ICU Medical Gets FDA Clearance for Infusion Pump-Software Combo
29 8월 2023 - 9:45PM
Dow Jones News
By Dean Seal
ICU Medical has received clearance from the U.S. Food and Drug
Administration for its Plum Duo infusion pump with LifeShield
infusion safety software.
The San Clemente, Calif.-based company said Tuesday that with
the clearance, the Plum Duo pump and LifeShield software will be
available to U.S. customers by early 2024.
The pump is the latest addition to the company's portfolio of
infusion devices and features a large touch screen, highly
intuitive user interface and two channels for delivering up to four
compatible IV medications.
The LifeShield software is a cloud-based software suite
providing tools for comprehensive drug library management.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
August 29, 2023 08:30 ET (12:30 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
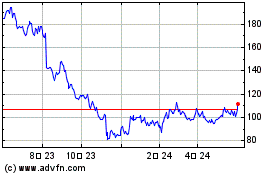
ICU Medical (NASDAQ:ICUI)
과거 데이터 주식 차트
부터 4월(4) 2024 으로 5월(5) 2024
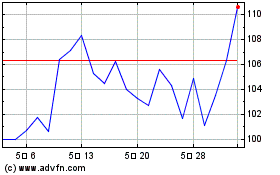
ICU Medical (NASDAQ:ICUI)
과거 데이터 주식 차트
부터 5월(5) 2023 으로 5월(5) 2024