Registration No. 333-276367
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
PANBELA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
2834
(Primary Standard Industrial
Classification Code Number)
|
88-2805017
(I.R.S. Employer
Identification No.)
|
712 Vista Blvd, Suite 305
Waconia, Minnesota 55387
(952) 479-1196
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Jennifer K. Simpson
Chief Executive Officer
712 Vista Blvd, Suite 305
Waconia, Minnesota 55387
(952) 479-1196
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| |
W. Morgan Burns
Joshua L. Colburn
W. Jason Deppen
Faegre Drinker Biddle & Reath LLP
90 South Seventh Street
2200 Wells Fargo Center
Minneapolis, Minnesota 55402-3901
(612) 766-7000
|
|
M. Ali Panjwani
Pryor Cashman LLP
7 Times Square
New York, New York 10036
(212) 421-4100
|
|
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. ☑
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
Non-accelerated filer ☑
|
Smaller reporting company ☑
|
| |
Emerging growth company ☐
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell securities, and we are not soliciting offers to buy these securities, in any state where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT TO COMPLETION
|
DATED JANUARY 25, 2024 |
Up to 8,000,000 Shares of Common Stock
Up to 8,000,000 Class E Common Warrants to purchase up to 2,660,000 Shares of Common Stock
Up to 8,000,000 Class F Common Warrants to purchase up to 14,000,000 Shares of Common Stock
Up to 8,000,000 Pre-Funded Warrants to purchase up to 8,000,000 Shares of Common Stock
Up to 24,660,000 Shares of Common Stock Underlying Warrants

This is a best efforts public offering of (a) up to 8,000,000 shares of our common stock, $0.001 par value per share, (b) up to 8,000,000 Class E Common Warrants to purchase up to 2,660,000 shares of our common stock (the “Class E Warrants”), and (c) up to 8,000,000 Class F Common Warrants to purchase up to 14,000,000 shares of our common stock (the “Class F Warrants” and, together with the Class E Warrants, the “common warrants”) at an assumed combined public offering price of $3.77 per share of common stock and accompanying warrants (equal to the last sale price of our common stock as reported by the Nasdaq Capital Market on January 23, 2024). Each share of our common stock is being sold together with a Class E Warrant to purchase 0.25 shares of our common stock and a Class F Warrant to purchase 1.75 shares of our common stock. Each common warrant is assumed to have an exercise price of $3.77 per share (100% of the public offering price per share and common warrants), will be exercisable upon issuance, and will expire five years from the date of issuance. After five business days, holders of Class E Warrants will have a separate option to elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal 1.33 times the number of shares of common stock that would be issuable upon a cash exercise.
We are also offering to those purchasers, if any, whose purchase of common stock in this offering would otherwise result in any such purchaser, together with its affiliates, beneficially owning more than 4.99% (or, at the election of such purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase pre-funded warrants in lieu of shares of our common stock that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of such purchaser, 9.99%) of our outstanding common stock. The purchase price for each pre-funded warrant will equal the per share public offering price for the common stock in this offering less the $0.001 per share exercise price of each such pre-funded warrant. Each pre-funded warrant will be exercisable upon issuance and will not expire prior to exercise. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis.
For purposes of clarity, each share of common stock or pre-funded warrant to purchase one share of common stock is being sold together with a Class E Warrant to purchase one share of common stock and a Class F Warrant to purchase one share of common stock.
Our common stock is listed on the Nasdaq Capital Market under the symbol “PBLA.” On January 23, 2024, the last reported sale price of our common stock on the Nasdaq Capital Market was $3.77 per share. The combined public offering prices per share and accompanying warrant or per pre-funded warrant and accompanying warrant will be determined between us and investors based on market conditions at the time of pricing and may be at a discount to the current market price of our common stock. Therefore, the recent market price and resulting assumed price used throughout this prospectus may differ substantially from the actual offering price. None of the common warrants or pre-funded warrants are listed on a national securities exchange. We do not intend to apply to list the common warrants or pre-funded warrants on any national securities exchange. Without an active trading market, the liquidity of the common warrants and pre-funded warrants may be limited.
Effective January 18, 2024, we effected a 1-for-20 reverse stock split of our outstanding shares of common stock. Unless specifically provided herein, the share and per share information that follows in this prospectus, other than in the historical financial statements and related notes included elsewhere in this prospectus, assumes the effect of the reverse stock split.
We have requested and been granted a hearing to appeal the staff determination letter we received from The Nasdaq Stock Market, LLC (“Nasdaq”) on November 28, 2023, which letter communicated that Nasdaq would suspend trading in our common stock and file a Form 25-NSE with the Securities and Exchange Commission, which would remove our common stock from listing and registration on Nasdaq, unless we appealed its delisting determination by requesting a hearing before the Nasdaq Hearings Panel. The suspension of trading and delisting of the company’s common stock has been stayed pending the conclusion of the hearing process. Consequently, the company’s common stock is expected to remain listed on the Nasdaq Capital Market at least until the panel renders a decision following the hearing. There can be no assurance that the panel will grant the company’s appeal for continued listing on The Nasdaq Capital Market. The determination letter communicated that our company (i) has not maintained a minimum closing bid price of $1.00 per share as required by Nasdaq Listing Rule 5550(a)(2) (the “Minimum Bid Price Requirement”) and (ii) was no longer in compliance with the minimum stockholders’ equity requirement for continued listing as required by Nasdaq Listing Rule 5550(b) (the “Minimum Stockholders’ Equity Requirement”). Effective January 18, 2024, we completed a 1-for-20 reverse stock split of our outstanding shares of common stock. The primary reason we effected a reverse stock split is to potentially increase the per share market price of our common stock to satisfy the Minimum Bid Price Requirement. On January 22, 2024, we received notice from Nasdaq indicating that, as of effective date of the reverse stock split, we were no longer in compliance with Nasdaq Listing Rule 5550(a)(4) (the “Minimum Float Requirement”), which requires a minimum of 500,000 publicly held shares, which serves as an additional basis for delisting the Company’s common stock. Additionally, it is our intention that the issuance of additional shares and net proceeds from this public offering will allow us to regain compliance with the Minimum Float Requirement and the Minimum Stockholders’ Equity Requirement. We believe that if we are unable to regain compliance with all applicable Nasdaq continued listing requirements, it is likely that our common stock will be delisted from the Nasdaq Capital Market.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 8 of this prospectus, and under similar headings in any amendments or supplements to this prospectus, including our most recent annual report on Form 10-K and any similar section contained in any documents that are incorporated by reference into this prospectus.
This offering will terminate on February 15, 2024, unless completed sooner or unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date, except that the shares of Common Stock underlying the Class E Warrants, Class F Warrants, and the pre-funded warrants will be offered on a continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended. We will deliver all securities to be issued in connection with this offering delivery versus payment upon receipt of investor funds received by us. Accordingly, there is no arrangement to receive or place investor funds in an escrow, trust or any similar account.
We have engaged Roth Capital Partners, LLC as our exclusive placement agent (“Roth” or the “placement agent”) to use its reasonable best efforts to solicit offers to purchase our securities in this offering. The placement agent has no obligation to purchase any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. Because there is no minimum offering amount required as a condition to closing in this offering the actual public offering amount, placement agent’s fee, and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth above and throughout this prospectus. We have agreed to pay the placement agent the placement agent fees set forth in the table below and to provide certain other compensation to the placement agent. See “Plan of Distribution” beginning on page 27 of this prospectus for more information regarding these arrangements.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| |
|
Per Share and
Common
Warrant
|
|
|
Per Pre-Funded
Warrant and
Common
Warrant
|
|
|
Total
|
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Placement Agent fees
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Net proceeds to us, before expenses(1)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
(1)
|
The above summary of offering proceeds does not give effect to any proceeds from the exercise of the common warrants or pre-funded warrants being issued in this offering.
|
Delivery of the shares of our common stock and pre-funded warrants to certain of the investors, together with accompanying common warrants, is expected to be made on or about , 2024, subject to customary closing conditions.
Roth Capital Partners
The date of this prospectus is , 2024
TABLE OF CONTENTS
| |
Page
|
|
ABOUT THIS PROSPECTUS
|
ii
|
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
iii
|
|
PROSPECTUS SUMMARY
|
1
|
|
THE OFFERING
|
8
|
|
RISK FACTORS
|
10
|
|
USE OF PROCEEDS
|
22
|
|
MARKET INFORMATION
|
23 |
|
CAPITALIZATION
|
23
|
|
DILUTION
|
24
|
|
DIVIDEND POLICY
|
25 |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
25 |
|
BUSINESS
|
36 |
|
MANAGEMENT
|
65 |
|
EXECUTIVE COMPENSATION
|
69 |
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
71 |
|
DESCRIPTION OF SECURITIES
|
72 |
|
PLAN OF DISTRIBUTION
|
78 |
|
LEGAL MATTERS
|
83 |
|
EXPERTS
|
83 |
|
WHERE YOU CAN FIND MORE INFORMATION
|
84 |
|
FINANCIAL STATEMENTS
|
F-1
|
ABOUT THIS PROSPECTUS
We incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus as well as additional information described under “Information Incorporated by Reference,” before deciding to invest in our securities.
You should rely only on the information contained in this prospectus. We have not, and the placement agent has not, authorized anyone to provide you with any information other than that contained in this prospectus. We take no responsibility for and can provide no assurance as to the reliability of any other information that others may give you. This prospectus may only be used where it is legal to offer and sell our securities. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date. We are not, and the placement agent is not, making an offer of these securities in any jurisdiction where the offer is not permitted.
For investors outside the United States: We have not and the placement agent has not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States must inform themselves about, and observe any restrictions relating to, the offering of securities and the distribution of this prospectus outside the United States.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We believe that the data obtained from these industry publications and third-party research, surveys and studies are reliable. We are ultimately responsible for all disclosure included in this prospectus.
You should rely only on the information contained in this prospectus, as supplemented and amended. We have not authorized anyone to provide you with information that is different. This prospectus may only be used where it is legal to sell these securities. The information in this prospectus may only be accurate on the date of this prospectus.
We urge you to read carefully this prospectus, as supplemented and amended, before deciding whether to invest in any of the securities being offered.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements contained in this prospectus other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, projected costs and our objectives for future operations, are forward-looking statements.
In some cases, you can identify forward-looking statements by the following words: “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. Forward-looking statements are not a guarantee of future performance or results and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. Forward-looking statements are based on information available at the time the statements are made and involve known and unknown risks, uncertainties and other factors that may cause our results, levels of activity, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements in this prospectus. These factors include:
| |
●
|
our lack of diversification and the corresponding risk of an investment in our company;
|
| |
●
|
potential deterioration of our financial condition and results due to failure to diversify;
|
| |
●
|
our ability to successfully complete acquisitions;
|
| |
●
|
our ability to integrate acquired companies and operations for new product candidates;
|
| |
●
|
our ability to obtain additional capital, on acceptable terms or at all, required to implement our business plan;
|
| |
●
|
final results of our Phase I clinical trial;
|
| |
●
|
progress and success of our randomized Phase II/III clinical trial;
|
| |
●
|
our ability to demonstrate safety and effectiveness of our product candidate;
|
| |
●
|
our ability to obtain regulatory approvals for our product candidate in the United States, the European Union, or other international markets;
|
| |
●
|
the market acceptance and future sales of our product candidate;
|
| |
●
|
the cost and delays in product development that may result from changes in regulatory oversight applicable to our product candidate;
|
| |
●
|
the rate of progress in establishing reimbursement arrangements with third-party payors;
|
| |
●
|
the effect of competing technological and market developments;
|
| |
●
|
the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims;
|
| |
●
|
our ability to maintain the listing of our common stock on a national securities exchange; and
|
| |
●
|
other risk factors included under the caption “Risk Factors” starting on page 8 of this prospectus.
|
You should read the matters described in “Risk Factors” and the other cautionary statements made in this prospectus as being applicable to all related forward-looking statements wherever they appear in this prospectus. We cannot assure you that the forward-looking statements in this prospectus will prove to be accurate and therefore you are encouraged not to place undue reliance on forward-looking statements. You should read this prospectus completely. Other than as required by law, we undertake no obligation to update or revise these forward-looking statements, even though our situation may change in the future.
We caution readers not to place undue reliance on any forward-looking statement that speaks only as of the date made and to recognize that forward-looking statements are predictions of future results, which may not occur as anticipated. Actual results could differ materially from those anticipated in the forward-looking statements and from historical results due to the risks and uncertainties described under the heading “Risk Factors” in this prospectus, as well as others that we may consider immaterial or do not anticipate at this time. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. Our expectations reflected in our forward-looking statements can be affected by inaccurate assumptions that we might make or by known or unknown risks and uncertainties, including those described under the heading “Risk Factors” in this prospectus. The risks and uncertainties described under the heading “Risk Factors” in this prospectus are not exclusive and further information concerning us and our business, including factors that potentially could materially affect our financial results or condition, may emerge from time to time. We assume no obligation to update forward-looking statements to reflect actual results or changes in factors or assumptions affecting such forward-looking statements. We advise stockholders and investors to consult any further disclosures we may make on related subjects in our subsequent annual reports on Form 10-K, quarterly reports on Form 10-Q, and current reports on Form 8-K that we file with or furnish to the U.S. Securities and Exchange Commission (the “SEC”).
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus, including our financial statements and the related notes and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in each case included elsewhere in this prospectus. Unless otherwise stated or the context requires otherwise, references in this prospectus to “Panbela,” the “Company,” “we,” “us,” “our” and similar references refer to Panbela Therapeutics, Inc. and its subsidiaries.
Business Overview
Panbela is a clinical stage biopharmaceutical company developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs. We are currently enrolling patients in our randomized double-blind placebo controlled clinical trial for the treatment of pancreatic cancer and we are a regulatory and commercial collaborator in a Phase III clinical trial funded by the National Cancer Institute (the “NCI”) for the study of colon cancer risk reduction and colon adenoma therapy (“CAT”), a preventative treatment approach for survivors of colorectal cancer or those who have high-risk colon polyps. In addition, the Company is designing a global protocol for a Phase III registration trial for familial adenomatous polyposis (“FAP”), a rare inherited condition that can cause the growth of thousands of colorectal adenomas (i.e., adenomatous polyps), which are recognized as a key risk factor for colon cancer. The global protocol will be submitted to the Federal Drug Administration (FDA) and European Medicines Agency (EMA) for agreement on the registration pathway. By leveraging Panbela’s extensive experience with FAP and in designing global registration trials, the team can develop a high-quality trial protocol that meets the standards of regulatory agencies and is designed to demonstrate the potential safety and efficacy of Flynpovi ™ efficiently and effectively in the treatment of FAP. We also support several investigator initiated trials and company sponsored preclinical trials including: (1) Phase II clinical trial for the treatment of early-onset type 1 diabetes funded by the Juvenile Diabetes Research Foundation; (2) Phase II clinical trial for treatment of gastric cancer funded by the NCI; (3) Phase I/II clinical trial for the treatment of non-small cell lung cancer (NSCLC) possessing the STK11 mutation; (4) Phase II program for the treatment of Metastatic Castration-Resistant Prostate Cancer; and (5) preclinical studies that we have sponsored in the orphan disease and cancer fields.
The Company’s lead assets are ivospemin (SBP-101), FlynpoviTM (eflornithine (CPP-1X) and sulindac), and eflornithine (CPP-1X) which provides a multi-targeted approach to reset dysregulated biology present in many types of diseases such as cancer and autoimmune disorders. Many tumors require greatly elevated levels of polyamines to support their growth and survival. These agents target the polyamine pathway at complementary junctions, which have been shown to be altered in disease. In particular, our lead assets have the potential to suppress and prevent tumor growth, enhance anti-tumor activity of other anti-cancer agents, and modulate the immune system.
Ivospemin is a proprietary polyamine analogue designed to induce polyamine metabolic inhibition. Ivospemin has demonstrated encouraging activity against metastatic disease in a clinical trial of patients with pancreatic cancer. The efficacy and safety results demonstrated in our completed Phase I clinical trial of ivospemin in combination with gemcitabine and nab-paclitaxel in the first line treatment of metastatic pancreatic cancer provides support for the current randomized, double-blind, placebo-controlled study of ivospemin in combination with gemcitabine and nab-paclitaxel in patients previously untreated for metastatic pancreatic cancer. We believe that ivospemin, if successfully developed, may represent a novel approach that effectively treats patients with pancreatic cancer and could become a dominant product in that market. Only three first-line treatment combinations, a single maintenance treatment for a subset (3-7%) of patients, and one second-line drug have been approved by the U.S. Food and Drug Administration (“FDA”) for pancreatic cancer in the last 25 years. Ivospemin has received Fast Track status and orphan drug designation status for pancreatic cancer in the United States as well as Europe.
On June 15, 2022 Panbela acquired Cancer Prevention Pharmaceuticals, Inc. (“CPP”), which added the Company’s second lead asset, eflornithine in multiple forms. First, an investigational new drug product, Flynpovi is a combination of the polyamine synthesis inhibitor eflornithine and the non-steroidal anti-inflammatory drug sulindac and then eflornithine as a single agent. Eflornithine is an enzyme-activated, irreversible inhibitor of the enzyme ornithine decarboxylase, the first rate-limiting enzyme in the biosynthesis of polyamines. Sulindac, a non-steroidal anti-inflammatory drug, facilitates the export and catabolism of polyamines. Flynpovi has a unique dual mechanism of action whereby it suppresses the synthesis of new polyamines and increases the export and catabolism of polyamines from the diet and microbiome. We believe Flynpovi is unique in that it is designed to treat the risk factors (e.g., polyps) that are hypothesized to lead to Familial Adenomatous Polyposis (“FAP”) surgeries and colon cancer and therefore may have the ability to prevent various types of colon cancer. In the FAP-310 Phase III trial, the efficacy and safety of the combination of Flynpovi (eflornithine and sulindac), as compared with either drug alone, in adults with FAP was conducted. While the study missed the primary composite endpoint (Burke et al. 2020), a post-hoc analysis showed that none of the patients in the combination arm progressed to a need for lower gastrointestinal (“LGI”) surgery for up to 48 months compared to 13.2% and 15.7% of patients in the sulindac and eflornithine arms (Balaguer et al. 2022). This data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy. Given the statistical significance of the LGI group, a new drug application (“NDA”) was filed with the FDA; however, since this was based on the results of an exploratory analysis, a complete response letter (“CRL”) was issued. To address the CRL, the Company is designing a Phase III registration trial and will advance this program while not increasing our current cash requirements. There are no currently approved pharmaceutical therapies for FAP
Additional programs are evaluating a single agent tablet eflornithine or high dose powder eflornithine sachet for several indications including prevention of gastric cancer, recent onset Type 1 diabetes, and STK-11 mutant NSCLC. Preclinical studies as well as Phase I or Phase II investigator-initiated trials suggest that eflornithine treatment is well tolerated and has potential activity. In December, US WorldMeds®, a Kentucky-based specialty pharmaceutical company to whom Panbela divested certain assets in its eflornithine pediatric neuroblastoma program, received FDA approval of their NDA for the use of eflornithine as a maintenance therapy for neuroblastoma in remission. This approval marks the first approval for eflornithine and any polyamine targeted therapy in a cancer indication.
Flynpovi has received Fast Track designation in the United States and orphan drug designation status for FAP in the United States and Europe. In addition, we have received orphan drug designation status for eflornithine as a single agent for Neuroblastoma in the United States and Europe and for gastric cancer in the United States.
Clinical Trials
Ivospemin (SBP-101)
In August 2015, the FDA accepted our Investigational New Drug (“IND”) application for our ivospemin product candidate. We have completed an initial clinical trial of ivospemin in patients with previously treated locally advanced or metastatic pancreatic cancer. This was a Phase I, first-in-human, dose-escalation, safety study. From January 2016 through September 2017, we enrolled twenty-nine patients into six cohorts, or groups, in the dose-escalation phase of the Phase I trial. No drug-related bone marrow toxicity or peripheral neuropathy was observed at any dose level. In addition to being evaluated for safety, 23 of the 29 patients were evaluable for preliminary signals of efficacy prior to or at the eight-week conclusion of their first cycle of treatment using the Response Evaluation Criteria in Solid Tumors (“RECIST”), the currently accepted standard for evaluating change in the size of tumors.
In 2018, we began enrolling patients in our second clinical trial, a Phase Ia/Ib study of the safety, efficacy, and pharmacokinetics of ivospemin administered in combination with two standard-of-care chemotherapy agents, gemcitabine and nab-paclitaxel. A total of 25 subjects were enrolled in four cohorts to evaluate the dosage level and schedule. An additional 25 subjects were enrolled in the expansion phase of the trial. Interim results were presented in January of 2022. Best response in evaluable subjects (cohorts 4 and Ib N=29) was a Complete Response in 1 (3%), Partial Response in 13 (45%), Stable Disease in 10 (34%) and Progressive Disease in 5 (17%). One subject did not have post baseline scans with RECIST tumor assessments. Median Progression Free Survival (“PFS”), now final at 6.5 months may have been negatively impacted by drug dosing interruptions to evaluate potential toxicity. Median overall survival in Cohort 4 + Phase Ib was 12.0 months when data was presented in January 2022 and is now final at 14.6 months. Two patients from cohort 2 have demonstrated long term survival. One at 30.3 months (final data) and one at 33.0 months and still alive. Seven subjects are still alive at this time, one from cohort 2 and six from cohort 4 plus Ib.
In January of 2022, the Company announced the initiation of a new pancreatic cancer clinical trial. Referred to as ASPIRE, the trial is a randomized double-blind placebo-controlled trial in combination with gemcitabine and nab-paclitaxel, a standard pancreatic cancer treatment regimen, in patients previously untreated for metastatic pancreatic cancer. The trial will be conducted globally at approximately 93 sites in the United States, Europe and Asia – Pacific.
The Aspire trial commenced early in 2022, while opening of clinical sites in the US and the rest of the world has been slower than originally anticipated, due in part to resource fatigue in the medical community, the Company expects all countries and sites to be open by in early 2024.
The trial was originally designed as a Phase II/III with a smaller sample size (150) to support the events required for interim analysis based on PFS and a primary endpoint of overall survival. In response to European and FDA regulatory feedback the study was amended to include the total trial sample size (600) and the design modified to utilize overall survival as the primary endpoint to be examined at interim analysis. PFS will also be analyzed to provide additional efficacy evidence. This amendment was supported by the final data from the Phase Ia/b first line metastatic pancreatic cancer trial which completed enrollment in December of 2020. The study will enroll 600 subjects and is anticipated to take 36 months for complete enrollment with the interim analysis available in mid-2024. The Independent Data Safety Monitoring Board (“DSMB”) has met twice, the most recently in November 2023, which evaluated the safety of 214 patients. Both meetings resulted in no safety concerns and the trial continuing without modification. On January 25, 2024, the Company announced that the trial had exceeded 50% enrollment. The Company projects that full enrollment will be completed by the first quarter of 2025 and that interim data analysis based on overall survival should be available by the middle of 2024.
If we can successfully complete all FDA recommended clinical studies, we intend to seek marketing authorization from the FDA, the European Medicines Agency (“EMA”) (European Union), Ministry of Health and Welfare (Japan) and TGA (Australia). The submission fees may be waived when ivospemin has been designated an orphan drug in each geographic region.
Additionally, in early April 2023, the Company announced a poster presentation highlighting the results for ivospemin as a polyamine metabolism modulator in ovarian cancer at the American Association for Cancer Research Annual Conference The poster concludes that the ivospemin treatment of C57Bl/6 mice injected with VDID8+ ovarian cancer cells significantly prolonged survival and decreased overall tumor burden. The results suggest that ivospemin may have a role in the clinical management of ovarian cancer, and the Company intends to continue pre-clinical and clinical studies in ovarian cancer.
Additional preclinical work is underway evaluating ivospemin (also known as SBP-101) and eflornithine (also known as CPP-1X or DFMO) in multiple myeloma (cell lines). Data published in the November 2023, supplemental issue of the Journal Blood investigated the effects of polyamine inhibition by ivospemin and CPP-1X on myeloma cell lines growth and viability in vitro. Results showed that ivospemin and CPP-1X treatment significantly decreased cell proliferation and induced apoptosis in a panel of multiple myeloma cell lines. When ivospemin and CPP-1X were combined an almost complete abolition of cell growth occurred. These results demonstrate the anti-neoplastic potential of ivospemin and CPP-1X and offer a compelling rationale for its clinical development as a potentially promising treatment option for multiple myeloma. The work reflects the Company’s on-going collaboration with researchers from The University of Texas MD Anderson Cancer Center for the evaluation of polyamine metabolic inhibitor therapies in combination with CAR-T cell therapies in preclinical models.
FLYNPOVI
In December 2009, the FDA accepted our IND application for the combination product, Flynpovi. Flynpovi showed promising results in an NCI supported randomized, placebo-controlled Phase IIb/III clinical trial to prevent recurrent colon adenomas, particularly high-risk pre-cancerous polyps in which 375 subjects who had resected sporadic adenoma were treated for 3 years with eflornithine (500 mg once a day) + sulindac (150 mg once a day [N = 191]) or matched placebo/placebo (N = 184). Results demonstrated a marked risk reduction (70%) in developing metachronous adenomas, 92% risk reduction in developing advanced adenomas, and 95% risk reduction in developing multiple adenomas with the active combination regimen compared to placebo (Meyskens et al. 2008). This combination regimen was generally well tolerated.
Given the similar mechanism of disease in sporadic and FAP-associated adenomatous polyposis, and the mechanism of action of Flynpovi in prevention of progressive polyposis in both the general population with sporadic adenomas and in patients with FAP, a Phase III program in FAP, and a Phase III program to study colon cancer risk reduction in partnership with the Southwest Oncology Group (SWOG) and the NCI were initiated.
In the FAP-310 Phase III study completed in 2019, the efficacy and safety of the combination of eflornithine and sulindac, as compared with either drug alone, in adults with familial adenomatous polyposis was conducted (Burke et al. 2020). The patients were randomly assigned in a 1:1:1 ratio to receive eflornithine, sulindac, or both once daily for up to 48 months. The primary end point, assessed in a time-to-event analysis, was disease progression, defined as a composite of major surgery, endoscopic excision of advanced adenomas, diagnosis of high-grade dysplasia in the rectum or pouch, or progression of duodenal disease. A total of 171 patients underwent randomization. Disease progression occurred in 18 of 56 patients (32%) in the eflornithine-sulindac group, 22 of 58 (38%) in the sulindac group, and 23 of 57 (40%) in the eflornithine group, with a hazard ratio of 0.71 (95% confidence interval [CI], 0.39 to 1.32) for eflornithine-sulindac as compared with sulindac (P = 0.29) and 0.66 (95% CI, 0.36 to 1.23) for eflornithine-sulindac as compared with eflornithine (Burke et al. 2020). Adverse and serious adverse events were similar across the treatment groups. In a post-hoc analysis, none of the patients in the combination arm progressed to a need for LGI surgery for up to 48 months compared with 7 (13.2%) and 8 (15.7%) patients in the sulindac and eflornithine arms (Balaguer et al. 2022). These data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy with HR = 0.00 (95% CI, 0.00-0.48; p = 0.005) for combination versus sulindac and HR = 0.00 (95% CI, 0.00-0.44; p = 0.003) for combination versus eflornithine. Given the statistical significance of the LGI group, an NDA was filed with the FDA. As the study failed to meet the primary endpoint, and the NDA was based on the results of an exploratory analysis, a complete response letter was issued. To address this deficiency concern, the Company must submit the results of one or more adequate and well-controlled clinical trials which demonstrate an effect on a clinical endpoint.
In collaboration with the NCI, and SWOG, a Phase III clinical trial has been initiated to study the benefits of Flynpovi as a therapeutic treatment for use by colon cancer survivors. The trial is named PACES for “Prevention of Adenomas and Cancer with eflornithine and sulindac.” The PACES trial is funded by the NCI and managed by the Southwest Oncology Group (“SWOG”). This is an ongoing double-blind placebo-controlled trial of Flynpovi to prevent recurrence of high risk adenomas and second primary colorectal cancers in patients with stage 0-III colon or rectal cancer, Phase III – Preventing Adenomas of the Colon With Eflornithine and Sulindac (“PACES”). The purpose of this study is to assess whether Flynpovi (compared to corresponding placebos) has a reduced rate of cancer or high-risk adenoma recurrence compared to comparator arms after three years of daily dosing. We have exclusive rights to the data that comes from the trial for regulatory and commercial purposes. The Company is evaluating its options for CAT in the European Union and Asia.
In April 2023, the Company announced that it regained the North American rights to develop and commercialize Flynpovi in patients with FAP, as a result of the termination of the license agreement between CPP and One-Two Therapeutics Assets Limited effective July 4, 2023.
Eflornithine (CPP-1X) and eflornithine sachets (CPP-1X-S)
For the single agent eflornithine, there is a Phase I/II trial in STK11 mutation patients with non-small cell lung cancer and Phase II trial in Recent Onset Type I diabetes with eflornithine have been initiated and are enrolling. Recently, a phase II trial evaluating eflornithine and High Dose Testosterone With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer started enrolling. Lastly, a Phase II trial evaluating eflornithine for the prevention of gastric cancer was completed in 2021 with data analysis ongoing.
Recent Developments
Nasdaq Staff Determination Letters
We have requested and been granted a hearing to appeal the staff determination letter we received from The Nasdaq Stock Market, LLC (“Nasdaq”) on November 28, 2023, which letter communicated that Nasdaq would suspend trading in our common stock and file a Form 25-NSE with the Securities and Exchange Commission, which would remove our common stock from listing and registration on Nasdaq, unless we appealed its delisting determination by requesting a hearing before the Nasdaq Hearings Panel. The suspension of trading and delisting of the company’s common stock has been stayed pending the conclusion of the hearing process. Consequently, the company’s common stock is expected to remain listed on the Nasdaq Capital Market at least until the panel renders a decision following the hearing. There can be no assurance that the panel will grant the company’s appeal for continued listing on The Nasdaq Capital Market. The determination letter communicated that our company (i) has not maintained a minimum closing bid price of $1.00 per share as required by Nasdaq Listing Rule 5550(a)(2) (the “Minimum Bid Price Requirement”) and (ii) was no longer in compliance with the minimum stockholders’ equity requirement for continued listing as required by Nasdaq Listing Rule 5550(b) (the “Minimum Stockholders’ Equity Requirement”). Effective January 18, 2024, we completed a 1-for-20 reverse stock split of our outstanding shares of common stock. The primary reason we effected a reverse stock split is to potentially increase the per share market price of our common stock to satisfy the Minimum Bid Price Requirement. On January 22, 2024, we received notice from Nasdaq indicating that, as of effective date of the reverse stock split, we were no longer in compliance with Nasdaq Listing Rule 5550(a)(4) (the “Minimum Float Requirement”), which requires a minimum of 500,000 publicly held shares, which serves as an additional basis for delisting the Company’s common stock. Additionally, it is our intention that the issuance of additional shares and net proceeds from this public offering will allow us to regain compliance with the Minimum Float Requirement and the Minimum Stockholders’ Equity Requirement. We believe that if we are unable to regain compliance with all applicable Nasdaq continued listing requirements, it is likely that our common stock will be delisted from the Nasdaq Capital Market.
Warrant Exercise Inducements & Private Placement of Class C Warrants
On November 2, 2023, we entered into warrant exercise inducement offer letters with certain holders of existing warrants to purchase our common stock, pursuant to which the holders agreed to exercise for cash their existing warrants to purchase 106,500 shares of our common stock, in the aggregate, at a reduced exercise price of $15.60 per share, in exchange for our agreement to issue new Class C common stock purchase warrants to purchase up to an aggregate of 213,000 shares of our common stock. The Company received aggregate gross proceeds of approximately $1.9 million from the exercise of the existing warrants and purchase of the new warrants. The new warrants had an initial exercise price of $15.60 per share and were exercisable upon the date of stockholder approval through the date that is five years from the date of any stockholder approvals necessary under the listing rules of Nasdaq. Our stockholders approved the issuance of the underlying shares of common stock at a special meeting held on December 19, 2023. We agreed to file a registration statement covering the resale of the shares issued or issuable upon the exercise of the new warrants and a registration statement on Form S-1 (File No. 333-275733) was declared effective by the SEC on December 20, 2023. As of January 23, 2024 there are 75,200 Class C Warrants outstanding.
Reverse Stock Split
Effective January 18, 2024, we completed a 1-for-20 reverse split of our outstanding shares of common stock. Unless specifically provided herein, the share and per-share information that follows in this prospectus, other than in the historical financial statements and related notes included elsewhere in this prospectus, assumes the effect of the reverse stock split.
Our primary objective in effecting the reverse stock split has been to attempt to raise the per-share trading price of our common stock to regain compliance with the Minimum Bid Price Requirement.
Because our Company has effectuated reverse stock splits over the prior two-year period with a cumulative ratio in excess of 250 shares to one, we are not eligible for any compliance cure period specified in Nasdaq Marketplace Rule 5810(c)(3)(A) and the Nasdaq staff issued a delisting determination letter in November 2023. While we intend to continue to actively monitor the bid price for our common stock and consider available options regain compliance with the Minimum Bid Price Requirement, there is no assurance that we will not be delisted from Nasdaq even though the reverse stock split has been effected.
Although we expect that the reverse stock split will allow us to maintain the bid price per share of our common stock above the $1.00 per share minimum price for any required number of days, thereby regaining compliance with the Minimum Bid Price Requirement, there can be no assurance that any reverse stock split will have that effect, initially or in the future, or that it would enable us to maintain the listing of our common stock on The Nasdaq Capital Market for any particular duration.
There can be no assurance that any reverse stock split will achieve any of the desired results. There also can be no assurance that the price per share of our common stock immediately after any reverse stock split would increase proportionately with the reverse stock split, or that any increase would be sustained for any period of time, as evidenced by the Company’s past reverse stock splits.
Warrant Exercise Inducements & Private Placement of Class D Warrants
On December 21, 2023, we entered into warrant exercise inducement offer letters with certain holders of existing warrants to purchase our common stock, pursuant to which the holders agreed to exercise for cash their existing warrants to purchase 127,800 shares of our common stock, in the aggregate, at their existing exercise price of $15.60 per share, in exchange for our agreement to issue new Class D common stock purchase warrants to purchase up to an aggregate of 255,600 shares of our common stock. The Company received aggregate gross proceeds of approximately $2.0 million from the exercise of the existing warrants. The new warrants had an initial exercise price of $19.00 per share and will only be exercisable contingent upon and after receiving stockholder approval as required by listing rules of Nasdaq and may be exercised until five years from the date of such stockholder approval, if any. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of our common stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities, upon any intervening reverse stock splits, and upon receipt of the stockholder approval. As of January 23, 2024 all of the new warrants remained outstanding but unexercisable pending stockholder approval. We have agreed to call a meeting to seek stockholder approval of the issuance of the shares of our common stock underlying the new warrants within six months and to file a registration statement covering the resale of the shares underlying the new warrants within sixty calendar days of December 21, 2023. Additionally, the Company agreed not to effect or agree to effect any variable rate transaction (as defined in the inducement letters) for one year, other than an at-the-market offering, which may be effected after six months.
Product Developments
Through January 23, 2024, we had:
| |
●
|
secured an orphan drug designation for ivospemin from the FDA;
|
| |
●
|
submitted and received acceptance from the FDA for an IND application for ivospemin;
|
| |
●
|
received Country approvals for the ASPIRE Trial in Australia, France, Italy and Spain; |
| |
●
|
completed a Phase Ia monotherapy safety study of ivospemin in the treatment of patients with metastatic pancreatic ductal adenocarcinoma;
|
| |
●
|
received “Fast Track” designation from the FDA for ivospemin for metastatic pancreatic cancer;
|
| |
●
|
completed enrollment and released interim results in our second trial a Phase Ia /Ib clinical study of ivospemin, a first-line study with ivospemin given in combination with a current standard of care in patients with pancreatic ductal adenocarcinoma who were previously untreated for metastatic disease; a total of 50 subjects were enrolled in this study, 25 in the Phase Ia and 25 in the Phase Ib or expansion phase;
|
| |
●
|
secured a two-year research agreement with Johns Hopkins School of Medicine led by Professor Robert Casero, an internationally recognized researcher in polyamine biology;
|
| |
●
|
completed process improvement measures expected to be scalable for commercial use and received issue notification for a patent covering this new shorter synthesis of ivospemin;
|
| |
●
|
initiated a randomized, double-blind, placebo-controlled study with ivospemin given in combination with gemcitabine and nab-paclitaxel in patients with pancreatic ductal adenocarcinoma who are previously untreated for metastatic disease;
|
| |
●
|
completed preclinical evaluation of ivospemin for use as neoadjuvant therapy in resectable pancreatic cancer prior to surgery;
|
| |
●
|
obtained early, preclinical, indication of tumor growth inhibition activity in ovarian cancer and presented the results at ASCO-GI conference;
|
| |
●
|
received USAN adoption of the nonproprietary name of ivospemin for SBP-101;
|
| |
●
|
acquired and integrated CPP, adding a second lead asset in multiple forms and an expansive clinical development program ranging from pre-clinical to registration level clinical trials;
|
| |
●
|
European Medicines Agency (EMA) Committee for Orphan Medicinal Products issued a positive opinion on Panbela’s application for orphan designation of ivospemin in combination with gemcitabine and nab-Paclitaxel in patients with metastatic pancreatic ductal adenocarcinoma;
|
| |
●
|
announced the initiation of phase II program through Indiana University for early onset Type I diabetes utilizing eflornithine; and;
|
| |
●
|
announced the initiation of the Phase I/II clinical trial for the treatment of non-small lung cancer (NSCLC) possessing the STK11 mutation through Moffitt Cancer Center;
|
| |
●
|
entered into a sponsored research agreement with The University of Texas MD Anderson Cancer Center for the evaluation of polyamine metabolic inhibitor therapies in combination with CAR-T cell therapies in preclinical models;
|
| |
●
|
announced the SWOG Cancer Research Network’s PACES S0820 Phase III trial passed a single planned futility analysis and will continue; |
| |
●
|
announced the approval of US WorldMeds NDA Approval for Eflornithine (DFMO) in Pediatric Neuroblastoma, first polyamine approval in oncology and |
| |
●
|
exceeded 50% enrollment in ASPIRE global clinical trial.
|
Risks Associated with Our Company
Our business is subject to many significant risks, as more fully described in the section titled “Risk Factors” immediately following this prospectus summary. You should read and carefully consider these risks, together with the risks set forth under the section titled “Risk Factors” and all of the other information in this prospectus, including the financial statements and the related notes included elsewhere in this prospectus, before deciding whether to invest in our securities. If any of the risks discussed in this prospectus actually occur, our business, financial condition or operating results could be materially and adversely affected. In particular, our risks include, but are not limited to, the following:
| |
●
|
our ability to obtain additional capital, on acceptable terms or at all, required to implement our business plan;
|
| |
●
|
our lack of diversification and the corresponding risk of an investment in our Company;
|
| |
●
|
our ability to maintain our listing on a national securities exchange;
|
| |
●
|
progress and success of our randomized Phase II/III clinical trial;
|
| |
●
|
our ability to demonstrate the safety and effectiveness of our product candidates: ivospemin ( SBP-101 ), Flynpovi, and eflornithine (CPP-1X);
|
| |
●
|
our ability to obtain regulatory approvals for our product candidates, SBP-101, Flynpovi and CPP-1X in the United States, the European Union or other international markets;
|
| |
●
|
the market acceptance and level of future sales of our product candidates, SBP-101, Flynpovi and CPP-1X ;
|
| |
●
|
the cost and delays in product development that may result from changes in regulatory oversight applicable to our product candidates, SBP-101, Flynpovi and CPP-1X ;
|
| |
●
|
the rate of progress in establishing reimbursement arrangements with third-party payors;
|
| |
●
|
the effect of competing technological and market developments;
|
| |
●
|
the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims; and
|
| |
●
|
other risk factors included under the caption “Risk Factors” starting on page 9 of this prospectus.
|
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined in Rule 12b-2 of the Exchange Act and have elected to take advantage of certain scaled disclosure available to smaller reporting companies.
Corporate History
The primary business underlying Panbela Therapeutics, Inc., was originally incorporated under the laws of the State of Delaware under the name “Sun BioPharma, Inc.” in September 2011. In 2015, it became a public company by completing a merger transaction with a wholly owned subsidiary of a public company then organized under the laws of the State of Utah. In 2016, it was reincorporated under the laws of the State of Delaware via a merger with our operating subsidiary. That company changed its name to “Panbela Therapeutics, Inc.” on December 2, 2020. On June 15, 2022, we became a successor issuer to Panbela Therapeutics, Inc. and adopted its name, pursuant to a holding company reorganization via merger by operation of Rule 12g-3(a) promulgated under the Exchange Act, resulting in our current structure – consisting of two wholly owned subsidiaries: Panbela Research, Inc. and Cancer Prevention Pharmaceuticals, Inc.
Corporate Information
Our corporate mailing address is 712 Vista Blvd, #305, Waconia, MN 55387. Our telephone number is (952) 479-1196, and our website is www.panbela.com. The information on our website is not part of this prospectus. We have included our website address as a factual reference and do not intend it to be an active link to our website. The information contained in or connected to our website is not incorporated by reference into, and should not be considered part of, this prospectus. The trade names, trademarks, and service marks of other companies appearing in this prospectus are the property of the respective holders.
THE OFFERING
|
Common stock offered by us
|
8,000,000 shares of our common stock, including shares of common stock issuable upon exercise of pre-funded warrants, plus up to 16,660,000 additional shares of our common stock issuable upon exercise of common warrants. |
| |
|
|
Common warrants offered by us
|
Class E Common Warrants to purchase up to 2,000,000 shares of our common stock (or 2,660,000 shares of common stock utilizing alternative cashless exercise) which will be exercisable during the period commencing on the date of their issuance and ending five years from such date at an exercise price of $ per share of common stock (100% of the public offering price per share and common warrants). After five business days, holders of Class E Warrants will have a separate option to elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal 1.33 times the number of shares of common stock that would be issuable upon a cash exercise.
Class F Common Warrants to purchase up to 14,000,000 shares of our common stock, which will be exercisable during the period commencing on the date of their issuance and ending five years from such date at an exercise price of $ per share of common stock (100% of the public offering price per share and common warrants).
|
| |
|
|
Pre-funded warrants offered by us
|
We are also offering to certain purchasers whose purchase of our common stock in this offering would otherwise result in the purchaser, together with its affiliates, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase pre-funded warrants (together with the common warrants, the “Warrants”) in lieu of common stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. Each pre-funded warrant will be exercisable for one share of common stock. The purchase price of each pre-funded warrant and the accompanying common warrants will equal the price at which the common stock and the accompanying common warrants are being sold to the public in this offering, minus $0.001, and the exercise price of each pre-funded warrant will be $0.001 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until exercised in full. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because we will issue common warrants to purchase 2 shares of common stock for each share of common stock and for each pre-funded warrant sold in this offering, the number of common warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold. |
| |
|
|
Assumed public offering price
|
$3.77 per share of common stock and accompanying common warrant, or $3.769 per pre-funded warrant and accompanying common warrant, as applicable, in each case assuming a public offering price equal to the last sale price of our common stock as reported by the Nasdaq Capital Market on January 23, 2024, which was $3.77. |
| |
|
|
Common stock outstanding before this offering
|
480,244 shares
|
| |
|
|
Common stock to be outstanding immediately after this offering
|
8,480,244 shares (assuming we sell only shares of common stock and no pre-funded warrants, and none of the common warrants issued in this offering are exercised). |
|
Use of proceeds
|
We estimate that the net proceeds from this offering will be up to approximately $27.8 million, based on an assumed combined public offering price of $3.77 per share of common stock and accompanying common warrants, after deducting the placement agent fees and estimated offering expenses payable by us. We intend to use the net proceeds from this offering for the continued clinical development of our product candidates ivospemin and eflornithine and for working capital, and other general corporate purposes, which may include repayment of debt. Because this is a best efforts offering with no minimum amount as a condition to closing, we may not sell all or any of the securities offered hereby. As a result, we may receive significantly less in net proceeds than we currently estimate. See “Use of Proceeds” on page 21. |
|
Risk Factors
|
You should read the “Risk Factors” section of this prospectus beginning on page 10 for a discussion of factors to consider carefully before deciding to invest in our securities.
|
| |
|
|
Nasdaq Capital Market trading symbol
|
“PBLA”
|
The number of shares of our common stock outstanding before and after this offering is based on an estimated 480,244 shares of our common stock outstanding as of January 23, 2024 and excludes:
| |
●
|
all shares issuable upon the exercise of warrants sold in this offering;
|
| |
● |
pending settlements of fractional shares for cash in connection with the reverse stock split effected as of January 18, 2024; |
| |
●
|
599 shares of common stock issuable upon the exercise of outstanding stock options as of the date of this prospectus at a weighted average exercise price of $13,297.06 per share; and
|
| |
●
|
340,952 shares of common stock issuable upon exercise of stock purchase warrants at a weighted average exercise price of $64.09 per share.
|
Unless otherwise indicated, all information in this prospectus assumes no exercise of the outstanding options or warrants.
RISK FACTORS
Any investment in our securities involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to purchase our securities. Our business, financial condition or results of operations could be materially adversely affected by these risks if any of them actually occur. This prospectus also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face as described below and elsewhere in this prospectus.
Risks Related to Our Business and Financial Position
We are a pre-revenue company with a history of negative operating cash flow.
We have experienced negative cash flows for our operating activities since inception, primarily due to the investments required to commercialize our primary drug candidates. Our financing cash flows historically have been positive due to proceeds from the sale of equity securities and promissory notes issuances. Our net cash used in operating activities was $15.3 million and $7.2 million for the years ended December 31, 2022 and 2021, respectively, and we had negative working capital of $6.0 million on December 31, 2022 and positive working capital of $9.6 million as December 31, 2021. For the quarter ended September 30, 2023 we had approximately $0.9 million in cash and approximately $7.0 million in negative working capital. Working capital is defined as current assets less current liabilities.
Our operations are subject to all the risks, difficulties, complications and delays frequently encountered in connection with the development of new products, as well as those risks that are specific to the pharmaceutical and biotechnology industries in which we compete. Investors should evaluate us considering the delays, expenses, problems and uncertainties frequently encountered by companies developing markets for new products, services and technologies. We may never overcome these obstacles.
As a result of our current limited financial liquidity, our auditors have expressed substantial doubt regarding our ability to continue as a “going concern.”
As a result of our current limited financial liquidity, our auditors’ report for our 2022 financial statements, which is incorporated by reference herein, contains a statement concerning our ability to continue as a “going concern.” Our limited liquidity could make it more difficult for us to secure additional financing or enter into strategic relationships on terms acceptable to us, if at all, and may materially and adversely affect the terms of any financing that we may obtain and our public stock price generally.
Our continuation as a “going concern” is dependent upon, among other things, achieving positive cash flow from operations and, if necessary, augmenting such cash flow using external resources to satisfy our cash needs. Our plans to achieve positive cash flow primarily include engaging in offerings of securities. Additional potential sources of funds include negotiating up-front and milestone payments on our current and potential future product candidates or royalties from sales of our products that secure regulatory approval and any milestone payments associated with such approved products. These cash sources could, potentially, be supplemented by financing or other strategic agreements. However, we may be unable to achieve these goals or obtain required funding on commercially reasonable terms, or at all, and therefore may be unable to continue as a going concern.
We may be unable to obtain the additional capital that is required to execute our business plan, which could restrict our ability to grow.
Our current capital and our other existing resources will be sufficient only to provide a limited amount of working capital and will not be sufficient to fund our expected continuing opportunities. Our capital at the end of the third quarter of 2023 and funds raised subsequent to September 30, 2023 will be sufficient to fund operations into the first quarter of 2024. We will require additional capital to continue to operate our business and complete our clinical development plans.
Future research and development, including clinical trial cost, capital expenditures and possible acquisitions, and our administrative requirements, such as salaries, insurance expenses and general overhead expenses, as well as legal compliance costs and accounting expenses, will require a substantial amount of additional capital and cash flow. There is no guarantee that we will be able to raise additional capital required to fund our ongoing business on commercially reasonable terms or at all.
We intend to pursue sources of additional capital through various financing transactions or arrangements, including collaboration arrangements, debt financing, equity financing or other means. We may not be successful in locating suitable financing transactions on commercially reasonable terms, in the time period required or at all, and we may not obtain the capital we require by other means. If we do not succeed in raising additional capital, our resources will not be sufficient to fund our operations going forward.
Any additional capital raised through the sale of equity may dilute the ownership percentage of our stockholders. This could also result in a decrease in the fair market value of our equity securities because our assets would be owned by a larger pool of outstanding equity. The terms of securities we issue in future capital transactions may be more favorable to our new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities which may have a further dilutive effect.
Our ability to obtain needed financing may be impaired by such factors as the capital markets, both generally and in the pharmaceutical and other drug development industries in particular, the limited diversity of our activities and/or the loss of key personnel. If the amount of capital we are able to raise from financing activities is not sufficient to satisfy our capital needs, even to the extent that we reduce our operations, we may be required to cease our operations.
We may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs, which may adversely impact our financial condition.
The markets for our product candidates are highly competitive and are subject to rapid scientific change, which could have a material adverse effect on our business, results of operations and financial condition.
The pharmaceutical and biotechnology industries in which we compete are highly competitive and characterized by rapid and significant technological change. We face intense competition from organizations such as pharmaceutical and biotechnology companies, as well as academic and research institutions and government agencies. Some of these organizations are pursuing products based on technologies similar to our technology. Other of these organizations have developed and are marketing products or are pursuing other technological approaches designed to produce products that are competitive with our product candidates in the therapeutic effect these competitive products have on the diseases targeted by our product candidates. Our competitors may discover, develop or commercialize products or other novel technologies that are more effective, safer or less costly than any that we may develop. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for our product candidates.
Many of our competitors are substantially larger than we are and have greater capital resources, research and development staffs and facilities than we have. In addition, many of our competitors are more experienced in drug discovery, development and commercialization, obtaining regulatory approvals and drug manufacturing and marketing.
