0001828673--12-31FalseQ30001828673us-gaap:AccountsPayableAndAccruedLiabilitiesMember2023-01-012023-12-310001828673us-gaap:SeniorNotesMembersrt:ChiefExecutiveOfficerMember2024-09-300001828673us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-310001828673us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Member2024-09-3000018286732023-09-300001828673us-gaap:RetainedEarningsMember2023-01-012023-03-310001828673hcwb:WugenLicenseMember2023-07-012023-09-300001828673us-gaap:SeniorNotesMembersrt:ChiefFinancialOfficerMember2024-09-3000018286732023-06-3000018286732024-11-110001828673hcwb:WugenLicenseMember2024-01-012024-09-300001828673us-gaap:RetainedEarningsMember2022-12-310001828673us-gaap:CommonStockMember2023-01-012023-03-310001828673us-gaap:CommonStockMember2023-04-012023-06-3000018286732023-01-012023-03-310001828673us-gaap:MoneyMarketFundsMember2023-12-310001828673us-gaap:RetainedEarningsMember2023-12-310001828673us-gaap:SeniorNotesMemberhcwb:DrWongMemberus-gaap:SubsequentEventMember2024-10-310001828673us-gaap:AdditionalPaidInCapitalMember2023-06-300001828673hcwb:CommonStockOptionsMember2024-01-012024-09-300001828673us-gaap:SeniorNotesMembersrt:MaximumMember2024-03-310001828673us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-09-300001828673us-gaap:RetainedEarningsMember2023-03-310001828673us-gaap:CommonStockMember2024-01-012024-03-310001828673us-gaap:AdditionalPaidInCapitalMember2023-09-300001828673us-gaap:FairValueInputsLevel2Member2023-12-310001828673us-gaap:SeniorNotesMemberus-gaap:SubsequentEventMember2024-10-310001828673us-gaap:RetainedEarningsMember2023-07-012023-09-3000018286732023-01-012023-09-3000018286732023-12-3100018286732024-03-310001828673us-gaap:AccountsPayableAndAccruedLiabilitiesMember2024-01-012024-09-300001828673hcwb:CommonStockOptionsMember2023-01-012023-09-300001828673us-gaap:AdditionalPaidInCapitalMember2023-07-012023-09-300001828673us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-09-300001828673us-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001828673us-gaap:CommonStockMember2023-07-012023-09-300001828673us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Member2023-12-310001828673hcwb:CogentBankMember2024-01-012024-09-300001828673us-gaap:SeniorNotesMembersrt:BoardOfDirectorsChairmanMember2024-09-300001828673us-gaap:SuretyBondMember2024-09-300001828673us-gaap:FairValueInputsLevel3Member2023-12-310001828673us-gaap:SeniorNotesMemberhcwb:BoardOfDirectorsAuditChairMember2024-09-300001828673us-gaap:RetainedEarningsMember2023-04-012023-06-300001828673us-gaap:CommonStockMember2024-03-310001828673us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-3000018286732024-07-012024-09-300001828673us-gaap:CommonStockMember2024-06-300001828673us-gaap:CommonStockMember2024-09-300001828673us-gaap:AdditionalPaidInCapitalMember2022-12-310001828673us-gaap:SeniorNotesMember2024-09-3000018286732023-07-012023-09-300001828673us-gaap:RetainedEarningsMember2023-06-3000018286732023-04-012023-06-300001828673us-gaap:CommonStockMember2023-12-3100018286732024-06-300001828673us-gaap:CommonStockMember2023-06-300001828673us-gaap:FairValueInputsLevel3Member2024-09-300001828673us-gaap:AdditionalPaidInCapitalMember2023-03-310001828673us-gaap:RetainedEarningsMember2024-07-012024-09-3000018286732023-01-012023-12-310001828673us-gaap:SuretyBondMember2024-01-012024-09-300001828673us-gaap:CommonStockMember2022-12-3100018286732024-04-012024-06-300001828673us-gaap:AdditionalPaidInCapitalMember2024-09-300001828673us-gaap:ResearchAndDevelopmentExpenseMember2024-07-012024-09-300001828673us-gaap:AdditionalPaidInCapitalMember2024-06-300001828673us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMember2024-09-300001828673us-gaap:RetainedEarningsMember2024-04-012024-06-300001828673us-gaap:MoneyMarketFundsMember2024-09-300001828673hcwb:WugenLicenseMember2024-09-300001828673us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMember2024-09-3000018286732024-01-012024-03-3100018286732024-01-012024-09-300001828673us-gaap:AdditionalPaidInCapitalMember2023-12-3100018286732022-03-010001828673us-gaap:SeniorNotesMemberus-gaap:CollateralPledgedMember2024-09-300001828673us-gaap:CommonStockMember2023-03-3100018286732024-01-100001828673hcwb:WugenLicenseMember2023-01-012023-09-300001828673us-gaap:CommonStockMember2023-09-300001828673us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-3100018286732022-12-310001828673us-gaap:AccountsPayableAndAccruedLiabilitiesMember2024-09-300001828673us-gaap:SeniorNotesMemberhcwb:BoardOfDirectorsMember2024-09-3000018286732023-03-310001828673us-gaap:RetainedEarningsMember2024-06-300001828673us-gaap:AdditionalPaidInCapitalMember2024-04-012024-06-300001828673us-gaap:FairValueInputsLevel1Member2024-09-300001828673hcwb:AccruedExpensesCurrentMember2023-12-310001828673us-gaap:SeniorNotesMemberus-gaap:SubsequentEventMemberhcwb:NewFinancingPlanMember2024-10-310001828673us-gaap:RetainedEarningsMember2024-03-310001828673us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMember2023-12-310001828673us-gaap:ResearchAndDevelopmentExpenseMember2023-07-012023-09-300001828673hcwb:CogentBankMember2024-09-300001828673us-gaap:RetainedEarningsMember2024-01-012024-03-310001828673us-gaap:FairValueInputsLevel1Member2023-12-310001828673us-gaap:FairValueInputsLevel2Member2024-09-300001828673us-gaap:SeniorNotesMembersrt:ExecutiveVicePresidentMember2024-09-3000018286732024-03-010001828673us-gaap:AdditionalPaidInCapitalMember2024-03-310001828673us-gaap:RetainedEarningsMember2023-09-300001828673us-gaap:AccountsPayableAndAccruedLiabilitiesMember2023-12-3100018286732024-09-300001828673srt:ScenarioForecastMember2024-12-310001828673hcwb:WugenLicenseMember2023-12-310001828673us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMember2023-12-310001828673hcwb:WugenLicenseMember2024-07-012024-09-300001828673us-gaap:SeniorNotesMember2024-01-012024-09-300001828673hcwb:CogentBankMember2022-08-150001828673us-gaap:RetainedEarningsMember2024-09-30xbrli:pureutr:sqftxbrli:sharesiso4217:USD
F
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
|
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2024
OR
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-40591
HCW Biologics Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
Delaware |
82-5024477 |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer
Identification No.) |
|
|
2929 N. Commerce Parkway Miramar, Florida |
33025 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (954) 842–2024
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange
on which registered |
Common Stock, par value $0.0001 per share |
HCWB |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
|
|
|
Emerging growth company |
☒ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of November 11, 2024, the registrant had 37,823,394 shares of common stock, $0.0001 par value per share, outstanding.
Table of Contents
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements.
HCW Biologics Inc.
Condensed Balance Sheets
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
|
September 30, |
|
|
|
2023 |
|
|
2024 |
|
|
|
|
|
|
Unaudited |
|
ASSETS |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
3,595,101 |
|
|
$ |
998,221 |
|
Accounts receivable, net |
|
|
1,535,757 |
|
|
|
651,840 |
|
Prepaid expenses |
|
|
1,042,413 |
|
|
|
356,156 |
|
Other current assets |
|
|
230,916 |
|
|
|
88,131 |
|
Total current assets |
|
|
6,404,187 |
|
|
|
2,094,348 |
|
Investments |
|
|
1,599,751 |
|
|
|
1,599,751 |
|
Property, plant and equipment, net |
|
|
20,453,184 |
|
|
|
22,833,904 |
|
Other assets |
|
|
56,538 |
|
|
|
28,476 |
|
Total assets |
|
$ |
28,513,660 |
|
|
$ |
26,556,479 |
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) |
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
6,167,223 |
|
|
$ |
22,666,107 |
|
Accrued liabilities and other current liabilities |
|
|
2,580,402 |
|
|
|
1,056,716 |
|
Short-term debt, net |
|
|
— |
|
|
|
6,340,511 |
|
Total current liabilities |
|
|
8,747,625 |
|
|
|
30,063,334 |
|
Debt, net |
|
|
6,304,318 |
|
|
|
6,462,769 |
|
Total liabilities |
|
|
15,051,943 |
|
|
|
36,526,103 |
|
Commitments and contingencies (Note 8) |
|
|
|
|
|
|
Stockholders’ equity (deficit): |
|
|
|
|
|
|
Common stock: |
|
|
|
|
|
|
Common, $0.0001 par value; 250,000,000 shares authorized
and 36,025,104 shares issued at December 31, 2023; 250,000,000 shares
authorized and 37,823,394 shares issued at September 30, 2024 |
|
|
3,603 |
|
|
|
3,782 |
|
Additional paid-in capital |
|
|
83,990,437 |
|
|
|
87,209,457 |
|
Accumulated deficit |
|
|
(70,532,323 |
) |
|
|
(97,182,863 |
) |
Total stockholders’ equity (deficit) |
|
|
13,461,717 |
|
|
|
(9,969,624 |
) |
Total liabilities and stockholders’ equity (deficit) |
|
$ |
28,513,660 |
|
|
$ |
26,556,479 |
|
See accompanying notes to the unaudited condensed interim financial statements.
HCW Biologics Inc.
Condensed Statements of Operations
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
|
|
Nine Months Ended
September 30, |
|
|
|
|
2023 |
|
|
2024 |
|
|
|
2023 |
|
|
2024 |
|
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
853,102 |
|
|
$ |
426,423 |
|
|
|
$ |
1,517,792 |
|
|
$ |
2,171,988 |
|
|
Cost of revenues |
|
|
(678,325 |
) |
|
|
(341,138 |
) |
|
|
|
(1,210,077 |
) |
|
|
(1,291,546 |
) |
|
Net revenues |
|
|
174,777 |
|
|
|
85,285 |
|
|
|
|
307,715 |
|
|
|
880,442 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
1,667,442 |
|
|
|
1,186,913 |
|
|
|
|
5,539,919 |
|
|
|
5,339,383 |
|
|
General and administrative |
|
|
1,509,936 |
|
|
|
1,639,152 |
|
|
|
|
5,106,674 |
|
|
|
4,799,437 |
|
|
Legal expenses |
|
|
2,075,279 |
|
|
|
949,455 |
|
|
|
|
4,610,091 |
|
|
|
15,761,531 |
|
|
Nonoperating loss |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
1,300,000 |
|
|
Total operating expenses |
|
|
5,252,657 |
|
|
|
3,775,520 |
|
|
|
|
15,256,684 |
|
|
|
27,200,351 |
|
|
Loss from operations |
|
|
(5,077,880 |
) |
|
|
(3,690,235 |
) |
|
|
|
(14,948,969 |
) |
|
|
(26,319,909 |
) |
|
Interest expense |
|
|
(95,514 |
) |
|
|
(223,363 |
) |
|
|
|
(284,465 |
) |
|
|
(383,029 |
) |
|
Other (expense) income, net |
|
|
234,753 |
|
|
|
11,310 |
|
|
|
|
919,688 |
|
|
|
52,397 |
|
|
Net loss |
|
$ |
(4,938,641 |
) |
|
$ |
(3,902,288 |
) |
|
|
$ |
(14,313,746 |
) |
|
$ |
(26,650,541 |
) |
|
Net loss per share, basic and diluted |
|
$ |
(0.14 |
) |
|
$ |
(0.10 |
) |
|
|
$ |
(0.40 |
) |
|
$ |
(0.71 |
) |
|
Weighted average shares outstanding, basic and diluted |
|
|
35,926,921 |
|
|
|
37,823,394 |
|
|
|
|
35,907,123 |
|
|
|
37,623,459 |
|
|
See accompanying notes to the unaudited condensed interim financial statements.
HCW Biologics Inc.
Condensed Statements of Changes in Stockholders’ Equity (Deficit)
For the Nine Months Ended September 30, 2023 and 2024
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity |
|
|
|
Common Stock |
|
|
Additional
Paid-In |
|
|
Accumulated |
|
|
Total
Stockholders’ |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity |
|
Balance, December 31, 2022 |
|
|
35,876,440 |
|
|
$ |
3,588 |
|
|
$ |
82,962,964 |
|
|
$ |
(45,538,046 |
) |
|
$ |
37,428,506 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
10,195 |
|
|
|
1 |
|
|
|
1,900 |
|
|
|
— |
|
|
|
1,901 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
259,206 |
|
|
|
— |
|
|
|
259,206 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(5,070,686 |
) |
|
|
(5,070,686 |
) |
Balance, March 31, 2023 |
|
|
35,886,635 |
|
|
$ |
3,589 |
|
|
$ |
83,224,070 |
|
|
$ |
(50,608,732 |
) |
|
$ |
32,618,927 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
40,086 |
|
|
|
4 |
|
|
|
7,708 |
|
|
|
— |
|
|
|
7,712 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
263,423 |
|
|
|
|
|
|
263,423 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,304,420 |
) |
|
|
(4,304,420 |
) |
Balance, June 30, 2023 |
|
|
35,926,721 |
|
|
$ |
3,593 |
|
|
$ |
83,495,201 |
|
|
$ |
(54,913,152 |
) |
|
$ |
28,585,642 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
600 |
|
|
|
— |
|
|
|
84 |
|
|
|
— |
|
|
|
84 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
219,848 |
|
|
|
— |
|
|
|
219,848 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,938,641 |
) |
|
|
(4,938,641 |
) |
Balance, September 30, 2023 |
|
|
35,927,321 |
|
|
$ |
3,593 |
|
|
$ |
83,715,133 |
|
|
$ |
(59,851,793 |
) |
|
$ |
23,866,933 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity (Deficit) |
|
|
|
Common Stock |
|
|
Additional
Paid-In |
|
|
Accumulated |
|
|
Total
Stockholders’ |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity (Deficit) |
|
Balance, December 31, 2023 |
|
|
36,025,104 |
|
|
$ |
3,603 |
|
|
$ |
83,990,437 |
|
|
$ |
(70,532,323 |
) |
|
$ |
13,461,717 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
12,572 |
|
|
|
1 |
|
|
|
2,254 |
|
|
|
— |
|
|
|
2,255 |
|
Issuance of Common Stock upon equity subscription |
|
|
1,785,718 |
|
|
|
178 |
|
|
|
2,499,827 |
|
|
|
— |
|
|
|
2,500,005 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
244,685 |
|
|
|
— |
|
|
|
244,685 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(7,468,061 |
) |
|
|
(7,468,061 |
) |
Balance, March 31, 2024 |
|
|
37,823,394 |
|
|
$ |
3,782 |
|
|
$ |
86,737,203 |
|
|
$ |
(78,000,384 |
) |
|
$ |
8,740,601 |
|
Issuance of Common Stock upon exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
239,821 |
|
|
|
— |
|
|
|
239,821 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(15,280,191 |
) |
|
|
(15,280,191 |
) |
Balance, June 30, 2024 |
|
|
37,823,394 |
|
|
$ |
3,782 |
|
|
$ |
86,977,024 |
|
|
$ |
(93,280,575 |
) |
|
$ |
(6,299,769 |
) |
Issuance of Common Stock upon exercise of stock options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
232,433 |
|
|
|
— |
|
|
|
232,433 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,902,288 |
) |
|
|
(3,902,288 |
) |
Balance, September 30, 2024 |
|
|
37,823,394 |
|
|
$ |
3,782 |
|
|
$ |
87,209,457 |
|
|
$ |
(97,182,863 |
) |
|
$ |
(9,969,624 |
) |
See accompanying notes to the unaudited condensed interim financial statements.
HCW Biologics Inc.
Condensed Statements of Cash Flows
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
|
|
|
2023 |
|
|
2024 |
|
Cash flows from operating activities: |
|
|
|
|
|
|
Net loss |
|
$ |
(14,313,746 |
) |
|
$ |
(26,650,541 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
Depreciation and amortization |
|
|
860,634 |
|
|
|
501,882 |
|
Stock-based compensation |
|
|
742,477 |
|
|
|
716,940 |
|
Unrealized loss (gain) on investments, net |
|
|
(248,445 |
) |
|
|
— |
|
Realized loss (gain) on investments, net |
|
|
(15,625 |
) |
|
|
— |
|
Changes in the carrying amount of right-of-use asset |
|
|
(1,045 |
) |
|
|
(418 |
) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Accounts receivable |
|
|
(292,383 |
) |
|
|
883,917 |
|
Deposit for interest reserve |
|
|
(5,250,000 |
) |
|
|
— |
|
Prepaid expenses and other assets |
|
|
(251,008 |
) |
|
|
829,043 |
|
Accounts payable and other liabilities |
|
|
392,802 |
|
|
|
12,383,171 |
|
Operating lease liability |
|
|
(242,990 |
) |
|
|
(56,541 |
) |
Net cash used in operating activities |
|
|
(18,619,329 |
) |
|
|
(11,392,547 |
) |
Cash flows from investing activities: |
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(2,486,950 |
) |
|
|
(148,205 |
) |
Proceeds for sale or maturities of short-term investments |
|
|
10,000,000 |
|
|
|
— |
|
Net cash provided by (used in) investing activities |
|
|
7,513,050 |
|
|
|
(148,205 |
) |
Cash flows from financing activities: |
|
|
|
|
|
|
Proceeds from issuance of common stock |
|
|
9,697 |
|
|
|
2,502,260 |
|
Proceeds from issuance of debt |
|
|
— |
|
|
|
6,530,000 |
|
Offering costs |
|
|
— |
|
|
|
— |
|
Debt repayment |
|
|
(8,981 |
) |
|
|
(88,388 |
) |
Net cash provided by financing activities |
|
|
716 |
|
|
|
8,943,872 |
|
Net (decrease) increase in cash and cash equivalents |
|
|
(11,105,563 |
) |
|
|
(2,596,880 |
) |
Cash and cash equivalents at the beginning of the period |
|
|
22,326,356 |
|
|
|
3,595,101 |
|
Cash and cash equivalents at the end of the period |
|
$ |
11,220,793 |
|
|
$ |
998,221 |
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
Cash paid for interest, net of amounts capitalized |
|
$ |
284,465 |
|
|
$ |
340,988 |
|
Noncash operating, investing and financing activities: |
|
|
|
|
|
|
Capital expenditures accrued, but not yet paid |
|
$ |
2,095,724 |
|
|
$ |
1,910,698 |
|
Purchases of property and equipment included in accounts payable and other liabilities |
|
$ |
— |
|
|
$ |
829,207 |
|
See accompanying notes to the unaudited condensed interim financial statements.
HCW Biologics Inc.
Notes to Condensed Interim Financial Statements
(Unaudited)
1. Organization and Summary of Significant Accounting Policies
Organization
HCW Biologics Inc. (the “Company”) is a biopharmaceutical company focused on discovering and developing novel immunotherapies to lengthen healthspan by disrupting the link between chronic, low-grade inflammation and age-related diseases. The Company believes age-related low-grade chronic inflammation, or “inflammaging,” is a significant contributing factor to several chronic diseases and conditions, such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and autoimmune diseases. The Company is located in Miramar, Florida and was incorporated in the state of Delaware in April 2018.
Liquidity and Going Concern
In accordance with FASB Accounting Standards Codification (“ASC”) 205-40, Presentation of Financial Statements – Going Concern (“Topic 205-40”), management is required to evaluate whether there are conditions and events, considered in the aggregate that raise substantial doubt about the Company’s ability to continue as a going concern for at least 12 months from the issuance date of the Company’s condensed interim financial statements. This evaluation does not take into consideration the potential mitigating effect of management’s plans that have not been fully implemented or are not within control of the Company as of the date the financial statements are issued. When substantial doubt exists under this methodology, management evaluates whether the mitigating effect of its plans sufficiently alleviates substantial doubt about the Company’s ability to continue as a going concern. The mitigating effect of management’s plans, however, is only considered if both (1) it is probable that the plans will be effectively implemented within one year after the date that the financial statements are issued, and (2) it is probable that the plans, when implemented, will mitigate the relevant conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued.
As of September 30, 2024, the Company had not generated any revenue from commercial product sales of its internally developed immunotherapeutic products for the treatment of cancer and other age-related diseases. During its development activities, the Company has sustained operating losses and expects to continue to incur operating losses for the foreseeable future. Since inception to September 30, 2024, the Company incurred cumulative net losses of $94.5 million. These losses reflects the events reported in the Form 8-K filed on May 1, 2024 with the Securities and Exchange Commission (“SEC”), involving a scheme that resulted in the misdirection of approximately $1.3 million held in Company accounts to a fraudulent account controlled by a third party. The Company has pursued relief through insurance claims and has reported the crime to legal authorities. Management expects to incur additional losses in the future to conduct product research and development and recognizes the need to raise additional capital to fully implement its business plan. As of September 30, 2024, the Company had $1.0 million in cash and cash equivalents.
On August 15, 2022, the Company entered into a loan and security agreement (the “2022 Loan Agreement”) with Cogent Bank, pursuant to which it received $6.5 million in proceeds to purchase a building that will become the Company's new headquarters. The loan is secured by a first priority lien on the building. As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction of its new manufacturing facilities and upgraded research laboratories. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements; however, as of the reporting date, the lender has not elected to do so. See Part I, Item 4. -- “Controls and Procedures.” As of September 30, 2024, the Company has reflected this loan as Short-term debt, net, to reflect that the lender has the right to accelerate the loan under a discretionary default provision. The Company continues to seek the financing required to complete the construction project, with the cooperation of the lender and lien holders.
To date, the Company has funded operations primarily through the sale of stock, issuance of senior secured notes and revenues generated from the Company’s exclusive worldwide license with Wugen, Inc. (“Wugen”), pursuant to which Wugen licensed limited rights to develop, manufacture, and commercialize cell therapy treatments for cancer based on two of the Company’s internally-developed multi-cytokine fusion protein molecules, and its manufacturing and supply arrangement with Wugen. In the three months ended September 30, 2023 and 2024, the Company recognized revenues generated from the supply of clinical and research grade material to Wugen of $853,102 and $426,423, respectively. In the nine months ended September 30, 2023 and 2024, the Company recognized revenues generated from the supply of clinical and research grade material to Wugen of $1.5 million and $2.2 million, respectively.
The Company launched a financing plan in the third quarter of 2024. The plan includes several capital-raising activities, such as issuance of secured notes, equity financings, as well as business development transactions, such as license agreements with guaranteed minimum payments. Through October 31, 2024, the Company issued an aggregate of $6.9 million in secured notes. On
September 25, 2024, the Company entered into a nonbinding term sheet with a party interested in licensing one of the Company’s preclinical molecules. The Company expects to close this license transaction in the fourth quarter of 2024. The proposed license agreement includes guaranteed minimum payments which are expected within the first year of the term. On November 13, 2024, we entered into an engagement letter with the Maxim Group to act as the exclusive placement agent for a multi-step equity financing. If the Company is not successful in raising additional capital through these activities, management intends to revise its business plan. If such revisions are insufficient, the Company may have to curtail or cease operations.
The Company has received written notices from the Listing Qualifications Staff of the Nasdaq Stock Market LLC (“Nasdaq”) informing the Company it is not in compliance with continued listing requirements on the Nasdaq Global Market for the market value of listed securities. Under Nasdaq Listing Rules, the Company has a period of 180 calendar days from the date of notice in which to regain compliance, and its first deadline is currently scheduled for December 16, 2024. The Company intend to take all reasonable measures available to us to regain compliance with the continued listing requirements for the Nasdaq Global Market, including utilizing the right to appeal to Nasdaq to extend the deadline to regain compliance based on a solid financial plan to do so. While the Company is exercising diligent efforts to maintain the listing of its common stock on Nasdaq, there can be no assurance that the Company will be able to regain or maintain compliance with the applicable continued listing standards set forth in the Nasdaq Listing Rules.
As of September 30, 2024, the conclusion of a going concern assessment was that there is substantial doubt about the Company’s ability to continue as a going concern. The Company considered its financing plans for the next 12 months, along with expected continuing operating losses and the burden of expenses incurred in connection with past legal proceedings. Management concluded that there were no mitigating circumstances which alleviated the substantial doubt over its ability to continue as a going concern.
As reported in the Company’s Form 8-K filed on July 18, 2024 and further described in Part II, Item 1. – “Legal Proceedings” below, as of July 13, 2024, the Company and Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, entered into a confidential Settlement Agreement and Release (the “Settlement Agreement”) with ImmunityBio and its affiliates. The Settlement Agreement includes mutual general releases by and among the parties thereto. No party is required to make any monetary payments to any other party or person under the Settlement Agreement and each party will bear its own expenses incurred in connection with the matter. The Company has substantially completed remediation procedures required to be in compliance with the terms of the Settlement Agreement.
The Company entered into the Settlement Agreement to avoid the costs, disruption and distraction of further litigation. In the accompanying condensed balance sheet as of September 30, 2024, the Company reported a balance of $14.4 million for legal fees incurred but not yet paid that were included within Accounts payable and an accrual of $35,000 for accrued legal fees within Accrued liabilities and other current liabilities. The Company is engaged in discussions with the law firms involved with this matter to arrange a reasonable payment plan with respect to those legal fees. With the execution of the Settlement Agreement, the Company resolved the attendant uncertainties for the outcome of the arbitration and additional complexities, and it launched its new financing plan.
The accompanying interim financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above. The Company believes that substantial doubt exists regarding its ability to continue as a going concern for at least 12 months from the date of issuance of the Company’s condensed interim financial statements and that the substantial doubt that existed in its going concern analysis was not alleviated.
Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed balance sheet as of September 30, 2024, the condensed statements of operations, and condensed statements of changes in stockholders’ equity (deficit) for the three and nine-month periods ended September 30, 2023 and 2024 and the condensed statements of cash flows for the nine months ended September 30, 2023 and 2024, and all related disclosures contained in the accompanying notes have been prepared in accordance with accounting principles that are generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and pursuant to Article 10 of Regulation S-X of the Securities Act of 1933, as amended (the “Securities Act”). Accordingly, they do not include all of the information and notes required by U.S. GAAP for complete financial statements. These unaudited condensed interim financial statements include only normal and recurring adjustments that the Company believes are necessary to fairly state the Company’s financial position and the results of its operations and cash flows. The results for the three and nine-month periods ended September 30, 2024 are not necessarily indicative of the results expected for the full fiscal year or any subsequent interim period. The condensed interim balance sheet at December 31, 2023 has been derived from the audited financial statements at that date but does not include all disclosures required by
U.S. GAAP for complete financial statements. Because all of the disclosures required by U.S. GAAP for complete financial statements are not included herein, these unaudited condensed interim financial statements and the notes accompanying them should be read in conjunction with the Company’s audited financial statements for the year ended December 31, 2023, which appear in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on May 15, 2024 (the “Annual Report”) and in other filings with the SEC.
Reclassification of Prior Period Presentation of Legal Expenses
Certain prior period amounts have been reclassified to distinguish between General and administrative expenses in the ordinary course of business and legal expenses incurred in connection with the arbitration and Settlement Agreement described in Notes 1. Reclassification of legal expenses incurred in connection with legal proceedings impacts the consolidated interim statements of operations. There is no effect on reporting results of operations from prior periods.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Management must apply significant judgment in this process. The Company evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors and adjusts those estimates and assumptions when facts and circumstances dictate. Actual results could differ from estimates.
Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurement (“Topic 820”), establishes a fair value hierarchy for those instruments measured at fair value that distinguishes between fair value measurements based on market data (observable inputs) and those based on the Company’s own assumptions (unobservable inputs). This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
Level 1: Observable inputs such as quoted prices in active markets;
Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and
Level 3: Unobservable inputs in which there is little or no market data, which require a reporting entity to develop its own assumptions.
Fair value measurements are classified based on the lowest level of input that is significant to the measurement. The Company’s assessment of the significance of a particular input to the fair value measurement requires judgment, which may affect the valuation of the assets and liabilities and their placement within the fair value hierarchy levels. The determination of the fair values takes into account the market for the Company’s financial assets and liabilities, the associated credit risk, and other factors as required. The Company considers active markets as those in which transactions for the assets or liabilities occur in sufficient frequency and volume to provide pricing information on an ongoing basis.
Revenue Recognition
The Company accounts for revenues in accordance with ASC 606, Revenue from Contracts with Customers (“Topic 606”). To determine revenue recognition for arrangements that fall within the scope of Topic 606, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the Company satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that it will collect the consideration it is entitled to in exchange for the goods or services transferred to the customer.
At contract inception, the Company assesses the goods or services promised within each contract, determines those that are performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. To date, the Company's revenues have been generated solely from transactions with Wugen. The Wugen License includes
licenses of intellectual property, cost reimbursements, upfront signing fees, milestone payments and royalties on future licensee’s product sales. In addition, the Company and Wugen have an agreement for supply of materials, from which the Company also recognizes revenues.
License Grants:
For out-licensing arrangements that include a grant of a license to the Company’s intellectual property, the Company considers whether the license grant is distinct from the other performance obligations included in the arrangement. For licenses that are distinct, the Company recognizes revenues from nonrefundable, upfront payments and other consideration allocated to the license when the license term has begun and the Company has provided all necessary information regarding the underlying intellectual property to the customer, which generally occurs at or near the inception of the arrangement.
Milestone and Contingent Payments:
At the inception of the arrangement and at each reporting date thereafter, the Company assesses whether it should include any milestone and contingent payments or other forms of variable consideration in the transaction price using the most likely amount method. If it is probable that a significant reversal of cumulative revenue would not occur upon resolution of the uncertainty, the associated milestone value is included in the transaction price. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of each such milestone and any related constraint and, if necessary, adjusts its estimate of the overall transaction price. Since milestone and contingent payments may become payable to the Company upon the initiation of a clinical study or filing for or receipt of regulatory approval, the Company reviews the relevant facts and circumstances to determine when the Company should update the transaction price, which may occur before the triggering event. When the Company updates the transaction price for milestone and contingent payments, the Company allocates the changes in the total transaction price to each performance obligation in the agreement on the same basis as the initial allocation. Any such adjustments are recorded on a cumulative catch-up basis in the period of adjustment, which may result in recognizing revenue for previously satisfied performance obligations in such period. The Company’s licensees will generally pay milestones payments subsequent to achievement of the triggering event.
Materials Supply:
The Company provides clinical and research grade materials so that licensees may develop products based on the licensed molecules. The Company plans to enter into commercialization supply agreements when licensees enter the commercial stage of their company. The amounts billed are recognized as revenue as the performance obligations are satisfied by the Company, once the Company determines that a contract exists.
On June 18, 2021, the Company entered into a master services agreement for the supply of materials for clinical development of licensed products. To meet all the criteria to qualify as a contract under Topic 606, the Company must enter into statements-of-work for purchases of clinical and research grade materials. The Company has determined that the manufacturing of the clinical and research materials supplied by the Company each represents a single performance obligation that is satisfied over time. The Company recognizes revenue using an input method based on the costs incurred relative to the total expected cost, which determines the extent of the Company's progress toward completion. As part of the accounting for these arrangements, the Company must develop estimates and assumptions that require judgement to determine the progress towards completion. The Company reviews its estimate of the progress toward completion based on the best information available to recognize the cumulative progress toward completion as of the end of each reporting period, and makes revisions to such estimates, if facts and circumstances change during each reporting period.
For the three and nine months ended September 30, 2023, the Company recognized $853,102 and $1.5 million in revenue related to the sale of development supply materials to Wugen, respectively. For the three and nine months ended September 30, 2024, the Company recognized $426,423 and $2.2 million in revenue related to sale of development supply materials to Wugen, respectively.
The Company holds a minority interest in Wugen which is accounted for using the measurement alternative whereby the investment is recorded at cost less impairment, adjusted for observable price changes in orderly transactions for an identical or similar investment of the same investee. No impairment has been recognized. As of September 30, 2024 and December 31, 2023, the Company included $1.6 million for the investment in Wugen in Investments in the accompanying condensed interim balance sheets. The Company used its equity interest in Wugen to collateralize the Secured Notes. See Note 3. Debt, Net.
Operating Leases
The Company determines if an arrangement is a lease at inception. Operating leases are included in Other assets, Accrued liabilities and other current liabilities, and Other liabilities on its condensed interim balance sheets. Operating lease Right of Use (“ROU”) assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over
the lease term at commencement date. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. The Company has a lease agreement with lease and non-lease components, which are accounted for separately. For short-term leases with a term of one year or less, the Company uses the practical expedient and does not record an ROU asset or lease liability for such short-term leases.
Net Loss Per Share
Basic loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during each period. Diluted loss per share of common stock includes the effect, if any, from the potential exercise of stock options and unvested shares of restricted stock, which would result in the issuance of incremental shares of common stock. For diluted net loss per share, the weighted-average number of shares of common stock is the same for basic net loss per share due to the fact that when a net loss exists, dilutive securities are not included in the calculation as the impact is anti-dilutive.
2. Accrued Liabilities and Other Current Liabilities
As of December 31, 2023, the Company had a balance of $2.6 million included in Accrued liabilities and other current liabilities in the audited balance sheet, consisting of $392,000 for construction expenses, $105,000 for manufacturing expenses, $1.1 million for legal fees, $262,000 for clinical expenses, $365,000 for bonus payable, $160,000 for salary expenses, $119,000 for the current portion of long-term debt, $28,500 for a lease liability and $68,500 for other liabilities.
As of September 30, 2024, the Company had a balance of $1.2 million included in Accrued liabilities and other current liabilities in the accompanying condensed interim balance sheet, consisting of $170,000 for legal fees, $422,000 for construction in progress, $49,000 for manufacturing expenses, $104,000 for property taxes, $117,000 for clinical expenses, $57,000 for bonus payable, $126,000 for the current portion of long-term debt and $90,000 for salary and benefits.
3. Debt, Net
Cogent Bank Loan
On August 15, 2022, the Company entered into the 2022 Loan Agreement with Cogent Bank, pursuant to which it received $6.5 million in proceeds to purchase a building that will become the Company's new headquarters. The loan is secured by a first priority lien on the building. The interest-only period was one year followed by 48 months of equal payments of principal and interest beginning on September 15, 2023 based on a 25-year amortization rate. The unamortized balance is due on August 15, 2027 (the “Maturity Date”), and bears interest at a fixed per annum rate equal to 5.75%. Upon the Maturity Date, a final payment of unamortized principal will be due. The Company has the option to prepay the outstanding balance of the loan prior to the Maturity Date without penalty.
As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction of its new manufacturing facilities and upgraded research laboratories. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements; however, as of the reporting date, the lender has not elected to do so. As of September 30, 2024, the Company has reflected this loan as Short-term debt, net, to reflect that the lender has the right to accelerate the loan under a discretionary default provision. As of September 30, 2024, $6.3 million is included in Short-term debt, net in the accompanying condensed interim balance sheet.
Senior Secured Notes
On March 31, 2024, the Company entered into a Note Purchase Agreement with the Purchasers (as defined in the Note Purchase Agreement), pursuant to which the Company may issue secured notes up to an aggregate principal amount up to $10.0 million (“Secured Notes”).
As of September 30, 2024, the Company received $6.5 million in funding from the issuance of Secured Notes, which is included within Debt, Net on the accompanying condensed interim balance sheet. Investors included Dr. Hing C. Wong, Founder and Chief Executive Officer, who invested $2.4 million; Rebecca Byam, Chief Financial Officer, who invested $220,000; Lee Flowers, Senior Vice President of Business Development, who invested $25,000; Scott T. Garrett, the Chairman of the Company’s board of directors, who invested $140,000; Gary M. Winer, a member of our board of directors, who invested $60,000; Rick S. Greene, a member of the board of directors, who invested $25,000, as well as unrelated parties.
