UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN
PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of September 2023.
Commission File Number: 001-39071
ADC Therapeutics SA
(Exact name of registrant as specified in its
charter)
Biopôle
Route de la Corniche 3B
1066 Epalinges
Switzerland
(Address of principal executive office)
Indicate by check mark whether the registrant files
or will file annual reports under cover of Form 20-F or Form 40-F:
INFORMATION CONTAINED IN THIS REPORT ON FORM
6-K
On September 11, 2023, ADC Therapeutics SA (the
“Company”) made available its updated investor presentation that it intends to use at the Morgan Stanley Global Healthcare
Conference and in the future, a copy of which is attached hereto as Exhibit 99.1. In addition, the Company announced that the implementation
of the new commercial model took longer than the Company expected and consequently, the disruption to the organization continued well
into the third quarter of 2023. As a result, the Company is withdrawing its 2023 revenue guidance.
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
ADC Therapeutics SA |
| Date: September 11, 2023 |
|
| |
|
| |
By: |
/s/ Peter J. Graham |
| |
Name: |
Peter J. Graham |
| |
Title: |
Chief Legal Officer |
EXHIBIT INDEX
| Exhibit No. |
Description |
| 99.1 |
Investor Presentation dated September 11, 2023 |
Exhibit 99.1

Morgan Stanley Global Healthcare Conference September 11, 2023

2 Forward - Looking Statements This presentation and any accompanying oral presentation have been prepared by ADC Therapeutics SA ("ADC Therapeutics“, “we” or “us”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or AD C Therapeutics or any officer, director, employee, agent or advisor of ADC Therapeutics. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Information provi ded in this presentation and any accompanying oral presentation speak only as of the date hereof. This presentation contains statements that constitute forward - looking statements within the meaning of the safe harbor provision s of the Private Securities Litigation Reform Act of 1995. Forward - looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described . Factors that may cause such differences include, but are not limited to: the success of the Company’s updated corporate strategy including operating efficiencies, capital allocation and portfolio prioritization; the Company’s ability to achieve a decrease in total operating expenses for 2023 and 2024, the ex pected cash runway into the middle of 2025, the effectiveness of the new commercial go - to - market strategy and the Company’s ability to continue to commercialize ZYNLONTA® in the United States and future revenue from the same; Swedish Orphan Biovitrum AB (Sobi®) ability to successfully commercialize ZYNLONTA® in the European Economic Area and m ark et acceptance, adequate reimbursement coverage, and future revenue from the same; our strategic partners’, including Mitsubishi Tanabe Pharma Corporation and Overland Pharmaceuticals, ability to obtain re gulatory approval for ZYNLONTA® in foreign jurisdictions, and the timing and amount of future revenue and payments to us from such partnerships; the Company’s ability to market its products in compliance with app lic able laws and regulations; the timing and results of the Company’s or its partners’ research projects or clinical trials including LOTIS 5, 7 and 9, ADCT 901, 601, and 602, the timing and outcome of regulatory su bmissions and actions by the FDA or other regulatory agencies with respect to the Company’s products or product candidates; projected revenue and expenses , the Company’s indebtedness including Healthcare Royalty Management and Oak Tree facilities and the restrictions imposed on the Company’s activities by such indebtedness, the ability to repay such indebtedness and the significant cash required to service such indebtedness; the Company’s ability to obtain financial and other resources for its research , development , clinical , and commercial activities and other statements regarding matters that are not historical facts , and involve predictions . These statements involve known and unknown risks , uncertainties and other factors that may cause actual results , performance , achievements or prospects to be materially different from any future results , performance , achievements or prospects expressed in or implied by such forward - looking statements . All statements other than statements of historical facts contained in this presentation, including statements regarding our f utu re catalysts, results of operations and financial position, business and commercialization strategy, market opportunities, revenue, products and product candidates, research pipeline, ongoing and planned preclinical stu dies and clinical trials, regulatory submissions and approvals, research and development costs, timing and likelihood of success, as well as plans and objectives of management for future operations are forward - looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements, or prospects to be materially different from any future res ult s, performance, achievements, or prospects expressed or implied by such forward looking statements. In some cases, you can identify forward looking statements by terminology such as “may”, “will”, “should”, “woul d”, “expect”, “intend”, “plan”, ”anticipate”, “believe”, “estimate”, “predict”, “potential”, “seem”, “seek”, “future”, “continue”, or ”appear” or the negative of these terms or similar expressions, although not all for war d - looking statements contain these words. Forward - looking statements are based on our management’s beliefs and assumptions and on information currently available to our m anagement. Such statements are subject to risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward - looking statements due to various factors, including those described in the “Risk Factor” section of the Company’s annual report on Form 20 - F and in the Company’s other filings with the US Securities and Exchange Commission. No assurance can be given that such future resul ts will be achieved. Investors are cautioned not to place to undue reliance on the forward - looking statements contained in this presentation. Such forward - looking statements contained in this presentation speak only as of the date of this presentation. We expressly disclaim any obligation or undertaking to update these forward - looking statements contained in this presentation to reflect any change in our expectations or any change i n events, conditions, or circumstances on which such statements are based unless required to do so by applicable law. No representations or warranties (expressed or implied) are made about the accuracy of a ny such forward - looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data d eri ved from third - party sources and our own internal estimates and research. While we believe these third - party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this presentation involve a number of assumpt ions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, although we believe our own internal research is reliable, such research has not been verified by any i nde pendent source.