We anticipate that the competition with our product candidates and technology will be based on a number of factors including product efficacy, safety, availability and price. The timing of market introduction of our planned future product candidates and competitive products will also affect competition among products. We expect the relative speed with which we can develop our product candidates, complete the required clinical trials, establish strategic partners and supply appropriate quantities of the product candidate for late stage trials, if required, to be important competitive factors. Our competitive position will also depend upon our ability to attract and retain qualified personnel, to obtain patent protection in non-U.S. markets, which we currently do not have, or otherwise develop proprietary products or processes and to secure sufficient capital resources for the period between technological conception and commercial sales or out-license to pharmaceutical partners. If we fail to develop and deploy a proposed product candidate in a successful and timely manner, we will in all likelihood not be competitive.
Our lack of diversification increases the risk of an investment in our Company and our financial condition and results of operations may deteriorate if we fail to diversify.
Our Board of Directors has centered our attention on our drug development activities, which are currently focused on a limited number of product candidates. Our ability to diversify our investments will depend on our access to additional capital and financing sources and the availability and identification of suitable opportunities.
Larger companies have the ability to manage their risk by diversification. However, we lack and expect to continue to lack diversification, in terms of both the nature and geographic scope of our business. As a result, we will likely be impacted more acutely by factors affecting pharmaceutical and biotechnology industries in which we compete than we would if our business were more diversified, enhancing our risk profile. If we cannot diversify our operations, our financial condition and results of operations could deteriorate.
Our business may suffer if we do not attract and retain talented personnel.
Our success will depend in large measure on the abilities, expertise, judgment, discretion, integrity and good faith of our management and other personnel in conducting our business. We have a small management team, and the loss of a key individual or inability to attract suitably qualified staff could materially adversely impact our business.
Our success depends on the ability of our management, employees, consultants and strategic partners, if any, to interpret market data correctly and to interpret and respond to economic market and other conditions in order to locate and adopt appropriate investment opportunities, monitor such investments, and ultimately, if required, to successfully divest such investments. Further, no assurance can be given that our key personnel will continue their association or employment with us or that replacement personnel with comparable skills can be found. We will seek to ensure that management and any key employees are appropriately compensated; however, their services cannot be guaranteed. If we are unable to attract and retain key personnel, our business may be adversely affected.
We may be required to defend lawsuits or pay damages for product liability claims.
Product liability is a major risk in testing and marketing biotechnology and pharmaceutical products. We may face substantial product liability exposure in human clinical trials and in the sale of products after regulatory approval. Product liability claims, regardless of their merits, could exceed policy limits, divert management’s attention and adversely affect our reputation and the demand for our product. In any such event, your investment in our securities could be materially and adversely affected.
Risks Related to the Development and Approval of New Drugs
Clinical trials required for our product candidate are expensive and time-consuming, and their outcome is highly uncertain. If any of our drug trials are delayed or yield unfavorable results, we will have to delay or may be unable to obtain regulatory approval for our product candidate.
We must conduct extensive testing of each product candidate before we can obtain regulatory approval to market and sell it. We need to conduct both preclinical animal testing and human clinical trials. Conducting these trials is a lengthy, time-consuming and expensive process. These tests and trials may not achieve favorable results for many reasons, including, among others, failure of the product candidate to demonstrate safety or efficacy, the development of serious or life-threatening adverse events, or side effects, caused by or connected with exposure to the product candidate, difficulty in enrolling and maintaining subjects in the clinical trial, lack of sufficient supplies of the product candidate or comparator drug, and the failure of clinical investigators, trial monitors, contractors, consultants, or trial subjects to comply with the trial protocol. A clinical trial may fail because it did not include a sufficient number of patients to detect the endpoint being measured or reach statistical significance. A clinical trial may also fail because the dose(s) of the investigational drug included in the trial were either too low or too high to determine the optimal effect of the investigational drug in the disease setting. Many clinical trials are conducted under the oversight of Independent Data Monitoring Committees (“IDMCs”) also known as DSMB’s. These independent oversight bodies are made up of external experts who review the progress of ongoing clinical trials, including available safety and efficacy data, and make recommendations concerning a trial’s continuation, modification, or termination based on interim, unblinded data. Any of our ongoing clinical trials may be discontinued or amended in response to recommendations made by responsible IDMCs based on their review of such interim trial results.
We will need to reevaluate our product candidates if they do not test favorably and either conduct new trials, which are expensive and time consuming, or abandon our drug development program. Even if we obtain positive results from preclinical or clinical trials, we may not achieve the same success in future trials. Many companies in the biopharmaceutical industry have suffered significant setbacks in clinical trials, even after promising results have been obtained in earlier trials. The failure of clinical trials to demonstrate safety and effectiveness for the desired indication could harm the development of our product candidate and our business, financial condition and results of operations may be materially harmed.
We face significant risks in our product candidate development efforts.
Our business depends on the successful development and commercialization of our product candidates. We are currently focused on developing our initial product candidate, ivospemin, for the treatment of PDA and are not permitted to market it in the United States until we receive approval of an NDA from the FDA, or in any foreign jurisdiction until we receive the requisite approvals from such jurisdiction. The process of developing new drugs and/or therapeutic products is inherently complex, unpredictable, time-consuming, expensive and uncertain. We must make long-term investments and commit significant resources before knowing whether our development programs will result in drugs that will receive regulatory approval and achieve market acceptance. A product candidate that appears to be promising at all stages of development may not reach the market for a number of reasons that may not be predictable based on results and data from the clinical program. A product candidate may be found ineffective or may cause harmful side effects during clinical trials, may take longer to progress through clinical trials than had been anticipated, may not be able to achieve the pre-defined clinical endpoints even though clinical benefit may have been achieved, may fail to receive necessary regulatory approvals, may prove impracticable to manufacture in commercial quantities at reasonable cost and with acceptable quality, or may fail to achieve market acceptance.
We cannot predict whether or when we will obtain regulatory approval to commercialize our product candidates and we cannot, therefore, predict the timing of any future revenues from this or other product candidates, if any. The FDA has substantial discretion in the drug approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. For example, the FDA:
| |
●
|
could determine that the information provided by us was inadequate, contained clinical deficiencies or otherwise failed to demonstrate the safety and effectiveness of any of our product candidates for any indication;
|
| |
●
|
may not find the data from clinical trials sufficient to support the submission of an NDA or to obtain marketing approval in the United States, including any findings that the clinical and other benefits of our product candidates outweigh their safety risks;
|
| |
●
|
may disagree with our trial design or our interpretation of data from preclinical studies or clinical trials or may change the requirements for approval even after it has reviewed and commented on the design for our trials;
|
| |
●
|
may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for the manufacturing of our product candidates;
|
| |
●
|
may approve our product candidates for fewer or more limited indications than we request or may grant approval contingent on the performance of costly post-approval clinical trials;
|
| |
●
|
may change its approval policies or adopt new regulations; or
|
| |
●
|
may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of our product candidates.
|
Any failure to obtain regulatory approval of our initial product candidate or future product candidates we develop, if any, would significantly limit our ability to generate revenues, and any failure to obtain such approval for all of the indications and labeling claims we deem desirable could reduce our potential revenues.
Our product candidates are based on a new formulation of an existing technology which has never been approved for the treatment of any cancer and, consequently, is inherently risky. Concerns about the safety and efficacy of our product candidate could limit our future success.
We are subject to the risks of failure inherent in the development of product candidates based on new technologies. These risks include the possibility that any product candidates we create will not be effective, that our current product candidate will be unsafe, ineffective or otherwise fail to receive the necessary regulatory approvals or that our product candidate will be hard to manufacture on a large scale or will be uneconomical to market.
Many pharmaceutical products cause multiple potential complications and side effects, not all of which can be predicted with accuracy and many of which may vary from patient to patient. Long term follow-up data may reveal additional complications associated with our product candidate. The responses of potential physicians and others to information about complications could materially affect the market acceptance of our product candidate, which in turn would materially harm our business.
Due to our reliance on third parties to conduct our clinical trials, we are unable to directly control the timing, conduct, expense and quality of our clinical trials, which could adversely affect our clinical data and results and related regulatory approvals.
We extensively outsource our clinical trial activities and expect to directly perform only a small portion of the preparatory stages for planned trials. We rely on independent third-party CROs to perform most of our clinical trials, including document preparation, site identification, screening and preparation, pre-study visits, training, program management and bio-analytical analysis. Many important aspects of the services performed for us by the CROs are out of our direct control. If there is any dispute or disruption in our relationship with our CROs, our clinical trials may be delayed. Moreover, in our regulatory submissions, we rely on the quality and validity of the clinical work performed by third-party CROs. If a CRO’s processes, methodologies or results are determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals could be adversely affected or invalidated.
We rely on third-party suppliers and other third parties for production of our product candidates and our dependence on these third parties may impair the advancement of our research and development programs and the development of our product candidates.
We rely on, and expect to continue to rely on, third parties for the supply of raw materials and manufacture of drug supplies necessary to conduct our preclinical studies and clinical trials. During 2021 the Company, in collaboration with our manufacturing partner confirmed a new shorter and less expensive synthesis of the active drug substance. However, delays in production by third parties could delay our clinical trials or have an adverse impact on any commercial activities. In addition, the fact that we are dependent on third parties for the manufacture of and formulation of our product candidates means that we are subject to the risk that the products may have manufacturing defects that we have limited ability to prevent or control. Although we oversee these activities to ensure compliance with our quality standards, budgets and timelines, we have had and will continue to have less control over the manufacturing of our product candidates than potentially would be the case if we were to manufacture our product candidates. Further, the third parties we deal with could have staffing difficulties, might undergo changes in priorities or may become financially distressed, which would adversely affect the manufacturing and production of our product candidates.
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials.
Pre-clinical studies and Phase I clinical trials are not primarily designed to test the efficacy of a product candidate in the general population, but rather to test initial safety, to study pharmacokinetics and pharmacodynamics, to study limited efficacy in a small number of study patients in a selected disease population, and to identify and attempt to understand the product candidate’s side effects at various doses and dosing schedules. Success in pre-clinical studies or completed clinical trials does not ensure that later studies or trials, including continuing pre-clinical studies and large-scale clinical trials, will be successful nor does it necessarily predict future results. Favorable results in early studies or trials may not be repeated in later studies or trials, and product candidates in later stage trials may fail to show acceptable safety and efficacy despite having progressed through earlier trials.
Risks Related to the Regulation of our Business
Federal and state pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
The Food and Drug Administration Modernization Act (the “FDMA”) established a public registry of open clinical trials involving drugs intended to treat serious or life-threatening diseases or conditions in order to promote public awareness of and access to these clinical trials. Under the FDMA, pharmaceutical manufacturers and other trial sponsors are required to post the general purpose of these trials, as well as the eligibility criteria, location and contact information of the trials. Failure to comply with any clinical trial posting requirements could expose us to negative publicity, fines and other penalties, all of which could materially harm our business.
In recent years, several states, including California, Vermont, Maine, Minnesota, New Mexico and West Virginia, have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs and file periodic reports on sales, marketing, pricing and other activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and available guidance is limited. Unless we are in full compliance with these laws, we could face enforcement actions and fines and other penalties and could receive adverse publicity, all of which could harm our business.
If any of the product candidates we develop become subject to unfavorable pricing regulations, third party reimbursement practices or healthcare reform initiatives, our ability to successfully commercialize our product candidates may be impaired.
Our future revenues, profitability and access to capital will be affected by the continuing efforts of governmental and private third-party payors to contain or reduce the costs of health care through various means. We expect several federal, state and foreign proposals to control the cost of drugs through government regulation. We are unsure of the impact recent health care reform legislation may have on our business or what actions federal, state, foreign and private payors may take in response to the recent reforms. Therefore, it is difficult to predict the effect of any implemented reform on our business. Our ability to commercialize our product candidates successfully will depend, in part, on the extent to which reimbursement for the cost of such product candidate and related treatments will be available from government health administration authorities, such as Medicare and Medicaid in the United States, private health insurers and other organizations. Significant uncertainty exists as to the reimbursement status of newly approved health care products, particularly for indications for which there is no current effective treatment or for which medical care typically is not sought. Adequate third-party coverage may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product research and development. If adequate coverage and reimbursement levels are not provided by government and third-party payors for use of our product candidates, our product candidates may fail to achieve market acceptance and our results of operations will be harmed.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
Legislative and regulatory actions affecting government prescription drug procurement and reimbursement programs occur relatively frequently. In the United States, the ACA was enacted in 2010 to expand healthcare coverage. Since then, numerous efforts have been made to repeal, amend or administratively limit the ACA in whole or in part. For example, the Tax Cuts and Jobs Act, signed into law by President Trump in 2017, repealed the individual health insurance mandate, which is considered a key component of the ACA. In December 2018, a Texas federal district court struck down the ACA on the grounds that the individual health insurance mandate is unconstitutional, although this ruling has been stayed pending appeal. The ongoing challenges to the ACA and new legislative proposals have resulted in uncertainty regarding the ACA's future viability and destabilization of the health insurance market. The resulting impact on our business is uncertain and could be material.
Efforts to control prescription drug prices could also have a material adverse effect on our business. For example, in 2018, President Trump and the Secretary of the U.S. Department of Health and Human Services (“HHS”) released the "American Patients First Blueprint" and have begun implementing certain portions. The initiative includes proposals to increase generic drug and biosimilar competition, enable the Medicare program to negotiate drug prices more directly and improve transparency regarding drug prices and ways to lower consumers' out-of-pocket costs. The Trump administration also proposed to establish an “international pricing index” that would be used as a benchmark to determine the costs and potentially limit the reimbursement of drugs under Medicare Part B. Among other pharmaceutical manufacturer industry-related proposals, Congress has proposed bills to change the Medicare Part D benefit to impose an inflation-based rebate in Medicare Part D and to alter the benefit structure to increase manufacturer contributions in the catastrophic phase. The volume of drug pricing-related bills has dramatically increased under the current Congress, and the resulting impact on our business is uncertain and could be material.
In addition, many states have proposed or enacted legislation that seeks to regulate pharmaceutical drug pricing indirectly or directly, such as by requiring biopharmaceutical manufacturers to publicly report proprietary pricing information or to place a maximum price ceiling on pharmaceutical products purchased by state agencies. For example, in 2017, California’s governor signed a prescription drug price transparency state bill into law, requiring prescription drug manufacturers to provide advance notice and explanation for price increases of certain drugs that exceed a specified threshold. Both Congress and state legislatures are considering various bills that would reform drug purchasing and price negotiations, allow greater use of utilization management tools to limit Medicare Part D coverage, facilitate the import of lower-priced drugs from outside the United States and encourage the use of generic drugs. The Inflation Reduction Act passed by Congress allows Medicare to negotiate the prices of the prescription drugs. Such initiatives and legislation may cause added pricing pressures on our products.
Changes to the Medicaid program at the federal or state level could also have a material adverse effect on our business. Proposals that could impact coverage and reimbursement of our products, including giving states more flexibility to manage drugs covered under the Medicaid program and permitting the re-importation of prescription medications from Canada or other countries, could have a material adverse effect by limiting our products’ use and coverage. Furthermore, state Medicaid programs could request additional supplemental rebates on our products as a result of an increase in the federal base Medicaid rebate. To the extent that private insurers or managed care programs follow Medicaid coverage and payment developments, they could use the enactment of these increased rebates to exert pricing pressure on our products, and the adverse effects may be magnified by their adoption of lower payment schedules.
Other proposed regulatory actions affecting manufacturers could have a material adverse effect on our business. It is difficult to predict the impact, if any, of any such proposed legislative and regulatory actions or resulting state actions on the use and reimbursement of our products in the United States, but our results of operations may be adversely affected.
Risks Related to our Intellectual Property
If we are unable to obtain, maintain and enforce our proprietary rights, we may not be able to compete effectively or operate profitably.
For ivospemin, we are party to a license agreement with the University of Florida Research Foundation (“UFRF”) and for Flynpovi, we are party to a license agreement with the Arizona Board of Regents of the University of Arizona. The patents underlying the licensed intellectual property and those of other biopharmaceutical companies are generally uncertain and involve complex legal, scientific and factual questions.
Our ability to develop and commercialize drugs depends in significant part on our ability to: (i) obtain and/or develop broad, protectable intellectual property; (ii) obtain additional licenses, if required, to the proprietary rights of others on commercially reasonable terms; (iii) operate without infringing upon the proprietary rights of others; (iv) prevent others from infringing on our proprietary rights; and (v) protect our corporate know-how and trade secrets.
Patents that we may acquire, and those that might be issued in the future, may be challenged, invalidated or circumvented, and the rights granted thereunder may not provide us with proprietary protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies or duplicate any technology we develop. Because of the extensive time required for development, testing and regulatory review of potential product candidates, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thus reducing any advantage of the patent.
Because patent applications in the U.S. and many foreign jurisdictions are typically not published until at least 12 months after filing, or in some cases not at all, and because publications of discoveries in the scientific literature often lag behind actual discoveries, neither we nor our licensors can be certain that either we or our licensors were the first to make the inventions claimed in issued patents or pending patent applications, or that we were the first to file for protection of the inventions set forth in these patent applications.
Additionally, UFRF previously elected to seek protection for certain elements of the licensed technology only in the United States, and the time to file for international patent protection has expired. This limits the strength of the Company’s intellectual property position in certain markets and could affect the overall value of the Company to a potential corporate partner.
Obtaining and maintaining our patent protection depends upon compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The United States Patent and Trademark Office and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
We may be exposed to infringement or misappropriation claims by third parties, which, if determined adversely to us, could cause us to pay significant damage awards.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. We may become a party to various types of patent litigation or other proceedings regarding intellectual property rights from time to time even under circumstances where we are not using and do not intend to use any of the intellectual property involved in the proceedings.
The cost of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the cost of such litigation or proceedings more effectively than we will be able to because our competitors may have substantially greater financial resources. If any patent litigation or other proceeding is resolved against us, we or our collaborators may be enjoined from developing, manufacturing, selling or importing our drugs without a license from the other party and we may be held liable for significant damages. We may not be able to obtain any required license(s) on commercially acceptable terms or at all.
Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
Confidentiality agreements with employees and others may not adequately prevent disclosure of our know-how, trade secrets and other proprietary information and may not adequately protect our intellectual property, which could impede our ability to compete.
Because we operate in the highly technical field of medical technology development, we rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with all of our employees, consultants and corporate partners, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties any confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements from these parties which provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology industry, we employ individuals who were previously employed at other biotechnology companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Associated with this Offering and Ownership of Our Common Stock
We could be delisted from Nasdaq, which would seriously harm the liquidity of our stock and our ability to raise capital.
During the first quarter of 2023 we cured previously identified minimum bid price and minimum stockholders’ equity deficiencies and regained compliance with all applicable listing standards of Nasdaq. On April 14, 2023, we had received a notification letter from Nasdaq’s Listing Qualifications Department indicating that for 30 consecutive business days our common stock did not maintain a minimum closing bid price of $1.00 per share as required by Nasdaq Listing Rule 5550(a)(2). Nasdaq granted us an extension until October 11, 2023 to regain compliance with the Minimum Bid Price Requirement for continued listing on the Nasdaq Capital Market. At our annual meeting of stockholders held on May 25, 2023, stockholders approved an amendment to our Restated Certificate of Incorporation to effect a reverse stock split of our outstanding common stock within a prescribed range. Pursuant to that authority, our Board of Directors approved a 1-for-30 reverse split ratio and our company effected the reverse stock split on June 1, 2023. The primary reason for the reverse stock split was to attempt to increase the per share market price of our common stock to exceed the Minimum Bid Price Requirement for continued listing on the Nasdaq Capital Market. On June 15, 2023, we received a notification letter from the Listing Qualifications Department of Nasdaq indicating that we regained compliance with the Minimum Bid Price Requirement as the closing bid price of our common stock met or exceeded $1.00 per share for a minimum of ten consecutive business days during the 180-calendar day grace period.
Subsequently, we have requested and been granted a hearing to present its plan to regain compliance with appeal the staff determination letter we received from Nasdaq on November 28, 2023, which letter communicated that Nasdaq would suspend trading in our common stock and file a Form 25-NSE with the Securities and Exchange Commission, which would remove our common stock from listing and registration on Nasdaq, unless we appealed its delisting determination by requesting a hearing before the Nasdaq Hearings Panel. The suspension of trading and delisting of the company’s common stock has been stayed pending the conclusion of the hearing process. Consequently, our common stock is expected to remain listed on the Nasdaq Capital Market at least until the panel renders a decision following the hearing. There can be no assurance that the panel will grant the company’s appeal for continued listing on The Nasdaq Capital Market. The determination letter communicated that our company (i) had not maintained a minimum closing bid price of $1.00 per share as required by the Minimum Bid Price Requirement and (ii) was not in compliance with the Minimum Stockholders’ Equity Requirement. Effective January 18, 2024, we completed a 1-for-20 reverse split of our outstanding shares of common stock. The primary reason we effected a reverse stock split is to potentially increase the per share market price of our common stock to satisfy the Minimum Bid Price Requirement. On January 22, 2024, we received notice from Nasdaq indicating that, as of effective date of the reverse stock split, we were no longer in compliance with Nasdaq Listing Rule 5550(a)(4) (the “Minimum Float Requirement”), which requires a minimum of 500,000 publicly held shares, which serves as an additional basis for delisting the Company’s common stock. Additionally, it is our intention that the issuance of additional shares and net proceeds from this public offering will allow us to regain compliance with the Minimum Float Requirement and the Minimum Stockholders’ Equity Requirement. We believe that if we are unable to regain compliance with all applicable Nasdaq continued listing requirements, then it is likely that our common stock will be delisted from the Nasdaq Capital Market.
While we have regained compliance in the past, if, for any reason, Nasdaq were to delist our securities from trading on The Nasdaq Capital Market and we were unable to obtain listing on another reputable national securities exchange, a reduction in some or all of the following may occur, each of which could materially adversely affect our stockholders:
| |
●
|
the liquidity and marketability of our common stock;
|
| |
●
|
the market price of our common stock;
|
| |
●
|
our ability to obtain financing for the continuation of our operations;
|
| |
●
|
the number of institutional and general investors that will consider investing in our common stock;
|
| |
●
|
the number of market makers in our common stock;
|
| |
●
|
the availability of information concerning the trading prices and volume of our common stock; and
|
| |
●
|
the number of broker-dealers willing to execute trades in shares of our common stock.
|
In addition, if we cease to be listed on The Nasdaq Capital Market, we may have to pursue trading on a less recognized or accepted market, such as the over the counter markets, our stock may be traded as a “penny stock”, which would make transactions in our stock more difficult and cumbersome, and we may be unable to access capital on favorable terms or at all, as companies trading on alternative markets may be viewed as less attractive investments with higher associated risks, such that existing or prospective institutional investors may be less interested in, or prohibited from, investing in our common stock. This may also cause the market price of our common stock to further decline.
We cannot assure that the reverse stock split, will increase our stock price for the required time period or maintain our listing on Nasdaq.
The reverse stock split has been accompanied by an increase the market price of our common stock; however, the ongoing effect of such a reverse stock split on the market price of our common stock cannot be predicted with any certainty, and the history of reverse stock splits for other companies is varied. Some investors may view the recent reverse stock split negatively.
While Nasdaq rules do not impose a specific limit on the number of times a listed company may effect a reverse stock split to maintain or regain compliance with the Minimum Bid Price Requirement, Nasdaq has stated that a series of reverse stock splits may undermine investor confidence in securities listed on Nasdaq, especially where the reverse stock splits follow dilutive transactions. Accordingly, Nasdaq may determine that it is not in the public interest to maintain our listing, even if we regain compliance with the Minimum Bid Price Requirement as a result of any reverse stock split. Because our Company has effectuated reverse stock splits over the prior two-year period with a cumulative ratio in excess of 250 shares to one, and common stock has failed to meet the Minimum Bid Price Requirement, we are not currently eligible for any compliance cure period specified in Nasdaq Marketplace Rule 5810(c)(3)(A) and, as discussed above, the Nasdaq staff has issued a delisting determination for noncompliance with the Minimum Bid Price Requirement.
Furthermore, the reverse stock split may not result in a per share price that attracts investors who do not trade in lower priced stocks. Although we believe the reverse stock split may enhance the marketability of our common stock to certain potential investors, we cannot assure you that our common stock will be more attractive to investors following the reverse stock split. Even though we have implemented a reverse stock split, the market price of our common stock may decrease due to factors unrelated to the reverse stock split, including our future performance or general market trends. If the trading price of the common stock declines, the percentage declines as an absolute number and as a percentage of our overall market capitalization may be greater than would occur in the absence of a reverse stock split.
the recent reverse stock split may decrease the liquidity of our common stock and result in higher transaction costs.
The liquidity of our common stock may be negatively impacted by a reverse stock split, given the reduced number of shares that would be outstanding after such a reverse stock split, particularly if the stock price does not increase as a result of the reverse stock split. Additionally, if the reverse stock split is implemented, it will increase the number of our stockholders who own “odd lots” of fewer than 100 shares of common stock. Brokerage commissions and other costs of transactions in odd lots are generally higher than the costs of transactions of more than 100 shares of common stock. Accordingly, the reverse stock split may not achieve the desired results of increasing marketability of our common stock as described above.
The reverse stock split was not accompanied by a decrease in our authorized shares.
Although the reverse stock split did not have any dilutive effect on our stockholders, the reduction in outstanding shares that resulted from the reverse stock split reduced the proportion of shares owned by our stockholders relative to the number of shares authorized for issuance, giving the Board of Directors an effective increase in the relative number of authorized shares available for issuance, in its discretion. The Board of Directors from time to time may deem it to be in the best interests of the Company and its stockholders to enter into transactions and other ventures that may include the issuance of shares of our common stock. If the Board of Directors authorizes the issuance of additional shares of common stock subsequent to a reverse stock split, the dilution to the ownership interest of our existing stockholders may be greater than would occur had such reverse stock split not been effected.
Raising additional capital may cause dilution to our stockholders or restrict our operations.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, stockholders’ ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect their rights as stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. Any of these events could adversely affect our ability to achieve our product development and commercialization goals and harm our business. We do not anticipate any adverse effects stemming from the lack of available credit facilities at this time.
Issuances of common stock in offerings or pursuant to the exercise of rights to purchase shares may cause the price of our common stock to decline and cause investors to lose a significant portion of their investment.
If our stockholders sell substantial amounts of our common stock in the public market or upon the expiration of any statutory holding period under Rule 144, or upon expiration of lock-up periods applicable to outstanding shares or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether sales have occurred or are occurring, also could make our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate more difficult. As of January 23, 2024, we had outstanding options to purchase 599 shares of our common stock at a weighted-average exercise price of $13,297.06 per share with a remaining contractual life of 8.7 years and outstanding warrants to purchase 340,952 of common stock at a weighted-average exercise price of $64.09 per share and an average remaining exercise period of 5.24 years.
Securities analysts may not initiate coverage or continue to cover our common stock, and this may have a negative impact on the market price of our common stock.
Common stock prices are often significantly influenced by the research and reports that securities analysts publish about companies and their business. We do not have any control over these analysts. There is no guarantee that securities analysts will cover, or continue to cover, our common stock. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of our common stock. If our common stock is covered by securities analysts and our stock is downgraded, our stock price will likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, we can lose visibility in the financial markets, which can cause our stock price or trading volume to decline.
If you purchase shares of common stock in this offering, you will incur immediate and substantial dilution in the book value of the shares of our common stock.
The proposed public offering price of the shares of our common stock is substantially higher than the net tangible book value per share of our common stock. Investors purchasing shares of common stock in this offering will pay a price per share that substantially exceeds the book value of our tangible assets after subtracting our liabilities. As a result, investors purchasing shares of common stock in this offering will incur immediate dilution of $0.209 per share, based on an assumed combined public offering price of $3.77 per share and accompanying common warrants (assuming a public offering price equal to the last sale price of our common stock as reported by the Nasdaq Capital Market on January 23, 2024, which was $3.77). Further, investors purchasing shares of common stock in this offering will contribute approximately 26% of the total amount invested by shareholders since our inception, but will own, as a result of such investment, approximately 94% of the shares of common stock outstanding immediately following this offering. As a result of the dilution to investors purchasing shares of common stock in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation. Further, because we may need to raise additional capital to fund our anticipated level of operations, we may in the future sell substantial amounts of common stock or securities convertible into or exchangeable for common stock. These future issuances of equity or equity-linked securities, together with the exercise of outstanding options and any additional shares issued in connection with acquisitions, if any, may result in further dilution to investors.
Holders of our warrants will have no rights as a common stockholder until they exercise their warrants and acquire our common stock.
Until you acquire shares of our common stock upon exercise of your warrants, you will have no rights with respect to shares of our common stock issuable upon exercise of your warrants. Upon exercise of your warrants, you will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date
The common warrants are speculative in nature.
The common warrants offered pursuant to this prospectus do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of our common stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the common warrants may exercise their right to acquire the common stock and pay an exercise price of $ per share (100% of the public offering price per share and common warrants), prior to five years from the date of issuance, after which date any unexercised common warrants will expire and have no further value. After five business days, Holders of Class E Warrants will have a separate option to elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal 1.33 times the number of shares of common stock that would be issuable upon a cash exercise. Moreover, following this offering, the market value of the common warrants is uncertain and there can be no assurance that the market value of the common warrants will equal or exceed their public offering price. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the common warrants, and, consequently, whether it will ever be profitable for holders of the common warrants to exercise those warrants.
There is no established public trading market for the warrants being offered in this offering.
There is no established public trading market for the common warrants or the pre-funded warrants being offered in this offering. We do not intend to apply to list the common warrants or the pre-funded warrants to be issued in this offering on any national securities exchange or to seek qualification of the common warrants or the pre-funded warrants for quotation on the over-the-counter markets. Without an active trading market, the liquidity of the common warrants and the pre-funded warrants will be limited without first exercising them.
This is a best efforts offering, no minimum amount of securities is required to be sold, and we may not raise the amount of capital we believe is required for our business plans.
The placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities being offered in this offering. The placement agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. This offering will terminate on February 15, 2024, unless completed sooner or unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. There is no required minimum number of securities or amount of proceeds that must be sold as a condition to completion of this offering. Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above. We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to fund for our operations as described in the “Use of Proceeds” section herein. Thus, we may not raise the amount of capital we believe is required for our operations in the short term and may need to raise additional funds, which may not be available or available on terms acceptable to us.
Our charter documents and Delaware law may inhibit a takeover that stockholders consider favorable.
The provisions of our certificate of incorporation and bylaws and applicable provisions of Delaware law may make it more difficult for or prevent a third party from acquiring control of us without the approval of our board of directors. These provisions:
| |
●
|
set limitations on the removal of directors;
|
| |
●
|
limit who may call a special meeting of stockholders;
|
| |
●
|
establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon at stockholder meetings;
|
| |
●
|
do not permit cumulative voting in the election of our directors, which would otherwise permit less than a majority of stockholders to elect directors;
|
| |
●
|
establish a classified board of directors limiting the number of directors that are elected each year; and
|
| |
●
|
provide our board of directors with the ability to designate the terms of and issue preferred stock without stockholder approval.
|
In addition, Section 203 of the Delaware General Corporation Law generally limits our ability to engage in any business combination with certain persons who own 15% or more of our outstanding voting stock or any of our associates or affiliates who at any time in the past three years have owned 15% or more of our outstanding voting stock unless our board of directors has pre-approved the acquisitions that lead to such ownership. These provisions may have the effect of entrenching our management team and may deprive stockholders of the opportunity to sell their shares to potential acquirers at a premium over prevailing prices. This potential inability to obtain a control premium could reduce the price of our common stock.
If we issue preferred stock, the rights of the holders of our common stock and the value of such common stock could be adversely affected.
Our Board of Directors is authorized to issue classes or series of preferred stock, without any action on the part of the stockholders. The Board of Directors also has the power, without stockholder approval, to set the terms of any such classes or series of preferred stock, including voting rights, dividend rights and preferences over the common stock with respect to dividends or upon the liquidation, dissolution or winding-up of our business and other terms. If we issue preferred stock in the future that has a preference over the common stock with respect to the payment of dividends or upon liquidation, dissolution or winding-up, or if we issue preferred stock with voting rights that dilute the voting power of the common stock, the rights of holders of the common stock or the value of the common stock would be adversely affected.
If we fail to maintain effective internal controls over financial reporting, the price of our common stock may be adversely affected.
We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. Any failure of these controls could also prevent us from maintaining accurate accounting records and discovering accounting errors and financial fraud. Management’s assessment of internal controls over financial reporting may identify weaknesses that need to be addressed or other potential matters that may raise concerns for investors. Any actual or perceived weaknesses that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
Even if this offering is completed, we will need to raise additional capital in the future to finance our operations, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
We have had recurring losses from operations, negative operating cash flow and have an accumulated deficit. We must raise additional funds in order to continue financing our operations. If additional capital is not available to us when needed or on acceptable terms, we may not be able to continue to operate our business pursuant to our business plan or we may have to discontinue our operations entirely. Any additional capital raised through the sale of equity or equity-backed securities may dilute our stockholders’ ownership percentages and could also result in a decrease in the market value of our equity securities. The terms of any securities issued by us in future capital transactions may be more favorable to new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect on the holders of any of our securities then outstanding.
If we are unable to secure additional funds when needed or on acceptable terms, we may be required to defer, reduce or eliminate significant planned expenditures, restructure, curtail or eliminate some or all of our operations, dispose of technology or assets, pursue an acquisition of our company by a third party at a price that may result in a loss on investment for our stockholders, file for bankruptcy or cease operations altogether. Any of these events could have a material adverse effect on our business, financial condition and results of operations. Moreover, if we are unable to obtain additional funds on a timely basis, there will be substantial doubt about our ability to continue as a going concern and increased risk of insolvency and up to a total loss of investment by our stockholders.
USE OF PROCEEDS
We estimate that we will receive net proceeds of up to approximately $27.8 million from the sale of the securities by us in this offering, based on an assumed combined public offering price of $3.77 per share and accompanying common warrants (based on the last sale price of our common stock as reported by the Nasdaq Capital Market on January 23, 2024, which was $3.77), after deducting the placement agent fees and estimated offering expenses payable by us, and excluding the proceeds, if any, received from the exercise of warrants issued in this offering.
We intend to use the net proceeds from the sale of any securities for (i) the continued clinical development of our initial product candidate ivospemin, (ii) cost of drug product for use in clinical development with collaboration partners of CPP assets, and (iii) general corporate purposes unless otherwise indicated in the applicable prospectus supplement. General corporate purposes may include the repayment of outstanding indebtedness, working capital, general and administrative expenses. We may also use a portion of the net proceeds to invest in or acquire businesses or technologies that we believe are complementary to our own, although we have no current plans, commitments or agreements with respect to any acquisitions as of the date of this prospectus supplement. We have not yet determined the amount of net proceeds to be used specifically for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds from the sale of these securities.
We are party to a guaranty (the “Guaranty”) pursuant to which we have agreed to guarantee the payment obligations of CPP, under a promissory note in favor of Sucampo GmbH dated as of June 15, 2022, (the “Note”), which had a principal balance of approximately $5.2 million as of September 30, 2023. CPP is required to make four remaining payments of $1 million, plus accrued but unpaid interest, on January 31st of each of 2024, 2025, 2026, with the remaining balance due on January 31, 2027. The first installment of $1.0 million plus accrued interest was paid on February 1, 2023.
Our expected use of net proceeds from this offering represents our intentions based on our present plans and business conditions, which could change as our plans and business conditions evolve. The amount and timing of our actual expenditures will depend on numerous factors, including the timing and success of clinical studies or clinical studies we may commence in the future, the timing of regulatory submissions and the feedback from regulatory authorities. As a result, our management will have broad discretion over the use of the net proceeds from this offering. Pending our use of the net proceeds from this offering, we may temporarily invest the net proceeds in investment-grade, interest-bearing securities.
We currently estimate the funds will allow us to make significant progress in the conduct of our new randomized double-blind, placebo-controlled clinical trial (known as the ASPIRE trial) for the treatment of pancreatic ductile adenocarcinoma. Continuation of the current trial, if the interim analysis is positive, will be required for FDA or other similar approvals. The cost and timing of additional clinical trials are highly dependent on the number of indications we pursue and the nature and size of the trials. It is estimated that the completion of the randomized clinical trial and other steps in the approval process for ivospemin in pancreatic cancer could cost between $60 and $80 million.
Predicting the cost necessary to develop product candidates can be difficult and we anticipate we will need additional funds to complete the development work generally required for obtaining regulatory approval to commercialize a drug. We have based these estimates on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect.
MARKET INFORMATION
Our common stock is listed on the Nasdaq Capital Market under the symbol “PBLA.” As of January 23, 2024, there were 49 holders of record of our common stock.
CAPITALIZATION
The following table presents a summary of our cash and cash equivalents and capitalization as of September 30, 2023:
| |
●
|
on an actual basis as of September 30, 2023;
|
| |
●
|
as adjusted for the issuance of 330,470 shares issued for exercises of warrants to purchase shares of common stock, which exercises occurred after September 30, 2023 and 132 shares issued primarily as the result of the cashless exchange for warrants which occurred after September 30, 2023;
|
| |
●
|
on an as adjusted basis to give effect to the issuance and sale of 8,000,000 shares of our common stock and common warrants to purchase up to 16,660,000 shares of our common stock in this offering at an assumed combined public offering price of $3.77 per share and accompanying common warrants less placement agent fees and estimated offering expenses payable by us, for total net proceeds of up to approximately $27.8 million (assuming no sale of pre-funded warrants). |
The unaudited as adjusted information below is prepared for illustrative purposes only and our capitalization following the completion of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read the following table in conjunction with Management’s Discussion and Analysis of Financial Condition and Results of Operations and the historical financial statements and related notes thereto incorporated herein by reference.
|
(in thousands)
|
|
Actual as of
September
30, 2023
(unaudited)
|
|
|
As of
September
30, 2023
(after
reflecting
subsequent events)
|
|
|
Offering
Adjustment
|
|
|
Pro Forma
as
Adjusted
|
|
|
Cash
|
|
$ |
907 |
|
|
$ |
5,817 |
|
|
$ |
27,772 |
|
|
$ |
33,589 |
|
| Common stock, $0.001 par value, 100,000,000 shares authorized; 149,656 shares issued and outstanding, actual; 480,244 shares issued and outstanding, after subsequent events; 8,480,244 shares issued and outstanding, pro forma as adjusted |
|
|
- |
|
|
|
0.4 |
|
|
|
8.0 |
|
|
|
8.4 |
|
|
Additional paid-in capital
|
|
|
106,029 |
|
|
|
110,939 |
|
|
|
27,764 |
|
|
|
138,703 |
|
|
Accumulated deficit
|
|
|
(109,883 |
)
|
|
|
(109,883 |
)
|
|
|
- |
|
|
|
(109,883 |
)
|
|
Accumulated comprehensive income
|
|
|
1,371 |
|
|
|
1,371 |
|
|
|
- |
|
|
|
1,371 |
|
|
Total stockholders’ equity
|
|
$ |
(2,483 |
)
|
|
$ |
2,427 |
|
|
$ |
27,772 |
|
|
|
30,199 |
|
Each $0.50 increase (decrease) in the assumed public offering price of $3.77 per share and common warrants would increase (decrease) each of cash, additional paid-in capital, total shareholders’ equity by approximately $3.7 million, assuming the number of shares of common stock and common warrants offered, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated placement agent fees and estimated offering expenses. Similarly, each increase (decrease) of 200,000 shares in the number of shares of common stock and common warrants offered would increase (decrease) cash, additional paid-in capital, total shareholders’ equity by approximately $0.7 million, assuming the assumed public offering price remains the same, and after deducting estimated placement agent fees and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
The number of shares of our common stock outstanding before and after this offering is based on 149,656 shares of our common stock outstanding as of September 30, 2023, and 330,588 shares issued primarily as the result of the exercise of warrants, primarily for cash, which occurred after September 30, 2023, and excludes:
| |
●
|
all shares issuable upon the exercise of warrants sold in this offering;
|
| |
|
|
| |
● |
pending settlements of fractional shares for cash in connection with the reverse stock split effected as of January 18, 2024; |
| |
●
|
599 shares of common stock issuable upon the exercise of outstanding stock options at a weighted average exercise price of $13,297.06 per share; and |
| |
●
|
340,952 shares of common stock issuable upon exercise of outstanding stock purchase warrants, including warrants issued subsequent to September 30, 2023 but not relating to this offering at a weighted average exercise price of $64.09 per share.
|
DILUTION
If you purchase shares of our common stock, your interest will be diluted immediately to the extent of the difference between the offering price per share you will pay in this offering and the as adjusted net tangible book value per share of our common stock after this offering. Net tangible book value per share represents our total tangible assets less total liabilities, divided by the number of shares of our common stock outstanding.
As of September 30, 2023, after giving effect to the issuance of 330,588 issued as the result of the exercise of warrants, primarily for cash, which occurred after September 30, 2023 our net tangible book value was approximately $2.4 million, or $5.054 per share of common stock.
After giving effect to the foregoing pro forma adjustments and the sale by us of all 8,000,000 shares of common stock at an assumed public offering price of $3.77 per share and accompanying common warrants and after deducting the placement agent fees and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2023, would have been approximately $30.2 million, or $3.561 per share. This represents an immediate decrease in as adjusted net tangible book value of approximately $1.493 per share to our existing stockholders, and an immediate dilution of $0.701 per share to purchasers of shares in this offering, as illustrated in the following table:
|
Assumed public offering price per share
|
|
$ |
3.770 |
|
|
Pro forma net tangible book value per share as of September 30, 2023
|
|
$ |
5.054 |
|
|
Increase per share attributable to new investors
|
|
$ |
(1.493) |
|
|
As adjusted net tangible book value per share after this offering
|
|
$ |
3.561 |
|
|
Dilution per share to new investors in the offering
|
|
$ |
0.209 |
|
A $0.50 increase or decrease in the assumed public offering price of $3.77 per share and common warrants would increase or decrease the dilution per share to new investors in the offering by approximately $0.06 per share, if the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated placement agent fees and estimated offering expenses.
The number of shares of our common stock outstanding before and after this offering is based on 149,656 shares of our common stock outstanding as of September 30, 2023, and 330,588 shares issued primarily as the result of the exercise of warrants, primarily for cash, which occurred after September 30, 2023:
| |
●
|
all shares issuable upon the exercise of warrants sold in this offering;
|
| |
●
|
pending settlements of fractional shares for cash in connection with the reverse stock split effected as of January 18, 2024;
|
| |
●
|
599 shares of common stock issuable upon the exercise of outstanding stock options at a weighted average exercise price of $13,297.06 per share; and
|
| |
●
|
340,952 shares of common stock issuable upon exercise of outstanding stock purchase warrants, including warrants issued subsequent to September 30, 2023 but not relating to this offering at a weighted average exercise price of $64.09 per share.
|
DIVIDEND POLICY
We have never declared or paid cash dividends on our capital stock. Following the completion of this offering, we intend to retain our future earnings, if any, to finance the further development and expansion of our business and do not expect to pay cash dividends in the foreseeable future. Payment of future cash dividends, if any, will be at the discretion of our Board of Directors after various factors, including our financial condition, operating results, current and anticipated cash needs, outstanding indebtedness, plans for expansion and restrictions imposed by lenders, if any.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the notes to those financial statements included elsewhere in this prospectus. This discussion contains forward-looking statements, which are based on our assumptions about the future of our business. Our actual results could differ materially from those contained in the forward-looking statements. Please read “Cautionary Note Regarding Forward-Looking Statements” included elsewhere in this prospectus for additional information regarding forward-looking statements used in this prospectus.
The Management Discussion and Analysis for the three and nine months ended September 30, 2023 versus the three and nine months ended September 30, 2022 and for the fiscal years ended December 31, 2022 and 2021 are historical. Share and per share amounts for three and nine months ended September 30, 2022 have been restated for comparison purposes for the 1-for-40 reverse stock split that was effected on January 13, 2023 or the 1-for-30 reverse stock split that was effected on June 1, 2023. No share or per share amounts for three and nine months ended September 30, 2023 versus the three and nine months ended September 30, 2022 and for the fiscal years ended December 31, 2022 and 2021 or for events which occurred subsequent to September 30, 2023 and included in the Management Discussion and analysis have been restated for the 1-for-20 reverse stock split that was effected on January 18, 2024.
Overview
Panbela Therapeutics, Inc. (“Panbela” and together with its direct and indirect subsidiaries, “we,” “us,” “our,” and the “Company”) is a clinical stage biopharmaceutical company developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs.
Our lead candidates are ivospemin (SBP-101) for which we have exclusively licensed the worldwide rights from the University of Florida Research Foundation, Inc. and Flynpovi (eflornithine (CPP-1X) and Sulindac). Flynpovi is delivered in an oral form. The Company has an exclusive worldwide license to commercialize Flynpovi from the Arizona Board of Regents of the University of Arizona.
As Panbela is focused on utilizing a polyamine platform to develop disruptive therapeutics for the treatment of patients with urgent unmet medical needs, we are engaged in two sponsored research agreements to evaluate the polyamines individually and combined for various diseases. At present, the collaboration with Johns Hopkins University School of Medicine has been focused on mechanism of action and solid tumors while the MD Anderson Cancer Center has been focused on the hematologic malignancies. An abstract about SBP-101 and CPP-1X (also known as DFMO or Eflornithine) research in multiple myeloma (cell lines), has been accepted for an online publication on the American Society of Hematology (ASH) meeting site in the November 2023 supplemental issue of the journal Blood.
Ivospemin (SBP-101)
In 2015, the FDA accepted our Investigational New Drug (“IND”) application for our ivospemin product candidate. In May of 2022 we were notified that the United States Adopted Names (“USAN”) had adopted ivospemin as a USAN for SBP-101. The USAN information on ivospemin was posted on the USAN Web site (www.ama-assn.org/go/usan).
We have completed an initial clinical trial of ivospemin in patients with previously treated locally advanced or metastatic pancreatic cancer. This was a Phase I, first-in-human, dose-escalation, safety study. From January 2016 through September 2017, we enrolled twenty-nine patients into six cohorts, or groups, in the dose-escalation phase of the Phase I trial. No drug-related bone marrow toxicity or peripheral neuropathy was observed at any dose level. In addition to being evaluated for safety, 23 of the 29 patients were evaluable for preliminary signals of efficacy prior to or at the eight-week conclusion of their first cycle of treatment using the Response Evaluation Criteria in Solid Tumors (“RECIST”), the currently accepted standard for evaluating change in the size of tumors.
In 2018, we began enrolling patients in our second clinical trial, a Phase Ia/Ib study of the safety, efficacy and pharmacokinetics of ivospemin administered in combination with two standard-of-care chemotherapy agents, gemcitabine and nab-paclitaxel. A total of 25 subjects were enrolled in four cohorts to evaluate the dosage level and schedule. An additional 25 subjects were enrolled in the expansion phase of the trial. Interim results were presented in January of 2022. Best response in evaluable subjects (cohorts 4 and Ib N=29) was a CR in 1 (3%), PR in 13 (45%), SD in 10 (34%) and PD in 5 (17%). One subject did not have post baseline scans with RECIST tumor assessments. Median PFS, now final at 6.5 months, may have been negatively impacted by drug dosing interruptions to evaluate potential toxicity. Median overall survival in cohort 4 + Phase Ib was 12.0 months when data was presented in January 2022 and is now final at 14.6 months. Two patients from cohort 2 have demonstrated long term survival. One at 30.3 months (final data) and one at 33.0 months and still alive at database lock on March 18, 2022. Seven subjects are still alive at database lock, one from cohort 2 and six from cohort 4 plus Phase Ib.
In January of 2022, the Company announced the initiation of a new clinical trial. Referred to as ASPIRE, the trial is a randomized double-blind placebo-controlled trial in combination with gemcitabine and nab-paclitaxel, a standard pancreatic cancer treatment regimen in patients previously untreated for metastatic pancreatic cancer. The trial will be conducted globally at approximately 94 sites in the United States, Europe and Asia – Pacific. The Company announced the first patient enrolled in the trial in Australia in August of 2022. In September 2022, the company announced that they had obtained regulatory approval to open sites in Spain, France and Italy. On September 30, 2023 there were 81 sites open in 10 countries.
While opening of clinical sites in the United States and the rest of the world has been slower than originally anticipated, due in part to resource fatigue in the medical community, the Company expects all countries and sites to be open by mid-2023.
The trial was originally designed as a Phase II/III with a smaller initial sample size. In response to European and FDA regulatory feedback, the study was amended to include the total trial sample size (600) and the design modified to utilize overall survival (the primary endpoint) to be examined at interim analysis as well. All Countries are open and the full complement of sites is expected to be open by year-end. The independent Data Safety Monitoring Board (DSMB) met for a prespecified safety analysis and recommended the trial continue without modification. The study is anticipated to take 36 months for complete enrollment of 600 subject with the interim analysis available in early 2024. On January 25, 2024, the Company announced that the trial had exceeded 50% enrollment. The Company projects that full enrollment will be completed by the first quarter of 2025 and that interim data analysis based on overall survival should be available by the middle of 2024.
In early April 2023 the Company announced a poster presentation highlighting the results for ivospemin as a polyamine metabolism modulator in ovarian cancer at the American Association for Cancer Research Annual Conference. The poster concludes that the ivospemin chemotherapy treatment of C57Bl/6 mice injected with VDID8+ ovarian cancer cells significantly prolonged survival and decreased overall tumor burden. The results suggest that ivospemin in combination with standard of care chemotherapy may have a role in the clinical management of ovarian cancer, and the Company intends to continue pre-clinical and clinical studies in ovarian cancer.
Additional clinical trials may be required for FDA or other country approvals. The cost and timing of additional clinical trials are highly dependent on the nature and size of the trials.
Flynpovi (eflornithine (CPP-1X) and sulindac)
In 2009, the FDA accepted our IND application for the combination product, Flynpovi, product candidate.