As of July 2, 2024, existing investors in Secured Notes unanimously agreed to an Amended and Restated Note Purchase Agreement and related documents (“Amended and Restated Note Purchase Agreement”). On September 30, 2024, existing investors approved an amendment to the Amended and Restated Note Purchase Agreement which extended the last closing date to October 31, 2024. No other terms were changed. Under the terms of the Amended and Restated Note Purchase Agreement, the Secured Notes continue to bear interest at a rate of 9% per annum, payable quarterly in arrears. The Secured Notes will mature on August 30, 2026 (the “Maturity Date”), on which date the principal balance, accrued but unpaid interest and other amounts owed under the terms of the Amended and Restated Note Purchase Agreement shall be due and payable. The Company pledged its equity ownership interest in Wugen, which was equivalent to a 5.6% ownership stake in that company as of September 30, 2024 (“Pledged Collateral”). The Pledged Collateral will be held and released according to the terms of the Escrow Agreement, as security for the Secured Notes.
If the Company elects to prepay the Senior Notes on or before December 31, 2024, there is a 5% prepayment penalty. The Secured Notes have a Mandatory Prepayment provision, according to which the Company is required to prepay the Secured Notes before the Maturity Date under certain circumstances. In the event of a Mandatory Prepayment, Secured Notes may receive a bonus payment based on the gross proceeds of the sale of the Pledged Collateral. If the Secured Notes are repaid on the Maturity Date, holders will receive a fixed bonus payment in addition to payment of outstanding principal and accrued and unpaid interest. If a bonus payment is paid, then there is no prepayment penalty. The Amended and Restated Note Purchase Agreement also contains default provisions, according to which, following an event of default, the Company may be required to distribute the Pledged Collateral to the Purchasers on a pro rata basis based on a $10.0 million issuance of Secured Notes, in full satisfaction of the indebtedness evidenced by the Secured Notes.
As of September 30, 2024, the fair value of the embedded derivative, which incorporated the likelihood of certain events occurring, was immaterial. Thus, as of September 30, 2024, the Company did not recognize the embedded derivative in the accompanying condensed financial statements.
The Company accounts for the bonus payment to be paid if the Secured Notes are repaid on the Maturity Date by accreting the bonus payment to the full amount due on the Maturity Date, utilizing the effective interest rate method. As of September 30, 2024, the Company did not recognize accretion of the bonus payment, which was not considered material to the accompanying condensed financial statements.
As of December 31, 2023 and September 30, 2024, the Company had 10,000,000 shares of preferred stock authorized and no such shares issued.
5. Net Loss Per Share
The following table summarizes the computation of the basic and diluted net loss per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
|
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(4,938,641 |
) |
|
$ |
(3,902,288 |
) |
|
$ |
(14,313,746 |
) |
|
$ |
(26,650,541 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding |
|
|
35,926,921 |
|
|
|
37,823,394 |
|
|
|
35,907,123 |
|
|
|
37,623,459 |
|
|
Net loss per share, basic and diluted |
|
$ |
(0.14 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.40 |
) |
|
$ |
(0.71 |
) |
|
The following table summarizes the outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per share because their inclusion would be anti-dilutive:
|
|
|
|
|
|
|
|
|
|
|
At September 30, |
|
|
|
2023 |
|
|
2024 |
|
Common stock options |
|
|
1,869,492 |
|
|
|
1,788,137 |
|
Potentially dilutive securities |
|
|
1,869,492 |
|
|
|
1,788,137 |
|
6. Fair Value of Financial Instruments
The carrying amount of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, U.S. government-backed securities with maturity dates up to one year, accounts payable and accrued liabilities, approximate fair value due to their short-term maturities.
Money market funds included in cash and cash equivalents and U.S. government-backed securities are measured at fair value based on quoted prices in active markets, which are considered Level 1 inputs. No transfers between levels occurred during the periods presented. The following table presents the Company’s assets, which were measured at fair value at December 31, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2023: |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
1,626,129 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,626,129 |
|
Total |
|
$ |
1,626,129 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,626,129 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At September 30, 2024: |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
83,814 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
83,814 |
|
Total |
|
$ |
83,814 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
83,814 |
|
The Company computes its quarterly income tax expense/(benefit) by using a forecasted annual effective tax rate and adjusts for any discrete items arising during the quarter. The Company did not have a provision for income taxes (current or deferred tax expense) as of December 31, 2023 and September 30, 2024. The Company will continue to maintain a 100% valuation allowance on total deferred tax assets. The Company believes it is more likely than not that the related deferred tax assets will not be realized. As a result, the Company’s effective tax rate will remain at 0.00% because no items either estimated or discrete items would impact the tax provision.
8. Commitments and Contingencies
Operating Leases
As of March 1, 2022, the Company entered a two-year lease, comprised of two operating leases, for approximately 12,250 square feet of space located in Miramar, Florida. Upon the commencement of those leases, the Company used its incremental borrowing rate of 6.0% to determine the amounts to recognize for a ROU asset and a lease liability.
On March 1, 2024, the Company entered a one-year lease for the same space, which expires on February 28, 2025. If a lease has a term that is 12 months or less in duration, the lease qualifies for a short-term lease exemption under ASC 842-20-25-2. The Company elected to take advantage of this exemption, and it will account for this lease on a straight-line basis over the lease term and will not recognize a ROU asset and a lease liability as a result. The remaining lease payments under the new short-term lease are $114,510. The Company has no obligations under financing leases.
For the three months ended September 30, 2023 and 2024, rent expense recognized by the Company was $42,400 and $49,500, respectively, of which $22,200 and $25,900, respectively, are included in research and development in the accompanying condensed interim statements of operations. For the nine months ended September 30, 2023 and 2024, rent expense recognized by the Company was $127,200 and $146,400, respectively, of which $66,600 and $75,300, respectively are included in research and development in the accompanying condensed interim statements of operations.
Contractual Commitments
The Company has commitments with a third-party manufacturing organization to supply us with clinical grade materials. As of September 30, 2024, it is under contract for obligations of $12,500 it expects to pay during the year ending December 31, 2024. As of December 31, 2023 and September 30, 2024, the Company had commitments to fund $4.4 million and $2.5 million, respectively, in construction costs related to the buildout of its new headquarters and manufacturing facility.
Project Financing
On January 10, 2024 (the “Termination Date”), the Company exercised its right to terminate its credit agreement (the “Credit Agreement”), dated April 21, 2023, with Prime Capital Ventures, LLC (the “Lender”), as permitted under the terms of the Credit Agreement. These funds were intended to finance the construction and renovation of the Company’s new headquarters (the “Property”). The termination followed repeated delays in funding and related concerns. There were no borrowings under the Credit Agreement as of the Termination Date, and the Company did not incur any penalties as a result of such termination under the terms of the Agreement. Upon exercising its right to terminate the Agreement, the Company was entitled to receive the return of the $5.3 million that the Company placed on deposit to establish an interest reserve account with the Lender. However, the Lender defaulted on its obligation to return the interest reserve deposit. Given the uncertainty of when or if funds will be recovered from the Lender, the Company recognized a reserve for a credit loss for $5.3 million as of December 31, 2023. The Company intends to pursue all available remedies to recover these funds, including legal actions, receivership and insurance.
The Company continues to seek financing to complete the construction and renovation of its Property, where it intends to build a new headquarters, including a biologics manufacturing facility, upgraded research laboratory facilities, vivarium, and upgraded office and common areas.
Nonoperating Loss
As reported in the Company’s Form 8-K filed on May 1, 2024 with the SEC, the Company became aware that it was the victim of a criminal scheme involving the impersonation of a purchaser upon the default on a legally binding commitment to purchase $8.0 million of secured notes from the Company. The scheme resulted in the misdirection of approximately $1.3 million held in Company accounts to a fraudulent account controlled by a third party. The Company is pursuing all available remedies to recover this loss. Given the limited success that these efforts have had to date for the recovery of funds, the Company recognized a loss of $1.3 million in the three- and nine-month periods ended September 30, 2024.
Legal
Legal Proceedings
From time to time, the Company is a party to or otherwise involved in legal proceedings, including suits, assessments, regulatory actions and investigations generally arising out of the normal course of business. In addition, the Company enters into agreements that may include indemnification provisions, pursuant to which the Company agrees to indemnify, hold harmless and defend the indemnified parties for losses suffered or incurred by the indemnified party. When the Company believes that the outcome of such a matter will result in a liability that is probable to be incurred and result in a potential loss, or range of loss, that can be reasonably estimated, the Company will accrue a liability and make the appropriate disclosure in the footnotes to the financial statements.
Arbitration, Settlement and General Release
On December 23, 2022, Altor BioScience, LLC and NantCell, Inc. (“Altor/NantCell”) initiated an arbitration against Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, in California alleging breach of contract and fiduciary duty, among other claims. On that same date, Altor/NantCell filed a lawsuit against the Company in federal court alleging misappropriation of trade secrets, inducement of breach of contract and breach of fiduciary duty, among other claims against the Company. On April 26, 2023, the parties stipulated that Altor/NantCell’s action against the Company would be consolidated with the Altor/NantCell arbitration demand against Dr. Wong. On April 27, 2023, the Court approved the parties’ stipulation and ordered the parties to arbitration. On May 1, 2023, Altor/NantCell filed a demand against the Company before JAMS. On May 3, 2023, Altor/NantCell dismissed the federal court action without prejudice and the Court ordered the case dismissed without prejudice and closed the case. Altor/NantCell’s proceeding against the Company proceeded in arbitration before JAMS and consolidated with the arbitration Altor/NantCell initiated against Dr. Wong (the “Arbitration”). On March 26, 2024, Altor/NantCell filed a complaint (the “Complaint”) against the Company in the Chancery Court of the State of Delaware for the contribution of legal fees and expenses advanced to Dr. Wong.
As reported in the Company’s Form 8-K filed on July 18, 2024 and described in Part II, Item 1. – “Legal Proceedings” below, as of July 13, 2024, the Company and Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, entered into a confidential Settlement Agreement and Release (the “Settlement Agreement”) with Altor BioScience, LLC (“Altor”), NantCell, Inc. (“NantCell”), and ImmunityBio, Inc. (the parent of Altor and NantCell, together with Altor and NantCell, “ImmunityBio”), to resolve the previously disclosed Arbitration before JAMS brought by Altor and NantCell as well as the Complaint Altor filed against the Company in the Chancery Court of the State of Delaware for the contribution of legal fees and expenses advanced to Dr. Wong. The Settlement Agreement includes mutual general releases by and among the parties thereto. No party is required to make any monetary payments to any other party or person under the Settlement Agreement and each party will bear its own expenses incurred in
connection with the matter. The Company has substantially completed remediation procedures required to be in compliance with the terms of the Settlement Agreement. The Settlement Agreement provides that, upon completion of these procedures, the parties mutually agreed to stipulate that the Arbitration and Complaint should be dismissed.
As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint.
Other Matters
As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction of its new manufacturing facilities and upgraded research laboratories. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements; however, as of the reporting date, to the Company’s knowledge, the lender has not elected to do so.
Inflationary Cost Environment, Banking Crisis, Supply Chain Disruption and the Macroeconomic Environment
The Company’s operations have been affected by many headwinds, including inflationary pressures, rising interest rates, ongoing global supply chain disruptions resulting from increased geopolitical tensions such as the war in the Middle East, the conflict between Russia and Ukraine, China-Taiwan relations, financial market volatility and currency movements. The Company has been impacted by inflation, and may continue to be so, when procuring materials required for the buildout of our new headquarters, the costs for recruiting and retaining employees and other employee-related costs. Management employs a number of strategies to effectively navigate these issues, including product redesign, alternate sourcing, and establishing contingencies in budgeting and timelines. Future developments in these and other areas present material uncertainty and risk with respect to the Company's clinical trials, IND-enabling activities, buildout of the new headquarters, as well as the Company's financial condition and results of operations. The extent and duration of such events and conditions, and resulting disruptions to our operations, are highly unpredictable.
9. Subsequent Events
Subsequent events have been evaluated through the date the financial statements were filed. In addition to the required recognition or disclosure disclosed in the footnotes herein, there were also the following subsequent events after the reporting date:
During October 2024, the Company issued $375,000 in Additional Secured Notes, including a $50,000 purchase by Dr. Wong on October 31, 2024.
On November 13, 2024, we entered into an engagement letter with the Maxim Group to act as the exclusive placement agent for a multi-step equity financing.
As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with (i) our unaudited condensed interim financial statements and related notes appearing elsewhere in this Quarterly Report on Form 10-Q and (ii) our audited financial statements and related notes and the discussion under the heading “Management's Discussion and Analysis of Financial Condition and Results of Operations” for the fiscal year ended December 31, 2023 included in the Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (the “SEC”) on May 15, 2024 (the “Annual Report”). Our historical results are not necessarily indicative of the results that may be expected for any period in the future. Unless the context requires otherwise, references in this Quarterly Report on Form 10-Q to the “Company,” “HCW Biologics,” “HCWB”, “we,” “us” and “our” refer to HCW Biologics Inc.
Forward-Looking Statements
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical facts contained in this quarterly report, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing and likelihood of success of our clinical trials, plans and objectives of management for future operations, adequacy of our cash resources and working capital, future economic conditions or performance, and future results of anticipated products, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this Quarterly Report on Form 10-Q are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in this report in Part II, Item 1A -“Risk Factors,” in this Quarterly Report on Form 10-Q and in other filings we make with the SEC from time to time. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. These forward-looking statements speak only as of the date hereof. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Overview
HCW Biologics Inc. is a clinical-stage biopharmaceutical company focused on discovering and developing novel immunotherapies to lengthen health span by disrupting the link between chronic, low-grade inflammation and age-related diseases. We believe age-related, chronic, low-grade inflammation, or “inflammaging,” is a significant contributing factor to several diseases and conditions, such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and autoimmune diseases. The induction and retention of low-grade inflammation in an aging human body is mainly the result of the accumulation of non-proliferative but metabolically active senescent cells, which can also be caused by persistent activation of protein complexes, known as inflammasomes, in innate immune cells.
These two elements share common mechanisms in promoting secretion of proinflammatory proteins and in many cases interact to drive senescence, and thus, inflammaging. Our novel approach is to reduce senescent cells and eliminate the proinflammatory factors they secrete systemically through multiple pathways. We believe our approach has the potential to fundamentally change the treatment of age-related diseases. Accumulation of senescent cells with senescence-associated proinflammatory factors has been implicated as a major source of chronic sterile inflammation leading to many aging-related pathologies. The key to our immunotherapeutic approach is elimination of senescent cells and the proinflammatory factors they secrete.
The Settlement Agreement among the Company, Dr. Wong, Altor, NantCell and ImmunityBio, which was entered into as of July 13, 2024, and is described in Part II, Item 1. – “Legal Proceedings” below, eliminated the uncertainty of the outcome of the previously disclosed arbitration proceedings and provided clarity for the future direction and emphasis of our clinical development
strategy. The settlement involved intellectual property the Company developed, including the proprietary TOBITM drug discovery platform and its unique Tissue-Factor scaffold used to create protein-fusion molecules that include several elements, such as multiple protein targets, including cytokines, single-chain antibodies, and ligands, as well as proprietary TOBI-based molecules.
The Backbone of the Company’s Clinical Development Program: Two Novel Proprietary Drug Discovery Platforms
Two Novel Drug Discovery Platforms. HCW Biologics created two novel drug discovery platforms, using two distinctly different scaffolds to anchor the composition of immunotherapeutic fusions. This results in the creation of molecules with different mechanisms of action, which opens up different pathways for treatment of diseases promoted by chronic inflammation.
TOBITM (Tissue factOr-Based fusIon) Platform. This patented drug discovery platform is built around a Tissue Factor scaffold. The TOBITM platform packs multiple protein targets, including cytokines, single-chain antibodies, and ligands, into a single fusion molecule. These molecules are capable of engaging immunostimulatory functions and addressing many signaling pathways simultaneously. Some of these fusion protein complexes have ex vivo and in vivo applications, although the Company is primarily focused on drugs developed using subcutaneous injection. Molecules created with the TOBITM platform are reproducible and suitable for cGMP manufacturing. We have manufactured quantities of four TOBI-based molecules in a cGMP manufacturing setting.
The Company’s clinical development program includes the following TOBI-based immunotherapeutics:
HCW9218. This is a clinical-stage bifunctional molecule that can impact senescence by reducing senescent cells (i.e., senescent-cell-reducing effect) and eliminating the proinflammatory factors they secrete (i.e., senomorphic effect). Subcutaneous administration of HCW9218 activates NK cells, innate lymphoid group-1, and CD8+ T cells, and neutralizes TGF-β. Our future HCW9218 program includes a Phase 2 study in ovarian cancer in a neoadjuvant setting. The primary focus for future clinical development of HCW9218 will be in other senescence-associated diseases and conditions beyond cancer.
The Company has exclusive rights to develop treatments for all other age-related diseases other than cancer using HCW9218. We will be able to use the Recommended Phase 2 Dose (“RP2D”) and other learnings based on findings in the Phase 1 studies which wrapped up at the beginning of 2024.
The Company has non-exclusive rights to develop HCW9218 treatments for ovarian cancer in a neoadjuvant setting. The University of Pittsburgh Medical Center (“UMPC”) is the sponsor for an ongoing Phase 2 clinical study to evaluate HCW9218 in the treatment of ovarian cancer in a neoadjuvant setting. For this study, HCW9218 will be administered in combination with neoadjuvant chemotherapy as a first-line treatment with neoadjuvant chemotherapy alone serving as a control arm. The Company has learned that UPMC may change the principal investigator for this study, due to some changes at the institution. Patient recruitment is in progress, but no patients have been enrolled in this study as of the reporting date. Under the terms of the Settlement Agreement, UPMC has until year end to launch this study.
The Settlement Agreement among the Company, Dr. Wong, Altor, NantCell and ImmunityBio, which was entered into as of July 13, 2024, and is described in Part II, Item 1. – “Legal Proceedings” below, eliminated the uncertainty of the outcome of the previously disclosed arbitration proceedings and provided clarity for the future direction and emphasis of our clinical development strategy. However, under the Settlement Agreement, the Company agreed to transfer the HCW9218 master cell line to ImmunityBio. In turn, ImmunityBio agreed to enter into a supply agreement with the Company by the end of January 2025 to ensure clinical supply of HCW9218 is available to carry on clinical studies.
HCW9302. HCW9302 is the basis for the Company’s autoimmune program. Subcutaneous administration of HCW9302 is designed to activate and expand Treg cells to reduce senescence by suppressing the activity of inflammasome-bearing immune cells and the inflammatory factors which they secrete.
The Company filed our IND application to the U.S. Food and Drug Administration (“FDA”), which is currently under review. The Company is seeking approval to conduct a clinical study to evaluate HCW9302 in an autoimmune indication. The Company cannot provide any assurance that the FDA will authorize it to initiate its planned clinical trials on a timely basis, or at all. In the event that the FDA does not accept the Company’s IND, the Company may also be required to seek feedback, and the feedback may be unfavorable. In the event that the Company does not receive feedback on a timely basis, or the Company is required to change the design of the clinical protocol or address other feedback, clinical development of the products would be delayed and the costs may increase. Moreover, if the FDA does not accept the Company’s IND, the Company may be required to conduct additional preclinical testing or other IND-enabling activities, which would result in further delay and additional costs.
We intend to explore utility of HCW9302 for other aging-related diseases, such as neurodegenerative diseases, in relevant animal models. Our plan is to identify the RP2D in patients based on a Phase 1b clinical trial to evaluate HCW9302 in an autoimmune indication, then expand to a neurodegenerative disease indication in a Phase 2 trial utilizing this RP2D.
HCW9206. HCW Biologics maintains the rights to the injectable form of HCW9206. This form of HCW9206 is preclinical, and we are beginning IND-enabling activities. Ex vivo rights have been licensed to Wugen. This form of HCW9206 is clinical stage, and Wugen is evaluating it in combination with HCW9201 in a Phase 1 clinical trial. HCW9206 has a unique design consisting of a multi-functional compound constructed with three powerful cytokines: IL-7, IL-15, and IL-21. It is designed to stimulate T cell proliferation and activation, enhance NK cell cytotoxicity, and improve overall immune surveillance against pathogens or tumors. HCW9206 is being considered to be the basis of our future oncology and anti-infectious disease programs.
HCW9201. This is a clinical-stage molecule currently being evaluated by Wugen in a Phase 1 clinical trial in Acute Myeloid Leukemia, as a cell-based treatment. We have not yet initiated any clinical trials to evaluate HCW9201 in other indications. We retain the rights for administration by subcutaneous injection. HCW9201 has a unique design that results in a multi-functional compound with three powerful cytokines, IL-12, IL-15, and IL-18, in a single protein complex. We are exploring intra-tumoral injection of HCW9201 for treatment of cancer.
New Drug Discovery Platform with Novel Protein-Based Scaffold. Our recently discovered a new drug development platform uses a novel protein scaffold that is not based on tissue factor, which differentiates it from TOBITM platform. With immunotherapeutic compounds that are constructed with this new platform without tissue factor, we expand the diseases and conditions that may be treated. The Company has already used this novel protein scaffold to construct several immunotherapeutic fusions, which we are testing to determine the most promising programs, including those that could be licensed to others.
This scaffold has the capability to construct multiple classes of immunotherapeutic compounds, including:
•Multifunctional, cytokine-based immunotherapeutic compounds.
•Constructs with immune-cell engagers targeting tissue factor and other cell-surface antigens associated with diseased cells.
•Multifunctional immunotherapeutic fusions which improve the performance of immune checkpoint inhibitors.
The Company is conducting rigorous preclinical animal studies using several different models to characterize these new compounds and identify those that indicate the greatest potential to evaluate in treatments of cancer and other diseases promoted by chronic inflammation. The clinical development plan is for at least one molecule constructed with this novel platform to be in clinical development in 2026, either through a Company-owned program or through an out-licensing program.
Business and Financing Strategies
The Company is exploring numerous additional molecules with substantial potential applications. The Company considers business development transactions, such as out-licensing agreements, to be fundamental elements of our financing strategy to provide future non-dilutive capital required to fund clinical development of the Company’s lead immunotherapeutic programs.
We signed our first out-license agreement at the end of 2020, which was an exclusive worldwide license (the “Wugen License”) with Wugen, Inc. (“Wugen”), a company that specializes in cell-based therapies for cancer. As part of the upfront license fee, the Company received shares of Wugen’s common stock that currently represents a 5.6% ownership interest based on fully converted issued and outstanding shares. Wugen licensed limited rights to develop, manufacture, and commercialize cell-based therapy treatments for cancer based on two of our internally-developed, multi-cytokine fusion protein molecules, HCW9201 and HCW9206. The licensed molecules are the basis for Wugen’s memory-like NK cell therapy program. Wugen also has a promising CAR-T program, based on its lead molecule WU-CART-007. The WU-CART007 program was recognized by two regulatory designations by the FDA and EU, respectively, that have expedited regulatory review. Wugen has been cleared to initiate a pivotal Phase 2 study in the first quarter of 2025 for its first-in-class, investigational, anti-CD7 CAR-T cell therapy, WU-CART-007, in patients with relapsed or refractory (R/R) T cell acute lymphoblastic leukemia or T cell lymphoblastic lymphoma (T-ALL/LBL).
We plan to continue to work with the National Institutes of Health and the National Cancer Institute (“NCI”) for collaborative agreements and assistance with funding research and clinical studies. In 2022, the Company entered a Cooperative Research and Development Agreement (“CRADA”), and under the CRADA we collaborated with NCI on a Phase 1b/2 clinical study in patients with advanced/metastatic and chemo-refractory/chemo-resistant pancreatic cancer. Our plan is to leverage these relationships to benefit the clinical development of HCW9206 and immunotherapeutic fusions we create in the future in cancer indications.
The Company remains committed to establishing some control over our clinical supply of materials, and the supply of licensed molecules for our licensees. The Company has retained manufacturing rights for the licensed molecules under the Wugen License and intends to use the same framework in future if we enter other licensing agreements. On September 9, 2024, the House of Representatives passed the BIOSECURE Act (the “Act”). With strong bi-partisan support, the bill is expected to become law in 2024. Somewhat unusually, the Act names specific companies as automatically qualifying as “biotechnology companies of concern.” The expectation is that this provision of the Act would effectively prohibit U.S. companies from doing business with five Chinese firms, including WuXi AppTec, Complete Genomics and MGI Tech. For life sciences companies, WuXi is one of the largest global providers of contract development and manufacturing services in the world. By some estimates, WuXi, an important partner with many biotech and pharma companies, is involved in developing 25% of the drugs used in the United States. The Act would effectively ban WuXi from conducting business in the United States, which is expected to disrupt the supply chain for life sciences companies. The result is expected to produce delays, price increases, and supply shortages for many years as the industry replaces WuXi’s capacity as well as other firms named in the Act.
Business Highlights
Remediation and Dismissal of Arbitration
•The Settlement Agreement among the Company, Dr. Wong, Altor, NantCell and ImmunityBio, which was entered into as of July 13, 2024, and is described in Part II, Item 1. – “Legal Proceedings” below.
•As required under the terms of the Settlement Agreement, the Company has substantially completed remediation procedures required to be in compliance with the terms of the Settlement Agreement.
•Under the provisions of the Settlement Agreement, upon completion of remediation procedures, the parties mutually agreed to stipulate that the Arbitration and Complaint should be dismissed. As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint.
Financing
•In the three-month period ended September 30, 2024, the Company launched a new financing plan. With the execution of the Settlement Agreement, we resolved the attendant uncertainties for the outcome of the arbitration and additional complexities that hampered our ability to raise capital.
•We have a multi-faceted financing plan, and have already taken the first steps to put on financing plan into action during the third quarter of 2024:
oLicensing non-core assets is a fundamental facet of our financing plan. On September 25, 2024, the Company entered into a nonbinding term sheet with a party interested in licensing one of the Company’s preclinical molecules. The proposed license is for one of the Company’s preclinical molecules created with our new platform. The Company has generated data from testing conducting in various animal models which have met requirements of the prospective licensee. Definitive agreements are being finalized and the closing is expected in the fourth quarter of 2024. The proposed license agreement includes guaranteed minimum payments which are expected within the first year of the term.
oWe have launched a multi-step equity financing plan. On November 13, 2024, we entered into an engagement letter with the Maxim Group to act as the exclusive placement agent for equity financings.
oThrough October 31, 2024, the Company issued $6.9 million Secured Notes, secured by the Company’s Wugen shares. We are authorized to issue up to $10.0 million in Secured Notes and we continue our fund-raising efforts to issue the fully authorized amount. Additional closings can take place by amending the purchase agreement.
oThe financing plan includes regaining compliance with all Listing Qualifications of the Nasdaq Stock Market LLC (“Nasdaq”) for the Nasdaq Global Market. We have a deadline regain compliance by December 16, 2024. We intend to take all reasonable measures available to us to regain compliance with the continued listing requirements for the Nasdaq Global Market, and we will utilize our right to appeal to Nasdaq to extend our deadline to regain compliance based on a solid financial plan that we are already beginning to execute.
•As of September 30, 2024, we believe that substantial doubt exists regarding our ability to continue as a going concern for at least 12 months from the date of issuance, without additional funding or financial support. After considering elements of our financing plan that were probable to occur within a year of the date of issuance, we concluded that substantial doubt was not alleviated in the going concern analysis.
Clinical Development
•There is an ongoing Investigator-sponsored Phase 2 clinical trial to evaluate HCW9218 in patients with metastatic advanced stage ovarian cancer in combination with neoadjuvant chemotherapy, sponsored by UPMC. (NCT05145569)
•We have filed our IND application to seek approval from the FDA to conduct clinical trials to evaluate HCW9302 in an autoimmune disease, and we are currently under review. In the review process, we will receive feedback from the FDA which may not be given on a timely basis, or we may be required to change the design of our clinical protocol in order to address the feedback, potentially resulting in delays and increased costs.
•The Company has filed provisional patents for its new drug discovery platform and several immunotherapeutic compounds constructed using this platform, based on a novel backbone different from Tissue Factor. This new drug discovery platform will open new pathways to create first-in-class immunotherapy for the treatment of diseases promoted by chronic inflammation.
Trends and Uncertainties
Inflationary Cost Environment, Banking Crisis, Supply Chain Disruption and the Macroeconomic Environment
Our operations have been affected by many headwinds, including inflationary pressures, rising interest rates, ongoing global supply chain disruptions resulting from increased geopolitical tensions such as the war between Russia and Ukraine, the war in the Middle East, China-Taiwan relations, financial market volatility and currency movements. These headwinds, specifically the supply chain disruptions, have adversely impacted our ability to procure certain services and materials, which in some cases impacts the cost and timing of clinical trials and IND-enabling activities. In addition, we have been impacted by inflation when procuring materials required for the buildout of our new headquarters, the costs for recruiting and retaining employees and other employee-related costs. Further, rising interest rates have also increased borrowing costs. The Company uses a number of strategies to effectively navigate these issues, including product redesign, alternate sourcing, and establishing contingencies in budgeting and timelines. However, the extent and duration of such events and conditions, and resulting disruptions to our operations, are highly unpredictable.
For discussion of risks related to potential impacts of supply chain, inflation, geopolitical and macroeconomic challenges on our operations, business results and financial condition, see Part II, Item 1A. – “Risk Factors” in the Company’s Annual Report.
Components of our Results of Operation
Revenues
We have no products approved for commercial sale and have not generated any revenue from commercial product sales of internally-developed immunotherapeutic products for the treatment of cancer and other age-related diseases. The principal source of our revenues to date have been generated from our Wugen License and Master Services Agreement (the “MSA”) with Wugen. See Note 1 to our condensed interim financial statements included elsewhere in this Quarterly Report for these definitions and more information.
We derive revenue from a license agreement granting rights to Wugen to further develop and commercialize products based on two of our internally-developed molecules. Consideration under our contract included a nonrefundable upfront payment, development, regulatory and commercial milestones, and royalties based on net sales of approved products. Additionally, HCW Biologics retained manufacturing rights and has agreed to provide Wugen with clinical and research grade materials for clinical development and commercialization of licensed products under separate agreements. We assessed which activities in the Wugen License should be considered distinct performance obligations that should be accounted for separately. We develop assumptions that require judgement to determine whether the license to our intellectual property is distinct from the research and development services or participation in activities under the Wugen License.
Performance obligations relating to the granting a license and delivery of licensed product and R&D know-how were satisfied when transferred upon the execution of the Wugen License on December 24, 2020. The Company recognized revenue for the related consideration at a point in time. The revenue recognized from a transaction to supply clinical and research grade materials entered into under the MSA and covered by a Statement of Work, represents one performance obligation that is satisfied over time. The Company recognizes revenue generated for supply of material for clinical development using an input method based on the costs incurred relative to the total expected cost, which determines the extent of the Company’s progress toward completion.
Operating Expenses
Our operating expenses are reported as research and development expenses and general and administrative expenses.
Research and Development
Our research and development expenses consist primarily of costs incurred for the development of our product candidates, which include:
•Employee-related expenses, including salaries, benefits, and stock-based compensation expense;
•Expenses related to manufacturing and materials, consisting primarily of expenses incurred primarily in connection with CMOs, which produce cGMP materials for clinical trials on our behalf;
•Expenses associated with preclinical activities, including research and development and other IND-enabling activities;
•Expenses incurred in connection with clinical trials; and
•Other expenses, such as facilities-related expenses, direct depreciation costs for capitalized scientific equipment, and allocation for overhead.
We expense research and development costs as they are incurred. Costs for contract manufacturing are recognized based on an evaluation of the progress to completion of specific tasks using information provided to us by our vendors. Payments for these activities are based on the terms of the agreement, and the pattern of payments for goods and services will change depending on the material. Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses and expensed as the related goods are delivered or the services are performed.
We expect research and development expenses to increase substantially for the foreseeable future as we continue the development of our product candidates. We cannot reasonably determine the nature, timing, and costs of the efforts that will be necessary to complete the development of, and obtain regulatory approval for, any of our product candidates. Product candidates in later stages of development generally have higher development costs than those in earlier stages. See “Risk Factors – Risks Related to the Development and Clinical Testing of Our Product Candidates,” in our Annual Report for a discussion of some of the risks and uncertainties associated with the development and commercialization of our product candidates. Any changes in the outcome of any of these risks and uncertainties with respect to the development of our product candidates in preclinical and clinical development could mean a significant change in the costs and timing associated with the development of these product candidates. For example, if the FDA or another regulatory authority were to delay our planned start of clinical trials or require us to conduct clinical trials or other testing beyond those that we currently expect or if we experience significant delays in enrollment in any of our planned clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development of that product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of employee-related expenses, including salaries, related benefits, and stock-based compensation expense for employees in the executive, legal, finance and accounting, human resources, and other administrative functions. General and administrative expenses also include third-party costs such as insurance costs, fees for professional services, such as legal fees in the ordinary course of business, auditing and tax services, facilities administrative costs, and other expenses.
We expect general and administrative expenses incurred in the normal course of business for other purposes, such as costs for recruitment and retention of personnel, service fees for consultants, advisors and accountants, as well as costs to comply with government regulations, corporate governance, internal control over financial reporting, insurance and other requirements for a public company, to continue to increase for the foreseeable future as we build our clinical programs.
Legal Expenses
Legal expenses consist of fees incurred by the Company in its own defense and that of officers and employees in connection with a legal matter brought against the Company and Dr. Hing C. Wong, our Founder and Chief Executive Officer, by a former employer of Dr. Wong.
During the period ended December 31, 2022, Altor/NantCell initiated legal proceedings against Dr. Wong and the Company. On April 26, 2023, the parties stipulated that Altor/NantCell’s action against the Company would be consolidated with the Altor/NantCell arbitration demand against Dr. Wong. On April 27, 2023, the U.S. District Court for the Southern District of Florida (the “Court”) with jurisdiction over the lawsuit against the Company approved the parties’ stipulation and ordered the parties to arbitration. On May 1, 2023, Altor/NantCell filed a demand against the Company before JAMS. On May 3, 2023, Altor/NantCell dismissed the federal court action without prejudice and the Court ordered the case dismissed without prejudice and closed the case. Proceedings against the Company and Dr. Wong were consolidated in the arbitration before JAMS (“Arbitration”). On March 26, 2024, Altor/NantCell filed a complaint (the “Complaint”) against the Company in the Chancery Court of the State of Delaware for the contribution of legal fees and expenses advanced to Dr. Wong. The arbitration hearing was held on May 20, 2024 to May 31, 2024, after which the parties entered into settlement negotiations.
As reported in the Company’s Form 8-K filed on July 18, 2024 and further described in Part II, Item 1. – “Legal Proceedings” below, as of July 13, 2024, the Company and Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, entered into a confidential Settlement Agreement and Release (the “Settlement Agreement”) with Altor BioScience, LLC (“Altor”), NantCell, Inc. (“NantCell”), and ImmunityBio, Inc. (the parent of Altor and NantCell, together with Altor and NantCell, “ImmunityBio”), to resolve the previously disclosed Arbitration before JAMS brought by Altor and NantCell as well as a Complaint Altor filed against the Company in the Chancery Court of the State of Delaware for the contribution of legal fees and expenses advanced to Dr. Wong. The Settlement Agreement includes mutual general releases by and among the parties thereto. No party is required to make any monetary payments to any other party or person under the Settlement Agreement and each party will bear its own expenses incurred in connection with the matter. The Company has substantially completed remediation procedures required to be in compliance with the terms of the Settlement Agreement. Under the Settlement Agreement, upon completion of these procedures, the parties mutually agreed to stipulate that the Arbitration and Complaint should be dismissed. In accordance with 17 CFR 229.601 (Item 601), the Company has included the Settlement Agreement in this Quarterly Report on Form 10-Q. As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint.
Nonoperating Loss
As reported in the Company’s Form 8-K filed on May 1, 2024 with the SEC, the Company became aware that it was the victim of a criminal scheme involving the impersonation of a purchaser upon the default on a legally binding commitment to purchase $8.0 million of secured notes from the Company. The scheme resulted in the misdirection of approximately $1.3 million held in Company accounts to a fraudulent account controlled by a third party. The Company is pursuing all available remedies to recover this loss. Given the limited success that these efforts have had to date for the recovery of funds, the Company recognized a loss of $1.3 million in the nine-month period ended September 30, 2024.