3 About ADC Therapeutics Validated PBD technology platform and growing next generation ‘toolbox’ Fully - integrated organization with specialized capabilities unique to ADCs Cash runway to mid - 2025 with multiple catalysts over next 12 - 15 months Pioneer in ADC field with approved product and multiple clinical - stage programs

4 Three Core Pillars to Unlock Value ADC: Antibody Drug Conjugate; DLBCL: Diffuse Large B - Cell Lymphoma; PBD: Pyrrolobenzodiazepine. Short - Mid Term Mid - long Term Maximizing the ZYNLONTA Opportunity Advancing the PBD - Based Pipeline Broadening the ADC Platform and Leadership ▪ Establish ZYNLONTA ® as 3L+ DLBCL standard of care ▪ E xpand treatable patient population in earlier lines as combination agent of choice ▪ Commercialize in U.S. and collaborate with strategic partners internationally ▪ Focus on 3 programs ADCT - 601 (AXL) ADCT - 901 (KAAG1) ADCT - 602 (CD22) ▪ Expand platform with new antibody constructs and payloads ▪ Advance differentiated next - generation assets 1 2 3

5 ▪ Strong balance sheet with $348M cash at end of 2Q 2023, including $75M payment from HCR ▪ Continue to prioritize nearer - term clinical catalysts and drive organizational efficiencies ▪ Cash runway expected to extend into mid - 2025 ▪ LOTIS - 5 updated data from safety run - in at SOHO 2023 showed no new safety signals and increased CR rate from 40% to 50% ▪ LOTIS - 7 has patients on trial and is actively enrolling in combination with bispecifics; trial design highlighted at SOHO 2023 ▪ Earlier - stage pipeline progressing – ADCT - 601 targeting AXL, ADCT - 901 targeting KAAG1 and ADCT - 602 targeting CD22 Recent Highlights (1) Submitted by Overland ADCT, seeking an indication for relapsed or refractory diffuse large B - cell lymphoma (DLBCL) after two or more lines of systemic therapy ; (2) by partner Mit subishi Tanabe Pharma Corporation; (3) on a non - IFRS basis or 32% on an IFRS measure including stock - based compensation; HCR: HealthCare Royalty; BLA: Biologics License Application; NMPA: China National Medical Products Administration ▪ New go - to - market model implemented to support growth ▪ Partner Sobi completed the first EU commercial sale of ZYNLONTA in May ▪ China BLA 1 for ZYNLONTA accepted for filing and granted priority review by NMPA; Phase 1/2 bridging study initiated 2 in Japan ZYNLONTA ® (loncastuximab tesirine - lpyl) Pipeline Corporate