In a Phase III study, the efficacy and safety of the combination of eflornithine and sulindac known as Flynpovi, as compared with either drug eflornithine or sulindac alone, in adults with familial adenomatous polyposis (“FAP”) was conducted. A total of 171 patients underwent randomization. Disease progression occurred in 18 of 56 patients (32%) in the Flynpovi group, 22 of 58 (38%) in the sulindac group, and 23 of 57 (40%) in the eflornithine group, with a hazard ratio of 0.71 (95% confidence interval [CI], 0.39 to 1.32) for Flynpovi as compared with sulindac (p = 0.29) and 0.66 (95% CI, 0.36 to 1.23) for Flynpovi as compared with eflornithine. In a post-hoc analysis, none of the patients in the Flynpovi arm progressed to a need for lower gastrointestinal (“LGI”) surgery for up to 48 months compared with 7 (13.2%) and 8 (15.7%) patients in the sulindac and eflornithine (CPP-1X) arms. These data corresponded to risk reductions for the need for LGI surgery approaching 100% between Flynpovi and either monotherapy with HR = 0.00 (95% CI, 0.00–0.48; p = 0.005) for Flynpovi versus sulindac and HR = 0.00 (95% CI, 0.00–0.44; p = 0.003) for Flynpovi versus eflornithine. Given the statistical significance of the LGI group, a new drug application (“NDA”) was filed with the FDA. As the study failed to meet the primary endpoint, and the NDA was based on the results of an exploratory analysis, a complete response letter was issued. To address this deficiency concern, the Company must submit the results of one or more adequate and well-controlled clinical trials which demonstrate an effect on a clinical endpoint.
In April of 2023 the Company regained the North American rights to develop and commercialize Flynpovi in patients with FAP, as a result of the termination of the licensing agreement between CPP and One-Two Therapeutics Assets Limited.
We also have an ongoing double-blind placebo-controlled trial of Flynpovi to prevent recurrence of high-risk adenomas and second primary colorectal cancers in patients with stage 0-III colon or rectal cancer, Phase III – Preventing Adenomas of the Colon with Eflornithine and Sulindac (“PACES”). The purpose of this study is to assess whether the combination of eflornithine and sulindac (compared to corresponding placebos) has efficacy against colorectal lesions with respect to high-grade dysplasia, adenomas with villous features, adenomas one cm or greater, multiple adenomas, any adenomas >/= 0.3 cm, total advanced colorectal events, or total colorectal events. The PACES trial is funded by the National Cancer Institute (“NCI”) in collaboration with Southwest Oncology Group (“SWOG”). The Company announced on June 28, 2023 that the PACES trial passed a pre-planned futility analysis.
Eflornithine (CPP-1X)/eflornithine sachets (CPP-1X-S)
In 2009 and 2018, the FDA accepted our IND applications for eflornithine.
There is a trial evaluating eflornithine sachets in STK11 mutation patients with non-small cell lung cancer scheduled to begin this year. For eflornithine tablets, a Phase II trial in early onset Type I diabetes was opened on January 11, 2023 in collaboration with Indiana University and the Juvenile Diabetes Research Foundation (“JDRF”). Two poster presentations were given discussing the Phase I T1D results, one at the Endocrine Society meeting and the other at the Immunology of Diabetes Society Meeting in June 2023. Additionally, eflornithine is being evaluated with high dose testosterone and enzalutamide in metastatic castration-resistant prostate cancer in a Phase II trial.
On July 17, 2023, the Company divested certain rights, titles and interests in its eflornithine pediatric neuroblastoma program. Included in these assets is an ongoing trial evaluating eflornithine sachets in relapsed refractory neuroblastoma supported by the Children’s Oncology Group (“COG”) /NCI Under the terms of the agreement with US World Meds®, the Company is entitled to receive up to approximately $9.5 million in non-dilutive funding in exchange for the sale of these assets. An initial payment of $400,000 was received by the Company at the time of closing, remaining payments will be receivable if the acquiring company successfully completes certain milestones related to clinical development, regulatory approval and commercial sales.
Financial Overview
On June 1, 2023, we effected a reverse stock split at a ratio of one-for-forty (1:30) shares of the Company’s common stock and on January 13, 2023, we effected a reverse stock split at a ratio of one-for-forty (1:40) shares of the Company’s common stock. All share and per share amounts of our common stock presented have been retroactively adjusted to reflect these reverse stock splits.
We have incurred losses of $109.9 million since 2011. For the nine months ended September 30, 2023 we incurred a net loss of $18.8 million. We also incurred negative cash flows from operating activities of approximately $21.8 million for this period. We expect to continue to incur substantial losses, which will generate negative net cash flows from operating activities, as we continue to pursue research and development activities and commercialize.
Our cash was approximately $0.9 million and $1.3 million as of September 30, 2023 and December 31, 2022, respectively. A decrease of $0.4 million in cash for the nine months ended September 30, 2023 was due to $21.8 million negative cash flow from operations offset in part to $21.4 million net financing activities. Due to drug shortages of Abraxane, which is utilized in addition to ivospemin for the current randomized clinical trial, the Company has become responsible for procuring this standard of care component to the clinical trial and in the quarter ended approximately $3.1 million was charged to research and development when the drug was made available to our clinical sites. The Company continues to explore all avenues to procure supply and prepayments of an additional approximately $0.5 million were required in the third quarter. These prepayments are required well in advance of delivery and will be held on the balance sheet as a prepaid expense and reflected in the periods cash used in operations. Net financing activities included a registered public offering of common stock, pre-funded warrants, and warrants with net proceeds of approximately $23.0 million. The Company also sold common stock through its at-the market sales arrangement, with net proceeds of approximately $1.6 million. In the same period, the Company also recorded $1.6 million in loan repayments.
We need to raise additional capital to continue our operations and execute our business plan past the third quarter of 2023 including completing required future trials and pursuing regulatory approvals in the United States, the European Union, and other international markets. Historically we have financed our operations principally from the sale of equity securities and debt. While we have been successful in the past in obtaining the necessary capital to support our operations and we are likely to seek additional financing through similar means, there is no assurance that we will be able to obtain additional financing under commercially reasonable terms and conditions, or at all. This risk would increase if our clinical data were not positive or if economic or market conditions deteriorate. The accompanying condensed consolidated financial statements have been prepared assuming that we will continue as a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business.
If we are unable to obtain additional financing when needed, we would need to scale back our operations, taking actions which may include, among other things, reducing use of outside professional service providers, reducing staff or staff compensation, significantly modifying, or delaying the development of our product candidates, licensing to third parties the rights to commercialize our product candidates, or ceasing operations.
The Company did not experience any significant disruptions to our operations as a result of the COVID-19 pandemic.
Warrant Exercise Inducements & Private Placement of Class C Warrants
On November 2, 2023, we entered into warrant exercise inducement offer letters with certain holders of existing warrants to purchase our common stock, pursuant to which the holders agreed to exercise for cash their existing warrants to purchase 2,130,000 shares of our common stock, in the aggregate, at a reduced exercise price of $0.78 per share, in exchange for our agreement to issue new Class C common stock purchase warrants to purchase up to an aggregate of 4,260,000 shares of our common stock. The Company received aggregate gross proceeds of approximately $1.9 million from the exercise of the existing warrants and purchase of the new warrants. The new warrants had an initial exercise price of $0.78 per share and were exercisable upon the date of stockholder approval through the date that is five years from the date of any stockholder approvals necessary under the listing rules of Nasdaq. Our stockholders approved the issuance of the underlying shares of common stock at a special meeting held on December 19, 2023. We agreed to file a registration statement covering the resale of the shares issued or issuable upon the exercise of the new warrants and a registration statement on Form S-1 (File No. 333-275733) was declared effective by the SEC on December 20, 2023. As of January 23, 2024 there are 1,504,000 Class C Warrants outstanding.
Warrant Exercise Inducements & Private Placement of Class D Warrants
On December 21, 2023, we entered into warrant exercise inducement offer letters with certain holders of existing warrants to purchase our common stock, pursuant to which the holders agreed to exercise for cash their existing warrants to purchase 2,556,000 shares of our common stock, in the aggregate, at their existing exercise price of $0.78 per share, in exchange for our agreement to issue new Class D common stock purchase warrants to purchase up to an aggregate of 5,112,000 shares of our common stock. The Company received aggregate gross proceeds of approximately $2.0 million from the exercise of the existing warrants. The new warrants had an initial exercise price of $0.95 per share and will only be exercisable contingent upon and after receiving stockholder approval as required by listing rules of Nasdaq and may be exercised until five years from the date of such stockholder approval, if any. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of our common stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities, upon any intervening reverse stock splits, and upon receipt of the stockholder approval. As of January 23, 2024 all of the new warrants remained outstanding but unexercisable pending stockholder approval. We have agreed to call a meeting to seek stockholder approval of the issuance of the shares of our common stock underlying the new warrants within six months and to file a registration statement covering the resale of the shares underlying the new warrants within sixty calendar days of December 21, 2023. Additionally, the Company agreed not to effect or agree to effect any variable rate transaction (as defined in the inducement letters) for one year, other than an at-the-market offering, which may be effected after six months.
Results of Operations
Comparison of the results of operations (in thousands):
| |
|
Three Months Ended September 30, |
|
|
|
|
|
Nine Months Ended September 30, |
|
|
|
|
|
| |
|
2023
|
|
|
2022
|
|
|
Percent
Change
|
|
|
2023
|
|
|
2022
|
|
|
Percent
Change
|
|
|
Operating Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative
|
|
$ |
1,107 |
|
|
$ |
1,294 |
|
|
|
-14.5 |
% |
|
$ |
4,102 |
|
|
$ |
4,349 |
|
|
|
-5.7 |
% |
|
Research and development
|
|
|
6,739 |
|
|
|
2,329 |
|
|
|
189.4 |
% |
|
|
14,501 |
|
|
|
24,563 |
|
|
|
-41.0 |
% |
|
Total operating expenses
|
|
|
7,846 |
|
|
|
3,623 |
|
|
|
116.6 |
% |
|
|
18,603 |
|
|
|
28,912 |
|
|
|
-35.7 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other expense, net
|
|
|
(4 |
) |
|
|
(835 |
) |
|
|
-99.5 |
% |
|
|
(353 |
) |
|
|
(1,390 |
) |
|
|
-74.6 |
% |
|
Income tax benefit
|
|
|
19 |
|
|
|
56 |
|
|
|
-66.1 |
% |
|
|
167 |
|
|
|
104 |
|
|
|
60.6 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$ |
(7,831 |
) |
|
$ |
(4,402 |
) |
|
|
77.9 |
% |
|
$ |
(18,789 |
) |
|
$ |
(30,198 |
) |
|
|
-37.8 |
% |
Research and development (“R&D”) and general and administrative (“G&A”) expenses include non-cash share-based compensation expense resulting from our issuance of stock options. We expense the fair value of equity awards over their vesting periods. The terms and vesting schedules for share-based awards vary by type of grant and the employment status of the grantee. The awards granted through September 30, 2023 vest upon performance or time-based conditions. We expect to record additional non-cash share-based compensation expense in the future, which may be significant.
The following table summarizes the stock-based compensation expense in our statements of comprehensive loss:
| |
|
Nine Months Ended September 30,
|
|
| |
|
2023
|
|
|
2022
|
|
|
General and administrative
|
|
$ |
554 |
|
|
$ |
697 |
|
|
Research and development
|
|
|
145 |
|
|
$ |
160 |
|
|
Total stock based compensation
|
|
$ |
699 |
|
|
$ |
857 |
|
Three months ended September 30, 2023 and September 30, 2022
General and administrative expense
Our G&A expenses decreased 14.5% to $1.1 million in the third quarter of 2023 down from $1.3 million in the third quarter of 2022. The decrease is due primarily to reduced legal and other professional services.
Research and development expense
Our R&D expenses increased 189.4% to $6.7 million in the third quarter of 2023 up from $2.3 million in the third quarter of 2022. The increase is primarily due to the cost of approximately $3.2 million or approximately 6 months’ supply of Abraxane a standard of care drug used in the ASPIRE clinical trial and first made available to the clinical sites during the three months ended September 30, 2023. The ASPIRE trial, and the need to supply standard of care drug to many sites, will continue to drive increased costs versus the prior year.
Other expense, net
Other expense, net, was approximately $4,000 for the three months ended September 30, 2023. Other expense for this period of $0.4 million from foreign currency exchange loss on the intercompany receivable balance and interest expense on one promissory note, was nearly offset by $0.4 million of other income from the gain on sale of assets and interest income on a money market account. Other expense, net, was approximately $0.9 million for the three months ended September 30, 2022. Other expense in the three months ended September 30, 2022, is related to foreign currency exchange loss on the intercompany receivable balance and interest expense on two promissory notes.
Income tax benefit
Income tax benefit decreased to $19,000 for the three months ended September 30, 2023 down from $56,000 for the three months ended September 30, 2022. Our income tax benefit is derived primarily from refundable tax credits associated with our R&D activities conducted in Australia. The ASPIRE trial is being conducted in several countries across the world, including five clinical sites in Australia as of September 30, 2023. Costs incurred to do research in Australia are available for this refundable credit.
Nine months ended September 30, 2023 and September 30, 2022
General and administrative expense
Our G&A expenses decreased 5.7% to $4.1 million in the nine months ended September 30, 2023 down from $4.3 million in the nine months ended September 30, 2022. The decrease is due primarily to professional services incurred in connection with the acquisition of CPP incurred in the nine months ended September 30, 2022.
Research and development expense
Our R&D expenses decreased 41.0% to $14.5 million in the nine months ended September 30, 2023 down $10.1 million from the nine months ended September 30, 2022. The decrease is primarily due to a $17.7 million IPR&D write-off in the second quarter of 2022 for the CPP acquisition. Exclusive of this one-time write-off, R&D increased $7.6 million in the first nine months of 2023 related to the cost of Abraxane and increased sites and subject enrollments in the ASPIRE trial. The ASPIRE trial will continue to drive increased costs versus the prior year.
Other expense, net
Other expense, net, was approximately $0.4 million for the nine months ended September 30, 2023. Other expenses for this period are related to foreign currency exchange loss on the intercompany receivable balance and interest expense on two promissory notes, partially offset by gain on sale of fixed assets and interest income on a money market account.
Other expense, net, was approximately $1.4 million for the nine months ended September 30, 2022, it is related to foreign currency exchange loss on the intercompany receivable balance.
Income tax benefit
Income tax benefit increased to $167,000 for the nine months ended September 30, 2023 up from $104,000 for the nine months ended September 30, 2022. Our income tax benefit is derived primarily from refundable tax credits associated with our R&D activities conducted in Australia which have started to increase versus last year due to the ASPIRE trial.
Comparison of the Results of Operations (in thousands) for the Years Ended December 31, 2022 and 2021
| |
|
Year Ended December 31, |
|
|
|
|
|
| |
|
2022
|
|
|
2021
|
|
|
Percent Change
|
|
|
Operating Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative
|
|
$ |
6,044 |
|
|
$ |
4,587 |
|
|
|
31.8 |
% |
|
Research and development
|
|
|
28,049 |
|
|
|
5,423 |
|
|
|
417.2 |
% |
|
Total operating expenses
|
|
|
34,093 |
|
|
|
10,010 |
|
|
|
240.6 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (expense) income, net
|
|
|
(956 |
) |
|
|
(613 |
) |
|
|
56.0 |
% |
|
Income tax benefit
|
|
|
116 |
|
|
|
488 |
|
|
|
-76.2 |
% |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$ |
(34,933 |
) |
|
$ |
(10,135 |
) |
|
|
244.7 |
% |
General and administrative (“G&A”) and research and development (“R&D”) expenses include non-cash stock-based compensation expense as a result of our issuance of stock options. The terms and vesting schedules for stock-based awards vary by type of grant and the employment status of the grantee. The awards granted through December 31, 2022 vest based upon time-based and performance conditions. We expect to record additional non-cash compensation expense in the future, which may be significant. The following table summarizes the stock-based compensation expense in our Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2022 and 2021 (in thousands):
| |
|
Year Ended December 31,
|
|
| |
|
2022
|
|
|
2021
|
|
| General and administrative |
|
$ |
889 |
|
|
$ |
1,083 |
|
|
Research and development
|
|
|
199 |
|
|
|
204 |
|
|
Total stock based compensation
|
|
$ |
1,088 |
|
|
$ |
1,287 |
|
General and administrative expense
G&A expenses increased 31.8% to $6.0 million in 2022, up from $4.6 million in 2021. The increase in G&A expenses is primarily the result of increased legal and financial advisor expenses.
Research and product development expense
Our R&D expenses increased 417.2% to $28.0 million in 2022, up from $5.4 million in 2021. After considering the non-cash write off of approximately $17.7 million of IPR&D, the remaining increase is due primarily to increased clinical trial costs related to our ivospemin (SBP-101) randomized trial. As we expand our clinical studies it is expected that R&D expenses will continue to increase. The write off of IPR&D is a one-time occurrence.
Other income (expense), net
Other expense, net, was $1.0 million and $0.6 million for the year ended December 31, 2022 and 2021 respectively and was composed primarily of foreign currency transaction loss.
Income tax benefit
Income tax benefit decreased to $116,000 in 2022, down from $488,000 in 2021. Our income tax benefit is derived primarily from refundable tax incentives associated with our R&D activities conducted in Australia which have significantly curtailed in 2022 as the Phase Ia/Ib trial completed and the ASPIRE trial had not yet opened all sites planned for in Australia.
Liquidity and Capital Resources
The following table summarizes our liquidity and capital resources as of September 30, 2023 and December 31, 2022, and our cash flow data for the nine months ended September 30, 2023 and 2022. It is intended to supplement the more detailed discussion that follows (in thousands):
|
Liquidity and Capital Resources
|
|
|
|
|
|
|
|
|
| |
|
September 30, 2023
|
|
|
December 31, 2022
|
|
|
Cash
|
|
$ |
907 |
|
|
$ |
1,285 |
|
|
Working capital (deficit)
|
|
$ |
(7,031 |
) |
|
$ |
(6,056 |
) |
|
Cash Flow Data
|
|
Nine Months Ended September 30,
|
|
| |
|
2023
|
|
|
2022
|
|
|
Cash Provided by (Used in):
|
|
|
|
|
|
|
|
|
|
Operating Activities
|
|
$ |
(22,169 |
) |
|
$ |
(10,273 |
) |
|
Investing Activities
|
|
|
400 |
|
|
|
(656 |
) |
|
Financing Activities
|
|
|
21,393 |
|
|
|
5 |
|
|
Effect of exchange rate changes on cash
|
|
|
(2 |
) |
|
|
(2 |
) |
|
Net increase (decrease) in cash
|
|
$ |
(378 |
) |
|
$ |
(10,926 |
) |
Working Capital
Our total cash and cash equivalents were $0.9 million and $1.3 million as of September 30, 2023, and December 31, 2022, respectively. We had $8.9 million in current liabilities and a working capital deficit of $7.0 million as of September 30, 2023, compared to $7.8 million in current liabilities and working capital deficit of $6.1 million as of December 31, 2022. Working capital is defined as current assets less current liabilities.
Cash Flows
Net Cash Used in Operating Activities
Net cash used in operating activities was approximately $21.8 million in the nine months ended September 30, 2023, compared to approximately $10.3 million in the nine months ended September 30, 2022. The net cash used in each of these periods primarily reflects the net loss for these periods and is partially offset by the effects of changes in operating assets and liabilities. For the nine months ended September 30, 2023, cash used in operating activities also included $5.5 million to fund long term deposits held by the CRO leading our randomized trial, offset in part by an increase in Accounts Payable as we slowed payments to the CRO for these Deposits. Also reflected in the increased cash used in operations is approximately $3.2 million to provide standard of care drug supply to the sites to support the Aspire Trial, and $0.5 million for prepayment of standard of care drug supply.
Net Cash Provided by Investing Activities
Cash provided by investing activities included the proceeds from the sale of intellectual property in the nine months ended September 30, 2023. In the nine months ended September 30, 2022, the cash incurred was related to banker and legal costs to acquire the in process research and development from CPP.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was approximately $21.4 million for the nine months ended September 30, 2023 with $5,000 provided by financing activities for the nine months ended September 30, 2022. The cash provided for the nine months ended September 30, 2023 represents the proceeds from the sale of common stock, pre-funded warrants and warrants, partially offset by the payments made on promissory notes. The cash provided for the nine months ended September 30, 2022 represents the proceeds from exercise of stock purchase warrants.
Capital Requirements
As we continue to pursue our operations and execute our business plan, including the completion of the clinical development plan for our initial product candidate, ivospemin, in pancreatic cancer, and pursuing regulatory approvals in the United States, the European Union and other international markets, we expect to continue to incur substantial and increasing losses, which will continue to generate negative net cash flows from operating activities.
Our future capital uses and requirements depend on numerous current and future factors. These factors include, but are not limited to, the following:
| |
●
|
the progress of clinical trials required to support our applications for regulatory approvals, including the completion of our global, randomized Phase II/III trial initiated in January of 2022;
|
| |
●
|
our ability to negotiate payment terms with critical vendors;
|
| |
●
|
the cost to implement development efforts for ivospemin in ovarian cancer and expand development efforts for assets acquired as the result of the acquisition of CPP;
|
| |
●
|
the cost, if any, to develop our product candidate, Flynpovi;
|
| |
●
|
the cost to develop eflornithine in various indications if early clinical trials underway now, and funded through third party collaborations, are successful;
|
| |
●
|
our ability to demonstrate the safety and effectiveness of our product candidates;
|
| |
●
|
our ability to obtain regulatory approval of our product candidates in the United States, the European Union or other international markets;
|
| |
●
|
the cost and delays in product development that may result from changes in regulatory oversight applicable to our product candidates;
|
| |
●
|
the market acceptance and level of future sales of our product candidates;
|
| |
●
|
the rate of progress in establishing reimbursement arrangements with third-party payors;
|
| |
●
|
the effect of competing technological and market developments; and
|
| |
●
|
the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims.
|
As of September 30, 2023, we did not have any existing credit facilities under which we could borrow funds. Historically we have financed our operations principally from the sale of equity securities and debt. While we have been successful in the past in obtaining the necessary capital to support our operations and we are likely to seek additional financing through similar means, there is no assurance that we will be able to obtain additional financing under commercially reasonable terms and conditions, or at all.
Indebtedness
CPP issued to Sucampo GmbH (“Lender”) an Amended and Restated Promissory Note (the “Note”) on June 15, 2022 for the principal sum of approximately $6.2 million (the “Principal”). The note bears simple interest on any outstanding Principal at a rate of 5% per annum. All unpaid Principal, together with any then unpaid and accrued interest, is payable as follows: (i) $1.0 million, plus all interest accrued but unpaid on or before each of January 31, 2024, January 31, 2025 and January 31, 2026; and (ii) all remaining Principal plus accrued but unpaid interest on or before January 31, 2027. The Company made the scheduled January 31, 2023 payment of $1.0 million plus accrued interest. The outstanding principal balance on September 30, 2023 was approximately $5.2 million. Accrued and unpaid interest as of September 30, 2023 totaled approximately $172,000.
Panbela has provided a Guarantee of payment in favor of the Lender for the full amount of the Note issued to the Lender.
Issuances of Common Stock and warrants during 2022 and 2021
On October 4, 2022, the Company completed a registered public offering and issued an aggregate of 177,175 shares of its common stock, pre-funded warrants to purchase up to an aggregate of 325,325 shares of common stock at an exercise price of $0.001 per shares and warrants to purchase up to an aggregate of 753,749 shares of its common stock. The exercise price on these warrants were repriced after a dilutive public offering that closed on January 30, 2023 to $1.51 per share. The securities were issued for a combined offering price of $12.00 per share of common stock and 1.5 warrants, or $11.999 per pre-funded warrant and 1.5 warrants. Net proceeds from the offering totaled approximately $5.3 million. All pre-funded warrants were exercised by December 31, 2022. The securities were offered pursuant to an effective registration statement on Form S-1.
On July 19, 2022, Panbela Therapeutics, Inc. (the “Company”), entered into a Sales Agreement with Roth Capital Partners, LLC (the “Agent”) to sell shares of the Company’s common stock having an aggregate gross sales price of up to $8,400,000, from time to time, through an “at-the-market” equity offering program (the “ATM Program”). During the last month of the year ended December 31, 2022, the Company sold 28,343 shares of common stock under the ATM Offering and generated approximately $93,000 in gross proceeds. The Company incurred financing costs of approximately $44,000, which were charged to additional paid in capital in December 2022 when the Company began selling shares under the ATM Offering. Under the ATM Program, the Company pays the Agent a commission equal to 3.0% of the aggregate gross proceeds of any sales of common stock under the ATM Program. Net proceeds for the year ended December 31, 2022 was approximately $46,000.
On July 2, 2021, the Company completed an underwritten public offering of 83,333 shares of its common stock for a purchase price of $120.00 per share. The gross proceeds from the offering was approximately $10.0 million. The net proceeds, after deducting the underwriter’s discount and other offering costs, was approximately $9.1 million.
Future Capital Requirements
We require additional funds to continue our operations and execute our business plan, including completion of required future trials and pursuing regulatory approvals in the United States, the European Union and other international markets. We historically have financed our operations principally from the sale of equity securities and debt. While we have been successful in the past in obtaining the necessary capital to support our operations and we are likely to seek additional financing through similar means, there is no assurance that we will be able to obtain additional financing under commercially reasonable terms and conditions, or at all. We believe that our existing cash and cash raised through public offerings in January 2023 will be sufficient to fund our operating expenses into the third quarter of 2023.
If we are unable to obtain additional financing when needed, we would need to scale back our operations, taking actions which may include, among other things, reducing use of outside professional service providers, reducing staff or staff compensation, significantly modifying or delaying the development of our product candidates, licensing to third parties the rights to commercialize our product candidate for pancreatic cancer or other applications that we would otherwise seek to pursue, or suspending operations.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, the interests of our current stockholders would be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our current stockholders. If we issue preferred stock, it could affect the rights of our stockholders or reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock may include voting rights, preferences as to dividends and liquidation, conversion and redemption rights, sinking fund provisions, and restrictions on our ability to merge with or sell our assets to a third party. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any of these events could adversely affect our ability to achieve our regulatory approvals and commercialization goals and harm our business.
Our future success is dependent upon our ability to obtain additional financing, Phase II/III clinical trials and required future trials, and our ability to obtain marketing approval for our product candidates in the United States, the European Union and other international markets. If we are unable to obtain additional financing when needed, if our Phase II/III clinical trial is not successful, if we do not receive regulatory approval required for future trials or if once these studies are concluded, we do not receive marketing approval for our SBP-101 product candidate, we would not be able to continue as a going concern and would be forced to cease operations. The financial statements included in this prospectus have been prepared assuming that we will continue as a going concern and do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties.
License Agreement
Pursuant to our exclusive license agreement with UFRF, which was last amended on October 4, 2019, we are required to pay royalties ranging from 2.5% to 5% of net sales of licensed products developed from the licensed technology for the shorter of: ten (10) years from the first commercial sale of a licensed product or the period of market exclusivity on a country-by-country basis. The latest amendment eliminated all future milestone payments. The Company remains committed to pay an annual license maintenance fee of $10,000.
Recent Accounting Pronouncements
See Note 4 to the Consolidated Financial Statements for the fiscal years ended December 31, 2021 and 2022 beginning on page F-16 below for a discussion of recent accounting pronouncements.
BUSINESS
PART I
Panbela Therapeutics, Inc. and its wholly owned subsidiaries Panbela Research, Inc., Cancer Prevention Pharma Limited (Ireland) and Cancer Prevention Pharmaceuticals, Inc. (collectively “we,” “us,” “our,” “Panbela” and the “Company”) exist for the primary purpose of developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs. Panbela Therapeutics Pty Ltd is a wholly owned subsidiary of Panbela Research, Inc. Cancer Prevention Pharmaceuticals, LLC., and Cancer Prevention Pharma Limited (UK and Wales) are wholly owned subsidiaries of Cancer Prevention Pharmaceuticals Inc. The original business entity predecessor to our Company was incorporated under the laws of the State of Delaware in 2011. The term “common stock” refers to our common stock, par value $0.001 per share.
Cancer Prevention Pharmaceuticals, Inc. (“CPP”) Acquisition
On June 15, 2022, Panbela acquired CPP, a private clinical stage company developing therapeutics to reduce the risk and recurrence of cancer and rare diseases, via merger for consideration consisting of (a) 164,689 shares of common stock, (b) 18,298 shares of common stock that remained subject to a holdback escrow (as defined in the Merger Agreement), (c) replacement options to purchase up to 39,918 shares of common stock at a weighted average exercise price of $14.00 per share, and (d) replacement warrants to purchase up to 8,451 shares of common stock at a weighted average exercise price of $165.80 per share, and post-closing contingent payments up to a maximum of $60 million, subject to satisfaction of milestones.
Holding Company Reorganization
Effective June 15, 2022, Panbela became a successor issuer to Panbela Research, Inc. (formerly known as Panbela Therapeutics, Inc., the “Predecessor”) pursuant to a holding company reorganization in which the Predecessor became a direct, wholly-owned subsidiary of Panbela. Panbela became a successor issuer to the Predecessor by operation of Rule 12g-3(a) promulgated under the Securities Exchange Act of 1934, as amended the (“Exchange Act”).
Business Overview
Panbela is a clinical stage biopharmaceutical company developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs. We are currently enrolling patients in our randomized double blind placebo controlled clinical trial for the treatment of pancreatic cancer a Phase III clinical trial funded by the National Cancer Institute (the “NCI”) for the study of colon cancer risk reduction and colon adenoma therapy (“CAT”), a preventative treatment approach for survivors of colorectal cancer or those who have high-risk colon polyps. In addition, we are designing a Phase III registration trial for familial adenomatous polyposis (“FAP”), a rare inherited condition that can cause the growth of thousands of colorectal adenomas (i.e., adenomatous polyps), which are recognized as a key risk factor for colon cancer. We also support several investigator initiated trials and company sponsored preclinical trials including: (1) Phase I and Phase II clinical trials for the treatment of early-onset type 1 diabetes funded by the Juvenile Diabetes Research Foundation; (2) Phase II clinical trial for the treatment of gastric cancer funded by the NCI; (3) Phase I/II clinical trial for the treatment of non-small cell lung cancer (NSCLC) possessing the STK11 mutation; and (4) preclinical studies that we have sponsored in the orphan disease and cancer fields.
The Company’s lead assets are ivospemin (SBP-101), FlynpoviTM (eflornithine (CPP-1X) and sulindac), and eflornithine (CPP-1X), which provide a multi-targeted approach to reset dysregulated biology present in many types of diseases such as cancer and autoimmunity. Many tumors require greatly elevated levels of polyamines to support their growth and survival. These agents target the polyamine pathway at complementary junctions, which have been shown to be altered in disease. In particular, our lead assets have the potential to suppress and prevent tumor growth, enhance anti-tumor activity of other anti-cancer agents, and modulate the immune system.
Ivospemin is a proprietary polyamine analogue designed to induce polyamine metabolic inhibition. Ivospemin has demonstrated encouraging activity against metastatic disease in a clinical trial of patients with pancreatic cancer. The efficacy and safety results demonstrated in our completed Phase I clinical trial of ivospemin in combination with gemcitabine and nab-paclitaxel in the first line treatment of metastatic pancreatic cancer provide support for the current randomized, double-blind, placebo-controlled study of ivospemin in combination with gemcitabine and nab-paclitaxel in patients previously untreated for metastatic pancreatic cancer. We believe that ivospemin, if successfully developed, may represent a novel approach that effectively treats patients with pancreatic cancer and could become a dominant product in that market. Only three first-line treatment combinations, a single maintenance treatment for a subset (3-7%) of patients, and one second-line drug have been approved by the U.S. Food and Drug Administration (the “FDA”) for pancreatic cancer in the last 25 years. Ivospemin has received Fast Track status and orphan drug designation status for pancreatic cancer in the United States and we have also received orphan drug designation in Europe.
Our June 2022 acquisition of CPP added the Company’s second lead asset, eflornithine, in multiple forms. First, an investigational new drug product, Flynpovi is a combination of the polyamine synthesis inhibitor eflornithine and the non-steroidal anti-inflammatory drug sulindac and then secondly, eflornithine as a single agent. Eflornithine is an enzyme-activated, irreversible inhibitor of the enzyme ornithine decarboxylase (“ODC”), the first rate-limiting enzyme in the biosynthesis of polyamines. Sulindac, a non-steroidal anti-inflammatory drug (“NSAID”), facilitates the export and catabolism of polyamines. Flynpovi has a unique dual mechanism of action whereby it suppresses the synthesis of new polyamines and increases the export and catabolism of polyamines from the diet and microbiome. We believe Flynpovi is unique in that it is designed to treat the risk factors (e.g., polyps) that are hypothesized to lead to FAP surgeries and colon cancer and therefore may have the ability to prevent various types of colon cancer. In the FAP-310 Phase III trial, the efficacy and safety of Flynpovi (eflornithine (CPP-1X) and sulindac), as compared with either drug alone, in adults with FAP was conducted. While the study missed the primary composite endpoint (Burke et al. 2020), a post-hoc analysis showed that none of the patients in the combination arm progressed to a need for lower gastrointestinal (“LGI”) surgery for up to 48 months compared to 13.2% and 15.7% of patients in the sulindac and eflornithine arms (Balaguer et al. 2022). These data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy. Given the statistical significance of the LGI group, a new drug application (“NDA”) was filed with the FDA; however, since this was based on the results of an exploratory analysis, a complete response letter (“CRL”) was issued. To address this deficiency concern, the Company must submit the results of one or more adequate and well-controlled clinical trials which demonstrate an effect on a clinical endpoint. There are no currently approved pharmaceutical therapies for FAP.
Additional programs are evaluating a single agent tablet eflornithine or high dose powder eflornithine sachets for several indications including prevention of gastric cancer, recent onset Type 1 diabetes, Metastatic Castration-Resistant Prostate Cancer and STK-11 mutant NSCLC. Preclinical studies as well as Phase I or Phase II investigator-initiated trials suggest that eflornithine treatment is well tolerated and has potential activity.
Flynpovi has received Fast Track designation in the United States and orphan drug designation status for FAP in the United States and Europe. In addition, we have received orphan drug designation status for eflornithine as a single agent for neuroblastoma in the United States and Europe and for gastric cancer in the United States.
Clinical Trials
Ivospemin (SBP-101)
In August 2015, the FDA accepted our Investigational New Drug (“IND”) application for our ivospemin (SBP-101) product candidate. We have completed an initial clinical trial of ivospemin in patients with previously treated locally advanced or metastatic pancreatic cancer. This was a Phase I, first-in-human, dose-escalation, safety study. From January 2016 through September 2017, we enrolled twenty-nine patients into six cohorts, or groups, in the dose-escalation phase of the Phase I trial. No drug-related bone marrow toxicity or peripheral neuropathy was observed at any dose level. In addition to being evaluated for safety, 23 of the 29 patients were evaluable for preliminary signals of efficacy prior to or at the eight-week conclusion of their first cycle of treatment using the Response Evaluation Criteria in Solid Tumors (“RECIST”), the currently accepted standard for evaluating change in the size of tumors. A summary of both the safety and preliminary signals of efficacy for this completed clinical trial is contained later in this “Business” section under ivospemin (SBP-101) Clinical Development – Pancreatic Cancer, Phase I Clinical Trial Design and Completion (ivospemin Monotherapy).
In 2018, we began enrolling patients in our second clinical trial, a Phase Ia/Ib study of the safety, efficacy and pharmacokinetics of ivospemin administered in combination with two standard-of-care chemotherapy agents, gemcitabine and nab-paclitaxel. A total of 25 subjects were enrolled in 4 cohorts to evaluate the dosage level and schedule. An additional 25 subjects were enrolled in the expansion phase of the trial. Interim results were presented in January of 2022. Best response in evaluable subjects (cohorts 4 and Ib N=29) was a Complete Response (“CR”) in 1 (3%), Partial Response (“PR”) in 13 (45%), Stable Disease (“SD”) in 10 (34%) and Progressive Disease (“PD”) in 5 (17%). One subject did not have post baseline scans with RECIST tumor assessments. Median Progression Free Survival (“PFS”), now final at 6.5 months, may have been negatively impacted by drug dosing interruptions to evaluate potential toxicity. Median overall survival in Cohort 4 + Phase Ib was 12.0 months when data was presented in January 2022 and is now final at 14.6 months. Two patients from cohort 2 have demonstrated long term survival: one at 30.3 months (final data) and one at 33.0 months and still alive as of March 18, 2022. Seven subjects were still alive at the data cutoff date of March 18, 2022, one from cohort 2 and six from cohort 4 plus Ib. Further details regarding the study design, safety and interim signals of efficacy are contained later in this “Business” section under ivospemin (SBP-101) Clinical Development – Pancreatic Cancer, Phase Ia/Ib Clinical Trial Interim Results (First Line Combination Therapy).
The safety results and tumor growth inhibition demonstrated in our Phase Ia/b study provides support for the randomized study of SBP-101 initiated in January of 2022. The trial, referred to as the ASPIRE trial, is a randomized double-blind placebo controlled trial in combination with gemcitabine and nab-paclitaxel in patients previously untreated for metastatic pancreatic cancer. The trial is being conducted globally at approximately 95 sites in the United States, Europe and Asia-Pacific. The ASPIRE trial commenced in 2022 and, while opening of clinical sites in the U.S. and the rest of the world has been slower than originally anticipated, due in part to resource fatigue in the medical community, the Company expects all countries and sites to be open by mid-2023.
The trial was originally designed as a Phase II/III with a smaller sample size (150) to support the events required for interim analysis based on PFS and a primary endpoint of overall survival. In response to European and FDA regulatory feedback, the study was amended to include the total trial sample size (600) and the design modified to utilize overall survival as the primary endpoint to be examined at interim analysis. PFS will also be analyzed to provide additional efficacy evidence. This amendment was supported by the final data from the Phase Ia/Ib first line metastatic pancreatic cancer trial which completed enrollment in December of 2020. The study will enroll 600 subjects and is anticipated to take 36 months for complete enrollment with the interim analysis available in mid- 2024. The Independent Data Safety Monitoring Board (“DSMB”) has met twice, the most recently in November 2023, which evaluated the safety of 214 patients. Both meetings resulted in no safety concerns and the trial continuing without modification. Further details regarding the study design and anticipated timing are contained later in this “Business” section under ivospemin (SBP-101) Clinical Development – Pancreatic Cancer, Randomized Clinical Trial design and anticipated timing (ASPIRE trial).
If we can successfully complete all FDA recommended clinical studies, we intend to seek marketing authorization from the FDA, the European Medicines Agency (“EMA”) (European Union), and TGA (Australia). The submission fees may be waived when ivospemin has been designated an orphan drug in each geographic region.
In early April 2022, the Company announced a poster presentation highlighting the results for ivospemin (also known as SBP-101) as a polyamine metabolism modulator in ovarian cancer at the American Association for Cancer Research Annual Conference which was subsequently published in June 2022 in the International Journal of Molecular Sciences (Holbert et al. 2022). The poster and publication conclude that the ivospemin treatment of C57Bl/6 mice injected with VDID8+ ovarian cancer cells significantly prolonged survival and decreased overall tumor burden. The results suggest that ivospemin may have a role in the clinical management of ovarian cancer, and the Company intends to continue pre-clinical and clinical studies in ovarian cancer. In April 2023, the Company announced a poster presentation highlighting additional preclinical work in ovarian cancer. The poster highlights the efficacy of SBP-101 in combination with standard of care chemotherapy agents used to treat platinum-resistant ovarian cancer. Treatment with gemcitabine, topotecan, and doxorubicin have been shown to significantly increase the in vitro toxicity of SBP-101 in both cisplatin-sensitive and cisplatin-resistant ovarian cancer cell lines. Paclitaxel and docetaxel have been shown to not have any added benefit in vitro to SBP-101 alone. The poster concludes that the treatment of C57Bl/6 mice containing VDID8+ ovarian cancer with SBP-101 in combination with doxorubicin significantly prolonged survival and decreased overall tumor burden.
Additional preclinical work is underway evaluating ivospemin and eflornithine (also known as CPP-1X or DFMO) in multiple myeloma (cell lines). Data published in the November supplemental issue of the Journal Blood investigated the effects of polyamine inhibition by ivospemin and CPP-1X on myeloma cell lines growth and viability in vitro. Results showed that ivospemin and CPP-1X treatment significantly decreased cell proliferation and induced apoptosis in a panel of multiple myeloma cell lines. When ivospemin and CPP-1X were combined an almost complete abolition of cell growth occurred. These results demonstrate the anti-neoplastic potential of ivospemin and CPP-1X and offer a compelling rationale for its clinical development as a potentially promising treatment option for multiple myeloma. The work reflects the company’s on-going collaboration with researchers from The University of Texas MD Anderson Cancer Center for the evaluation of polyamine metabolic inhibitor therapies in combination with CAR-T cell therapies in preclinical models.
Flynpovi
December 2009, the FDA accepted our IND application for the combination product, Flynpovi. Flynpovi showed promising results in an NCI supported randomized, placebo-controlled Phase IIb/III clinical trial to prevent recurrent colon adenomas, particularly high-risk pre-cancerous polyps in which 375 subjects who had resected sporadic adenoma were treated for 3 years with eflornithine (500 mg once a day) + sulindac (150 mg once a day [N = 191]) or matched placebo/placebo (N = 184). Results demonstrated a marked risk reduction (70%) in developing metachronous adenomas, 92% risk reduction in developing advanced adenomas, and 95% risk reduction in developing multiple adenomas with the active combination regimen compared to placebo (Meyskens et al. 2008). This combination regimen was generally well tolerated.
Given the similar mechanism of disease in sporadic and FAP-associated adenomatous polyposis, and the mechanism of action of Flynpovi in prevention of progressive polyposis in both the general population with sporadic adenomas and in patients with FAP, a Phase III program in FAP and a Phase III program to study colon cancer risk reduction in partnership with the Southwest Oncology Group (“SWOG”) and the NCI were initiated.
In the FAP-310 Phase III study completed in 2019, the efficacy and safety of the combination of eflornithine and sulindac, as compared with either drug alone, in adults with familial adenomatous polyposis was conducted (Burke et al. 2020). The patients were randomly assigned in a 1:1:1 ratio to receive eflornithine, sulindac, or both once daily for up to 48 months. The primary end point, assessed in a time-to-event analysis, was disease progression, defined as a composite of major surgery, endoscopic excision of advanced adenomas, diagnosis of high-grade dysplasia in the rectum or pouch, or progression of duodenal disease. A total of 171 patients underwent randomization. Disease progression occurred in 18 of 56 patients (32%) in the eflornithine-sulindac group, 22 of 58 (38%) in the sulindac group, and 23 of 57 (40%) in the eflornithine group, with a hazard ratio of 0.71 (95% confidence interval [CI], 0.39 to 1.32) for eflornithine-sulindac as compared with sulindac (P = 0.29) and 0.66 (95% CI, 0.36 to 1.23) for eflornithine-sulindac as compared with eflornithine (Burke et al. 2020). Adverse and serious adverse events were similar across the treatment groups. In a post-hoc analysis, none of the patients in the combination arm progressed to a need for lower gastrointestinal (“LGI”) surgery for up to 48 months compared with 7 (13.2%) and 8 (15.7%) patients in the sulindac and eflornithine arms (Balaguer et al. 2022). These data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy with HR = 0.00 (95% CI, 0.00-0.48; p = 0.005) for combination versus sulindac and HR = 0.00 (95% CI, 0.00-0.44; p = 0.003) for combination versus eflornithine. Given the statistical significance of the LGI group, an NDA was filed with the FDA. As the study failed to meet the primary endpoint, and the NDA was based on the results of an exploratory analysis, a complete response letter was issued. To address this deficiency concern, the Company must submit the results of one or more adequate and well-controlled clinical trials which demonstrate an effect on a clinical endpoint.
In collaboration with the NCI, and SWOG, a Phase III clinical trial has been initiated to study the benefits of Flynpovi as a therapeutic treatment for use by colon cancer survivors. The trial is named PACES for “Prevention of Adenomas and Cancer with eflornithine and sulindac.” The PACES trial is funded by the NCI and managed by SWOG. This is an ongoing Phase III double blind placebo-controlled trial of Flynpovi to prevent recurrence of high risk adenomas and second primary colorectal cancers in patients with stage 0-III colon or rectal cancer. The purpose of this study is to assess whether Flynpovi (compared to corresponding placebos) has a reduced rate of cancer or high-risk adenoma recurrence compared to comparator arms after three years of daily dosing. We have exclusive rights to the data that comes from the trial for regulatory and commercial purposes. The Company is evaluating its options for colorectal adenoma therapy (CAT) in the European Union and Asia.
In April 2023, the Company announced that it regained the North American rights to develop and commercialize Flynpovi in patients with FAP, as a result of the termination of the licensing agreement between CPP and with One-Two Therapeutics Assets Limited effective July 4, 2023.
Eflornithine (CPP-1X) and eflornithine sachets (CPP-1X-S)
For the single agent eflornithine, there is a trial ongoing evaluating eflornithine sachets (CPP-1X-S) in a Phase I/II trial in STK11 mutation patients with non-small cell lung cancer and Phase II trial in Recent Onset Type I diabetes with eflornithine are began this year. Lastly, a Phase II trial evaluating eflornithine for the prevention of gastric cancer was completed in 2021 with data analysis ongoing.
Through January 23, 2024, we had:
| |
●
|
secured an orphan drug designation for ivospemin from the FDA;
|
| |
●
|
submitted and received acceptance from the FDA for an IND application for ivospemin;
|
| |
●
|
received Country approvals for the Aspire Trial in Australia, France, Italy and Spain;
|
| |
●
|
completed a Phase Ia monotherapy safety study of ivospemin in the treatment of patients with metastatic pancreatic ductal adenocarcinoma;
|
| |
●
|
received “Fast Track” designation from the FDA for ivospemin for metastatic pancreatic cancer;
|
| |
●
|
completed enrollment and released interim results in our second trial a Phase Ia /Ib clinical study of ivospemin, a first-line study with ivospemin given in combination with a current standard of care in patients with pancreatic ductal adenocarcinoma who were previously untreated for metastatic disease; a total of 50 subjects were enrolled in this study, 25 in the Phase Ia and 25 in the Phase Ib or expansion phase;
|
| |
●
|
secured a two-year research agreement with Johns Hopkins School of Medicine led by Professor Robert Casero, an internationally recognized researcher in polyamine biology;
|
| |
●
|
completed process improvement measures expected to be scalable for commercial use and received issue notification for a patent covering this new shorter synthesis of ivospemin;
|
| |
●
|
initiated a randomized, double-blind, placebo-controlled study with ivospemin given in combination with gemcitabine and nab-paclitaxel in patients with pancreatic ductal adenocarcinoma who are previously untreated for metastatic disease;
|
| |
●
|
completed preclinical evaluation of ivospemin for use as neoadjuvant therapy in resectable pancreatic cancer prior to surgery;
|
| |
●
|
obtained early, preclinical, indication of tumor growth inhibition activity in ovarian cancer and presented the results at ASCO-GI conference;
|
| |
●
|
received USAN adoption of the nonproprietary name of ivospemin for SBP-101;
|
| |
●
|
acquired and integrated CPP, adding a second lead asset in multiple forms and an expansive clinical development program ranging from pre-clinical to registration level clinical trials;
|
| |
●
|
European Medicines Agency (“EMA”) Committee for Orphan Medicinal Products issued a positive opinion on Panbela’s application for orphan designation of ivospemin in combination with gemcitabine and nab-Paclitaxel in patients with metastatic pancreatic ductal adenocarcinoma;
|
| |
●
|
announced the initiation of phase II program through Indiana University for early onset Type I diabetes utilizing eflornithine;
|
| |
●
|
announced the initiation of the Phase I/II clinical trial for the treatment of non-small lung cancer (“NSCLC”) possessing the STK11 mutation through Moffitt Cancer Center;
|
| |
●
|
entered into a sponsored research agreement with The University of Texas MD Anderson Cancer Center for the evaluation of polyamine metabolic inhibitor therapies in combination with CAR-T cell therapies in preclinical models;
|
| |
●
|
announced the SWOG Cancer Research Network’s PACES S0820 Phase III trial passed a single planned futility analysis and will continue; |
| |
●
|
announced the approval of US WorldMeds NDA Approval for Eflornithine (“DFMO”) in Pediatric Neuroblastoma, first polyamine approval in oncology; and |
| |
●
|
Exceeded 50% enrollment in ASPIRE global clinical trial.
|
Pancreatic Cancer
Pancreatic cancer afflicts approximately 151,000 people in Europe (Epidemiology in Europe and Recommendations for Screening in High-Risk Populations, Partyka, et al July 2023) , approximately 64,000 people in the United States annually (American Cancer Society. Cancer Facts & Figures 2023. Atlanta, GA: American Cancer Society; 2023 and Overview of Pancreatic Cancer) and 293,000 people worldwide – excluding Europe and United States (GLOBOCAN 2020). It has been identified as the fourth leading cause of death from cancer in Europe (GLOBOCAN 2020) and the third leading cause of death from cancer in the United States (SEER Cancer Statistics Factsheets 2021). On average, Pancreatic Ductal Adenocarcinoma (“PDA”) represents approximately 95% of all pancreatic cancers diagnosed in a given calendar year. Considering that the median overall survival for previously untreated patients with good performance status is between 8.5 months (Von Hoff 2013) and 11.1 months (Conroy 2011) with the two most commonly available treatment regimens, effective treatment for PDA has remained a major unmet medical need.
Pancreatic cancer is generally not diagnosed early because the initial clinical signs and symptoms are vague and non-specific. The most common presenting symptoms include weight loss, epigastric (upper central region of the abdomen) and/or back pain, and jaundice. The back pain is typically dull, constant, and of visceral origin radiating to the back, in contrast to the epigastric pain which is vague and intermittent. Less common symptoms include nausea, vomiting, diarrhea, anorexia, and new onset diabetes (which can be an early signal) or glucose intolerance (Hidalgo 2010).
Surgery remains the only treatment option with curative intent, although only about 20% of patients are candidates for surgical resection at the time of the diagnosis. Patients who undergo radical surgery still have a limited survival rate, averaging 23 months (Macarulla T, et al Clin Transl Oncol 2017).
For the minority of patients who present with resectable disease, surgery is the treatment of choice. Depending on the location of the tumor, the operative procedures may involve cephalic pancreatoduodenectomy, referred to as a “Whipple procedure” distal pancreatectomy or total pancreatectomy. Pancreatic enzyme deficiency and diabetes are frequent complications of both the disease and these surgical procedures. Up to 70% of patients with pancreatic cancer present with biliary obstruction that can be relieved by percutaneous or endoscopic stent placement. However, even if the tumor is fully resected, the outcome in patients with pancreatic cancer has been disappointing (Hidalgo 2010, Seufferlein 2012). Post-operative administration of chemotherapy improved progression-free and overall survival in three large randomized clinical trials (Hidalgo 2010), but median post-surgical survival in patients treated in all three trials was similar, only 20-22 months. Pre-operative (neo-adjuvant) chemotherapy is of increasing interest, with the goal of improved successful resections and long-term outcomes.