Interest Expense
Interest expense includes interest paid on debt. This includes interest due on the Cogent Bank loan and senior secured notes issued by the Company.
Other Income, Net
Other income, net consists of interest earned on our cash, cash equivalents, unrealized gains and losses related to our investments in U.S. government-backed securities, and other income and expenses related to non-operating activities.
Results of Operations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
|
Nine Months Ended
September 30, |
|
|
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
853,102 |
|
|
$ |
426,423 |
|
|
$ |
1,517,792 |
|
|
$ |
2,171,988 |
|
|
Cost of revenues |
|
|
(678,325 |
) |
|
|
(341,138 |
) |
|
|
(1,210,077 |
) |
|
|
(1,291,546 |
) |
|
Net revenues |
|
|
174,777 |
|
|
|
85,285 |
|
|
|
307,715 |
|
|
|
880,442 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
1,667,442 |
|
|
|
1,186,913 |
|
|
|
5,539,919 |
|
|
|
5,339,383 |
|
|
General and administrative |
|
|
1,509,936 |
|
|
|
1,639,152 |
|
|
|
5,106,674 |
|
|
|
4,799,437 |
|
|
Legal expenses |
|
|
2,075,279 |
|
|
|
949,455 |
|
|
|
4,610,091 |
|
|
|
15,761,531 |
|
|
Nonoperating loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,300,000 |
|
|
Total operating expenses |
|
|
5,252,657 |
|
|
|
3,775,520 |
|
|
|
15,256,684 |
|
|
|
27,200,351 |
|
|
Loss from operations |
|
|
(5,077,880 |
) |
|
|
(3,690,235 |
) |
|
|
(14,948,969 |
) |
|
|
(26,319,909 |
) |
|
Interest expense |
|
|
(95,514 |
) |
|
|
(223,363 |
) |
|
|
(284,465 |
) |
|
|
(383,029 |
) |
|
Other income, net |
|
|
234,753 |
|
|
|
11,310 |
|
|
|
919,688 |
|
|
|
52,397 |
|
|
Net loss |
|
$ |
(4,938,641 |
) |
|
$ |
(3,902,288 |
) |
|
$ |
(14,313,746 |
) |
|
$ |
(26,650,541 |
) |
|
Comparison of the Three Months ended September 30, 2023 and September 30, 2024
Revenues
The Company recognized revenues of $853,102 and $426,423 for the three months ended September 30, 2023 and 2024, respectively. Revenues were derived exclusively from the sale of licensed molecules to Wugen. Under the terms of the supply agreement between Wugen and the Company, the Company earns an industry-standard gross margin. Occasionally, Wugen acquires product which is part of inventory we made for our own use. In these instances, we do not apply the standard costs since the cost of manufacturing these materials would have already been expensed in a prior period.
Research and Development Expenses
The following table summarizes our research and development expenses for the three months ended September 30, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
|
|
|
|
|
|
|
|
2023 |
|
|
2024 |
|
|
$ Change |
|
|
% Change |
|
Salaries, benefits and related expenses |
|
$ |
692,607 |
|
|
$ |
652,867 |
|
|
$ |
(39,740 |
) |
|
|
(6 |
)% |
Manufacturing and materials |
|
|
206,150 |
|
|
|
47,748 |
|
|
|
(158,402 |
) |
|
|
(77 |
)% |
Preclinical expenses |
|
|
306,344 |
|
|
|
144,746 |
|
|
|
(161,598 |
) |
|
|
(53 |
)% |
Clinical trials |
|
|
343,821 |
|
|
|
164,139 |
|
|
|
(179,682 |
) |
|
|
(52 |
)% |
Other expenses |
|
|
118,520 |
|
|
|
177,413 |
|
|
|
58,893 |
|
|
|
50 |
% |
Total research and development expenses |
|
$ |
1,667,442 |
|
|
$ |
1,186,913 |
|
|
$ |
(480,529 |
) |
|
|
(29 |
)% |
Research and development expenses decreased by $480,529, or 29%, from $1.7 million for the three months ended September 30, 2023 to $1.2 million for the three months ended September 30, 2024. The decrease was primarily attributable to a decrease in expenses for manufacturing and materials, preclinical activities and clinical studies.
Salaries, benefits, and related expenses decreased by $39,740, or 6%, from $692,607 for the three months ended September 30, 2023 to $652,867 for the three months ended September 30, 2024. The decrease reflects cost cutting measures put in place in the second quarter of 2024, which resulted in a $11,572 decrease in salaries and related taxes and a $29,801 decrease in costs related to employee benefits for the three-month period ended September 30, 2024.
Manufacturing and materials expense decreased by $158,402, or 77%, from $206,150 for the three months ended September 30, 2023 to $47,748 for the three months ended September 30, 2024. In the three months ended September 30, 2023, costs were incurred primarily for master cell bank characterization for HCW9101H, a high-producing cell line of a key component of the manufacturing process for the Company's proprietary molecules including those licensed to Wugen, as well as ancillary activities such as shipping, insurance and storage. In the three months ended September 30, 2024, costs were associated with ancillary activities such as shipping and storage.
Expenses associated with preclinical activities decreased by $161,598, or 53%, from $306,344 for the three months ended September 30, 2023 to $144,746 for the three months ended September 30, 2024. In the three months ended September 30, 2023 and 2024, costs were incurred primarily for IND-enabling studies to prepare for the submission of an IND application to evaluate HCW9302 in a clinical study.
Expenses associated with clinical activities decreased by $179,682, or 52%, from $343,821 for the three months ended September 30, 2023 to $164,139 for the three months ended September 30, 2024. The decrease in costs was primarily attributable a $188,637 decrease in the expenses associated with patient fees, partially offset by a $9,290 increase in consulting and other professional fees.
Subject to our ability to successfully execute our plans to obtain financing, we anticipate expenses related to clinical activities will increase substantially in the future, in the event that we successfully advance our immunotherapeutic fusion molecules in clinical development. If we are unable to complete planned capital-raising transactions and business development transactions for out-licensing, we may have to curtail or cease operations.
Other expenses, which include overhead allocations, increased by $58,893, or 50%, from $118,520 for the three months ended September 30, 2023 to $177,413 for the three months ended September 30, 2024. This increase is primarily attributable to a change in allocation of overhead to cost of good sold and a $62,031 decrease in travel-related expenses partially offset by a $9,786 increase in costs associated with IT, office supplies and other office equipment.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the three months ended September 30, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
|
|
|
|
|
|
|
|
2023 |
|
|
2024 |
|
|
$ Change |
|
|
% Change |
|
Salaries, benefits and related expenses |
|
$ |
775,956 |
|
|
$ |
619,070 |
|
|
$ |
(156,886 |
) |
|
|
(20 |
)% |
Professional services |
|
|
207,815 |
|
|
|
376,910 |
|
|
|
169,095 |
|
|
|
81 |
% |
Facilities and office expenses |
|
|
175,694 |
|
|
|
134,580 |
|
|
|
(41,114 |
) |
|
|
(23 |
)% |
Depreciation |
|
|
63,083 |
|
|
|
69,370 |
|
|
|
6,287 |
|
|
|
10 |
% |
Rent and occupancy expense |
|
|
36,372 |
|
|
|
49,160 |
|
|
|
12,788 |
|
|
|
35 |
% |
Other expenses |
|
|
251,016 |
|
|
|
390,062 |
|
|
|
139,046 |
|
|
|
55 |
% |
Total general and administrative expenses |
|
$ |
1,509,936 |
|
|
$ |
1,639,152 |
|
|
$ |
129,216 |
|
|
|
9 |
% |
General and administrative expenses related to the ordinary course of business increased by $129,216, or 9%, from $1.5 million for the three months ended September 30, 2023 to $1.6 million for the three months ended September 30, 2024. The increase is primarily attributable to an increase in professional services, mainly legal fees related to patents, and an increase in other expenses, mainly costs related to insurance, offset by a decrease in salaries and benefits.
Salaries, benefits and related expenses decreased by $156,886, or 20%, from $775,956 for the three months ended September 30, 2023 to $619,070 for the three months ended September 30, 2024. The decrease reflects cost cutting measures put in place in the second quarter of 2024, which resulted in a $152,332 decrease in salaries and related taxes and a $15,511 decrease in costs related to employee benefits for the three-month period ended September 30, 2024. These costs were offset by an increase of $10,957 in expenses related to stock-based compensation.
Professional services increased by $169,095, or 81%, from $207,815 for the three months ended September 30, 2023 to $376,910 for the three months ended September 30, 2024. Professional services include corporate legal services, legal services for procuring patents, as well as other professional services, such as auditing and tax advisory fees. The increase is primarily attributable to a $133,263 increase legal fees for services incurred in connection with procuring patents and a $35,832 increase in costs related to other professional services such as auditing and tax advisory fees. As a result of the Settlement Agreement, the Company agreed to maintain patent protection for rights retained under the agreement.
Facilities and office expenses decreased by $41,114, or 23%, from $175,694 for the three months ended September 30, 2023 to $134,580 for the three months ended September 30, 2024, primarily due to a $62,791 decrease in software and other licensing fees, partially offset by a $13,558 increase in other IT related expenses and a $10,818 increase in facilities expenses such as electricity and waste disposal.
Other expenses increased by $139,046, or 55%, from $251,016 for the three months ended September 30, 2023 to $390,046 for the three months ended September 30, 2024. The increase is primarily attributable to a $115,668 increase in insurance-related costs and a $37,730 increase in finance costs.
Legal Expenses
Legal expenses were $2.1 million in the three months ended September 30, 2023 and $1.0 million for the three months ended September 30, 2024. In the three months ended September 30, 2023, costs were incurred in connection with several legal proceedings which culminated by consolidating Altor/NantCell’s action against the Company with the Altor/NantCell arbitration demand against Dr. Wong. Thereafter, proceedings against the Company and Dr. Wong were consolidated in the arbitration before JAMS.
In the three months ended September 30, 2024, the Company reached a Settlement Agreement with the other parties to the arbitration and undertook the necessary steps for remediation and transfer of information required under the terms of the agreement. As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint. While the Company has relief from the future burden of ongoing legal expenses related to these proceedings, we incurred significant legal expenses for our defense and for the defense of officers and employees. We require a reasonable payment plan to prevent these expenses from overwhelming the Company’s resources. We are engaged in discussions with the law firms involved with this matter to work out payment plans.
Interest Expense
On August 15, 2022, we entered into a loan and security agreement with Cogent Bank to partially fund our purchase of the property we acquired on that same date. We borrowed $6.5 million under this agreement. Amounts outstanding on the loan accrue interest at a rate per annum equal to 5.75%. We were obligated to make interest-only payments on this loan from September 2022 through August 2023 and principal and interest payments in 47 equal monthly installments, based on a 25-year maturity schedule, commencing September 15, 2023. We paid $95,514 and $93,936 in cash for interest on the Cogent Bank loan for the three months ended September 30, 2023 and 2024, respectively. For the three months ended September 30, and September 30, 2024, interest was expensed.
As of September 30, 2024, the Company had issued $6.5 million in senior secured notes which bear interest at an annual rate of 9%, payable quarter in arrears. We paid $87,386 in cash interest on the senior secured notes for the three months ended September 30, 2024. There were no senior secured notes outstanding in 2023.
Other Income, Net
Other income, net decreased from $234,753 for the three months ended September 30, 2023 to $11,310 for the three months ended September 30, 2024. The decrease is primarily attributable to a decline in interest earned from money market deposits and a decrease in unrealized gains from investments in U.S. government-backed securities. In addition, for the three months ended September 30, 2023, Other income included rental income. On August 15, 2022, the Company entered into a short-term, market-rate lease with the former owner of the building we purchased on the same date, which terminated in the year ended December 31, 2023. We received rental income of $60,003 for the three months ended September 30, 2023.
Comparison of the Nine Months ended September 30, 2023 and September 30, 2024
Revenues
The Company recognized $1.5 million and $2.2 million of revenues for the nine months ended September 30, 2023 and 2024, respectively. Revenues were derived exclusively from the sale of licensed molecules to Wugen. Under the terms of the supply
agreement between Wugen and the Company, the Company earns an industry-standard gross margin. Occasionally, Wugen acquires product which is part of inventory we made for our own use. In these instances, we do not apply the standard costs since the cost of manufacturing these materials would have already been expensed in a prior period.
Research and Development Expenses
The following table summarizes our research and development expenses for the nine months ended September 30, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
September 30, |
|
|
|
|
|
|
|
|
|
2023 |
|
|
2024 |
|
|
$ Change |
|
|
% Change |
|
Salaries, benefits and related expenses |
|
$ |
2,195,265 |
|
|
$ |
2,189,250 |
|
|
$ |
(6,015 |
) |
|
|
(0 |
)% |
Manufacturing and materials |
|
|
623,785 |
|
|
|
1,375,830 |
|
|
|
752,045 |
|
|
|
121 |
% |
Preclinical expenses |
|
|
1,367,725 |
|
|
|
688,310 |
|
|
|
(679,415 |
) |
|
|
(50 |
)% |
Clinical trials |
|
|
788,116 |
|
|
|
495,910 |
|
|
|
(292,206 |
) |
|
|
(37 |
)% |
Other expenses |
|
|
565,028 |
|
|
|
590,083 |
|
|
|
25,055 |
|
|
|
4 |
% |
Total research and development expenses |
|
$ |
5,539,919 |
|
|
$ |
5,339,383 |
|
|
$ |
(200,536 |
) |
|
|
(4 |
)% |
Research and development expenses decreased by $200,536, or 4%, from $5.5 million for the nine months ended September 30, 2024 to $5.3 million for the nine months ended September 30, 2024, primarily due to an increase in manufacturing and materials expenses, partially offset by a decrease in preclinical and clinical expenses.
Salaries, benefits, and related expenses decreased by $6,015, or 0%, from $2.2 million for the nine months ended September 30, 2023 to $2.2 million for the nine months ended September 30, 2024. This decrease was primarily attributable to a difference in the costs allocated to cost of goods sold, as well as a $121,139 decrease in salaries, benefits and related expenses as a result of cost cutting measures put in place in the second quarter of 2024, consisting of a $83,893 decrease in salaries, bonuses and related taxes and a $29,185 decrease in costs related to employee benefits, for the nine-month period ended September 30, 2024.
Manufacturing and materials expense increased by $752,045, or 121%, from $623,785 for the nine months ended September 30, 2023 to $1.4 million for the nine months ended September 30, 2024. In the nine months ended September 30, 2023, costs were incurred primarily for production activities associated with the master cell bank characterization for a high production cell line for HCW9101; a 200L cGMP manufacturing run of HCW9302; and ancillary activities such as shipping, insurance and storage. In the nine months ended September 30, 2024, costs were primarily attributable to the costs of production and materials related to manufacturing the high producing cell-line of HCW9101, which is used in the manufacturing process for all TOBI-based molecules.
Expenses associated with preclinical activities decreased by $679,415, or 50%, from $1.4 million for the nine months ended September 30, 2023 to $688,310 for the nine months ended September 30, 2024. In the nine months ended September 30, 2023, costs were incurred to complete the toxicology study and for additional studies required for submission of the HCW9302 IND. In the nine months ended September 30, 2024, toxicology and other IND-enabling studies were winding down, and the IND application was finalized, submitted and is now under review.
Expenses associated with clinical activities decreased by $292,206, or 37%, from $788,116 for the nine months ended September 30, 2023 to $495,910 for the nine months ended September 30, 2024. The decrease was primarily attributable to a $341,271 decrease in patient fees, partially offset by a $61,080 increase costs for post-clinical studies that we conducted through collaborations. In the nine months ended September 30, 2023, there were two ongoing clinical trials, including a Phase 1 study sponsored by the University of Minnesota to evaluate HCW9218 in the treatment of various solid tumors, and a Company-sponsored Phase 1b clinical trial to evaluate HCW9218 in chemo-refractory/chemo-resistant pancreatic cancer. In the nine months ended September 30, 2024, we completed enrollment in the Phase 1/1b clinical trials and the majority of our activities were focused on post-clinical correlative studies which we conducted through collaborations. As a result of the Settlement Agreement, ImmunityBio has the exclusive right to the use of HCW9218 in the treatment of pancreatic cancer. We will continue to evaluate HCW9218 in combination with neoadjuvant chemotherapy in the treatment of ovarian cancer.
Other expenses, which include overhead allocations, increased by $25,055, or 4%, from $565,028 for the nine months ended September 30, 2023 to $590,083 for the nine months ended September 30, 2024. The decrease in other expenses is primarily attributable to a decrease in the allocation of depreciation to cost of goods sold, partially offset by a $38,055 decrease in travel-related expenses.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the nine months ended September 30, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
September 30, |
|
|
|
|
|
|
|
|
|
2023 |
|
|
2024 |
|
|
$ Change |
|
|
% Change |
|
Salaries, benefits and related expenses |
|
$ |
2,408,622 |
|
|
$ |
1,855,660 |
|
|
$ |
(552,962 |
) |
|
|
(23 |
)% |
Professional services |
|
|
1,008,807 |
|
|
|
960,680 |
|
|
|
(48,127 |
) |
|
|
(5 |
)% |
Facilities and office expenses |
|
|
439,373 |
|
|
|
542,890 |
|
|
|
103,517 |
|
|
|
24 |
% |
Depreciation |
|
|
188,454 |
|
|
|
203,050 |
|
|
|
14,596 |
|
|
|
8 |
% |
Rent expense |
|
|
118,296 |
|
|
|
155,860 |
|
|
|
37,564 |
|
|
|
32 |
% |
Other expenses |
|
|
943,122 |
|
|
|
1,081,297 |
|
|
|
138,175 |
|
|
|
15 |
% |
Total general and administrative expenses |
|
$ |
5,106,674 |
|
|
$ |
4,799,437 |
|
|
$ |
(307,237 |
) |
|
|
(6 |
)% |
General and administrative expenses related to the ordinary course of business decreased by $307,237, or 6%, from $5.1 million for the nine months ended September 30, 2023 to $4.8 million for the nine months ended September 30, 2024. The decrease is primarily attributable to a decrease in salaries and bonuses as a result of cost-cutting measures put in place in the second quarter of 2024.
Salaries, benefits and related expenses decreased by $552,962, or 23%, from $2.4 million for the nine months ended September 30, 2023 to $1.9 million for the nine months ended September 30, 2024. The decrease is primarily attributable to a $212,014 decrease in salaries and related taxes, a $19,372 decrease in expenses for employee benefits, and a $304,175 decrease resulting from the waiver of performance-based bonuses earned in 2022 but deferred.
Professional services decreased by $48,127, or 5%, from $1,008,807 for the nine months ended September 30, 2023 to $960,680 for the nine months ended September 30, 2024. Professional services include corporate legal services, legal services for procuring patents, as well as other professional services, such as auditing and tax advisory fees. The decrease is primarily attributable to a $69,797 decrease in legal fees incurred in connection with procuring patents, partially offset by a $21,670 increase in fees for other professional services such as audit and tax advisory fees. As a result of the Settlement Agreement, the Company agreed to maintain patent protection for rights retained under the agreement.
Facilities and office expenses increased by $103,517, or 24%, from $439,373 for the nine months ended September 30, 2023 to $542,890 for the nine months ended September 30, 2024, primarily due to a $78,185 increase in software and other licensing fees and a $21,157 increase in costs for waste disposal.
Other expenses increased by $138,175, or 15%, from $943,122 for the nine months ended September 30, 2023 to $1.1 million for the nine months ended September 30, 2024. The increase is primarily attributable to a $227,470 increase in financing expenses primarily related to our search for a lender to complete the construction of the Company’s new headquarters and a $30,528 increase in insurance-related costs, partially offset by a $102,505 decrease in Delaware franchise taxes.
Legal Expenses
Legal expenses were $4.6 million in the nine months ended September 30, 2023 and $15.8 million for the nine months ended September 30, 2024. In the nine months ended September 30, 2023, costs were incurred in connection with several legal proceedings which culminated by consolidating Altor/NantCell’s action against the Company with the Altor/NantCell arbitration demand against Dr. Wong. Thereafter, proceedings against the Company and Dr. Wong were consolidated in the arbitration before JAMS.
In the nine months ended September 30, 2024, preparations for the arbitration were taking place with witness preparation and depositions. The arbitration hearing was held from May 20, 2024 to May 31, 2024, which required a large team of lawyers to represent the Company; Dr. Wong; Dr. Peter Rhode, our Chief Scientific Officer and Vice President of Clinical Affairs and an officer of the Company; as well as other employees. The hearing was followed by an extended period of intense negotiations which culminated in a Settlement Agreement entered into by the Company and Dr. Wong as of July 13, 2024 with Altor/NantCell and its parent, ImmunityBio. While the Company has relief from the future burden of ongoing legal expenses related to these proceedings, we incurred significant legal expenses for our defense and for the defense of officers and employees. We require a reasonable payment plan to prevent these expenses from overwhelming the Company’s resources. We are engaged in discussions with the law firms involved with this matter.
Interest Expense
On August 15, 2022, we entered into a loan and security agreement with Cogent Bank to partially fund our purchase of the property we acquired on that same date. We borrowed $6.5 million under this agreement. Amounts outstanding on the loan accrue interest at a rate per annum equal to 5.75%. We were obligated to make interest-only payments on this loan from September 2022 through August 2023 and principal and interest payments in 47 equal monthly installments, based on a 25-year maturity schedule, commencing September 15, 2023. We paid $284,465 and $282,098 in cash for interest related to the Cogent Bank loan for the nine months ended September 30, 2023 and 2024, respectively. For the nine months ended September 30,2023, interest was expensed. Interest was capitalized the last quarter of 2023 and ceased in the second quarter of 2024. In all other periods, interest was expensed.
As of September 30, 2024, the Company had issued $6.5 million in senior secured notes which bear interest at an annual rate of 9%, payable quarter in arrears. We paid $152,679 in cash interest on the senior secured notes for the nine months ended September 30, 2024. There were no senior secured notes outstanding in 2023.
Other Income, Net
Other income, net decreased from $919,688 for the nine months ended September 30, 2023, to $52,397 for the nine months ended September 30, 2024. The decrease is primarily attributable to a decrease interest earned for money market deposits and unrealized gains for investments in U.S. government-backed securities. Other income included rental income for the nine months ended September 30, 2023. On August 15, 2022, the Company entered into a short-term, market-rate lease with the former owner of the building we purchased on the same date, which terminated in the year ended December 31, 2023. We received rental income of $178,910 for the nine months ended September 30, 2023.
Liquidity and Capital Resources
Sources of Liquidity
As of September 30, 2024, we concluded that there is substantial doubt over as to whether the Company has sufficient capital to operate for the next 12 months from the issuance date of this Quarterly Report based on our liquidity, revenues and ongoing operating losses, as well as the legal fees incurred in conjunction with an arbitration with ImmunityBio prior to the Settlement Agreement executed in the third quarter of 2024. As of September 30, 2024, our principal source of liquidity was $1.0 million in cash and cash equivalents. To date, the Company has funded operations primarily through the sale of stock, issuance of senior secured notes and revenues generated the Wugen License, pursuant to which Wugen licensed limited rights to develop, manufacture, and commercialize cell therapy treatments for cancer based on two of the Company’s internally-developed multi-cytokine fusion protein molecules, and its manufacturing and supply arrangement with Wugen. As of September 30, 2024, the Company had not generated any revenue from commercial product sales of its internally-developed immunotherapeutic products for the treatment of cancer and other age-related diseases. In the course of its development activities, the Company has sustained operating losses and expects to continue to incur operating losses for the foreseeable future.
On August 15, 2022, the Company entered into the 2022 Loan Agreement with Cogent Bank, pursuant to which it received $6.5 million in proceeds to purchase a building that will become the Company's new headquarters. The loan is secured by a first priority lien on the building. As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction of its new manufacturing facilities and upgraded research laboratories. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements; however, as of the reporting date, the lender has not elected to do so. As of September 30, 2024, the Company has reflected this loan as Short-term debt, net, to reflect that the lender has the right to accelerate the loan under a discretionary default provision. The Company continues to seek the financing required to complete the construction project, with the cooperation of the lender and lien holders. See Part I, Item 4. -- “Controls and Procedures.”
In 2024, we have raised $9.4 million in financings. On February 20, 2024, we completed a $2.5 million private placement of common stock in which we sold an aggregate of 1,785,718 shares to certain of our officers and directors, at a purchase price of $1.40 per share. As of September 30, 2024, we received $6.5 million from the issuance of Secured Notes, and subsequent to the reporting period, the Company issued an additional $375,000 in Secured Notes. Of the total issuance of Secured Notes, the Company issued $2.9 million to members of the Company’s board of directors and officers, including $2.4 million purchased by Dr. Hing C. Wong, Founder and CEO, $220,000 purchased by Rebecca Byam, Chief Financial Officer, $140,000 purchased by Scott T. Garrett, Chairman of the board of directors, $60,000 purchased by Gary M. Winer, member of the board of directors, $25,000 purchased by Lee Flowers, Senior Vice President for Business Development, and $25,000 purchased by Rick S. Greene, member of the board of directors. The Company expects the funds raised through the issuance of Secured Notes to be sufficient capital to reach a milestone in the fourth quarter of 2024. Management has made some reductions in costs and there may be further reductions or restructuring in the future after we complete the reassessment of our clinical development strategy.
A benefit of reaching a Settlement Agreement and concluding the Arbitration is a release from restrictions related to protection of privileged information, which hampered investor ability to conduct due diligence. As a result, we believe that other avenues of financing are now available to us, namely, capital-raising activities for equity and equity-like investments as well as business development transactions such as out-licensing agreements for rights for our proprietary molecules. However, there can be no assurance of our success in our capital-raising activities. If we are not successful in raising additional capital, we have the ability to revise our business plan and reduce costs. If such revisions are insufficient, we may have to curtail or cease operations.
The Company and Dr. Wong, our Founder and Chief Executive Officer, entered into a Settlement Agreement and Release on a previously reported Arbitration as of July 13, 2024. See Part II., Item 1. - “Legal Proceedings.” While the Settlement Agreement has resolved uncertainty regarding the outcome of these proceedings and the ongoing legal expenses, the Company incurred significant legal expenses in the period leading up to the Settlement Agreement. As of September 30, 2024, we reported a balance of $14.4 million for legal fees incurred but not yet paid in the accompany condensed balance sheet. In order not to overwhelm the Company’s resources, a reasonable payment plan will be required. The Company is engaged in discussions with the law firms involved with this matter.
We considered elements of our financing plan that were probable and likely to be implemented within the next year to determine if financing activities currently underway are sufficient mitigate the substantial doubt in our going concern analysis. The Company launched a financing plan in the third quarter of 2024. The plan includes several capital-raising activities, such as issuance of secured notes, equity financings, as well as business development transactions, such as license agreements with guaranteed minimum payments. Through October 31, 2024, the Company had issued an aggregate of $6.9 million in secured notes. On September 25, 2024, the Company entered into a nonbinding term sheet with a party interested in licensing one of the Company’s preclinical molecules. The Company expects to close this license transaction in the fourth quarter of 2024. The proposed terms of the license include a guaranteed minimum payments which are expected within the first year of the term. On November 13, 2024, we entered into an engagement letter with the Maxim Group to act as the exclusive placement agent for a multi-step equity financing. If the Company is not successful in raising additional capital through these activities, management intends to revise its business plan and reduce costs. If such revisions are insufficient, the Company may have to curtail or cease operations.
The accompanying interim financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above. The Company believes that substantial doubt exists regarding its ability to continue as a going concern for at least 12 months from the date of issuance of the Company’s condensed interim financial statements, without additional funding or financial support. After considering management’s plan for financing and funds raised that are probable to occur within one year, as well as that the Company expects to continue to incur losses from operations for the foreseeable future, management concluded that the substantial doubt that existed in its going concern analysis was not alleviated.
Because of the numerous risks and uncertainties associated with the clinical development and commercialization of immunotherapeutics, we are unable to estimate the exact amount of capital requirements to pursue these activities. Our funding requirements will depend on many factors, including, but not limited to:
•timing, progress, costs, and results of our ongoing preclinical studies and clinical trials of our immunotherapeutic products;
•costs, timing, and outcome of regulatory review of our product candidates;
•number of trials required for regulatory approval;
•whether we enter into any cooperative, collaboration or co-development agreements and the terms of such agreements;
•whether we raise additional funding through bank loan facilities, other debt arrangements, out-licensing or joint ventures, cooperative agreements or strategic collaborations;
•effect of competing technology and market developments;
•cost of maintaining, expanding, and enforcing our intellectual property rights;
•impact of arbitration, litigation, regulatory inquiries, or investigations, as well as costs to indemnify our officers and directors against third-party claims related to our patents and other intellectual property:
•cost and timing of buildout of new headquarters, including risks of cost overruns and delays, and ability to obtain additional financing, if needed; and
•costs and timing of future commercialization activities, including product manufacturing, marketing, sales, and distribution, for any of our product candidates for which we receive regulatory approval.
A change in the outcome of any of these or other factors with respect to the clinical development and commercialization of our product candidates could significantly change the costs and timing associated with the development of that product candidate. Further, our operating plan may change, and we may need additional funds to meet operational needs and capital requirements for clinical trials and other research and development expenditures.
Comparison of the Cash Flows for the Nine Months Ended September 30, 2023 and September 30, 2024
The following table summarizes our cash flows for the nine months ended September 30, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended
September 30, |
|
|
|
2023 |
|
|
2024 |
|
Cash used in operating activities |
|
$ |
(18,619,329 |
) |
|
$ |
(11,392,547 |
) |
Cash provided by (used in) investing activities |
|
|
7,513,050 |
|
|
|
(148,205 |
) |
Cash provided by financing activities |
|
|
716 |
|
|
|
8,943,872 |
|
Net (decrease) increase in cash and cash equivalents |
|
$ |
(11,105,563 |
) |
|
$ |
(2,596,880 |
) |
Operating Activities
Net cash used in operating activities were $18.6 million for the nine months ended September 30, 2023 and $11.4 million for the nine months ended September 30, 2024.
Cash used in operating activities for the nine months ended September 30, 2023 consisted primarily of a net loss of $14.3 million, as well as a deposit $5.3 million used to establish an interest reserve for future interest payments, as required under the terms of the Credit Agreement intended to be used to fund the construction and renovation of the Company’s new headquarters. In addition, other uses of cash include a $292,383 increase in accounts receivable and a $251,008 increase in prepaid expenses and other current assets, partially offset by cash provided by a $392,802 net increase in accounts payable and other current liabilities. Further offset to the use of cash resulted from net noncash adjustments of $1.1 million, consisting primarily of $860,634 of cash provided by an adjustment for depreciation and amortization, $742,477 of cash provided by an adjustment for stock-based compensation, reduced by $248,445 of cash used for an adjustment for unrealized gains on investments.
Cash used in operating activities for the nine months ended September 30, 2024 consisted primarily of net loss for the period of $26.7 million, partially offset by a $12.4 million increase in accounts payable and other liabilities, a decrease of $883,917 in accounts receivable and a decrease of $829,043 in prepaid expenses and other current assets. Further offset to the use of case resulted from noncash adjustments of $1.2 million, consisting primarily of $501,882 of cash provided by an adjustment for depreciation and amortization and $716,940 of cash provided by an adjustment for stock-based compensation.
Investing Activities
Cash used by investing activities for the nine months ended September 30, 2023, consisted of $10.0 million of cash provided when short-term investments reached maturity, partially offset by $2.5 million of cash used to purchase equipment.
Cash used by investment activities for the nine months ended September 30, 2024 consisted of $148,205 used for purchases of property and equipment.
Financing Activities
Cash provided by financing activities for the nine months ended September 30, 2023 resulted from issuance of common stock upon exercise of vested employee stock options, partially offset by a principal repayment under the 2022 Loan Agreement related to the acquisition financing used to purchase a building.
Cash provided by financing activities for the nine months ended September 30, 2024 resulted from a $2.5 million private placement of the Company’s common stock and $6.5 million from the issuance of Secured Notes, partially offset a $88,388 principal repayment under the 2022 Loan Agreement related to the acquisition financing used to purchase a building.
Critical Accounting Policies, Significant Judgements and Use of Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our unaudited condensed interim financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of
contingent assets and liabilities at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgements about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgements and estimates.
Revenue Recognition
We recognize revenue under the guidance of Topic 606. To determine the appropriate amount of revenue to be recognized for arrangements determined to be within the scope of Topic 606, we perform the following five steps: (i) identification of the contract(s) with the customer, (ii) identification of the promised goods or services in the contract and determination of whether the promised goods or services are performance obligations, (iii) measurement of the transaction price, (iv) allocation of the transaction price to the performance obligations, and (v) recognition of revenue when (or as) we satisfy each performance obligation. We only apply the five-step model to contracts when it is probable that we will collect the consideration we are entitled to in exchange for the goods or services we transfer to our customer. See Note 1 to our condensed interim financial statements appearing elsewhere in this Quarterly Report on Form 10-Q for more information.
Other than the above, there have been no material changes to our critical accounting policies and estimates from those described under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations— Critical Accounting Policies, Significant Judgements and Use of Estimates” in our Annual Report.
Recent Accounting Pronouncements
See Note 1 to our Annual Report.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
As of September 30, 2024, we had cash and cash equivalents of $1.0 million including cash, cash equivalents and market investments. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. We are exposed to market risk related to the marketability of our Wugen common stock reported within Investments in the accompanying condensed interim balance sheet. Until such time as these shares become publicly traded, we will have limited access to liquidity for these securities.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
As of September 30, 2024, our management, with participation of our principal executive officer and principal financial officer, performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a – 15(e) under the Exchange Act). While the Company implemented an Authorization Matrix and a Remediation Plan in the second quarter of 2024, our management believes the strengthened controls designed to address the material weaknesses identified in prior periods have not been in place for a sufficient time to conclude on whether or not they have been operating effectively as of September 30, 2024. Our management will continue to evaluate the effectiveness of the internal controls over financing reporting, including the Authorization Matrix and Remediation Plan, in future reporting periods.
The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) under the Exchange Act). Internal control over financial reporting is a process designed under the supervision and
with the participation of our management, including our principal executive officer and our principal financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States.
As of September 30, 2024, our management assessed the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework. Based on this assessment, two material weaknesses over financial reporting were identified (described below). A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that a reasonable possibility exists that a material misstatement of our annual or condensed interim financial statements would not be prevented or detected on a timely basis.
Two of the material weaknesses were identified in a prior period, as the Company reported in a Current Report on Form 8-K filed with the SEC on May 1, 2024. The Company was a victim of a criminal scheme involving the impersonation of a purchaser of Secured Notes. The scheme resulted in the misdirection of approximately $1.3 million held in Company accounts to a fraudulent account controlled by a third party and a default on a legally binding commitment to purchase Secured Notes. As a result of the default and the related misdirection of funds, management re-evaluated the effectiveness of our disclosure controls and procedures and internal control over financial reporting as of December 31, 2023. Based on this assessment, management identified material weaknesses in two areas, including the methods used to review, evaluate and accept financing proposals from investors and lenders and the process used to enter unusual significant transactions. As a result of the material weakness to protect the Company’s assets from fraud committed by third parties, there was a $1.3 million loss recognized on the Company’s interim condensed financial statements.
During the period ended June 30, 2024, a Remediation Plan was implemented. See “Remediation Plan for Material Weaknesses in Internal Control over Financial Reporting.” Our principal executive officer and principal financial officer concluded there was not yet enough evidence due to the infrequency of significant transactions to assess the effectiveness of internal controls during the period ended September 30, 2024. We will continue to assess the effectiveness of the internal controls in each reporting period, especially those related to significant or unusual transactions contained in the Remediation Plan.
As of September 30, 2024, the Company identified two additional material weaknesses in internal controls over financing reporting related to the classification of the Cogent Loan and accounting for the Secured Notes. On August 15, 2022, the Company entered into the 2022 Loan Agreement with Cogent Bank, pursuant to which we received $6.5 million in proceeds to purchase a building. The loan is secured by a first priority lien on the building. As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction and improvements on the building. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements. The Company did not identify and account for the loan as Short-term debt, net, to reflect that the lender has the right to accelerate the loan under a discretionary default provision as of September 30, 2024. As a result, we will enhance the controls we use to evaluate triggering events in debt agreements, to appropriately classify debt as current or noncurrent in the correct period.
