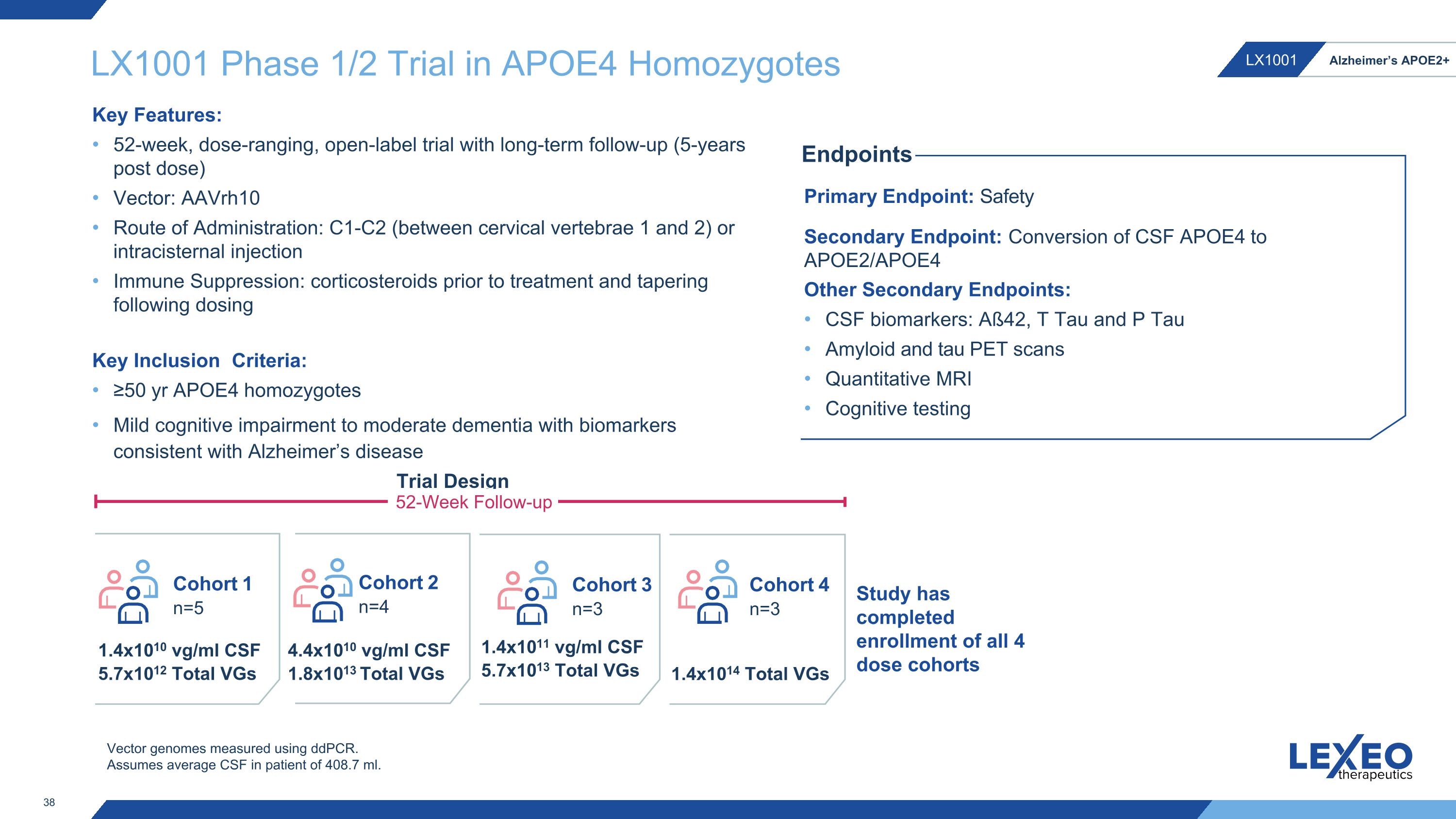
LX1001 Phase 1/2 Trial in APOE4 Homozygotes Key Inclusion Criteria: ≥50 yr APOE4 homozygotes Mild cognitive impairment to moderate dementia with biomarkers consistent with Alzheimer’s disease Cohort 1 n=5 Cohort 2 n=4 Cohort 3 n=3 1.4x1010 vg/ml CSF 5.7x1012 Total VGs 4.4x1010 vg/ml CSF 1.8x1013 Total VGs 1.4x1011 vg/ml CSF 5.7x1013 Total VGs Primary Endpoint: Safety Secondary Endpoint: Conversion of CSF APOE4 to APOE2/APOE4 Other Secondary Endpoints: CSF biomarkers: Aß42, T Tau and P Tau Amyloid and tau PET scans Quantitative MRI Cognitive testing Endpoints Trial Design 52-Week Follow-up Key Features: 52-week, dose-ranging, open-label trial with long-term follow-up (5-years post dose) Vector: AAVrh10 Route of Administration: C1-C2 (between cervical vertebrae 1 and 2) or intracisternal injection Immune Suppression: corticosteroids