For patients who present with unresectable, locally advanced or metastatic disease, which represent a majority of PDA patients, management options range from chemotherapy alone to combined forms of treatment with radiation therapy and chemotherapy. However, due to the increased toxicity of combined treatment, randomized trials of such combined regimens have had low enrollment, precluding a firm conclusion as to any advantage of adding radiation to chemotherapy (Hidalgo 2010).
Gemcitabine was the first chemotherapeutic agent approved for the treatment of patients with PDA in the modern regulatory era, providing a median survival duration of 5.65 months (Burris 1997). Gemcitabine monotherapy was the standard of care for patients with metastatic pancreatic cancer until combination therapy with gemcitabine plus erlotinib (Tarceva®) was shown to increase median survival by two weeks. This modest benefit was tempered by a significant side effect profile and high cost, limiting its adoption as a standard treatment regimen. Subsequently, the multidrug chemotherapy combination FOLFIRINOX was shown to provide a median survival benefit of 4.3 months (OS = 11.1 months) over gemcitabine alone (6.8 months), but its significant side effect profile limits the regimen to select patients with a good performance status and often requires supplementation with WBC growth factor therapy. Nab-paclitaxel (Abraxane®) received marketing authorization for use in combination with gemcitabine (FDA approved 2013) after showing an increase in overall survival of seven weeks compared to gemcitabine alone (Von Hoff 2013). Thus, combination therapies have demonstrated a modest survival benefit compared to gemcitabine alone as summarized in the table below (Thota 2014).
Current First-Line Treatment Approaches: Survival & Toxicity Profiles Across Three Major Positive Clinical Trials
| |
|
Gemcitabine vs.
Gemcitabine/Erlotinib
Phase 3 trial
|
|
|
ACCORD 11 Trial
|
|
|
Metastatic Pancreatic
Adenocarcinoma Clinical
Trial (MPACT)
|
|
| |
|
Gemcitabine
|
|
|
Gemcitabine/
Erlotinib
|
|
|
Gemcitabine
|
|
|
FOLFIRINOX
|
|
|
Gemcitabine
|
|
|
Gemcitabine/
Nab-Paclitaxel
|
|
|
One-Year Survival
|
|
|
17% |
|
|
|
23% |
|
|
|
20.6% |
|
|
|
48.4% |
|
|
|
22% |
|
|
|
35% |
|
|
Median Overall Survival (months)
|
|
|
5.91 |
|
|
|
6.24 |
|
|
|
6.8 |
|
|
|
11.1 |
|
|
|
6.7 |
|
|
|
8.5 |
|
|
Median Progression-Free Survival (months)
|
|
|
3.55 |
|
|
|
3.75 |
|
|
|
3.3 |
|
|
|
6.4 |
|
|
|
3.7 |
|
|
|
5.5 |
|
|
Overall Response Rate
|
|
|
8% |
|
|
|
8.6% |
|
|
|
9.4% |
|
|
|
31.6% |
|
|
|
7% |
|
|
|
23% |
|
|
Toxicity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neutropenia
|
|
|
– |
|
|
|
– |
|
|
|
21% |
|
|
|
45.7% |
|
|
|
27% |
|
|
|
38% |
|
|
Febrile neutropenia
|
|
|
– |
|
|
|
– |
|
|
|
1.2% |
|
|
|
5.4% |
|
|
|
1% |
|
|
|
3% |
|
|
Thrombocytopenia
|
|
|
– |
|
|
|
– |
|
|
|
3.6% |
|
|
|
9.1% |
|
|
|
9% |
|
|
|
13% |
|
|
Diarrhea
|
|
|
2% |
|
|
|
6% |
|
|
|
1.8% |
|
|
|
12.7% |
|
|
|
1% |
|
|
|
6% |
|
|
Sensory neuropathy
|
|
|
– |
|
|
|
– |
|
|
|
0% |
|
|
|
9% |
|
|
|
1% |
|
|
|
17% |
|
|
Fatigue
|
|
|
15% |
|
|
|
15% |
|
|
|
17.8% |
|
|
|
23.6% |
|
|
|
7% |
|
|
|
17% |
|
|
Rash
|
|
|
6% |
|
|
|
1% |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
Stomatitis
|
|
<1%
|
|
|
|
0% |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
Infection
|
|
|
17% |
|
|
|
16% |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
Source: Thota R et al., Oncology 2014; Jan 28(1):70–74
Other drugs are currently under investigation, but none have received marketing authorization as a first-line treatment of PDA since the approval of Abraxane, Lynparza® (olaparib) was approved in December 2019 for maintenance therapy of patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) metastatic pancreatic adenocarcinoma whose disease has not progressed on at least 16 weeks of a first-line platinum-and chemotherapy regimen.
Familial adenomatous polyposis
Familial adenomatous polyposis (“FAP”) is a rare and potentially life threatening genetic condition occurring in approximately one in 10,000 individuals in the United States. FAP is caused primarily by mutations in the adenomatous polyposis coli (APC) tumor suppressor gene. APC mutations are usually inherited as autosomal dominant genetic traits, but as many as 25% of those afflicted with FAP with an identical germline mutation have no family history. Only 1 in 10,000 people will develop FAP. Estimated annual prevalence in the U.S. is approximately 30,000 and in Europe approximately 50,000. If untreated, patients will develop hundreds to thousands of polyps throughout the colon and rectum. FAP often develops in the early teens and results in a nearly 100% lifetime risk of colorectal cancer by age forty if untreated. No approved FAP drug is on the market.
Most patients are asymptomatic for years until the adenomas are large and numerous, and cause rectal bleeding or even anemia, or cancer develops. Generally, cancers start to develop a decade after the appearance of the polyps. Nonspecific symptoms may include constipation or diarrhea, abdominal pain, palpable abdominal masses and weight loss.
Cancer prevention and maintaining a good quality of life are the main goals of management of patients with FAP. By the late teens or early twenties, colorectal cancer prophylactic surgery is advocated. Prophylactic surgery often requires total abdominal colectomy with ileal-rectal anastomoses (“IRA”) and subsequent frequent endoscopic surveillance, with polypectomy and cautery/laser ablation as needed. Patients with extensive rectal involvement must undergo total proctocolectomy with ileal pouch-anal reconstruction. Despite this, approximately 50% of patients who have had total proctocolectomy with ileal pouch-anal reconstruction will develop adenomatous polyps in the neo-rectum (ileal pouch). Duodenal cancer and desmoids are the two main causes of mortality after total colectomy; they need to be identified early and treated. Upper endoscopy is necessary for surveillance to reduce the risk of ampullary and duodenal cancer. Patients with progressive tumors and unresectable disease may respond or stabilize with a combination of cytotoxic chemotherapy and surgery (when possible, to perform). Individuals with FAP carry a 100% risk of CRC; however, this risk is reduced significantly when patients enter a screening-treatment program.
A major unmet need in the treatment of patients with FAP is a therapeutic means to defer or obviate the need for major surgical interventions, particularly colectomy with IRA or proctocolectomy with an ileal surgical pouch (IPAA). Such interventions often require temporary or permanent ileostomy, and with it, long-term or permanent quality of life (QoL) deficits such as frequent bowel movements (average 6 per day), nocturnal fecal incontinence and, in female patients, reduced reproductive potential. It is critical to find non-surgical alternatives that will delay or obviate the need of repeated endoscopic and surgical procedures to maintain patient QoL. For those patients who have an intact colon in particular, pharmacotherapy offers the opportunity to meaningfully control or delay polyposis progression and offer a greater choice over when or if they undergo prophylactic colectomy/proctocolectomy in order to optimize QoL.
This potential benefit is in fact likely the most powerful potential benefit possible since the long-term course of FAP essentially mandates ultimate colectomy for most patients. The value to a younger patient in safely delaying such a radical procedure by years cannot be overstated.
There are currently no approved and marketed pharmacotherapeutic treatments for patients with FAP. While in 1999 celecoxib was conditionally approved by the FDA for the treatment of FAP based on reductions of polyp number observed in a randomized double-blind placebo controlled study conducted in patients with FAP, it was subject to the marketing authorization holder, Pfizer, providing additional data. In 2011, the FDA requested that Pfizer voluntarily withdraw the FAP indication for CELEBREX (celecoxib) Capsules from the market because the post-marketing study intended to verify clinical benefit and required as a condition of approval under subpart H was never completed. In a letter in 2011, Pfizer requested that the FDA withdraw the FAP indication for CELEBREX (celecoxib) Capsules from the market. Effective in 2012, the approval for the FAP indication for CELEBREX (celecoxib) Capsules was withdrawn. Celecoxib was also authorized for FAP treatment centrally by the European Commission after the EMA’s scientific review in October 2003 under “exceptional circumstances”. Authorization was granted subject to specific obligations during product life-cycle, chiefly to provide further data on its efficacy and safety; however, the applicant/authorization holder could not fulfill this central post-authorization obligation. According to publicly available information, the post- authorization study was initiated in the first quarter of 2004 and the EU Centralized Marketing Authorisation was withdrawn because the holder was unable to provide the data as required.
Ovarian Cancer
Worldwide Ovarian Cancer has annual incidence of approximately 314,000 and annual deaths of approximately 207,000 (Globocan 2020). In the United States, Ovarian represents approximately 1% of all new cancer cases at approximately 22,000 (American Cancer Society. Cancer Facts & Figures 2021. Atlanta, GA: American Cancer Society; 2021) and the five year survival rate for metastatic disease is approximately 29% (SEER fact sheet Ovarian 2022). According to the American Cancer Society, ovarian cancer is the fifth leading cause of cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system.
Nearly 70 % of the patients are diagnosed with advanced-stage due to the failure of screening methods for detecting early-stage disease (Giornelli 2016; Partridge et al. 2009; Bast et al. 2007; Gohagan et al. 2000; Chudecka-Głaz 2015). Thus, most patients will relapse within the first 2 years after diagnosis, even after an optimal primary cytoreductive surgery and six cycles of the standard adjuvant chemotherapy with carboplatin/paclitaxel.
The second line chemotherapy depends mainly on the disease-free interval (“DFI”) (time between completion of first line chemotherapy and clinical relapse); or progression-free interval (“PFI”) (time between the last chemotherapy given for relapsed disease and progression). There are three classifications: Platinum-refractory/resistant with relapse during platinum treatment (refractory) or with a DFI/PFI <6 months (resistant), Platinum-sensitive relapse occurring >12 m of last platinum-based chemotherapy, or partially sensitive to platinum with disease-free survival (“DFS”)/ PFS between 6 and 12 months from the last platinum-based chemotherapy.
According to Pignata et al. 2017, in platinum-sensitive patients, treatment with platinum-based combinations is associated with a PFS advantage compared with single agents or non-platinum combinations. For patients with partially sensitive relapse (PFI between 6 and 12 months), two options are available: platinum doublets or non-platinum therapy (single agent or combination). Last, patients with resistant or refractory relapse (PFI < 6 months) disease there are few options. For these patients, monotherapy with a non-platinum drug or participation in clinical trials is indicated.
Colorectal Cancer
According to United States Cancer Statistics published by the American Cancer Society, in the United States in 2022, it is estimated that CRC will be the third most commonly occurring cancer among males and females and the third leading cause of cancer-related deaths. High-risk adenomatous polyps are considered the key risk factor for CRC. In 2015, the disease will be responsible for an estimated 52,000 deaths in the United States. An even higher rate of incidence occurs in the European Union, where approximately 255,000 people per year die from CRC according to the Globocan 2020 Fact Sheets.
Globally, there are approximately 1,931,000 new diagnoses each year (approximately 180,000 expected in North America in 2020). Rates of presentation are also becoming significant in Asia (China and Japan). Colorectal adenomas (or “polyps”) are considered the key risk factor for CRC. The general consensus in the medical and scientific communities is that these polyps are the precursors to more than 90% of all colorectal cancers.
Colon cancer represents nearly three-fourths of all colorectal cancers in the U.S. Despite potentially curative treatment with surgery (with or without adjuvant chemotherapy), local stage and locally advanced stage colon cancer patients remain at considerable risk for colorectal adenomas, distant recurrence, secondary colonic tumor formation, and colorectal cancer related mortality. Polypectomy appears to be an effective way to decrease mortality from colon cancer but widespread adoption of this approach is limited by both cost and patient acceptability (Newcomb et al. 1992; Selby et al. 1992). Certain types of colorectal polyps have increased risk of progression to colorectal cancer. High-risk polyps (polyps with villous histology, size ≥ 1 cm, high grade dysplasia, or multiple adenomas defined as 3 or more) have become the focus of colorectal tumorigenesis research due to the higher rate of malignant potential for these lesion (Lotfi et al. 1986; Spencer et al. 1984; Winawer et al. 1993; Martinez et al. 2009). The current standard of care for resected colon cancer patient (beyond surgery, and adjuvant chemotherapy when indicated) is surveillance monitoring with clinical exams, laboratory analyses, and colonoscopic evaluation. However, data suggest that colonoscopy does not predict death from colorectal cancer uniformly throughout the colon – in fact, right-sided colorectal cancers were not observed to gain any mortality benefit from colonoscopy (Baxter et al. 2009). Other potential problems with colonoscopy include (rarely) perforations, infection, bleeding, and non-adherence with current recommendations. Safe and effective chemopreventive interventions, therefore, offer great potential to complement and improve upon the current colon cancer surveillance paradigm. Unlike other therapies used to treat CAT, Flynpovi is a non-surgical and non-invasive option that has the potential to both improve patient quality of life and reduce higher healthcare system-wide expense burdens.
Proprietary Technology
Function and Characteristics of Polyamines
Polyamines are metabolically distinct entities within human cells that bind to and facilitate DNA replication, RNA transcription and processing, and protein (such as pancreatic enzymes) synthesis. Human cells contain three essential and naturally occurring polyamines – putrescine, spermidine, and spermine. Polyamines perform many functions necessary for cellular proliferation, apoptosis and protein synthesis. The critical balance of polyamines within cells is maintained by several enzymes such as ornithine decarboxylase (“ODC”) and spermidine/spermine N1 acetyl transferase (“SSAT”). All of these homeostatic enzymes are short-lived, rapidly inducible intracellular proteins that serve to regulate native polyamine pools tightly and continuously. These enzymes constantly maintain polyamines within a very narrow range of concentration inside the cell.
Polyamine metabolism and cancer
Polyamines are required for cell proliferation. It is believed that many cancers, especially oncogene-driven cancers, might be sensitive to interference with polyamine metabolism. The natural polyamines putrescine, spermidine and spermine are intimately involved in growth-related processes, wound healing, and the development of cancer. Under normal conditions, the pool of polyamines is tightly controlled through regulation of synthesis, catabolism, and transport mechanisms (Gerner and Meyskens 2004). The loss of this tight control can result in an excessive accumulation of polyamines, which favors malignant transformation of cells. Consequently, with the loss of growth control in cancer cells, the transformed cells may be more sensitive to polyamine depletion than normal cells. Thus, the polyamine metabolic pathway is a rational target for therapeutic intervention (Casero 2018).
Immune systems require multiple soluble and cellular components, including polyamines, for a normal immune function. As such, polyamines are important modulators of the immune response, particularly in the tumor microenvironment where they are found in high concentrations. High levels of polyamines are present in tumor cells and in autoreactive B- and T-cells in autoimmune diseases. Dysregulation of polyamines can result in tumor immune evasion, elevated cell stress, and increased autoimmunity. By resetting the polyamine pathway through therapeutic interventions, there is the potential to restore normal immune functions.
Pharmacotherapeutic Approaches to Reset the Polyamine Pathway
The Company’s lead assets are ivospemin and Flynpovi, which provide a multi-targeted approach to reset dysregulated biology present in many types of diseases such as cancer and autoimmunity. For instance, many tumors require greatly elevated levels of polyamines to support their growth and survival. These agents target the polyamine pathway at complementary junctions which have been shown to be altered is disease. In particular, these agents have the potential to suppress and prevent tumor growth, enhance anti-tumor activity of other anti-cancer agents, and modulate the immune system.

Polyamine Analogue - ivospemin (SBP-101)
Many tumors, including pancreatic cancer, display an increased uptake rate of polyamines. Polyamine analogues such as ivospemin are structurally similar to naturally occurring polyamines and are recognized by the cell’s polyamine uptake system, allowing these compounds to gain ready entrance to the cell. We believe that pancreatic acinar cells, because of their extraordinary protein synthesis capacity, exhibit enhanced uptake of polyamines and polyamine analogues. Because of this preferential uptake by pancreatic acinar cells, polyamine analogues such as ivospemin disrupt the cell’s polyamine balance and biosynthetic network, and induce programmed cell death, or apoptosis, via processes including caspase 3 activation and poly ADP ribose polymerase (PARP) cleavage. Proof of concept has been demonstrated in multiple human pancreatic cancer models, both in vivo and in vitro, that pancreatic ductal adenocarcinoma exhibits sensitivity to ivospemin.
Ivospemin is a proprietary polyamine analogue, which we believe accumulates in the exocrine pancreas acinar cells due to its unique chemical structure. ivospemin was discovered and extensively studied by Professor Raymond J. Bergeron at the University of Florida College of Pharmacy.
As laboratory studies suggest, the primary mechanism of action for ivospemin has been demonstrated to include the enhanced uptake of the compound in the exocrine pancreas; therefore, pancreatic cancer was logical for the initial development of this compound. Sufficiently high dosing in animal models leads to correspondingly depressed levels of native polyamines, with caspase 3 activation, PARP cleavage and apoptotic destruction (programmed cell death) of the exocrine pancreatic acinar and ductal cells without an inflammatory response. Importantly, pancreatic islet cells, which secrete insulin, are structurally and functionally dissimilar to acinar cells and are not impacted by ivospemin. In animal models at two independent laboratories, ivospemin has demonstrated significant suppression of transplanted human pancreatic cancer cells, including metastatic pancreatic cancer growth.
We believe that ivospemin exploits the natural affinity of the exocrine pancreas, the liver and kidney, and pancreatic ductal adenocarcinoma cells while leaving the insulin-producing islet cells unharmed. Most current cancer therapies, including chemotherapy, radiation, and surgery, are associated with significant side effects that further reduce the patient’s quality of life. However, based on data evaluated from clinical studies to date, we believe that the adverse effects of ivospemin in causing bone marrow suppression or peripheral neuropathy do not overlap with or exacerbate those seen with typical chemotherapy options. The dose-limiting toxicities observed in cohort five of our first Phase I study, as noted below, were not observed at lower doses and are not expected to overlap with the adverse events of bone marrow suppression and peripheral neuropathy commonly associated with standard chemotherapy. The dose and dosing schedule evaluated in the expansion phase of the recently completed Phase Ia/Ib is below the maximum tolerable dose (MTD) and at this dose level, neither the exocrine nor the endocrine human pancreas is expected to be affected by ivospemin, resulting in no treatment impact on pancreatic enzyme or insulin levels. This dose level and dosing schedule in the new ASPIRE trial will be the same as evaluated in the expansion phase of the Ia/Ib study.
Ornithine Decarboxylase Inhibitor - eflornithine (CPP-1X)
Ornithine decarboxylase is the first and rate-limiting enzyme in the biosynthesis of polyamines which catalyzes the conversion of ornithine to putrescine and regulates the biosynthesis of polyamines in mammalian as well as many other eukaryotic cells. Eflornithine, also known as α-difluoromethylornithine (DFMO), is an ornithine analogue. Eflornithine irreversibly binds to ODC1 and prevents the natural ODC1 substrate, ornithine, from accessing the active site of the enzyme (Meyskens and Gerner 1999). The administration of eflornithine decreases both ODC activity and polyamine concentrations. In genetic mouse models with an APC gene mutation, the administration of eflornithine reduces intestinal carcinogenesis, decreasing the concentration of polyamines through transport and catabolism and inhibiting tumor development (Babbar et al. 2003).
Treatment of animals with eflornithine results in inhibition of ODC activity, especially in tissues and organs with rapidly dividing cells. Polyamine biosynthesis has been shown to be critical for eukaryotic cellular growth and differentiation, and inhibition of polyamine biosynthesis can stimulate or inhibit cellular differentiation depending on the model studied (Gerner and Meyskens 2004). Accordingly, eflornithine has promoted or inhibited cell differentiation in a variety of models.
Polyamine biosynthesis is also a critical step in experimental chemical-induced carcinogenesis, cell transformation, and tumor cell proliferation, and there is a growing body of evidence that eflornithine's inhibitory effect on cell proliferation and tumorigenesis may involve a complex inter-relationship between oncogenes, polyamine metabolism, and ODC activity. MYC is an oncogene that encodes a transcription factor that is required for the proliferation of normal cells but when overexpressed can lead to aberrant cell growth (Gerner and Meyskens 2004). Additionally, c-Myc is a transcriptional activator of the ODC gene (Pena et al. 1993) (Bello-Fernandez, Packham, and Cleveland 1993). Furthermore, eflornithine has been shown to decrease N-Myc mRNA in neuroblastoma cells and c-Myc mRNA in human colon carcinoma cells (Celano et al. 1988) and spermidine preferentially stimulated transcription and expression of c-Myc, but not c-Fos (Tabib and Bachrach 1999). Taken together, these results suggest that polyamines play a feedback role in the regulation of expression of certain oncogenes at the level of transcription.
Mice with a mutation of the adenomatous polyposis coli (Apc) tumor suppressor gene develop intestinal tumors in numbers similar to those found in patients with FAP. Mutations of the Apc gene increases the activity of ODC and leads to increased intestinal polyamine levels. Studies in animal models of FAP indicate that eflornithine alone is effective in reducing the number of intestinal tumors (Erdman et al. 1999b) and colonic tumor burden (Yerushalmi et al. 2006). Eflornithine may lower polyamine levels in colorectal mucosa and skin cells (Gerner and Meyskens 2004).
The major clinical evidence for benefit of eflornithine derives from prospective, randomized, placebo-controlled clinical studies of eflornithine monotherapy in patients with elevated risk for developing certain forms of cancer (prostate and basal cell skin cancer). In a randomized, placebo-controlled, clinical study in subjects with a history of resected colon polyps, eflornithine reduced polyamines in rectal mucosal tissue. This marker study is especially relevant to patients with FAP, in whom target tissues include intestinal and colonic mucosa (Meyskens et al. 1998).
Eflornithine has received regulatory approvals as a high dose, intravenously delivered medication for the treatment of a form of African sleeping sickness, and as a topical agent for the treatment of hirsutism (excess hair growth on body parts where hair growth is usually absent or minimal). No oral dosage form of eflornithine has ever received regulatory approval in any indication.
Activator of Spermidine/Spermine N-Acetyltransferase (“SSAT1”) – Sulindac
Transport of polyamines is maintained by the peroxisome-proliferator activated receptor-g (“PPARg”). This receptor positively regulates SSAT transcription facilitating polyamine acetylation and transport of polyamines out of the cell. Under normal conditions, the K-Ras molecule has no activity on PPARg. However, mutation of the K-Ras gene produces a product that inhibits PPARg’s effect on SSAT translation resulting in elevated polyamine pools and tumorigenesis (Babbar et al. 2003). NSAIDs, such as sulindac, act through PPARg to enhance transcriptional of SSAT which increases catabolism and export of polyamines.
Sulindac is a member of the arylalkanoic acid class of NSAIDs and is a non-selective inhibitor of cyclooxygenases involved in prostaglandin synthesis. To understand potential mechanisms of action of sulindac, patterns of gene expression resulting from treatment with sulindac sulfone, a sulindac metabolite lacking cyclooxygenase inhibitory activity, were measured in human colon tumor-derived cells (Babbar et al. 2003). Sulindac sulfone inhibited cell growth, and induced apoptosis and the expression of spermidine/spermine N-acetyltransferase (SSAT1), a polyamine catabolic enzyme implicated in polyamine export (Xie, Gillies, and Gerner 1997). Sulindac sulfone induction of SAT1 expression occurs via a cyclooxygenase-independent transcriptional activation of SAT1 at a specific peroxisomal proliferator activated receptor gamma (PPARγ) responsive element (“PPRE”) in the SAT1 gene. Treatment of cells with sulindac sulfone induces SAT1 expression and stimulates polyamine export.
Experimental findings in human cell and mouse models indicate that sulindac and other NSAIDS activate polyamine catabolism (Gerner and Meyskens 2009). Thus, NSAIDs complement inhibitors of polyamine synthesis, like eflornithine, to reduce tissue polyamine levels. In cell culture, sulindac metabolites reduce cell survival in vitro in a dose-dependent manner at doses above 150 µM at 24-hour exposure times (Lawson et al. 2000).
Experiments in both mouse and rat models of colon cancer have demonstrated a preventative effect for sulindac (Babbar et al. 2003). Sulindac blocked tumor formation in the multiple intestinal neoplasia (Min) mouse, a murine model of APC mutation-associated intestinal carcinogenesis, mimicking FAP. In the Min mouse, tumor-preventing doses of sulindac inhibited tissue levels of prostaglandin-E2 and COX-2 (Boolbol et al. 1996). In other nonclinical studies, sulindac had an inhibitory effect on bladder, lung, and forestomach tumor formation in rat and mouse models (Kelloff, Boone, et al. 1994, Kelloff, Crowell, et al. 1994).
Dual Targeting - Flynpovi
The ability to decrease the polyamine pools by a dual mechanism of action, i.e., suppressed synthesis and enhanced catabolism and export, led to the hypothesis that Flynpovi would complement one another in the prevention of tumor development in a patient population where elevated polyamine pools lead to enhanced tumorigenesis. Eflornithine is the irreversible inhibitor of ODC which is responsible for de novo synthesis of polyamines and sulindac regulates SSAT which plays a role in polyamine export and catabolism. Hence the combination, Flynpovi, inhibits the generation of new polyamines and also removes polyamines obtained from the diet and microbiome.
The ability of Flynpovi to reduce polyamines in the GI tract has been demonstrated in both the preclinical and clinical settings. In the study by Igantenko et al, the effect of eflornithine alone and in combination with NSAIDs sulindac or celecoxib on intestinal tumor number and grade and polyamine content was evaluated in ApcMin/+ mice (Ignatenko et al. 2008). Administration of eflornithine in combination with sulindac was superior to each single agent at significantly (P< 0.05) decreasing putrescine, spermidine, and total intestinal polyamine concentrations to below baseline levels in the ApcMin/+ mice. Additionally, in this study with the exception of the 0.5% eflornithine treatment group, all treatment groups developed significantly (P<0.05) fewer tumors/animal than the control group. The combination treatment of 2% eflornithine and sulindac suppressed intestinal tumorigenesis to a level that was not statistically significantly different from that for sulindac alone. Although sulindac alone produced a significant decrease in the number of intestinal tumors in ApcMin/+ mice, it did not reduce the percentage of high‑grade adenomas. However, the combination of eflornithine and sulindac significantly (P<0.05) decreased the number of high-grade adenomas compared to the sulindac alone group.
The ability of the eflornithine and sulindac treatment group to suppress high grade adenomas is a key finding as it is the high grade adenomas in this model which correlate to the high grade adenomas seen in FAP patients that are indicators for excisional and surgical events clinically. These data support the rationale for treatment of FAP patients with eflornithine combined with sulindac to reduce intestinal polyamine contents and the incidence of high-grade intestinal adenomas.
More importantly, combination treatment with Flynpovi dramatically reduces the incidence of metachronous colorectal adenomas in patients with prior sporadic adenomas (Meyskens et al. 2008). Meyskens and colleagues performed a Phase IIb/III, double-blind pharmacoprevention of Sporadic Colorectal Adenomas Study (PSCA Study) in which 375 subjects who had resected sporadic adenoma were treated for 3 years with eflornithine (500 mg once a day) + sulindac (150 mg once a day [N = 191]) or matched placebo/placebo (N = 184). Results demonstrated a marked risk reduction (70%) in developing metachronous adenomas, 92% risk reduction in developing advanced adenomas, and 95% risk reduction in developing multiple adenomas with the active combination regimen compared to placebo. This combination regimen was generally well tolerated.
The mechanism of disease in sporadic and FAP-associated adenomatous polyposis, and the mechanism of eflornithine and NSAID action in prevention of progressive polyposis in both the general population with sporadic adenomas and in patients with FAP, led to the development of the FAP-310 trial in patients with FAP associated with APC germline mutations.
The FAP-310 Phase III study that evaluated the efficacy and safety of the combination of eflornithine and sulindac, as compared with either drug alone, in adults with familial adenomatous polyposis was conducted (Burke et al. 2020). The patients were randomly assigned in a 1:1:1 ratio to receive eflornithine, sulindac, or both once daily for up to 48 months. In a post-hoc analysis, none of the patients in the combination arm progressed to a need for lower gastrointestinal (LGI) surgery for up to 48 months compared with 7 (13.2%) and 8 (15.7%) patients in the sulindac and eflornithine arms (Balaguer et al. 2022). These data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy with HR = 0.00 (95% CI, 0.00-0.48; P= 0.005) for combination versus sulindac and HR = 0.00 (95% CI, 0.00-0.44; P= 0.003) for combination versus eflornithine.
Development Plan for Ivospemin (SBP-101)
Development of ivospemin for the pancreatic cancer indication has included a pre-clinical and a clinical phase. The pre-clinical phase, which was substantially completed during 2015, consisted of four primary components: chemistry, manufacturing and controls (“CMC”), preclinical (laboratory and animal) pharmacology studies, preclinical toxicology studies, and regulatory submissions in Australia and the United States.
Preparation of the ivospemin IND for pancreatic cancer required collaboration by our manufacturing, preclinical toxicology, pharmacokinetic, and metabolism experts, our regulatory affairs project management, and our in-house clinical expertise. In August 2015, the FDA accepted our application.
In Australia, a Human Research Ethics Committee (“HREC”) application was submitted with subsequent Clinical Trial Notification (“CTN”) to the Therapeutic Goods Administration (“TGA”).
Our initial clinical trial in previously treated patients with locally advanced or metastatic pancreatic cancer was a Phase I, first-in-human, dose-escalation, safety study conducted at clinical sites in both Australia and the United States. We engaged expert clinicians who treat pancreatic cancer at major cancer treatment centers in Melbourne and Adelaide, Australia as well as the Mayo Clinic Scottsdale and HonorHealth in Scottsdale, Arizona. These Key Opinion Leaders, with proven performance in pancreatic cancer studies, agreed to participate as investigators for our Phase I First-in-Human study.
Enrollment in our initial Phase I safety trial of ivospemin in previously treated pancreatic cancer patients commenced in January 2016 and was completed in September 2017. Results from this trial are discussed in ivospemin (SBP-101) Clinical Development – Pancreatic Cancer, Phase I Clinical Trial Design and Completion (ivospemin Monotherapy) below.
We completed enrollment of patients in our second clinical trial in December 2020. This second clinical trial was a Phase Ia/Ib study of the safety, efficacy and pharmacokinetics of ivospemin administered in combination with two standard-of-care chemotherapy agents, gemcitabine and nab-paclitaxel. A total of 25 subjects were enrolled in four cohorts of Phase Ia and an additional 25 subjects were enrolled in the expansion Phase Ib by December of 2020. Safety and interim efficacy results from this trial are discussed in ivospemin (SBP-101) Clinical Development - Pancreatic Cancer, Phase Ia/Ib Clinical Trial Interim Results (First Line Combination Therapy) below.
In January of 2022, we initiated our third clinical trial. This new trial is a randomized, double blind, placebo controlled study of safety and efficacy of ivospemin administered in combination with two standard-of-care chemotherapy agents, gemcitabine and nab-paclitaxel. Trial design and expected timing are discussed in Clinical Development - Pancreatic Cancer, Randomized Clinical Trial Design and Anticipated Timing (ASPIRE trial).
In addition, we are exploring ivospemin for neoadjuvant treatment in appropriate pancreatic cancer patients. There is also preclinical data to suggest that ivospemin may have potential therapeutic uses for cancers other than pancreatic. In February 2021, we entered into a research agreement with the Johns Hopkins University School of Medicine. The collaboration has focused on the further development of Panbela’s investigative agent ivospemin, including activity in cell lines outside of pancreatic cancer, biomarkers informing diagnostics and potential combination with checkpoint inhibitors. In December 2021, the Company announced positive preclinical data supporting the activity of ivospemin in ovarian cancer cell lines which was presented and published in 2022 (Holbert et al. 2022). Further data resulting from the ongoing relationship with Johns Hopkins University School of Medicine is expected.
Ivospemin (SBP-101) Clinical Development – Pancreatic Cancer
Our clinical development in Pancreatic Cancer thus far includes:
| |
●
|
a Phase I SBP-101 Monotherapy study completed in 2017;
|
| |
●
|
a Phase Ia/Ib SBP-101 First Line Combination Therapy study, which completed enrollment in late 2020; and
|
| |
●
|
a Randomized, Double Blind Placebo Controlled First Line Combination Therapy study was initiated in January of 2022.
|
Details of these programs follow.
Phase I Clinical Trial Design and Completion (ivospemin Monotherapy)
We have completed an initial clinical trial of ivospemin in patients with previously treated locally advanced or metastatic pancreatic cancer. This was a Phase I, first-in-human, dose-escalation, safety study. From January 2016 through September 2017, we enrolled twenty-nine patients into six cohorts, or groups, in the dose-escalation phase of the Phase I trial. No drug-related bone marrow toxicity or peripheral neuropathy was observed at any dose level. In addition to being evaluated for safety, 23 of the 29 patients were evaluable for preliminary signals of efficacy prior to or at the eight-week conclusion of their first cycle of treatment using the Response Evaluation Criteria in Solid Tumors (“RECIST”), the currently accepted standard for evaluating change in the size of tumors.
The absence of adverse events which could potentially overlap with adverse events typically observed in the use of conventional chemotherapeutic agents, supports the case for combination of ivospemin with conventional chemotherapeutic agents, such as gemcitabine, nab-paclitaxel, or even FOLFIRINOX.
Phase Ia/Ib Clinical Trial Interim Results (First Line Combination Therapy)
In 2018, we began enrolling patients in our second clinical trial, a Phase Ia/Ib study of the safety, efficacy and pharmacokinetics of ivospemin administered in combination with two standard-of-care chemotherapy agents, gemcitabine and nab-paclitaxel. A total of 25 subjects were enrolled in 4 cohorts to evaluate the dosage level and schedule. An additional 25 subjects were enrolled in the expansion phase of the trial. Interim results were presented in January of 2022. Best response in evaluable subjects (cohorts 4 and Ib N=29) was a CR in 1 (3%), PR in 13 (45%), SD in 10 (34%) and PD in 5 (17%). One subject did not have post baseline scans with RECIST tumor assessments. Median PFS, now final at 6.5 months, may have been negatively impacted by drug dosing interruptions to evaluate potential toxicity. Median overall survival in Cohort 4 + Phase Ib was 12.0 months when data was presented in January 2022 and is now final at 14.6 months. Two patients from cohort 2 have demonstrated long term survival: one at 30.3 months (final data) and one at 33.0 months and still alive.
Figure 4. Evaluation of SBP 101 Phase Ib First-line combo-therapy Safety Trial -
Best Overall Response
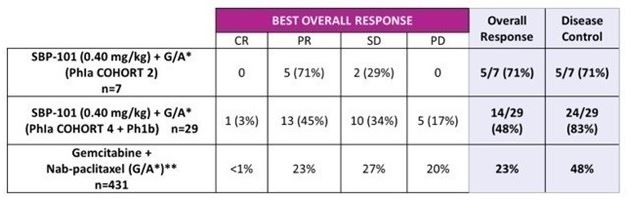
Source: Singhal, N., Poster Presentation, ASCO GI 2022
Randomized Clinical Trial design and anticipated timing (ASPIRE trial)
In January of 2022, the Company announced the initiation of a new clinical trial. Referred to as ASPIRE, the trial is a randomized double-blind placebo-controlled trial in combination with gemcitabine and nab-paclitaxel, a standard pancreatic cancer treatment regimen in patients previously untreated for metastatic pancreatic cancer. The trial will be conducted globally at approximately 95 sites in the United States, Europe and Asia - Pacific.
While opening of clinical sites in the United States and the rest of the world has been slower than originally anticipated, due in part to resource fatigue in the medical community, the Company expects all countries and sites to be open by early -2024.
The trial was originally designed as a Phase II/III trial with a smaller sample size (150) to support the events required for interim analysis based on PFS and a primary endpoint of overall survival (OS). In response to European and FDA regulatory feedback, the study was amended to include the total trial sample size (600) and the design modified to utilize overall survival as the primary endpoint to be examined at interim analysis. PFS will also be analyzed to provide additional efficacy evidence. This amendment was supported by the final data from the Phase Ia/Ib first line metastatic pancreatic cancer trial which completed enrollment in December of 2020. The study will enroll 600 subjects and is anticipated to take 36 months for complete enrollment.
On January 25, 2024, the Company announced that the trial had exceeded 50% enrollment. The Company projects that full enrollment will be completed by the first quarter of 2025 and that interim data analysis based on overall survival should be available by the middle of 2024.
If we can successfully complete all FDA recommended clinical studies, we intend to seek marketing authorization from the FDA, the EMA (European Union), Ministry of Health and Welfare (Japan) and TGA (Australia). The submission fees may be waived when ivospemin (SBP-101) has been designated an orphan drug in each geographic region, as described under “Orphan Drug Status.”
Development Plan for Flynpovi and Eflornithine (CPP-1X)
In December 2009, the FDA accepted CPP’s IND application for the combination product, Flynpovi, product candidate and in November 2009 and August 2018, the FDA accepted IND applications for eflornithine.
The Development plan executed by CPP of Flynpovi for FAP and colon cancer prevention has included both a pre-clinical/non-clinical and a clinical phase. The non-clinical phase consisted of four primary components: CMC, preclinical (laboratory and animal) pharmacology studies, preclinical toxicology studies, and regulatory submissions in the U.S. and Europe. Similarly, the development plan for eflornithine and eflornithine sachets in several different indications included much of the same primary components and regulatory submission in the U.S.
Clinical Development –Flynpovi
Our clinical development of Flynpovi thus far includes:
| |
●
|
The PACES Phase III Trial
|
FAP-310 Phase III Trial
In the FAP-310 Phase III study, the efficacy and safety of the combination of Flynpovi (ES combo), as compared with either drug eflornithine or sulindac alone, in adults with FAP was conducted. A total of 171 patients underwent randomization. Disease progression occurred in 18 of 56 patients (32%) in the Flynpovi, 22 of 58 (38%) in the sulindac group, and 23 of 57 (40%) in the eflornithine group, with a hazard ratio of 0.71 (95% CI, 0.39 to 1.32) for Flynpovi as compared with sulindac (P = 0.29) and 0.66 (95% CI, 0.36 to 1.23) for Flynpovi as compared with eflornithine. In a post-hoc analysis, none of the patients in the combination arm progressed to a need for LGI surgery for up to 48 months compared with 7 (13.2%) and 8 (15.7%) patients in the sulindac and eflornithine arms. These data corresponded to risk reductions for the need for LGI surgery approaching 100% between combination and either monotherapy with HR = 0.00 (95% CI, 0.00–0.48; P = 0.005) for combination versus sulindac and HR = 0.00 (95% CI, 0.00–0.44; P = 0.003) for combination versus eflornithine.
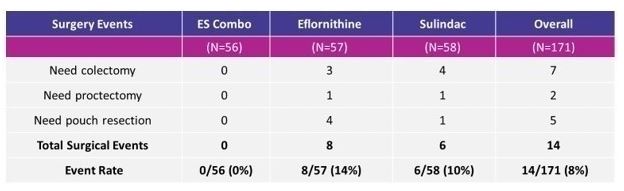
Given the statistical significance of the LGI group, an NDA was filed with the FDA. As the study failed to meet the primary endpoint, and the NDA was based on the results of an exploratory analysis, a complete response letter was issued. To address this deficiency concern, the Company must submit the results of one or more adequate and well-controlled clinical trials which demonstrate an effect on a clinical endpoint.
Phase III Clinical Trial in Colon Cancer Survivors
In collaboration with the NCI, and SWOG, a Phase III clinical trial has been initiated to study the benefits of Flynpovi as a therapeutic treatment for use by colon cancer survivors. The trial is named PACES for “Prevention of Adenomas and Cancer with eflornithine and sulindac.” The PACES trial is funded by the NCI and managed by SWOG. This is an ongoing double blind placebo-controlled trial of Flynpovi to prevent recurrence of high risk adenomas and second primary colorectal cancers in patients with stage 0-III colon or rectal cancer, Phase III PACES. The purpose of this study is to assess whether the Flynpovi, combination of eflornithine and sulindac, (compared to corresponding placebos) has a reduced rate of cancer or high-risk adenoma recurrence compared to comparator arms after three years of daily dosing. We have exclusive rights to the data that comes from the trial for regulatory and commercial purposes.
Clinical Development –Eflornithine (CPP – 1X)
Our clinical development of eflornithine thus far includes:
| |
●
|
Phase II Gastric Cancer Prevention Trial
|
| |
●
|
Phase I and Phase II Recent Onset Type 1 Diabetes Trials
|
| |
●
|
Phase I/II STK-11 Mutant NSCLC Trial
|
Phase II Gastric Cancer Prevention Trial
H. pylori is the most common bacterial infection in humans and causes gastritis in all individuals. Gastritis progresses along the “Correa cascade” from gastritis to the precancerous stages of atrophic gastritis (loss of specialized gastric epithelium) and intestinal metaplasia (“IM”), to gastric adenocarcinoma (Correa 1992). In response to H. pylori infection the host elicits a robust innate and adaptive immune response, which results in mucosal inflammation but fails to eradicate the organism. Several studies have demonstrated that the failure of the immune response may be related to dysregulated L-arginine metabolism and polyamines including the upregulation of ornithine decarboxylase (“ODC”) by macrophages (Chaturvedi et al. 2010; Chaturvedi, de Sablet, Coburn, et al. 2012) (Chaturvedi, de Sablet, Peek, et al. 2012), (Chaturvedi et al. 2011) (Xu et al. 2004) (Chaturvedi et al. 2014) (Chaturvedi et al. 2004). Levels of polyamines are increased in H. pylori-induced gastritis in mice, and oral DFMO treatment reduces gastric polyamine levels, and severity of both H. pylori colonization and gastritis (Chaturvedi et al. 2010). In the gerbil model of H. pylori-induced gastric cancer, polyamine levels correlate with levels of gastritis, DNA damage, and progression to dysplasia/carcinoma. In this model, eflornithine reduces polyamine levels and DNA damage, and reduces rates of dysplasia and carcinoma by >50% (Chaturvedi et al. 2014).
In collaboration with investigators at Vanderbilt University and funding by the NCI, the investigator-initiated Phase II trial performed in Honduras and Puerto Rico was a randomized, double-blinded study comparing once daily eflornithine versus placebo for an up to 18-month treatment period in patients with gastric premalignant lesions. This trial has completed and is undergoing data analysis. The Company has received orphan drug designation for the use of eflornithine for the treatment of gastric cancer in the United States.
Phase I and II Recent Onset Type 1 Diabetes (T1D) Trials
T1D is an organ-specific autoimmune disease characterized by chronic immune-mediated destruction of pancreatic β-cells, leading to partial, or in most cases, absolute insulin deficiency. The majority of cases result from autoimmune mediated pancreatic β-cell destruction, which occurs at a variable rate. Patients become clinically symptomatic when approximately 90% of pancreatic β-cells are destroyed. Therefore, preserving β-cell function is a target for promising treatments (Couper et al. 2014). The activity of ODC is upregulated in early diabetic kidney disease, contributing to renal hypertrophy and hyperfiltration (Pedersen et al. 1992; Deng et al. 2003). In vivo studies in experimental models of recent-onset T1D evaluating eflornithine demonstrate roles in suppressing the development of renal hypertrophy and hyperplasia, decreasing the incidence of diabetes, augmenting the survival and regeneration of β-cell populations, decreasing insulinitis, and maintaining an immune-tolerant balance of T-cell subpopulations.
The Company collaborated with investigators at Indiana University on a JDRF funded Phase I study to evaluate the safety and efficacy of increasing doses of eflornithine in patients with recent onset Type 1 diabetes. The completed Phase I trial demonstrated that a 3-month course of oral eflornithine was well tolerated with a favorable adverse event profile in children and adults with recent-onset T1D. Urinary polyamine data showed that eflornithine treatment inhibited ornithine decarboxylase activity effectively, reflected by a dose dependent reduction in urinary putrescine values. Furthermore, although not powered to detect metabolic efficacy, subjects treated with 750 mg/m2/day and 1000 mg/m2/day of eflornithine exhibited higher C-peptide AUC 6 months after treatment indicative of improved β cell function compared to placebo. These data suggest that eflornithine may improve β cell function alone and in combination regimens to treat or prevent type 1 diabetes that also include immunotherapy. A larger Phase II study fully powered to detect an effect of eflornithine treatment on maintenance of C-peptide is being planned with the goal of initiating in early 2023.
Phase I/II STK-11 Mutant NSCLC Trial
STK11 is the fourth-most frequently mutated gene in lung adenocarcinoma, with loss of function occurring in up to 30% of all cases (Laderian et al. 2020). Patients with LKB1 loss have reduced infiltrates of cytotoxic T-cells and respond poorly to anti PD1 or anti-PDL-1 therapies regardless of the PDL-1 status. CheckMate-057 trial lung tumors harboring co-mutations in KRAS and STK11 had an inferior response to PD-1 axis inhibitors (Skoulidis et al. 2018). These results suggest that STK11-mutated tumors were found to have a cold immune microenvironment regardless of KRAS status.
Bioinformatic analyses using two well-annotated lung adenocarcinoma datasets identified upregulation of ornithine decarboxylase (the target for eflornithine). Additionally, LKB1-loss tumors show a significant up-regulation of several solute transporters (SLC7A2, SLC14A2, and SLC16A4). SLC7A2 is known to be responsible for the membrane transport of cationic amino acids arginine, lysine, and ornithine. Furthermore, LKB1 loss the arginine pathway in which arginine is converted to ornithine and urea (by arginase) and ornithine is converted to putrescine (by ODC1). Together, the results suggested that ODC1 may be a key metabolic driver in LKB1-loss lung cancer.
In other model systems eflornithine treatment has been shown to modulate the tumor microenvironment. A previously studied cohort revealed that ODC1 may be instrumental to immune suppression (Chamaillard et al. 1997). Since eflornithine is an ODC1 inhibitor, it is hypothesized that inhibiting the metabolic enzyme ODC1 using eflornithine will increase the number of tumor-infiltrating lymphocytes (“TILs”) in LKB1-loss tumors and restore benefit of PD(L)-1 blockade to these patients.
The Company is currently planning a Phase I/II investigator-initiated trial to assess eflornithine in patients with STK-11 mutant NSCLC with the goal of starting in early 2023.
Neuroblastoma Trial
Neuroblastoma, a rare cancer originating from immature nerve cells, contributes to nearly 15% of pediatric cancer deaths.[1] Panbela Therapeutics' subsidiary, Cancer Prevention Pharmaceuticals, has extensively collaborated with leading neuroblastoma research groups such as the Neuroblastoma Medulloblastoma Translational Research Consortium (“NMTRC”) (now Beat Childhood Cancer), New Advances in Neuroblastoma Therapy (“NANT”), the Children’s Oncology Group (“COG”), and the National Cancer Institute (NCI) in the clinical development of eflornithine as a treatment for neuroblastoma
In July of 2023 the Company announced it had divested certain assets in its eflornithine pediatric neuroblastoma program to US WorldMeds®1 (“USWM”), a Kentucky-based specialty pharmaceutical company. Under the terms of the agreement, Panbela is entitled to receive up to approximately $9.5 million non-dilutive funding in exchange for the sale of certain assets within its pediatric neuroblastoma program for eflornithine. Panbela will receive payments upon USWM’s successful completion of milestones related to eflornithine's clinical development, regulatory approval, and commercial sales.
In December of 2023 the Company announced the USWM received FDA approval of their NDA for the use of eflornithine as a maintenance therapy for neuroblastoma in remission.
Total Development Costs
The development of ivospemin involves a preclinical and a clinical development phase. We have completed our initial preclinical development work for pancreatic cancer as well as two Phase I clinical trials. The Phase II/III trial has just been initiated. Additional clinical trials will likely be required for FDA or other approvals in foreign jurisdictions if the results of the first-line clinical trial of our ivospemin product candidate justify continued development. The cost and timing of additional clinical trials is highly dependent on the nature and size of the trials.
The development of Flynpovi also has involved preclinical and clinical development work for FAP and colon cancer prevention. The Company intends to secure consensus from the FDA and the European Medicines Agency (“EMA”) on a global registration trial.
Orphan Drug Status
The Orphan Drug Act provides special status to drugs which are intended for the safe and effective treatment, diagnosis or prevention of rare diseases that affect fewer than 200,000 people in the United States, or that affect more than 200,000 persons but for which a manufacturer is not expected to recover the costs of developing and marketing such a drug. Orphan drug designation has the advantage of reducing drug development costs by: (i) streamlining the FDA’s approval process, (ii) providing tax breaks for expenses related to the drug development, (iii) allowing the orphan drug manufacturer to receive assistance from the FDA in funding the clinical testing necessary for approval of an orphan drug, and (iv) facilitating drug development efforts. More significantly, the orphan drug manufacturer’s ability to recover its investment in developing the drug is also greatly enhanced by the FDA granting the manufacturer seven years of exclusive U.S. marketing rights upon approval. Designation of a product candidate as an orphan drug therefore may provide its sponsor with the opportunity to adopt a faster and less expensive pathway to commercializing its product.
We obtained U.S. Orphan Drug Status for ivospemin in 2014. On December 14, 2022, we announced that the EMA Committee for Orphan Medicinal Products issued a positive opinion on Panbela’s application for orphan designation of ivospemin (SBP-101) in combination with gemcitabine and nab Paclitaxel in patients with metastatic pancreatic ductal adenocarcinoma.
We have obtained orphan drug designation status for Flynpovi and eflornithine for FAP in the United States (2013 and 2011 respectively) and Europe (2013 and 2011 respectively). In addition, we have received orphan drug designation status for eflornithine as a single agent for neuroblastoma in the United States (2010) and Europe (2011) and for gastric cancer (2015) in the United States.