The second material weakness identified as of September 30, 2024 related to accounting for complex transactions. This involved appropriately accounting for the Secured Notes and disclosing the amended terms that were executed during the third quarter of 2024. The Secured Notes were deemed to be a hybrid instrument, consisting of a debt host with embedded derivatives requiring bifurcation and accounting for separately. Prior to correcting the initial accounting treatment for the Secured Notes, as amended, the Company neglected to identify and account for the embedded derivatives. In addition, the disclosures for the Secured Notes would not have identified the embedded derivatives. The aggregation of these factors could have resulted in a material misstatement in the Company’s financial statements. As of September 30, 2024, there was no impact to the financial statements related to correcting the accounting treatment for embedded derivatives. Another amended term for the Secured Notes is a fixed bonus payment that holders will receive if the Secured Notes are repaid on the Maturity Date. The Company determined that the fixed bonus payment should be accreted to the principal owed to holders over the term. As of and for the three and nine months ended September 30, 2024, the Company did not accrete the fixed bonus payment. Accretion during this reporting period does not materially misstate the amount owed to the holders. In subsequent periods, the Company will accrete the fixed bonus payment as it will be material. If the Company did not correct the accounting treatment, we would understate our obligations. Over the term, this could have resulted in a material misstatement in the Company’s financial statements. Management resolved to allow for more time and resources to determine proper accounting for complex transactions.
Remediation Plan for Material Weakness in Internal Control over Financial Reporting
We are committed to establishing and maintaining a strong internal control environment. In response to the identified material weakness as described above, the Company’s Board of Directors and its Audit Committee have conducted an internal investigation to determine the root cause of the material weaknesses, with advice from outside advisors. Along with the advice of outside advisors, the board of directors worked with management to evaluate internal controls over financial reporting based on criteria set forth in “Internal Control – Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission. As a result, the following two actions were taken by the board of directors:
On May 13, 2024, the board of directors adopted a Delegation of Management Authorities (“Authorization Matrix”), which provides for authorization thresholds for significant and unusual transactions, budget and strategic plans, audit and policies, personnel actions, contracts, litigation, major projects, credit or loans, and consulting agreements.
On June 11, 2024, the board of directors adopted a Remediation Plan designed to implement and strengthen controls and prevent re-occurrence of fraud on the Company:
1.All lenders, investors or others involved in financial transactions or investing in the Company will undergo a standard due diligence process, including a background check and document verification.
a.The Company will obtain the appropriate background checks, including identity and verification, criminal, civil, and financial searches.
b.The Company will use third parties to verify Know Your Client (“KYC”) documents, including, verification process with the banks providing letters.
2.On May 13, 2024, the Board adopted a Delegation of Management Authorities (the “Authority Matrix”).
3.All Company transactions are required to be performed with U.S. dollars via Company check or wire transfer unless board approval is received.
4.Company funds are not permitted to be transferred to a personal account. All Company accounts will be in the Company name.
5.All significant theft or fraud will be reported to the Board and authorities immediately, and no less than 24 hours after the Company becomes aware of the occurrence of the theft or fraud.
6.The Company shall continue ongoing cybersecurity training for all employees.
7.The Company will annually review with employees, the IT/equipment use policy in Section 404 of the Employee Handbook.
8.Communications with third parties relating to Company business shall take place on Company email or authorized Company accounts. For record keeping purposes, any communications relating to loans, investments, and financing arrangements in the Company shall take place (if electronically) via electronic mail through Company email accounts, or, if necessary to chat, via regular SMS chat on Company authorized phones/accounts.
9.The Company will provide information on its financial condition (including proof of liquidity) only as approved by two executive employees.
Inherent Limitations of Internal Controls
While we strive to create a stronger control environment, we recognize that it is impossible for our internal controls over financial reporting to prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. While we are committed to continuously improve and strengthen our control environment, over time, our internal controls over financial reporting may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Projections of any evaluation of effectiveness to future periods are subject to the risk that internal controls over financial reporting may become inadequate because of
changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act during the three months ended September 30, 2024 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
From time to time, the Company is a party to or otherwise involved in legal proceedings, including suits, assessments, regulatory actions and investigations generally arising out of the normal course of business. Such proceedings can be costly, time consuming, and unpredictable. Therefore, no assurance can be given on the outcome of any proceeding or the potential impact on our results of operations or financial condition.
During the period ended December 31, 2022, Altor/NantCell initiated legal proceedings against Dr. Wong and the Company. On April 26, 2023, the parties stipulated that Altor/NantCell’s action against the Company would be consolidated with the Altor/NantCell arbitration demand against Dr. Wong. On April 27, 2023, the U.S. District Court for the Southern District of Florida (the “Court”) with jurisdiction over the lawsuit against the Company approved the parties’ stipulation and ordered the parties to arbitration. On May 1, 2023, Altor/NantCell filed a demand against the Company before JAMS. On May 3, 2023, Altor/NantCell dismissed the federal court action without prejudice and the Court ordered the case dismissed without prejudice and closed the case. Proceedings against the Company and Dr. Wong were consolidated in the arbitration before JAMS. The arbitration hearing was held from May 20, 2024 to May 31, 2024, after which the parties entered into settlement negotiations.
As reported in the Company’s Form 8-K filed on July 18, 2024, as of July 13, 2024, the Company and Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, entered into a confidential Settlement Agreement and Release (the “Settlement Agreement”) with Altor BioScience, LLC (“Altor”), NantCell, Inc. (“NantCell”), and ImmunityBio, Inc. (the parent of Altor and NantCell, together with Altor and NantCell, “ImmunityBio”), to resolve the previously disclosed arbitration before JAMS brought by Altor and NantCell (the “Arbitration”) as well as a complaint Altor filed against the Company in the Chancery Court of the State of Delaware for the contribution of legal fees and expenses advanced to Dr. Wong (“Complaint”). The Settlement Agreement includes mutual general releases by and among the parties thereto. No party is required to make any monetary payments to any other party or person under the Settlement Agreement and each party will bear its own expenses incurred in connection with the matter. The Company has completed the required remediation procedures. As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint. In accordance with 17 CFR 229.601 (Item 601), the Company has included the Settlement Agreement in the Quarterly Report on this Form 10-Q.
Pursuant to the Settlement Agreement, the Company transferred and assigned to ImmunityBio ownership of certain intellectual property (including issued patents, pending patent applications, and know-how) for TOBITM-based molecules for use in the oncology field. The Company retains the worldwide, perpetual, irrevocable, fully paid-up, royalty-free, exclusive right and license to exploit HCW9218 for all age-related diseases other than cancer, with the exception of the treatment of ovarian cancer, which is also retained by the Company and is currently being studied in a Phase 2 clinical trial at the University of Pittsburgh Medical Center. The Company also retains the right to develop treatments for all indications with respect to HCW9302 and HCW9206, which, along with HCW9218, are the lead product candidates in the Company’s clinical development pipeline. ImmunityBio has the exclusive right to pursue oncology indications with all of the TOBITM-based molecules designed with a TGF-β domain, including HCW9218, with the exception of the treatment of ovarian cancer with HCW9218 in combination with neoadjuvant chemotherapy, which is retained by the Company. Under the Settlement Agreement, ImmunityBio also receives an exclusive license to exploit fusion proteins, molecules and/or antibodies created utilizing the TOBITM Platform directed to the receptors of PDL-1, IL-7, IL-12, IL-18, and IL-21, and one additional target to be selected by ImmunityBio within six months from the date of the Settlement Agreement, at its sole discretion, in the oncology field. The Company’s ownership and rights with respect to HCW9302, HCW9206 and HCW9201 are expressly excluded from the rights transferred to ImmunityBio for oncology indications. In addition, ImmunityBio received a non-exclusive license to exploit HCW9201 administered by injection for oncology indications.
The Company retains ownership and control of the TOBITM platform and TOBI-based molecules, with no restrictions under the Settlement Agreement on our ability to use the TOBITM platform for protein-fusion molecules for non-oncology indications. We have rights to pursue oncology indications, in particular using HCW9302, HCW9206 and HCW9201. Further, the Company retains ownership of the Wugen license and shares of Wugen common stock transferred to the Company as the upfront licensing fee from Wugen for granting the Wugen license. For our lead molecule, HCW9218, we maintain the non-exclusive right to use HCW9218 in combination with neoadjuvant chemotherapy in ovarian cancer, in addition to exclusive rights for clinical development and use of HCW9218 in the treatment of all non-oncological diseases. We retain ownership of our lead molecule, HCW9302, which expands Treg cells and is designed to treat autoimmune diseases and other proinflammatory diseases, including cancer, and the ownership of HCW9206, a preclinical molecule which we are developing for the treatment of cancer and other age-related diseases. The Company agreed to provide ImmunityBio with a right of first refusal to enter a licensing agreement for oncology indications for HCW9206. We have no restrictions on the development of HCW9206 for our own clinical development activities, including oncology indications. Under the terms of the Settlement Agreement, ImmunityBio will own the cell line and supply for HCW9218, and the
parties agreed that within six months from the date of the Settlement Agreement they will enter into a supply agreement providing the Company with a continuing supply of HCW9218 molecules. The Company also retains in vivo rights to HCW9201, a combination of IL-12, IL-15, and IL-18 in a single protein complex which is designed to stimulate activation and proliferation signals in human NK cells. The Company retains ownership of the cell lines for HCW9302, HCW9206 and HCW9201, and thus will retain independent control over manufacturing and supply for these compounds.
Item 1A. Risk Factors.
There have been no material changes to the risk factors previously disclosed by us in our Annual Report. The risk factors included the Annual Report continue to apply to us and describe risks and uncertainties that could cause actual results to differ materially from the results expressed or implied by the forward-looking statements contained in this Quarterly Report. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business, financial condition and results of operations.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
Unregistered Sales of Equity Securities
On February 20, 2024 (the “Purchase Date”), we entered into subscription agreements (the “Subscription Agreements”) with certain officers and directors of the Company, including our Founder and Chief Executive Officer, our Chief Financial Officer and the Chairman of the Company’s Board of Directors, pursuant to which the Company sold an aggregate of 1,785,718 shares (the “Shares”) of our common stock, par value $0.0001 per share (the “Common Stock”), at a purchase price of $1.40 per share for an aggregate purchase price of $2.5 million. The per share purchase price represents a 25% premium to the per share closing price of the Common Stock as reported on the Nasdaq Global Market on the Purchase Date and a 19% premium to the 5-day volume weighted average closing price per share of the Common Stock as reported on the Nasdaq Global Market for the period ending on the Purchase Date.
The Shares issued pursuant to the Subscription Agreements were not registered under the Securities Act of 1933, as amended, in reliance upon exemptions provided by Section 4(a)(2) of the Securities Act of 1933, as amended.
Issuer Repurchases of Equity Securities
None.
Item 3. Defaults Upon Senior Securities.
Not Applicable.
Item 4. Mine Safety Disclosures.
Not Applicable.
Item 5. Other Information.
Insider Adoption or Termination of Trading Arrangements
During the fiscal quarter ended September 30, 2024, none of our directors or officers informed us of the adoption, modification or termination of a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as those terms are defined in Regulation S-K, Item 408.
Secured Notes Issuance
The following information is being included in this Item 5 in lieu of filing such information on a Current Report on Form 8-K under Item 2.03. Creation of a Direct Financial Obligation or an Obligation under an Off-Balance Sheet Arrangement of a Registrant and Item 8.01:
On March 28, 2024, the Company entered into a senior secured note purchase agreement (the “Note Purchase Agreement”) with the Purchasers (as defined in the Note Purchase Agreement), pursuant to which we agreed to issue senior secured notes in an aggregate principal amount of up to $10.0 million (“Secured Notes”) to certain accredited investors, including unrelated parties as well
as officers and directors of the Company. As of March 31, 2024, the Company had an initial closing and issued $2.0 million in Initial Secured Notes. As of June 30, 2024, all existing investors approved an Amended and Restated Note Purchase Agreement (“Amended and Restated Note Purchase Agreement”), with terms described below. As of September 30, 2024, the Amended and Restated Note Purchase Agreement was amended to extend the last closing date to issue Additional Secured Notes to October 31, 2024. No other terms were amended. The material terms of the Additional Secured Notes are identical to the terms of the Initial Secured Notes.
As of September 30, 2024, the Company issued an aggregate of $6.5 million of Secured Notes, with $2.8 million from the Company’s officers and members of the board of directors, including $2.4 million purchased by Dr. Hing C. Wong, Founder and CEO, $220,000 purchased by Rebecca Byam, Chief Financial Officer, $140,000 purchased by Scott T. Garrett, Chairman of the board of directors, $60,000 purchased by Gary M. Winer, member of the board of directors, $25,000 purchased by Lee Flowers, Senior Vice President for Business Development, and $25,000 purchased by Rick S. Greene, member of the board of directors.
During October 2024, the Company issued $375,000 of Additional Secured Notes, including $50,000 purchased by Dr. Wong on October 31, 2024.
The Senior Notes bear interest at a rate of 9% per annum, payable quarterly in arrears, and mature on August 30, 2026 (the “Maturity Date”), on which date the principal balance, accrued but unpaid interest, and other amounts that may be due under the terms of the Amended and Restated Note Purchase Agreement shall be due and payable. The Secured Notes may be prepaid on or prior to December 31, 2024, but will be subject to a 5% prepayment penalty (“Premium Amount”). Thereafter, the Senior Notes may be repaid upon a Mandatory Redemption event or at the end of the term.
As a condition to entering into the Amended and Restated Note Purchase Agreement, the Company, Mercedes M. Sellek, P.A. (“Escrow Agent”), and the Purchasers entered into that certain Escrow Agreement and Amended and Restated Pledge Agreement, dated July 2, 2024, pursuant to which the Company agreed to pledge our equity ownership interest in Wugen, which was equivalent to a 5.6% ownership stake in that company as of September 30, 2024 (the “Pledged Collateral”), to be held and released by Escrow Agent according to the terms of the Escrow Agreement, as security for the Secured Notes.
Upon a qualifying event Mandatory Redemption involving a transaction such as an acquisition, merger or initial public offering in which the Pledged Collateral can be sold or liquidated prior to the Maturity Date, subject to certain limitations (such as a threshold price per share in the case of an initial public offering), the Company agreed to repay all indebtedness (including accrued interest) related to the Secured Notes plus a Bonus Payment (as defined in the Amended and Restated Note Purchase Agreement). If there is no such Mandatory Redemption prior to the Maturity Date, the Company agreed to pay the holders of Secured Note a Bonus Payment under certain circumstances.
Upon a bona fide equity offering (as defined in the Amended and Restated Note Purchase Agreement), Senior Note holders have the right to convert to shares of the Company’s common stock (as defined in the Amended and Restated Note Purchase Agreement). The Amended and Restated Note Purchase Agreement sets forth preliminary terms that are subject to final documentation.
Upon an Event of Default (as defined in the Amended and Restated Note Purchase Agreement), the Company will have a thirty (30) day cure period (the “Cure Period”), and if the Event of Default is not so cured at the end of the Cure Period, the Company is required to distribute the Pledged Collateral to the Purchasers on a pro rata basis, determined based on the issuance of $10.0 million in Secured Notes, in full satisfaction of the indebtedness evidenced by the Secured Notes.
The foregoing descriptions of the Amended and Restated Note Purchase Agreement, Amended and Restated Senior Notes, Escrow Agreement and Amended and Restated Pledge Agreement do not purport to be complete and are qualified in their entirety by reference to the full text of the Form of Amended and Restated Senior Secured Note Purchase Agreement, Form of Senior Secured Promissory Note, Form of the Amended and Restated Pledge Agreement and Form of Amended and Restated Escrow Agreement, copies of which are filed as Exhibit 10.1, Exhibit 10.2, Exhibit 10.3 and Exhibit 10.4, respectively, to this Quarterly Report and are incorporated herein by reference.
The issuance of the Additional Secured Notes was exempt from the registration requirements of the Securities Act of 1933, as amended, in accordance with Section 4(a)(2), as a transaction by an issuer not involving a public offering. In addition, our Board of Directors and the Audit Committee of our Board of Directors reviewed the transaction under our policy for Related Party Transactions (the “Policy”) and determined that the issuance of the Additional Secured Notes was in compliance with the Policy.
Item 6. Exhibits.
The exhibits filed or furnished as part of this Quarterly Report on Form 10-Q are set forth on the Exhibit Index, which Exhibit Index is incorporated herein by reference.
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit Number |
|
|
|
Incorporated by Reference |
|
Filed Herewith |
|
|
Description |
|
Form |
|
File No. |
|
Exhibit No. |
|
Filing Date |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 |
|
Form of Amended and Restated Senior Secured Note Purchase Agreement, dated July 2, 2024, by and between the Company and the Purchaser party thereto |
|
10-Q |
|
001-40591 |
|
10.1 |
|
8/14/2024 |
|
|
10.2 |
|
Form of Senior Secured Promissory Note by and between the Company and the Holder party thereof |
|
10-Q |
|
001-40591 |
|
10.2 |
|
8/14/2024 |
|
|
10.3 |
|
Form of Amended and Restated Pledge Agreement, dated July 2, 2024, by and among the Company, Escrow Agent and Noteholder parties thereto |
|
10-Q |
|
001-40591 |
|
10.3 |
|
8/14/2024 |
|
|
10.4 |
|
Form of Amended and Restated Escrow Agreement, dated July 2, 2024, by and among the Company, Escrow Agent and Noteholder parties thereto |
|
10-Q |
|
001-40591 |
|
10.4 |
|
8/14/2024 |
|
|
10.5 |
|
Form of First Amendment to the Amended and Restated Senior Secured Note Purchase Agreement, dated September 30, 2024, by and between the Company and Purchaser parties thereto |
|
|
|
|
|
|
|
|
|
X |
10.6# |
|
Settlement Agreement and Release, dated July 13, 2024, by and between the Company and Altor BioScience, LLC, NantCell, Inc., and ImmunityBio, Inc. |
|
|
|
|
|
|
|
|
|
X |
31.1 |
|
Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1* |
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
32.2* |
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
101 |
|
The following materials from the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, formatted in Inline XBRL (eXtensible Business Reporting Language): (i) the Condensed Interim Balance Sheets as of December 31, 2023 and September 30, 2024 (unaudited); (ii) the Condensed Interim Statements of Operations for the three and nine months ended September 30, 2023 (unaudited) and September 30, 2024 (unaudited); (iv) the Condensed Interim Statements of Changes in Stockholders’ Equity for the nine months ended September 30, 2023 (unaudited) and September 30, 2024 (unaudited); (v) the Condensed Interim Statements of Cash Flows for the nine months ended September 30, 2023 (unaudited) and September 30, 2024 (unaudited); and (vi) the notes to the Condensed Interim Financial Statements (unaudited). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
* |
This certification is deemed not filed for purpose of Section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act. |
# |
Certain information in this document has been excluded pursuant to Item 601(a)(5) or (a)(6) of Regulation S-K. The Registrant agrees to furnish supplementally such information to the SEC upon request. |
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
HCW Biologics Inc. |
|
|
|
|
Date: November 14, 2024 |
|
By: |
/s/ Hing C. Wong |
|
|
|
Hing C. Wong |
|
|
|
Chief Executive Officer |
|
|
|
(Principal Executive Officer) |
|
|
|
|
Date: November 14, 2024 |
|
By: |
/s/ Rebecca Byam |
|
|
|
Rebecca Byam |
|
|
|
Chief Financial Officer |
|
|
|
(Principal Financial and Accounting Officer) |
Exhibit 10.5
HCW BIOLOGICS INC.
FIRST AMENDMENT TO AmendED AND RESTATED
SENIOR SECURED NOTE PURCHASE AGREEMENT
This First Amendment to Amended and Restated Senior Secured Note Purchase Agreement(this “Amendment”) is made as of September 30, 2024 (the “Effective Date”) by and between HCW Biologics Inc., a Delaware corporation (the “Company”), and each of the purchasers listed on Exhibit B attached thereto (each a “Purchaser” and together the “Purchasers”).
RECITALS
The Company and certain Purchasers entered into that certain Senior Secured Note Purchase Agreement dated as of March 28, 2024 (the “Original Agreement”), and that certain Amended and Restated Senior Secured Note Purchase Agreement dated as of July 2, 2024 (the “Agreement”).
The Company and the Purchasers desire to amend the Agreement to extend the period during which Closings thereunder may take place. Capitalized terms not otherwise defined herein have the meaning given them in the Note.
Therefore, the parties hereby agree as follows:
1.Section 1(b)(i) of the Agreement is hereby amended to delete and replace the last sentence thereof with the following: “In no event shall any such Closing take place later than October 31, 2024.”
2.In all other respects the Agreement shall remain in full force and effect.
3.This Amendment may be executed in any number of counterparts, each of which when so executed and delivered shall be deemed an original, and all of which together shall constitute one and the same agreement. Execution by via Docusign, Box Sign or similar system or execution of a facsimile or scanned copy will have the same force and effect as execution of an original, and a facsimile or scanned signature will be deemed an original and valid signature.
The parties have executed this First Amendment to Amended and Restated Senior Secured Note Purchase Agreement as of the date first written above.
the company:
HCW BIOLOGICS INC.
By: /s/ HING C. WONG_____________
Name: Hing C. Wong
Title: Chief Executive Officer
The Purchasers:
HING C. WONG
By: /s/ Hing C. Wong_______________
Name: Hing C. Wong
CHRIS CHEUNG & LING CHEUNG
By: /s/ Chris Cheung and Ling Cheung__
Name: Chris Cheung & Ling Cheung
Michael poon & manwah wong
By: Michael Poon & Manwah Wong____
Name: Michael Poon & Manwah Wong
Ho cheunG wong
By: /s/ Ho Cheung Wong______________
Name: Ho Cheung Wong
hoi sang ‘KELLY’ yeung
By: /s/ Hoi Sang Yeung______________
Name: Hoi Sang ‘Kelly’ Yueng
r. kemp riechman trustee revocable trust of roland kemp riechmann
By:
Name: R. Kemp Riechman
Title: Trustee
Benjamin j. patz
By: /s/ Benjamin J. Patz________________
Name: Benjamin J. Patz
Rebecca byam
By: /s/ Rebecca Byam_________________
Name: Rebecca Byam
Gary winer
By: /s/ Gary Winer____________________
Name: Gary Winer
Scott Garrett
By: /s/ Scott T. Garrett_________________
Name: Scott Garrett
O’NEILl aaf llc
By: /s/ George O’Neill Jr.______________
Name: George D. O’neill Jr.
Title: Manager
LEE FLOWERS
By: /s/ Lee D. Flowers_________________
Name: Lee Flowers
RICK GREENE
By: /s/ Rick Greene___________________
Name: Rick Greene
Exhibit 10.6
CERTAIN INFORMATION HAS BEEN REDACTED FROM THIS EXHIBIT IN ACCORDANCE WITH ITEM 601(B)(10)(IV) OF REGULATION S-K BECAUSE SUCH INFORMATION (1) IS NOT MATERIAL AND (2) IS THE TYPE OF INFORMATION THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. INFORMATION THAT HAS BEEN SO REDACTED FROM THIS EXHIBIT IS MARKED AS FOLLOWS: “XXXXXXXXXX” TO INDICATE THE OMISSION.
SETTLEMENT AGREEMENT AND RELEASE
This Settlement Agreement and Release dated July 13, 2024 (“Settlement” or “Agreement”) is made and entered into by and among the following parties and by and through their respective counsel: (i) Altor BioScience, LLC (“Altor”), (ii) NantCell, Inc. (“NantCell” and, together with Altor, “Claimants”), (iii) HCW Biologics, Inc. (“HCW”), and (iv) Dr. Hing C. Wong (“Dr. Wong” and, together with HCW, “Respondents,” and Respondents, together with Claimants, the “Settling Parties”). The parties to this Agreement are referred to herein collectively as the “Parties” and each a “Party.” The Settlement is intended by the Settling Parties to fully, finally, and forever resolve, discharge, and settle the Settled Claims subject to the terms and conditions hereof.
I.BRIEF OVERVIEW OF THE CLAIMS
A.On December 23, 2022, Dr. Wong filed a statement of claims against Claimants in JAMS Arbitration for declaratory relief, seeking a declaration that Dr. Wong was not liable to Claimants for breach of contract and other causes of action.[1]
B.Also on December 23, 2022, Claimants filed suit against HCW in the District Court for the Southern District of Florida for misappropriation of trade secrets and other causes of action.[2]
C. On January 9, 2023, Claimants filed a demand for arbitration against Dr. Wong in JAMS Arbitration for breach of contract and other causes of action.[3]
D.On May 1, 2023, Claimants filed a demand for arbitration against HCW for misappropriation of trade secrets and other causes of action.[4]
E.Ultimately, the federal action was dismissed in favor of the three arbitrations, which were consolidated into one action, referred to as JAMS Reference No. XXXXXXXXXX.
F.On April 1, 2024, Claimants filed an action against HCW in the Court of Chancery of the State of Delaware.[5]
II.CLAIMS OF CLAIMANTS AND BENEFITS OF SETTLEMENT; RESPONDENTS DENIAL OF WRONGDOING AND LIABILITY
The Settling Parties wish to settle the Actions solely as between the Claimants, on the one hand, and Respondents, on the other hand, by entering into this Settlement, solely to avoid the costs, disruption, and distraction of further litigation, and without admitting the validity of any allegations made in the Actions, or any liability with respect thereto, have concluded that it is desirable that the claims against them be settled and dismissed on the terms reflected in this Settlement. Further, entry into this Settlement by Claimants is not an admission as to the lack of merit of any of the claims asserted by any of them in the Actions, and entry into this Settlement by Respondents is not an admission as to the merit of any of the claims asserted against them in the Actions.
III. TERMS OF SETTLEMENT AGREEMENT AND RELEASE
NOW, THEREFORE, in consideration of the mutual covenants and agreements contained herein and other valuable consideration, the receipt and sufficiency of which are hereby acknowledged and agreed, the Parties hereby agree as follows:
1.Definitions. As used in this Settlement, the following terms have the meanings specified below.
a.“Actions” refer to the civil litigation and arbitrations referenced in Section I above.
b.“Active Ingredient” means any clinically active material that provides pharmacological activity in a pharmaceutical product (excluding formulation components such as coatings, stabilizers, excipients or solvents, adjuvants or controlled release technologies).
c.“Additional Molecule” means any fusion protein developed by HCW utilizing the TOBI Platform as of the Effective Date other than the TGFb Molecules. Notwithstanding the foregoing, “Additional Molecule” specifically excludes HCW9302.
d.“Affiliate” means any Person that Controls, is Controlled by, or is under common Control with another Person.
e.XXXXXX XXX XX XXXX XXXXXXXXXX XXXXX XXXX XXXXXXX XXX XXXXXXXX XXXXXXXXXX XXXXX XXXXXX XXXXXXXXX XXXXX XX XX XXXX XXX XXXX XX XXX XXXXXXX XXXXXXXX XXX XXX.
f.“Cellular Therapy Products” means any pharmaceutical or biological product, process or therapy that contains or comprises cells (including without limitation, cytokine-induced memory-like Natural Killer Cells or T-Cells) that have been engineered, modified, or otherwise manipulated ex vivo, as an Active Ingredient, either alone or in combination with other Active Ingredients.
Settlement Agreement and Release
Page 2
Notwithstanding the foregoing, for purposes of this Agreement, Cellular Therapy Products shall not include Treg Products.
g.“cGMP” means the then-current good manufacturing practices required by the FDA, as set forth in the FD&C Act, as amended, and the regulations promulgated thereunder, for the manufacture and testing of pharmaceutical materials, and comparable applicable law related to the manufacture and testing of pharmaceutical materials in jurisdictions outside the United States, including the quality guidelines promulgated by the ICH designated ICH Q7A, titled “Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical ingredients” and the regulations promulgated thereunder, in each case as they may be updated from time to time.
h.“CMC” means chemistry, manufacturing and controls processes with respect to any product or investigational agent, including the chemistry, manufacturing and controls section of any regulatory materials for such product or investigational agent.
i.“Commercialization” means any and all activities, other than manufacturing, directed to the preparation for sale of, or sale of the referenced products, including activities related to marketing, promoting, distributing, and importing the products, and interacting with Regulatory Agencies regarding any of the foregoing. When used as a verb, “to Commercialize” and “Commercializing” means to engage in Commercialization, and “Commercialized” has a corresponding meaning.
j.“Control” means the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of a Person, whether through the ability to exercise voting power, by contract or otherwise provided that with respect to any Intellectual Property Rights or information, “control” means that the applicable Person owns or has a license to such item or right and has the ability to grant to a party a license, sublicense, or rights of access and use under such item or right without (a) violating the terms or conditions of any agreement or other arrangement between such Person and any Third Party in existence as of the time such party would be required hereunder to grant such license, sublicense, or rights of access and use, and (b) paying any consideration to any Third Party. “Controlling” and “Controlled” have meanings correlative thereto.
k.“Development” means (i) with respect to ImmunityBio, all activities related to discovery, research, development, creation and prosecution of Intellectual Property Rights, pre-clinical and other non-clinical testing, test method development and stability testing, toxicology, formulation, process development, manufacturing scale-up, qualification and validation, quality assurance/quality control, clinical studies, including manufacturing in support thereof, statistical analysis and report writing, the preparation and submission of Drug Approval Applications, regulatory affairs with respect to the foregoing and all other activities necessary or reasonably useful or otherwise requested or required by a Regulatory Agency as a condition or in support of obtaining or maintaining a Regulatory Approval and (ii) with respect to HCW, all activities related to discovery, research, development, pre-clinical and other non-clinical testing, test method development and stability testing, toxicology, clinical studies, statistical analysis and report writing, the preparation and submission of Drug Approval Applications, regulatory affairs with respect to the foregoing and all other activities necessary or reasonably useful or otherwise requested or required by a Regulatory Agency as a condition or in support of obtaining or maintaining a Regulatory Approval and, solely with respect to Non-TGFb Products, subject to ImmunityBio’s rights under Paragraphs 2(d), 2(e), 2(f) or 5(e), all activities related to the creation and prosecution of Intellectual Property Rights generated after the Effective Date (other than with respect to a Non-TGFb Product in the Oncology Field that is Directed To a Licensed Target created by either Party utilizing the TOBI Platform). When used as a verb, “Develop” means to engage in Development.
l.“Directed To” means, with respect to any fusion protein, molecule and/or antibody and a biological target and/or its receptor or Licensed Target, that such fusion protein, molecule and/or antibody binds to, inhibits, modulates or otherwise interacts with such biological target and/or its receptor or Licensed Target.
m.“Drug Approval Application” means a New Drug Application submitted pursuant to Section 505 of the FD&C Act, a Biologics License Application, or any corresponding foreign application (in each case, including any amendment or supplement thereto) for any investigational agent.
Settlement Agreement and Release
Page 3
n.“Effective Date” means the date of full execution of this Settlement by all of the Settling Parties.
o.“EirGenix” means EirGenix, Inc.
p.“Exclusive Licensed Field” means (i) with respect to TGFb Products covered by Group A Patents, Group B Patents or HCW Additional Assigned Patents, all Indications other than those in the Oncology Field and (ii) with respect to Non-TGFb Products covered by Group B Patents or HCW Additional Assigned Patents, all Indications other than neoadjuvant ovarian cancer Indications. For clarity, the “Exclusive Licensed Field” for Licensed Products that contain both TGFb Molecules and non-TGFb molecules shall be limited to Indications other than those in the Oncology Field.
q.“Exploit” or “Exploitation” means (i) with respect to ImmunityBio, the making, having made, using, having used, selling, having sold, offering for sale or otherwise disposing of, a product or investigational agent, including all discovery, research, Development (including the conduct of clinical trials), manufacturing, registration, modification, enhancement, improvement, labeling, storage, formulation, exportation, importation, optimization, transportation, distribution, promotion, marketing and Commercialization activities related thereto and (ii) with respect to HCW, using, having used, selling, having sold, offering for sale or otherwise disposing of, a product or investigational agent, including all discovery, research, Development (including the conduct of clinical trials), registration, labeling, storage, exportation, importation, transportation, distribution, promotion, marketing and Commercialization activities related thereto and, solely with respect to Non-TGFb Products (other than any Non-TGFb Product that is Directed To a Licensed Target created by either Party utilizing the TOBI Platform) and any product exclusively licensed by HCW to Wugen under the Wugen Agreement as of the Effective Date, the making or having made of a product or investigational agent and all manufacturing, modification, enhancement, improvement, formulation, and optimization activities related thereto.
r.“FDA” means the United States Food and Drug Administration or any successor federal agency thereto.
s.“Group A Patents” means the Group A Patent Rights as set forth in Schedule 1and all Patent Rights thereof and thereto.
t.“Group B Patents” means the Group B Patent Rights as set forth in Schedule 1and all Patent Rights thereof and thereto.
u.“Group C Patents” means the Group C Patent Rights as set forth in Schedule 1and all Patent Rights thereof and thereto.
v.“ImmunityBio” means ImmunityBio, Inc., a Delaware corporation.
w.“Improvements” means any improvements, enhancements or modifications to the products developed using the TOBI Platform and Directed To any Licensed Target, that are conceived, made, reduced to practice or developed by Respondents or any of their Affiliates after the Effective Date.
x.“IND” means (a) an Investigational New Drug Application as defined in the Federal Food, Drug, & Cosmetic Act (“FD&C Act”) and applicable regulations promulgated thereunder by the FDA, and (b) the equivalent application to the applicable Regulatory Agency in any other regulatory jurisdiction, the filing of which is necessary to initiate or conduct clinical testing of a pharmaceutical product in humans in such jurisdiction.
y.“Indication” means a class of human disease or condition for which a separate marketing authorization application (including any extensions or supplements) is required to be filed with a Regulatory Agency.
z.“Intellectual Property Rights” means any and all (A) patents, divisionals, applications, utility models, industrial rights and similar intellectual property rights registered or applied for in the United States and all other countries throughout the world (including all reissues, divisions, continuations, continuations-in-part, renewals, extensions and reexaminations thereof) (collectively, “Patent Rights”); (B) rights in trademarks, service marks, trade dress, logos, domain names, rights of publicity, trade names and corporate names (whether or not registered) in the United States and all other countries throughout the world, including all registrations and applications for registration of the foregoing
Settlement Agreement and Release
Page 4
and all goodwill related thereto; (C) copyrights (whether or not registered) and rights in works of authorship, databases and mask works, and registrations and applications for registration thereof in the United States and all other countries throughout the world, including all renewals, extensions, reversions or restorations associated with such copyrights, now or hereafter provided by law, regardless of the medium of fixation or means of expression; (D) right in inventions, practices, methods, protocols, formulas, know-how, know-how related to manufacturing, testing, characterization and/or similar processes, specifications, formulae, software, algorithms, CMC information, formulations, expertise, test data, stability data, other study data and procedures, trade secrets, processes, assays, techniques and results of experimentation and testing, and other scientific, technical or regulatory information (including raw data) in the United States and all other countries throughout the world (collectively, “Know-How”); (E) other intellectual property or proprietary rights in the United States and all other countries throughout the world, including all neighboring rights and sui generis rights; (F) rights to apply for, file, register establish, maintain, extend or renew any of the foregoing; (G) rights to enforce and protect any of the foregoing, including the right to bring legal actions for past, present and future infringement, misappropriation or other violations of any of the foregoing; and (H) rights to transfer and grant licenses and other rights with respect to any of the foregoing.
aa.“Knowledge” means actual knowledge of XXX, XX. XXXX, XXXXX XXXXX, XXXX XXXX, XXX XXX, XXXXXXX XXX, XXX XXXX, XXXXXX XXX, XXXXX XXXXXXXX, XXXXXX XXXXXXXXXX, XXX/XX XXX XXX XXXXX XXXXXXXXXX XXXXX XXXXXXX, after performing due inquiry with respect to the applicable facts and information.
bb.“Licensed Field” means both the Exclusive Licensed Field and the Non-Exclusive Licensed Field.
cc.“Licensed Target” means the biological target and/or receptors of each of (a) PDL-1, (b) IL-7, (c) IL-12, (d) IL-18, (e) IL-21 and (f) such additional biological target selected pursuant to Paragraph 5(b).
dd.“Licensed Products” means (a) TGFb Products, and (b) Non-TGFb Products; provided that, the foregoing “(b)” expressly excludes any product that comprises or is Directed To, as applicable, subject matter that is subject to ImmunityBio’s exclusive rights pursuant to Paragraph 5.
ee.“Non-Exclusive Licensed Field” means neoadjuvant ovarian cancer Indications solely to the extent (i) used in combination with standard of care chemotherapy and (ii) limited to the treatment plan and the indication (if any) as submitted to the FDA by HCW as of the Effective Date.
ff.“Non-TGFb Product” means any pharmaceutical product in any form that does not (i) contain a TGFb Molecule or (ii) otherwise relate to human transforming growth factor receptors or TGFb traps.
gg.“Oncology Field” means all uses for oncology diseases, disorders or conditions in humans or animals, including prophylactic or therapeutic treatment, delay or prevention of any oncology diseases, disorders or conditions in humans and animals.
ii.“Person” means an individual, corporation, partnership, limited liability company, association, trust or other entity or organization, but not including a government or political subdivision or any agency or instrumentality of such government or political subdivision.
jj.“Regulatory Agency” means the FDA and any other governmental authority with responsibility for the approval of the marketing and sale of pharmaceuticals or biologics or other regulation of pharmaceuticals or biologics.
kk.“Regulatory Approval” means all approvals (including, without limitation, where applicable, Drug Approval Applications, pricing and reimbursement approval, labeling approval and schedule classifications), licenses, registrations, certificates, permits or authorizations of any Regulatory Agency necessary for the manufacture, use, storage, import, export, transport, offer for sale, or sale of any product or investigational agent, together with all amendments, supplements and updates thereto and all benefits arising therefrom, including any orphan drug exclusivities or other non-patent exclusivities.