prior to treatment and tapering following dosing Vector genomes measured using ddPCR. Assumes average CSF in patient of 408.7 ml. Cohort 4 n=3 1.4x1014 Total VGs Study has completed enrollment of all 4 dose cohorts
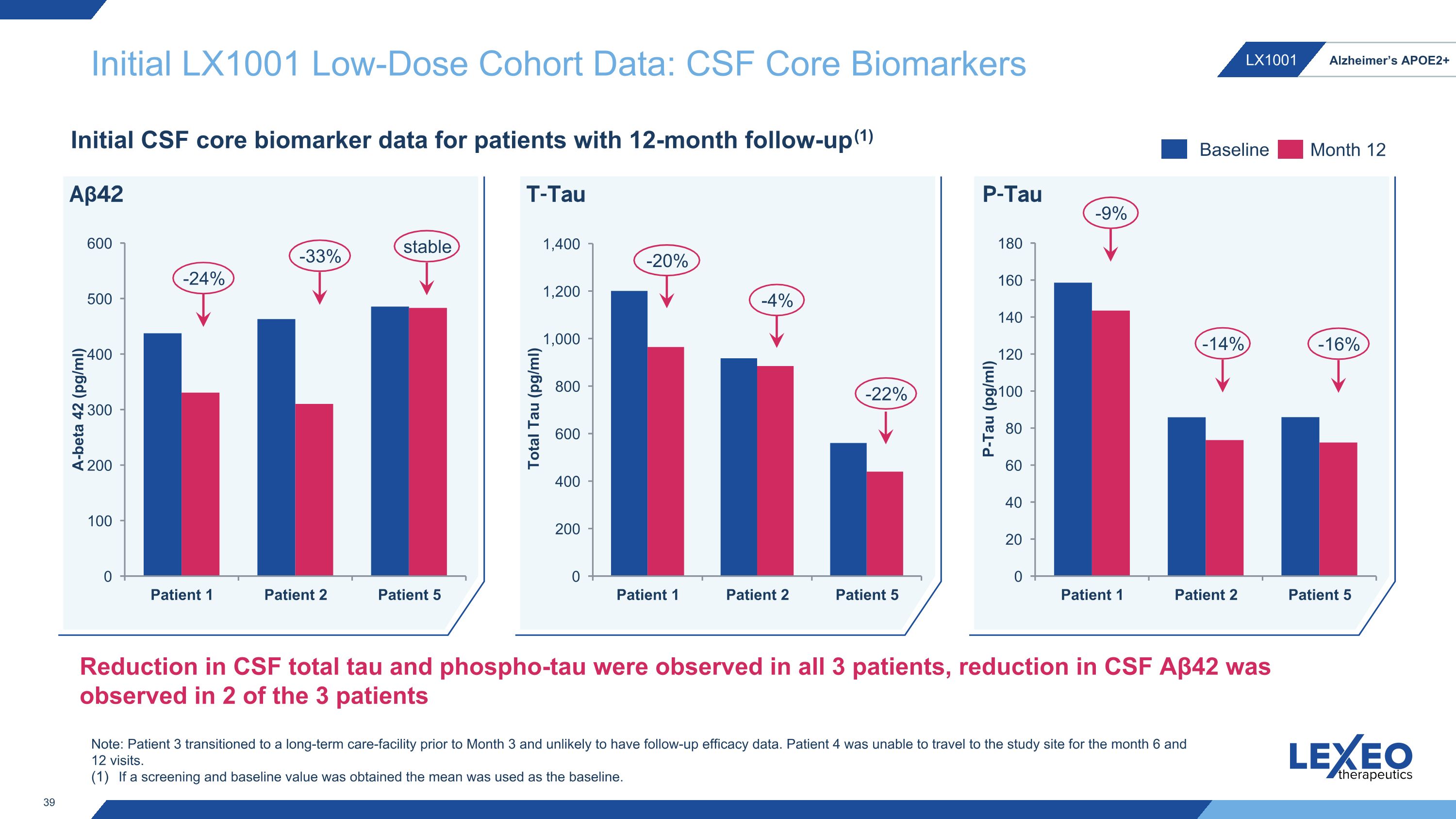
Initial LX1001 Low-Dose Cohort Data: CSF Core Biomarkers Initial CSF core biomarker data for patients with 12-month follow-up(1) -20% -4% -9% -14% T-Tau P-Tau -22% -16% Baseline Month 12 -24% -33% Aβ42 stable Reduction in CSF total tau and phospho-tau were observed in all 3 patients, reduction in CSF Aβ42 was observed in 2 of the 3 patients Note: Patient 3 transitioned to a long-term care-facility prior to Month 3 and unlikely to have follow-up efficacy data. Patient 4 was unable to travel to the study site for the month 6 and 12 visits. If a screening and baseline value was obtained the mean was used as the baseline.