Fast Track
In June 2020, we received Fast Track Designation from the FDA for development of ivospemin for the treatment of first-line patients with metastatic PDA when administered in combination with gemcitabine and nab-paclitaxel.
Additionally, in September 2017, we received Fast Track Designation from the FDA for the development of Flynpovi for the treatment of FAP.
With the designation of Fast Track Designation, we, or our North American partners, may engage in more frequent interactions with the FDA, and the FDA may review sections of a New Drug Application (“NDA”) before the application is complete. This rolling review is available if we provide, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, Fast Track Designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Intellectual Property
As the result of efforts at our contract manufacturer Syngene International Ltd to refine the synthetic process, a new shorter synthetic process has been developed on which a patent (US 11,098,005 B2) “METHODS FOR PRODUCING (65,155)-3,8,13,18-TETRAAZAICOSANE-6, 15-DIOL” issued on Aug. 24, 2021 and was assigned to Panbela. The patent claims cover a novel process for the production of ivospemin and reduces the number of synthetic steps from nineteen to six.
For Flynpovi, there is a composition of matter patent for the fixed dose combination of eflornithine and sulindac that is broadly nationalized, providing potential protection through 2037. Additionally, we hold several Method of Use patents for Flynpovi and/or eflornithine for the treatment of Familial Adenomatous Polyposis, neuroblastoma, and the Treatment of Recent Onset Type 1 Diabetes.
We are evaluating other opportunities to provide additional intellectual property.
Human Capital Management
As of January 23, 2024, we had eight employees, seven of which were full time. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We believe our relationship with our employees is good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing, and integrating our existing and new employees, advisors, and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to motivate such individuals to perform to the best of their abilities and achieve our objectives and lead to the success of the Company and increase value to our stockholders.
We value diversity of backgrounds and perspectives in our workforce and our policy is that we do not discriminate based on race, religion, creed, color, national origin, ancestry, physical disability, mental disability, medical condition, genetic information, marital status, sex, gender, gender identity, gender expression, age, military and veteran status, sexual orientation or any other protected characteristic as established by federal, state or local laws.
We believe that operational responsibilities can be handled by our current employees, independent consultants and our global CRO. We have historically used the services of independent consultants and contractors to perform various professional services. We believe that this use of third-party service providers enhances our ability to minimize general and administrative expenses. We intend to periodically evaluate our staffing and talent requirements and expect to add employees if that becomes a more appropriate resourcing alternative.
Competition
The development and commercialization of new products to treat cancer is intensely competitive and subject to rapid and significant technological change. While we believe that our knowledge, experience and scientific resources provide us with competitive advantages, we face substantial competition from major pharmaceutical companies, specialty pharmaceutical companies, and biotechnology companies worldwide. Many of our competitors have significantly greater financial, technical, and human resources. Smaller and early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. As a result, our competitors may discover, develop, license or commercialize products before or more successfully than we do.
We face competition with respect to our current product candidates and will face competition with respect to future product candidates, from segments of the pharmaceutical, biotechnology and other related markets that pursue approaches to targeting molecular alterations and signaling pathways associated with cancer. Our competitors may obtain regulatory approval of their products more rapidly than we do or may obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are more effective, more convenient, less costly, or possessing better safety profiles than our products, and these competitors may be more successful than us in manufacturing and marketing their products.
In addition, we may need to develop our product candidates in collaboration with diagnostic companies, and we will face competition from other companies in establishing these collaborations. Our competitors will also compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Furthermore, we also face competition more broadly across the market for cost-effective and reimbursable cancer treatments. The most common methods of treating patients with cancer are surgery, radiation and drug therapy, including chemotherapy, immunotherapy, hormone therapy and targeted drug therapy or a combination of such methods. There are a variety of available drug therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. While our product candidates, if any are approved, may compete with these existing drug and other therapies, to the extent they are ultimately used in combination with or as an adjunct to these therapies, our product candidates may be approved as companion treatments and not be competitive with current therapies. Some of these drugs are branded and subject to patent protection, and others are available on a generic basis. Insurers and other third-party payors may also encourage the use of generic products or specific branded products. We expect that if our product candidates are approved, they will be priced at a premium over competitive generic, including branded generic, products. As a result, obtaining market acceptance of, and gaining significant share of the market for, any of our product candidates that we successfully introduce to the market will pose challenges. In addition, many companies are developing new therapeutics and we cannot predict what the standard of care will be as our product candidate progresses through clinical development.
Commercialization
We have not established a sales, marketing or product distribution infrastructure nor have we devoted significant management resources to planning such an infrastructure because our lead product candidate is still in early clinical development. We currently anticipate that we will partner with a larger pharmaceutical organization having the expertise and capacity to perform these functions.
Flynpovi will be commercialized, if approved, in North America by One-Two.
Manufacturing and Suppliers
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We currently rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for preclinical and clinical testing as well as for commercial manufacture of any products that we may commercialize. If needed, we intend to engage, by entering into a supply agreement or through another arrangement, third-party manufacturers to provide us with additional SBP-101 clinical supply. We identified and qualified manufacturers to provide the active pharmaceutical ingredient and fill-and-finish services for our initial product candidate prior to our submission of an NDA to the FDA and expect to continue utilizing this approach for any future product candidates.
Securing the manufacture of Flynpovi for further clinical studies and for commercialization in North America, if the product is approved, is the responsibility of One-Two, who was granted a non-exclusive license to manufacture for North America.
Material Agreements
The Standard Exclusive License Agreement (“License Agreement”) dated December 22, 2011, between us and UFRF grants us an exclusive license to the proprietary technology covered by issued United States Patents Nos. US 5,962,533, which expired in February 2016, and US 6,160,022 which expired in July 2020 and Know-How as defined by the License Agreement, with reservations by UFRF for academic or government uses. Under this agreement, we had agreed to pay various royalties, expenses and milestone payments to UFRF. The License Agreement was amended in December 2016 (“First Amendment”) and again in October 2019 (“Second Amendment”). Under the Second Amendment all minimum royalty payments and milestone payments defined in the License Agreement were eliminated. In addition, the period for payment of royalties was changed to be the shorter of (i) ten (10) years from first commercial sale or (ii) the period of market exclusivity on a country-by-country basis. UFRF may also terminate this license for standard and similar causes such as material breach of the agreement, bankruptcy, failure to pay royalties and other customary conditions. The agreement allows for UFRF to terminate if the first commercial sale is not made by December 31, 2025.
Government Regulation
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug and Cosmetic Act and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the United States typically involves preclinical laboratory and animal tests, the submission to the FDA of an IND which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including the Investigator’s Brochure, information about product chemistry, manufacturing and controls, potential perceived side effects and risks, and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice (“GCP”), an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board (“IRB”) for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase I, the initial introduction of the drug into healthy human subjects/patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence of effectiveness. Phase II usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase II evaluations, pivotal, or Phase III trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In many cases the FDA requires two adequate and well-controlled Phase III clinical trials to demonstrate the efficacy of the drug. A single Phase III trial with other confirmatory evidence may be sufficient in instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome, and confirmation of the result in a second trial would be practically or ethically impossible. After an NDA is approved, a Phase IV trial may be undertaken to evaluate safety over a long period of time, quality of life or cost effectiveness.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the United States. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, toxicology, manufacture, controls and any proposed labeling. The cost of preparing and submitting an NDA is substantial, and the fees are typically increased annually.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of NDAs to encourage timeliness. Most applications for standard review drug products are reviewed within twelve months from submission; most applications for priority review drugs are reviewed within eight months from submission. Priority review can be applied to drugs that the FDA determines offer major advances in treatment or provide a treatment where no adequate therapy exists. If priority review is achieved, the FDA’s goal is to act on the application within six months. The review process for both standard and priority review may be extended by the FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission. The FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an outside advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current good manufacturing practice (“cGMP”), a quality system regulating manufacturing, is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy (“REMS”) to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use (“ETASU”). ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Fast Track Designation and Accelerated Approval
The FDA is required to facilitate the development, and expedite the review, of drugs that are (1) intended for the treatment of a serious or life-threatening disease or (2) condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the Fast Track program, the sponsor of a new product candidate may request that the FDA designate the product candidate for a specific indication as a Fast Track drug concurrent with, or after, the filing of the IND for the product candidate. The FDA must determine if the product candidate qualifies for Fast Track Designation within 60 days of receipt of the sponsor’s request.
Under the Fast Track program and the FDA’s accelerated approval regulations, the FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase IV or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to priority review by the FDA.
If a submission is granted Fast Track Designation, the sponsor may engage in more frequent interactions with the FDA, and the FDA may review sections of the NDA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, Fast Track Designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Breakthrough Therapy Designation
The FDA is also required to expedite the development and review of the application for approval of drugs that are intended to treat a serious or life-threatening disease or condition where preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Under the Breakthrough Therapy program, the sponsor of a new product candidate may request that the FDA designate the product candidate for a specific indication as a breakthrough therapy. The FDA must determine if the product candidate qualifies for Breakthrough Therapy designation within 60 days of receipt of the sponsor’s request.
Orphan Drug Designation and Exclusivity
The Orphan Drug Act provides incentives for the development of products intended to treat rare diseases or conditions. Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug available in the United States for this type of disease or condition will be recovered from sales of the product. If a sponsor demonstrates that a drug is intended to treat a rare disease or condition, the FDA will grant orphan designation for that product for the orphan disease indication, assuming that the same drug has not already been approved for the indication for which the sponsor is seeking orphan designation. If the same drug has already been approved for the indication for which the sponsor is seeking orphan designation, the sponsor must present a plausible hypothesis of clinical superiority in order to obtain orphan designation. Orphan designation must be requested before submitting an NDA. After the FDA grants orphan designation, the FDA discloses the identity of the therapeutic agent and its potential orphan use.
Orphan designation may provide manufacturers with benefits such as research grants, tax credits, Prescription Drug User Fee Act (“PDUFA”) application fee waivers and eligibility for orphan drug exclusivity. If a product that has orphan designation subsequently receives the first FDA approval of the active moiety for that disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which for seven years prohibits the FDA from approving another product with the same active ingredient for the same indication, except in limited circumstances. Orphan drug exclusivity will not bar approval of another product under certain circumstances, including if a subsequent product with the same active ingredient for the same indication is shown to be clinically superior to the approved product on the basis of greater efficacy or safety, or providing a major contribution to patient care, or if the company with orphan drug exclusivity is not able to meet market demand. Further, the FDA may approve more than one product for the same orphan indication or disease as long as the products contain different active ingredients. Moreover, competitors may receive approval of different products for the indication for which the orphan drug has exclusivity or obtain approval for the same product but for a different indication for which the orphan drug has exclusivity.
In the European Union, orphan drug designation also entitles a party to financial incentives such as reduction of fees or fee waivers and 10 years of market exclusivity is granted following drug or biological product approval. This period may be reduced to 6 years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Orphan drug designation must be requested before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports are required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase IV testing, risk evaluation and mitigation strategies, or REMS, and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control, drug manufacture, packaging and labeling procedures must continue to conform to current good manufacturing practices, or cGMPs, after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the Agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality-control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Additional Regulations and Environmental Matters
In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including but not limited to, the Centers for Medicare and Medicaid Services, or CMS, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, or DOJ, and individual U.S. Attorney offices within the DOJ, and state and local governments. For example, sales, marketing and scientific/educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the false claims laws, and our activities may implicate the privacy provisions of the Health Insurance Portability and Accountability Act (“HIPAA”) and similar state laws, each as amended.
The federal Anti-Kickback Statute prohibits, among other things, any person or entity, from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers on the other. There are statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. While we reasonably believe our practices to be in compliance with the Anti-Kickback Statute, our practices may not in all cases meet all the criteria for protection under a statutory exception or regulatory safe harbor.
Additionally, the intent standard under the Anti-Kickback Statute was amended by the Affordable Care Act (“ACA”) to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it to have committed a violation. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act (as further discussed below).
The Civil Monetary Penalties statute authorizes the imposition of severe financial penalties against any person or entity who, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
The federal False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to, or approval by, the federal government or knowingly making, using, or causing to be made or used, a false record or statement material to a false or fraudulent claim to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, and thus non-reimbursable, uses.
HIPAA created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up by trick, scheme or device, a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of, or payment for, healthcare benefits, items or services.
Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
We may be subject to data privacy and security regulations by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) and its implementing regulations, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Additionally, the federal Physician Payments Sunshine Act within the ACA, and its implementing regulations, require that certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) report information related to certain payments or other transfers of value made or distributed to physicians, other specified health care professionals and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians, other specified health care professionals and teaching hospitals and report annually certain ownership and investment interests held by physicians and other specified health care professionals and their immediate family members. Some states have analogous laws requiring manufacturers to report certain transfers of value to covered individuals and entities. To distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of drug and biological products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical and biotechnology companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical and biotechnology companies for use in sales and marketing, and to prohibit certain other sales and marketing practices. All our activities are potentially subject to federal and state consumer protection and unfair competition laws.
If our operations are found to be in violation of any of the federal and state healthcare laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government programs, such as Medicare and Medicaid, injunctions, private “qui tam” actions brought by individual whistleblowers in the name of the government, or refusal to allow us to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend, in part, on the extent to which third-party payors provide coverage and establish adequate reimbursement levels for such products. In the United States, third-party payors include federal and state healthcare programs, privately managed care providers, health insurers and other organizations. The process for determining whether a third-party payor will provide coverage for a product may be separate from the process for setting the price of a product or for establishing the reimbursement rate that such a payor will pay for the product. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all the FDA-approved products for a particular indication. Third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical products, therapies and services, in addition to questioning their safety and efficacy. We may need to conduct expensive pharmaco-economic studies to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain the FDA approvals. Our product candidates may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage for the product. This is also true of Medicare reimbursement, where different vendors process payments, so that coverage by one vendor does not assure that all other vendors will provide coverage. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. In addition, the United States federal government position on matters related to drug pricing is evolving and uncertain, and any changes could have a material impact on drug pricing generally in the United States, including for our product candidates if approved.
Different pricing and reimbursement schemes exist in other countries. In the EU, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines but monitor and control company profits. The National Institute for Health and Care Excellence (“NICE”) in the United Kingdom also requires consideration of cost-benefit analysis. The downward pressure on health care costs has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country.
The marketability of any product candidates for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on healthcare pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Available Information
Our website is located at www.Panbela.com. The information contained on or connected to our website is not a part of this prospectus. We have included our website address as a factual reference and do not intend it to be an active link to our website.
We make available, free of charge, through our website materials we file or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act, including our annual report on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K and amendments to those reports. These materials are posted to our website as soon as reasonably practical after we electronically file them with or furnish them to the SEC.
Members of the public may read and copy any materials we file with the SEC at its Public Reference Room at 450 Fifth Street, NW, Washington, DC 20549. Information on the operation of the Public Reference Room is available by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements and other information about us and other issuers that file electronically at http://www.sec.gov.
MANAGEMENT
Information about our Executive Officers
Jennifer K. Simpson, Ph.D., MSN, CRNP, age 55, has served as President and Chief Executive Officer and as a director of our Company since July 2020. Prior to joining the Company, Dr. Simpson served as President and Chief Executive Officer and as a member of the board of directors of Delcath Systems, Inc. (Nasdaq: DCTH) from 2015 to June 2020. She had previously held various other leadership roles at Delcath since 2012. From 2011 to 2012, Dr. Simpson served as Vice President, Global Marketing, Oncology Brand Lead at ImClone Systems, Inc. (a wholly owned subsidiary of Eli Lilly and Company), where she was responsible for all product commercialization activities and launch preparation for one of the late-stage assets. From 2009 to 2011, Dr. Simpson served as Vice President, Product Champion and from 2008 to 2009 as the Associate Vice President, Product Champion for ImClone’s product Ramucirumab. From 2006 to 2008, Dr. Simpson served as Product Director, Oncology Therapeutics Marketing at Ortho Biotech (now Janssen Biotech), a Pennsylvania-based biotech company that focuses on innovative solutions in immunology, oncology and nephrology. Earlier in her career, Dr. Simpson spent over a decade as a hematology/oncology nurse practitioner and educator. Dr. Simpson has served on the board of directors and nominating and corporate governance committee of Eagle Pharmaceuticals, Inc. since August 2019 and on the board of Directors of CytRx Corporation since July 2021. Dr. Simpson earned a Ph.D. in Epidemiology from the University of Pittsburgh, an M.S. in Nursing from the University of Rochester, and a B.S. in Nursing from the State University of New York at Buffalo.
Susan Horvath, age 64, has served as our Vice President and Chief Financial Officer since April 2018. Ms. Horvath has held both finance and operating positions within pharmaceutical, healthcare and consumer organizations. In addition to her position with the Company, Ms. Horvath sits on the board of directors and provides financial consulting services for Photonic Pharma, LLC, a privately held company focused on efficiencies in early stage drug discovery. Prior to joining the Panbela team, Ms. Horvath served as Chief Financial Officer of Eyebobs, LLC, a private company focused on eyewear for corrective vision, from 2016 to January 2018; Vice President and Chief Financial Officer of Tenacious Holdings, Inc. (d/b/a ergodyne), a privately held, safety products company, from 2014 to 2016; Chief Financial Officer and Vice President of Human Resources at Healthsense, Inc., a next generation technology (SaaS) and remote monitoring company focused on providing safety and improving quality of life while reducing overall costs of healthcare for seniors and fragile adults, from 2011 to 2014; Chief Financial Officer, Vice President of Operations & Human Resources of Hemosphere, Inc., an early commercialization stage medical device company, from 2008 to 2010; and Vice President & Team Leader International of CNS, Inc, a publicly traded consumer health care products company focused on the development and marketing of strong consumer brands, from 2004 to 2007. Ms. Horvath holds a Bachelor of Science degree in Accounting from the University of Illinois, Champaign, and is a Certified Management Accountant and Certified Public Accountant, inactive.
Information about our Board of Directors
Our business is overseen by a Board of Directors divided into three classes as nearly equal in number as possible, and directors typically are elected to a designated class for a term of three years. The following sets forth certain information regarding the current members of our Board of Directors:
Class II Directors - Term Expiring in 2024
Michael T. Cullen, M.D., M.B.A., age 77, has served as Chairman of the Board and a non-employee director of our Company since his retirement as an employee of the Company in May 2021. Dr. Cullen had served as Executive Chairman and as a director of our Company since its co-founding in November 2011. Dr. Cullen brings 33 years of pharmaceutical experience to our Company, including expertise in working with development-stage companies in planning, designing and advancing drug candidates from preclinical through clinical development. Dr. Cullen served as our President and Chief Executive Officer between October 2018 and July 2020. He previously served as our Chief Medical Officer and President from November 2011 to June 2015. Dr. Cullen provided due diligence consulting to the pharmaceutical industry from 2009 to 2011, after one year in transition consulting to Eisai Pharmaceuticals. He developed several oncology drugs as Chief Medical Officer for MGI Pharma Inc. from 2000 to 2008, and previously at G.D. Searle, SunPharm Corporation, and as Vice President for Clinical Consulting at IBAH Inc., the world’s fifth largest contract research organization, where he provided consulting services on business strategy, creating development plans, regulatory matters and designing clinical trials for several development stage companies in the pharmaceutical industry. Dr. Cullen was also a co-founder and Chief Executive Officer of IDD Medical, a pharmaceutical start-up company. Dr. Cullen joined 3M Pharmaceuticals in 1988 and contributed to the development of cardiovascular, pulmonary, rheumatology and immune-response modification drugs. Over the course of his career Dr. Cullen has been instrumental in obtaining the approval of ten drugs, including three since 2004: Aloxi®, Dacogen® and Lusedra®. Board-certified in Internal Medicine, Dr. Cullen practiced from 1977 to 1988 at Owatonna Clinic, Owatonna, MN, where he served as president. Dr. Cullen earned his MD and BS degrees from the University of Minnesota and his M.B.A. from the University of St. Thomas and completed his residency and Board certification in Internal Medicine through the University of North Carolina in Chapel Hill and Wilmington, NC.
D. Robert Schemel, age 68, has served as a director since September 2015. Mr. Schemel had previously served as a director of Sun BioPharma Research, Inc. since March 2012. Mr. Schemel has over 39 years’ experience in the agriculture industry. From 1973-2005, Mr. Schemel owned and operated a farming operation in Kandiyohi County, Minnesota, building a 5,000-acre operation producing corn, soybeans and sugar beets. Mr. Schemel has extensive experience in serving on boards of directors. From 1992-1996 he served as a board member for ValAdCo and then from 1996-2003 he served as the Chairman of the Board for Phenix Biocomposites.
Class III Directors -Terms Expiring in 2025
Arthur J. Fratamico, age 58, has served as a director of our Company since December of 2019. He is a registered pharmacist with over 30 years of experience in the pharmaceutical industry and has been the Chief Executive Officer of Radiant Biotherapeutics, which is advancing a novel antibody platform that is focused on the development of Multabodies, which are multi-valent and multi-specific antibodies since May 2021. Prior to Radiant, Mr. Fratamico served as Chief Business Officer at Galera Therapeutics, Inc., a biopharmaceutical company dedicated to discovering and developing novel dismutase mimetics with the goal of transforming cancer radiotherapy, since January 2017. Prior to joining Galera, Mr. Fratamico served as Chief Business Officer of Vitae Pharmaceuticals, Inc., a Nasdaq-listed clinical-stage biotechnology company, from May 2014 until its sale to Allergan in December 2016. Prior to Vitae Pharmaceuticals, he held similar executive roles with a number of biotechnology companies leading their business development efforts, including facilitating the sales of Gemin X Pharmaceuticals, Inc. and MGI Pharma, Inc. In addition to being responsible for numerous licensing transactions and acquisitions, he also directed corporate strategy and managed external corporate communications. He also served in several senior marketing, product planning and new product development positions. Mr. Fratamico earned a bachelor’s degree in pharmacy from the Philadelphia College of Pharmacy and Science and an M.B.A. from Drexel University.
Jeffrey S. Mathiesen, age 63, has served as a director of our Company since September 2015. Mr. Mathiesen also serves as a director and audit committee chairman of NeuroOne Medical Technologies Corporation, a publicly traded medical device company. Since June 2021, Mr. Mathiesen has served as Chief Financial Officer, Treasurer and Secretary of Helius Medical Technologies, Inc. (Nasdaq: HSDT), a publicly traded medical device company, developing noninvasive platform technologies focused on neurological wellness, and he served as director since May 2022 and previously served as director and Audit Committee Chair from June 2020 through June 2021. Additionally, Mr. Mathiesen previously served as a director and Audit Committee Chair of Healthcare Triangle, Inc. (Nasdaq: HCTI), a publicly traded provider of cloud and data transformation platform and solutions for healthcare and life sciences, from March 2022 to December 2022 and as a director and audit committee chairman of eNeura, Inc., a privately held medical technology company providing therapy for both acute treatment and prevention of migraine, from July 2018 to February 2020. Mr. Mathiesen has served as Advisor to the CEO of Teewinot Life Sciences, a privately held biopharmaceutical company focused on the biosynthetic production of pure pharmaceutical grade cannabinoids from October 2019 to December 2019, and as Chief Financial Officer from March 2019 to October 2019. In August 2020, Teewinot Life Sciences filed a voluntary petition under Chapter 11 of the United States Bankruptcy Code. Previously he served as Chief Financial Officer of Gemphire Therapeutics Inc., a publicly traded biopharmaceutical company from September 2015 to September 2018. From August 2015 to September 2015, he was a consultant to Gemphire. He served as Chief Financial Officer of Sunshine Heart, Inc., a publicly traded medical device company, from March 2011 to January 2015. Mr. Mathiesen has held executive positions with publicly traded companies dating back to 1993, including vice president and chief financial officer positions. Mr. Mathiesen holds a B.S. in Accounting from the University of South Dakota and is also a Certified Public Accountant.
Class I Directors -Terms Expiring in 2026
Daniel J. Donovan, age 59, has served as a director since June 2022. He had served as a director and Chief Business Officer, a non-employee position, of CPP from 2011 until immediately before the completion of its acquisition by Panbela in June 2022. He has served as chief executive officer of rareLife Solutions, Inc., a private company since he founded it in 2014. He served on the Board of Directors at Intensity Therapeutics since January of 2023 and is a member of the audit committee. Before rareLife, Mr. Donovan founded Envision Pharma in 2001, serving as managing director then president until 2011. Envision Pharma was acquired by United BioSource Corporation in 2008, where Mr. Donovan served as Senior Vice President Strategy and Market Development and was a member of the leadership team. Mr. Donovan began his career at Pfizer serving in a variety of positions of increasing responsibility, ranging from sales to market research and marketing in the U.S. and internationally, culminating in his position as Director and European Team Leader. During his time at Pfizer, he played a pivotal role in the commercialization of some of the pharmaceutical industry’s most successful product launches.
Jeffrey E. Jacob, age 62, has served as a director since June 2022. He served as Chief Executive Officer of CPP from 2009 until immediately before the completion of its acquisition by Panbela in June 2022. He is also the principal of Tucson Pharma Ventures LLC, an Arizona-based biopharmaceutical consulting and investment firm, a role he’s held since 2004. In 2004, Mr. Jacob founded Systems Medicine Inc., a startup company applying systems biology, predictive pharmacogenomics, and clinical trial design innovations to the development of new cancer drugs and served as its chief executive officer until its sale in 2007, after which he served as a divisional chief executive officer until late 2008. Between 1987 and 2004, Mr. Jacob was employed by Research Corporation Technologies, most recently as Senior Vice President. During that time, he led the transformation of Research Corporation Technologies from a patent development and licensing organization to an early stage-technology incubation and venture deployment firm. He has served as a member of the board of directors of Research Corporation Technologies and currently serves as its chair. He is also a founding board member and previously served as the chief program officer of Critical Path Institute. Mr. Jacob holds a master’s degree in engineering and a master’s degree in technology and policy from the Massachusetts Institute of Technology and a bachelor’s degree in engineering from the University of Arizona.
Jennifer K. Simpson Ph.D., MSN, CRNP, has served as our President and Chief Executive Officer and as a director of our Company since July 2020. See “Information about our Executive Officers” above for further information regarding Dr. Simpson’s background and experience.
Director Independence
The continued listing rules of The Nasdaq Stock Market, LLC (the “Nasdaq Rules”) require that a majority of our Board of Directors be “independent directors” as that term is defined in the Nasdaq Rules. Our Board has determined that each of our non-employee directors, namely Messrs. Donovan, Fratamico, Mathiesen, and Schemel, are “independent directors.”
DIRECTOR COMPENSATION
The following table sets forth certain information regarding compensation of the persons who served as our non-employee directors during the most recently completed fiscal year. Share amounts have been restated for the historical 1-for-30 and the 1-for-40 reverse splits.
|
Name
|
|
Fees Earned
or
Paid in Cash
($)
|
|
|
Option
Awards
($)
|
|
|
Total
($)
|
|
|
Michael T. Cullen (a)
|
|
|
72,500 |
|
|
|
3,642 |
|
|
|
76,142 |
|
|
Arthur J. Fratamico (b)
|
|
|
49,000 |
|
|
|
3,642 |
|
|
|
52,642 |
|
|
Jeffrey S. Mathiesen (c)
|
|
|
81,500 |
|
|
|
3,642 |
|
|
|
85,142 |
|
|
D. Robert Schemel (d)
|
|
|
57,500 |
|
|
|
3,642 |
|
|
|
61,142 |
|
| Daniel J. Donovan (e) |
|
|
52,500 |
|
|
|
3,642 |
|
|
|
56,142 |
|
|
Jeffrey J. Jacob (f)
|
|
|
40,000 |
|
|
|
3,642 |
|
|
|
43,642 |
|
|
(a)
|
Dr. Cullen held options to purchase an aggregate of 27 shares as of December 31, 2023. |
|
(b)
|
Mr. Fratamico held options to purchase an aggregate of 14 shares as of December 31, 2023. |
|
(c)
|
Mr. Mathiesen held options to purchase an aggregate of 14 shares as of December 31, 2023. |
|
(d)
|
Mr. Schemel held options to purchase an aggregate of 14 shares as of December 31, 2023. |
|
(e)
|
Mr. Donovan held options to purchase an aggregate of 14 shares as of December 31, 2023. |
|
(f)
|
Mr. Jacob held options to purchase an aggregate of 24 shares as of December 31, 2023. |
During 2023, Panbela reimbursed non-employee directors for out-of-pocket expenses incurred in connection with attending meetings of our Board of Directors and its committees.
Directors who are also our employees or service providers receive no additional cash compensation for service on our Board of Directors. Dr. Simpson served as President and Chief Executive Officer throughout fiscal 2022.
In February 2022, the Compensation Committee approved an update to the cash compensation for non-employee directors. The annual amounts described below were effective January 1, 2022 and remain in place unless and until they are changed by the committee.
|
Annual Retainers
(all amounts in $)
|
|
General
|
|
|
Audit Committee
|
|
|
Nominating &
Governance
Committee
|
|
|
Compensation
Committee
|
|
|
Nonemployee director
|
|
|
40,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chair(a)
|
|
|
32,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lead independent director(a)
|
|
|
22,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Committee chair
|
|
|
|
|
|
|
15,000 |
|
|
|
7,500 |
|
|
|
10,000 |
|
|
Committee member
|
|
|
|
|
|
|
7,500 |
|
|
|
4,000 |
|
|
|
5,000 |
|
|
(a)
|
Paid in addition to nonemployee director retainer.
|
EXECUTIVE COMPENSATION
Compensation of Named Executive Officers
The following disclosure focuses on our named executive officers. For fiscal 2023, our “named executive officers” consisted of our only executive officers, Dr. Simpson and Ms. Horvath.
Base salaries for each of our named executive officers were initially established based on arm’s-length negotiations with the applicable executive. The Compensation Committee of our Board of Directors reviews our executive officers’ salaries annually. When negotiating or reviewing base salaries, the Compensation Committee considers market competitiveness based on the experience of its members, the executive’s expected future contribution to our success and the relative salaries and responsibilities of our other executives.
Summary Compensation Table
The following table provides information regarding the compensation earned by our named executive officers for the periods presented (collectively referred to as the “Executives”):
|
Name and Principal Positions
|
|
Year
|
|
Salary
($)
|
|
|
Option
Awards(a)
($)
|
|
Stock
Awards
($)
|
|
Nonequity
Incentive Plan
Compensation
(b)
($)
|
|
|
Total
($)
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jennifer K. Simpson
|
|
2023
|
|
|
527,000 |
|
|
|
44,921 |
|
‐
|
|
‐
|
|
|
|
571,921 |
|
|
President and Chief Executive Officer
|
|
2022
|
|
|
506,000 |
|
|
‐
|
|
‐
|
|
|
188,324 |
|
|
|
694,324 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Susan Horvath
|
|
2023
|
|
|
333,000 |
|
|
|
15,535 |
|
‐
|
|
‐
|
|
|
|
348,535 |
|
|
Chief Financial Officer and Vice President of Finance
|
|
2022
|
|
|
320,200 |
|
|
‐
|
|
‐
|
|
|
103,459 |
|
|
|
423,459 |
|
|
(a)
|
The values of option awards in this table represent the fair value of such awards granted during the fiscal year, as computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718. The assumptions used to determine the valuation of the awards are discussed in Note 9 to the consolidated financial statements. No equity awards were awarded for fiscal 2022.
|
|
(b)
|
Represents payments made in 2023 under Panbela’s 2022 Cash Incentive Programs as described further below. The Compensation Committee has not certified actual performance for purposes of determining the cash incentive payments earned under the 2023 Cash Incentive Program.
|
Cash Incentive Compensation
For 2023, the Compensation Committee established performance objectives for each of the Executives based on clinical development and financial milestones. Each Executive’s potential payment upon satisfaction of the objectives was equal to the target set forth in the Executive’s employment agreement as described further below. The 2023 incentive, if any, is expected to be paid in the first quarter of 2024, subject to a final determination by the Compensation Committee.
Outstanding Equity Awards as of December 31, 2023 (Adjusted for the 1 for 20, 1 for 30 and 1 for 40 reverse stock splits)
| |
|
|
|
Option Awards
|
|
Name
|
|
Grant
Date
|
|
Number of
securities
underlying
unexercised
options (#)
exercisable
|
|
|
Number of
securities
underlying
unexercised
options (#) un-
exercisable
|
|
|
Option
exercise
price
($)
|
|
Option
expiration
Date
|
|
Jennifer K. Simpson
|
|
7/17/2020
|
|
|
8 |
|
|
|
– |
|
|
|
239,760 |
|
7/17/2030
|
| |
|
3/30/2021
|
|
|
4 |
|
|
2(a)
|
|
|
|
98,160 |
|
3/30/2031
|
| |
|
3/27/2023
|
|
|
– |
|
|
165(b) |
|
|
|
300 |
|
3/27/2033
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Susan Horvath
|
|
4/17/2018
|
|
|
1 |
|
|
|
– |
|
|
|
138,000 |
|
4/17/2028
|
| |
|
5/21/2019
|
|
|
2 |
|
|
|
– |
|
|
|
70,800 |
|
5/21/2029
|
| |
|
9/24/2019
|
|
|
1 |
|
|
|
– |
|
|
|
120,000 |
|
9/24/2029
|
| |
|
6/24/2020
|
|
|
1 |
|
|
|
– |
|
|
|
119,520 |
|
6/24/2030
|
| |
|
3/30/2021
|
|
|
1 |
|
|
–
|
|
|
|
98,160 |
|
3/30/2031
|
| |
|
3/27/2023
|
|
|
– |
|
|
57(b) |
|
|
|
300 |
|
3/27/2033
|
|
(a)
|
Scheduled to vest with respect to all remaining shares on March 30, 2024.
|
|
(b)
|
Scheduled to vest in three substantially equal installments on March 27 in each of 2024, 2025 and 2026. |
Employment Agreements
We are party to employment agreements with each of the Executives. In addition to the specific terms summarized below, each Executive is eligible to participate in the other compensation and benefit programs generally available to our employees, including our other executive officers, if any. Each such employment agreement also includes customary non-competition and non-solicitation covenants and a requirement that the Executive sign a supplemental agreement regarding confidentiality and assignment of intellectual property.
In accordance with the employment agreements, the base salary of each Executive is reviewed annually by the Compensation Committee of our Board of Directors. Pursuant to the employment agreements, the committee may authorize an increase for the applicable year but may not reduce an Executive’s base salary below its then-current level other than with the Executive’s consent or pursuant to a general wage reduction in respect of substantially all of our executive officers.
President and Chief Executive Officer
Under her employment agreement, Dr. Simpson is eligible to receive an annual performance-based cash bonus with a target amount equal to no less than 50% of her base salary. Payment of the bonus amount is subject to achievement of metrics to be established by our Board of Directors and her continued employment with Panbela through the end of the applicable cash bonus period.
Vice President of Finance and Chief Financial Officer
Under her employment agreement, Ms. Horvath is eligible to receive an annual performance-based cash bonus with a target amount equal to no less than 40% of her base salary. Payment of the bonus amount is subject to achievement of metrics to be established by our Board of Directors and her continued employment with Panbela through the end of the applicable cash bonus period.
Potential Payments Upon Termination or Change-in-Control
Under their respective employment agreements, if Dr. Simpson’s or Ms. Horvath’s employment is terminated by us for any reason other than for “cause” (as defined in the applicable employment agreement) or her for “good reason” (as defined in the applicable employment agreement), then either Dr. Simpson or Ms. Horvath will be eligible to receive an amount equal to their respective annualized salary plus an amount equal to a prorated portion of their cash bonus target, if any, for the year in which the termination occurred, in addition to other amounts accrued on or before the date of termination. If any such termination occurs within six months prior or two years after a “change of control” (as defined in the applicable employment agreement), then Dr. Simpson or Ms. Horvath, as applicable, would instead receive an amount equal to her respective annualized salary, plus an amount equal to her full cash bonus target for the year in which the termination occurred.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information with respect to the beneficial ownership of our outstanding common stock as of January 23, 2024 by (i) each of our named executive officers identified in the Summary Compensation Table below; (ii) each of our directors; (iii) all of our executive officers, directors and director nominees as a group; and (iv) each other beneficial owner of 5% or more of our outstanding common stock. Ownership percentages are based on an estimated 480,244 shares of common stock outstanding as of the close of business on the same date. Beneficial ownership is determined in accordance with the rules of the SEC. To our knowledge and subject to applicable community property laws, each of the holders of stock listed below has sole voting and investment power as to the stock owned unless otherwise noted. The table below includes the number of shares underlying rights to acquire common stock that are exercisable within 60 days from January 23, 2024. Except as otherwise noted below, the address for each director or officer listed in the table is c/o Panbela Therapeutics, Inc., 712 Vista Blvd #305, Waconia, Minnesota 55387.
|
Name
|
|
Amount and
Nature
of
Beneficial
Ownership
|
|
|
Percentage of
Outstanding
Shares*
|
|
|
Executive Officers and Directors
|
|
|
|
|
|
|
|
|
|
Jennifer K. Simpson
|
|
|
33 |
(a)
|
|
|
* |
|
|
Susan Horvath
|
|
|
27 |
(b)
|
|
|
* |
|
|
Michael T. Cullen
|
|
|
60 |
(c)
|
|
|
* |
|
|
Daniel J. Donovan
|
|
|
25 |
(d)
|
|
|
* |
|
|
Arthur J. Fratamico
|
|
|
14 |
(e)
|
|
|
* |
|
|
Jeffrey E. Jacob
|
|
|
27 |
(f)
|
|
|
* |
|
|
Jeffrey S. Mathiesen
|
|
|
14 |
(g)
|
|
|
* |
|
|
D. Robert Schemel
|
|
|
48 |
(h)
|
|
|
* |
|
|
All directors and current executive officers as a group (8 persons)
|
|
|
248 |
(i)
|
|
|
* |
|
|
(a)
|
Includes 12 shares subject to stock options and 14 shares subject to warrants. |
|
(b)
|
Includes 6 shares subject to stock options and 14 shares subject to warrants. |
|
(c)
|
Includes 27 shares subject to stock options, and 1 shares subject to warrants. Also includes 147 shares and 10 shares subject to warrants in each case held by the Cullen Living Trust. |
|
(d)
|
Includes 14 shares subject to stock options. Also includes 3 shares held by Westport Boys, LLC (“Westport”), 6 shares held by GDB Investments, LLP (“GDB”). Mr. Donovan is a managing member of Westport and a designated member of GDB. Mr. Donovan disclaims beneficial ownership of the securities owned by Westport and GDB except to the extent of his pecuniary interest therein. |
|
(e)
|
Includes 14 shares subject to stock options. |
|
(f)
|
Includes 24 shares subject to options, 2 shares subject to warrants held jointly with spouse. |
|
(g)
|
Includes 14 shares subject to options. |
|
(h)
|
Includes 14 shares subject to stock options, 19 shares and 12 shares subject to warrants held by spouse |
|
(i)
|
Includes 125 shares subject to stock options and 53 shares subject to warrants. |
DESCRIPTION OF SECURITIES
The summary of the general terms and provisions of the common stock, par value $0.001 per share (“Common Stock”), of Panbela set forth below does not purport to be complete and is subject to and qualified by reference to the Corporation’s Restated Certificate of Incorporation, as amended (the “Certificate”), and Restated Bylaws of the Corporation, as amended (the “Bylaws”). For additional information, please read the Certificate, Bylaws and the applicable provisions of the General Corporation Law of Delaware (the “DGCL”).
Authorized Shares
The Corporation is authorized to issue up to 110,000,000 shares of capital stock, of which 100,000,000 constitute shares of Common Stock and 10,000,000 constitute shares of preferred stock, par value $0.001 per share (“Preferred Stock”).
Common Stock
No outstanding shares of common stock is entitled to preference over any other share, and each share is equal to any other share in all respects. Holders of shares of common stock are entitled to one vote for each share held of record at each meeting of shareholders. Holders of shares of common stock are not entitled to any preemptive, subscription, conversion, redemption or sinking fund rights. The absence of preemptive rights could result in a dilution of the interest of shareholders should additional common shares be issued.
Subject to any prior rights of any Preferred Stock then outstanding, holders of common stock are entitled to receive dividends in the form of cash, property or shares of capital stock of the Corporation, when and as declared by the board of directors, provided there are sufficient net profits or surplus legally available for that purpose. In any distribution of capital assets, such as liquidation, whether voluntary or involuntary, holders of shares of common stock are entitled to receive pro rata the assets remaining after creditors have been paid in full. All of the issued and outstanding shares of common stock are non-assessable.
Anti-Takeover Provisions
The Charter Documents and the DGCL contain certain provisions that may discourage an unsolicited takeover of the Company or make an unsolicited takeover of the Company more difficult. The following are some of the more significant anti-takeover provisions that are applicable to the Company:
Delaware Anti-Takeover Law
In general, Section 203 of the DGCL prohibits a Delaware corporation with a class of voting stock listed on a national securities exchange or held of record by 2,000 or more stockholders from engaging in a Business Combination (as defined below) with an Interested Stockholder (as defined below) for a three-year period following the time that this stockholder becomes an interested stockholder, unless the Business Combination is approved in a prescribed manner. A “Business Combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the Interested Stockholder. An “Interested Stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of Interested Stockholder status, 15% or more of the corporation’s voting stock. Under Section 203, a Business Combination between a corporation and an Interested Stockholder is prohibited for three years unless it satisfies one of the following conditions:
| |
●
|
Before the stockholder became an Interested Stockholder, the board of directors approved either the Business Combination or the transaction which resulted in the stockholder becoming an Interested Stockholder;
|
| |
●
|
Upon consummation of the transaction which resulted in the stockholder becoming an Interested Stockholder, the Interested Stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances; or
|
| |
●
|
At or after the time the stockholder became an Interested Stockholder, the Business Combination was approved by the board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the Interested Stockholder.
|
Requirements for Advance Notification of Stockholder Nominations and Proposals
The Bylaws establish advance-notice procedures with respect to stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors.
Special Meetings of Stockholders
The Certificate and Bylaws provide that a special meeting of stockholders may be called only by the board of directors, the Chairman of the Board or the Chief Executive Officer of the Corporation.
Classified Board of Directors
The Certificate provides that directors are divided into three classes and elected for staggered terms. At each annual meeting, approximately one third of the directors will be elected to serve a three-year term. Directors serving staggered terms can be removed from office only for cause and only by the affirmative vote of the holders of 75% or more of the outstanding shares of stock then entitled to vote at an election of directors.
Authority of the Board of Directors
The board of directors has the power to issue any or all of the shares of the Corporation’s capital stock, including the authority to establish one or more series of Preferred Stock and to fix the powers, preferences, rights and limitations of such class or series, without seeking stockholder approval. The board of directors has the authority to adopt and change Bylaws, subject to the right of holders of at least 66.67% of the voting power of all then-outstanding shares entitled to vote generally in the election of directors to adopt, amend or repeal Bylaws.
Preferred Stock
Our Board of Directors has the authority, without first obtaining the approval of our stockholders, to establish one or more series of preferred stock and to fix:
| |
●
|
the number of shares of such series;
|
| |
●
|
the designations, preferences and relative rights, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences; and
|
| |
●
|
any qualifications, limitations or restrictions.
|
We believe that the ability of our Board of Directors to issue one or more series of preferred stock provides flexibility in structuring possible future financings and acquisitions, and in meeting other corporate needs that may arise. The authorized shares of preferred stock, as well as authorized and unissued shares of common stock, are available for issuance without action by the holders of common stock, unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded.
Our Board of Directors may authorize, without stockholder approval, the issuance of preferred stock with voting and conversion rights that could adversely affect the voting power and other rights of holders of common stock. Although our Board of Directors has no current intention of doing so, it could issue a series of preferred stock that could, depending on the terms of such series, impede the completion of a merger, tender offer or other takeover attempt of our Company. Our Board of Directors could also issue preferred stock having terms that could discourage an acquisition attempt through which an acquirer may be able to change the composition of our Board of Directors, including a tender offer or other transaction that some, or a majority, of the stockholders might believe to be in their best interests or in which stockholders might receive a premium for their stock over the then-current market price. Any issuance of preferred stock therefore could have the effect of decreasing the market price of our common stock.
Our Board of Directors will make any determination to issue such shares based on its judgment as to the best interests of our Company and stockholders. We have no current plans to issue any preferred stock.
Options
The 2016 Plan initially authorized the issuance of up to 116 shares of our common stock pursuant to awards granted thereunder and 164,246 shares have been added pursuant to its annual evergreen feature. As of January 23, 2024, options to purchase 553 shares of our common stock were outstanding under the 2016 Plan with a weighted average price of $13,297.06 per share. Approximately 163,757 shares of common stock remained available for future grants under the 2016 Plan as of the same date.
As of January 23, 2024, options to purchase 5 shares of our common stock remained outstanding under the 2011 Plan with a weighted average price of $76,200.00 per share. We ceased making awards under the 2011 Plan upon stockholder approval of the 2016 Plan.
As of January 23, 2024, options to purchase 41 shares of our common stock remained outstanding under CPP’s 2010 Equity Incentive Plan with a weighted average price of $6,743.41, all of which were assumed by us in connection with the acquisition of CPP.
Warrants Outstanding
As of January 23, 2024, we had issued and outstanding warrants to purchase 340,952 shares of common stock and no warrants to purchase shares of preferred stock outstanding. As of the same date, the outstanding warrants had a weighted average exercise price of $64.09 per share and an average remaining exercise period of 5.24 years. To the extent the offering is deemed to involve a sale of Common Stock at a price less than the exercise price of any of the following warrants, then each such warrant’s exercise price will be adjusted according to its respective terms. Under no circumstances (other than a forward stock split) will any outstanding warrants be exercisable for a greater number of shares of Common Stock.
October 2022 Warrants
In October 2022, we issued common stock purchase warrants to purchase up to an aggregate of 1,256 shares of our Common Stock (the “October 2022 Warrants”) in connection with a public offering of shares of our Common Stock and warrants. The October 2022 Warrants had an initial exercise price of $108,960.00 per share (after adjusting for subsequent reverse stock splits), were exercisable upon issuance and through October 4, 2027. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. As of January 23, 2024 there remained 285 shares underlying outstanding October 2022 Warrants, each having an adjusted exercise price of $12.39 per share.
January 2023 Warrants
In January 2023, we issued common stock purchase warrants to purchase up to an aggregate of 3,091 shares of our Common Stock (the “January 2023 Warrants”) in connection with a public offering of shares of our Common Stock and warrants. The January Warrants had an initial exercise price of $1,650.00 per share (after adjusting for subsequent reverse stock splits), were exercisable upon issuance and through January 30, 2028. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. On or after the thirty-day anniversary of their issuance, a holder of common warrants may also provide notice and elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal to the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise and (y) 0.75. As of January 23, 2024 there remained 128 shares underlying outstanding January 2023 Warrants, each having an adjusted cash exercise price of $12.39 per share.
Class A and B Warrants
In June 2023, we issued Class A common stock purchase warrants to purchase up to an aggregate of 113,500 shares of our Common Stock (the “Class A Warrants”) and Class B common stock purchase warrants to purchase up to an aggregate of 113,500 shares of our Common Stock (the “Class B Warrants” and, together with the Class A Warrant, the “June Warrants”) in connection with a public offering of shares of our Common Stock and warrants. The June Warrants had an initial exercise price of $15.60 per share (after adjusting for the subsequent reverse stock split), were exercisable upon issuance and through June 16, 2028. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. With respect to the Class A Warrants only, on or after the thirty-day anniversary of their issuance, a holder of common warrants may also provide notice and elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal to the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise and (y) 0.24. As of January 23, 2024 there remained 2,595 shares underlying outstanding Class A Warrants and 7,000 shares underlying outstanding Class B Warrants, each having an adjusted cash exercise price of $12.39 per share.
Class C Warrants
On November 2, 2023, we issued Class C common stock purchase warrants to purchase up to an aggregate of 213,000 shares of our Common Stock (the “Class C Warrants”) in connection with the inducement of exercises of existing warrants to purchase shares of our Common Stock. The Class C Warrants had an initial exercise price of $15.60 per share (after adjusting for the subsequent reverse stock split) and were exercisable upon the date of stockholder approval through the date that is five years from the date of any stockholder approvals necessary under the listing rules of Nasdaq. Our stockholders approved the issuance of the underlying shares of Common Stock at a special meeting held on December 19, 2023. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities. As of January 23, 2024 there remained 75,200 shares underlying outstanding Class C Warrants, each having a cash exercise price of $15.60 per share.
We agreed to file a registration statement covering the resale of the shares underlying the Class C Warrants issued or issuable upon the exercise of the Class C Warrants and a registration statement on Form S-1 (File No. 333-275733) was declared effective by the SEC on December 20, 2023.
Class D Warrants
On December 21, 2023, we issued Class D common stock purchase warrants to purchase up to an aggregate of 255,600 shares of our Common Stock (the “Class D Warrants”) in connection with the inducement of exercises of existing Class C Warrants. The Class D Warrants had an initial exercise price of $19.00 per share (after adjusting for the subsequent reverse stock split) and will only be exercisable contingent upon and after receiving stockholder approval as required by listing rules of Nasdaq and may be exercised until five years from the date of such stockholder approval, if any. The exercise price is separately subject to reduction in the event of certain future dilutive issuances of shares of Common Stock by the Company, including pursuant to common stock equivalents and convertible or derivative securities, upon any intervening reverse stock splits, and upon receipt of the stockholder approval. As of January 23, 2024 all of the Class D Warrants remained outstanding and unexercisable pending stockholder approval.
We have agreed to file a registration statement covering the resale of the shares underlying the Class D Warrants within sixty (60) calendar days of December 21, 2023. Additionally, the Company agreed not to effect or agree to effect any variable rate transaction (as defined in the inducement letters) for one year, other than an at-the-market offering, which may be effected after six months.
Class E and F Warrants
See the description under the heading “Common Warrants” below for a discussion of certain terms of the Class E and F Warrants proposed for issuance pursuant to this offering.
Pre-Funded Warrants
The following summary of certain terms and provisions of pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration and Exercise Price. Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.001. The pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The pre-funded warrants will be issued separately from the accompanying common warrants and may be transferred separately immediately thereafter.