Settlement Agreement and Release
Page 5
ll.“ROFR Information Package” means (a) the following information Controlled by any Respondent at the time a ROFR Information Package is delivered that summarizes material data relating to the Additional Molecule including (i) structure, (ii) characterization, (iii) data and information supporting the promotion and activation of immune cells, (iv) clinical readouts, (v) pre-IND and IND enabling studies conducted or ongoing, (vi) the competitive advantages of using such fusion proteins for products, (vii) any other information provided to any prospective Third Party licensee, (b) physical samples of such fusion proteins of sufficient quality and quantity to permit ImmunityBio to conduct due diligence studies thereof, and (c) as applicable, the terms of any proposed Third Party license for any Additional Molecule (excluding the identity of the applicable Third Party).
mm.“SRS” means Shareholder Representative Services LLC.
nn.“T Cells” means a T-lymphocyte.
oo.“TGFb Assigned Patents” means the Group A Patents and Group B Patents.
pp.“TGFb Know-How” means all Know-How Controlled by the Respondents or any of their Affiliates as of the Effective Date or at any time thereafter that is necessary or reasonably useful for the Exploitation of the TGFb Molecules or any TGFb Products.
qq.“TGFb Molecules” means any molecules Controlled by any Respondent as of the Effective Date or thereafter that were generated through the use of the TOBI Platform related to the human transforming growth factor receptor and TGFb traps, including, without limitation, HCW9218, HCW9219, HCW9209 and any derivatives thereof or therefrom.
rr.“TGFb Product” means any pharmaceutical product in any form that contains a TGFb Molecule.
ss.“Third Party” means any Person other than the Claimants, Respondents, or its or their respective Affiliates.
tt.“TOBI Platform” means HCW’s TOBI™ immunotherapeutic drug design and discovery platform and any modifications, improvements and thereto.
uu.“Transferred Assets” means (i) the Transferred Intellectual Property Rights, (ii) Transferred Regulatory Materials, (iii) Transferred Invention Records and Prosecution Files, (iv) Transferred Inventory and (v) Transferred Contracts.
vv.“Transferred Contracts” means the contracts set forth on Schedule 3.
ww.“Transferred Intellectual Property Rights” means (i) the TGFb Assigned Patents, (ii) the TGFb Know-How, and (iii) other than the Group C Patents, all other Intellectual Property Rights (A) as of the Effective Date, necessary or reasonably useful for, and (B) any time thereafter, necessary for or otherwise specific to, the Exploitation of the TGFb Molecules or any TGFb Products Controlled by Respondents or any of their Affiliates (such Patent Rights in (iii), the “HCW Additional Assigned Patents”). For clarity, all rights, title, and interest in and to compositions of matter, all formulations, all methods of treatment, and all methods of manufacture necessary for or reasonably useful for ImmunityBio’s Exploitation of the TGFb Molecules or any TGFb Products that exist as of the Effective Date are included in the Transferred Intellectual Property Rights, and all formulations, all methods of treatment, and all methods of manufacture necessary for or otherwise specific to ImmunityBio’s Exploitation of the TGFb Molecules or any TGFb Products that exist after the Effective Date are included in the Transferred Intellectual Property Rights.
xx.“Transferred Invention Records and Prosecution Files” means (i) all records, books, documents and files pertaining to and/or demonstrating the inventorship of any Transferred Intellectual Property Rights and (ii) any other documents and materials relating to the prosecution, defense, maintenance, validity and enforceability of the Transferred Intellectual Property Rights.
yy.“Transferred Inventory” means the inventory of TGFb Molecules and any other biological materials necessary or reasonably useful to Develop the TGFb Molecules, to the extent provided for in Schedule 2(a)(iii).
Settlement Agreement and Release
Page 6
zz.“Transferred Regulatory Materials” means all U.S. and foreign regulatory applications, submissions and approvals (including all INDs and Drug Approval Applications and foreign counterparts thereof), and all Regulatory Approvals for TGFb Molecules, and all correspondence with the FDA and other Regulatory Agencies relating to the TGFb Molecules or any of the foregoing regulatory applications, submission and approvals, that, in each case, are in the possession of or Controlled by, or held by Respondents or any of their Affiliates as of the Effective Date, whether generated, filed or held by or for Respondents or any of their Affiliates or licensees.
aaa.“Treg Products” means any pharmaceutical or biological product that contains or comprises Tregs that have been engineered, modified, or otherwise manipulated ex vivo, as an Active Ingredient, and the primary mechanism of action of such product is through the activities of such Tregs.
bbb.“Tregs” means regulatory T Cells that are a subpopulation of T Cells which negatively regulate the immune system, maintain tolerance to self-antigens, suppress immune system in cancers, abrogate autoimmune disease or alleviate inflammation.
ccc.“Wugen” means Wugen, Inc., a Delaware corporation.
“Wugen Agreement” means the Exclusive License Agreement entered into as of December 24, 2020 by and between Wugen and HCW, in the form as attached hereto as Exhibit A.
[1] Dr. Hing C. Wong v. Altor BioScience, LLC; NantCell, Inc. JAMS Arbitration Ref. No. XXXXXXXXXX.
[2] Altor BioScience, LLC., et al., v. HCW Biologics, Inc., Case No. 22-CV-62404-RAR (S.D. Fla. Dec. 23, 2022).
[3] Altor BioScience, LLC; NantCell, Inc. v. Hing C. Wong, JAMS Arbitration Ref. No. XXXXXXXXXX.
[4] Altor BioScience, LLC; NantCell, Inc. v. HCW Biologics, JAMS Arbitration Ref. No. XXXXXXXXXX.
[5] Altor BioScience, LLC and NantCell, Inc. v. HCW Biologics, Inc., C.A. No. 2024-0310-PAF (Del. Ch. Apr. 1, 2024).
2. All TGFb Molecules.
a.Assignment. Respondents hereby assign to ImmunityBio their entire right, title and interest in and to all Transferred Assets effective as of the Effective Date. In furtherance of the foregoing:
i.Assurances. Respondents agree to execute the Patent Assignment as set forth on Exhibit B hereto, as well as the Power of Attorney as set forth on Exhibit C hereto, each as of the Effective Date, and further agree going forward to cooperate with and assist ImmunityBio, and perform all acts deemed necessary or desirable by ImmunityBio, to apply for, obtain, establish, perfect, maintain, evidence, enforce or otherwise protect any of the full benefits, enjoyment, right, title and interest throughout the world in the TGFb Assigned Patents and TGFb Molecules. Such acts may include, but are not limited to, execution of assignments of title and other documents and assistance or cooperation in legal proceedings. Should ImmunityBio be unable to secure Respondents’ signature on any such document, in connection with and effective by the Power of Attorney attached hereto as Exhibit C, Respondents hereby irrevocably designate and appoint ImmunityBio and its duly authorized representatives as Respondents’ agents and attorneys-in-fact, with full power of substitution and delegation, to undertake such acts in Respondents’ name as if executed and delivered by Respondents (which appointment is coupled with an interest), and Respondents waive and quitclaim to ImmunityBio any and all claims of any nature whatsoever that they may have or may later have for infringement of any Transferred Intellectual Property Rights;
ii.Unassigned Assets. In the event that any Party becomes aware of any Transferred Assets (a) that come into existence after the Effective Date and/or (b) was otherwise not
Settlement Agreement and Release
Page 7
assigned in accordance with Paragraph 2, including due to error or because such assignment would require an authorization, approval, consent or waiver from a Third Party, then such Party will notify the other Party and Respondents will take all actions necessary to effect the assignment of such unassigned Transferred Assets to ImmunityBio, including executing any additional legal instruments reflecting assignment as ImmunityBio deems necessary or useful and/or obtaining any required authorization, approval, consent or waiver from an applicable Third Party.
iii.Technology Transfer. Within XXXXXX XXX XXXX XXXXX XXX XXXXXXXXX XXXX, HCW shall (a) provide ImmunityBio with complete and accurate copies of the TGFb Know-How and all other Transferred Assets as provided for in Schedule 2(a)(iii) and (b) deliver to ImmunityBio the Transferred Inventory. HCW shall preserve the TGFb Know-How in the same state as immediately prior to the Effective Date until such TGFb Know-How is transferred in accordance with this Paragraph 2(a)(iii). ImmunityBio will be responsible for all reasonable, necessary and documented transportation costs for the shipment of any materials to ImmunityBio required under Schedule 2(a)(iii). Within XXXXXX XXX XXXX of any assignment of Transferred Assets under Paragraph 2(a)(ii), Respondents shall provide and transfer copies of (if applicable) such Transferred Assets to ImmunityBio, and Respondents or any of their Affiliates shall provide reasonable technical assistance with respect to any such additional Transferred Assets to the extent necessary to permit ImmunityBio’s Exploitation thereof. HCW grants to ImmunityBio a right of reference to any regulatory documentation, submissions or approvals with any Regulatory Agency, including in connection with the Transferred Regulatory Materials, submitted in HCW’s or its Affiliate’s name (or in the name of any of their respective designees). ImmunityBio grants to HCW a right of reference to the Transferred Regulatory Materials solely in connection with HCW’s Exploitation of HCW9218 in the Licensed Field.
iv.Respondents’ Non-Compete. On the Effective Date and thereafter, Respondents shall not, directly or indirectly, generate any derivatives of TGFb Molecules for use in the Oncology Field other than the Non-Exclusive Licensed Field.
b.Retained Liabilities; Indemnification. For the avoidance of doubt, the transfer provided in Paragraph 2(a) above is a transfer of assets only and the Settling Parties acknowledge and agree that ImmunityBio is not assuming any liabilities of any Respondents or any of their Affiliates arising, accruing or existing as of and prior to the Effective Date, which such liabilities are expressly retained by Respondent (“HCW Retained Liabilities”). For avoidance of doubt, HCW Retained Liabilities includes, without limitation, third party claims arising from infringing activity caused by Exploitation of the Transferred Intellectual Property Rights and for liabilities, including amounts accrued and/or owed, arising out of any Transferred Contract, in all cases prior to the Effective Date. Respondents shall defend, indemnify, and hold harmless ImmunityBio and its Affiliates and their respective officers, directors, employees, agents, successors and assigns (the “ImmunityBio Indemnitees”) from and against any and all losses, damages, liabilities, actually incurred expenses and costs, including reasonable legal expense and attorneys’ fees (“Losses”) to which any ImmunityBio Indemnitee may become subject as a result of any
Settlement Agreement and Release
Page 8
claim, demand, action or other proceeding by any Third Party (“Third Party Claim”) arising out of, based on, or resulting from any HCW Retained Liabilities.
c.License to HCW. Subject to the terms and conditions of this Agreement, including the contingency referenced in this Paragraph 2(c) below, ImmunityBio hereby grants HCW (i) a worldwide, perpetual (subject to this Paragraph 2(c)), fully-paid up, royalty-free, exclusive license, with the right to sublicense, under the TGFb Assigned Patents, the HCW Additional Assigned Patents and the TGFb Know-How, to Exploit Licensed Products, as applicable, in the Exclusive Licensed Field and (ii) a worldwide, perpetual (subject to this Paragraph 2(c)), fully-paid up, royalty-free, non-exclusive license, with the right to sublicense, under the TGFb Assigned Patents, the HCW Additional Assigned Patents and the TGFb Know-How, to Exploit Licensed Products in the Non-Exclusive Licensed Field. HCW hereby grants ImmunityBio a right of first refusal to regain exclusive rights through termination of the license rights granted to HCW pursuant to this Paragraph 2(c) to Exploit Licensed Products for use in the Non-Exclusive Licensed Field, following the procedures as set forth in Paragraph 6 hereof, mutatis mutandis. HCW agrees to provide full access to all safety and clinical data generated in connection with Licensed Products, whether for the Exclusive or Non-Exclusive Licensed Field or otherwise, to ImmunityBio. Notwithstanding the foregoing, in the event that HCW does not initiate (i.e., dose a first patient) a clinical trial of a TGFb Molecule for use in the Non-Exclusive Licensed Field prior to December 31, 2024, then the applicable license granted to HCW as described in this Paragraph 2(c)(ii) shall automatically terminate effective as of such date.
d.Prosecution and Maintenance.
i.Rights. For the avoidance of doubt, ImmunityBio shall control and have the first right, but not the obligation, to prepare, file, prosecute, and maintain the TGFb Assigned Patents worldwide. Further, ImmunityBio shall have the first right, but not the obligation, to conduct any opposition, re-issuance, post-grant review, inter-partes review, reexamination request, nullity action, interference, or other similar post-grant proceedings and any appeals therefrom relating to the TGFb Assigned Patents worldwide. Notwithstanding the foregoing, and solely with respect to claims contained in a TGFb Assigned Patent that specifically cover the Exploitation of a Licensed Product in the Exclusive Licensed Field (an “Exclusive Licensed Field-Specific Claim”), ImmunityBio shall file, at HCW’s expense, any additional related applications HCW deems reasonably necessary to further protect a Licensed Product in the Exclusive Licensed Field, provided that, ImmunityBio may elect not to file such related application(s) to the extent ImmunityBio in good faith believes such application may have any adverse impact on any TGFb Assigned Patents, HCW Additional Assigned Patents or ImmunityBio TGFb Products. ImmunityBio shall provide HCW with a copy of any draft of a material and substantive filing directed to an Exclusive Licensed Field-Specific Claim reasonably in advance of ImmunityBio’s filing of such draft to permit HCW, using counsel of HCW’s choosing, an opportunity to review and provide reasonable comments and/or instructions thereto within XXXXXXX XXX XXXX of HCW’s receipt of the applicable draft (or a shorter period reasonably designated by ImmunityBio if XXXXXXX XXX XXXX is not practicable given the filing deadline). ImmunityBio will incorporate in good faith all reasonable comments and effect all reasonable instructions related directly to such Exclusive Licensed Field-Specific Claim thereto provided by HCW during the applicable comment period and in connection with the filing thereof; provided that, ImmunityBio may elect not to incorporate such reasonable comments to the extent ImmunityBio believes such reasonable comments may have any adverse impact on any TGFb Assigned Patents or ImmunityBio TGFb Products. Notwithstanding anything to the contrary herein, solely for purposes of this Paragraph 2(d), Paragraph 2(e) and Paragraph 2(f), the HCW Additional Assigned Patents shall be treated as TGFb Assigned Patents, mutatis mutandis.
ii.Costs. HCW and ImmunityBio shall XXXX XXXX XXXX XX XXX third party expenses (including reasonable attorneys’ fees) incurred in connection with the prosecution or maintenance activities that pertain to Patent Rights comprised of an Exclusive Licensed Field-Specific Claim and a claim that is not an Exclusive Licensed Field-Specific Claim. With respect to all third party expenses (including reasonable attorneys’ fees) incurred or to be incurred by ImmunityBio in connection with any prosecution or maintenance activities that pertain solely to an Exclusive Licensed Field-Specific Claim (a) HCW shall reimburse ImmunityBio for all such expenses, and (b) without limiting the foregoing, HCW shall, within HCW’s XXXXXXX XXX XXX (or shorter, as applicable) review and comment period, advance to ImmunityBio (or its designee) all such expenses. To the extent HCW does not advance such
Settlement Agreement and Release
Page 9
expenses, ImmunityBio shall have no obligation to take any action with respect to such Exclusive Licensed Field-Specific Claim.
iii.Step-In Right. ImmunityBio may cease prosecution and/or maintenance of any Exclusive Licensed Field-Specific Claim on a country-by-country basis by providing HCW written notice reasonably in advance (but at least XXXXX XXX XXXX before the applicable deadline with a relevant patent authority). If ImmunityBio elects to cease prosecution or maintenance of the relevant Exclusive Licensed Field-Specific Claim in a country, HCW, shall have the right, but not the obligation, at its sole discretion and cost, to continue prosecution or maintenance of such Exclusive Licensed Field-Specific Claim and in such country with counsel of its choosing.
i.Rights. If either Party becomes aware of any existing or threatened infringement of any TGFb Assigned Patent (“Infringement”), it shall promptly notify the other Party in writing to that effect. Respondents shall share with ImmunityBio all information available to it regarding such alleged Infringement, pursuant to a mutually agreeable “common interest agreement” executed by the Parties under which the Parties agree to their shared, mutual interest in the outcome of any suit or other action to enforce the TGFb Assigned Patent against such Infringement. ImmunityBio shall have (a) with respect to any Infringement of the TGFb Assigned Patents, the sole right; provided that, such Infringement is not subject to clause (b), and (b) with respect to any Infringement that is solely of an Exclusive Licensed Field-Specific Claim, the first right, and in each case of clause (a) and (b), but not the obligation, to bring an appropriate suit or other action against any Person engaged in the Infringement of any TGFb Assigned Patent or Exclusive Licensed Field-Specific Claim, as applicable, XX XXXXXXXXXXXXX XXXX XXX XXXXXXX. If the applicable Infringement is solely with respect to an Exclusive Licensed Field-Specific Claim and ImmunityBio notifies HCW in writing that it does not intend to commence a suit or other action to enforce the applicable Exclusive Licensed Field-Specific Claim against such Infringement or to take other action to secure the abatement of such Infringement, or fails to take any such action after a period of XXXXX XXXX XXXXXXXX XXXX following either Party’s receipt of the notice of Infringement pursuant to this Paragraph 2(e)(i) then, HCW shall have the right, but not the obligation, to commence such a suit or take such action, at XXXXX XXXX XXX XXXXXXX and using counsel of its choosing.
ii.Recoveries. Any amounts recovered in connection with an Infringement suit or action under Paragraph 2(e)(i) shall first be used to reimburse ImmunityBio and HCW for their costs and expenses incurred in connection with such Infringement suit or action and all remaining amounts shall be divided as follows: (a) with respect to an Infringement suit or action that relates to only claims that are not Exclusive Licensed Field-Specific Claims, XXXXXXXXXXX XXXXXXX XXX XXXXXXX XXXXXXX XXXXXX; (b) with respect to an Infringement suit or action (1) that relates to both claims that are not Exclusive Licensed Field-Specific Claims and Exclusive Licensed Field-Specific Claims or (2) that is controlled by ImmunityBio and relates to only Exclusive Licensed Field-Specific Claims, XXXXXXXXXXX XXX XXX XXXX XXXXXX XXXXX XXXXXXX XXXXX;and (c) with respect to an Infringement suit or action that is controlled by HCW and relates to only Exclusive Licensed Field-Specific Claims, XXXXXXXXXXX XXX XXX XXXX XXXXXXX XX XXXX XXXXX XX XXXXXXXXXX XXXXXXXXXX XX XXXXXXXXXX XX XXXXX XX XXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXX XXX XXXXXXXXXXXXX.
f.Cooperation. Each Party shall cooperate with the Party controlling the prosecution and/or enforcement of any TGFb Assigned Patent, at the controlling Party’s request and, subject to Paragraph 2(d)(ii) expense. To the extent that HCW exercises or intends to exercise its rights under Paragraphs 2(d) or 2(e), (i) XXX XXXX XXXX XXX XX XXX XXXXX XXX XXXXXXXX incurred in connection therewith and (ii) notwithstanding Paragraphs 2(d) and 2(e), if ImmunityBio reasonably believes that HCW’s initiation or continued prosecution, maintenance or enforcement, as applicable, of any such Exclusive Licensed Field-Specific Claim may have an adverse impact on any TGFb Assigned Patents or ImmunityBio TGFb Products, then HCW shall not have the right to continue the prosecution, maintenance or enforcement, as applicable, of such Exclusive Licensed Field-Specific Claim.
g.License to ImmunityBio. Respondents hereby grant ImmunityBio (i) a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, non-exclusive license, with the right to grant and authorize sublicenses through multiple tiers, under (A) the Group C Patents, and (B) any other
Settlement Agreement and Release
Page 10
Intellectual Property Rights Controlled by Respondents or any of their Affiliates after the Effective Date and reasonably useful for the Exploitation of TGFb Molecules or TGFb Products, in each of (A) and (B), for all uses in the Oncology Field; and (ii) a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, exclusive license (subject only to ImmunityBio’s license to HCW under Paragraph 2(c)(ii) to Exploit Licensed Products in the Non-Exclusive Licensed Field), with the right to grant and authorize sublicenses through multiple tiers, subject to and in accordance with Paragraph 2(a)(ii), any other Transferred Intellectual Property Rights that are unable to be assigned and until such time that such Transferred Intellectual Property Rights, as applicable, are assigned, for all uses in the Oncology Field.
h.Publicity. HCW agrees to update its corporate website pipeline to be consistent with this arrangement limiting HCW9218 to the Licensed Field.
3. NK Cell Memory Like Activation Technology.
a.Subject to the terms and conditions of this Agreement, Respondents hereby grant ImmunityBio a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, non-exclusive license, with the right to sublicense, under any Intellectual Property Rights Controlled by the Respondents or any of their Affiliates as of the Effective Date and thereafter, to Exploit HCW9201 (IL12/IL15/IL18) solely as a subcutaneous injection (in vivo). For clarity, the foregoing license grant excludes any rights exclusively granted to Wugen under the Wugen Agreement as of the Effective Date.
b.Within XXXXXX XXX XXXX XX XXX XXXXXXXXX XXXX (or such longer period as requested by ImmunityBio), HCW shall perform a manufacturing technology transfer to ImmunityBio or its designee sufficient to permit ImmunityBio or its designee to manufacture HCW9201, which shall comprise a transfer of complete and accurate copies of all Know-How Controlled by the Respondents or any of their Affiliates as of the Effective Date that is necessary or reasonably useful in exercising ImmunityBio’s rights under the license grant set forth in Paragraph 3(a) including, without limitation, transfer of the materials and documentation as set forth in Schedule 2(a)(iii), as well as any inventory and materials pertaining to HCW9201 (replacing HCW9218 and HCW 9101 with HCW9201, as appropriate) as set forth in Schedule 2(a)(iii).
c.Respondents shall defend, indemnify, and hold harmless ImmunityBio Indemnitees from and against any and all Losses to which any ImmunityBio Indemnitee may become subject as a result of any Third Party Claim arising out of, based on, or resulting from ImmunityBio’s exercise of the licenses granted under Paragraph 3(a), including the Exploitation of HCW9201, solely to the extent such Third Party Claim (i) alleges infringement or misappropriation of such Third Party’s Intellectual Property Rights as a direct result of ImmunityBio’s exercise of the licenses granted under Paragraph 3(a), (ii) arises in connection with the rights granted to Wugen under the Wugen Agreement or any amendments or modifications thereto, or (iii) arises in connection with the gross negligence or willful misconduct of Respondents or any of their Affiliates in connection with the performance of Respondents’ obligations under Paragraph 3(b).
4. All Affinity Purification and Column Technology.
a.Subject to the terms and conditions of this Agreement, Respondents hereby grant ImmunityBio a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, non-exclusive license, with the right to sublicense, under any Intellectual Property Rights Controlled by the Respondents or any of their Affiliates as of the Effective Date and thereafter, to Exploit (i) the HCW9101 master cell bank XXXXXXXXX XX XXX XX XXX XXX/XX XXX XXXXX XXXXX XXXXXXXX XXXXXXXXXXXXX XXXXXXXXXXXXXXX to manufacture and purify cGMP HCW9218 and (ii)
Settlement Agreement and Release
Page 11
master and research cell banks for the TGFb Molecules for manufacturing and purification of tissue factor based fusion proteins. Within XXXXXX XXX XXXX XXXXX XXX XXXXXXXXX XXXX, HCW shall provide ImmunityBio with complete and accurate copies of all Know-How Controlled by the Respondents or any of their Affiliates as of the Effective Date or thereafter and necessary or reasonably useful in exercising ImmunityBio’s rights under the license grant set forth in this Paragraph 4(a) including, without limitation, transfer the materials and documentation as set forth in Schedule 2(a)(iii), as well as any inventory and materials as set forth in Schedule 2(a)(iii). For clarity, such technology transfer shall include, without limitation, Know-How sufficient for XXXXXXXXXXX XX XXX XXXXXXXX XX XXX XXXXX XXX XXXXXXXXXXXXX XX XXX XXXXXX, XXXXX XXX XXXXXXXX XXXXXX and shall be subject to the same ongoing obligations and procedure as set forth in Paragraph 2(a)(ii), mutatis mutandis, including with respect to any applicable Improvements and ongoing support.
b.Respondents shall, as of the Effective Date, execute and deliver to each of ImmunityBio and EirGenix a signed letter in the form attached as Exhibit D. Further, Respondents hereby consent and agree to the assignment as set forth under the terms of this Agreement of the Transferred Contracts to ImmunityBio, subject only to the consent of EirGenix (or other third party thereto), as required. Further, nothing in this Agreement shall be interpreted as limiting or restricting ImmunityBio from entering into a direct contractual relationship with HCW’s Third Party contract manufacturing organization(s), including EirGenix, and Respondents represent that Respondents have not taken, and will not take, any action (or inaction) to interfere with or prevent ImmunityBio’s exercise of ImmunityBio’s rights with respect to EirGenix. Nothing in this Agreement shall be construed as an attem pt or agreement to assign or transfer any Transferred Contract to ImmunityBio which by its terms is not assignable or transferable without a consent or is cancelable by a Third Party in the event of an assignment or transfer (a “Non-Assignable Contract”), unless and until such consent shall have been obtained. Respondents and ImmunityBio shall obtain as expeditiously as possible any consent that may be required for the assignment or transfer of the Non-Assignable Contract to ImmunityBio, and Respondents shall take all such actions as may be necessary to effect the assignment and transfer of the Non-Assignable Contract. Unless and until any such consent that may be required is obtained, Respondents shall establish an arrangement reasonably satisfactory to ImmunityBio under which ImmunityBio would obtain the rights, claims and benefits under such Non-Assignable Contract (including by means of any subcontracting, sublicensing or subleasing arrangement) or under which Respondents would enforce for the benefit of ImmunityBio, any and all rights, claims and benefits of Respondents against a third party thereto.
c.For the avoidance of doubt, HCW shall control and have the first right, but not the obligation, to prepare, file, prosecute, and maintain the patents licensed to ImmunityBio pursuant to Paragraph 4(a) (other than the TGFb Assigned Patents which are subject to the assignment under Paragraph 2) (the “9101 Patents”) worldwide. Further, HCW shall have the first right, but not the obligation, to
Settlement Agreement and Release
Page 12
conduct any opposition, re-issuance, post-grant review, inter-partes review, reexamination request, nullity action, interference, or other similar post-grant proceedings and any appeals therefrom relating to the 9101 Patents. HCW shall provide ImmunityBio with a copy of the draft prepared for the filing of any claim contained in a 9101 Patent (a “9101 Specific Claim”) before the filing of such 9101 Specific Claim and will consider in good faith comments thereto provided by ImmunityBio in connection with the filing thereof. HCW shall provide ImmunityBio with regular updates on the prosecution of the 9101 Specific Claims. HCW may cease prosecution and/or maintenance of any 9101 Specific Claim on a country-by-country basis by providing ImmunityBio written notice reasonably in advance (but at least XXXXX XXX XXXX before the applicable deadline with a relevant patent authority). If HCW elects to cease prosecution or maintenance of the relevant 9101 Specific Claim in a country, ImmunityBio shall have the right, but not the obligation, at its sole discretion and cost, to continue prosecution or maintenance of such 9101 Specific Claim and in such country.
5. ImmunityBio’s Choice of Six (6) Licensed Targets and Associated TOBI Platform Products.
a.Respondents hereby grant to ImmunityBio, with respect to each Licensed Target, a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, exclusive (even as to Respondents) license, with the right to sublicense through multiple tiers, under all Intellectual Property Rights Controlled by Respondents or any of their Affiliates as of the Effective Date and any Improvements thereto, to Exploit Licensed Targets and any products (other than the fusion proteins referred to as HCW9206 and HCW9302), including any fusion proteins, molecules and/or antibodies therein, created by Respondents prior to the Effective Date or by either Party thereafter utilizing the TOBI Platform Directed To a Licensed Target (“TOBI Platform Products”), solely in the Oncology Field; provided that, the foregoing license grant excludes the right to Exploit any Cellular Therapy Products ex vivo to the extent exclusively licensed to Wugen pursuant to the Wugen Agreement as of the Effective Date. For clarity, from and after the Effective Date, Respondents shall not, and shall cause its Affiliates, acquirors and/or sublicensees not to, institute or prosecute, any claim demand, action or other proceeding for damages, costs, expenses or compensation, or for an enjoinment, injunction or any other equitable remedy, against ImmunityBio or any of its Affiliates, acquirors and/or sublicensees alleging that the Exploitation of Licensed Targets or TOBI Platform Products in any way infringes any Intellectual Property Rights owned or Controlled by Respondents or any of its Affiliates, acquirors or sublicensees, including, without limitation, those covering HCW9206 or HCW9302.
b.ImmunityBio shall choose one (1) additional Licensed Target at its sole discretion within the next six (6) months following the Effective Date (“Target Evaluation Period”).
c.In connection with ImmunityBio’s right to choose one (1) additional Licensed Target at its sole discretion during the Target Evaluation Period, with respect to any biological target, Respondents shall provide ImmunityBio (i) all material data, results, presentations and other information related to such biological target and any fusion proteins, molecules and/or antibodies Directed To such target that is necessary or reasonably useful for ImmunityBio to evaluate whether to select such target to be a Licensed Target and (ii) at ImmunityBio’s reasonable request, direct access to personnel of Respondents for the purpose of evaluating and discussing the information described in clause (i). In the event that ImmunityBio selects a new biological target to be a Licensed Target pursuant to this Paragraph 5, it shall provide written notice of such selection to the Respondents. Upon expiration of the Target Evaluation Period, ImmunityBio shall have no further right under this Paragraph 5 to select biological targets not already selected to be a Licensed Target.
d.The Licensed Targets (i) shall be exclusive to ImmunityBio (even as to Respondents) as to any fusion protein, molecule and/or antibody created utilizing the TOBI Platform in the Oncology Field, such that Respondents cannot Develop, manufacture Commercialize, or Exploit, or license, authorize, appoint or otherwise enable any other Third Party to Develop, manufacture, Commercialize or Exploit, any fusion protein, molecule and/or antibody created utilizing the TOBI Platform Directed To such Licensed Target or that is a derivative of a pre-existing fusion protein, molecule and/or antibody created utilizing the TOBI Platform Directed To such Licensed Target, in either case, in the Oncology Field (but excluding the fusion proteins referred to as HCW9206 and HCW9302) and (ii) the
Settlement Agreement and Release
Page 13
sequence of such selected Licensed Target shall be the sole confidential information of ImmunityBio and subject to terms and conditions of Paragraph 19 of this Settlement.
ImmunityBio’s right to file, prosecute, maintain, and enforce any licensed Intellectual Property Rights granted under the license set forth in Paragraph 5(a) shall be as set forth in Paragraphs 2(d) through (f) (inclusive), mutatis mutandis.
6. Right of First Refusal for ImmunityBio.
a.Respondents hereby grant to ImmunityBio an exclusive option and right of first refusal to obtain an exclusive license to any Additional Molecule in the Oncology Field (the “ROFR”). If Respondents or any of their Affiliates desires to enter into any transaction with a Third Party for the license, transfer, or other disposition of any Additional Molecule for use in the Oncology Field (each, a “ROFR Transaction”), HCW will provide prompt written notice to ImmunityBio (“ROFR Notice”). Such ROFR Notice provided by HCW to ImmunityBio shall be accompanied by a ROFR Information Package and a Non-Disclosure Agreement to govern the treatment of the ROFR Information Package. ImmunityBio may exercise its ROFR by providing HCW with written notice thereof (“Exercise Notice”) by the date that is XXXXX XXX XXXXfollowing ImmunityBio’s receipt of a ROFR Notice by HCW. The Exercise Notice shall identify the Additional Molecule with respect to which ImmunityBio has an interest in exercising its ROFR. Following HCW’s receipt of such Exercise Notice, the Parties will enter into good faith negotiations for a license for a period not to exceed XXXXXX XXXX XXXX. The financial terms of any such license shall reasonably reflect the scope and content of the license grant, including its exclusivity, the Additional Molecule, the licensed Indication and the licensed territory. Notwithstanding the foregoing, and even if such aggregate XXX XXXXXXX XXX XXXXX XXXXX XXX period has expired, neither Respondents nor any of their Affiliates shall grant licenses or other rights to any Additional Molecule to any Third Party without first providing ImmunityBio with notice that a Third Party license is sought, including disclosure to ImmunityBio of all material terms of such Third Party’s offer, and giving ImmunityBio the opportunity to match such Third Party offer and exercise its ROFR with respect to such Additional Molecule. If ImmunityBio does not exercise its ROFR as provided in this Paragraph 6, or if the Parties do not complete negotiations for a license related to any Additional Molecule for which such Third Party license is sought within the time periods set forth in this Paragraph 6, then Respondents will be free to grant such Third Party license to the applicable Third Party for such Additional Molecule provided that such license shall be on terms no more favorable to such Third Party than the license terms last offered by ImmunityBio in writing; provided further that, if Respondents or any of their Affiliates desire to enter into a new ROFR Transaction, including, for clarity, for the same Additional Molecule, the procedure set forth in this Paragraph 6 shall apply to each such new ROFR Transaction. For the avoidance of doubt, Respondents agree that they will not, directly or indirectly, Develop, make, have made, use or Commercialize any Additional Molecule for use in the Oncology Field, without first complying with the procedures described in this Paragraph 6.
7. Other Provisions Regarding the License Grants.
a.Except as explicitly set forth in this Settlement, none of the Respondents nor Claimants shall be deemed by estoppel or implication to have granted any other party (including Claimants and Respondents, respectively) any license or other right to any intellectual property of such Respondent.
b.No later than XXX XXXXXXX XXXXXX XXXXX XXXX XXXXX XXX XXXXXXXXX XXXX, the Parties shall define and finalize the actions that the Parties shall employ with respect to Licensed Products in any Licensed Field to protect patients and promote their well-being in a written pharmacovigilance agreement (the “Pharmacovigilance Agreement”) for the Development and Commercialization of the Licensed Products in any Licensed Field globally. The Pharmacovigilance Agreement shall include mutually acceptable guidelines and procedures for the receipt, investigation, recording, communication, and exchange (as between the Parties) of adverse event reports, pregnancy reports, and any other information concerning the safety of the Licensed Products in any Licensed Field, and other routine pharmacovigilance reporting requirements. Such guidelines and procedures shall be in accordance with, and enable the Parties to fulfill, local and national regulatory reporting obligations under applicable laws. Furthermore, such agreed procedure shall be consistent with relevant ICH guidelines,
Settlement Agreement and Release
Page 14
except where said guidelines may conflict with existing local regulatory reporting requirements, in which case the local reporting requirements shall prevail. As between the Parties, ImmunityBio shall be responsible for preparing all adverse event reports and responses to safety issues and requests of Regulatory Agencies relating to Licensed Products in any Licensed Field. Each Party hereby agrees to comply with its respective obligations under such Pharmacovigilance Agreement and to cause its Affiliates and (sub)licensees to comply with such obligations.
