/NOT FOR DISTRIBUTION TO UNITED
STATES NEWSWIRE SERVICES OR FOR DISSEMINATION IN
THE UNITED STATES. ANY FAILURE TO
COMPLY WITH THIS RESTRICTION MAY CONSTITUTE A VIOLATION OF
UNITED STATES SECURITIES LAWS/
Another double-digit increase in revenue
quarter over quarter as the company continues to execute on its
business plan to commercialize its brands in different geographic
markets and product segments.
Successful launch of the RHO Phyto line of
advanced medical cannabis products nationwide in Canada in partnership with Medical Cannabis by
Shoppers Drug Mart Inc.
Good Preparation Practices certification and
authorization by the National Institute for Drug and Food
Surveillance ("INVIMA") for the sale of compounded pharmaceutical
products to service medical prescriptions of individual patients in
Colombia.
TORONTO, Nov. 12, 2020 /CNW/ - Avicanna Inc.
("Avicanna" or the "Company) (TSX: AVCN) (OTCQX:
AVCNF) (FSE: 0NN) a biopharmaceutical company focused on the
development, manufacturing and commercialization of plant-derived
cannabinoid-based products announces results for the third quarter
ended September 30, 2020.
Aras Azadian, Chief Executive
Officer of Avicanna, commented "We are thrilled to report the
tremendous progress our team continues to make in our
commercialization and research efforts. During the third quarter,
we were able to demonstrate the superiority of our technologies and
products with several strategic commercial partnerships but also
with an incredibly successful launch of our medical products in the
Canadian market."
Third Quarter Highlights
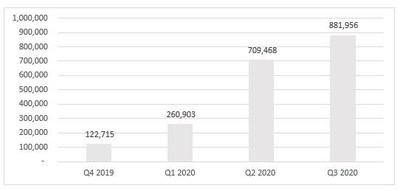
- The Company continued to make strides in its strategic
commercial initiatives, leading to an increase in revenue of
approximately 24% from Q2-2020, continuing the trend of
double-digit growth, quarter over quarter from Q4-2019. In
addition, the Company was able to reach a major milestone with the
launch of certain products from its RHO Phyto product line in
Canada and further diversified its
revenue streams. Below is a summary of the revenue trend over the
last four quarters.
- Avicanna launched the first product of its RHO Phyto branded
line of medical cannabis products on the Medical Cannabis by
Shoppers™ ("Shoppers") platform on August
12, 2020. As of the date of this MD&A, Avicanna has
launched its oil drops and sublingual sprays, for a total of four
SKUs, through the Shoppers online platform, which offers nationwide
service to Canadians.
- Certain of the RHO Phyto products are participating in the
Medical Cannabis Real-World Evidence study ("MC-RWE study") at the
University Health Network ("UHN"). This first-of-its-kind Canadian
study is led by Dr. Hance Clarke, Director of Pain Services at
Toronto General Hospital, and will
examine the efficacy of a select group of medical cannabis products
including Avicanna's RHO Phyto line of products on patient reported
outcomes of pain, sleep and anxiety. This specific study is aligned
and in parallel with Avicanna's comprehensive clinical program of
other real-world evidence studies involving the RHO Phyto products
and clinical trials on its pharmaceutical pipeline with
world-class, Toronto-based medical
institutions.
- The Company hosted its third annual symposium, "Medical
Cannabis 2.0", through a virtual format on July 21, 2020. The presentations focused on the
evolution of medical cannabis including the Avicanna led
advancements in R&D for novel cannabinoid delivery forms and
formulations. Presenters including Dr. Ruth
Ross (Professor and Chair, Department of Pharmacology &
Toxicology, Faculty of Medicine, University of
Toronto, Senior Scientist, Campbell Family Mental Health
Research Institute, Centre for Addiction and Mental Health) and Dr.
Hance Clarke (Staff Anesthesiologist, Director Pain Services,
Director Good Hope Ehlers Danlos Clinic, Medical Director of The
Pain Research Unit, Department of Anesthesia and Pain Management,
Toronto General Hospital, University
Health Network, Associate Professor, Department of Anesthesia,
University of Toronto). Over 1,000
participants attended the symposium.
- The Company announced on September 14,
2020 that through Avicanna LATAM S.A.S., the Company's
pharmacy in Bogota has been
certified with Good Preparation Practices and authorized by INVIMA
for the sale of compounded pharmaceutical products to service
medical prescriptions of individual patients in Colombia. This is the final step in the
Company's fully integrated seed to patient business model in
Colombia, which includes
cultivation, extraction and manufacturing of pharmaceutical
products for the emerging medical market of 50 million people.