Exercisability. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). Purchasers of the pre-funded warrants in this offering may elect to deliver their exercise notice following the pricing of the offering and prior to the issuance of the pre-funded warrants at closing to have their pre-funded warrants exercised immediately upon issuance and receive shares of common stock underlying the pre-funded warrants upon closing of this offering. A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s pre-funded warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants in this offering may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding common stock. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. If, at the time a holder exercises its pre-funded warrants, a registration statement registering the issuance of the shares of common stock underlying the pre-funded warrants under the Securities Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Transferability. Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
Exchange Listing. There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized trading system. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder. Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their pre-funded warrants.
Fundamental Transaction. In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction.
Common Warrants
The following summary of certain terms and provisions of the common warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the common warrants, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of common warrants for a complete description of the terms and conditions of the common warrants.
Duration and Exercise Price. Each common warrant offered hereby will have an initial exercise price per share equal to $ (100% of the public offering price per share and common warrants). The common warrants will be immediately exercisable and will expire on the fifth anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The exercise price is separately subject to reduction in the event of (i) certain future dilutive issuances of shares of common stock by us, including pursuant to common stock equivalents and convertible or derivative securities and (ii) any reverse stock split. The common warrants will be issued separately from the common stock and will be held separately immediately thereafter. A Class E Warrant to purchase 0.25 shares of our common stock and a Class F Warrant to purchase 1.75 shares of our common stock will be issued for every share of common stock or pre-funded warrant to purchase one share purchased in this offering.
Exercisability. The common warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the common warrant to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s common warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the common warrants. No fractional shares of common stock will be issued in connection with the exercise of a common warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. If, at the time a holder exercises its common warrants, a registration statement registering the issuance of the shares of common stock underlying the common warrants under the Securities Act is not then effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the common warrants.
Alternative Cashless Exercise. On or after the thirty-day anniversary of their issuance, a holder of Class E Warrants may also provide notice and elect an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise and (y) 1.33.
Transferability. Subject to applicable laws, a common warrant in book entry form may be transferred at the option of the holder through the facilities of the Depository Trust Company and common warrants in physical form may be transferred upon surrender of the common warrant to the warrant agent together with the appropriate instruments of transfer. Pursuant to a warrant agency agreement between us and VStock Transfer, as warrant agent, the common warrants initially will be issued in book-entry form and will be represented by one or more global certificates deposited with The Depository Trust Company (“DTC”) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Exchange Listing. There is no established public trading market for the common warrants, and we do not expect a market to develop. In addition, we do not intend to list the common warrants on any securities exchange or nationally recognized trading system. Without an active trading market, the liquidity of the common warrants will be limited.
Right as a Stockholder. Except as otherwise provided in the common warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the common warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their common warrants.
Fundamental Transaction. In the event of a fundamental transaction, as described in the form of common warrant, and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the common warrants will be entitled to receive upon exercise of the common warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the common warrants immediately prior to such fundamental transaction.
Transfer Agent, Registrar, and Warrant Agent
The transfer agent and registrar for our common stock, warrant agent for the October 2022 Warrants, January 2022 Warrants, and Class A and B Warrants, and the proposed warrant agent for the Class E and F Warrants is VStock Transfer, which can be contacted at 18 Lafayette Place, Woodmere, New York, 11598, info@vstocktransfer.com, or +1 (212) 828-8436.
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options and warrants, or the anticipation of these sales, could adversely affect prevailing market prices from time to time and could impair our ability to raise equity capital in the future.
Upon the closing of this offering, we will have a total of 8,480,244 shares of our common stock outstanding and a total of 24,480,244 shares of our common stock outstanding if the common warrants sold in this offering are exercised for cash in full, based on the 480,244 shares of our common stock outstanding as of January 23, 2024. Of these outstanding shares, all of the shares sold in the offering will be freely tradable, except that any shares purchased in this offering by our affiliates, as that term is defined in Rule 144 under the Securities Act, would only be able to be sold in compliance with the limitations described below.
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares upon expiration of the lock-up agreements described below, without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described below, within any three-month period, a number of shares that does not exceed the greater of:
| |
●
|
1% of the number of shares of our common stock then outstanding, which will equal approximately 95,024 shares immediately after this offering; or |
| |
●
|
if and when our common stock is listed on the Nasdaq Capital Market, the average weekly trading volume of our common stock on such market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
|
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information or holding period provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required by that rule to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701, subject to the market standoff agreements and lock-up agreements described below.
Stock Options and Warrants
See “Description of Securities” above for a discussion of our outstanding and options warrant to purchase shares of common stock
PLAN OF DISTRIBUTION
Pursuant to a placement agency agreement, dated as of , 2024, we have engaged Roth Capital Partners, LLC to act as our lead placement agent to solicit offers to purchase the securities offered by this prospectus on a reasonable best efforts basis. The placement agent is not purchasing or selling any securities, nor is it required to arrange for the purchase and sale of any specific number or dollar amount of securities, other than to use its “reasonable best efforts” to arrange for the sale of the securities by us. Therefore, we may not sell the entire amount of securities being offered, or any at all. The placement agent may engage one or more subagents or selected dealers in connection with this offering.
We expect to enter into a securities purchase agreement directly with the institutional investors, at the investor’s option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase of our securities in this offering.
The placement agency agreement provides that the placement agent’s obligations are subject to the conditions contained in the placement agency agreement.
This offering will terminate on February 15, 2024, unless completed sooner or unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date, except that the shares of Common Stock underlying the Class E Warrants, Class F Warrants, and the pre-funded warrants will be offered on a continuous basis until their respective full exercise or expiration. We will deliver the securities being issued to the investors upon receipt of investor funds for the purchase of the securities offered pursuant to this prospectus. Accordingly, there is no arrangement to receive or place investor funds in an escrow, trust or any similar account. We expect to deliver the securities being offered pursuant to this prospectus on or about , 2024. There is no minimum number of securities or amount of proceeds that is a condition to closing of this offering.
Placement Agent Fees, Commissions and Expenses
Upon the closing of this offering, we will pay the placement agent a cash transaction fee equal to 7.0% of the aggregate gross proceeds to us from the sale of the securities in the offering. In addition, we will reimburse the placement agent for its out-of-pocket expenses incurred in connection with this offering, including the fees and expenses of the counsel for the placement agent, up to $100,000.
The following table shows the public offering price, placement agent fees and proceeds, before expenses, to us, assuming the purchase of all the securities we are offering.
| |
|
Per Share and
Common
Warrant
|
|
|
Per Pre-Funded
Warrant and
Common
Warrant
|
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
Placement Agent fees
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to us, before expenses
|
|
$
|
|
|
|
$
|
|
|
We estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding placement agent fees, will be approximately $315,000, all of which are payable by us. This figure includes the placement agent’s accountable expenses, including, but not limited to, legal fees for placement agent’s legal counsel, that we have agreed to pay at the closing of the offering up to an aggregate expense reimbursement of $100,000.
Lock-Up Agreements
We and our executive officers and directors expect to enter into lock up agreements with the representative prior to the commencement of this offering pursuant to which each of these persons or entities, for a period of 90 days from the effective date of the registration statement of which this prospectus forms a part, without the prior consent of the representative, agree not to (a) offer, sell, contract to sell, hypothecate, pledge or otherwise dispose of (or enter into any transaction which is designed to, or might reasonably be expected to, result in the disposition (whether by actual disposition or effective economic disposition due to cash settlement or otherwise), directly or indirectly, or establish or increase a put equivalent position or liquidate or decrease a call equivalent position within the meaning of Section 16 of the Exchange Act, any shares of our common stock or securities convertible, or exercisable into, shares of our common stock;; (b) file or cause to be filed any registration statement with the SEC relating to the offering of any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of our capital stock or issue, enter into any agreement to issue, or announce the issuance or any proposed issuance of, any of the foregoing; or (c) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our capital stock, whether any such transaction described in (a), (b) or (c) above is to be settled by delivery of shares of our capital stock or such other securities, in cash or otherwise.
Indemnification
We have agreed to indemnify the placement agent against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the placement agent may be required to make for these liabilities.
Determination of Offering Price and Warrant Exercise Price
The actual public offering price of the securities we are offering, and the exercise price of the common warrants and pre-funded warrants that we are offering, will be negotiated between us, the placement agent and the investors in the offering based on the trading price of our common stock prior to the offering, among other things. Other factors that will be considered in determining the public offering price of the securities we are offering, as well as the exercise price of the common warrants and pre-funded warrants that we are offering include our history and prospects, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, the general conditions of the securities markets at the time of the offering and such other factors as are deemed relevant, including the Nasdaq Minimum Price (as defined in Nasdaq Listing Rule 5635(d)).
Other Compensation
If within six (6) months following the termination or expiration of our engagement with the placement agent, we complete any sale of equity or equity-linked securities for which the placement agent is not acting as underwriter or placement agent (other than the exercise by any person or entity of any options, warrants or other convertible securities) to any of the investors that the placement agent introduced to us or with which the placement agent conducted discussions on our behalf, subject to specified exceptions, then we are required to pay to the placement agent a commission as described in this section, in each case only with respect to the portion of such financing received from such investors.
Regulation M
The placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, the placement agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the placement agent acting as principal. Under these rules and regulations, the placement agent (i) may not engage in any stabilization activity in connection with our securities and (ii) may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.
Electronic Distribution
A prospectus in electronic format may be made available on a website maintained by the placement agent. In connection with the offering, the placement agent or selected dealers may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
Other than the prospectus in electronic format, the information on the placement agent’s website and any information contained in any other website maintained by the placement agent is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent in its capacity as placement agent and should not be relied upon by investors.
Certain Relationships
The placement agent and its affiliates may in the future provide, from time to time, investment banking and financial advisory services to us in the ordinary course of business, for which they may receive customary fees and commissions.
The Nasdaq Capital Market Listing
Our common stock is listed on The Nasdaq Capital Market under the symbol “PBLA.”
Offer Restrictions Outside the United States
European Economic Area
In relation to each member state of the European Economic Area, no offer of securities which are the subject of the offering has been, or will be made to the public in that Member State, other than under the following exemptions under the Prospectus Directive:
| |
(a)
|
to any legal entity which is a qualified investor as defined in the Prospectus Directive;
|
| |
(b)
|
to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the Representatives for any such offer; or
|
| |
(c)
|
in any other circumstances falling within Article 3(2) of the Prospectus Directive,
|
provided that no such offer of securities referred to in (a) to (c) above shall result in a requirement for the Company or the placement agent to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person located in a Member State to whom any offer of securities is made or who receives any communication in respect of an offer of securities, or who initially acquires any shares of our securities will be deemed to have represented, warranted, acknowledged and agreed to and with the placement agent and the Company that (1) it is a “qualified investor” within the meaning of the law in that Member State implementing Article 2(1)(e) of the Prospectus Directive; and (2) in the case of any shares of our securities acquired by it as a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, the securities acquired by it in the offer have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Member State other than qualified investors, as that term is defined in the Prospectus Directive, or in circumstances in which the prior consent of the placement agent has been given to the offer or resale; or where our securities have been acquired by it on behalf of persons in any Member State other than qualified investors, the offer of those securities to it is not treated under the Prospectus Directive as having been made to such persons.
The Company, the placement agent and their respective affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgments and agreements.
This prospectus has been prepared on the basis that any offer of our securities in any Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly, any person making or intending to make an offer in that Member State of our securities which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the Company or the placement agent to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the placement agent have authorized, nor do they authorize, the making of any offer of securities in circumstances in which an obligation arises for the Company or the placement agent to publish a prospectus for such an offer.
For the purposes of this provision, the expression an “offer of our securities to the public” in relation to any of our securities in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (as amended) and includes any relevant implementing measure in each Member State. The above selling restriction is in addition to any other selling restrictions set out below.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to Prospective Investors in Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to our securities or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company or our securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of our securities will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of our securities has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes ("CISA"). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of our securities.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority (“DFSA”). This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The securities to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this prospectus, you should consult an authorized financial advisor.
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (“ASIC”), in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the “Corporations Act”) and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of our securities may only be made to persons (the “Exempt Investors”) who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the securities without disclosure to investors under Chapter 6D of the Corporations Act.
The securities applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring our securities must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Hong Kong
The securities have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the securities has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold, directly or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the relevant time. For the purposes of this paragraph, “Japanese Person” shall mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of securities may not be circulated or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| |
(a)
|
a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
|
| |
(b)
|
a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor,
|
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the securities pursuant to an offer made under Section 275 of the SFA except:
| |
(a)
|
to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA;
|
| |
(b)
|
where no consideration is or will be given for the transfer;
|
| |
(c)
|
where the transfer is by operation of law;
|
| |
(d)
|
as specified in Section 276(7) of the SFA; or
|
| |
(e)
|
as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
|
Notice to Prospective Investors in Canada
The securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the placement agent is not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering
LEGAL MATTERS
The validity of the securities offered by this prospectus will be passed upon for us by Faegre Drinker Biddle & Reath LLP. Pryor Cashman LLP is acting as counsel for the placement agent in connection with certain legal matters related to this offering.
EXPERTS
The financial statements of Panbela as of December 31, 2022 and 2021 and for the two years in the period ended December 31, 2022 incorporated in this prospectus by reference from the Company's Annual Report on Form 10-K have been audited by Cherry Bekaert LLP, an independent registered public accounting firm, as stated in their report, which is incorporated herein by reference. Such financial statements have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act that registers the distribution of the securities offered under this prospectus. The registration statement, including the attached exhibits and schedules, contains additional relevant information about us and the securities. The rules and regulations of the SEC allow us to omit from this prospectus certain information included in the registration statement.
We are subject to the informational requirements of the Securities Exchange Act and are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. Any information we file with the SEC, including the documents incorporated by reference into this prospectus, is also available on the SEC’s website at www.sec.gov. We also make these documents publicly available, free of charge, on our website at www.panbela.com as soon as reasonably practicable after filing such documents with the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
The Financial Statements the three and nine months ended September 30, 2023 versus the three and nine months ended September 30, 2022 and for the fiscal years ended December 31, 2022 and 2021 are historical. Share and per share amounts have been restated for comparison purposes for the 1-for-40 reverse stock split that was effected on January 13, 2023 or the 1-for-30 reverse stock split that was effected on June 1, 2023. No share or per share amounts for three and nine months ended September 30, 2023 versus the three and nine months ended September 30, 2022 and for the fiscal years ended December 31, 2022 and 2021 have been restated for the 1-for-20 reverse stock split that was effected on January 18, 2024.
Item 1. Financial Statements.
Panbela Therapeutics, Inc.
Condensed Consolidated Balance Sheets
(In thousands, except share amounts)
| |
|
September 30, 2023
|
|
|
December 31, 2022
|
|
| |
|
(Unaudited)
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$ |
907 |
|
|
$ |
1,285 |
|
|
Prepaid expenses and other current assets
|
|
|
824 |
|
|
|
443 |
|
|
Income tax receivable
|
|
|
155 |
|
|
|
49 |
|
|
Total current assets
|
|
|
1,886 |
|
|
|
1,777 |
|
|
Other non-current assets
|
|
|
8,742 |
|
|
|
3,201 |
|
|
Total assets
|
|
$ |
10,628 |
|
|
$ |
4,978 |
|
| |
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$ |
6,612 |
|
|
$ |
2,865 |
|
|
Accrued expenses
|
|
|
1,133 |
|
|
|
2,993 |
|
|
Accrued interest payable
|
|
|
172 |
|
|
|
325 |
|
|
Note payable
|
|
|
- |
|
|
|
650 |
|
|
Debt, current portion
|
|
|
1,000 |
|
|
|
1,000 |
|
|
Total current liabilities
|
|
|
8,917 |
|
|
|
7,833 |
|
| |
|
|
|
|
|
|
|
|
|
Debt, net of current portion
|
|
|
4,194 |
|
|
|
5,194 |
|
|
Total non-current liabilities
|
|
|
4,194 |
|
|
|
5,194 |
|
| |
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
13,111 |
|
|
|
13,027 |
|
| |
|
|
|
|
|
|
|
|
| Stockholders' deficit: |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 10,000,000 authorized; no shares issued or outstanding as of September 30, 2023 and December 31, 2022
|
|
|
- |
|
|
|
- |
|
|
Common stock, $0.001 par value; 100,000,000 authorized; 2,996,753 and 34,761 shares issued, and 2,996,334 and 34,761 outstanding as of September 30, 2023 and December 31, 2022, respectively
|
|
|
3 |
|
|
|
- |
|
|
Treasury Stock at cost; 419 and 0 shares as of September 30, 2023 and December 31, 2022, respectively
|
|
|
- |
|
|
|
- |
|
|
Additional paid-in capital
|
|
|
106,026 |
|
|
|
82,286 |
|
|
Accumulated deficit
|
|
|
(109,883 |
) |
|
|
(91,094 |
) |
|
Accumulated comprehensive income
|
|
|
1,371 |
|
|
|
759 |
|
|
Total stockholders' deficit
|
|
|
(2,483 |
) |
|
|
(8,049 |
) |
|
Total liabilities and stockholders' deficit
|
|
$ |
10,628 |
|
|
$ |
4,978 |
|
Share and per share data have been adjusted for all periods presented to reflect the one-for-thirty reverse stock split effective June 1, 2023 and the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to condensed consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
(Unaudited)
| |
|
Three Months Ended September 30,
|
|
|
Nine Months Ended September 30,
|
|
| |
|
2023
|
|
|
2022
|
|
|
2023
|
|
|
2022
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative
|
|
$ |
1,107 |
|
|
$ |
1,294 |
|
|
$ |
4,102 |
|
|
$ |
4,349 |
|
|
Research and development
|
|
|
6,739 |
|
|
|
2,329 |
|
|
|
14,501 |
|
|
|
24,563 |
|
|
Operating loss
|
|
|
(7,846 |
) |
|
|
(3,623 |
) |
|
|
(18,603 |
) |
|
|
(28,912 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
49 |
|
|
|
6 |
|
|
|
114 |
|
|
|
10 |
|
|
Gain on sale of intellectual property
|
|
|
400 |
|
|
|
- |
|
|
|
400 |
|
|
|
- |
|
|
Interest expense
|
|
|
(71 |
) |
|
|
(87 |
) |
|
|
(245 |
) |
|
|
(107 |
) |
|
Other expense
|
|
|
(382 |
) |
|
|
(754 |
) |
|
|
(622 |
) |
|
|
(1,293 |
) |
|
Total other expense
|
|
|
(4 |
) |
|
|
(835 |
) |
|
|
(353 |
) |
|
|
(1,390 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income tax benefit
|
|
|
(7,850 |
) |
|
|
(4,458 |
) |
|
|
(18,956 |
) |
|
|
(30,302 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit
|
|
|
19 |
|
|
|
56 |
|
|
|
167 |
|
|
|
104 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(7,831 |
) |
|
|
(4,402 |
) |
|
|
(18,789 |
) |
|
|
(30,198 |
) |
|
Foreign currency translation adjustment
|
|
|
381 |
|
|
|
727 |
|
|
|
612 |
|
|
|
1,240 |
|
|
Comprehensive loss
|
|
$ |
(7,450 |
) |
|
$ |
(3,675 |
) |
|
$ |
(18,177 |
) |
|
$ |
(28,958 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share
|
|
$ |
(2.69 |
) |
|
$ |
(257.36 |
) |
|
$ |
(14.35 |
) |
|
$ |
(2,255.96 |
) |
|
Weighted average shares outstanding - basic and diluted
|
|
|
2,914,600 |
|
|
|
17,107 |
|
|
|
1,309,137 |
|
|
|
13,386 |
|
Share and per share data have been adjusted for all periods presented to reflect the one-for-thirty reverse stock split effective June 1, 2023 and the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to condensed consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Condensed Consolidated Statements of Stockholders’ (Deficit) Equity
(In thousands, except share amounts)
(Unaudited)
| |
|
|
|
|
|
|
|
|
For the Nine Months Ended September 30, 2023
|
|
| |
|
Common Stock |
|
|
Treasury Stock |
|
|
Additional Paid-In
|
|
|
Accumulated
|
|
|
Accumulated Other
|
|
|
Total Stockholders'
|
|
| |
|
Shares
|
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Comprehensive Income
|
|
|
(Deficit) Equity
|
|
|
Balance as of January 1, 2023
|
|
|
34,761 |
|
|
$ |
- |
|
|
|
- |
|
|
|
- |
|
|
$ |
82,286 |
|
|
$ |
(91,094 |
) |
|
$ |
759 |
|
|
$ |
(8,049 |
) |
|
Proceeds from sale of Common Stock
|
|
|
237,191 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
15,359 |
|
|
|
- |
|
|
|
- |
|
|
|
15,359 |
|
|
Cash paid for fractional shares
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(4 |
) |
|
|
- |
|
|
|
- |
|
|
|
(4 |
) |
|
Warrant exchange cashless
|
|
|
264,124 |
|
|
|
1 |
|
|
|
- |
|
|
|
- |
|
|
|
(1 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
180 |
|
|
|
- |
|
|
|
- |
|
|
|
180 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(5,125 |
) |
|
|
- |
|
|
|
(5,125 |
) |
|
Foreign currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
164 |
|
|
|
164 |
|
|
Balance as of March 31, 2023
|
|
|
536,076 |
|
|
$ |
1 |
|
|
|
- |
|
|
$ |
- |
|
|
$ |
97,820 |
|
|
$ |
(96,219 |
) |
|
$ |
923 |
|
|
$ |
2,525 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from sale of Common Stock
|
|
|
2,008,881 |
|
|
$ |
2 |
|
|
|
- |
|
|
|
- |
|
|
$ |
7,711 |
|
|
|
- |
|
|
|
- |
|
|
$ |
7,713 |
|
|
Cash paid for fractional shares
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(5 |
) |
|
|
- |
|
|
|
- |
|
|
|
(5 |
) |
|
Warrant exchange cashless
|
|
|
67,694 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Adjustment for fractional shares
|
|
|
(613 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
329 |
|
|
|
- |
|
|
|
- |
|
|
|
329 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(5,833 |
) |
|
|
- |
|
|
|
(5,833 |
) |
|
Foreign currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
67 |
|
|
|
67 |
|
|
Balance as of June 30, 2023
|
|
|
2,612,038 |
|
|
$ |
3 |
|
|
|
- |
|
|
$ |
- |
|
|
$ |
105,855 |
|
|
$ |
(102,052 |
) |
|
$ |
990 |
|
|
$ |
4,796 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from sale of Common Stock
|
|
|
3,017 |
|
|
$ |
- |
|
|
|
- |
|
|
|
- |
|
|
$ |
3 |
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
Incremental offering costs
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(22 |
) |
|
|
- |
|
|
|
- |
|
|
|
(22 |
) |
|
Prefunded warrants exercised
|
|
|
165,000 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Prefunded warrant exchange cashless
|
|
|
95,954 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Warrant exchange cashless
|
|
|
120,744 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Treasury Stock
|
|
|
(419 |
) |
|
|
- |
|
|
|
419 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
190 |
|
|
|
- |
|
|
|
- |
|
|
|
190 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(7,831 |
) |
|
|
- |
|
|
|
(7,831 |
) |
|
Foreign currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
381 |
|
|
|
381 |
|
|
Balance as of September 30, 2023
|
|
|
2,996,334 |
|
|
$ |
3 |
|
|
|
419 |
|
|
$ |
- |
|
|
$ |
106,026 |
|
|
$ |
(109,883 |
) |
|
$ |
1,371 |
|
|
$ |
(2,483 |
) |
Share and per share data have been adjusted for all periods presented to reflect the one-for-thirty reverse stock split effective June 1, 2023 and the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to condensed consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Condensed Consolidated Statements of Stockholders’ (Deficit) Equity
(In thousands, except share amounts)
(Unaudited)
| |
|
|
|
|
|
|
|
|
For the Nine Months Ended September 30, 2022
|
|
| |
|
Common Stock |
|
|
Treasury Stock |
|
|
Additional Paid-In
|
|
|
Accumulated
|
|
|
Accumulated Other
|
|
|
Total Stockholders'
|
|
| |
|
Shares |
|
|
Amount
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Comprehensive Income
|
|
|
Equity (Deficit)
|
|
|
Balance as of January 1, 2022
|
|
|
10,995 |
|
|
$ |
- |
|
|
|
- |
|
|
|
- |
|
|
$ |
66,240 |
|
|
$ |
(56,160 |
) |
|
$ |
133 |
|
|
$ |
10,213 |
|
|
Vesting of restricted stock
|
|
|
4 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
334 |
|
|
|
- |
|
|
|
- |
|
|
|
334 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(3,666 |
) |
|
|
- |
|
|
|
(3,666 |
) |
|
Foreign currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(299 |
) |
|
|
(299 |
) |
|
Balance as of March 31, 2022
|
|
|
10,999 |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
$ |
66,574 |
|
|
$ |
(59,826 |
) |
|
$ |
(166 |
) |
|
$ |
6,582 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock - CPP
|
|
|
6,099 |
|
|
$ |
- |
|
|
|
- |
|
|
|
- |
|
|
$ |
9,605 |
|
|
|
- |
|
|
|
- |
|
|
$ |
9,605 |
|
|
Vesting of restricted stock
|
|
|
4 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
293 |
|
|
|
- |
|
|
|
- |
|
|
|
293 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(22,130 |
) |
|
|
- |
|
|
|
(22,130 |
) |
|
Foreign currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
812 |
|
|
|
812 |
|
|
Balance as of June 30, 2022
|
|
|
17,102 |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
$ |
76,472 |
|
|
$ |
(81,956 |
) |
|
$ |
646 |
|
|
$ |
(4,838 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of options, cashless
|
|
|
12 |
|
|
$ |
- |
|
|
|
- |
|
|
|
- |
|
|
$ |
- |
|
|
|
- |
|
|
|
- |
|
|
$ |
- |
|
|
Exercise of warrants for cash
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
5 |
|
|
|
- |
|
|
|
- |
|
|
|
5 |
|
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
230 |
|
|
|
- |
|
|
|
- |
|
|
|
230 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(4,402 |
) |
|
|
- |
|
|
|
(4,402 |
) |
|
Foreign currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
727 |
|
|
|
727 |
|
|
Balance as of September 30, 2022
|
|
|
17,114 |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
$ |
76,707 |
|
|
$ |
(86,358 |
) |
|
$ |
1,373 |
|
|
$ |
(8,278 |
) |
Share and per share data have been adjusted for all periods presented to reflect the one-for-thirty reverse stock split effective June 1, 2023 and the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to condensed consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(Unaudited)
| |
|
Nine Months Ended September 30,
|
|
| |
|
2023
|
|
|
2022
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$ |
(18,789 |
) |
|
$ |
(30,198 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Write off of in process research and development (IPR&D)
|
|
|
- |
|
|
|
17,737 |
|
|
Stock-based compensation
|
|
|
699 |
|
|
|
857 |
|
|
Non-cash interest expense
|
|
|
172 |
|
|
|
97 |
|
|
Gain on sale of intellectual property
|
|
|
(400 |
) |
|
|
- |
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Income tax receivable
|
|
|
(112 |
) |
|
|
302 |
|
|
Prepaid expenses and other current assets
|
|
|
(381 |
) |
|
|
(451 |
) |
|
Other non-current assets
|
|
|
(5,541 |
) |
|
|
(2,561 |
) |
|
Accounts payable
|
|
|
4,370 |
|
|
|
5,392 |
|
|
Accrued liabilities
|
|
|
(2,187 |
) |
|
|
(1,448 |
) |
|
Net cash used in operating activities
|
|
|
(22,169 |
) |
|
|
(10,273 |
) |
| |
|
|
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
Investment in IPR&D
|
|
|
- |
|
|
|
(660 |
) |
|
Proceeds from sale of intellectual property
|
|
|
400 |
|
|
|
- |
|
|
Cash acquired in merger
|
|
|
- |
|
|
|
4 |
|
|
Net cash provided by (used) in investing activities
|
|
|
400 |
|
|
|
(656 |
) |
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
Proceeds from sale of common stock and warrants, net of $2.1 million of offering costs
|
|
|
23,052.00 |
|
|
|
- |
|
|
Cash paid for fractional shares
|
|
|
(9.00 |
) |
|
|
- |
|
|
Proceeds from exercise of stock purchase warrants
|
|
|
- |
|
|
|
5 |
|
|
Principal payments on notes
|
|
|
(1,650 |
) |
|
|
- |
|
|
Net cash provided by financing activities
|
|
|
21,393 |
|
|
|
5 |
|
| |
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash
|
|
|
(2 |
) |
|
|
(2 |
) |
| |
|
|
|
|
|
|
|
|
|
Net change in cash
|
|
|
(378 |
) |
|
|
(10,926 |
) |
|
Cash and cash equivalents at beginning of period
|
|
|
1,285 |
|
|
|
11,867 |
|
|
Cash and cash equivalents at end of period
|
|
$ |
907 |
|
|
$ |
941 |
|
| |
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information:
|
|
|
|
|
|
|
|
|
|
Cash paid during period for interest
|
|
$ |
398 |
|
|
$ |
9 |
|
| |
|
|
|
|
|
|
|
|
|
Supplemental disclosure of non-cash transactions:
|
|
|
|
|
|
|
|
|
|
Fair value of common stock, stock options and stock warrants issued as consideration for asset acquisition
|
|
$ |
- |
|
|
$ |
9,605 |
|
Share and per share data have been adjusted for all periods presented to reflect the one-for-thirty reverse stock split effective June 1, 2023 and the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to condensed consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Notes to Condensed Consolidated Financial Statements
1. Business
Panbela Therapeutics, Inc. (“Panbela”) and its direct wholly owned subsidiaries: Panbela Research, Inc. (“Panbela Research”) Cancer Prevention Pharmaceuticals, Inc. (“CPP”) and Cancer Prevention Pharma (Ireland) Limited exist for the primary purpose of developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs. Panbela Therapeutics Pty Ltd is a wholly owned subsidiary of Panbela Research organized under the laws of Australia. Cancer Prevention has two wholly owned dormant subsidiaries: Cancer Prevention Pharma Limited, a United Kingdom entity, and Cancer Prevention Pharmaceuticals, LLC, an Arizona limited liability company. Panbela Therapeutics, Inc., together with its direct and indirect subsidiaries is referred to as “we,” “us,” “our,” and the “Company.”
The primary objective of our pipeline is the utilization of pharmacotherapies to reduce or normalize increased disease-associated polyamines using complementary pharmacotherapies. Our lead candidates are ivospemin (SBP-101) for which we have exclusively licensed the worldwide rights from the University of Florida Research Foundation, Inc., Flynpovi™ a combination of eflornithine (CPP-1X) and sulindac and eflornithine (CPP-1X) alone in tablet or sachet form. We have exclusively licensed rights from the Arizona Board of Regents of the University of Arizona to commercialize Flynpovi, and a sublicense agreement to develop and commercialize Flynpovi in North America was terminated by the licensee on April 4, 2023.
Reverse stock splits
Effective June 1, 2023, Panbela effected a 1-for-30 reverse stock split of its outstanding shares of common stock. Effective January 13, 2023, Panbela effected a 1-for-40 reverse stock split of its outstanding shares of common stock. Unless specifically provided otherwise herein, all share and per share amounts of our common stock presented have been retroactively adjusted to reflect the reverse stock splits. See Note 7 for more information.
2. Risks and Uncertainties
The Company operates in a highly regulated and competitive environment. The development, manufacturing and marketing of pharmaceutical products require approval from, and are subject to ongoing oversight by, the Food and Drug Administration (the “FDA”) in the United States, the Therapeutic Goods Administration in Australia, the European Medicines Agency in the European Union, and comparable agencies in other countries. Obtaining approval for a new pharmaceutical product is never certain, may take many years, and is normally expected to involve substantial expenditures.
We have incurred losses of $109.9 million since our inception in 2011. For the nine months ended September 30, 2023, we incurred a net loss of $18.8 million. We also incurred negative cash flows from operating activities of approximately $22.2 million for this period. As we continue to pursue development activities and seek commercialization, we expect to incur substantial losses, which are likely to generate negative net cash flows from operating activities. We generated approximately $21.4 million positive cash flows from financing activities related to proceeds from the sale of common stock, pre-funded warrants, and warrants, partially offset by the payments made on two promissory notes. As of September 30, 2023, we had cash of approximately $0.9 million, working capital deficit of $7.0 million, (working capital is defined as current assets less current liabilities), and stockholders’ deficit of $2.5 million. The Company’s principal sources of cash have historically included the issuance of convertible debt and equity securities.
The accompanying condensed consolidated financial statements have been prepared assuming that we will continue as a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The condensed consolidated financial statements do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties. Our current independent registered public accounting firm included a paragraph emphasizing this going concern uncertainty in their audit report regarding our 2022 financial statements dated March 16, 2023. Our ability to continue as a going concern, realize the carrying value of our assets and discharge our liabilities in the ordinary course of business is dependent upon a number of factors, including our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our product candidates in the United States, Australia, the European Union or other markets and ultimately our ability to market and sell our product candidates. These factors, among others, raise substantial doubt about our ability to continue operations as a going concern. See Note 4 titled “Liquidity and Business Plan.”
In January of 2022, the Company announced the opening of a global randomized Phase II/III clinical trial, which is being conducted in the United States, Europe and Asia Pacific (APAC). The Company does not expect any disruption to the conduct of this new clinical trial associated with COVID-19. The trial is reliant on adequate supply of gemcitabine and Abraxane (nab-paclitaxel) which can be subject to supply shortages.
3. Basis of Presentation
We have prepared the accompanying interim condensed consolidated financial statements in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the Securities and Exchange Commission (“SEC”). Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. These interim condensed consolidated financial statements reflect all adjustments consisting of normal recurring accruals, which, in the opinion of management, are necessary to present fairly our consolidated financial position, consolidated results of operations and consolidated cash flows for the periods and as of the dates presented. Our fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2022, was derived from audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. These interim condensed consolidated financial statements should be read in conjunction with the annual consolidated financial statements and the notes thereto included in our most recent filed Annual Report on Form 10-K and our subsequent filings with the SEC. The nature of our business is such that the results of any interim period may not be indicative of the results to be expected for the entire year.
4. Liquidity and Business Plan
On June 21, 2023, the Company completed a registered public offering of common stock, pre-funded warrants and warrants which resulted in net proceeds of approximately $7.7 million.
On January 30, 2023, the Company completed a registered public offering of common stock, pre-funded warrants and warrants which resulted in net proceeds of approximately $13.8 million. Also, in the first quarter of 2023, the Company received net proceeds of approximately $1.6 million from the sale of common stock via the Company’s ATM Program (See Note 7). No sales occurred under the ATM Program during the second quarter of 2023.
We need to raise additional capital to support our current business plans. We may seek to raise additional funds through various sources, such as equity and debt financing, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This risk would increase if our clinical data were not positive or economic and market conditions deteriorate.
Our future success is dependent upon our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our product candidates ivospemin, Flynpovi and eflornithine in the United States or other markets and ultimately our ability to market and sell product candidates. If we are unable to obtain additional financing when needed, if our clinical trials are not successful or if we are unable to obtain marketing approval, we would not be able to continue as a going concern and would be forced to cease operations and liquidate our company.
There can be no assurances that we will be able to obtain additional financing on commercially reasonable terms, or at all. The sale of additional convertible debt or equity securities would likely result in dilution to our current stockholders.
5. Summary of Significant Accounting Policies
Principles of consolidation
The accompanying condensed consolidated financial statements include the assets, liabilities, and expenses of the Company. All significant intercompany transactions and balances have been eliminated in consolidation.
Use of estimates
The preparation of condensed consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amount of expenses during the reporting period. Actual results could differ from those estimates, particularly given the significant social and economic disruptions and uncertainties with the ongoing pandemic and control responses.
Research and development costs
Research and development costs include expenses incurred in the conduct of our clinical trials; for third-party service providers performing various testing and accumulating data related to our preclinical studies; sponsored research agreements; developing and scaling the manufacturing process necessary to produce sufficient amounts of drug product for our product candidates for use in our pre-clinical studies and human clinical trials; consulting resources with specialized expertise related to execution of our development plan for our product candidates; personnel costs, including salaries, benefits and share-based compensation; and costs to license and maintain licensed intellectual property.
We charge research and development costs, including clinical trial costs, to expense when incurred. Our human clinical trials are, and will be, performed at clinical trial sites and are administered jointly by us with assistance from contract research organizations (“CROs”). Costs of setting up clinical trial sites are accrued upon execution of the study agreement. Expenses related to the performance of clinical trials generally are accrued based on contracted amounts and the achievement of agreed upon milestones, such as patient enrollment, patient follow-up, etc. We monitor levels of performance under each significant contract, including the extent of patient enrollment and other activities through communications with the clinical trial sites and CROs, and adjust the estimates, if required, on a quarterly basis so that clinical expenses reflect the actual effort expended at each clinical trial site and by each CRO.
The cost to secure certain third-party drug product for the clinical trials, which is often paid for in advance of delivery, is charged to research and development when it is received and available to be shipped to clinical sites.
Research and development costs for 2022 include IPR&D. This asset was acquired from the security holders of CPP and written off to research and development immediately subsequent to the asset acquisition.
All material CRO contracts are terminable by us upon written notice, and we are generally only liable for actual effort expended by the CROs and certain non-cancelable expenses incurred at any point of termination.
We expense costs associated with obtaining licenses for patented technologies when it is determined there is no alternative future use of the intellectual property subject to the license.
Stock-based compensation
In accounting for stock-based incentive awards, we measure and recognize the cost of employee and non-employee services received in exchange for awards of equity instruments based on the fair value of those awards on the grant date. Calculating stock-based compensation expense requires the input of highly subjective assumptions, which represent our best estimates and involve inherent uncertainties and the application of management’s judgment. Compensation cost is recognized ratably using the straight-line attribution method over the vesting period, which is considered to be the requisite service period. Compensation expense for performance-based stock option awards is recognized when “performance” has occurred or is probable of occurring.
The fair value of stock-based awards is estimated at the date of grant using the Black-Scholes option pricing model. The determination of the fair value of stock-based awards is affected by our stock price, as well as assumptions regarding a number of complex and subjective variables. Risk free interest rates are based upon U.S. Treasury rates appropriate for the expected term of each award. Expected volatility rates are based on historical company share price volatility. The assumed dividend yield is zero, as we do not expect to declare any dividends in the foreseeable future. The expected term of options granted is determined using the “simplified” method. Under this approach, the expected term is presumed to be the mid-point between the average vesting date and the end of the contractual term.
Foreign currency translation adjustments
The functional currency of Panbela Therapeutics Pty Ltd is the Australian Dollar. Accordingly, assets and liabilities, and equity transactions of Panbela Therapeutics Australia Pty Ltd, are translated into U.S. dollars at period-end exchange rates. Revenues and expenses are translated at the average exchange rate in effect for the period. The resulting translation gains and losses are recorded as a component of accumulated comprehensive loss presented within the stockholders’ equity. During the nine-month periods ended September 30, 2023 and 2022, any reclassification adjustments from accumulated other comprehensive loss to operations were inconsequential.
Comprehensive loss
Comprehensive loss consists of our net loss and the effects of foreign currency translation.
Net loss per share
Basic net loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is based on the weighted average of common shares outstanding during the period plus dilutive potential common shares calculated using the treasury stock method. Such potentially dilutive shares are excluded when the effect is anti-dilutive or reduce a net loss per share. The Company’s potentially dilutive shares, which include outstanding common stock options, and warrants, have not been included in the computation of diluted net loss per share for all periods as the result would be anti-dilutive.
The following table sets forth the potential shares of common stock that were not included in the calculation of diluted net loss per share as their effects would have been anti-dilutive as of the dates indicated:
| |
|
September 30,
|
|
| |
|
2023
|
|
|
2022
|
|
|
Employee and non-employee stock options
|
|
|
13,455 |
|
|
|
3,352 |
|
|
Common stock issuable under common stock purchase warrants
|
|
|
4,068,826 |
|
|
|
4,538 |
|
| |
|
|
4,082,281 |
|
|
|
7,890 |
|
6. Notes Payable
Sucampo promissory note
As of September 30, 2023, CPP had a balance outstanding of approximately $5.4 million for principal and interest under an amended and restated promissory note (the “Sucampo Note). The note was issued with an initial principal amount of approximately $6.2 million in favor of Sucampo GmbH dated as of June 15, 2022. The principal balance outstanding as of September 30, 2023 of approximately $5.2 million under the Sucampo Note bears simple interest at a rate of 5% per annum. All unpaid principal, together with any then unpaid and accrued interest is payable as follows: (i) $1.0 million, plus all interest accrued but unpaid on or before each of January 31, 2024, January 31, 2025, and January 31, 2026; and (ii) all remaining principal plus accrued but unpaid interest on or before January 31, 2027. On February 1, 2023, Panbela paid the balance due of $1.0 million plus accrued and unpaid interest of approximately $295,000. As of September 30, 2023, the Company was current in all payments due under the Sucampo Note and the accrued and unpaid interest on this note was approximately $172,000. Panbela has agreed to guarantee CPP’s payment obligations under the Sucampo Note pursuant to a Guaranty dated as of June 15, 2022.
Tillotts promissory note
As of December 31, 2022, CPP had a balance outstanding of approximately $0.7 million representing principal and interest under an amended promissory note issued with an initial principal amount of approximately $650,000 in favor of Tillotts Pharma AG. The principal balance and accrued and unpaid interest were paid in full on January 31, 2023.
7. Stockholders’ Equity
Public offering of common stock and warrants June 2023
On June 21, 2023, the Company completed a registered public offering and issued an aggregate of 586,000 shares of its common stock, pre-funded warrants to purchase up to an aggregate of 1,684,000 shares of common stock at an exercise price of $0.001 per share and warrants to purchase up to an aggregate of 4,540,000 shares of its common stock at an exercise price of $3.75 per share. The securities were issued for a combined offering price of $3.75 per share of common stock and warrants to purchase two shares, or $3.749 per pre-funded warrant and warrants to purchase two shares. Net proceeds from the offering totaled approximately $7.7 million. As of September 30, 2023, all pre-funded warrants had been exercised. The securities were offered pursuant to an effective registration statement on Form S-1.
Of the remaining warrants, warrants to purchase 2,270,000 shares of common stock, have an alternative cashless exercise provision pursuant to which the holder may provide notice and receive an aggregate number of shares equal to the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise and (y) 0.24. This feature became available on June 28, 2023 and as of June 30, 2023 no shares were issued with this alternative cashless exercise. As of September 30, 2023 120,744 shares of common stock had been issued for warrants to purchase 503,100 shares of common stock.
As of the same date warrants to purchase up to an aggregate of 4,036,900 shares of common stock remained outstanding with an exercise price of $3.75 per shares, of which warrants to purchase 1,766,900 were eligible for alternative cashless exercise.
Public offering of common stock and warrants January 2023
On January 30, 2023, the Company completed a registered public offering and issued an aggregate of 161,407 shares of its common stock, pre-funded warrants to purchase up to an aggregate of 61,090 shares of common stock at an exercise price of $0.001 per share and warrants to purchase up to an aggregate of 444.999 shares of its common stock at an exercise price as adjusted, of $2.239 per share. The securities were issued for a combined offering price of $67.50 per share of common stock and warrants to purchase two shares, or $67.47 per pre-funded warrant and warrants to purchase two shares. Net proceeds from the offering totaled approximately $13.8 million. The securities were offered pursuant to an effective registration statement on Form S-1.
All of the pre-funded warrants were exercised by February 3, 2023. The remaining warrants have an alternative cashless exercise provision pursuant to which the holder may provide notice and receive an aggregate number of shares equal to the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise and (y) 0.75. This feature became available on March 1, 2023 and as of as of September 30, 2023, 331,824 shares of common stock had been issued for warrants to purchase 442,434 shares. Warrants to purchase 2,566 shares of common stock remained outstanding with an exercise price as adjusted, of $2.239 per share.
At-the-market program
We are party to a Sales Agreement dated July 29, 2022, pursuant to which Roth Capital Partners, LLC (the “Agent”) may sell shares of the Company’s common stock having an aggregate gross sales price of up to $8.4 million, from time to time, through an “at-the-market” equity offering program (the “ATM Program”). Under the Sales Agreement, Roth is entitled to a commission equal to 3.0% of the aggregate gross proceeds of any sales of common stock under the ATM Program.
During January 2023, the Company sold 14,694 shares of common stock under the ATM Program for approximately $1.6 million in gross proceeds.
During September 2023, the Company sold 3,017 shares of common stock under the ATM program for approximately $3,900 in gross proceeds.
In connection with the warrant transactions that occurred subsequent to the end of the period, the Company is restricted from selling under the ATM Program until at least May 2, 2024 (See Note 10).
Reverse stock splits
On May 25, 2023, the Company held a special meeting of its stockholders at which the stockholders approved a proposal to effect an amendment to the Company's certificate of incorporation, as amended, to implement a reverse stock split within a range of a ratio of one-for-five (1:5) up to a ratio of one-for-one hundred (1:100). On June 1, 2023, the Company's Board of Directors approved the implementation of the reverse stock split of the Company's Common Stock at a ratio of one-for-thirty (1:30). As a result of the reverse stock split, every thirty (30) shares of the Company's Common Stock either issued and outstanding immediately prior to the effective time was, automatically and without any action on the part of the respective holders thereof, combined and converted into one (1) share of the Company's common stock. No fractional shares were issued in connection with the reverse stock split. Stockholders who otherwise were entitled to receive a fractional share in connection with the reverse stock split instead were eligible to receive a cash payment, which was not material in the aggregate, instead of shares. On June 1, 2023, the Company filed a Certificate of Amendment of its Certificate of Incorporation, as amended with the Secretary of State of Delaware effecting a one-for-thirty (1:30) reverse stock split of the shares of the Company’s common stock, issued and outstanding, effective June 1, 2023.
On November 29, 2022, the Company held a special meeting of its stockholders at which the stockholders approved a proposal to effect an amendment to the Company's certificate of incorporation, as amended, to implement a reverse stock split at a ratio of one-for-forty (1:40). On January 13, 2023, the Company's Board of Directors approved the implementation of the reverse stock split of the Company's Common Stock. As a result of the reverse stock split, every forty (40) shares of the Company's Common Stock either issued and outstanding immediately prior to the effective time was, automatically and without any action on the part of the respective holders thereof, combined and converted into one (1) share of the Company's common stock. No fractional shares were issued in connection with the reverse stock split. Stockholders who otherwise were entitled to receive a fractional share in connection with the reverse stock split instead were eligible to receive a cash payment, which was not material in the aggregate, instead of shares. On January 13, 2023, the Company filed a Certificate of Amendment of its Certificate of Incorporation, as amended with the Secretary of State of Delaware effecting a one-for-forty (1:40) reverse stock split of the shares of the Company’s common stock, issued and outstanding, effective January 13, 2023.
Shares reserved
The following shares of common stock were reserved for future issuance as of the date indicated:
| |
|
September 30, 2023
|
|
|
Stock options outstanding
|
|
|
13,455 |
|
|
Shares available for grant under equity incentive plan
|
|
|
- |
|
|
Warrants outstanding
|
|
|
4,068,826 |
|
| |
|
|
4,082,281 |
|
8. Stock-based Compensation
2016 Omnibus Incentive Plan
The Panbela Therapeutics, Inc. 2016 Omnibus Incentive Plan (the “2016 Plan”) was adopted by our Board of Directors in March 2016 and approved by our stockholders in May 2016. The 2016 Plan permits the granting of incentive and non-statutory stock options, restricted stock, stock appreciation rights, performance units, performance shares and other stock awards to eligible employees, directors and consultants. We grant options to purchase shares of common stock under the 2016 Plan at no less than the fair market value of the underlying common stock as of the date of grant. Options granted under the 2016 Plan have a maximum term of ten years. As of September 30, 2023, options to purchase 12,054 shares of common stock were outstanding under the 2016 Plan and no shares remained available for future awards. The weighted average exercise price is $1,105.88 and the average remaining life is approximately 9.0 years.
2011 Stock Option Plan
Our Board of Directors ceased making awards under the Panbela Therapeutics, Inc. 2011 Stock Option Plan (the “2011 Plan”) upon the original receipt of stockholder approval for the 2016 Plan in May 2016. Awards outstanding under the 2011 Plan remain outstanding in accordance with and pursuant to the terms thereof. As of September 30, 2023, options to purchase 171 shares of common stock remained outstanding under the 2011 Plan. The weighted average exercise price is $3,721.58 and the average remaining life is approximately 1.3 years.
CPP’s 2010 Equity Incentive Plan
The Company has assumed all remaining rights and obligations with respect to CPP’s 2010 Equity Incentive Plan (the “CPP Plan”) through the issuance of replacement options. As of September 30, 2023, options to purchase 1,230 shares of common stock remained outstanding under the CPP Plan, with a weighted average exercise price of $361.56 per share, and the average remaining contractual life was 6.8 years.
Stock-based compensation expense
General and administrative (“G&A”) and research and development (“R&D”) expenses include non-cash stock-based compensation expense as a result of our issuance of stock options. The terms and vesting schedules for stock-based awards vary by type of grant and the employment status of the grantee. The awards granted through September 30, 2023 vest based upon time-based and performance conditions. There was approximately $0.2 million unamortized stock-based compensation expense related to options granted to employees, directors, and consultants as of September 30, 2023. The unamortized expense will be recognized over the next 12 months.