6 Deep Pipeline in Hematology and Solid Tumors Anticipated milestones set forth in this chart are subject to further future adjustment based on, among other factors, the im pac t of the COVID - 19 pandemic. NTE: Non - Transplant Eligible. The Company is exploring partnerships for Camidanlumab tesirine (Cami) targeting CD25 for the treatment of r/r Hodgkin lympho ma. Preclinical Phase 1a Phase 1b Phase 2 ZYNLONTA ® | Targeting CD19 ADCT - 602 | Targeting CD22 Expected Milestones LOTIS - 5: Complete enrollment in 2024 LOTIS - 7: Initial data in 2024 Initial data from Phase 1 study in 1H 2024 PBD - Based Phase 3 / Confirmatory* ZYNLONTA Confirmatory LOTIS - 5 with rituximab in 2L NTE DLBCL LOTIS - 2 in 3L+ patients with r/r DLBCL Multiple programs New clinical candidates FDA approved LOTIS - 7 in non - Hodgkin Lymphoma Expanded platform Various Solid Tumors ADCT - 601 | Targeting AXL Initial data from Phase 1 study in 1H 2024 Acute Lymphoblastic Leukemia Additional data from Phase 1 study in 1H 2024 Various Solid Tumors ADCT - 901 | Targeting KAAG1

7 Three Horizons to Grow ZYNLONTA to $500M - $1B in Potential Peak Sales 1. Cerner Enviza's CancerMPact – Epi Database, Patient Metrics & Treatment Architecture, NH Lymphoma (2022) 2 . Clarivate's Disease Market Landscape & Forecast, Non - Hodgkin's Lymphoma and Chronic Lymphocytic Leukemia, Epidemiology (2022) 3. Francesco Merli, et. Al., Simplified Geriatric Assessment in Older Patients With Diffuse Large B - Cell Lymphoma: The Prospecti ve Elderly Project of the Fondazione Italiana Linfomi Journal of Clinical Oncology 39, no. 11(2021), https://ascopubs.org/doi/fu ll/10.1200/JCO.20.02465. >11,000 patients total in the U.S.: ~5,000 2L SCT ineligible patients 1,2 + >6,000 3L+ patients >6,000 patients in the U.S. 1,2 > 18,000 (2L and 3L+ patients in the U.S.) 1,2 3L/3L+ monotherapy Approved Horizon 2 2L in chemo - free combination with rituximab in development Horizon 3 Early - line novel combinations In development Horizon 1 ▪ High unmet medical need remains ▪ ZYNLONTA positioned for growth in academic and community settings ▪ Community offers greatest potential due to limited CAR - T use ▪ ZYNLONTA + rituximab chemo - free combination being studied in 2L SCT ineligible ▪ Rituximab - based combinations most commonly - used regimen ▪ ZYNLONTA + bispecific antibodies in development ▪ Potential for ZYNLONTA to add efficacy without additional toxicity

8 ZYNLONTA: Well - positioned for long - term growth ▪ Deep and durable single - agent efficacy ▪ Fast time to response (median 41 days 1 ) ▪ Manageable safety profile with no CRS ▪ Simple dosing, no REMS or inpatient stay ▪ Data from EHA and ICML: at 2 - year follow - up, median DoR not reached for patients in CR Product profile ideally suited across treatment settings ▪ Commercial organization covers 90%+ of approved DLBCL market opportunity ▪ Aligned to local healthcare ecosystems to drive synergies between academic/ community ▪ Focused on establishing ZYNLONTA as standard of care in 3L+ DLBCL Optimal go - to - market model implemented 1. Based on pivotal LOTIS - 2 trial. Full prescribing information available at www.ZYNLONTA.com , including warnings and precautions. CR: Complete Response; CRS: Cytokine Release Syndrome; DoR : Duration of response; REMS: Risk Evaluation and Mitigation Strategies; EHA: European Hematology Association; ICML: International Conference on Malignant Lymphoma