- Avicanna announced on July 24,
2020 that its research collaborators had received two
independent peer-reviewed grants from the Natural Sciences and
Engineering Research Council of Canada ("NSERC"). In addition to a recent
MITACS award, Dr. Christine Allen, a
Professor at the University of Toronto,
was awarded an NSERC grant for development of cannabinoid-based
pharmaceutical formulations for the treatment of COVID-19 induced
lung inflammation. Avicanna further expanded its existing
neurobiological research collaboration with Dr. Jibran Khokhar, a Professor at the University of Guelph, with a 2-year NSERC grant to
investigate the neural basis of cannabis-induced toxicosis. The
NSERC grants are being used to expand the investigators'
collaborative research with Avicanna at little to no additional
cost to the Company.
- Avicanna and Red White &
Bloom Brands Inc. ("RWB") entered into a distribution agreement
(the "Distribution Agreement") on August 11,
2020, for the exclusive distribution of Avicanna's
hemp-based CBD derma-cosmetic and topical products, branded as Pura
H&W™, by RWB in the United
States and certain other markets. Under the Distribution
Agreement, which has an initial five-year term, RWB will
exclusively distribute the Pura H&W™ brand and certain other
white label brands at RWB's direction. In exchange for this
exclusivity, RWB is required to pay Avicanna an upfront exclusivity
fee in the amount of CAD$250,000 in
cash, along with minimum purchase requirements for the rights to be
the exclusive distributor of Avicanna's Pura H&W branded
cosmetic products in the US. Under the Distribution Agreement, RWB
also has the right to purchase Avicanna's cosmetic products for
distribution into the United
States and certain other territories under brands of RWB's
choosing. The initial product offerings under the Distribution
Agreement includes body and face lotions, cosmetic creams, gels and
serums, as well as soaps and bath bombs.
- The Company issued an aggregate of 1,952,410 units (the "August
Units") at a price of $1.40 per
August Unit, for aggregate gross proceeds of approximately
$2.7 million on August 18, 2020. Each August Unit was comprised
of one Common Share and one-half of one Common Share purchase
warrant, each whole warrant exercisable into one Common Share at a
price of $2.00 per share until
August 18, 2022, subject to
acceleration rights.
- The Company announced on August 24,
2020 that it completed exports of CBG and CBD isolates into
the USA and CBD isolate into
Germany. The Company also
commenced a pilot tracking system for the export of its active
pharmaceutical ingredient products in partnership with TruTrace
Technologies Inc.
- The Company announced on September 4,
2020 that it completed further exports of CBD water soluble
formula into the USA and CBD-based
cosmetics into the United Kingdom.
The Colombian Ministry of Health also granted SMGH a commercial and
industrial fabrication quota to produce psychoactive THC
derivatives.
- The Company announced that it entered into an agreement with a
US distributor partner on September 29,
2020, whereby the Company plans to develop certain
hemp-derived cannabinoid-based products, including sublingual and
sustained release tablets intended for the sleep market for such US
distributor. Under the agreement, the Company is developing the
intellectual property that forms the basis of the products for a
development fee. The Company will receive an ongoing royalty
payment based on the gross revenue of the products, and the Company
will have the opportunity to supply cannabinoid for the manufacture
of the products.
- During the quarter, the Company incurred general and
administrative (G&A") expenses that total $2,954,438. While the Company's G&A expenses
did marginally increase from the previous quarter, there were
several expenses incurred that were one time in nature. Below is a
summary of the adjusted EBITDA of the Company over the last four
quarters, indicating its continued improvement.
|
Q1
2020
|
Q2
2020
|
Q3
2020
|
|
Revenue
|
260,903
|
709,468
|
881,956
|
|
Adjusted
EBITDA*
|
(3,063,524)
|
(3,641,496)
|
(2,608,063)
|
|
|
* Adjusted EBITDA is
a Non-GAAP measure. Please refer below for a reconciliation of Net
Income (Loss) to EBITDA and Adjusted EBITDA.
|
- Subsequent to quarter end, on November
2, 2020, the Company closed a non-brokered convertible
debenture financing, pursuant to which it issued convertible
debentures (the "Debentures") with an aggregate Face Principal
Amount (as defined below) of $1,100,000 (the "Debenture Financing"). The
Debentures bear interest at 8.0% per annum and will mature on the
date that is 12 months from the date of issuance, with the first
year of interest payable in advance on the date of issuance and
capitalized and added into the principal amount (such aggregate
amount being, the "Face Principal Amount"). With these funds the
Company was able to strengthen its balance sheet and provide the
necessary working capital to make a large commercial push in the
first quarter of 2021.