8. Proceedings Against Claimants and ImmunityBio.
a.XXX. XX. XXXX XXXXXX XX XXXXXX XXXX XXX XXX XXXXXXXX XXXXX, XXX XXX XX XXXXXXXXXXX XX XXXXXX XX XXX XXX, XX XXXXX XXXXXXX XX XXX XXXXXX XXXXXXX XX XXXXX XXX XX XXXX XXXXXXXXX XXXXX XXXX XXXXXXXX XXXXXXXX XX XXX XX XXXXX XXXXXXX XXXXX, XXXXXXXXX. XX. XXXX XXXX XXXXXX XXX XXXXX XX XXXXXXXXXXX XXX XXXX XX XXXXXXXX - XX XXX - XXXXXXXX XX XXX XXX XXX XXXXXX XXXXX XXXXXXXXXXXX.
9. Forensic and Other Remediation of Certain Information and Data Repositories.
a.WithinXXXXXX XXXX XXXXXXXX XXXX XX XXX XXXXXXXXX XXXX, each Respondent (i.e., HCW and Dr. Wong) and each HCW employee who previously worked for Altor or NantCell and is still employed by or acting as a consultant for HCW shall affirm, by signing a declaration under oath in the form attached to this Agreement as Exhibit E, stating that: (1) they do not possess, are not using, and will not use any confidential and/or proprietary information of Claimants (“Claimants’ Confidential Materials”), including but not limited to (i) emails (and/or attachments) sent to or from their Altor or NantCell email addresses during their time at Altor or NantCell and (ii) documents stored on Altor’s private corporate servers and copies thereof; (2) they have conducted a reasonably diligent search of all Information Sources in their possession, have destroyed any Claimants’ Confidential Materials in their possession (other than HCW backups or archives that are not ordinarily accessible and which Dr. Wong agrees not to access and HCW agrees not to permit its employees to access), and have concluded that they no longer possess or have access to any Claimants’ Confidential Materials; (3) they understand they have an ongoing obligation to promptly destroy any Claimants’ Confidential Materials without using or disclosing such materials, if they later discover any such materials in their possession; and (4) they have not provided and will not provide any Claimants’ Confidential Materials to any third party. Each of Respondents and each HCW employee who previously worked for Altor or NantCell and is still employed by or acting as a consultant for HCW shall return such signed declaration to Claimants within thirty (30) calendar days after the Effective Date.
b.For the purposes of this Agreement, “Information Sources” shall include, but not be limited to, (i) HCW file servers, (ii) HCW email systems or email servers, (iii) HCW laptop or desktop computers, (iv) Altor laptops or desktop computers, (v) Altor lab notebooks or physical notebooks used for Altor work while at Altor, (vi) portable storage devices (including but not limited to USB devices) of Respondents or HCW employees who previously worked for Altor or NantCell, (vii) personal emails, files, or text messages of Dr. Wong and other HCW employees who previously worked for Altor or NantCell, and (viii) any backups and archives of the foregoing. A “reasonably diligent search” shall include, but not be limited to, diligent searches to identify all emails containing Altor or NantCell domain names, all documents bearing the logo of or referencing that it is an Altor or NantCell document, all documents describing or relating to Altor’s or NantCell’s research, and all documents previously downloaded from Altor or NantCell company servers.
c.For the purposes of this Agreement, “Claimants’ Confidential Materials” does not include information that (i) is or becomes generally available to the public other than as a result of disclosure by Respondents in violation of this Paragraph 9, so long as Respondents first obtained the information after they had become generally available to the public, or (ii) becomes available to Respondents on a non-confidential basis from a source that has the right to disclose such information. For the avoidance of doubt, “Claimants’ Confidential Materials” includes all materials, if any, in Respondents’
Settlement Agreement and Release
Page 15
or their representatives’ or Affiliates’ possession that were trade secret, confidential, proprietary, and/or internal to Claimants and were obtained as a result of, or in connection with, Dr. Wong’s or other HCW employees’ prior employment with Altor or NantCell.
d.Within XXXXXX XXXX XXXXXXXX XXXX XX XXX XXXXXXXXX XXXX, HCW’s counsel and XXXXXXXX XXXXXXXX XXXXXshall destroy or oversee the destruction of the following portable storage devices (including any copies, backups, or archives) which constitute Information Sources: (i) XXXXXXX XXXXXX XX XX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXXXXXXXXXXXXXX, (ii) XXXXXXX XXXXXX XXXXX XX XX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXXXXXXXXXXXXXX, (iii) XX XXXXXXXXXX XXX XX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXXXXXXXXXXXXXXXXXXXXXXX, and (iv) XXXXXXX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXX XXXXXXXXX XXXXXX.
e.Within XXXXXX XXXX XXXXXXXX XXXX XX XXX XXXXXXXXX XXXX, HCW’s counsel shall oversee the forensic remediation process referenced in Paragraph 78-79 of the Rebuttal Expert Report XX XXXXXX XXXXX, dated April 23, 2024.
f.Claimants contend that a hard drive with serial number XXXXXXXXXXXXXXXXXXXXXXXX, a hard drive with serial number XXXXXXXXXXXXXX, and potential copies of the XXXXXXX XXXXX may also contain Claimants’ confidential information and trade secrets. Respondents agree that if any of these devices are located, they will either be (i) destroyed promptly, or (ii) provided to Respondents’ counsel who will promptly notify Claimants’ counsel within XXXXXXX XXXX XXXXXXXX XXXX of Respondents’ discovery of such devices.
g.Nothing in this Agreement shall limit or affect the Parties’ ongoing obligations under employment, confidentiality, and non-disclosure agreements previously executed in connection with Dr. Wong’s or other HCW employees’ prior employment with Altor or NantCell, to the extent any such ongoing obligations exist.
10. HCW Retained Rights.
a.Subject to the terms and conditions of this Agreement, HCW will retain freedom to develop the TOBI Platform for all indications and uses.
11. Legal Fees.
a.XXX XXXXXXXXX XX XXXXX, XXX XXXXXXX XXX XXXXXXXXXXX XXX XXXXX XXX XXXXX XXXX XXX XXXXXXXX XXXX XXX XX, XXXX XX, XXXXXXXXX XXX XXXXXX XXXXXXXX XXXX XXXX XXXX XXXXXXXXX XXX XXXXXXXXXXX XX XX. XXXX. XXX XXXXXX, XXXXXXXXXXX XXXX XXX XX XXXXXXX XX XXXXXXXXXX XX XXX XXXXXXXXXX XX XXX XXXXXXXX XX XX. XXXXXX XXXXXXX XXXX XXXX XXX XXXX XXXX XX XX XXX XX, XXXX, XXX XXXXXXXXXXX XXXX XXX XXXX XX XXXXXX XXXX XXXXXX XXXXXXXXXX XXX XXXXXXX XX XXX XXXX XX XX XXX XXX XXXX. All Parties will be responsible for their share of expenses, including expenses of the Arbitrator, invoiced by JAMS. ImmunityBio agrees to release its pending claim against HCW for contribution of funds it has advanced that was filed in the Delaware Court of Chancery, captioned Altor Bioscience, LLC, et al. v. HCW Biologics, Inc., C.A. No. 2024-0310-PAF, and to dismiss that action. Other than as set forth in this Paragraph 11, each Party shall bear its own expenses, costs, and fees in connection with the Action and this Agreement, including any expenses, costs, or fees incurred by its attorneys, experts, advisors, agents, or representatives.
12. Representations and Warranties of Respondents. Each Respondent, as to himself, herself, or itself, as applicable, hereby represents and warrants to, and covenants and agrees with, Claimants, as of the date hereof, as follows:
a.Respondents have the right to grant the rights, transfers and assignments granted herein, without the need for any assignments, releases, consents, approvals, immunities or other rights not yet obtained;
Settlement Agreement and Release
Page 16
b.The Transferred Assets are solely and exclusively owned by Respondents or its Affiliates and are free of and not subject to any restrictions or to any mortgages, liens, pledges, security interests, encumbrances or encroachments;
c.Each of the patents and trademarks included in the Transferred Intellectual Property Rights is valid, enforceable and subsisting and has not lapsed, expired, been cancelled or become abandoned and all applicable fees have been paid on or before the due date for payment;
d.Schedule 1 attached hereto is complete, true and accurate and contains all patents and patent applications Controlled by the Respondents, whether published or unpublished, that are related to the TGFb Molecules or necessary or reasonably useful for the research, Development, Commercialization, use, sale or Exploitation of the TGFb Molecules;
e.To the Knowledge of the Respondents, except for the Transferred Intellectual Property Rights, no other rights or licenses (including other Intellectual Property Rights) are necessary to use, Develop, manufacture, import Commercialize or Exploit the TGFb Molecules;
f.To the Knowledge of the Respondents, except for the Transferred Intellectual Property Rights, no other rights or licenses (including other Intellectual Property Rights) are necessary to use, Develop, manufacture, import, Commercialize or Exploit HCW9201 for use as a subcutaneous injection;
g.The materials, inventory, documentation and other technology transferred to ImmunityBio as set forth in Schedule 2(a)(iii), and pursuant to Paragraphs 2(a)(iii), 3 and 4, contain all that are needed to manufacture the TGFb Molecules and HCW9101 on a level consistent with Respondents and/or its Third Party contract manufacturers, including without limitation EirGenix, as of the Effective Date, and Respondents are not withholding anything necessary or reasonably useful for the manufacturing of the TGFb Molecules and HCW9101.
h.The Transferred Contracts set forth on Schedule 3 are all Third Party agreements to which HCW is a Party that are necessary for the use, Development, manufacture, importation, Commercialization of Exploitation of the Transferred Assets. HCW has made available to ImmunityBio prior to the Effective Date a true, complete and correct copy of each Transferred Contract as in effect on the date of this Agreement. HCW is not currently and, to the Knowledge of Respondents, no other party to a Transferred Contract is, in breach of or default of the terms of any Transferred Contract. Each Transferred Contract is a legal, valid and binding obligation of HCW or its Affiliates and is in full force and effect.
i.The Wugen Agreement attached as Exhibit Ais a true and complete copy as of the Effective Date.
As of the Effective Date, the Wugen Agreement has not been amended or modified and Wugen has not exercised any of Wugen’s option rights thereunder.
13. Release by Claimants.
a.Each of the Claimants and their respective present or former agents, affiliates, successors, assigns, predecessors, parents, subsidiaries, representatives, trustees, executors, heirs, spouses, marital communities, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively the “Claimant Releasing Parties”), without any other action being required to be taken, do hereby completely, finally, and fully forever release, remise, acquit, compromise, settle, extinguish, relinquish, and forever discharge without limitation, Respondents and their respective predecessors, successors-in-interest, direct and/or indirect parents, direct and/or indirect subsidiaries, affiliates, representatives, agents, trustees, executors, heirs, spouses, marital communities, assigns, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively, the “Respondent Released Parties”) (except for XXXXXXXX XXXXX XXXXXXXXXXXXX XX., XXX., XXXXXXXX XXXXXX XXXXXXX XXX., XXXX XXXXXX XXXXXXXXXX XXXXXXX, XXXX XXXXXXXXX, XXXXXXX XXXXXXX XXX., XXXXXXXX XXXXXXX, XXXXX XXX, XXX XXXX XX , which are expressly excluded from and shall not constitute Respondent Released Parties) from any and all causes of action, suits, charges, debts, dues, sums of money, accounts, reckonings, bonds, bills, specialties, covenants, contracts, appraisal rights, torts,
Settlement Agreement and Release
Page 17
controversies, agreements, promises, variances, trespasses, damages, judgments, executions, claims, and demands whatsoever, whether asserted or unasserted, contingent or remote, known or unknown, and whether arising in law, admiralty, or equity that the Claimant Releasing Parties or any of them had, now have, or that their successors or assigns hereinafter can, shall, or may have, for, upon, or by reason of any matter, cause, or thing whatsoever that have been or could have been asserted by the Claimants, in any forum, including class, derivative, individual, or other claims, whether state, federal, or foreign, common law, statutory, or regulatory, including, without limitation, claims under the federal securities laws (collectively, the “Claimants’ Settled Claims”); provided, however, that the Claimants’ Settled Claims shall not include (i) the right of the Claimants to enforce the terms of this Agreement, or (ii) any rights or claims that arise after the Effective Date.
14. Release by Respondents.
a.Respondents and their respective present or former agents, affiliates, successors, assigns, predecessors, parents, subsidiaries, representatives, trustees, executors, heirs, spouses, marital communities, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively the “Respondent Releasing Parties,” and together with the Claimant Releasing Parties, the “Releasing Parties”), without any other action being required to be taken, do hereby completely, finally, and fully forever release, remise, acquit, compromise, settle, extinguish, relinquish, and forever discharge without limitation, the Claimants and their respective predecessors, successors-in-interest, parents, subsidiaries, affiliates, representatives, agents, trustees, executors, heirs, spouses, marital communities, assigns, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively, the “Claimant Released Parties”) from any and all causes of action, suits, charges, debts, dues, sums of money, accounts, reckonings, bonds, bills, specialties, covenants, contracts, appraisal rights, torts, controversies, agreements, promises, variances, trespasses, damages, judgments, executions, claims, and demands whatsoever, whether asserted or unasserted, contingent or remote, known or unknown, and whether arising in law, admiralty, or equity that the Respondent Releasing Parties or any of them had, now have, or that their successors or assigns hereinafter can, shall, or may have, for, upon, or by reason of any matter, cause, or thing whatsoever that have been or could have been asserted by Respondents, in any forum, including class, derivative, individual, or other claims, whether state, federal, or foreign, common law, statutory, or regulatory (collectively, the “Respondents’ Settled Claims,” and together with the Claimants’ Settled Claims, the “Settled Claims”); provided, however, that the Respondents’ Settled Claims shall not include (i) the claims referenced in Paragraph 8 of this Settlement, subject to the terms therein, (ii) the right of Respondents to enforce the terms of this Settlement, or (iii) any rights or claims that arise after the Effective Date.
15. Release of Unknown Claims. The Parties understand and agree that the releases described herein shall extend to claims that the Releasing Parties do not know or suspect to exist at the time of the release, which if known, might have affected the Releasing Parties’ decisions to enter into the releases. The Releasing Parties shall be deemed to relinquish, to the extent applicable and to the full extent permitted by law, the provisions, rights, and benefits of Section 1542 of the California Civil Code, which states that:
A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR
Settlement Agreement and Release
Page 18
AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.
The Releasing Parties shall be deemed to waive any and all provisions, rights and benefits conferred by any law of any state or territory of the United States, or principle of common law, that is similar, comparable, or equivalent to Section 1542 of the California Civil Code. The Releasing Parties acknowledge that they may discover facts in addition to or different from those that they now know or believe to be true with respect to the subject matter of this Agreement, but that it is their intention to fully, finally, and forever settle and release any and all claims released in Paragraphs 13-14, whether known or unknown, suspected or unsuspected, which now exist or heretofore existed or may hereafter exist and without regard to the subsequent discovery or existence of such additional or different facts. Each Party acknowledges that the foregoing waiver was separately bargained for, is an integral element of this Agreement, and was relied upon by the other Parties in entering into this Agreement.
16. Dismissal of Arbitration and Delaware Action. Upon execution of this Agreement by all Parties, the Parties will inform the Arbitrator in the pending JAMS arbitration (Altor BioScience, LLC, et al. vs. Hing C. Wong, et al. - JAMS Ref No. XXXXXXXXXX (the “Arbitration”)) that the Arbitration has settled, and ask the Arbitrator to refrain from further work pending dismissal. Claimants and Respondents will cooperate to arrange for prompt dismissal of (i) the Arbitration; and (ii) the pending action in the Delaware Court of Chancery, Altor Bioscience, LLC v. HCW Biologics, Inc., C.A. No. 2024-0310-PAF, after Respondents’ compliance with Paragraphs 2 through 9 above. To that end, counsel for the Parties shall work together to submit stipulations of dismissal in the Arbitration and the pending action in the Delaware Court of Chancery no earlier than XXXXXX XXX XXXX XXXXX XXX XXXXXXXXX XXXX, but as soon as practicable thereafter.
17. No Assignment. The Parties represent that they have not assigned or transferred, or purported to assign or transfer, to any person or entity any claim released in this Agreement.
18. No Admission of Liability. This Agreement constitutes a compromise of disputed claims. This Agreement shall not be deemed a presumption, a concession, or an admission by any Party of any fault, liability, or wrongdoing as to any facts, claims, or defenses that have been or might have been alleged or asserted in the Action, and shall not be interpreted, construed, deemed, invoked, offered, received in evidence, or otherwise used by any person in any claim, action, proceeding, or settlement negotiation, except for any litigation or proceeding arising out of or relating to the terms of this Agreement, whether civil, criminal, or administrative, for any purpose other than as provided expressly herein. Notwithstanding the preceding sentence, this Agreement, proof of its execution, and payment of consideration under its terms shall be admissible to prove settlement and release of the claims set forth herein if such shall be necessary. In the event that this Agreement is rendered null and void for any reason,
Settlement Agreement and Release
Page 19
the existence of or the provisions contained in this Agreement shall not be deemed to prejudice in any way the respective positions of the Parties.
19. Disclosure and Confidentiality. This Settlement (its existence, and its terms) and all documents, communications, drafts and other materials of any kind relating to their negotiation, the circumstances leading thereto, or the implementation thereof shall be and remain confidential and shall not be disclosed to any other person without the Parties’ prior, express, written consent, except as required by applicable law, rule or regulation, or to comply with or enforce the terms of the Settlement itself. There will be no press releases, web site announcements or other public statements by any Party or any of their representatives regarding this Settlement or any of the matters referenced herein or therein other than as required by law. Notwithstanding the foregoing, each of the Parties may disclose the terms of this Settlement to his, her, or its respective attorneys, accountants, insurers, and/or regulators, and the Parties may disclose the terms as necessary in any required SEC or other regulatory filings or communications.
20. Entire Agreement; No Reliance. This Agreement constitutes the entire agreement of the Parties and replaces, cancels, and supersedes any and all prior agreements and understandings between them pertaining to the subject matter hereof. There is no separate agreement, representation, or other inducement between the Parties for the execution of this Agreement. In entering into this Agreement, no Party is relying upon any representation, commitment, warranty, or promise by any other Party unless expressly set forth in this Agreement. The Parties agree this provision is an “anti-reliance” provision under Delaware law precluding claims based on any representation, commitment, warranty, or promise whatsoever not set forth in this Agreement.
21. Counterparts. This Agreement may be executed in any number of counterparts, by original or electronically transmitted signature, all of which shall be considered one and the same agreement, and shall become effective when all such counterparts have been signed by each of the Parties and delivered to all of the other Parties.
22. Mutual Drafting. The Parties to this Agreement agree that they have thoroughly discussed all aspects of this Agreement with their attorneys, that they have read and fully understand all of the provisions of this Agreement, and that they are voluntarily entering into this Agreement. The Parties further agree that this Agreement has been jointly negotiated and prepared and that no Party shall be deemed to have prepared this Agreement for purposes of construing its terms.
23. Authorization. Each of the individuals executing this Agreement hereto on behalf of one or more of the Parties warrants and represents that he or she has been duly authorized and empowered to
Settlement Agreement and Release
Page 20
execute this Agreement on behalf of each such respective Party and to bind each such respective Party to the terms hereof.
24. Further Actions. The Parties and their attorneys agree to cooperate fully and to use their best efforts to effectuate expeditiously the terms and conditions of this Agreement, including the execution of all related documents, as soon as practicable. Counsel for the Parties are expressly authorized to enter into such changes, modifications, or amendments of this Agreement as to which they mutually agree as long as such changes are in writing.
25. No Waiver. Any failure by any Party to insist upon the strict performance by any other Party of any of the provisions of this Agreement shall not be deemed a waiver of any of the provisions hereof, and such Party, notwithstanding such failure, shall have the right thereafter to insist upon the strict performance of any and all of the provisions of this Agreement to be performed by such other Party.
26. Choice of Law and Forum. This Agreement and any and all disputes arising out of or relating in any way to this Agreement, whether in contract, tort, or otherwise, shall be governed by, and construed in accordance with, the laws of the state of Delaware, without regard to conflicts of law principles. Each of the Parties (a) irrevocably submits to the personal jurisdiction of any state or federal court sitting in Wilmington, Delaware, as well as to the jurisdiction of all courts to which an appeal may be taken from such courts, in any suit, action, or proceeding arising out of or relating to this Agreement and/or the Settlement, (b) agrees that all claims in respect of such suit, action, or proceeding shall be brought, heard, and determined exclusively in the Court (provided, however, that in the event that subject matter jurisdiction is unavailable in the Court, then all such claims shall be brought, heard, and determined exclusively in any other state or federal court sitting in Wilmington, Delaware), (c) agrees that it shall not attempt to deny or defeat such personal jurisdiction by motion or other request for leave from such court, (d) agrees not to bring any action or proceeding arising out of or relating to this Agreement in any other court, and (e) expressly waives and agrees not to plead or to make any claim that any such action or proceeding is subject (in whole or in part) to a jury trial. Each of the Parties waives any defense of inconvenient forum to the maintenance of any action or proceeding brought in accordance with this paragraph. Each of the Parties further agrees to waive any bond, surety, or other security that might be required of any other party with respect to any action or proceeding, including an appeal thereof. Each of the Parties further consents and agrees that process in any suit, action, or proceeding may be served on such Party by certified mail, return receipt requested, addressed to such Party or such Party’s registered agent in the state of its incorporation or organization, or in any other manner provided by law. Each of the Parties further consents and agrees that process in any suit, action, or proceeding may be served by mailing or emailing such written notice to:
If to Claimants:
XXXXX XXXXXXX XXXXXXXX X XXXXXXXX, XXX
XXXXX X. XXXXXX, XX.
XXXXXXXXXXXXXXXXXXXXXXXXXX
XXX X. XXXXXXXXX XX. XXXX XXXXX
XXX XXXXXXX, XXXXXXXXXX XXXXX-XXXX
If to Respondents:
XXXXXX, XXX
XXXXX XXXXXXXXX
XXXXXXXXXXXXXXXXXXXXX
XX XXXXXX XXXXX
Settlement Agreement and Release
Page 21
XXX XXXX, XXX XXXX XXXXX-XXXX
XXXXXX, XXXXXXXXXX X XXXXXXXXX XXX
XXXX XX
XXXXXXXXXXXXXX
XXX X. XXXXX XXX.
XXXXX XXX
27. Binding Effect. This Agreement shall be binding upon and inure to the benefit of the Parties and their respective heirs, executors, administrators, successors, and assigns, and upon any corporation or other entity with which any Party hereto may merge or consolidate. The releases in Paragraphs 13 through 15 of this Agreement shall inure to the benefit of, and be enforceable by, the respective released persons described therein.
28. Severability. If any provision in this Agreement is held by a court of competent jurisdiction to be invalid, void, or unenforceable, the remaining provisions shall nevertheless continue in full force and continue to be binding on the Parties without being impaired or invalidated in any way.
29. Notice/Cure/Settlement Conference. Before raising any potential breaches of this Settlement with any court, the Parties will meet and confer within XXXX XXX XXXXXXXX XXXX of notice of the breach to negotiate a resolution. Notice pursuant to this requirement shall be in writing and shall be deemed duly given: (i) upon actual receipt; (ii) XXXX XXX XXXXXXXX XXXX after mailing by first class, certified, or registered U.S. mail, postage prepared and addressed as indicated in Paragraph 26, return receipt requested; (iii) if given by email, once such notice or other communication is transmitted to the email address(es) specified in this Settlement, or (iv) if sent through a nationally-recognized overnight delivery service that guarantees next day delivery and addressed as indicated in this Settlement, the business day following its delivery to such service in time for next day delivery. The Party alleged to be in breach shall have XXXXX XXX XXXXXXXX XXXX in which to cure the breach. If no resolution can be negotiated or if the breach is not cured withinXXXXX XXX XXXXXXXX XXXX, or cannot be cured, the non-breaching Party may file an action seeking to enforce this Settlement.
30. Enforcement. Nothing herein shall be construed to limit or prejudice in any way any Party’s rights to seek enforcement of the terms of this Agreement against the breaching Party, including specifically, rights to sue for breach of contract and for specific performance and/or to seek appropriate legal and/or equitable relief to enforce this Agreement. The Parties agree that any Party found to have breached this Agreement shall reimburse the non-breaching Party for the actual and reasonable attorneys’ fees, costs, and expenses that the non-breaching Party incurs in connection with the enforcement of this Agreement or any claim, damages, or litigation relating to any breach of this Agreement.
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed and delivered as of the 13th day of July, 2024.
[SIGNATURE PAGES FOLLOW]
Settlement Agreement and Release
Page 22
ALTOR BIOSCIENCE, LLC
By:
Name: Richard Adcock
Title: President and Chief Executive Officer
NANTCELL, INC.
By:
Name: Richard Adcock
Title: President and Chief Executive Officer
IMMUNITYBIO, INC.
By:
Name: Richard Adcock
Title: President and Chief Executive Officer
HCW BIOLOGICS, INC.
By:
Name: Dr. Hing C. Wong
Title: Chief Executive Officer
DR. HING C. WONG
By:
Name: Dr. Hing C. Wong
Settlement Agreement and Release
Page 23
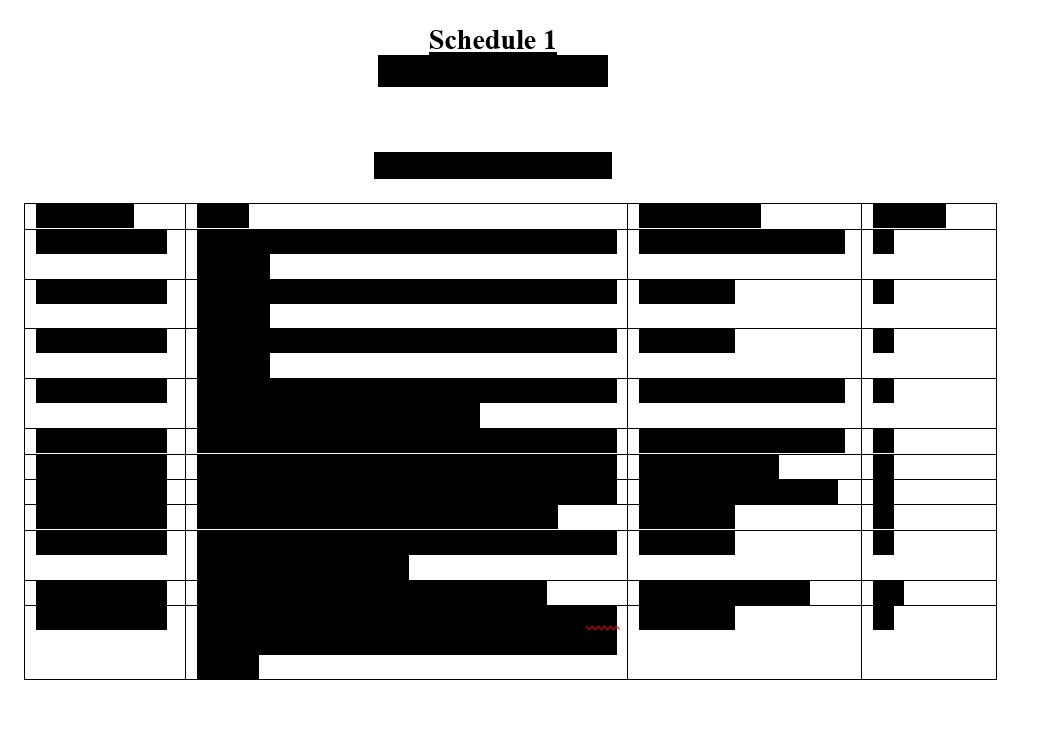
Settlement Agreement and Release
Page 24
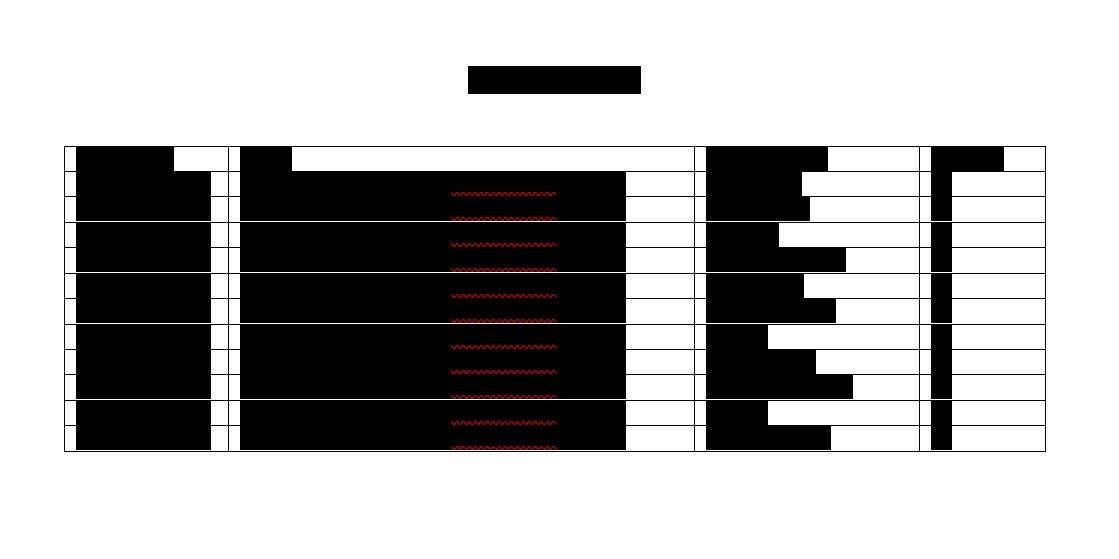
Settlement Agreement and Release
Page 25

Settlement Agreement and Release
Page 26
Settlement Agreement and Release
Page 27

Settlement Agreement and Release
Page 28

Settlement Agreement and Release
Page 29

Settlement Agreement and Release
Page 30

Settlement Agreement and Release
Page 31

Settlement Agreement and Release
Page 32
Exhibit 31.1
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Hing C. Wong, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of HCW Biologics Inc. for the quarter ended September 30, 2024;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
/s/ Hing C. Wong |
Hing C. Wong |
Founder and Chief Executive Officer (Principal Executive Officer) |
Date: November 14, 2024
Exhibit 31.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Rebecca Byam, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of HCW Biologics Inc. for the quarter ended September 30, 2024;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
/s/ Rebecca Byam |
Rebecca Byam |
Chief Financial Officer (Principal Financial Officer) |
Date: November 14, 2024
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of HCW Biologics Inc. (the “Company”) on Form 10-Q for the period ended September 30, 2024 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1)The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and result of operations of the Company.
|
|
|
|
Date: November 14, 2024 |
|
By: |
/s/ Hing C. Wong |
|
|
|
Hing C. Wong |
|
|
|
Founder and Chief Executive Officer (Principle Executive Officer) |
Exhibit 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of HCW Biologics Inc. (the “Company”) on Form 10-Q for the period ended September 30, 2024 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
(1)The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and result of operations of the Company.
|
|
|
|
Date: November 14, 2024 |
|
By: |
/s/ Rebecca Byam |
|
|
|
Rebecca Byam |
|
|
|
Chief Financial Officer (Principal Financial Officer) |
v3.24.3
Cover Page - shares
|
9 Months Ended |
|
Sep. 30, 2024 |
Nov. 11, 2024 |
| Cover [Abstract] |
|
|
| Document Type |
10-Q
|
|
| Amendment Flag |
false
|
|
| Document Quarterly Report |
true
|
|
| Document Transition Report |
false
|
|
| Document Period End Date |
Sep. 30, 2024
|
|
| Document Fiscal Year Focus |
2024
|
|
| Document Fiscal Period Focus |
Q3
|
|
| Entity Registrant Name |
HCW Biologics Inc.
|
|
| Entity Central Index Key |
0001828673
|
|
| Current Fiscal Year End Date |
--12-31
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
| Entity Tax Identification Number |
82-5024477
|
|
| Entity Address, Address Line One |
2929 N
|
|
| Entity Address, Address Line Two |
Commerce Parkway
|
|
| Entity Address, City or Town |
Miramar
|
|
| Entity Address, State or Province |
FL
|
|
| Entity Address, Postal Zip Code |
33025
|
|
| City Area Code |
954
|
|
| Local Phone Number |
842–2024
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
| Entity Small Business |
true
|
|
| Entity Emerging Growth Company |
true
|
|
| Entity Ex Transition Period |
true
|
|
| Entity Shell Company |
false
|
|
| Title of 12(b) Security |
Common Stock
|
|
| Trading Symbol |
HCWB
|
|
| Security Exchange Name |
NASDAQ
|
|
| Entity Common Stock, Shares Outstanding |
|
37,823,394
|
| Entity File Number |
001-40591
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Balance Sheets (Unaudited) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 998,221
|
$ 3,595,101
|
| Accounts receivable, net |
651,840
|
1,535,757
|
| Prepaid expenses |
356,156
|
1,042,413
|
| Other current assets |
88,131
|
230,916
|
| Total current assets |
2,094,348
|
6,404,187
|
| Investments |
1,599,751
|
1,599,751
|
| Property, plant and equipment, net |
22,833,904
|
20,453,184
|
| Other assets |
28,476
|
56,538
|
| Total assets |
26,556,479
|
28,513,660
|
| Current liabilities: |
|
|
| Accounts payable |
22,666,107
|
6,167,223
|
| Accrued liabilities and other current liabilities |
1,056,716
|
2,580,402
|
| Short-term debt, net |
6,340,511
|
|
| Total current liabilities |
30,063,334
|
8,747,625
|
| Debt, net |
6,462,769
|
6,304,318
|
| Total Liabilities |
36,526,103
|
15,051,943
|
| Commitments and contingencies (Note 8) |
|
|
| Stockholders' equity (deficit): |
|
|
| Common Stock Value |
3,782
|
3,603
|
| Additional paid-in capital |
87,209,457
|
83,990,437
|
| Accumulated deficit |
(97,182,863)
|
(70,532,323)
|
| Total stockholders' equity (deficit) |
(9,969,624)
|
13,461,717
|
| Total liabilities and stockholders' equity (deficit) |
$ 26,556,479
|
$ 28,513,660
|
| X |
- DefinitionAmount of liabilities incurred to vendors for goods and services received, and accrued liabilities classified as other, payable within one year or the normal operating cycle, if longer.