9 LOTIS - 5: Developing ZYNLONTA to be the Combination Agent of Choice in Earlier Lines of Therapy DLBCL: Diffuse Large B - Cell Lymphoma; ASCT: Autologous Stem - Cell Transplant; ORR: Overall Response Rate; CR: Complete Response; SOHO: Society of Hematologic Oncology; IDMC: Independent Data Monitoring Committee 2L DLBCL ASCT ineligible Phase 3 confirmatory trial in combination with rituximab Enrollment ongoing in randomized portion of the trial, target of 350 patients Updated safety lead - in results at SOHO 2023: ORR of 80%, CR of 50% with no new safety signals Enrollment completion expected in 2024 IDMC in late July: No notable safety signals and recommended continuation of trial as planned Patient population Study summary Status Support Next milestone Other notes

10 LOTIS - 5 Trial Design Phase 3 trial of loncastuximab tesirine in combination with rituximab 1. Defined as time between randomization and the first documentation of recurrence or progression, or death from any cause. Treatment Period Follow - Up Period Non - randomized Safety Run - in Target N=20 Loncastuximab tesirine 150 ߤ g/kg + rituximab 375 mg/m 2 Q3W for 2 cycles Loncastuximab tesirine 75 ߤ g/kg + rituximab 375 mg/m 2 Q3W for up to 6 additional cycles For both parts of the study, irrespective of disease status, patients will be followed for up to 4 years after EOT until withdrawal of consent, loss to follow - up, or death — whichever occurs first Lonca (150 ߤ g/kg) + rituximab (375 mg/m 2 ) Q3W for 2 cycles Lonca (75 ߤ g/kg) + rituximab (375 mg/m 2 ) Q3W for up to 6 additional cycles R - GemOx : rituximab 375 mg/m 2 + gemcitabine 1000 mg/m 2 + oxaliplatin 100 mg/m 2 Q2W for up to 8 cycles End of Treatment Randomized 1:1 Target N=330 KEY INCLUSION/EXCLUSTION CRITERIA ▪ Adults with a pathologic diagnosis of R/R DLBCL (Including DLBCL transformed from indolent lymphoma or HGBCL, with MYC and BCL2 and/or BCL6 rearrangements) ▪ R/R disease following ≥1 multi - agent systemic treatment regiment ▪ Not a candidate for SCT based on performance status, advanced age, and/or significant medical comorbidities (as considered by the investigator) ▪ If patient had received previous CD19 directed therapy, biopsy proven CD19 expression required ▪ ECOG performance status of 0 - 2 ▪ Excludes previous treatment with Lonca or R - GemOx PRIMARY ENDPOINTS ▪ PFS 1 by independent central review KEY SECONDARY ENDPOINTS ▪ OS, ORR, CRR, DoR

11 Updated Safety Run - In Results of LOTIS - 5 1 1. Kwiatek M, et al. Updated results of the safety run - in of the Phase 3 LOTIS - 5 trial: Novel combination of loncastuximab tesirine with r ituximab (Lonca - R) versus immunochemotherapy in patients with R/R DLBCL. Poster presented at the Tenth Annual Meeting of the Society of Hematologic Oncology (SOHO 2023). Patient population (n=20) ▪ Patients median age 74.5 years (range 35 - 93) and received median of 1 previous therapy (range 1 - 7) ▪ Median number of doses administered was 5 (range 1 - 8), and median duration of follow - up was 10.8 months (range 1.9 - 21.9) Safety ▪ No new safety signals identified ▪ 11 (55%) patients had grade ≥3 treatment - emergent adverse events (TEAEs) ▪ Most common were increased gamma - glutamyltransferase (5 patients [25%]) and neutropenia (3 patients [15%]). April 10, 2023 data cutoff February 28, 2022 data cutoff Overall response rate 80% 75% Complete response 50% 40% Partial response 30% 35% Median duration of response 8.0 months N/A Median progression - free survival 8.3 months N/A Efficacy