Summary of Operations ($CDN)
|
Three Months
Ended
|
Nine Months
Ended
|
|
September 30,
2020
|
September 30,
2019
|
September 30,
2020
|
September 30,
2019
|
|
|
|
|
|
|
|
|
$
|
$
|
|
Revenues
|
881,956
|
4,943
|
1,852,327
|
45,537
|
|
Impairment on
inventory
|
(612,105)
|
-
|
(612,105)
|
-
|
|
Inventory Production
Costs expensed to Cost of Sales
|
(439,099)
|
-
|
(673,387)
|
-
|
|
Fair value changes in
biological assets included in inventory sold
|
(37,818)
|
-
|
(607,370)
|
-
|
|
Unrealized
gain/(loss) on changes in fair value of biological
assets
|
(1,103,910)
|
-
|
723,361
|
-
|
|
General and
administrative
|
2,954,438
|
5,673,540
|
9,064,843
|
12,604,022
|
|
Share-based
compensation
|
839,954
|
262,498
|
2,455,916
|
1,982,066
|
|
Depreciation and
amortization
|
419,914
|
326,983
|
1,259,742
|
512,100
|
|
Impairment of
goodwill
|
2,520,382
|
-
|
3,207,227
|
|
|
Total
Expenses
|
(6,734,688)
|
(6,263,021)
|
(15,987,728)
|
(15,098,188)
|
|
Other income
(loss)
|
378,512
|
72,748
|
(2,117,968)
|
473,646
|
|
Net loss before
taxes
|
(7,667,152)
|
(6,185,330)
|
(17,422,870)
|
(14,579,005)
|
|
Net loss after
taxes
|
(7,667,152)
|
(6,185,330)
|
(17,422,870)
|
(14,579,005)
|
|
Weighted average
number of Common Shares outstanding – basic and diluted
|
26,566,915
|
21,830,153
|
25,348,330
|
19,298,899
|
|
Loss per share –
basic and diluted
|
(0.35)
|
(0.33)
|
(0.82)
|
(0.84)
|
Summary of Balance Sheet ($CDN)
|
|
As at September
30,
2020
|
As at December
31,
2019
|
|
|
|
|
|
Assets
|
|
$
|
$
|
|
Cash
|
|
101,088
|
441,757
|
|
Amounts
receivable
|
|
2,442,488
|
1,202,924
|
|
Prepaid
assets
|
|
652,287
|
704,632
|
|
Biological
assets
|
|
233,644
|
117,367
|
|
Inventory
|
|
2,084,414
|
1,484,371
|
|
Right to use
asset
|
|
392,516
|
539,710
|
|
Property and
equipment
|
|
19,951,265
|
22,622,322
|
|
Intangible
assets
|
|
10,504,182
|
11,063,900
|
|
Derivative
asset
|
|
1,501,034
|
3,780,000
|
|
Investments
|
|
72
|
72
|
|
Goodwill
|
|
-
|
3,207,227
|
|
Total
Assets
|
|
37,862,990
|
45,164,282
|
|
Liabilities and
Equity
|
|
|
|
|
Amounts
payable
|
|
4,815,132
|
5,177,634
|
|
Due to related
party
|
|
4,952,124
|
3,319,116
|
|
Convertible
debentures
|
|
757,400
|
715,626
|
|
Derivative
liability
|
|
-
|
23,434
|
|
Lease
liability
|
|
417,975
|
555,339
|
|
Term loan
|
|
-
|
-
|
|
Deferred
revenue
|
|
3,074,752
|
3,323,518
|
|
Deferred tax
liability
|
|
2,173,834
|
2,173,834
|
|
Total
Liabilities
|
|
16,191,217
|
15,288,501
|
|
Shareholder's
equity
|
|
21,671,773
|
29,875,781
|
|
Total Liabilities
and Shareholder's Equity
|
|
37,862,990
|
45,164,282
|
About Avicanna
Avicanna is a diversified and vertically integrated Canadian
biopharmaceutical company focused on the research, development and
commercialization of plant-derived cannabinoid-based products for
the global consumer, medical, and pharmaceutical market
segments.
Avicanna is an established leader in cannabinoid research and
development, which it primarily conducts at its R&D
headquarters in the Johnson & Johnson Innovation Centre, JLABS
@ Toronto, Canada and in
collaboration with leading Canadian academic and medical
institutions. In addition to its developing pharmaceutical
pipeline, Avicanna's team of experts have developed and
commercialized several industry leading product lines,
including:
- Pura H&W: an advanced and clinically tested line of CBD
consumer dermacosmetic products; and,
- RHO Phyto: an advanced line of medical cannabis products
containing varying ratios of CBD and THC currently available
nation-wide across Canada in
partnership with Medical Cannabis by Shoppers™, a subsidiary of
Shoppers Drug Mart. RHO Phyto is the first strictly medical
formulary of advanced "Cannabis 2.0" products, containing oils,
sprays, capsules, creams, and gels, all developed with scientific
rigour, manufactured under GMP standards and supported by
pre-clinical data.