| Name: |
us-gaap_AccountsPayableAndOtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe total amount of investments that are intended to be held for an extended period of time (longer than one operating cycle). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of current assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe total amount due to the entity within one year of the balance sheet date (or one operating cycle, if longer) from outside sources, including trade accounts receivable, notes and loans receivable, as well as any other types of receivables, net of allowances established for the purpose of reducing such receivables to an amount that approximates their net realizable value. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_ReceivablesNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionReflects the total carrying amount as of the balance sheet date of debt having initial terms less than one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_ShortTermBorrowings |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Balance Sheets (Parenthetical) (Unaudited) - $ / shares
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Condensed Balance Sheets [Line Items] |
|
|
| Common Stock, Par or Stated Value Per Share |
$ 0.0001
|
$ 0.0001
|
| Common Stock, Shares Authorized |
250,000,000
|
250,000,000
|
| Common Stock, Shares, Issued |
37,823,394
|
36,025,104
|
| X |
- DefinitionSeries A redeemable preferred stock
| Name: |
hcwb_CondensedBalanceSheetsLineItems |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
Condensed Statements of Operations (Unaudited) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Revenues: |
|
|
|
|
| Revenues |
$ 426,423
|
$ 853,102
|
$ 2,171,988
|
$ 1,517,792
|
| Cost of revenues |
(341,138)
|
(678,325)
|
(1,291,546)
|
(1,210,077)
|
| Net revenues |
85,285
|
174,777
|
880,442
|
307,715
|
| Operating expenses: |
|
|
|
|
| Research and development |
1,186,913
|
1,667,442
|
5,339,383
|
5,539,919
|
| General and administrative |
1,639,152
|
1,509,936
|
4,799,437
|
5,106,674
|
| Legal expenses |
949,455
|
2,075,279
|
15,761,531
|
4,610,091
|
| Nonoperating loss |
0
|
0
|
1,300,000
|
0
|
| Total operating expenses |
3,775,520
|
5,252,657
|
27,200,351
|
15,256,684
|
| Loss from operations |
(3,690,235)
|
(5,077,880)
|
(26,319,909)
|
(14,948,969)
|
| Interest expense |
(223,363)
|
(95,514)
|
(383,029)
|
(284,465)
|
| Other (expense) income, net |
11,310
|
234,753
|
52,397
|
919,688
|
| Net loss |
$ (3,902,288)
|
$ (4,938,641)
|
$ (26,650,541)
|
$ (14,313,746)
|
| Net loss per share, basic |
$ (0.1)
|
$ (0.14)
|
$ (0.71)
|
$ (0.4)
|
| Net loss per share, diluted |
$ (0.1)
|
$ (0.14)
|
$ (0.71)
|
$ (0.4)
|
| Weighted average shares outstanding, basic |
37,823,394
|
35,926,921
|
37,623,459
|
35,907,123
|
| Weighted average shares outstanding, diluted |
37,823,394
|
35,926,921
|
37,623,459
|
35,907,123
|
| X |
- DefinitionThe aggregate cost of goods produced and sold and services rendered during the reporting period. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
| Name: |
us-gaap_CostOfRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 9: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense classified as operating and nonoperating. Includes, but is not limited to, cost of borrowing accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-24
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense provided in the period for legal costs incurred on or before the balance sheet date pertaining to resolved, pending or threatened litigation, including arbitration and mediation proceedings. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(6))
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_LegalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net amount of other operating income and expenses, the components of which are not separately disclosed on the income statement, from items that are associated with the entity's normal revenue producing operations.
| Name: |
us-gaap_OtherOperatingIncomeExpenseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenuesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Statements of Changes in Stockholders' Equity (Deficit) (Unaudited) - USD ($)
|
Total |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Accumulated Deficit [Member] |
| Beginning balance (in shares) at Dec. 31, 2022 |
|
35,876,440
|
|
|
| Beginning balance , Value at Dec. 31, 2022 |
$ 37,428,506
|
$ 3,588
|
$ 82,962,964
|
$ (45,538,046)
|
| Issuance of Common Stock upon exercise of stock options, Shares |
|
10,195
|
|
|
| Issuance of Common Stock upon exercise of stock options , Value |
1,901
|
$ 1
|
1,900
|
|
| Stock-based compensation , Value |
259,206
|
|
259,206
|
|
| Net Income (Loss) |
(5,070,686)
|
|
|
(5,070,686)
|
| Ending balance (in shares) at Mar. 31, 2023 |
|
35,886,635
|
|
|
| Ending balance , Value at Mar. 31, 2023 |
32,618,927
|
$ 3,589
|
83,224,070
|
(50,608,732)
|
| Beginning balance (in shares) at Dec. 31, 2022 |
|
35,876,440
|
|
|
| Beginning balance , Value at Dec. 31, 2022 |
37,428,506
|
$ 3,588
|
82,962,964
|
(45,538,046)
|
| Net Income (Loss) |
(14,313,746)
|
|
|
|
| Ending balance (in shares) at Sep. 30, 2023 |
|
35,927,321
|
|
|
| Ending balance , Value at Sep. 30, 2023 |
23,866,933
|
$ 3,593
|
83,715,133
|
(59,851,793)
|
| Beginning balance (in shares) at Mar. 31, 2023 |
|
35,886,635
|
|
|
| Beginning balance , Value at Mar. 31, 2023 |
32,618,927
|
$ 3,589
|
83,224,070
|
(50,608,732)
|
| Issuance of Common Stock upon exercise of stock options, Shares |
|
40,086
|
|
|
| Issuance of Common Stock upon exercise of stock options , Value |
7,712
|
$ 4
|
7,708
|
|
| Stock-based compensation , Value |
263,423
|
|
263,423
|
|
| Net Income (Loss) |
(4,304,420)
|
|
|
(4,304,420)
|
| Ending balance (in shares) at Jun. 30, 2023 |
|
35,926,721
|
|
|
| Ending balance , Value at Jun. 30, 2023 |
28,585,642
|
$ 3,593
|
83,495,201
|
(54,913,152)
|
| Issuance of Common Stock upon exercise of stock options, Shares |
|
600
|
|
|
| Issuance of Common Stock upon exercise of stock options , Value |
84
|
|
84
|
|
| Stock-based compensation , Value |
219,848
|
|
219,848
|
|
| Net Income (Loss) |
(4,938,641)
|
|
|
(4,938,641)
|
| Ending balance (in shares) at Sep. 30, 2023 |
|
35,927,321
|
|
|
| Ending balance , Value at Sep. 30, 2023 |
23,866,933
|
$ 3,593
|
83,715,133
|
(59,851,793)
|
| Beginning balance (in shares) at Dec. 31, 2023 |
|
36,025,104
|
|
|
| Beginning balance , Value at Dec. 31, 2023 |
13,461,717
|
$ 3,603
|
83,990,437
|
(70,532,323)
|
| Issuance of Common Stock upon exercise of stock options, Shares |
|
12,572
|
|
|
| Issuance of Common Stock upon exercise of stock options , Value |
2,255
|
$ 1
|
2,254
|
|
| Issuance of Common Stock upon equity subscription,Shares. |
|
1,785,718
|
|
|
| Issuance of Common Stock upon equity subscription |
2,500,005
|
$ 178
|
2,499,827
|
|
| Stock-based compensation , Value |
244,685
|
|
244,685
|
|
| Net Income (Loss) |
(7,468,061)
|
|
|
(7,468,061)
|
| Ending balance (in shares) at Mar. 31, 2024 |
|
37,823,394
|
|
|
| Ending balance , Value at Mar. 31, 2024 |
8,740,601
|
$ 3,782
|
86,737,203
|
(78,000,384)
|
| Beginning balance (in shares) at Dec. 31, 2023 |
|
36,025,104
|
|
|
| Beginning balance , Value at Dec. 31, 2023 |
13,461,717
|
$ 3,603
|
83,990,437
|
(70,532,323)
|
| Net Income (Loss) |
(26,650,541)
|
|
|
|
| Ending balance (in shares) at Sep. 30, 2024 |
|
37,823,394
|
|
|
| Ending balance , Value at Sep. 30, 2024 |
(9,969,624)
|
$ 3,782
|
87,209,457
|
(97,182,863)
|
| Beginning balance (in shares) at Mar. 31, 2024 |
|
37,823,394
|
|
|
| Beginning balance , Value at Mar. 31, 2024 |
8,740,601
|
$ 3,782
|
86,737,203
|
(78,000,384)
|
| Stock-based compensation , Value |
239,821
|
|
239,821
|
|
| Net Income (Loss) |
(15,280,191)
|
|
|
(15,280,191)
|
| Ending balance (in shares) at Jun. 30, 2024 |
|
37,823,394
|
|
|
| Ending balance , Value at Jun. 30, 2024 |
(6,299,769)
|
$ 3,782
|
86,977,024
|
(93,280,575)
|
| Stock-based compensation , Value |
232,433
|
|
232,433
|
|
| Net Income (Loss) |
(3,902,288)
|
|
|
(3,902,288)
|
| Ending balance (in shares) at Sep. 30, 2024 |
|
37,823,394
|
|
|
| Ending balance , Value at Sep. 30, 2024 |
$ (9,969,624)
|
$ 3,782
|
$ 87,209,457
|
$ (97,182,863)
|
| X |
- DefinitionIssuance of common stock upon equity subscription.
| Name: |
hcwb_IssuanceOfCommonStockUponEquitySubscription |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIssuance of Common Stock upon equity subscription, shares.
| Name: |
hcwb_IssuanceOfCommonStockUponEquitySubscriptionShares |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued as a result of the exercise of stock options. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
Condensed Statements of Cash Flows (Unaudited) - USD ($)
|
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Cash flows from operating activities: |
|
|
| Net Income (Loss) |
$ (26,650,541)
|
$ (14,313,746)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Depreciation and amortization |
501,882
|
860,634
|
| Stock-based compensation |
716,940
|
742,477
|
| Unrealized loss (gain) on investments, net |
0
|
(248,445)
|
| Realized loss (gain) on investments, net |
0
|
(15,625)
|
| Changes in the carrying amount of right-of-use asset |
(418)
|
(1,045)
|
| Changes in operating assets and liabilities: |
|
|
| Accounts receivable |
883,917
|
(292,383)
|
| Deposit for interest reserve |
0
|
(5,250,000)
|
| Prepaid expenses and other assets |
829,043
|
(251,008)
|
| Accounts payable and other liabilities |
12,383,171
|
392,802
|
| Operating lease liability |
(56,541)
|
(242,990)
|
| Net cash used in operating activities |
(11,392,547)
|
(18,619,329)
|
| Cash flows from investing activities: |
|
|
| Purchases of property and equipment |
(148,205)
|
(2,486,950)
|
| Proceeds for sale or maturities of short-term investments |
0
|
10,000,000
|
| Net cash provided by (used in) investing activities |
(148,205)
|
7,513,050
|
| Cash flows from financing activities: |
|
|
| Proceeds from issuance of common stock |
2,502,260
|
9,697
|
| Proceeds from issuance of debt |
6,530,000
|
0
|
| Offering costs |
0
|
0
|
| Debt repayment |
(88,388)
|
(8,981)
|
| Net cash provided by financing activities |
8,943,872
|
716
|
| Net (decrease) increase in cash and cash equivalents |
(2,596,880)
|
(11,105,563)
|
| Cash and cash equivalents at the beginning of the period |
3,595,101
|
22,326,356
|
| Cash and cash equivalents at the end of the period |
998,221
|
11,220,793
|
| Supplemental disclosure of cash flow information: |
|
|
| Cash paid for interest, net of amounts capitalized |
340,988
|
284,465
|
| Noncash operating, investing and financing activities: |
|
|
| Capital expenditures accrued, but not yet paid |
1,910,698
|
2,095,724
|
| Purchases of property and equipment included in accounts payable and other liabilities |
$ 829,207
|
$ 0
|
| X |
- DefinitionFuture cashflow related to fixed asset and other liabilities.
| Name: |
hcwb_AccruedFixedAssetAndOtherLiablities |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionChange in deposit for interest reserve.
| Name: |
hcwb_ChangeInDepositForInterestReserve |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionChanges in carrying amount of right of use asset.
| Name: |
hcwb_ChangesInCarryingAmountOfRightOfUseAsset |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsNoncashItemsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFuture cash outflow to pay for purchases of fixed assets that have occurred. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-5
| Name: |
us-gaap_CapitalExpendituresIncurredButNotYetPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NoncashInvestingAndFinancingItemsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for cost incurred directly with the issuance of an equity security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow during the period from additional borrowings in aggregate debt. Includes proceeds from short-term and long-term debt. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net cash inflow or outflow in aggregate debt due to repayments and proceeds from additional borrowings.
| Name: |
us-gaap_ProceedsFromRepaymentsOfDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from sales of all investments, including securities and other assets, having ready marketability and intended by management to be liquidated, if necessary, within the current operating cycle. Includes cash flows from securities classified as trading securities that were acquired for reasons other than sale in the short-term. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of realized gain (loss) on investment. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
| Name: |
us-gaap_RealizedInvestmentGainsLosses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_UnrealizedGainLossOnInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
Pay vs Performance Disclosure - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Sep. 30, 2023 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Pay vs Performance Disclosure |
|
|
|
|
|
|
|
|
| Net Income (Loss) |
$ (3,902,288)
|
$ (15,280,191)
|
$ (7,468,061)
|
$ (4,938,641)
|
$ (4,304,420)
|
$ (5,070,686)
|
$ (26,650,541)
|
$ (14,313,746)
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNon Rule 10b5-1 Arr Modified.
| Name: |
hcwb_NonRule10B51ArrModifiedFlag |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRule 10b5-1 Arr Modified.
| Name: |
hcwb_Rule10B51ArrModifiedFlag |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Organization and Summary of Significant Accounting Policies
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Organization and Summary of Significant Accounting Policies |
1. Organization and Summary of Significant Accounting Policies Organization HCW Biologics Inc. (the “Company”) is a biopharmaceutical company focused on discovering and developing novel immunotherapies to lengthen healthspan by disrupting the link between chronic, low-grade inflammation and age-related diseases. The Company believes age-related low-grade chronic inflammation, or “inflammaging,” is a significant contributing factor to several chronic diseases and conditions, such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and autoimmune diseases. The Company is located in Miramar, Florida and was incorporated in the state of Delaware in April 2018. Liquidity and Going Concern In accordance with FASB Accounting Standards Codification (“ASC”) 205-40, Presentation of Financial Statements – Going Concern (“Topic 205-40”), management is required to evaluate whether there are conditions and events, considered in the aggregate that raise substantial doubt about the Company’s ability to continue as a going concern for at least 12 months from the issuance date of the Company’s condensed interim financial statements. This evaluation does not take into consideration the potential mitigating effect of management’s plans that have not been fully implemented or are not within control of the Company as of the date the financial statements are issued. When substantial doubt exists under this methodology, management evaluates whether the mitigating effect of its plans sufficiently alleviates substantial doubt about the Company’s ability to continue as a going concern. The mitigating effect of management’s plans, however, is only considered if both (1) it is probable that the plans will be effectively implemented within one year after the date that the financial statements are issued, and (2) it is probable that the plans, when implemented, will mitigate the relevant conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. As of September 30, 2024, the Company had not generated any revenue from commercial product sales of its internally developed immunotherapeutic products for the treatment of cancer and other age-related diseases. During its development activities, the Company has sustained operating losses and expects to continue to incur operating losses for the foreseeable future. Since inception to September 30, 2024, the Company incurred cumulative net losses of $94.5 million. These losses reflects the events reported in the Form 8-K filed on May 1, 2024 with the Securities and Exchange Commission (“SEC”), involving a scheme that resulted in the misdirection of approximately $1.3 million held in Company accounts to a fraudulent account controlled by a third party. The Company has pursued relief through insurance claims and has reported the crime to legal authorities. Management expects to incur additional losses in the future to conduct product research and development and recognizes the need to raise additional capital to fully implement its business plan. As of September 30, 2024, the Company had $1.0 million in cash and cash equivalents. On August 15, 2022, the Company entered into a loan and security agreement (the “2022 Loan Agreement”) with Cogent Bank, pursuant to which it received $6.5 million in proceeds to purchase a building that will become the Company's new headquarters. The loan is secured by a first priority lien on the building. As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction of its new manufacturing facilities and upgraded research laboratories. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements; however, as of the reporting date, the lender has not elected to do so. See Part I, Item 4. -- “Controls and Procedures.” As of September 30, 2024, the Company has reflected this loan as Short-term debt, net, to reflect that the lender has the right to accelerate the loan under a discretionary default provision. The Company continues to seek the financing required to complete the construction project, with the cooperation of the lender and lien holders. To date, the Company has funded operations primarily through the sale of stock, issuance of senior secured notes and revenues generated from the Company’s exclusive worldwide license with Wugen, Inc. (“Wugen”), pursuant to which Wugen licensed limited rights to develop, manufacture, and commercialize cell therapy treatments for cancer based on two of the Company’s internally-developed multi-cytokine fusion protein molecules, and its manufacturing and supply arrangement with Wugen. In the three months ended September 30, 2023 and 2024, the Company recognized revenues generated from the supply of clinical and research grade material to Wugen of $853,102 and $426,423, respectively. In the nine months ended September 30, 2023 and 2024, the Company recognized revenues generated from the supply of clinical and research grade material to Wugen of $1.5 million and $2.2 million, respectively. The Company launched a financing plan in the third quarter of 2024. The plan includes several capital-raising activities, such as issuance of secured notes, equity financings, as well as business development transactions, such as license agreements with guaranteed minimum payments. Through October 31, 2024, the Company issued an aggregate of $6.9 million in secured notes. On September 25, 2024, the Company entered into a nonbinding term sheet with a party interested in licensing one of the Company’s preclinical molecules. The Company expects to close this license transaction in the fourth quarter of 2024. The proposed license agreement includes guaranteed minimum payments which are expected within the first year of the term. On November 13, 2024, we entered into an engagement letter with the Maxim Group to act as the exclusive placement agent for a multi-step equity financing. If the Company is not successful in raising additional capital through these activities, management intends to revise its business plan. If such revisions are insufficient, the Company may have to curtail or cease operations. The Company has received written notices from the Listing Qualifications Staff of the Nasdaq Stock Market LLC (“Nasdaq”) informing the Company it is not in compliance with continued listing requirements on the Nasdaq Global Market for the market value of listed securities. Under Nasdaq Listing Rules, the Company has a period of 180 calendar days from the date of notice in which to regain compliance, and its first deadline is currently scheduled for December 16, 2024. The Company intend to take all reasonable measures available to us to regain compliance with the continued listing requirements for the Nasdaq Global Market, including utilizing the right to appeal to Nasdaq to extend the deadline to regain compliance based on a solid financial plan to do so. While the Company is exercising diligent efforts to maintain the listing of its common stock on Nasdaq, there can be no assurance that the Company will be able to regain or maintain compliance with the applicable continued listing standards set forth in the Nasdaq Listing Rules. As of September 30, 2024, the conclusion of a going concern assessment was that there is substantial doubt about the Company’s ability to continue as a going concern. The Company considered its financing plans for the next 12 months, along with expected continuing operating losses and the burden of expenses incurred in connection with past legal proceedings. Management concluded that there were no mitigating circumstances which alleviated the substantial doubt over its ability to continue as a going concern. As reported in the Company’s Form 8-K filed on July 18, 2024 and further described in Part II, Item 1. – “Legal Proceedings” below, as of July 13, 2024, the Company and Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, entered into a confidential Settlement Agreement and Release (the “Settlement Agreement”) with ImmunityBio and its affiliates. The Settlement Agreement includes mutual general releases by and among the parties thereto. No party is required to make any monetary payments to any other party or person under the Settlement Agreement and each party will bear its own expenses incurred in connection with the matter. The Company has substantially completed remediation procedures required to be in compliance with the terms of the Settlement Agreement. The Company entered into the Settlement Agreement to avoid the costs, disruption and distraction of further litigation. In the accompanying condensed balance sheet as of September 30, 2024, the Company reported a balance of $14.4 million for legal fees incurred but not yet paid that were included within Accounts payable and an accrual of $35,000 for accrued legal fees within Accrued liabilities and other current liabilities. The Company is engaged in discussions with the law firms involved with this matter to arrange a reasonable payment plan with respect to those legal fees. With the execution of the Settlement Agreement, the Company resolved the attendant uncertainties for the outcome of the arbitration and additional complexities, and it launched its new financing plan. The accompanying interim financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above. The Company believes that substantial doubt exists regarding its ability to continue as a going concern for at least 12 months from the date of issuance of the Company’s condensed interim financial statements and that the substantial doubt that existed in its going concern analysis was not alleviated. Summary of Significant Accounting Policies Basis of Presentation The accompanying unaudited condensed balance sheet as of September 30, 2024, the condensed statements of operations, and condensed statements of changes in stockholders’ equity (deficit) for the three and nine-month periods ended September 30, 2023 and 2024 and the condensed statements of cash flows for the nine months ended September 30, 2023 and 2024, and all related disclosures contained in the accompanying notes have been prepared in accordance with accounting principles that are generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and pursuant to Article 10 of Regulation S-X of the Securities Act of 1933, as amended (the “Securities Act”). Accordingly, they do not include all of the information and notes required by U.S. GAAP for complete financial statements. These unaudited condensed interim financial statements include only normal and recurring adjustments that the Company believes are necessary to fairly state the Company’s financial position and the results of its operations and cash flows. The results for the three and nine-month periods ended September 30, 2024 are not necessarily indicative of the results expected for the full fiscal year or any subsequent interim period. The condensed interim balance sheet at December 31, 2023 has been derived from the audited financial statements at that date but does not include all disclosures required by U.S. GAAP for complete financial statements. Because all of the disclosures required by U.S. GAAP for complete financial statements are not included herein, these unaudited condensed interim financial statements and the notes accompanying them should be read in conjunction with the Company’s audited financial statements for the year ended December 31, 2023, which appear in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on May 15, 2024 (the “Annual Report”) and in other filings with the SEC. Reclassification of Prior Period Presentation of Legal Expenses Certain prior period amounts have been reclassified to distinguish between General and administrative expenses in the ordinary course of business and legal expenses incurred in connection with the arbitration and Settlement Agreement described in Notes 1. Reclassification of legal expenses incurred in connection with legal proceedings impacts the consolidated interim statements of operations. There is no effect on reporting results of operations from prior periods. Use of Estimates The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Management must apply significant judgment in this process. The Company evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors and adjusts those estimates and assumptions when facts and circumstances dictate. Actual results could differ from estimates. Fair Value Measurements Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurement (“Topic 820”), establishes a fair value hierarchy for those instruments measured at fair value that distinguishes between fair value measurements based on market data (observable inputs) and those based on the Company’s own assumptions (unobservable inputs). This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows: Level 1: Observable inputs such as quoted prices in active markets; Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and Level 3: Unobservable inputs in which there is little or no market data, which require a reporting entity to develop its own assumptions. Fair value measurements are classified based on the lowest level of input that is significant to the measurement. The Company’s assessment of the significance of a particular input to the fair value measurement requires judgment, which may affect the valuation of the assets and liabilities and their placement within the fair value hierarchy levels. The determination of the fair values takes into account the market for the Company’s financial assets and liabilities, the associated credit risk, and other factors as required. The Company considers active markets as those in which transactions for the assets or liabilities occur in sufficient frequency and volume to provide pricing information on an ongoing basis. Revenue Recognition The Company accounts for revenues in accordance with ASC 606, Revenue from Contracts with Customers (“Topic 606”). To determine revenue recognition for arrangements that fall within the scope of Topic 606, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the Company satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that it will collect the consideration it is entitled to in exchange for the goods or services transferred to the customer. At contract inception, the Company assesses the goods or services promised within each contract, determines those that are performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. To date, the Company's revenues have been generated solely from transactions with Wugen. The Wugen License includes licenses of intellectual property, cost reimbursements, upfront signing fees, milestone payments and royalties on future licensee’s product sales. In addition, the Company and Wugen have an agreement for supply of materials, from which the Company also recognizes revenues. License Grants: For out-licensing arrangements that include a grant of a license to the Company’s intellectual property, the Company considers whether the license grant is distinct from the other performance obligations included in the arrangement. For licenses that are distinct, the Company recognizes revenues from nonrefundable, upfront payments and other consideration allocated to the license when the license term has begun and the Company has provided all necessary information regarding the underlying intellectual property to the customer, which generally occurs at or near the inception of the arrangement. Milestone and Contingent Payments: At the inception of the arrangement and at each reporting date thereafter, the Company assesses whether it should include any milestone and contingent payments or other forms of variable consideration in the transaction price using the most likely amount method. If it is probable that a significant reversal of cumulative revenue would not occur upon resolution of the uncertainty, the associated milestone value is included in the transaction price. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of each such milestone and any related constraint and, if necessary, adjusts its estimate of the overall transaction price. Since milestone and contingent payments may become payable to the Company upon the initiation of a clinical study or filing for or receipt of regulatory approval, the Company reviews the relevant facts and circumstances to determine when the Company should update the transaction price, which may occur before the triggering event. When the Company updates the transaction price for milestone and contingent payments, the Company allocates the changes in the total transaction price to each performance obligation in the agreement on the same basis as the initial allocation. Any such adjustments are recorded on a cumulative catch-up basis in the period of adjustment, which may result in recognizing revenue for previously satisfied performance obligations in such period. The Company’s licensees will generally pay milestones payments subsequent to achievement of the triggering event. Materials Supply: The Company provides clinical and research grade materials so that licensees may develop products based on the licensed molecules. The Company plans to enter into commercialization supply agreements when licensees enter the commercial stage of their company. The amounts billed are recognized as revenue as the performance obligations are satisfied by the Company, once the Company determines that a contract exists. On June 18, 2021, the Company entered into a master services agreement for the supply of materials for clinical development of licensed products. To meet all the criteria to qualify as a contract under Topic 606, the Company must enter into statements-of-work for purchases of clinical and research grade materials. The Company has determined that the manufacturing of the clinical and research materials supplied by the Company each represents a single performance obligation that is satisfied over time. The Company recognizes revenue using an input method based on the costs incurred relative to the total expected cost, which determines the extent of the Company's progress toward completion. As part of the accounting for these arrangements, the Company must develop estimates and assumptions that require judgement to determine the progress towards completion. The Company reviews its estimate of the progress toward completion based on the best information available to recognize the cumulative progress toward completion as of the end of each reporting period, and makes revisions to such estimates, if facts and circumstances change during each reporting period. For the three and nine months ended September 30, 2023, the Company recognized $853,102 and $1.5 million in revenue related to the sale of development supply materials to Wugen, respectively. For the three and nine months ended September 30, 2024, the Company recognized $426,423 and $2.2 million in revenue related to sale of development supply materials to Wugen, respectively. The Company holds a minority interest in Wugen which is accounted for using the measurement alternative whereby the investment is recorded at cost less impairment, adjusted for observable price changes in orderly transactions for an identical or similar investment of the same investee. No impairment has been recognized. As of September 30, 2024 and December 31, 2023, the Company included $1.6 million for the investment in Wugen in Investments in the accompanying condensed interim balance sheets. The Company used its equity interest in Wugen to collateralize the Secured Notes. See Note 3. Debt, Net. Operating Leases The Company determines if an arrangement is a lease at inception. Operating leases are included in Other assets, Accrued liabilities and other current liabilities, and Other liabilities on its condensed interim balance sheets. Operating lease Right of Use (“ROU”) assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. The Company has a lease agreement with lease and non-lease components, which are accounted for separately. For short-term leases with a term of one year or less, the Company uses the practical expedient and does not record an ROU asset or lease liability for such short-term leases. Net Loss Per Share Basic loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during each period. Diluted loss per share of common stock includes the effect, if any, from the potential exercise of stock options and unvested shares of restricted stock, which would result in the issuance of incremental shares of common stock. For diluted net loss per share, the weighted-average number of shares of common stock is the same for basic net loss per share due to the fact that when a net loss exists, dilutive securities are not included in the calculation as the impact is anti-dilutive. |
| X |
- DefinitionThe entire disclosure for the business description and accounting policies concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Accounting policies describe all significant accounting policies of the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Accrued Liabilities and Other Current Liabilities
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Accrued Liabilities and Other Current Liabilities |
2. Accrued Liabilities and Other Current Liabilities As of December 31, 2023, the Company had a balance of $2.6 million included in Accrued liabilities and other current liabilities in the audited balance sheet, consisting of $392,000 for construction expenses, $105,000 for manufacturing expenses, $1.1 million for legal fees, $262,000 for clinical expenses, $365,000 for bonus payable, $160,000 for salary expenses, $119,000 for the current portion of long-term debt, $28,500 for a lease liability and $68,500 for other liabilities. As of September 30, 2024, the Company had a balance of $1.2 million included in Accrued liabilities and other current liabilities in the accompanying condensed interim balance sheet, consisting of $170,000 for legal fees, $422,000 for construction in progress, $49,000 for manufacturing expenses, $104,000 for property taxes, $117,000 for clinical expenses, $57,000 for bonus payable, $126,000 for the current portion of long-term debt and $90,000 for salary and benefits.
|
| X |
- DefinitionThe entire disclosure for accounts payable and accrued liabilities at the end of the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 720
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483384/720-30-45-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Debt, Net
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Debt Disclosure [Abstract] |
|
| Debt, Net |
3. Debt, Net
Cogent Bank Loan On August 15, 2022, the Company entered into the 2022 Loan Agreement with Cogent Bank, pursuant to which it received $6.5 million in proceeds to purchase a building that will become the Company's new headquarters. The loan is secured by a first priority lien on the building. The interest-only period was one year followed by 48 months of equal payments of principal and interest beginning on September 15, 2023 based on a 25-year amortization rate. The unamortized balance is due on August 15, 2027 (the “Maturity Date”), and bears interest at a fixed per annum rate equal to 5.75%. Upon the Maturity Date, a final payment of unamortized principal will be due. The Company has the option to prepay the outstanding balance of the loan prior to the Maturity Date without penalty. As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction of its new manufacturing facilities and upgraded research laboratories. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements; however, as of the reporting date, the lender has not elected to do so. As of September 30, 2024, the Company has reflected this loan as Short-term debt, net, to reflect that the lender has the right to accelerate the loan under a discretionary default provision. As of September 30, 2024, $6.3 million is included in Short-term debt, net in the accompanying condensed interim balance sheet. Senior Secured Notes On March 31, 2024, the Company entered into a Note Purchase Agreement with the Purchasers (as defined in the Note Purchase Agreement), pursuant to which the Company may issue secured notes up to an aggregate principal amount up to $10.0 million (“Secured Notes”). As of September 30, 2024, the Company received $6.5 million in funding from the issuance of Secured Notes, which is included within Debt, Net on the accompanying condensed interim balance sheet. Investors included Dr. Hing C. Wong, Founder and Chief Executive Officer, who invested $2.4 million; Rebecca Byam, Chief Financial Officer, who invested $220,000; Lee Flowers, Senior Vice President of Business Development, who invested $25,000; Scott T. Garrett, the Chairman of the Company’s board of directors, who invested $140,000; Gary M. Winer, a member of our board of directors, who invested $60,000; Rick S. Greene, a member of the board of directors, who invested $25,000, as well as unrelated parties. As of July 2, 2024, existing investors in Secured Notes unanimously agreed to an Amended and Restated Note Purchase Agreement and related documents (“Amended and Restated Note Purchase Agreement”). On September 30, 2024, existing investors approved an amendment to the Amended and Restated Note Purchase Agreement which extended the last closing date to October 31, 2024. No other terms were changed. Under the terms of the Amended and Restated Note Purchase Agreement, the Secured Notes continue to bear interest at a rate of 9% per annum, payable quarterly in arrears. The Secured Notes will mature on August 30, 2026 (the “Maturity Date”), on which date the principal balance, accrued but unpaid interest and other amounts owed under the terms of the Amended and Restated Note Purchase Agreement shall be due and payable. The Company pledged its equity ownership interest in Wugen, which was equivalent to a 5.6% ownership stake in that company as of September 30, 2024 (“Pledged Collateral”). The Pledged Collateral will be held and released according to the terms of the Escrow Agreement, as security for the Secured Notes. If the Company elects to prepay the Senior Notes on or before December 31, 2024, there is a 5% prepayment penalty. The Secured Notes have a Mandatory Prepayment provision, according to which the Company is required to prepay the Secured Notes before the Maturity Date under certain circumstances. In the event of a Mandatory Prepayment, Secured Notes may receive a bonus payment based on the gross proceeds of the sale of the Pledged Collateral. If the Secured Notes are repaid on the Maturity Date, holders will receive a fixed bonus payment in addition to payment of outstanding principal and accrued and unpaid interest. If a bonus payment is paid, then there is no prepayment penalty. The Amended and Restated Note Purchase Agreement also contains default provisions, according to which, following an event of default, the Company may be required to distribute the Pledged Collateral to the Purchasers on a pro rata basis based on a $10.0 million issuance of Secured Notes, in full satisfaction of the indebtedness evidenced by the Secured Notes. As of September 30, 2024, the fair value of the embedded derivative, which incorporated the likelihood of certain events occurring, was immaterial. Thus, as of September 30, 2024, the Company did not recognize the embedded derivative in the accompanying condensed financial statements. The Company accounts for the bonus payment to be paid if the Secured Notes are repaid on the Maturity Date by accreting the bonus payment to the full amount due on the Maturity Date, utilizing the effective interest rate method. As of September 30, 2024, the Company did not recognize accretion of the bonus payment, which was not considered material to the accompanying condensed financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for information about short-term and long-term debt arrangements, which includes amounts of borrowings under each line of credit, note payable, commercial paper issue, bonds indenture, debenture issue, own-share lending arrangements and any other contractual agreement to repay funds, and about the underlying arrangements, rationale for a classification as long-term, including repayment terms, interest rates, collateral provided, restrictions on use of assets and activities, whether or not in compliance with debt covenants, and other matters important to users of the financial statements, such as the effects of refinancing and noncompliance with debt covenants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/470/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Preferred Stock
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Preferred Stock |
4. Preferred Stock As of December 31, 2023 and September 30, 2024, the Company had 10,000,000 shares of preferred stock authorized and no such shares issued.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for terms, amounts, nature of changes, rights and privileges, dividends, and other matters related to preferred stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/505/tableOfContent
| Name: |
us-gaap_PreferredStockTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Net Loss Per Share
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Net Loss Per Share |
5. Net Loss Per Share The following table summarizes the computation of the basic and diluted net loss per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
|
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(4,938,641 |
) |
|
$ |
(3,902,288 |
) |
|
$ |
(14,313,746 |
) |
|
$ |
(26,650,541 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding |
|
|
35,926,921 |
|
|
|
37,823,394 |
|
|
|
35,907,123 |
|
|
|
37,623,459 |
|
|
Net loss per share, basic and diluted |
|
$ |
(0.14 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.40 |
) |
|
$ |
(0.71 |
) |
|
The following table summarizes the outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per share because their inclusion would be anti-dilutive:
|
|
|
|
|
|
|
|
|
|
|
At September 30, |
|
|
|
2023 |
|
|
2024 |
|
Common stock options |
|
|
1,869,492 |
|
|
|
1,788,137 |
|
Potentially dilutive securities |
|
|
1,869,492 |
|
|
|
1,788,137 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Fair Value of Financial Instruments
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value of Financial Instruments |
6. Fair Value of Financial Instruments The carrying amount of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, U.S. government-backed securities with maturity dates up to one year, accounts payable and accrued liabilities, approximate fair value due to their short-term maturities. Money market funds included in cash and cash equivalents and U.S. government-backed securities are measured at fair value based on quoted prices in active markets, which are considered Level 1 inputs. No transfers between levels occurred during the periods presented. The following table presents the Company’s assets, which were measured at fair value at December 31, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2023: |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
1,626,129 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,626,129 |
|
Total |
|
$ |
1,626,129 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,626,129 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At September 30, 2024: |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
83,814 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
83,814 |
|
Total |
|
$ |
83,814 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
83,814 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 107
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-107
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 940
-SubTopic 820
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478119/940-820-50-1
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Income Taxes
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
7. Income Taxes The Company computes its quarterly income tax expense/(benefit) by using a forecasted annual effective tax rate and adjusts for any discrete items arising during the quarter. The Company did not have a provision for income taxes (current or deferred tax expense) as of December 31, 2023 and September 30, 2024. The Company will continue to maintain a 100% valuation allowance on total deferred tax assets. The Company believes it is more likely than not that the related deferred tax assets will not be realized. As a result, the Company’s effective tax rate will remain at 0.00% because no items either estimated or discrete items would impact the tax provision.