12 LOTIS - 7: Potential to Change the Lymphoma Treatment Paradigm Relapsed/refractory B - cell Non - Hodgkin Lymphoma Phase 1 trial of ZYNLONTA in combination with glofitamab, mosunetuzumab Actively enrolling patients in combination with bispecifics Malignant B - cells show broad/consistent expression of CD20 (targeted by Roche bispecifics) and CD19 (targeted by ZYNLONTA) Initial data expected in 2024 Patient population Study summary Status Support Next milestone

13 LOTIS - 7 Trial Design Phase 1b trial of Lonca in combination with other anticancer agents in R/R B - NHL Treatment Period (cycles of 21 days) R/R DLBCL, HGBCL, FL, MZL For arm C only: MCL and BL Lonca IV Q3W at escalating doses (from 90 µg/kg to 150 µg/kg) + Glofitamab (2.5 mg on C1D8, 10 mg on C1D15, and 10 or 30 mg for C2 - 12 D1) Lonca IV Q3W at escalating doses (from 90 µg/kg to 150 µg/kg) + Polatuzumab vedotin (1.8 mg/kg on D1 of each 21 - day cycle) End of Treatment Follow - up period for ≤2 years from EOT. Participants may continue treatment for up to one year or until disease progression, unacceptable toxicity, or other discontinuation criteria, whichever occurs first. Estimated enrollment, N=200 Part 1 3+3 Dose escalation Part 2 Dose expansion Screening Period (≤28 d) PRIMARY ENDPOINTS ▪ Safety and tolerability of loncastuximab in combination with polatuzumab vedotin , glofitamab or mosunetuzumab ▪ MTD and/or RDE for the combination of agents (dose - escalation, Part 1) SECONDARY ENDPOINTS ▪ Efficacy: ORR, DOR, CRR, PFS, RFS, OS ▪ Pharmacokinetics ▪ Immunogenicity Lonca IV Q3W at escalating doses (from 90 µg/kg to 150 µg/kg) + Mosunetuzumab (5 mg on C1D1, 15 r 45 mg on C1D8, and 45 mg for C1D15 and C2 - 8 D1) Arm C Arm E Arm F KEY INCLUSION/EXCLUSTION CRITERIA ▪ Adult patients with pathologic diagnosis or R/R disease B - NHL (2016 WHO classification) who have failed or been intolerant to any approved therapy and have received ≥2 systemic treatment regiments ▪ ECOG performance status of 0 to 2 ▪ Measurable disease as defined by the 2014 Lugano Classification ▪ Excludes patients with clinically significant third space fluid accumulation ▪ Excludes previous treatment with study drugs (applied to relevant arm and/or cohort of the specific drug administered)

14 ADCT - 601 targeting AXL Differentiation ▪ AXL thought to be overexpressed in various solid tumors ▪ In pancreatic PDX model, ADCT - 601 showed superior activity vs. Genmab’s ADC at same dose ▪ Sensitivity to ADCT - 601 increased in TKI resistant EGFR mutant NSCLC cell lines, confirming role of AXL upregulation in EGFR TKI resistance Humanized anti - AXL Antibody – Site Specific Conjugation – PL1601: Potential best - in - class ADC against a target expressed across multiple solid tumor segments of significant unmet medical need Status/ Next Steps ▪ Phase 1b study: Initial focus on single - agent activity with expansion potential to combinations ▪ IHC assay being finalized ▪ Initial data from Phase 1 study expected in 1H 2024