With ongoing clinical trials on its dermacosmetic (Pura
H&W), medical cannabis (RHO Phyto) and a pipeline of
pharmaceutical products, Avicanna's dedication to researching the
important role that cannabinoids play in an increasingly wider
scope of products has been at the core of the Company's vision
since its inception. Furthermore, Avicanna's commitment to
education is demonstrated through its annual medical symposium, the
Avicanna Academy educational platform, and the My Cannabis Clinic
patient program through its subsidiary company.
Avicanna manages its own supply chain including cultivation and
extraction through its two majority-owned subsidiaries, Sativa
Nativa S.A.S. and Santa Marta Golden Hemp S.A.S., both located in
Santa Marta, Colombia. Through these sustainable,
economical, and industrial scale subsidiaries, Avicanna cultivates,
processes, and commercializes a range of cannabis and hemp
cultivars dominant in CBD, CBG, THC, and other cannabinoids for use
as active pharmaceutical ingredients. Avicanna's Avesta Genetica
program specializes in the development and optimization of rare
cultivars for commercial production along with feminized seeds for
global export. In June 2020, Avicanna
made history with a shipment of hemp seeds to the United States of America by completing the
first ever export of hemp seeds from Colombia.
SOURCE Avicanna Inc.
Stay Connected
For more information about Avicanna,
visit www.avicanna.com, call 1-647-243-5283, or contact
Setu Purohit, President by email
at info@avicanna.com.
Cautionary Note Regarding Forward-Looking Information and
Statements
This press release contains certain "forward-looking
information" within the meaning of applicable Canadian securities
legislation (each such statement a "forward-looking
statement"). Such forward-looking statements are not
representative of historical facts or information or current
condition, but instead represent only Avicanna's current beliefs
and assumptions regarding future events, plans or objectives, many
of which, by their nature, are inherently uncertain and
outside of Avicanna's control. Generally, such forward-looking
statements can be identified by the use of forward-looking
terminology such as "plans", "expects" or "does not expect", "is
expected", "budget", "scheduled", "estimates", "forecasts",
"intends", "anticipates" or "does not anticipate", or "believes",
or variations of such words and phrases or may contain statements
that certain actions, events or results "may", "could", "would",
"might" or "will be taken", "will continue", "will occur" or "will
be achieved". The forward-looking information and forward-looking
statements contained herein may include, the Company's
revenues to continue to increase through fiscal 2021, the Company's
anticipated activities and results of its various commercial
initiatives, its availability and sufficiency of working capital,
the anticipated growth of the Company's business in the first
quarter of 2021 and the anticipated further development and
improvement of products by the Company.
By identifying such information and statements in this
manner, Avicanna is alerting the reader that such information and
statements are subject to known and unknown risks, uncertainties
and other factors that may cause the actual results, level of
activity, performance or achievements of Avicanna to be materially
different from those expressed or implied by such information and
statements. In addition, in connection with the forward-looking
statements contained in this press release, Avicanna has made
certain assumptions.
Among others, the key factors that could cause actual results
to differ materially from those projected in the forward-looking
information and statements are the following: decreases in the
prevailing prices for cannabis and cannabis products in the markets
in which the Company operates; adverse changes in applicable laws;
adverse changes in the application or enforcement of current laws,
including those related to taxation; increasing costs of compliance
with extensive government regulation; changes in general economic,
business and political conditions, including changes in the
financial markets ; risks related to licensing, including the
ability to obtain the requisite licenses or renew existing licenses
for the Company's existing and proposed operations; dependence upon
third party service providers, skilled labor and other key inputs;
risks inherent in the agricultural and retail business;
intellectual property risks; risks related to litigation;
dependence upon senior management; and the other risks disclosed in
the Company's long form final prospectus dated July 8, 2019 and the Company's annual information
form dated April 15, 2020. Should one
or more of these risks, uncertainties or other factors materialize,
or should assumptions underlying the forward-looking information or
statements prove incorrect, actual results may vary materially from
those described herein as intended, planned, anticipated, believed,
estimated or expected.
Although Avicanna believes that the assumptions and factors
used in preparing, and the expectations contained in, the
forward-looking statements are reasonable, undue reliance should
not be placed on such statements, and no assurance or guarantee can
be given that such forward-looking statements will prove to be
accurate, as actual results and future events could differ
materially from those anticipated in such statements.
SOURCE Avicanna Inc.