Stock-based compensation expense for each of the periods presented is as follows (in thousands):
| |
|
Nine Months Ended September 30,
|
|
| |
|
2023
|
|
|
2022
|
|
|
General and Administrative
|
|
$ |
554 |
|
|
$ |
697 |
|
|
Research and Development
|
|
|
145 |
|
|
|
160 |
|
| |
|
$ |
699 |
|
|
$ |
857 |
|
Details of options granted, exercised, cancelled, or forfeited during the nine months ended September 30, 2023 follows:
| |
|
Shares Underlying
Options
|
|
|
Weighted
Average
Exercise Price
Per Share
|
|
|
Aggregate
Intrinsic Value
|
|
|
Balance at January 1, 2023
|
|
|
3,287 |
|
|
$ |
4,369 |
|
|
$ |
- |
|
|
Granted
|
|
|
10,240 |
|
|
|
15 |
|
|
|
|
|
|
Exercised
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
Cancelled
|
|
|
(72 |
) |
|
|
1,424 |
|
|
|
|
|
|
Forfeitures or expirations
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
Balance at September 30, 2023
|
|
|
13,455 |
|
|
$ |
1,071 |
|
|
$ |
- |
|
Information about stock options outstanding, vested and expected to vest as of September 30, 2023 is as follows:
| |
|
|
|
|
Outstanding, Vested and Expected to Vest |
|
|
Options Vested and Exercisable |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per Share Exercise Price
|
|
|
Shares
|
|
|
Weighted Average
Remaining
Contractual Life
(Years)
|
|
|
Weighted
Average
Exercise Price
|
|
|
Options
Exercisable
|
|
|
Weighted Average
Remaining
Contractual Life
(Years)
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
$15 |
|
|
|
|
10,240 |
|
|
|
9.50 |
|
|
$ |
15 |
|
|
|
1,662 |
|
|
|
9.50 |
|
| |
$264 |
|
|
|
|
1,150 |
|
|
|
7.26 |
|
|
$ |
264 |
|
|
|
1,150 |
|
|
|
7.26 |
|
| $1,764 |
- |
$3,540 |
|
|
|
582 |
|
|
|
5.01 |
|
|
$ |
3,215 |
|
|
|
569 |
|
|
|
4.94 |
|
| $3,810 |
- |
$4,908 |
|
|
|
498 |
|
|
|
5.57 |
|
|
$ |
4,493 |
|
|
|
398 |
|
|
|
5.08 |
|
| $5,004 |
- |
$7,320 |
|
|
|
428 |
|
|
|
6.14 |
|
|
$ |
6,102 |
|
|
|
428 |
|
|
|
6.14 |
|
| $9,720 |
- |
$18,120 |
|
|
|
557 |
|
|
|
4.81 |
|
|
$ |
12,987 |
|
|
|
557 |
|
|
|
4.81 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals
|
|
|
|
13,455 |
|
|
|
8.66 |
|
|
$ |
1,071 |
|
|
|
4,764 |
|
|
|
7.19 |
|
Assumptions used to calculate the fair market value of options granted in the nine month period ended September 30, 2023 include:
| |
|
2023
|
|
|
Common stock fair value
|
|
|
$15.00 |
|
|
Risk-free interest rate
|
|
|
3.59% |
- |
3.60% |
|
|
Expected dividend yield
|
|
|
|
|
|
|
|
Expected Option life
|
|
|
5.08 |
- |
5.50 |
|
|
Expected stock price volatility
|
|
|
131.0% |
- |
138.0% |
|
9. Gain on Sale of Intellectual Property
On July 17, 2023, the Company divested certain rights, titles and interests in its eflornithine pediatric neuroblastoma program. Under the terms of the agreement, the Company is entitled to receive up to approximately $9.5 million in non-dilutive funding in exchange for the sale of these assets. An initial payment of $400,000 was received by the Company at the time of closing, remaining payments will be receivable if the acquiring company successfully completes certain milestones related to clinical development, regulatory approval and commercial sales. At the time of closing, the successful completion of these milestones is not probable and the Company did not recognize these future payments on the date of the sale as they did not have any realized or realizable value. It was determined that the contract was not with a customer and did not represent the sale of a business and therefore the initial payment was recognized as a gain on sale of intellectual property which was reflected in other income during the three months ended September 30, 2023.
10. Subsequent Events
On November 2, 2023, the Company entered into warrant exercise inducement offer letters (the “Inducement Letters”) with certain holders (the “Holders”) of its existing warrants to purchase shares of common stock (the “Existing Warrants”), pursuant to which the Holders agreed to exercise for cash their Existing Warrants to purchase 2,130,000 shares of the Company’s common stock, in the aggregate, at a reduced exercise price of $0.78 per share, in exchange for the Company’s agreement to issue new warrants (the “Inducement Warrants”) on substantially the same terms as the Existing Warrants described below, to purchase up to 4,260,000 shares of the Company’s common stock (the “Inducement Warrant Shares”) and a cash payment of $0.125 per Existing Warrant Share which was paid in full upon the exercise of the Existing Warrants. The Company received aggregate gross proceeds of approximately $1.9 million from the exercise of the Existing Warrants by the Holders and the sale of the Inducement Warrants. The Company incurred $115,659 in investment banking fees relating to the transaction, in addition to reimbursement for certain expenses.
The Company has agreed to file a registration statement on Form S-3 covering the resale of the Inducement Warrants Shares issued or issuable upon the exercise of the Inducement Warrants (the “Resale Registration Statement”) within twenty (20) calendar days of the date of the Inducement Letters. In the Inducement Letters, the Company agreed not to issue any shares of common stock or common stock equivalents or to file any other registration statement with the SEC (in each case, subject to certain exceptions) until the Company receives stockholder approval. The Company also has agreed not to effect or agree to effect any variable rate transaction (as defined in the Inducement Letters) until one year from the date of the Inducement Letters, other than an at-the-market offering, which may be effected after the date that is six months from the date of the Inducement Letters.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
Panbela Therapeutics, Inc.
Waconia, MN
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Panbela Therapeutics, Inc. and Subsidiaries (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of operations and comprehensive loss, stockholders’ (deficit) equity, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years then ended in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt about the Company's Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has recurring losses and negative cash flows from operations that raise substantial doubt about its ability to continue as a going concern. Management’s evaluations of the events and conditions and management’s plans regarding those matters are described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter – Asset Purchase
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the Company’s Audit Committee and that: (i) relates to accounts or disclosures that are material to the financial statements and (ii) involved especially challenging, subjective, or complex judgements. The communication of critical audit matters does not alter, in any way, our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Critical Audit Matter Description
As described in Note 6 to the financial statements, on June 15, 2022, the Company completed an acquisition of Cancer Prevention Pharmaceuticals, Inc. (“CPP”) in a transaction accounted for as an asset purchase (the “Transaction”). The net assets acquired were recorded at fair value and included acquired in-process research and development (“IPR&D”) associated with CPP’s product candidates and was recorded as a research and development expense in the Company’s consolidated statement of operations.
Auditing the Company’s accounting for the Transaction was complex because of the judgment involved in evaluating whether the Transaction met the criteria of a business combination or an asset acquisition among other accounting considerations. The subjective considerations included whether substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets, the most significant of which is the IPR&D asset.
How the Critical Audit Matter Was Addressed in the Audit
To test the Company’s accounting for the Transaction, we performed the following audit procedures:
| |
●
|
We evaluated the Company's application of the relevant accounting guidance as defined in ASC Topic 805 – Business Combinations.
|
| |
●
|
We assessed the completeness and accuracy of the net asset acquired, including the evaluation of any implied goodwill.
|
| |
●
|
We recalculated the fair value of the non-cash consideration paid in the Transaction, including the common stock options.
|
| |
●
|
We also assessed the appropriateness of the related disclosures in the consolidated financial statements.
|
We have served as the Company’s auditors since 2014.
/s/ Cherry Bekaert
Tampa, Florida
March 16, 2023
Panbela Therapeutics, Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
| |
|
December 31, 2022
|
|
|
December 31, 2021
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$ |
1,285 |
|
|
$ |
11,867 |
|
|
Prepaid expenses and other current assets
|
|
|
443 |
|
|
|
91 |
|
|
Income tax receivable
|
|
|
49 |
|
|
|
321 |
|
|
Total current assets
|
|
|
1,777 |
|
|
|
12,279 |
|
|
Other noncurrent assets
|
|
|
3,201 |
|
|
|
593 |
|
|
Total assets
|
|
$ |
4,978 |
|
|
$ |
12,872 |
|
| |
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' (DEFICIT) EQUITY
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$ |
2,865 |
|
|
$ |
640 |
|
|
Accrued expenses
|
|
|
2,993 |
|
|
|
2,020 |
|
|
Accrued interest payable
|
|
|
325 |
|
|
|
- |
|
|
Note payable
|
|
|
650 |
|
|
|
- |
|
|
Debt, current portion
|
|
|
1,000 |
|
|
|
- |
|
|
Total current liabilities
|
|
|
7,833 |
|
|
|
2,660 |
|
| |
|
|
|
|
|
|
|
|
|
Debt, net of current portion
|
|
|
5,194 |
|
|
|
- |
|
|
Total non current liabilities
|
|
|
5,194 |
|
|
|
- |
|
| |
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
13,027 |
|
|
|
2,660 |
|
| |
|
|
|
|
|
|
|
|
|
Stockholders' (deficit) equity:
|
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 10,000,000 authorized; no shares issued or outstanding as of December 31, 2022 and December 31, 2021
|
|
|
- |
|
|
|
- |
|
|
Common stock, $0.001 par value; 100,000,000 authorized; 1,049,644 and 335,961 shares issued and outstanding, as of December 31, 2022 and December 31, 2021, respectively
|
|
|
1 |
|
|
|
- |
|
|
Additional paid-in capital
|
|
|
82,285 |
|
|
|
66,240 |
|
|
Accumulated deficit
|
|
|
(91,094 |
) |
|
|
(56,161 |
) |
|
Accumulated comprehensive income
|
|
|
759 |
|
|
|
133 |
|
|
Total stockholders' (deficit) equity
|
|
|
(8,049 |
) |
|
|
10,212 |
|
|
Total liabilities and stockholders' (deficit) equity
|
|
$ |
4,978 |
|
|
$ |
12,872 |
|
Share and per share data have been adjusted for all periods presented to reflect the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to the consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
| |
|
Year Ended December 31,
|
|
| |
|
2022
|
|
|
2021
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
General and administrative
|
|
$ |
6,044 |
|
|
$ |
4,587 |
|
|
Research and development
|
|
|
28,049 |
|
|
|
5,423 |
|
|
Operating loss
|
|
|
(34,093 |
) |
|
|
(10,010 |
) |
| |
|
|
|
|
|
|
|
|
|
Other (expense) income:
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
14 |
|
|
|
1 |
|
|
Interest expense
|
|
|
(288 |
) |
|
|
(12 |
) |
|
Other expense
|
|
|
(682 |
) |
|
|
(602 |
) |
|
Total other expense
|
|
|
(956 |
) |
|
|
(613 |
) |
| |
|
|
|
|
|
|
|
|
|
Loss before income tax benefit
|
|
|
(35,049 |
) |
|
|
(10,623 |
) |
| |
|
|
|
|
|
|
|
|
|
Income tax benefit
|
|
|
116 |
|
|
|
488 |
|
| |
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(34,933 |
) |
|
|
(10,135 |
) |
|
Foreign currency translation adjustment
|
|
|
626 |
|
|
|
517 |
|
|
Comprehensive loss
|
|
$ |
(34,307 |
) |
|
$ |
(9,618 |
) |
| |
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share
|
|
$ |
(67.91 |
) |
|
$ |
(34.64 |
) |
|
Weighted average shares outstanding - basic and diluted
|
|
|
514,369 |
|
|
|
292,607 |
|
Share and per share data have been adjusted for all periods presented to reflect the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to the consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
(In thousands, except share amounts)
| |
|
For the Years ended December 31, 2022 and 2021 |
|
| |
|
|
|
|
Additional
|
|
|
|
|
|
Accumulated
|
|
|
Total
|
|
| |
|
Common Stock |
|
|
Paid in |
|
|
Accumulated |
|
|
Comprehensive |
|
|
Stockholders' |
|
| |
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Loss
|
|
|
Equity (Deficit)
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2021
|
|
|
241,495 |
|
|
$ |
- |
|
|
$ |
54,857 |
|
|
$ |
(46,026 |
) |
|
$ |
(384 |
) |
|
$ |
8,447 |
|
|
Public Offering - Issuance of common stock
|
|
|
83,319 |
|
|
|
- |
|
|
|
9,054 |
|
|
|
- |
|
|
|
- |
|
|
|
9,054 |
|
|
Exercise of warrants for cash
|
|
|
5,723 |
|
|
|
- |
|
|
|
1,042 |
|
|
|
- |
|
|
|
- |
|
|
|
1,042 |
|
|
Exercise of warrants, cashless
|
|
|
4,763 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Vesting of restricted stock
|
|
|
557 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Exercise of options, cashless
|
|
|
104 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Stock-based compensation
|
|
|
- |
|
|
|
- |
|
|
|
1,287 |
|
|
|
- |
|
|
|
- |
|
|
|
1,287 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(10,135 |
) |
|
|
- |
|
|
|
(10,135 |
) |
|
Foreign currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
517 |
|
|
|
517 |
|
|
Balance at December 31, 2021
|
|
|
335,961 |
|
|
|
- |
|
|
|
66,240 |
|
|
|
(56,161 |
) |
|
|
133 |
|
|
|
10,212 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Issuance of common stock, options and warrants - CPP acquisition |
|
|
182,988 |
|
|
|
- |
|
|
|
9,605 |
|
|
|
- |
|
|
|
- |
|
|
|
9,605 |
|
|
Public offering - issuance of common stock and warrants
|
|
|
502,500 |
|
|
|
1 |
|
|
|
5,302 |
|
|
|
- |
|
|
|
- |
|
|
|
5,303 |
|
|
Sale of common stock
|
|
|
28,345 |
|
|
|
- |
|
|
|
46 |
|
|
|
- |
|
|
|
- |
|
|
|
46 |
|
|
Vesting of restricted stock
|
|
|
269 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Exercise of options, cashless
|
|
|
372 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Exercise of warrants for cash
|
|
|
25 |
|
|
|
- |
|
|
|
5 |
|
|
|
- |
|
|
|
- |
|
|
|
5 |
|
|
Share Based Compensation
|
|
|
- |
|
|
|
- |
|
|
|
1,088 |
|
|
|
- |
|
|
|
- |
|
|
|
1,088 |
|
|
Net Loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(34,933 |
) |
|
|
- |
|
|
|
(34,933 |
) |
|
Adjustment for fractional shares not issued, stock split
|
|
|
(816 |
) |
|
|
- |
|
|
|
(1 |
) |
|
|
- |
|
|
|
- |
|
|
|
(1 |
) |
|
Foreign Currency translation adjustment
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
626 |
|
|
|
626 |
|
|
Balance at December 31, 2022
|
|
|
1,049,644 |
|
|
$ |
1 |
|
|
$ |
82,285 |
|
|
$ |
(91,094 |
) |
|
$ |
759 |
|
|
$ |
(8,049 |
) |
Share and per share data have been adjusted for all periods presented to reflect the one-for-forty reverse stock split effective January 13, 2023.
The accompanying notes to the consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| |
|
Year Ended December 31,
|
|
| |
|
2022
|
|
|
2021
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$ |
(34,933 |
) |
|
$ |
(10,135 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Write off of in process research and development (IPR&D)
|
|
|
17,737 |
|
|
|
- |
|
|
Stock-based compensation
|
|
|
1,088 |
|
|
|
1,287 |
|
|
Non-cash interest expense
|
|
|
273 |
|
|
|
- |
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Income tax receivable
|
|
|
212 |
|
|
|
51 |
|
|
Prepaid expenses and other current assets
|
|
|
(127 |
) |
|
|
247 |
|
|
Deposits held for clinical trial costs
|
|
|
(2,561 |
) |
|
|
(540 |
) |
|
Accounts payable
|
|
|
2,249 |
|
|
|
631 |
|
|
Accrued liabilities
|
|
|
786 |
|
|
|
1,214 |
|
|
Net cash used in operating activities
|
|
|
(15,276 |
) |
|
|
(7,245 |
) |
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
Investment in IPR&D
|
|
|
(660 |
) |
|
|
- |
|
|
Cash acquired in merger
|
|
|
4 |
|
|
|
- |
|
|
Net cash used in investing activities
|
|
|
(656 |
) |
|
|
- |
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
| Proceeds from sale of common stock, net of fees and offering costs of $44 |
|
|
46 |
|
|
|
- |
|
|
Proceeds from public offering of common stock and warrants net of underwriters discount and offering costs of $727
|
|
|
5,303 |
|
|
|
- |
|
|
Proceeds from public offering of common stock net of underwriters discount and offering costs of $946
|
|
|
- |
|
|
|
9,054 |
|
|
Proceeds from exercise of warrants
|
|
|
5 |
|
|
|
1,042 |
|
|
Net cash provided by financing activities
|
|
|
5,354 |
|
|
|
10,096 |
|
|
Effect of exchange rate changes on cash
|
|
|
(4 |
) |
|
|
(6 |
) |
|
Net change in cash
|
|
|
(10,582 |
) |
|
|
2,845 |
|
|
Cash and cash equivalents at beginning of year
|
|
|
11,867 |
|
|
|
9,022 |
|
|
Cash and cash equivalents at end of year
|
|
$ |
1,285 |
|
|
$ |
11,867 |
|
|
Supplemental disclosure of cash flow information:
|
|
|
|
|
|
|
|
|
|
Cash paid during period for interest
|
|
$ |
15 |
|
|
$ |
12 |
|
|
Supplemental disclosure of non-cash transactions:
|
|
|
|
|
|
|
|
|
|
Fair value of common stock, stock options and stock warrants issued as consideration for asset acquisition
|
|
$ |
9,605 |
|
|
$ |
- |
|
The accompanying notes to the consolidated financial statements are an integral part of these statements.
Panbela Therapeutics, Inc.
Notes to Consolidated Financial Statements
Panbela Therapeutics, Inc. (“Panbela”) and its direct wholly-owned subsidiaries: Cancer Prevention Pharmaceuticals, Inc. (“CPP”) and Panbela Research, Inc. (“Panbela Research”) and their respective subsidiaries, all of which are wholly owned exist for the primary purpose of developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs. Panbela Therapeutics Pty Ltd is a wholly-owned subsidiary of Panbela Research organized under the laws of Australia. CPP has two wholly owned dormant subsidiaries: Cancer Prevention Pharma Limited, a United Kingdom entity, and Cancer Prevention Pharmaceuticals, LLC, an Arizona limited liability company. Panbela, together with its direct and indirect subsidiaries is referred to as “we,” “us,” “our,” and the “Company.”
The primary objective of our pipeline is the utilization of pharmacotherapies to reduce or normalize increased disease-associated polyamines using complementary pharmacotherapies. Our lead candidates are ivospemin (SBP-101) for which we have exclusively licensed the worldwide rights to from the University of Florida Research Foundation, Inc. and Flynpovi™ a combination of eflornithine (CPP-1X) and sulindac. We have exclusively licensed rights from the Arizona Board of Regents of the University of Arizona to commercialize Flynpovi, these rights are subject to a sublicense agreement to develop and commercialize Flynpovi in North America.
Acquisition of CPP
On June 15, 2022, we completed the previously announced strategic business reorganization and acquisition of CPP pursuant to the agreement and plan of merger, dated as of February 21, 2022 (the “Merger Agreement”), by and among Panbela, CPP, Panbela Research, Canary Merger Subsidiary I, Inc. (“Merger Sub I”), and Canary Merger Subsidiary II, Inc. (“Merger Sub II”). Pursuant to the terms of the Merger Agreement, (i) Merger Sub I, then a wholly-owned subsidiary of Panbela, which was itself a wholly-owned subsidiary of Panbela Research, merged with and into Panbela Research (the “First Merger”), with Panbela Research surviving the First Merger, and (ii) Merger Sub II, then a wholly-owned subsidiary of Panbela, merged with and into CPP (the “Second Merger” and, together with the First Merger, the “Mergers”), with CPP surviving the Second Merger. As a result of the Mergers, each of Panbela Research and CPP became a wholly owned subsidiary of Panbela. In addition, in connection with the consummation of the Mergers, then “Panbela Therapeutics, Inc.” was renamed to “Panbela Research, Inc.” and then “Canary Merger Holdings, Inc.” was renamed to “Panbela Therapeutics, Inc.” See Note 6, “Asset Acquisition,” for additional information.
Reverse Stock Split
Effective January 13, 2023, Panbela effected a 1-for-40 reverse stock split of its outstanding shares of common stock. Unless specifically provided otherwise herein, all share and per share amounts of our common stock presented have been retroactively adjusted to reflect the reverse stock split. See Note 9 for more information.
|
2.
|
Risks and Uncertainties
|
The Company operates in a highly regulated and competitive environment. The development, manufacturing and marketing of pharmaceutical products require approval from, and are subject to ongoing oversight by, the Food and Drug Administration (“FDA”) in the United States, the Therapeutic Goods Administration in Australia, the European Medicines Agency in the European Union, and comparable agencies in other countries. Obtaining approval for a new pharmaceutical product is never certain, may take many years, and is normally expected to involve substantial expenditure.
We have incurred losses of $91.1 million since our inception in 2011. For the year ended December 31, 2022, we incurred a net loss of $34.9 million. Included in the net loss for the year ended December 31, 2022 was $17.7 million of in-process research and development (“IPR&D”) written off as research and development (“R&D”) expense as the result of the acquisition of CPP. We incurred negative cash flows from operating activities of approximately $15.3 million for this period. As we continue to pursue development activities and seek commercialization of our lead assets, we expect to incur substantial losses, which are likely to generate negative net cash flows from operating activities. As of December 31, 2022, we had cash of $1.3 million, negative working capital of $6.0 million (current assets less current liabilities), and stockholders’ deficit of $8.0 million. The Company’s principal sources of cash have historically included the issuance of equity securities and convertible debt. CPP’s principal sources of cash have historically also included issuance of equity securities, convertible debt and development partners.
The accompanying Consolidated Financial Statements have been prepared assuming that we will continue as a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business and do not include any adjustments relating to the recoverability or classification of assets or the amounts of liabilities that might result from the outcome of these uncertainties. Our ability to continue as a going concern, realize the carrying value of our assets and discharge our liabilities in the ordinary course of business is dependent upon a number of factors, including our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for our ivospemin (SBP-101), eflornithine (CPP-1X) and eflornithine sachets (CPP-1X-S) product candidates in the United States, Australia, the European Union or other markets, and Flynpovi outside of North America and ultimately our ability to market and sell our product candidates. These factors, among others, raise substantial doubt about our ability to continue operations as a going concern. See Note 3 titled “Liquidity and Business Plan.”
December 2023 marked 33 months since the World Health Organization declared the spread of a novel strain of coronavirus (“COVID-19”) a global pandemic. While there is still some uncertainty of the situation, we continue to be unable to make any prediction as to the ultimate impact COVID-19 will have on the Company's business, financial condition, results of operation and cash flows. During the spring of 2021, the Company also experienced a delay in the manufacturing of the active product substance, which is manufactured in India. There was also a delay in the final manufacturing steps which are completed in the United States, in part related to COVID-19. To date there has been no disruption in supply for our clinical or preclinical testing. In January of 2022, the Company announced the opening of a global randomized Phase II/III clinical trial, which is expected to be conducted in the United States, Europe and Australia. While regulatory approval and site openings have been slower than expected the Company does not expect any significant delays in enrollment or data. The Company’s administrative operations have been decentralized since inception so the Company experienced no administrative disruptions or additional costs due to the pandemic or related restrictions.
|
3.
|
Liquidity and Management Plans
|
We will need to seek additional sources of funds to support our current business plans. We may seek to raise additional funds through various sources, such as equity and debt financings, or through strategic collaborations and license agreements. We can give no assurances that we will be able to secure additional sources of funds to support our operations, or if such funds are available to us, that such additional financing will be sufficient to meet our needs or on terms acceptable to us. This risk would increase if our clinical data were not positive or economic and market conditions deteriorate.
If we are unable to obtain additional financing when needed, we would need to scale back our operations taking actions that may include, among other things, reducing use of outside professional service providers, reducing staff or staff compensation, significantly modify or delay the development of our product candidates, license to third parties the rights to commercialize our product candidates as therapies in cancer or auto-immune diseases or other applications that we would otherwise seek to pursue, or cease operations.
Subsequent to December 31, 2022, the Company completed a registered public offering of common stock and warrants to purchase shares of common stock for gross proceeds of approximately $15.0 million. See Note 13 for additional information regarding the offering.
The company also sold shares of common stock via an At the Market (ATM) facility. During the quarter ended December 31, 2022, 28,343 shares were sold for gross proceeds of approximately $93,000. Subsequent to December 31, 2022 the Company completed the additional sale of common stock via the ATM for gross proceeds of approximately $1.6 million. See Note 13 for additional information regarding the offering.
On October 4, 2022, the Company completed a registered public offering and issued an aggregate of 177,175 shares of its common stock, pre-funded warrants to purchase up to an aggregate of 325,325 shares of common stock at an exercise price of $0.001 per share and warrants to purchase up to an aggregate of 753,749 shares of its common stock at an exercise price of $12.00 per share. The securities were issued for a combined offering price of $12.00 per share of common stock and 1.5 warrants, or $11.999 per pre-funded warrant and 1.5 warrants. Net proceeds from the offering totaled approximately $5.3 million. All pre-funded warrants were exercised by December 31, 2022 for additional proceeds of $13,000.
Much of the development efforts under way regarding the asset acquired in the CPP acquisition are funded by a licensing partner, see Note 8, “License Agreement for the Development and Commercialization of Flynpovi”, or collaborations with outside organizations including National Cancer Institute (“NCI”) and the Juvenile Diabetes Research Foundation (“JDRF”).
On July 2, 2021, the Company completed an underwritten public offering of 83,333 shares of common stock at $120.00 per share which resulted in net proceeds of approximately $9.1 million. In the first quarter of 2021, the Company received net proceeds of approximately $1.0 million from the exercise of common stock warrants; a total 5,598 shares of common stock were issued
It is expected that our cash will last into the third quarter of 2023.
Our future success is dependent upon our ability to obtain additional financing, the success of our development efforts, our ability to obtain marketing approval for ivospemin (SBP-101), eflornithine (CPP-1X) and eflornithine sachets (CPP-1X-S) in the United States or other markets and Flynpovi outside of the United States and ultimately our ability to market and sell our product candidates. If we are unable to obtain additional financing when needed, if our clinical trials are not successful or if we are unable to obtain marketing approval, we would not be able to continue as a going concern and would be forced to cease operations and liquidate our company.
There can be no assurances that we will be able to obtain additional financing on commercially reasonable terms, or at all. The sale of additional equity securities or convertible debt would likely result in dilution to our current stockholders.
|
4.
|
Summary of Significant Accounting Policies
|
Basis of presentation
We have prepared the accompanying Consolidated Financial Statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Our fiscal year ends on December 31.
Principles of consolidation
The accompanying Consolidated Financial Statements include the assets, liabilities and expenses of Panbela Therapeutics, Inc. and our direct and indirect subsidiaries. All significant intercompany transactions and balances have been eliminated in consolidation.
Use of estimates
The preparation of Consolidated Financial Statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Business Combinations and Asset Acquisition
We account for acquired businesses using the acquisition method of accounting, which requires that the assets acquired, and liabilities assumed be recorded at the date of acquisition at their respective fair values if the acquisition meets the definition of a business combination. If the acquisition does not meet the definition of a business combination, then it is accounted for as an asset acquisition and the purchase consideration is allocated to the acquired assets.
ASC 805, Business Combinations, provides a model for determining whether an acquisition represents a business combination. In order to be a business, the integrated set of activities of the acquired entity needs to have an input and a substantive process that together significantly contribute to the ability to create outputs. The acquired entity must also pass the “Screen Test” which involves determining whether the acquisition represents an in-substance asset acquisition based on whether the fair value of the gross assets acquired is “substantially all” concentrated in a single asset or group of similar assets. This evaluation excludes certain acquired assets such as cash, deferred taxes, and goodwill associated with deferred taxes, but includes all other gross assets, including any consideration transferred in excess of the identified assets.
Concentration of credit risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash. Cash is deposited in demand accounts at commercial banks. At times, such deposits may be in excess of insured limits. As of December 31, 2022, $1.0 million of the Company’s cash was in excess of insured limits. The Company has not experienced any losses on its deposits of cash.
Cash and cash equivalents
Cash equivalents include short-term, highly liquid investments with maturities of three months or less.
Other noncurrent assets
Other noncurrent assets are comprised primarily of long-term deposits with contract research organizations (“CROs”). These amounts are recognized as operating expenses or research and development expense as the trial is completed.
Research and development costs
Research and development costs include expenses incurred in the conduct of our human clinical trials, for third-party service providers performing various testing and accumulating data related to our preclinical studies; sponsored research agreements; developing and scaling the manufacturing process necessary to produce sufficient amounts of the SBP-101, Flynpovi and CPP-1X compounds for use in our pre-clinical studies and human clinical trials; consulting resources with specialized expertise related to execution of our development plan for our product candidates; personnel costs, including salaries, benefits and share-based compensation; and costs to license and maintain our licensed intellectual property.
We charge research and development costs, including clinical trial costs, to expense when incurred. Our human clinical trials are performed at clinical trial sites and are administered jointly by us with assistance from CROs. Costs of setting up clinical trial sites are accrued upon execution of the study agreement. Expenses related to the performance of clinical trials generally are accrued based on contracted amounts and the achievement of agreed upon milestones, such as site openings, patient enrollment, patient follow-up, etc. We monitor levels of performance under each significant contract, including the extent of patient enrollment and other activities through communications with the clinical trial sites and CROs, and adjust the estimates, if required, on a quarterly basis so that clinical expenses reflect the actual effort expended at each clinical trial site and by each CRO.
Research and development costs also include IPR&D. This asset was acquired from the security holders of CPP and written off to research and development expense immediately subsequent to the asset acquisition.
We expense costs associated with obtaining licenses for patented technologies when it is determined there is no alternative future use of the intellectual property subject to the license.
Clinical Trial Accruals
Costs for preclinical studies and clinical trial activities are recognized based on an evaluation of the vendors’ progress towards completion of specific tasks, using data such as clinical site activations, patient enrollment or information provided to the Company by its vendors regarding their actual costs incurred. Payments for these activities are based on the terms of individual contracts and payment timing may differ significantly from the period in which the services are performed. The Company determines accrual estimates through reports from and discussions with applicable personnel and outside service providers as to the progress or state of completion, or the services completed. The Company’s estimates of accrued expenses as of each balance sheet date are based on the facts and circumstances known at the time.
Stock-based compensation
In accounting for stock-based incentive awards, we measure and recognize the cost of employee and non-employee services received in exchange for awards of equity instruments based on the grant date fair value of those awards. Compensation cost is recognized ratably using the straight-line attribution method over the vesting period, which is considered to be the requisite service period. We record forfeitures in the periods in which they occur. The compensation expense for performance-based stock option awards is recognized when “performance” has occurred or is probable of occurring.
The fair value of stock-based awards is estimated at the date of grant using the Black-Scholes option pricing model. The determination of the fair value of stock-based awards is affected by our stock price, as well as assumptions regarding a number of complex and subjective variables. Risk free interest rates are based upon U.S. Treasury rates appropriate for the expected term of each award. Expected volatility rates are based primarily on the volatility rates of the Company’s common stock. The assumed dividend yield is zero, as we do not expect to declare any dividends in the foreseeable future. The expected term of options granted is determined using the “simplified” method. Under this approach, the expected term is presumed to be the mid-point between the average vesting date and the end of the contractual term.
The fair value of restricted stock units is calculated as the fair value of the underlying common stock as of the date of grant.
Income taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the Consolidated Financial Statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted rates, for each of the jurisdictions in which the Company operates, expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. Valuation allowances are established when necessary to reduce deferred tax assets to the amount that is more likely than not to be realized. The Company has provided a full valuation allowance against the gross deferred tax assets as of December 31, 2022 and 2021. The Company’s policy is to classify interest and penalties related to income taxes as income tax expense in the Consolidated Statements of Operations and Comprehensive Loss.
Foreign currency translation
The functional currency of Panbela Therapeutics Pty Ltd is the Australian Dollar (“AUD”). Accordingly, assets and liabilities, and equity transactions of Panbela Therapeutics Pty Ltd are translated into U.S. dollars at period-end exchange rates. Expenses are translated at the average exchange rate in effect for the period. The resulting translation gains and losses are recorded as a component of accumulated comprehensive gain (loss) in the Consolidated Statements of Operations and Comprehensive Loss. During the years ended December 31, 2022 and 2021, any reclassification adjustments from accumulated other comprehensive gain to operations were inconsequential.
The Company records transactions denominated in foreign currencies at the exchange rate in effect on that date. Fluctuations between the transaction date and settlement date are recognized as transaction gain/loss.
Net loss per share
We compute net loss per share by dividing our net loss (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Shares issued during the period and shares reacquired during the period, if any, are weighted for the portion of the period that they were outstanding. The computation of diluted earnings per share, or EPS, is similar to the computation of basic EPS except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued. Our diluted EPS is the same as basic EPS due to common equivalent shares being excluded from the calculation, as their effect is anti-dilutive.
The following outstanding potential common shares were not included in the diluted net loss per share calculations as their effects were not dilutive:
| |
|
December 31,
|
|
| |
|
2022
|
|
|
2021
|
|
|
Employee and non-employee stock options
|
|
|
100,556 |
|
|
|
61,582 |
|
|
Restricted stock units
|
|
|
- |
|
|
|
269 |
|
|
Common stock issuable under common stock purchase warrants
|
|
|
889,910 |
|
|
|
127,710 |
|
| |
|
|
990,466 |
|
|
|
189,561 |
|
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued ASU No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, which simplifies accounting for convertible instruments by removing major separation models required under current U.S. GAAP. The ASU removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception and it also simplifies the diluted earnings per share calculation in certain areas. This ASU is effective for annual reporting periods beginning after December 15, 2021, including interim periods within those fiscal years. The Company has adopted the ASU for the year ended December 31, 2022. This update permits the use of either the modified retrospective or fully retrospective method of transition. The Company has determined that the impact this ASU did not have material effect on its consolidated financial statements.
Accrued expenses consisted of the following (in thousands):
| |
|
December 31,
|
|
| |
|
2022
|
|
|
2021
|
|
|
Clinical trial and related expenses
|
|
$ |
1,760 |
|
|
$ |
992 |
|
|
Incentive compensation
|
|
|
550 |
|
|
|
434 |
|
|
Severance pay and related payroll taxes
|
|
|
448 |
|
|
|
- |
|
|
Professional services
|
|
|
147 |
|
|
|
235 |
|
|
Other
|
|
|
88 |
|
|
|
359 |
|
|
Total accrued liabilities
|
|
$ |
2,993 |
|
|
$ |
2,020 |
|
On June 15, 2022, the Company completed the previously announced strategic business reorganization and acquisition of CPP through the Mergers. Under the terms of the Merger Agreement, the holders of CPP’s outstanding capital stock immediately prior to the Merger received shares of common stock of Panbela upon closing of the Merger. The stockholders of Panbela Research retained a majority of the outstanding shares of Panbela, the post-merger holding company. CPP stockholders will be eligible to receive contingent payments totaling a maximum of $60 million from milestone and royalty payments associated with the potential approval and commercialization of eflornithine, the lead asset.
We performed the “screen test,” to determine if substantially all of the fair value of the gross assets acquired in the Mergers is concentrated in a single identifiable asset or group of similar identifiable assets. CPP’s lead asset, eflornithine in three forms, including Flynpovi (eflornithine (CPP-1X) and sulindac), eflornithine (CPP-1X), and eflornithine sachets (CPP-1X-S), were identified as the single identifiable asset consisting of IPR&D. Accordingly, our acquisition of CPP has been recorded as an asset acquisition.
The contract consideration for the assets acquired includes certain contingent consideration which at the acquisition date is neither probable of occurring nor reasonably estimable. As such, the value of this contingent consideration has been excluded from the allocation of the purchase price below. Acquisition-related transaction costs incurred have been recorded as additional investment in IPR&D.
The following is a summary of the purchase consideration and the allocation of that purchase consideration in connection with the CPP asset acquisition:
| |
|
Shares
|
|
|
Value (in thousands)
|
|
| |
|
|
|
|
|
|
|
|
|
Common stock issued to CPP shareholders
|
|
|
182,978 |
|
|
$ |
7,839 |
|
|
Common stock underlying options continued
|
|
|
39,904 |
|
|
|
1,637 |
|
|
Common stock underlying warrants replaced
|
|
|
8,452 |
|
|
|
129 |
|
|
Total non cash consideration
|
|
|
|
|
|
$ |
9,605 |
|
|
Transaction costs incurred
|
|
|
|
|
|
$ |
658 |
|
| |
|
|
|
|
|
|
|
|
|
Total Consideration
|
|
|
|
|
|
$ |
10,263 |
|
|
In process research and development *
|
|
$ |
17,737 |
|
|
Cash
|
|
|
4 |
|
|
Other current assets
|
|
|
230 |
|
|
Accounts payable and accrued expenses
|
|
|
(811 |
) |
|
Accrued interest and notes payable
|
|
|
(6,897 |
) |
| |
|
$ |
10,263 |
|
* In accordance with FASB ASC Topic 730, Research and Development this asset was immediately expensed upon the closing of the merger.
Sucampo Promissory Note
As of December 31, 2022, CPP had a balance outstanding of approximately $6.4 million principal and interest under an amended and restated promissory note (the “Sucampo Note”) issued with an initial principal amount of approximately $6.2 million in favor of Sucampo GmbH dated as of June 15, 2022. The principal balance outstanding under the Sucampo Note bears simple interest at a rate of 5% per annum. All unpaid principal, together with any then unpaid and accrued interest is payable as follows: (i) $1.0 million, plus all interest accrued but unpaid on or before each of January 31,2023, January 31, 2024, January 31, 2025 and January 31, 2026; and (ii) all remaining principal plus accrued but unpaid interest on or before January 31, 2027. If CPP or its parent, Panbela, receives cash proceeds from any issuance or offering of debt or equity before January 31, 2023, then CPP will be required to make a concurrent mandatory prepayment from such cash proceeds in an amount equal to the lesser of (i) $1.0 million plus all interest accrued but unpaid on the Sucampo Note through the date of payment; and (ii) 10% of such cash proceeds. The amount payable by CPP on January 31, 2023 would have been reduced on a dollar-for-dollar basis by the amount of any such prepayment. The Company completed an equity raise on October 4, 2022 which then gave rise to an obligation to pay 10% of the $6.0 million gross proceeds or $600,000, which was due by November 4, 2022. As the Company had not made this payment as of December 31, 2022, the note was in default as of the payment date and began incurring a penalty interest of an additional 5% as of November 4, 2022. On February 1, 2023 Panbela paid the balance due of $1.0 million plus accrued and unpaid interest of approximately $295,000. As a result of this payment the Company is now current with all payments due and is no longer in default. As of December 31, 2022, the accrued and unpaid interest on this note was approximately $243,000. Panbela has agreed to guarantee CPP’s payment obligations under the Sucampo Note pursuant to a Guaranty dated as of June 15, 2022.
Tillotts Promissory Note
As of December 31, 2022, CPP had a balance outstanding of approximately $0.7 million representing principal and interest under an amended promissory note (the “Tillotts Note”) issued with an initial principal amount of approximately $650,000 in favor of Tillotts Pharma AG. The principal balance outstanding under the Tillotts Note bears simple interest at a rate of 4% per annum. Accrued and unpaid interest as of December 31, 2022 was approximately $82,000. All outstanding amounts under the amended Tillotts Note matured and were paid in full on January 31, 2023.
|
8.
|
License Agreement for the Development and Commercialization of Flynpovi
|
CPP is party to a license agreement with One-Two Therapeutics Assets Limited (“One-Two”) dated as of July 16, 2021. Under the agreement, One-Two has licensed CPP’s North American development and commercialization rights for Flynpovi. The agreement also calls for CPP to receive a milestone payment upon regulatory approval of Flynpovi by the U.S. Food and Drug Administration (“FDA”) and royalties on net sales of Flynpovi in the licensed territories. Payment of the milestone payment and net sales royalties shall be reduced on a dollar-for-dollar basis by amounts funded by One-Two for One-Two’s direct employee, clinical and regulatory costs associated with any development activities necessary to secure FDA approval. The Company is not responsible for any costs, as they are incurred, associated with the development and regulatory approval of Flynpovi in North America.
|
9.
|
Commitments and Contingencies
|
License agreement with the University of Florida Research Foundation
On December 22, 2011, we entered into an exclusive license agreement with the University of Florida Research Foundation (“UFRF”). This license agreement was amended on December 12, 2016 (“First Amendment”) and again on October 3, 2019 (“Second Amendment”). The license agreement requires the Company to pay royalties to UFRF ranging from 2.5% to 5% of net sales of licensed products developed from the licensed technology. The Second Amendment eliminated all minimum annual royalties and modified the duration of royalty payments to the shorter of (1) ten years from first commercial sale of licensed products or (2) the expiration of the period of regulatory exclusivity on a country-by-country basis. All future milestone payments contemplated in the original agreement were eliminated in the Second Amendment.
The amended license agreement remains subject to customary and usual termination provisions. The Company must also pay an annual license maintenance fee of $10,000.
License Agreement with the University of Arizona
CPP is party to a license agreement with the Arizona Board of Regents of the University of Arizona (the “University”). Pursuant to an Inter-institutional Agreement, the Regents of the University of California on behalf of the University of California, Irvine, has agreed to license certain patents, provisional patents, clinical trial data and other intellectual property related to the chemoprevention of cancer, the prevention of polyps and other technologies to CPP. The University has the right to administer the joint patent rights held between the University and the University of California, Irvine. The license agreement gives CPP exclusive rights to commercialize products based on intellectual property. In exchange for the intellectual property, CPP paid the University certain fees and reimbursements of patent costs and granted the university a warrant to acquire shares of CPP. As a result of the Mergers, the warrant was replaced with a warrant to purchase 2,772 shares of common stock of Panbela at a price of $11.20 per share.
CPP also agreed to pay the University additional milestone payments totaling up to $90,000 upon the achievement of certain research, development and regulatory milestones. Future milestone payments are considered to be contingent consideration and will be accrued when probable of being paid. As of December 31, 2022, no milestone payments were probable of being paid.
Public offering of common stock and warrants
On October 4, 2022, the Company completed a registered public offering and issued an aggregate of 177,175 shares of its common stock, pre-funded warrants to purchase up to an aggregate of 325,325 shares of common stock at an exercise price of $0.001 per share and warrants to purchase up to an aggregate of 753,749 shares of its common stock at an exercise price of $12.00 per share. The securities were issued for a combined offering price of $12.00 per share of common stock and 1.5 warrants, or $11.999 per pre-funded warrant and 1.5 warrants. Net proceeds from the offering totaled approximately $5.3 million. All pre-funded warrants were exercised by December 31, 2022. The securities were offered pursuant to an effective registration statement on Form S-1.
At-the-Market Program
On July 19, 2022, Panbela Therapeutics, Inc. (the “Company”), entered into a Sales Agreement with Roth Capital Partners, LLC (the “Agent”) to sell shares of the Company’s common stock having an aggregate gross sales price of up to $8,400,000, from time to time, through an “at-the-market” equity offering program (the “ATM Program”).
During the last month of the year ended December 31, 2022, the Company sold 28,343 shares of common stock under the ATM Offering and generated approximately $93,000 in gross proceeds. The Company incurred financing costs of approximately $44,000, which were charged to additional paid in capital December 2022 when the Company began selling shares under the ATM Offering. Under the ATM Program, the Company pays Roth a commission equal to 3.0% of the aggregate gross proceeds of any sales of common stock under the ATM Program. Net proceeds for the year ended December 31, 2022 was approximately $46,000.
Public offering of common stock
On July 2, 2021, the Company completed an underwritten public offering of 83,333 shares of its common stock for a purchase price of $120.00 per share. The gross proceeds from the offering were approximately $10.0 million. The net proceeds, after deducting the underwriter’s discount and other offering costs, were approximately $9.1 million. The securities were offered pursuant to an effective registration statement on Form S-3.
Exercise of Warrants to purchase common stock
During the year ended December 31, 2021 the company issued 25 shares for a total of approximately $4,500 in proceeds resulting from the exercise of warrants at 181.60 per share.
During the year ended December 31, 2021, the Company issued a total of 5,723 shares of common stock for a total of approximately $1.0 million in proceeds, resulting from exercises of outstanding warrants. Most of the warrants exercised, a total of 5,598, were exercised at $181.60 per share and the shares of common stock were issued pursuant to an effective registration on Form S-1.
During the same period, the Company issued an additional 4,762 shares of common stock resulting from the net, cashless, exercise of outstanding warrants to purchase 13,403 shares. Warrants to purchase a total of 14,333 shares of common stock, all having an exercise price of $5.00 per share, expired during the year ended December 31, 2021. Additionally, warrants to purchase 2,714 shares of common stock, with an exercise price of $15.00 expired during the year ended December 31, 2021.
Reverse Stock Split
On November 29, 2022, the Company held a special meeting of its stockholders at which the stockholders approved a proposal to effect an amendment to the Company's certificate of incorporation, as amended, to implement a reverse stock split at a ratio of one-for-forty (1:40). On January 13, 2023, the Company's Board of Directors approved the implementation of the reverse stock split of the Company's Common Stock. As a result of the reverse stock split, every forty (40) shares of the Company's Common Stock either issued and outstanding immediately prior to the effective time was, automatically and without any action on the part of the respective holders thereof, combined and converted into one (1) share of the Company's common stock. No fractional shares were issued in connection with the reverse stock split. Stockholders who otherwise were entitled to receive a fractional share in connection with the reverse stock split instead were eligible to receive a cash payment, which was not material in the aggregate, instead of shares. On January 13, 2023, the Company filed a Certificate of Amendment of its Certificate of Incorporation, as amended with the Secretary of State of Delaware effecting a one-for-forty (1:40) reverse stock split of the shares of the Company’s common stock, issued and outstanding, effective January 13, 2023. The Company’s common stock began trading on a reverse split adjusted basis when the market opened Friday, January 13, 2023.
Shares reserved
|
Shares of common stock reserved for future issuance were as follows as of December 31, 2022:
|
|
| |
|
|
|
|
|
Stock options outstanding
|
|
|
100,556 |
|
|
Shares available for grant under equity incentive plan
|
|
|
50,511 |
|
|
Common shares issuable under outstanding common stock purchase warrants
|
|
|
889,910 |
|
| |
|
|
1,040,977 |
|
|
11.
|
Stock-Based Compensation
|
2016 Omnibus Incentive Plan
The 2016 Omnibus Incentive Plan, as last amended effective April 9, 2020 (the “2016 Plan”), has been approved by our Board of Directors and ratified by our stockholders. The 2016 Plan permits the granting of incentive and non-statutory stock options, restricted stock, stock appreciation rights, performance units, performance shares and other stock awards to eligible employees, directors and consultants. We grant options to purchase shares of common stock under the 2016 Plan with an exercise price no less than the fair market value of the underlying common stock as of the date of grant. Options granted under the 2016 Plan have a maximum term of ten years. Under the 2016 Plan, a total of 106,825 shares of common stock have been reserved for issuance. As of December 31, 2022, options to purchase 55,495 shares of common stock were outstanding under the 2016 Plan, with a weighted average exercise price of $241.65 per share, and the average remaining contractual life was approximately 6.5 years. At the same date 50,511 shares remained available for future awards.
2011 Stock Option Plan
Prior to approval of the 2016 Plan, stock-based awards were granted under the 2011 Stock Option Plan (the “2011 Plan”). In conjunction with stockholder approval of the 2016 Plan, the Board terminated the 2011 Plan, although awards outstanding under the 2011 Plan will remain outstanding in accordance with and pursuant to the terms thereof. Options granted under the 2011 Plan have a maximum term of ten years and generally vest over zero to two years for employees. As of December 31, 2022, options to purchase 5,600 shares of common stock remained outstanding under the 2011 Plan, with a weighted average exercise price of $118.92 per share, and the average remaining contractual life was approximately 2.0 years.
CPP’s 2010 Equity Incentive Plan
As a result of the Mergers, the Company has assumed all remaining rights and obligations with respect to CPP’s 2010 Equity Incentive Plan (the “CPP Plan”) through the issuance of replacement options. As of December 31, 2022, options to purchase 39,461 shares of common stock remained outstanding under the CPP Plan, with a weighted average exercise price of $14.013 per share, and the average remaining contractual life was 7.2 years.
We recognize stock-based compensation based on the fair value of each award as estimated using the Black-Scholes option valuation model. Ultimately, the actual expense recognized over the vesting period will only be for those shares that actually vest.
A summary of option activity is as follows:
| |
|
Shares Underlying
Options
|
|
|
Weighted
Average
Exercise Price
Per Share
|
|
|
Aggregate
Intrinsic Value
|
|
|
Balance at January 1, 2021
|
|
|
53,438 |
|
|
$ |
270.92 |
|
|
$ |
388,461 |
|
|
Granted
|
|
|
13,851 |
|
|
|
154.55 |
|
|
|
|
|
|
Exercised
|
|
|
(197 |
) |
|
|
35.20 |
|
|
|
|
|
|
Cancelled
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
Forfeitures
|
|
|
(5,510 |
) |
|
|
410.75 |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2021
|
|
|
61,582 |
|
|
$ |
233.00 |
|
|
$ |
8,821 |
|
|
Options continued from CPP plan
|
|
|
39,904 |
|
|
|
14.00 |
|
|
|
|
|
|
Exercised
|
|
|
(443 |
) |
|
|
8.80 |
|
|
|
|
|
|
Cancelled
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
Forfeitures
|
|
|
(487 |
) |
|
|
562.97 |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2022
|
|
|
100,556 |
|
|
$ |
145.50 |
|
|
$ |
- |
|
Stock-based compensation expense for each of the periods presented is as follows (in thousands):
| |
|
Year ended December 31, |
| |
|
2022
|
|
|
2021
|
|
| General and administrative |
|
$ |
889 |
|
|
$ |
1,083 |
|
|
Research and development
|
|
|
199 |
|
|
|
204 |
|
|
Total stock based compensation
|
|
$ |
1,088 |
|
|
$ |
1,287 |
|
A summary of the status of our unvested shares during the two years ended and as of December 31, 2022 is as follows:
| |
|
Shares Under
Option
|
|
|
Weighted Average
Grant Date Fair Value
|
|
|
Unvested at January 1, 2021
|
|
|
12,653 |
|
|
$ |
166.80 |
|
|
Granted
|
|
|
13,851 |
|
|
|
118.93 |
|
|
Vested
|
|
|
(7,153 |
) |
|
|
144.41 |
|
|
Forfeitures
|
|
|
(1,250 |
) |
|
|
119 |
|
|
Unvested at December 31, 2021
|
|
|
18,101 |
|
|
$ |
137.41 |
|
|
Granted
|
|
|
- |
|
|
|
- |
|
|
Vested
|
|
|
(8,184 |
) |
|
|
135.03 |
|
|
Forfeitures
|
|
|
(487 |
) |
|
|
562.93 |
|
|
Unvested at December 31, 2022
|
|
|
9,430 |
|
|
$ |
139.04 |
|
Information about stock options outstanding, vested and expected to vest as of December 31, 2022, is as follows:
| |
|
|
|
|
Outstanding, Vested and Expected to Vest
|
|
|
Options Vested and Exercisable
|
|
|
Per Share Exercise Price
|
|
|
Shares
|
|
|
Weighted
Average
Remaining
Contractual
Life (Years)
|
|
|
Weighted
Average
Exercise Price
|
|
|
Options
Exercisable
|
|
|
Weighted
Average
Remaining
Contractual Life
(Years)
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| $8.80 |
-
|
$58.80 |
|
|
|
39,810 |
|
|
|
7.12 |
|
|
$ |
14.31 |
|
|
|
39,810 |
|
|
|
7.12 |
|
| $90.40 |
-
|
$91 |
|
|
|
1,578 |
|
|
|
8.02 |
|
|
$ |
90.46 |
|
|
|
625 |
|
|
|
6.99 |
|
| $100 |
- |
166.80 |
|
|
|
30,595 |
|
|
|
6.38 |
|
|
$ |
135.94 |
|
|
|
24,383 |
|
|
|
5.90 |
|
| $180 |
-
|
$244 |
|
|
|
11,676 |
|
|
|
5.94 |
|
|
$ |
208.29 |
|
|
|
10,738 |
|
|
|
5.81 |
|
| $324 |
-
|
$404 |
|
|
|
12,051 |
|
|
|
6.19 |
|
|
$ |
365.14 |
|
|
|
10,724 |
|
|
|
6.02 |
|
| $604 |
|
|
|
4,846 |
|
|
|
3.95 |
|
|
$ |
604.00 |
|
|
|
4,846 |
|
|
|
3.95 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals
|
|
|
|
100,556 |
|
|
|
6.62 |
|
|
$ |
145.50 |
|
|
|
91,126 |
|
|
|
6.34 |
|
As of December 31, 2022, total compensation expense related to unvested employee stock options not yet recognized was $0.8 million, which is expected to be allocated to expenses over a weighted-average period of 1.1 years.