15 ADCT - 601 targeting AXL AXL POTENTIAL INDICATIONS AXL is a recently characterized target for ADC development with significant malignant expression and efficient internalization. ADCT - 601 enables a best - in - class approach to this novel target. Zammarachi , F. Mol Cancer Ther 21(4) April 2022.. 1. Seer 2022 – Survival Statistics; Cancer.org – Survival Rates. 2. Metastatic calculations include both new ly incident metastatic cases and recurrent metastatic cases in 2022. 3. Newly incident metastatic cases sourced from Seer 202 2; Recurrent Metastatic cases calculated from DRG Clarivate Epidemiology Reports, Cerner Enviza's CancerMPact – Epi Database, Patient Metrics & Treatment Architecture (2022), and Seer 2022. 4. Sarcoma includes select STS subtypes Synovial Sarcoma, Leiomyosarcoma, Und if ferentiated Pleomorphic Sarcoma, Liposarcoma and Osteosarcoma. Subtype frequency sourced from Zhang. International Journal of Immunopathology and Pharmacology . 2 012; Dantas - Barbosa. BJC. 2017; SEER 2022. 5. Number reflects advanced stage and early - stage high risk cases. RCC: Renal Cell Ca rcinoma; NSCLC: Non - small cell lung cancer. ADCT - 601 showed superior activity compared to the MMAE - based HuMax - AXL ADC (Genmab) when used at the same dose (0.3 mg/kg, qdx1) 0 5 10 15 20 25 30 35 40 45 0 500 1000 1500 2000 2500 Days M e a n T u m o r V o l u m e ( m m 3 ) ± S E M Vehicle, qd x 1 ADCT-601, 0.075 mg/kg, qdx1 ADCT-601, 0.15 mg/kg, qdx1 ADCT-601, 0.3 mg/kg, qdx1 HuMax-AXL-ADC, 0.3 mg/kg, qdx1 HuMax-AXL-ADC, 4 mg/kg, qdx1 5 - year OS 1 ~20 K RCC 20% 0% 40% 150 50 ~110 K NSCLC ~42 K Pancreatic ~19 K Ovarian 5 100 Sarcoma 4 , ~3 K Pre - clinical Data ADCT - 601 Monotherapy (Pancreatic) Overview U.S. Metastatic Cases, 2022 2,3 (Drug - treatable) 0

16 ADCT - 601 Trial Design Focus on NSCLC and sarcoma with potential to expand into other tumor types 13 mg RDE Dose expansion by tumor types with optimization based on patient selection 15 mg Continued d ose escalation PRIMARY ENDPOINTS ▪ Safety and tolerability SECONDARY ENDPOINTS ▪ Overall Response Rate (ORR) ▪ Duration of Response (DOR) ▪ Progression - Free Survival (PFS) ▪ Overall Survival (OS) KEY INCLUSION/EXCLUSTION CRITERIA ▪ Pathologic diagnosis of solid tumor malignancy that is locally advanced or metastatic at time of screening ▪ Participants who are refractory to or intolerant to available standard therapies known to provide clinical benefit for their condition per Investigator judgment ▪ Excludes patients with recent infection requiring intravenous (IV) antibiotics, IV antiviral, or IV antifungal treatment within 4 weeks of Cycle 1 Day 1, symptomatic central nervous system metastases or evidence of leptomeningeal disease, clinically significant third space fluid accumulation 11 mg 7.5 mg RDE: Recommended dose for expansion Potential to expand in combination after expansion

17 ADCT - 901 targeting KAAG1 1. Van der Eynde. J Exp Med. 1999. Differentiation ▪ KAAG1 an attractive ADC target with over - expression in various solid tumors 1 ▪ ADCT - 901 showed robust in vivo efficacy and improved survival in KAAG1 (+) TNBC, OC, and RCC models ▪ Potential to combine with gemcitabine, o laparib and VEGF inhibition demonstrated by synergy and/or improved survival in preclinical in vivo models Status/ Next Steps ▪ Finalizing protocol amendment to explore different dosing schedules ▪ Finalizing IHC assay ▪ Initial data from Phase 1 study expected in 1H 2024 Humanized anti - KAAG1 Antibody – Stochastic Conjugation – Tesirine: Potential first - in - class ADC against a novel target expressed across multiple solid tumor segments of significant unmet medical need