|
| X |
- DefinitionThe entire disclosure for income tax. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 231
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482663/740-10-55-231
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477891/740-270-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/740/tableOfContent
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-14
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-21
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Commitments and Contingencies
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
8. Commitments and Contingencies Operating Leases As of March 1, 2022, the Company entered a two-year lease, comprised of two operating leases, for approximately 12,250 square feet of space located in Miramar, Florida. Upon the commencement of those leases, the Company used its incremental borrowing rate of 6.0% to determine the amounts to recognize for a ROU asset and a lease liability. On March 1, 2024, the Company entered a one-year lease for the same space, which expires on February 28, 2025. If a lease has a term that is 12 months or less in duration, the lease qualifies for a short-term lease exemption under ASC 842-20-25-2. The Company elected to take advantage of this exemption, and it will account for this lease on a straight-line basis over the lease term and will not recognize a ROU asset and a lease liability as a result. The remaining lease payments under the new short-term lease are $114,510. The Company has no obligations under financing leases. For the three months ended September 30, 2023 and 2024, rent expense recognized by the Company was $42,400 and $49,500, respectively, of which $22,200 and $25,900, respectively, are included in research and development in the accompanying condensed interim statements of operations. For the nine months ended September 30, 2023 and 2024, rent expense recognized by the Company was $127,200 and $146,400, respectively, of which $66,600 and $75,300, respectively are included in research and development in the accompanying condensed interim statements of operations. Contractual Commitments The Company has commitments with a third-party manufacturing organization to supply us with clinical grade materials. As of September 30, 2024, it is under contract for obligations of $12,500 it expects to pay during the year ending December 31, 2024. As of December 31, 2023 and September 30, 2024, the Company had commitments to fund $4.4 million and $2.5 million, respectively, in construction costs related to the buildout of its new headquarters and manufacturing facility. Project Financing On January 10, 2024 (the “Termination Date”), the Company exercised its right to terminate its credit agreement (the “Credit Agreement”), dated April 21, 2023, with Prime Capital Ventures, LLC (the “Lender”), as permitted under the terms of the Credit Agreement. These funds were intended to finance the construction and renovation of the Company’s new headquarters (the “Property”). The termination followed repeated delays in funding and related concerns. There were no borrowings under the Credit Agreement as of the Termination Date, and the Company did not incur any penalties as a result of such termination under the terms of the Agreement. Upon exercising its right to terminate the Agreement, the Company was entitled to receive the return of the $5.3 million that the Company placed on deposit to establish an interest reserve account with the Lender. However, the Lender defaulted on its obligation to return the interest reserve deposit. Given the uncertainty of when or if funds will be recovered from the Lender, the Company recognized a reserve for a credit loss for $5.3 million as of December 31, 2023. The Company intends to pursue all available remedies to recover these funds, including legal actions, receivership and insurance. The Company continues to seek financing to complete the construction and renovation of its Property, where it intends to build a new headquarters, including a biologics manufacturing facility, upgraded research laboratory facilities, vivarium, and upgraded office and common areas. Nonoperating Loss As reported in the Company’s Form 8-K filed on May 1, 2024 with the SEC, the Company became aware that it was the victim of a criminal scheme involving the impersonation of a purchaser upon the default on a legally binding commitment to purchase $8.0 million of secured notes from the Company. The scheme resulted in the misdirection of approximately $1.3 million held in Company accounts to a fraudulent account controlled by a third party. The Company is pursuing all available remedies to recover this loss. Given the limited success that these efforts have had to date for the recovery of funds, the Company recognized a loss of $1.3 million in the three- and nine-month periods ended September 30, 2024. Legal Legal Proceedings From time to time, the Company is a party to or otherwise involved in legal proceedings, including suits, assessments, regulatory actions and investigations generally arising out of the normal course of business. In addition, the Company enters into agreements that may include indemnification provisions, pursuant to which the Company agrees to indemnify, hold harmless and defend the indemnified parties for losses suffered or incurred by the indemnified party. When the Company believes that the outcome of such a matter will result in a liability that is probable to be incurred and result in a potential loss, or range of loss, that can be reasonably estimated, the Company will accrue a liability and make the appropriate disclosure in the footnotes to the financial statements. Arbitration, Settlement and General Release On December 23, 2022, Altor BioScience, LLC and NantCell, Inc. (“Altor/NantCell”) initiated an arbitration against Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, in California alleging breach of contract and fiduciary duty, among other claims. On that same date, Altor/NantCell filed a lawsuit against the Company in federal court alleging misappropriation of trade secrets, inducement of breach of contract and breach of fiduciary duty, among other claims against the Company. On April 26, 2023, the parties stipulated that Altor/NantCell’s action against the Company would be consolidated with the Altor/NantCell arbitration demand against Dr. Wong. On April 27, 2023, the Court approved the parties’ stipulation and ordered the parties to arbitration. On May 1, 2023, Altor/NantCell filed a demand against the Company before JAMS. On May 3, 2023, Altor/NantCell dismissed the federal court action without prejudice and the Court ordered the case dismissed without prejudice and closed the case. Altor/NantCell’s proceeding against the Company proceeded in arbitration before JAMS and consolidated with the arbitration Altor/NantCell initiated against Dr. Wong (the “Arbitration”). On March 26, 2024, Altor/NantCell filed a complaint (the “Complaint”) against the Company in the Chancery Court of the State of Delaware for the contribution of legal fees and expenses advanced to Dr. Wong. As reported in the Company’s Form 8-K filed on July 18, 2024 and described in Part II, Item 1. – “Legal Proceedings” below, as of July 13, 2024, the Company and Dr. Hing C. Wong, the Company’s Founder and Chief Executive Officer, entered into a confidential Settlement Agreement and Release (the “Settlement Agreement”) with Altor BioScience, LLC (“Altor”), NantCell, Inc. (“NantCell”), and ImmunityBio, Inc. (the parent of Altor and NantCell, together with Altor and NantCell, “ImmunityBio”), to resolve the previously disclosed Arbitration before JAMS brought by Altor and NantCell as well as the Complaint Altor filed against the Company in the Chancery Court of the State of Delaware for the contribution of legal fees and expenses advanced to Dr. Wong. The Settlement Agreement includes mutual general releases by and among the parties thereto. No party is required to make any monetary payments to any other party or person under the Settlement Agreement and each party will bear its own expenses incurred in connection with the matter. The Company has substantially completed remediation procedures required to be in compliance with the terms of the Settlement Agreement. The Settlement Agreement provides that, upon completion of these procedures, the parties mutually agreed to stipulate that the Arbitration and Complaint should be dismissed. As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint. Other Matters As of September 30, 2024, certain subcontractors have filed mechanics liens related to unpaid invoices issued in connection with the Company’s construction of its new manufacturing facilities and upgraded research laboratories. The 2022 Loan Agreement contains a provision for a discretionary default in the event that the Company fails to pay sums due in connection with construction of any improvements; however, as of the reporting date, to the Company’s knowledge, the lender has not elected to do so. Inflationary Cost Environment, Banking Crisis, Supply Chain Disruption and the Macroeconomic Environment The Company’s operations have been affected by many headwinds, including inflationary pressures, rising interest rates, ongoing global supply chain disruptions resulting from increased geopolitical tensions such as the war in the Middle East, the conflict between Russia and Ukraine, China-Taiwan relations, financial market volatility and currency movements. The Company has been impacted by inflation, and may continue to be so, when procuring materials required for the buildout of our new headquarters, the costs for recruiting and retaining employees and other employee-related costs. Management employs a number of strategies to effectively navigate these issues, including product redesign, alternate sourcing, and establishing contingencies in budgeting and timelines. Future developments in these and other areas present material uncertainty and risk with respect to the Company's clinical trials, IND-enabling activities, buildout of the new headquarters, as well as the Company's financial condition and results of operations. The extent and duration of such events and conditions, and resulting disruptions to our operations, are highly unpredictable.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant arrangements with third parties, which includes operating lease arrangements and arrangements in which the entity has agreed to expend funds to procure goods or services, or has agreed to commit resources to supply goods or services, and operating lease arrangements. Descriptions may include identification of the specific goods and services, period of time covered, minimum quantities and amounts, and cancellation rights. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/440/tableOfContent
| Name: |
us-gaap_CommitmentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Subsequent Events
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
9. Subsequent Events Subsequent events have been evaluated through the date the financial statements were filed. In addition to the required recognition or disclosure disclosed in the footnotes herein, there were also the following subsequent events after the reporting date: During October 2024, the Company issued $375,000 in Additional Secured Notes, including a $50,000 purchase by Dr. Wong on October 31, 2024. On November 13, 2024, we entered into an engagement letter with the Maxim Group to act as the exclusive placement agent for a multi-step equity financing. As of the filing date, the parties to the Settlement Agreement are working together to finalize the notices and related final documentation to obtain dismissal of the Arbitration and Complaint.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Organization and Summary of Significant Accounting Policies (Policies)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation |
Basis of Presentation The accompanying unaudited condensed balance sheet as of September 30, 2024, the condensed statements of operations, and condensed statements of changes in stockholders’ equity (deficit) for the three and nine-month periods ended September 30, 2023 and 2024 and the condensed statements of cash flows for the nine months ended September 30, 2023 and 2024, and all related disclosures contained in the accompanying notes have been prepared in accordance with accounting principles that are generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and pursuant to Article 10 of Regulation S-X of the Securities Act of 1933, as amended (the “Securities Act”). Accordingly, they do not include all of the information and notes required by U.S. GAAP for complete financial statements. These unaudited condensed interim financial statements include only normal and recurring adjustments that the Company believes are necessary to fairly state the Company’s financial position and the results of its operations and cash flows. The results for the three and nine-month periods ended September 30, 2024 are not necessarily indicative of the results expected for the full fiscal year or any subsequent interim period. The condensed interim balance sheet at December 31, 2023 has been derived from the audited financial statements at that date but does not include all disclosures required by U.S. GAAP for complete financial statements. Because all of the disclosures required by U.S. GAAP for complete financial statements are not included herein, these unaudited condensed interim financial statements and the notes accompanying them should be read in conjunction with the Company’s audited financial statements for the year ended December 31, 2023, which appear in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on May 15, 2024 (the “Annual Report”) and in other filings with the SEC.
|
| Reclassification of Prior Period Presentation of Legal Expenses |
Reclassification of Prior Period Presentation of Legal Expenses Certain prior period amounts have been reclassified to distinguish between General and administrative expenses in the ordinary course of business and legal expenses incurred in connection with the arbitration and Settlement Agreement described in Notes 1. Reclassification of legal expenses incurred in connection with legal proceedings impacts the consolidated interim statements of operations. There is no effect on reporting results of operations from prior periods.
|
| Use of Estimates |
Use of Estimates The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Management must apply significant judgment in this process. The Company evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors and adjusts those estimates and assumptions when facts and circumstances dictate. Actual results could differ from estimates.
|
| Fair Value Measurements |
Fair Value Measurements Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurement (“Topic 820”), establishes a fair value hierarchy for those instruments measured at fair value that distinguishes between fair value measurements based on market data (observable inputs) and those based on the Company’s own assumptions (unobservable inputs). This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows: Level 1: Observable inputs such as quoted prices in active markets; Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and Level 3: Unobservable inputs in which there is little or no market data, which require a reporting entity to develop its own assumptions. Fair value measurements are classified based on the lowest level of input that is significant to the measurement. The Company’s assessment of the significance of a particular input to the fair value measurement requires judgment, which may affect the valuation of the assets and liabilities and their placement within the fair value hierarchy levels. The determination of the fair values takes into account the market for the Company’s financial assets and liabilities, the associated credit risk, and other factors as required. The Company considers active markets as those in which transactions for the assets or liabilities occur in sufficient frequency and volume to provide pricing information on an ongoing basis.
|
| Revenue Recognition |
Revenue Recognition The Company accounts for revenues in accordance with ASC 606, Revenue from Contracts with Customers (“Topic 606”). To determine revenue recognition for arrangements that fall within the scope of Topic 606, the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the Company satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that it will collect the consideration it is entitled to in exchange for the goods or services transferred to the customer. At contract inception, the Company assesses the goods or services promised within each contract, determines those that are performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. To date, the Company's revenues have been generated solely from transactions with Wugen. The Wugen License includes licenses of intellectual property, cost reimbursements, upfront signing fees, milestone payments and royalties on future licensee’s product sales. In addition, the Company and Wugen have an agreement for supply of materials, from which the Company also recognizes revenues. License Grants: For out-licensing arrangements that include a grant of a license to the Company’s intellectual property, the Company considers whether the license grant is distinct from the other performance obligations included in the arrangement. For licenses that are distinct, the Company recognizes revenues from nonrefundable, upfront payments and other consideration allocated to the license when the license term has begun and the Company has provided all necessary information regarding the underlying intellectual property to the customer, which generally occurs at or near the inception of the arrangement. Milestone and Contingent Payments: At the inception of the arrangement and at each reporting date thereafter, the Company assesses whether it should include any milestone and contingent payments or other forms of variable consideration in the transaction price using the most likely amount method. If it is probable that a significant reversal of cumulative revenue would not occur upon resolution of the uncertainty, the associated milestone value is included in the transaction price. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of each such milestone and any related constraint and, if necessary, adjusts its estimate of the overall transaction price. Since milestone and contingent payments may become payable to the Company upon the initiation of a clinical study or filing for or receipt of regulatory approval, the Company reviews the relevant facts and circumstances to determine when the Company should update the transaction price, which may occur before the triggering event. When the Company updates the transaction price for milestone and contingent payments, the Company allocates the changes in the total transaction price to each performance obligation in the agreement on the same basis as the initial allocation. Any such adjustments are recorded on a cumulative catch-up basis in the period of adjustment, which may result in recognizing revenue for previously satisfied performance obligations in such period. The Company’s licensees will generally pay milestones payments subsequent to achievement of the triggering event. Materials Supply: The Company provides clinical and research grade materials so that licensees may develop products based on the licensed molecules. The Company plans to enter into commercialization supply agreements when licensees enter the commercial stage of their company. The amounts billed are recognized as revenue as the performance obligations are satisfied by the Company, once the Company determines that a contract exists. On June 18, 2021, the Company entered into a master services agreement for the supply of materials for clinical development of licensed products. To meet all the criteria to qualify as a contract under Topic 606, the Company must enter into statements-of-work for purchases of clinical and research grade materials. The Company has determined that the manufacturing of the clinical and research materials supplied by the Company each represents a single performance obligation that is satisfied over time. The Company recognizes revenue using an input method based on the costs incurred relative to the total expected cost, which determines the extent of the Company's progress toward completion. As part of the accounting for these arrangements, the Company must develop estimates and assumptions that require judgement to determine the progress towards completion. The Company reviews its estimate of the progress toward completion based on the best information available to recognize the cumulative progress toward completion as of the end of each reporting period, and makes revisions to such estimates, if facts and circumstances change during each reporting period. For the three and nine months ended September 30, 2023, the Company recognized $853,102 and $1.5 million in revenue related to the sale of development supply materials to Wugen, respectively. For the three and nine months ended September 30, 2024, the Company recognized $426,423 and $2.2 million in revenue related to sale of development supply materials to Wugen, respectively.
|
| Investments |
Investments The Company holds a minority interest in Wugen which is accounted for using the measurement alternative whereby the investment is recorded at cost less impairment, adjusted for observable price changes in orderly transactions for an identical or similar investment of the same investee. No impairment has been recognized. As of September 30, 2024 and December 31, 2023, the Company included $1.6 million for the investment in Wugen in Investments in the accompanying condensed interim balance sheets. The Company used its equity interest in Wugen to collateralize the Secured Notes. See Note 3. Debt, Net.
|
| Operating Leases |
Operating Leases The Company determines if an arrangement is a lease at inception. Operating leases are included in Other assets, Accrued liabilities and other current liabilities, and Other liabilities on its condensed interim balance sheets. Operating lease Right of Use (“ROU”) assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. The Company has a lease agreement with lease and non-lease components, which are accounted for separately. For short-term leases with a term of one year or less, the Company uses the practical expedient and does not record an ROU asset or lease liability for such short-term leases.
|
| Net Loss Per Share |
Net Loss Per Share Basic loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during each period. Diluted loss per share of common stock includes the effect, if any, from the potential exercise of stock options and unvested shares of restricted stock, which would result in the issuance of incremental shares of common stock. For diluted net loss per share, the weighted-average number of shares of common stock is the same for basic net loss per share due to the fact that when a net loss exists, dilutive securities are not included in the calculation as the impact is anti-dilutive.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for investment in financial asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 12
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-19
| Name: |
us-gaap_InvestmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for reclassification affecting comparability of financial statement. Excludes amendment to accounting standards, other change in accounting principle, and correction of error. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 205
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483504/205-10-50-1
| Name: |
us-gaap_PriorPeriodReclassificationAdjustmentDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Net Loss Per Share (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Summary of Basic and Diluted Net Income (Loss) Per Common Share |
The following table summarizes the computation of the basic and diluted net loss per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
|
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(4,938,641 |
) |
|
$ |
(3,902,288 |
) |
|
$ |
(14,313,746 |
) |
|
$ |
(26,650,541 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding |
|
|
35,926,921 |
|
|
|
37,823,394 |
|
|
|
35,907,123 |
|
|
|
37,623,459 |
|
|
Net loss per share, basic and diluted |
|
$ |
(0.14 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.40 |
) |
|
$ |
(0.71 |
) |
|
|
| Summary of Outstanding Potentially Dilutive Securities |
The following table summarizes the outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per share because their inclusion would be anti-dilutive:
|
|
|
|
|
|
|
|
|
|
|
At September 30, |
|
|
|
2023 |
|
|
2024 |
|
Common stock options |
|
|
1,869,492 |
|
|
|
1,788,137 |
|
Potentially dilutive securities |
|
|
1,869,492 |
|
|
|
1,788,137 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of an entity's basic and diluted earnings per share calculations, including a reconciliation of numerators and denominators of the basic and diluted per-share computations for income from continuing operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Fair Value of Financial Instruments (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Summary of assets and liabilities that are measured at fair value on a recurring basis |
The following table presents the Company’s assets, which were measured at fair value at December 31, 2023 and September 30, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2023: |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
1,626,129 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,626,129 |
|
Total |
|
$ |
1,626,129 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,626,129 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At September 30, 2024: |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
83,814 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
83,814 |
|
Total |
|
$ |
83,814 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
83,814 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets and liabilities, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Organization and Summary of Significant Accounting Policies - Additional Information (Detail) - USD ($)
|
3 Months Ended |
9 Months Ended |
|
|
|
|
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Oct. 31, 2024 |
Mar. 31, 2024 |
Dec. 31, 2023 |
Aug. 15, 2022 |
| Revenue recognized |
$ 426,423
|
$ 853,102
|
$ 2,171,988
|
$ 1,517,792
|
|
|
|
|
| Investment impairment charges |
|
|
0
|
|
|
|
|
|
| Legal fees incurred but not yet paid |
14,400,000
|
|
14,400,000
|
|
|
|
|
|
| Accrued Legal Fees |
35,000
|
|
35,000
|
|
|
|
|
|
| Cash and cash equivalents |
998,221
|
|
998,221
|
|
|
|
$ 3,595,101
|
|
| Loss contingency loss in period |
1,300,000
|
|
1,300,000
|
|
|
|
|
|
| Research and development |
|
|
94,500,000
|
|
|
|
|
|
| Common Stock Value |
3,782
|
|
3,782
|
|
|
|
3,603
|
|
| Senior Secured Notes [Member] |
|
|
|
|
|
|
|
|
| Debt instrument, face amount |
6,500,000
|
|
6,500,000
|
|
|
|
|
|
| Senior Secured Notes [Member] | Subsequent Event [Member] |
|
|
|
|
|
|
|
|
| Total issuance of Secured Notes |
|
|
|
|
$ 375,000
|
|
|
|
| Secured debt |
|
|
|
|
375,000
|
|
|
|
| Senior Secured Notes [Member] | Subsequent Event [Member] | New Financing Plan [Member] |
|
|
|
|
|
|
|
|
| Total issuance of Secured Notes |
|
|
|
|
6,900,000
|
|
|
|
| Secured debt |
|
|
|
|
$ 6,900,000
|
|
|
|
| Cogent Bank Member |
|
|
|
|
|
|
|
|
| Proceeds to purchase |
|
|
|
|
|
|
|
$ 6,500,000
|
| Maximum [Member] | Senior Secured Notes [Member] |
|
|
|
|
|
|
|
|
| Debt instrument, face amount |
|
|
|
|
|
$ 10,000,000
|
|
|
| Wugen License |
|
|
|
|
|
|
|
|
| Revenue recognized |
426,423
|
$ 853,102
|
2,200,000
|
$ 1,500,000
|
|
|
|
|
| Investments |
1,600,000
|
|
1,600,000
|
|
|
|
$ 1,600,000
|
|
| Surety Bond |
|
|
|
|
|
|
|
|
| Total issuance of Secured Notes |
8,000,000
|
|
8,000,000
|
|
|
|
|
|
| Loss contingency loss in period |
|
|
1,300,000
|
|
|
|
|
|
| Secured debt |
$ 8,000,000
|
|
$ 8,000,000
|
|
|
|
|
|
| X |
- DefinitionCumulative losses to conduct product research and development in future.
| Name: |
hcwb_CumulativeLossesToConductProductResearchAndDevelopmentInFuture |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionInvestment impairment charges.
| Name: |
hcwb_InvestmentImpairmentCharges |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLegal fees incurred but not yet paid.
| Name: |
hcwb_LegalFeesIncurredButNotYetPaid |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for professional fees, such as for legal and accounting services received. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_AccruedProfessionalFeesCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482900/835-30-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all investments. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 944
-SubTopic 80
-Name Accounting Standards Codification
-Section 55
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480078/944-80-55-14
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 944
-SubTopic 80
-Name Accounting Standards Codification
-Section 55
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480078/944-80-55-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1)(h))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1)(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_Investments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionMaximum borrowing capacity under the credit facility without consideration of any current restrictions on the amount that could be borrowed or the amounts currently outstanding under the facility. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityMaximumBorrowingCapacity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of loss pertaining to the specified contingency that was charged against earnings in the period, including the effects of revisions in previously reported estimates. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-1
| Name: |
us-gaap_LossContingencyLossInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date, including the current and noncurrent portions, of collateralized debt obligations (with maturities initially due after one year or beyond the operating cycle, if longer). Such obligations include mortgage loans, chattel loans, and any other borrowings secured by assets of the borrower. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_SecuredDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=us-gaap_SeniorNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=hcwb_NewFinancingPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CreditFacilityAxis=hcwb_CogentBankMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
hcwb_AgreementAxis=hcwb_WugenLicenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LossContingenciesByNatureOfContingencyAxis=us-gaap_SuretyBondMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Accrued Liabilities and Other Current Liabilities - Additional Information (Detail) - USD ($)
|
3 Months Ended |
9 Months Ended |
12 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
| Payables And Accruals [Line Items] |
|
|
|
|
|
| Accrued liabilities and other current liabilities |
$ 1,056,716
|
|
$ 1,056,716
|
|
$ 2,580,402
|
| Accounts Payable and Accrued Liabilities, Current |
1,200,000
|
|
1,200,000
|
|
2,600,000
|
| Legal Fees |
949,455
|
$ 2,075,279
|
15,761,531
|
$ 4,610,091
|
|
| Accrued Expenses Current [Member] |
|
|
|
|
|
| Payables And Accruals [Line Items] |
|
|
|
|
|
| short term lease liability |
|
|
|
|
28,500
|
| Accounts Payable and Accrued Liabilities [Member] |
|
|
|
|
|
| Payables And Accruals [Line Items] |
|
|
|
|
|
| Salaries and Employee Benefits |
90,000
|
|
90,000
|
|
|
| Construction expenses |
|
|
|
|
392,000
|
| Manufacturing expenses |
|
|
49,000
|
|
105,000
|
| Construction in progress |
422,000
|
|
422,000
|
|
|
| Legal Fees |
|
|
170,000
|
|
1,100,000
|
| Property taxes |
104,000
|
|
104,000
|
|
|
| Clinical expenses |
117,000
|
|
117,000
|
|
262,000
|
| Bonus payable |
57,000
|
|
57,000
|
|
365,000
|
| Salary expense |
|
|
|
|
160,000
|
| Current portion of long term debt |
$ 126,000
|
|
$ 126,000
|
|
119,000
|
| Other liabilities |
|
|
|
|
$ 68,500
|
| X |
- Definition
+ References
+ Details
| Name: |
hcwb_PayablesAndAccrualsLineItems |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities incurred to vendors for goods and services received, and accrued liabilities classified as other, payable within one year or the normal operating cycle, if longer.
| Name: |
us-gaap_AccountsPayableAndOtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable for incentive compensation awarded to employees and directors or earned by them based on the terms of one or more relevant arrangements. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedBonusesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of the obligations incurred through that date and payable for employees' services provided. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 8
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-8
| Name: |
us-gaap_AccruedSalariesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of structure or a modification to a structure under construction. Includes recently completed structures or modifications to structures that have not been placed into service. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_ConstructionInProgressGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable for the acquisition of merchandise, materials, supplies and services pertaining to construction projects such as a housing development or factory expansion not classified as trade payables. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_ConstructionPayableCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense provided in the period for legal costs incurred on or before the balance sheet date pertaining to resolved, pending or threatened litigation, including arbitration and mediation proceedings. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(6))
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_LegalFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as current. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe aggregate costs incurred in the production of goods for sale. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_ManufacturingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_OtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
hcwb_AccruedExpensesCurrentAxis=hcwb_AccruedExpensesCurrentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=us-gaap_AccountsPayableAndAccruedLiabilitiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Debt, Net - Additional Information (Details) - USD ($)
|
9 Months Ended |
|
|
|
Sep. 30, 2024 |
Mar. 31, 2024 |
Dec. 31, 2023 |
Aug. 15, 2022 |
| Line of Credit Facility [Line Items] |
|
|
|
|
| Debt |
$ 6,462,769
|
|
$ 6,304,318
|
|
| Wugen License |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Pledged collateral percentage |
5.60%
|
|
|
|
| Senior Secured Notes [Member] |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Maturity date |
Aug. 30, 2026
|
|
|
|
| Principal amount |
$ 6,500,000
|
|
|
|
| Interest rate |
9.00%
|
|
|
|
| Penalty percentage |
5.00%
|
|
|
|
| Senior Secured Notes [Member] | Collateral Pledged |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Principal amount |
$ 10,000,000
|
|
|
|
| Senior Secured Notes [Member] | Maximum [Member] |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Principal amount |
|
$ 10,000,000
|
|
|
| Senior Secured Notes [Member] | Chief Executive Officer [Member] |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Accredited investors principal amount |
2,400,000
|
|
|
|
| Senior Secured Notes [Member] | Chief Financial Officer |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Accredited investors principal amount |
220,000
|
|
|
|
| Senior Secured Notes [Member] | Chairman of Board Of Directors [Member] |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Accredited investors principal amount |
140,000
|
|
|
|
| Senior Secured Notes [Member] | Board Of Directors |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Accredited investors principal amount |
60,000
|
|
|
|
| Senior Secured Notes [Member] | Senior Vice President [Member] |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Accredited investors principal amount |
25,000
|
|
|
|
| Senior Secured Notes [Member] | Board Of Directors Audit Chair |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Accredited investors principal amount |
$ 25,000
|
|
|
|
| Cogent Bank Member |
|
|
|
|
| Line of Credit Facility [Line Items] |
|
|
|
|
| Proceeds to purchase |
|
|
|
$ 6,500,000
|
| Line Of credit facility frequency of payments |
The interest-only period was one year followed by 48 months of equal payments of principal and interest
|
|
|
|
| Maturity date |
Aug. 15, 2027
|
|
|
|
| Credit facility interest rate during period |
5.75%
|
|
|
|
| Debt, current portion |
$ 6,300,000
|
|
|
|
| X |
- DefinitionDebt prepayment penalty percentage.
| Name: |
hcwb_DebtPrepaymentPenaltyPercentage |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPledged collateral percentage.
| Name: |
hcwb_PledgedCollateralPercentage |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482900/835-30-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDate when the debt instrument is scheduled to be fully repaid, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of principal of investment owned. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478795/946-210-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477439/946-210-55-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478795/946-210-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
| Name: |
us-gaap_InvestmentOwnedBalancePrincipalAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDescription of the frequency of periodic payments, which may be presented in a variety of ways (for example, monthly, quarterly, annually). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityFrequencyOfPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe effective interest rate during the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityInterestRateDuringPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum borrowing capacity under the credit facility without consideration of any current restrictions on the amount that could be borrowed or the amounts currently outstanding under the facility. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityMaximumBorrowingCapacity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt classified as current. Excludes lease obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermDebtCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
hcwb_AgreementAxis=hcwb_WugenLicenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=us-gaap_SeniorNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CollateralAxis=us-gaap_CollateralPledgedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ChiefExecutiveOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ChiefFinancialOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_BoardOfDirectorsChairmanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=hcwb_BoardOfDirectorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ExecutiveVicePresidentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=hcwb_BoardOfDirectorsAuditChairMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CreditFacilityAxis=hcwb_CogentBankMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued for nonredeemable preferred shares and preferred shares redeemable solely at option of issuer. Includes, but is not limited to, preferred shares issued, repurchased, and held as treasury shares. Excludes preferred shares classified as debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
Net Loss Per Share - Summary of Basic and Diluted Net Income (Loss) Per Common Share (Detail) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Sep. 30, 2023 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Numerator: |
|
|
|
|
|
|
|
|
| Net Income (Loss) |
$ (3,902,288)
|
$ (15,280,191)
|
$ (7,468,061)
|
$ (4,938,641)
|
$ (4,304,420)
|
$ (5,070,686)
|
$ (26,650,541)
|
$ (14,313,746)
|
| Denominator: |
|
|
|
|
|
|
|
|
| Weighted average shares outstanding, basic |
37,823,394
|
|
|
35,926,921
|
|
|
37,623,459
|
35,907,123
|
| Weighted average shares outstanding, diluted |
37,823,394
|
|
|
35,926,921
|
|
|
37,623,459
|
35,907,123
|
| Net loss per share, basic |
$ (0.1)
|
|
|
$ (0.14)
|
|
|
$ (0.71)
|
$ (0.4)
|
| Net loss per share, diluted |
$ (0.1)
|
|
|
$ (0.14)
|
|
|
$ (0.71)
|
$ (0.4)
|
| X |
- References
+ Details
| Name: |
hcwb_DenominatorAbstract |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
hcwb_NumeratorAbstract |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Net Loss Per Share - Summary of Outstanding Potentially Dilutive Securities (Detail) - shares
|
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Potentially dilutive securities |
1,788,137
|
1,869,492
|
| Common Stock Options [Member] |
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Potentially dilutive securities |
1,788,137
|
1,869,492
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=hcwb_CommonStockOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Fair Value of Financial Instruments - Summary of Assets and Liabilities Measured at Fair Value on a Recurring Basis (Detail) - USD ($)
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Assets: |
|
|
| Assets |
$ 83,814
|
$ 1,626,129
|
| Money Market Funds [Member] |
|
|
| Assets: |
|
|
| Assets |
83,814
|
1,626,129
|
| Level 1 [Member] |
|
|
| Assets: |
|
|
| Assets |
83,814
|
1,626,129
|
| Level 1 [Member] | Money Market Funds [Member] |
|
|
| Assets: |
|
|
| Assets |
83,814
|
1,626,129
|
| Level 2 [Member] |
|
|
| Assets: |
|
|
| Assets |
0
|
0
|
| Level 2 [Member] | Money Market Funds [Member] |
|
|
| Assets: |
|
|
| Assets |
0
|
0
|
| Level 3 [Member] |
|
|
| Assets: |
|
|
| Assets |
0
|
0
|
| Level 3 [Member] | Money Market Funds [Member] |
|
|
| Assets: |
|
|
| Assets |
$ 0
|
$ 0
|
| X |
- DefinitionFair value portion of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_AssetsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsFairValueDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_FairValueByAssetClassAxis=us-gaap_MoneyMarketFundsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
v3.24.3
Commitments and Contingencies - Additional Information (Detail)
|
3 Months Ended |
9 Months Ended |
12 Months Ended |
|
|
|
|
|
Sep. 30, 2024
USD ($)
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2024
USD ($)
|
Sep. 30, 2023
USD ($)
|
Dec. 31, 2023
USD ($)
|
Dec. 31, 2024
USD ($)
|
Mar. 01, 2024 |
Jan. 10, 2024
USD ($)
|
Mar. 01, 2022
ft²
|
| Operating Leased Assets [Line Items] |
|
|
|
|
|
|
|
|
|
| Area of land | ft² |
|
|
|
|
|
|
|
|
12,250
|
| Lease term |
|
|
|
|
|
|
1 year
|
|
2 years
|
| Lease termination date |
|
|
Feb. 28, 2025
|
|
|
|
|
|
|
| Operating Lease, Weighted Average Discount Rate, Percent |
|
|
|
|
|
|
|
|
6.00%
|
| Short-term lease |
|
|
$ 114,510
|
|
|
|
|
|
|
| Operating Leases, Rent Expense |
$ 49,500
|
$ 42,400
|
146,400
|
$ 127,200
|
|
|
|
|
|
| Commitment fund |
|
|
2,500,000
|
|
$ 4,400,000
|
|
|
|
|
| Deposit for interest reserve |
|
|
|
|
|
|
|
$ 5,300,000
|
|
| Deposit for credit loss reserve |
|
|
|
|
$ 5,300,000
|
|
|
|
|
| Loss contingency loss in period |
1,300,000
|
|
1,300,000
|
|
|
|
|
|
|
| Surety Bond [Member] |
|
|
|
|
|
|
|
|
|
| Operating Leased Assets [Line Items] |
|
|
|
|
|
|
|
|
|
| Loss contingency loss in period |
|
|
1,300,000
|
|
|
|
|
|
|
| Secured debt |
8,000,000
|
|
8,000,000
|
|
|
|
|
|
|
| Forecast Member |
|
|
|
|
|
|
|
|
|
| Operating Leased Assets [Line Items] |
|
|
|
|
|
|
|
|
|
| Future payment obligations |
|
|
|
|
|
$ 12,500
|
|
|
|
| Research and Development Expense [Member] |
|
|
|
|
|
|
|
|
|
| Operating Leased Assets [Line Items] |
|
|
|
|
|
|
|
|
|
| Operating Leases, Rent Expense |
$ 25,900
|
$ 22,200
|
$ 75,300
|
$ 66,600
|
|
|
|
|
|
| X |
- DefinitionDeposit account for credit loss reserve.
| Name: |
hcwb_DepositAccountForCreditLossReserve |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDeposit account for interest reserve
| Name: |
hcwb_DepositAccountForInterestReserve |
| Namespace Prefix: |
hcwb_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of contractual obligation, including, but not limited to, long-term debt, lease obligation, purchase obligation, and other commitments. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
| Name: |
us-gaap_ContractualObligation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of rent expense incurred for leased assets, including but not limited to, furniture and equipment, that is not directly or indirectly associated with the manufacture, sale or creation of a product or product line.
| Name: |
us-gaap_LeaseAndRentalExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionDate which lease or group of leases is set to expire, in YYYY-MM-DD format.
| Name: |
us-gaap_LeaseExpirationDate1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of loss pertaining to the specified contingency that was charged against earnings in the period, including the effects of revisions in previously reported estimates. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-1
| Name: |
us-gaap_LossContingencyLossInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OperatingLeasedAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date, including the current and noncurrent portions, of collateralized debt obligations (with maturities initially due after one year or beyond the operating cycle, if longer). Such obligations include mortgage loans, chattel loans, and any other borrowings secured by assets of the borrower. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_SecuredDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of short-term lease cost, excluding expense for lease with term of one month or less. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_ShortTermLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_LossContingenciesByNatureOfContingencyAxis=us-gaap_SuretyBondMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementScenarioAxis=srt_ScenarioForecastMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionAmount of principal of investment owned. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478795/946-210-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477439/946-210-55-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478795/946-210-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
| Name: |
us-gaap_InvestmentOwnedBalancePrincipalAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date, including the current and noncurrent portions, of collateralized debt obligations (with maturities initially due after one year or beyond the operating cycle, if longer). Such obligations include mortgage loans, chattel loans, and any other borrowings secured by assets of the borrower. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_SecuredDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongtermDebtTypeAxis=us-gaap_SeniorNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_InvestmentIssuerNameAxis=hcwb_DrWongMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
HCW Biologics (NASDAQ:HCWB)
과거 데이터 주식 차트
부터 12월(12) 2024 으로 1월(1) 2025

HCW Biologics (NASDAQ:HCWB)
과거 데이터 주식 차트
부터 1월(1) 2024 으로 1월(1) 2025