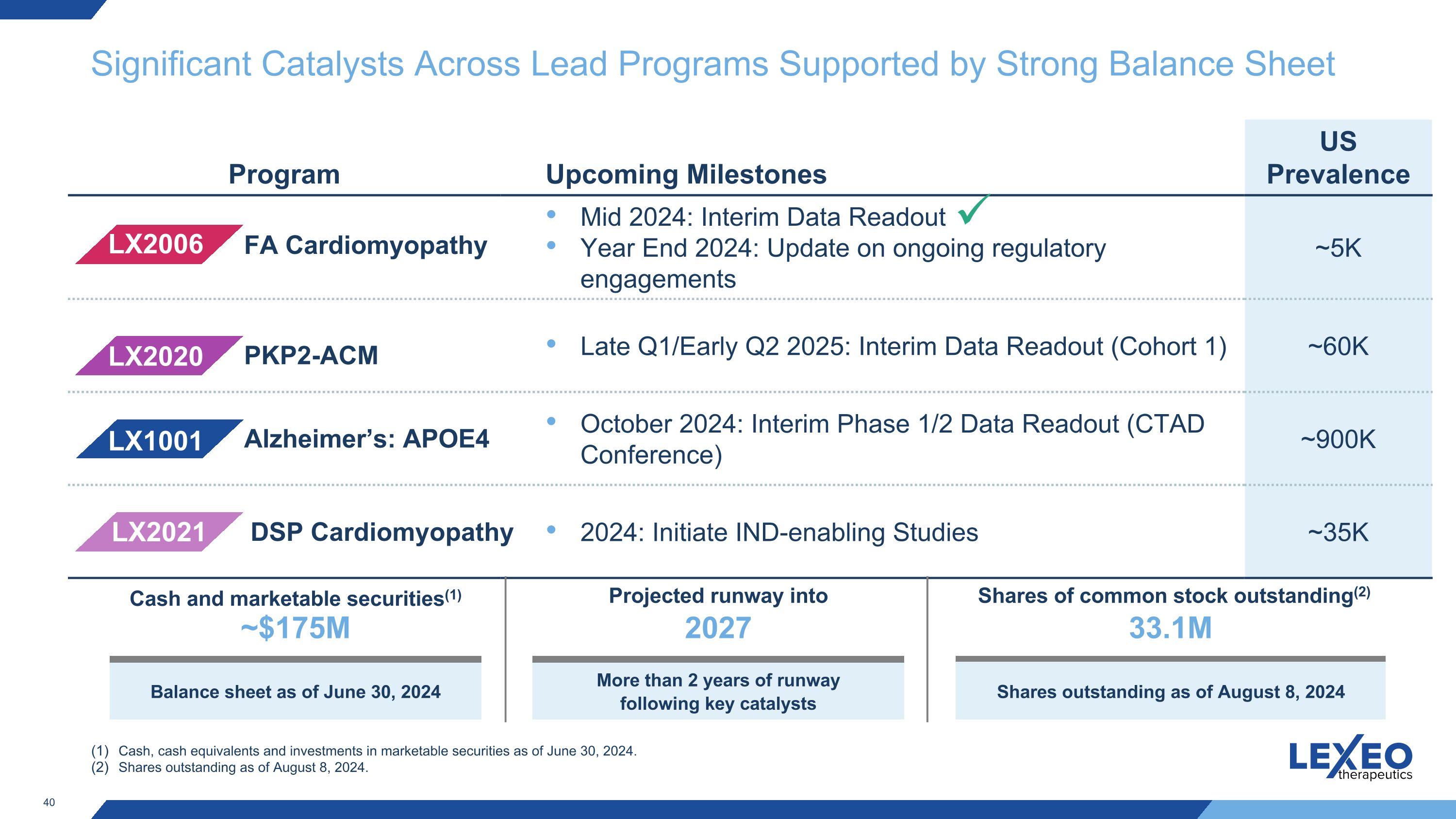
Significant Catalysts Across Lead Programs Supported by Strong Balance Sheet Program Upcoming Milestones US Prevalence Mid 2024: Interim Data Readout Year End 2024: Update on ongoing regulatory engagements ~5K Late Q1/Early Q2 2025: Interim Data Readout (Cohort 1) ~60K October 2024: Interim Phase 1/2 Data Readout (CTAD Conference) ~900K 2024: Initiate IND-enabling Studies ~35K LX1001 LX2006 LX2020 LX2021 Alzheimer’s: APOE4 FA Cardiomyopathy PKP2-ACM DSP Cardiomyopathy Cash, cash equivalents and investments in marketable securities as of June 30, 2024. Shares outstanding as of August 8, 2024. Cash and marketable securities(1) ~$175M Balance sheet as of June 30, 2024 Projected runway into 2027 More than 2 years of runway following key catalysts Shares of common stock outstanding(2) 33.1M Shares outstanding as of August 8, 2024

Thank you 41
Lexeo Therapeutics (NASDAQ:LXEO)
과거 데이터 주식 차트
부터 10월(10) 2024 으로 11월(11) 2024

Lexeo Therapeutics (NASDAQ:LXEO)
과거 데이터 주식 차트
부터 11월(11) 2023 으로 11월(11) 2024