18 ADCT - 602 targeting CD22 1. Data on file. 2. Aldoss . BCJ. 2021; Cerner Enviza's CancerMPact – Epi Database, Patient Metrics & Treatment Architecture, ALL (2022) ; Seer 2022 – Acut e Lymphocytic Leukemia; Physician Interviews. Differentiation ▪ ADCT - 602 a modified version of anti - CD22 monoclonal antibody, epratuzumab ▪ ADCT - 602 showed r obust efficacy at low concentration in xenograft in vivo models and outperformed Genentech’s ADC against CD22 1 ▪ Notably, ADCT - 602 shows no indication of VOD at any dose studied to date Status/ Next Steps ▪ Ph 1 in collaboration with MD Anderson ▪ Data at ASH 2022: - 4 patients achie ved MRD( - ) remission - 1 additional patient had marrow blast clearance without count recovery ▪ Continue expanding at current dosing level as well as dose escalating ▪ New sites to enhance enrollment ▪ Additional data from Phase 1 study expected in 1H 2024 Opportunity ▪ ~7,000 incident ALL patients in the U.S., of which >600 adult B - ALL patients start new therapy in 3L+ 1 - CD22 expressed in >90% of B - cell ALL 2 ▪ Incremental areas of potential application in NHL, CLL Humanized anti - CD22 Antibody – Site Specific Conjugation – Tesirine: Optimized CD22 targeted ADC enabling utilization across multiple ALL segments of unmet need without veno - occlusive disease (VOD) complication

19 ADCT Proven Track Record 3 clinical POC+ assets Success with PBD payload ADCT Foundation P urpose - built organization with specialized capabilities unique to ADCs 3 2 1 1) Discovery | Intelligent choice of targeting moiety + linker + payload permutation 3) CMC | High quality, consistent and scalable drug manufacturing for complex, highly potent molecules 2) Early Development | Efficiently m ove from research to early clinic with optimized therapeutic window ADCT Technology Platform D iverse toolbox of complementary technologies to address challenges of each target • Conditional Binding • Bispecifics • Biparatopics • Cleavable linkers • Reducible linkers • Non - cleavable linkers • pH sensitive linkers • Site - specific conjugation • DNA alkylating: Camptothecins, next gen PBD • Immune agonists • Dual payloads • Others not disclosed Linker & Conjugation Targeting Moiety Payload Comprehensive Toolbox to Unlock ADC Opportunity Validated and integrated capabilities

20 Multiple Value - Driving Catalysts Expected in 2023/4 2H 2023 2024 2H 2023 □ ZYNLONTA: European launch by partner Sobi in 2Q 2023 □ ZYNLONTA: Updated safety lead - in data from LOTIS - 5 in 2H 2023 1H 2024 □ ADCT - 601 (AXL): Initial data from Phase 1 study □ ADCT - 901 (KAAG1): Initial data from Phase 1 study □ ADCT - 602 (CD22): Additional data from Phase 1 study 2024 □ Complete enrollment of LOTIS - 5 study □ Initial data from novel bispecific combinations from LOTIS - 7 study ▪ Cash runway to mid - 2025 to support company through value - generating milestones ▪ Ongoing conversations regarding potential partnerships and licensing ▪ Advancing next - generation preclinical programs

Thank you
ADC Therapeutics (NYSE:ADCT)
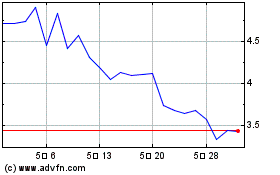
과거 데이터 주식 차트
부터 4월(4) 2024 으로 5월(5) 2024
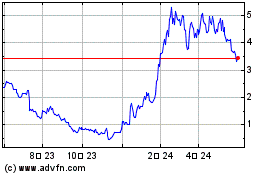
ADC Therapeutics (NYSE:ADCT)
과거 데이터 주식 차트
부터 5월(5) 2023 으로 5월(5) 2024