No new options were granted during the year ended December 31, 2022. On June 15, 2022, as a component of the purchase consideration for the acquisition of CPP the Company provided fully vested replacement options to purchase up to 39,904 shares of common stock at a weighted average exercise price of $14.00 per share.
Non-employee stock-based compensation
We account for stock options granted to nonemployees in accordance with Accounting Standards Update (“ASU”) 2019-07, “Compensation – Stock Compensation (Topic 718). In connection with stock options granted to nonemployees, we recorded $281,000 and $222,000 for nonemployee stock-based compensation during 2022 and 2021, respectively.
Restricted stock units
The number and weighted average grant date fair value of restricted non vested common stock for the most recent completed fiscal years is as follows:
| |
|
Number of
Restricted Shares
|
|
|
Weighted Average
Grant Date Fair
Value
|
|
|
Restricted nonvested at January 1, 2021
|
|
|
287 |
|
|
$ |
138.00 |
|
|
Granted in 2021
|
|
|
539 |
|
|
|
166.80 |
|
|
Vested in 2021
|
|
|
(557 |
) |
|
|
152.00 |
|
|
Restricted nonvested at December 31, 2021
|
|
|
269 |
|
|
$ |
138.00 |
|
|
Granted in 2022
|
|
|
- |
|
|
|
- |
|
|
Vested in 2022
|
|
|
(269 |
) |
|
|
67.60 |
|
|
Restricted nonvested at December 31, 2022
|
|
|
- |
|
|
$ |
- |
|
As of December 31, 2022, there was no compensation expense yet to be recognized related to unvested restricted stock units.
Stock compensation expense includes expense related to restricted stock units of approximately $43,000 and $106,000 during the years ended December 31,2022 and December 31, 2021, respectively.
We have incurred net operating losses since our inception. We have not reflected the benefit of net operating loss carryforwards in the accompanying financial statements and have established a full valuation allowance against our deferred tax assets.
On December 31, 2022 and 2021, the Company had an income tax receivable of approximately $49,000 and $321,000 respectively, comprised of refundable tax incentives related to research and development activities of our subsidiary Panbela Therapeutics Pty Ltd.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes as well as operating losses and tax credit carryforwards.
The significant components of our deferred tax assets and liabilities are as follows (in thousands):
| |
|
2022
|
|
|
Tax Expense:
|
|
|
|
|
|
Current
|
|
|
(116 |
) |
|
Deferred
|
|
|
- |
|
|
Noncurrent
|
|
|
- |
|
| |
|
|
(116 |
) |
| |
|
December 31, |
|
|
Deferred tax assets (liabilities)
|
|
2022
|
|
|
2021
|
|
| |
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards
|
|
$ |
17,564 |
|
|
$ |
9,986 |
|
|
Research credit carryforwards
|
|
|
338 |
|
|
|
235 |
|
|
Stock-based compensation
|
|
|
1,928 |
|
|
|
1,734 |
|
|
Section 174 amortization
|
|
|
1,921 |
|
|
|
- |
|
|
Other
|
|
|
68 |
|
|
|
66 |
|
|
Deferred tax assets
|
|
|
21,819 |
|
|
|
12,021 |
|
|
Valuation allowance
|
|
|
(21,819 |
) |
|
|
(12,021 |
) |
|
Net deferred tax asset
|
|
$ |
- |
|
|
$ |
- |
|
Realization of the future tax benefits is dependent on our ability to generate sufficient taxable income within the carry-forward period. Because of our history of operating losses, management believes that the deferred tax assets arising from the above-mentioned future tax benefits are currently not likely to be realized and, accordingly, we have provided a full valuation allowance.
A reconciliation of the statutory tax rates and the effective tax rates is as follows:
| |
|
|
Year Ended December 31,
|
|
| |
|
2022
|
|
|
2021
|
|
|
Statutory rate
|
|
|
21.0 |
% |
|
|
21.0 |
% |
|
Permanent differences
|
|
|
(10.8 |
) |
|
|
2.1 |
|
|
Change in effective tax rate
|
|
|
0.0 |
|
|
|
(0.4 |
) |
|
States
|
|
|
0.1 |
|
|
|
- |
|
|
Valuation allowance
|
|
|
(13.0 |
) |
|
|
(24.3 |
) |
|
Foreign research incentives
|
|
|
0.1 |
|
|
|
3.1 |
|
|
Other
|
|
|
2.9 |
|
|
|
1.6 |
|
|
Effective rate
|
|
|
0.3 |
% |
|
|
3.1 |
% |
Net operating losses and tax credit carryforwards as of December 31, 2022, are as follows:
|
(In Thousands)
|
|
Amount
|
|
Expiration Years
|
|
Net operating losses--federal
|
|
|
23,068 |
|
Expires beginning 2031
|
|
2018 to 2022 net operating losses -- federal
|
|
|
23,977 |
|
Never expires
|
|
Tax credits--federal
|
|
|
338 |
|
Beginning 2041
|
Utilization of the net operating loss carryforwards and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by the IRC, and similar state provisions. We have not performed a detailed analysis to determine whether an ownership change under Section 382 of the IRC has occurred. The effect of an ownership change would be the imposition of an annual limitation on the use of net operating loss carryforwards attributable to periods before the change.
The Company is subject to taxation in the United States and Australia. Tax returns for the year ended December 31, 2017 and thereafter are subject to examinations by federal and state tax authorities. Tax returns of Panbela Therapeutics Pty Ltd for the year ended December 31, 2015 and thereafter are subject to examination by the Australian tax authorities.
During the month of January 2023, the Company sold 440,830 shares of common stock under the ATM Program. Net proceeds were approximately $1.6 million.
On January 30, 2023, the Company completed a registered public offering and issued an aggregate of 4,842,224 shares of its common stock, pre-funded warrants to purchase up to an aggregate of 1,832,776 shares of common stock at an exercise price of $0.001 per share and warrants to purchase up to an aggregate of 13,350,000 shares of its common stock at an exercise price of $2.75 per share. The securities were issued for a combined offering price of $2.25 per share of common stock and 2 warrants, or $2.249 per pre-funded warrant and 2 warrants. Net proceeds from the offering totaled approximately $13.7 million. All pre-funded warrants were exercised by February 2, 2023. The securities were offered pursuant to an effective registration statement on Form S-1.
Up to 8,000,000 Shares of Common Stock
Up to 8,000,000 Class E Common Warrants to purchase up to 2,660,000 Shares of Common Stock
Up to 8,000,000 Class F Common Warrants to purchase up to 14,000,000 Shares of Common Stock
Up to 8,000,000 Pre-Funded Warrants to purchase up to 8,000,000 Shares of Common Stock
Up to 24,660,000 Shares of Common Stock Underlying Warrants
Panbela Therapeutics, Inc.
PROSPECTUS
Roth Capital Partners
, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
|
Item 13.
|
Other Expenses of Issuance and Distribution.
|
The following table sets forth the costs and expenses payable by Panbela Therapeutics, Inc. (the “Company”) in connection with the offering and sale of the common stock being registered. All amounts shown are estimates, except the Securities and Exchange Commission (the “Commission”) registration fee.
|
U.S. Securities and Exchange Commission registration fee
|
|
$
|
8,856 |
|
|
FINRA filing fee
|
|
$
|
25,000 |
|
|
Accounting fees and expenses
|
|
$
|
40,000
|
|
|
Legal fees and expenses
|
|
$
|
200,000
|
|
|
Transfer agent, registrar, and warrant agent fees
|
|
$
|
20,000
|
|
|
Printing expenses
|
|
$
|
10,000
|
|
|
Miscellaneous
|
|
$
|
11,144
|
|
|
Total
|
|
$
|
315,000 |
|
|
Item 14.
|
Indemnification of Directors and Officers.
|
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive officer, employee or agent of the corporation or is or was serving at the request of the corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation.
The Company’s certificate of incorporation and amended and restated bylaws limit the liability of its directors to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| |
●
|
breach of their duty of loyalty to the Company or its stockholders;
|
| |
●
|
act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
|
| |
●
|
unlawful payment of dividends or redemption of shares as provided in Section 174 of the Delaware General Corporation Law; or
|
| |
●
|
transaction from which the directors derived an improper personal benefit.
|
These limitations of liability do not apply to liabilities arising under federal securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission. The Company’s amended and restated bylaws provide that it will indemnify its directors and executive officers, and may indemnify other officers, employees and other agents, to the fullest extent permitted by law.
As permitted by the Delaware General Corporation Law, the Company has entered into indemnification agreements with each of the Company’s directors and executive officers that require the Company to indemnify such persons against expenses, judgments, penalties, fines, settlements and other amounts actually and reasonably incurred, including expenses of a derivative action, in connection with an actual or threatened proceeding if any of the Company’s directors or executive officers may be made a party because he or she is or was one of the Company’s directors. The Company will be obligated to pay such amounts only if the director acted in good faith and in a manner that he or she reasonably believed to be in or not opposed to the Company’s best interests. With respect to any criminal proceeding, the Company will be obligated to pay such amounts only if the director had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification.
Section 145(g) of the Delaware General Corporation Law permits a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee, or agent of the corporation arising out of his or her actions in connection with their services to the Company, regardless of whether its amended and restated bylaws permit indemnification. The Company has purchased and intends to maintain insurance on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
|
Item 15.
|
Recent Sales of Unregistered Securities.
|
During the six months ended June 30, 2021, the Company issued 162 shares of Common Stock as a result of exercises of outstanding warrants. Of the shares of Common Stock issued, 158 shares were issued pursuant to net, cashless, exercises of warrants to purchase 5446 shares and the remaining 5 shares were issued for $25,000 cash. We relied on exemptions from registration set forth in Section 4(a)(2) of the Securities Act, without the use of any general solicitations or advertising to market or otherwise offer the securities for sale and all participants were “accredited investors,” as defined in Rule 501 of Regulation D as promulgated by the SEC under the Securities Act.
On June 15, 2022, pursuant to an agreement and plan of merger dated as of February 21, 2022 (the “Merger Agreement”), the Company sold and issued the following securities to the holders of CPP securities: (a) 5,489 shares of Common Stock, (b) 609 shares of Common Stock that remained subject to a holdback escrow (as defined in the Merger Agreement), (iv) replacement options to purchase up to 1,330 shares of Common Stock at a weighted average purchase price of $420.00 per share, and (v) replacement warrants to purchase up to 2810 shares of Common Stock at a weighted average purchase price of $4974.00 per share. Effective June 15, 2023, all of the shares in the holdback escrow were released to the sellers. We relied on exemptions from registration set forth in Section 4(a)(2) of the Securities Act, without the use of any general solicitations or advertising to market or otherwise offer the securities for sale and all participants were “accredited investors,” as defined in Rule 501 of Regulation D as promulgated by the SEC under the Securities Act.
On November 2, 2023, the Company entered into warrant exercise inducement offer letters with certain holders of its existing warrants to purchase shares of Common Stock, pursuant to which the holders agreed to exercise for cash their existing warrants to purchase 2,130,000 shares of Common Stock, in the aggregate, at a reduced exercise price of $0.78 per share, in exchange for the Company’s agreement to issue new Class C Warrants on substantially the same terms as the existing warrants, to purchase up to 4,260,000 shares of Common Stock (the “Class C Warrant Shares”) and a cash payment of $0.125 per existing warrant share which was paid in full upon the exercise of the existing warrants. The terms of exercise of the Class C Warrants are further described under the heading “Description of Securities – Warrants Outstanding – Class C Warrants” above. The Company received aggregate gross proceeds of approximately $1.9 million from the exercise of the existing warrants by the holders and the sale of the Class C Warrants. The Company issued the Class C Warrants pursuant to the exemption from the registration requirements of the Securities Act available under Section 4(a)(2) and Rule 506(b) of Regulation D promulgated thereunder and the Class C Warrant Shares have been or will be issued pursuant to the same exemption or pursuant to the exemption provided by Section 3(a)(9) of the Securities Act.
On December 21, 2023, the Company entered into warrant exercise inducement offer letters with the holders of its existing Class C Warrants, pursuant to which the holders agreed to exercise for cash their existing Class C Warrants to purchase an aggregate of 2,556,000 shares of Common Stock, in the aggregate, at the existing exercise price of $0.78 per share, in exchange for the Company’s agreement to issue new Class D Warrants on substantially the same terms as the Class C Warrants, to purchase up to 5,112,000 shares of Common Stock (the “Class D Warrant Shares”). The terms of exercise of the Class D Warrants are further described under the heading “Description of Securities – Warrants Outstanding – Class D Warrants” above. The Company received aggregate gross proceeds of approximately $2.0 million from the exercise of the Class C Warrants by the holders. The Company issued the Class C Warrant Shares and the Class D Warrants pursuant to the exemption from the registration requirements of the Securities Act available under Section 4(a)(2) and Rule 506(b) of Regulation D promulgated thereunder and the Class D Warrant Shares have been or will be issued pursuant to the same exemption or pursuant to the exemption provided by Section 3(a)(9) of the Securities Act.
|
Item 16.
|
Exhibits and Financial Statement Schedules.
|
(a) Exhibits
|
Exhibit
No.
|
|
Description |
|
4.2
|
|
Form of Common Stock Warrant issued December 2018 and January 2019 (incorporated by reference to Exhibit 10.3 to current report on Form 8-K filed December 28, 2018)
|
|
4.3
|
|
Form of Common Stock Warrant issued August through October 2019 (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed August 29, 2019)
|
|
4.4
|
|
Form of Warrants issued May 22, June 5, June 15, and June 22, 2020 (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed June 11, 2020)
|
|
4.5
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated September 1, 2020 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed September 1, 2020)
|
|
4.6
|
|
Form of Common Stock Purchase Warrant (included in Exhibit 4.6)
|
|
4.7
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated as of October 4, 2022 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on October 4, 2022)
|
|
4.8
|
|
Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.3 to current report on Form 8-K filed October 4, 2022)
|
|
4.9
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated as of January 30, 2023 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on January 31. 2023)
|
|
4.10
|
|
Warrant Agency Agreement with VStock Transfer, LLC dated as of June 21, 2023 (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on June 21, 2023)
|
|
4.11
|
|
Form of Class A Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.3 to current report on Form 8-K filed on June 21, 2023)
|
|
4.12
|
|
Form of Class B Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.4 to current report on Form 8-K filed on June 21, 2023)
|
|
4.13
|
|
Form of Class C Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on November 3, 2023)
|
|
4.14
|
|
Form of Class D Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to current report on Form 8-K filed on December 21, 2023)
|
|
4.15+
|
|
Form of Warrant Agency Agreement with VStock Transfer, LLC
|
|
4.16+
|
|
Form of Pre-Funded Warrant
|
|
4.17++
|
|
Form of Class E Common Stock Purchase Warrant
|
|
4.18+
|
|
Form of Class F Common Stock Purchase Warrant
|
|
5.1++
|
|
Opinion of Faegre Drinker Biddle & Reath LLP
|
|
10.1*
|
|
2011 Stock Option Plan, as amended through January 1, 2015 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed September 11, 2015)
|
|
10.2*
|
|
Form of Incentive Stock Option Agreement for awards under 2011 Plan (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed September 11, 2015)
|
|
10.3*
|
|
Form of Non-Qualified Stock Option Agreement for awards under 2011 Plan (incorporated by reference to Exhibit 10.3 to current report on Form 8-K filed September 11, 2015)
|
|
10.4*
|
|
2016 Omnibus Incentive Plan as amended and restated through April 9, 2020 (incorporated by reference to Exhibit 99.1 to current report on Form 8-K filed May 26, 2020)
|
|
10.5*
|
|
Form of Incentive Stock Option Agreement for awards under 2016 Plan (incorporated by reference to Exhibit 10.4 to quarterly report on Form 10-Q for quarter ended June 30, 2016)
|
|
10.6*
|
|
Form of Non-Qualified Stock Option Agreement for awards under 2016 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.5 to quarterly report on Form 10-Q for quarter ended June 30, 2016)
|
|
10.7*
|
|
Form of Performance-Based Stock Option Agreement for awards under 2016 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.7 to annual report on Form 10-K for fiscal year ended December 31, 2016)
|
|
10.8**
|
|
Standard Exclusive License Agreement with University of Florida Research Foundation, Inc., dated December 22, 2011 (incorporated by reference to Exhibit 10.5 to current report on Form 8-K filed September 11, 2015)
|
|
10.9
|
|
Form of First Amendment to License Agreement with University of Florida Research Foundation, Inc. dated December 12, 2016 (incorporated by reference to Exhibit 10.10 to annual report on Form 10-K for fiscal year ended December 31, 2019)
|
|
10.10
|
|
Second Amendment to License Agreement with University of Florida Research Foundation, Inc., dated October 3, 2019 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed October 9, 2019)
|
|
Exhibit
No.
|
|
Description |
|
10.11*
|
|
Employment Agreement with Susan Horvath, dated April 17, 2018 (incorporated by reference to Exhibit 10.4 to quarterly report on Form 10-Q for quarter ended March 31, 2018)
|
|
10.12*
|
|
Employment Agreement with Jennifer K. Simpson, dated July 15, 2020 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed July 16, 2020)
|
|
10.13
|
|
First Amendment to Seed Capital Accelerator Loan Agreement and Seed Capital Loan Note dated October 13, 2017 (incorporated by reference to Exhibit 10.1 to quarterly report on Form 10-Q for quarter ended March 31, 2019)
|
|
10.14
|
|
Second Amendment to Seed Capital Accelerator Loan Agreement and Seed Capital Loan Note dated April 5, 2019 (incorporated by reference to Exhibit 10.2 to quarterly report on Form 10-Q for quarter ended March 31, 2019
|
|
10.15
|
|
Third Amendment to Seed Capital Accelerator Loan Agreement and Seed Capital Loan Noted dated December 31,2019 (incorporated by reference to Exhibit 10.25 to annual report on Form 10-K for fiscal year ended December 31, 2019)
|
|
10.16
|
|
Form of Securities Purchase Agreement, dated December 21 and 31, 2018, January 14, 25, and 31, 2019 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed December 28, 2018)
|
|
10.17*
|
|
Cancer Prevention Pharmaceuticals, Inc. 2010 Equity Incentive Plan, as amended (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed June 16, 2022)
|
|
10.18*
|
|
Form of Stock Option Assumption Notice (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed June 16, 2022)
|
|
10.19
|
|
Form of Replacement Warrant (incorporated by reference to Exhibit 10.3 to current report on Form 8-K filed June 16, 2022)
|
|
10.20
|
|
Convertible Promissory Note in favor of Sucampo GmbH (f/k/a Sucampo AG), dated as of September 6, 2017, as amended through April 7, 2022 (incorporated by reference to Exhibit 10.4 to current report on Form 8-K filed June 16, 2022)
|
|
10.21
|
|
Guaranty in favor of Sucampo GmbH (f/k/a Sucampo AG), dated June 15, 2022 (incorporated by reference to Exhibit 10.5 to current report on Form 8-K filed June 16, 2022)
|
|
10.22
|
|
Separation and Release Agreement with Jeffrey E. Jacobs, dated June 15, 2022 (incorporated by reference to Exhibit 10.6 to current report on Form 8-K filed June 16, 2022)
|
|
10.23
|
|
Form of Securities Purchase Agreement (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed on October 4, 2022)
|
|
10.24
|
|
Placement Agency Agreement with Roth Capital Partners, LLC dated as of September 29, 2022 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed on October 4, 2022)
|
|
10.25
|
|
Placement Agency Agreement with Roth Capital Partners, LLC dated as of June 16, 2023 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed June 21, 2023)
|
|
10.26
|
|
Form of Securities Purchase Agreement by and between Panbela Therapeutics, Inc. and the purchasers named therein (incorporated by reference to Exhibit 10.2 to current report on Form 8-K filed June 21, 2023)
|
|
10.27
|
|
Form of Inducement Letters dated November 2, 2023 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed November 3, 2023).
|
|
10.28
|
|
Form of Inducement Letters dated December 21, 2023 (incorporated by reference to Exhibit 10.1 to current report on Form 8-K filed December 21, 2023)
|
|
10.29+
|
|
Form of Placement Agency Agreement with Roth Capital Partners, LLC
|
|
10.30++
|
|
Form of Securities Purchase Agreement
|
|
21.1+
|
|
List of Subsidiaries
|
| 23.1+ |
|
Consent of Independent Registered Public Accounting Firm
|
|
23.2++
|
|
Consent of Faegre Drinker Biddle & Reath LLP (included in Exhibit 5.1)
|
|
24.1+
|
|
Powers of Attorney (see signature page)
|
|
107++
|
|
Filing Fee Table
|
|
*
|
Management compensatory plan or arrangement required to be filed as an exhibit to this prospectus.
|
|
**
|
Portions of exhibit omitted pursuant to order granting confidential treatment issued by the Securities and Exchange Commission.
|
(b) Financial Statement Schedules.
All schedules are omitted as the required information is inapplicable or the information is presented in the financial statements or related notes.
Schedule II. Valuation and Qualifying Accounts
All other schedules are omitted as the required information is inapplicable or the information is presented in the financial statements or related notes.
The registrant hereby undertakes:
| |
(1)
|
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
| |
(i)
|
To include any prospectus required by Section 10(a)(3) of the Securities Act;
|
| |
(ii)
|
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
|
| |
(iii)
|
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in this registration statement;
|
provided, however, that paragraphs (a)(1)(i), (ii) and (iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section 15(d) of the Exchange Act, that are incorporated by reference in the registration statement.
| |
(2)
|
That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered herein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
| |
(3)
|
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
|
| |
(4)
|
That, for the purpose of determining liability under the Securities Act to any purchaser: each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
|
| |
(5)
|
That, for the purpose of determining liability under the Securities Act to any purchaser the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective; and for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
| |
(6)
|
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
|
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Minneapolis, State of Minnesota on January 25, 2024.
| |
PANBELA THERAPEUTICS, INC.
|
| |
|
| |
By:
|
/s/ Jennifer K. Simpson
|
| |
|
Jennifer K. Simpson
President and Chief Executive Officer
|
Power of Attorney
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
| |
|
|
|
|
|
/s/ Jennifer K. Simpson
|
|
President and Chief Executive Officer
|
|
January 25, 2024 |
|
Jennifer K. Simpson
|
|
(Principal Executive Officer), and Director
|
|
|
| |
|
|
|
|
|
/s/ Susan Horvath
|
|
Vice President of Finance, Chief Financial Officer, Treasurer
|
|
January 25, 2024 |
|
Susan Horvath
|
|
and Secretary (Principal Financial and Accounting Officer)
|
|
|
| |
|
|
|
|
| * |
|
Chair of the Board and Director
|
|
January 25, 2024 |
|
Michael T. Cullen
|
|
|
|
|
| |
|
|
|
|
| * |
|
Director
|
|
January 25, 2024 |
|
Daniel J. Donovan
|
|
|
|
|
| |
|
|
|
|
| * |
|
Director
|
|
January 25, 2024 |
|
Arthur J. Fratamico
|
|
|
|
|
| |
|
|
|
|
| * |
|
Director
|
|
January 25, 2024 |
|
Jeffrey E. Jacob
|
|
|
|
|
| |
|
|
|
|
| * |
|
Director
|
|
January 25, 2024 |
|
Jeffrey S. Mathiesen
|
|
|
|
|
| |
|
|
|
|
| * |
|
Director
|
|
January 25, 2024 |
|
D. Robert Schemel
|
|
|
|
|
* Jennifer K. Simpson, by signing her name hereto, does hereby sign this document on behalf of each of the above-named directors of the registrant pursuant to powers of attorney duly executed by such persons.
|
By:
|
/s/ Jennifer K. Simpson |
| |
Jennifer K. Simpson |
| |
Attorney-in-Fact
|
Exhibit 4.17
CLASS E COMMON STOCK PURCHASE WARRANT
PANBELA THERAPEUTICS, INC.
|
Warrant Shares:
|
Initial Exercise Date: , 2024
|
CUSIP:
ISIN:
THIS CLASS E COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, or its assigns (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the date hereof (the “Initial Exercise Date”) and on or prior to 5:00 p.m. (New York City time) on , 2029 (the “Termination Date”) but not thereafter, to subscribe for and purchase from Panbela Therapeutics, Inc., a Delaware corporation (the “Company”), up to shares (as subject to adjustment hereunder, the “Warrant Shares”) of Common Stock. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b). This Warrant shall initially be issued and maintained in the form of a security held in book-entry form and the Depository Trust Company or its nominee (“DTC”) shall initially be the sole registered holder of this Warrant, subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agency Agreement, in which case this sentence shall not apply.
Section 1. Definitions. In addition to the terms defined elsewhere in this Warrant, the following terms have the meanings indicated in this Section 1:
“Affiliate” means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with a Person, as such terms are used in and construed under Rule 405 under the Securities Act.
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported on the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Warrants then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
“Board of Directors” means the board of directors of the Company.
“Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”, “non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The City of New York generally are open for use by customers on such day.
“Commission” means the United States Securities and Exchange Commission.
“Common Stock” means the Common Stock of the Company, par value $0.001 per share, and any other class of securities into which such securities may hereafter be reclassified or changed.
1 Note to Draft: To be the fifth (5th) anniversary of the Initial Exercise Date.
“Common Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Exempt Issuance” means the issuance of (a) shares of Common Stock, options, restricted stock units or other equity-based awards to employees, officers or directors of the Company or its subsidiaries pursuant to any compensation plan duly adopted for such purpose, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose for services rendered to the Company, (b) securities upon the exercise or exchange of or conversion of any securities issued pursuant to the Purchase Agreement and/or other securities exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of the Purchase Agreement, provided that such securities have not been amended since the date of this Agreement to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock splits or combinations) or to extend the term of such securities, (c) securities issued pursuant to acquisitions or strategic transactions (including, without limitation, joint venture, co-marketing, co-development or other collaboration agreements) approved by a majority of the disinterested directors of the Company, provided that such securities are issued as “restricted securities” (as defined in Rule 144) and carry no registration rights that require or permit the filing of any registration statement in connection therewith during the 90 day period following the Initial Exercise Date, and provided that any such issuance shall only be to a Person (or to the equityholders of a Person) which is, itself or through its subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of the Company and shall provide to the Company additional benefits in addition to the investment of funds, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities, and (d) shares of Common Stock, options, restricted stock units or other equity-based awards issued to consultants approved by a majority of the disinterested directors of the Company, provided that such securities are issued as “restricted securities” (as defined in Rule 144) and carry no registration rights that require or permit the filing of any registration statement in connection therewith during the 90 day period following the Initial Exercise Date.
“Minimum Exercise Price” means 50% of the Nasdaq Minimum Price (as defined in Nasdaq Listing Rule 5635(d) or its successor) calculated as of the date of the Purchase Agreement as proportionately adjusted to reflect any Stock Dividend or Splits in the manner set forth in Section 3(a).
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Purchase Agreement” means the Securities Purchase Agreement, dated as of , 2024, between the Company and the purchasers signatory thereto.
“Registration Statement” means the Company’s registration statement on Form S-1 (File No. 333-276367).
“Rule 144” means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Subsidiary” means any subsidiary of the Company and shall, where applicable, also include any direct or indirect subsidiary of the Company formed or acquired after the date hereof.
“Trading Day” means a day on which the Common Stock is traded on a Trading Market.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market or the New York Stock Exchange (or any successors to any of the foregoing).
“Transfer Agent” means VStock Transfer, the current transfer agent of the Company, with a mailing address of 18 Lafayette Place, Woodmere, New York 11598, and any successor transfer agent of the Company.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported on the Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the holders of a majority in interest of the Warrants then outstanding and reasonably acceptable to the Company, the reasonable and documented fees and expenses of which shall be paid by the Company.
“Warrant Agency Agreement” means that certain warrant agency agreement, dated on or about the Initial Exercise Date, between the Company and the Warrant Agent.
“Warrant Agent” means the Transfer Agent and any successor warrant agent of the Company.
“Warrants” means this Warrant and other Common Stock purchase warrants issued by the Company pursuant to the Registration Statement.
Section 2. Exercise.
(a) Exercise of Warrant. Subject to the provisions of Section 2(e), exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company and, for informational purposes only, the Warrant Agent of a duly executed PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise in the form annexed hereto (the “Notice of Exercise”). Within the earlier of (i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period (as defined in Section 2(d)(i) herein) following the date of exercise as aforesaid, the Holder shall deliver the aggregate Exercise Price for the shares specified in the applicable Notice of Exercise by wire transfer or cashier’s check drawn on a United States bank unless the cashless exercise procedure specified in Section 2(c) below is specified in the applicable Notice of Exercise. For clarification purposes, any reference to a cashless exercise in this Warrant shall include, without limitation, an “alternative cashless exercise,” as contemplated in Section 2(c) below. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within five (5) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise within one (1) Business Day of receipt of such notice. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise delivered on or prior to 4:00 p.m. (New York City time) on the Trading Date prior to the Initial Exercise Date, which may be delivered at any time after the time of execution of the Purchase Agreement, the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Initial Exercise Date and the Initial Exercise Date shall be the Warrant Share Delivery Date for purposes hereunder, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received by such Warrant Share Delivery Date. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated on the face hereof. Notwithstanding the foregoing in this Section 2(a), a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing this Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions), shall effect exercises made pursuant to this Section 2(a) by delivering to DTC (or such other clearing corporation, as applicable) the appropriate instruction form for exercise, complying with the procedures to effect exercise that are required by DTC (or such other clearing corporation, as applicable), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agency Agreement, in which case this sentence shall not apply. For purposes of Regulation SHO, a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing this Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions) shall be deemed to have exercised its interest in this Warrant upon instructing its broker that is a DTC Participant to exercise its interest in such Warrant, provided that in each such case payment of the applicable aggregate Exercise Price (other than in the case of a cashless exercise) is received within the earlier of (i) two (2) trading days and (ii) the number of trading days comprising the Standard Settlement Period, in each case following such instruction.
(b) Exercise Price. The exercise price per share of Common Stock under this Warrant shall be $ per share, subject to adjustment hereunder (the “Exercise Price”).
(c) Cashless Exercise. If at the time of exercise hereof there is no effective registration statement registering or the prospectus contained therein is not available for the issuance of the Warrant Shares to the Holder, then this Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where:
(A) =as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2(a) hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2(a) hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b) of Regulation NMS promulgated under the federal securities laws) on such Trading Day, (ii) at the option of the Holder, either (y) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise or (z) the Bid Price of the Common Stock on the principal Trading Market as reported by Bloomberg L.P. as of the time of the Holder’s execution of the applicable Notice of Exercise if such Notice of Exercise is executed during “regular trading hours” on a Trading Day and is delivered within two (2) hours thereafter (including until two (2) hours after the close of “regular trading hours” on a Trading Day) pursuant to Section 2(a) hereof or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof after the close of “regular trading hours” on such Trading Day;
(B) = the Exercise Price of this Warrant, as adjusted hereunder; and
(X) = the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise.
If Warrant Shares are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act, the Warrant Shares shall take on the registered characteristics of the Warrants being exercised. The Company agrees not to take any position contrary to this Section 2(c). Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant to this Section 2(c).
Notwithstanding anything to the contrary herein, the Holder may also effect an “alternative cashless exercise” on or after the fifth Business Day following the Initial Exercise Date. In such event, the aggregate number of Warrant Shares issuable in such alternative cashless exercise pursuant to any given Notice of Exercise electing to effect an alternative cashless exercise shall equal the product of (x) the aggregate number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise and (y) .
(d) Mechanics of Exercise.
(i) Delivery of Warrant Shares Upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale of the Warrant Shares by Holder or (B) this Warrant is being exercised via cashless exercise, and otherwise by physical delivery of a certificate, registered in the Company’s share register in the name of the Holder or its designee, for the number of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise by the date that is the earliest of (i) two (2) Trading Days after the delivery to the Company of the Notice of Exercise, (ii) one (1) Trading Day after delivery of the aggregate Exercise Price to the Company and (iii) the number of Trading Days comprising the Standard Settlement Period after the delivery to the Company of the Notice of Exercise (such date, the “Warrant Share Delivery Date”). Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received within the earlier of (i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period following delivery of the Notice of Exercise. If the Company fails for any reason to deliver to the Holder the Warrant Shares subject to a Notice of Exercise by the Warrant Share Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the third Trading Day after the Warrant Share Delivery Date) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise. The Company agrees to maintain a transfer agent that is a participant in the FAST program so long as this Warrant remains outstanding and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed in a number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of delivery of the Notice of Exercise.
(ii) Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
(iii) Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
(iv) Compensation for Buy-In on Failure to Timely Deliver Warrant Shares Upon Exercise. In addition to any other rights available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with the provisions of Section 2(d)(i) above pursuant to an exercise on or before the Warrant Share Delivery Date (other than any such failure that is solely due to any action or inaction by the Holder with respect to such exercise), and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall (A) pay in cash to the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying (1) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue times (2) the price at which the sell order giving rise to such purchase obligation was executed, and (B) at the option of the Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honored (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of this Warrant to purchase shares of Common Stock with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
(v) No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round down to the next whole share.
(vi) Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that, in the event that Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
(vii) Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
(e) Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates, and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, nonexercised portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion of the unexercised or nonconverted portion of any other securities of the Company (including, without limitation, any other Common Stock Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2(e), beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith. To the extent that the limitation contained in this Section 2(e) applies, the determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be the Holder’s determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such determination and shall have no liability for exercises of this Warrant that are not in compliance with the Beneficial Ownership Limitation, provided this limitation of liability shall not apply if the Holder has detrimentally relied on outstanding share information provided by the Company or the Transfer Agent. In addition, a determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. For purposes of this Section 2(e), in determining the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent periodic or annual report filed with the Commission, as the case may be, (B) a more recent public announcement by the Company or (C) a more recent written notice by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the written or oral request of a Holder, the Company shall within two Trading Days confirm orally and in writing to the Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution Parties since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% (or, upon election by a Holder prior to the issuance of any Warrants, 9.99%) of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of this Warrant. The Holder, upon notice to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(e), provided that the Beneficial Ownership Limitation in no event exceeds 9.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2(e) shall continue to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity with the terms of this Section 2(e) to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
Section 3. Certain Adjustments.
(a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
(b) Adjustment Upon Issuance of Shares of Common Stock. If and whenever on or after the date of the Purchase Agreement, the Company issues or sells (or enters into any agreement to grant, issue or sell), or in accordance with this Section 3(b) is deemed to have issued or sold, any shares of Common Stock and/or Common Stock Equivalents (including the issuance or sale of shares of Common Stock owned or held by or for the account of the Company, but excluding any Exempt Issuance issued or sold or deemed to have been issued or sold) for a consideration per share (the “New Issuance Price”) less than a price equal to the Exercise Price in effect immediately prior to such issuance or sale or deemed issuance or sale (such Exercise Price then in effect is referred to herein as the “Applicable Price”) (the foregoing a “Dilutive Issuance”), then simultaneously with the consummation (or, if earlier, the announcement) of each such Dilutive Issuance, the Exercise Price then in effect shall be reduced to an amount equal to the New Issuance Price. For all purposes of the foregoing (including, without limitation, determining the adjusted Exercise Price and the New Issuance Price under this Section 3(b)), the following shall be applicable:
(i) Issuance of Options. If the Company in any manner grants or sells any rights, warrants or options to subscribe for or purchase shares of preferred stock and/or Common Stock or Common Stock Equivalents (“Options”) and the lowest price per share for which one share of Common Stock is at any time issuable upon the exercise of any such Option or upon conversion, exercise or exchange of any Common Stock Equivalents issuable upon exercise of any such Option or otherwise pursuant to the terms thereof is less than the Applicable Price, then such share of Common Stock shall be deemed to be outstanding and to have been issued and sold by the Company at the time of the granting or sale of such Option for such price per share. For purposes of this Section 3(b)(i), the “lowest price per share for which one share of Common Stock is issuable upon the exercise of any such Options or upon conversion, exercise or exchange of any Common Stock Equivalents issuable upon exercise of any such Option or otherwise pursuant to the terms thereof” shall be equal to (1) the lower of (x) the sum of the lowest amounts of consideration (if any) received or receivable by the Company with respect to any one share of Common Stock upon the granting or sale of such Option, upon exercise of such Option and upon conversion, exercise or exchange of any Common Stock Equivalents issuable upon exercise of such Option or otherwise pursuant to the terms thereof and (y) the lowest exercise price set forth in such Option for which one share of Common Stock is issuable upon the exercise of any such Options or upon conversion, exercise or exchange of any Common Stock Equivalents issuable upon exercise of any such Option or otherwise pursuant to the terms thereof minus (2) the sum of all amounts paid or payable to the holder of such Option (or any other Person) upon the granting, issuance or sale of such Option, upon exercise of such Option and upon conversion, exercise or exchange of any Common Stock Equivalents issuable upon exercise of such Option or otherwise pursuant to the terms thereof plus the value of any other consideration received or receivable by, or benefit conferred on, the holder of such Option (or any other Person). Except as contemplated below, no further adjustment of the Exercise Price shall be made upon the actual issuance of such shares of Common Stock or of such Common Stock Equivalents upon the exercise of such Options or otherwise pursuant to the terms of or upon the actual issuance of such shares of Common Stock upon conversion, exercise or exchange of such Common Stock Equivalents. This Section 3(b)(i) shall not apply to any Exempt Issuance.
(ii) Issuance of Common Stock Equivalents. If the Company in any manner issues or sells any Common Stock Equivalents and the lowest price per share for which one share of Common Stock is at any time issuable upon the conversion, exercise or exchange thereof or otherwise pursuant to the terms thereof is less than the Exercise Price, then such share of Common Stock shall be deemed to be outstanding and to have been issued and sold by the Company at the time of the issuance or sale of such Common Stock Equivalents for such price per share. For the purposes of this Section 3(b)(ii), the “lowest price per share for which one share of Common Stock is issuable upon the conversion, exercise or exchange thereof or otherwise pursuant to the terms thereof” shall be equal to (1) the lower of (x) the sum of the lowest amounts of consideration (if any) received or receivable by the Company with respect to one share of Common Stock upon the issuance or sale of the Common Stock Equivalent and upon conversion, exercise or exchange of such Common Stock Equivalent or otherwise pursuant to the terms thereof and (y) the lowest conversion price set forth in such Common Stock Equivalent for which one share of Common Stock is issuable upon conversion, exercise or exchange thereof or otherwise pursuant to the terms thereof minus (2) the sum of all amounts paid or payable to the holder of such Common Stock Equivalent (or any other Person) upon the issuance or sale of such Common Stock Equivalent plus the value of any other consideration received or receivable by, or benefit conferred on, the holder of such Common Stock Equivalent (or any other Person). Except as contemplated below, no further adjustment of the Exercise Price shall be made upon the actual issuance of such shares of Common Stock upon conversion, exercise or exchange of such Common Stock Equivalents or otherwise pursuant to the terms thereof, and if any such issuance or sale of such Common Stock Equivalents is made upon exercise of any Options for which adjustment of the Warrant has been or is to be made pursuant to other provisions of this Section 3(b), except as contemplated below, no further adjustment of the Exercise Price shall be made by reason of such issuance or sale.
(iii) Change in Option Price or Rate of Conversion. If the purchase or exercise price provided for in any Options, the additional consideration, if any, payable upon the issue, conversion, exercise or exchange of any Common Stock Equivalents, or the rate at which any Common Stock Equivalents are convertible into or exercisable or exchangeable for shares of Common Stock increases or decreases at any time (other than proportional changes in conversion or exercise prices, as applicable, in connection with an event referred to in Section 3(a)), the Exercise Price in effect at the time of such increase or decrease shall be adjusted to the Exercise Price which would have been in effect at such time had such Options or Common Stock Equivalents provided for such increased or decreased purchase price, additional consideration or increased or decreased conversion rate, as the case may be, at the time initially granted, issued or sold. For purposes of this Section 3(b)(iii), if the terms of any Option or Common Stock Equivalents that was outstanding as of the date of the Purchase Agreement are increased or decreased in the manner described in the immediately preceding sentence, then such Option or Common Stock Equivalents and the shares of Common Stock deemed issuable upon exercise, conversion or exchange thereof shall be deemed to have been issued as of the date of such increase or decrease. No adjustment pursuant to this Section 3(b) shall be made if such adjustment would result in an increase of the Exercise Price then in effect.
(iv) Calculation of Consideration Received. If any Option and/or Common Stock Equivalents and/or Adjustment Right is issued in connection with the issuance or sale or deemed issuance or sale of any other securities of the Company (as determined by the Holder, the “Primary Security”, and such Option and/or Common Stock Equivalents and/or Adjustment Right, the “Secondary Securities” and together with the Primary Security, each a “Unit”), together comprising one integrated transaction, the aggregate consideration per share of Common Stock with respect to such Primary Security shall be deemed to be the lowest of (x) the purchase price of such Unit, (y) if such Primary Security is an Option and/or Common Stock Equivalent, the lowest price per share for which one share of Common Stock is at any time issuable upon the exercise or conversion of the Primary Security in accordance with Section 3(b)(i) or 3(b)(ii) above and (z) the lowest VWAP on any Trading Day during the five (5) Trading Day period (the “Adjustment Period”) immediately following the public announcement of such Dilutive Issuance (for the avoidance of doubt, if such public announcement is released prior to the opening of the applicable Trading Market on a Trading Day, such Trading Day shall be the first Trading Day in such five Trading Day period and if this Warrant is exercised, on any given Exercise Date during any such Adjustment Period, solely with respect to such portion of this Warrant converted on such applicable Exercise Date, such applicable Adjustment Period shall be deemed to have ended on, and included, the Trading Day immediately prior to such Exercise Date). If any shares of Common Stock, Options or Common Stock Equivalents are issued or sold or deemed to have been issued or sold for cash, the consideration received therefor will be deemed to be the net amount of consideration received by the Company therefor. If any shares of Common Stock, Options or Common Stock Equivalents are issued or sold for a consideration other than cash, the amount of such consideration received by the Company will be the fair value of such consideration, except where such consideration consists of publicly traded securities, in which case the amount of consideration received by the Company for such securities will be the arithmetic average of the VWAPs of such security for each of the five (5) Trading Days immediately preceding the date of receipt. If any shares of Common Stock, Options or Common Stock Equivalents are issued to the owners of the non-surviving entity in connection with any merger in which the Company is the surviving entity, the amount of consideration therefor will be deemed to be the fair value of such portion of the net assets and business of the non-surviving entity as is attributable to such shares of Common Stock, Options or Common Stock Equivalents (as the case may be). The fair value of any consideration other than cash or publicly traded securities will be determined jointly by the Company and the Holder. If such parties are unable to reach agreement within ten (10) days after the occurrence of an event requiring valuation (the “Valuation Event”), the fair value of such consideration will be determined within five (5) Trading Days after the tenth (10th) day following such Valuation Event by an independent, reputable appraiser jointly selected by the Company and the Holder. The determination of such appraiser shall be final and binding upon all parties absent manifest error and the fees and expenses of such appraiser shall be borne by the Company. For purposes of hereof, “Adjustment Right” means any right granted with respect to any securities issued in connection with, or with respect to, any issuance or sale (or deemed issuance or sale in accordance with this Section 3(b)) of shares of Common Stock (other than rights of the type described in Section 3(c) and Section 3(d) hereof) that could result in a decrease in the net consideration received by the Company in connection with, or with respect to, such securities (including, without limitation, any cash settlement rights, cash adjustment or other similar rights).
(v) Record Date. If the Company takes a record of the holders of shares of Common Stock for the purpose of entitling them (A) to receive a dividend or other distribution payable in shares of Common Stock, Options or in Common Stock Equivalents or (B) to subscribe for or purchase shares of Common Stock, Options or Common Stock Equivalents, then such record date will be deemed to be the date of the issuance or sale of the shares of Common Stock deemed to have been issued or sold upon the declaration of such dividend or the making of such other distribution or the date of the granting of such right of subscription or purchase (as the case may be).
(vi) Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3(a) above, if at any time the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
(c) Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares of Common Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the participation in such Distribution (provided, however, that, to the extent that the Holder's right to participate in any such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
(d) Fundamental Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly, in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company or any Subsidiary, directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially all of its assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders of 50% or more of the outstanding Common Stock or 50% or more of the voting power of the common equity of the Company, (iv) the Company, directly or indirectly, in one or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or (v) the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another Person or group of Persons whereby such other Person or group acquires 50% or more of the outstanding shares of Common Stock or 50% or more of the voting power of the common equity of the Company (each a “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to any limitation in Section 2(e) on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2(e) on the exercise of this Warrant). For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. Notwithstanding anything to the contrary, in the event of a Fundamental Transaction, the Company or any Successor Entity (as defined below) shall, at the Holder’s option, exercisable at any time concurrently with, or within 30 days after, the consummation of the Fundamental Transaction (or, if later, the date of the public announcement of the applicable Fundamental Transaction), purchase this Warrant from the Holder by paying to the Holder an amount of cash equal to the Black Scholes Value (as defined below) of the remaining unexercised portion of this Warrant on the date of the consummation of such Fundamental Transaction; provided, however, that, if the Fundamental Transaction is not within the Company's control, including not approved by the Company's Board of Directors, the Holder shall only be entitled to receive from the Company or any Successor Entity the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of this Warrant, that is being offered and paid to the holders of shares of Common Stock of the Company in connection with the Fundamental Transaction, whether that consideration be in the form of cash, shares or any combination thereof, or whether the holders of shares of Common Stock are given the choice to receive from among alternative forms of consideration in connection with the Fundamental Transaction; provided, further, that if holders of shares of Common Stock of the Company are not offered or paid any consideration in such Fundamental Transaction, such holders of shares of Common Stock will be deemed to have received shares of common stock of the Successor Entity (which Entity may be the Company following such Fundamental Transaction) in such Fundamental Transaction. “Black Scholes Value” means the value of this Warrant based on the Black-Scholes Option Pricing Model obtained from the “OV” function on Bloomberg, L.P. (“Bloomberg”) determined as of the day of consummation of the applicable Fundamental Transaction for pricing purposes and reflecting (A) a risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement of the applicable contemplated Fundamental Transaction and the Termination Date, (B) an expected volatility equal to the greater of 100% and the 100 day volatility obtained from the HVT function on Bloomberg (determined utilizing a 365 day annualization factor) as of the Trading Day immediately following the public announcement of the applicable contemplated Fundamental Transaction, (C) the underlying price per share used in such calculation shall be the greater of (i) the sum of the price per share being offered in cash, if any, plus the value of any non-cash consideration, if any, being offered in such Fundamental Transaction and (ii) the highest VWAP during the period beginning on the Trading Day immediately preceding the public announcement of the applicable contemplated Fundamental Transaction (or the consummation of the applicable Fundamental Transaction, if earlier) and ending on the Trading Day of the Holder’s request pursuant to this Section 3(d), (D) a remaining option time equal to the time between the date of the public announcement of the applicable contemplated Fundamental Transaction and the Termination Date and (E) a zero cost of borrow. The payment of the Black Scholes Value will be made by wire transfer of immediately available funds (or such other consideration) within five (5) Business Days of the Holder’s election (or, if later, on the date of consummation of the Fundamental Transaction). The Company shall cause any successor entity in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”) to assume in writing all of the obligations of the Company under this Warrant in accordance with the provisions of this Section 3(d) pursuant to written agreements in form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay) prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant which is exercisable for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock (but taking into account the relative value of the shares of Common Stock pursuant to such Fundamental Transaction and the value of such shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic value of this Warrant immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall be added to the term “Company” under this Warrant (so that from and after the occurrence or consummation of such Fundamental Transaction, each and every provision of this Warrant and the other Transaction Documents referring to the “Company” shall refer instead to each of the Company and the Successor Entity or Successor Entities, jointly and severally), and the Successor Entity or Successor Entities, jointly and severally with the Company, may exercise every right and power of the Company prior thereto and the Successor Entity or Successor Entities shall assume all of the obligations of the Company prior thereto under this Warrant and the other Transaction Documents with the same effect as if the Company and such Successor Entity or Successor Entities, jointly and severally, had been named as the Company herein.
(e) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
(f) Notice to Holder.
(i) Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly deliver to the Holder by email a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment. Notwithstanding any other provision of this Warrant, as to any Warrant not held in certificated form, where this Warrant provides for notice of any event to a Holder, such notice shall be sufficiently given if given to DTC (or any successor depository) pursuant to the procedures of DTC (or such successor depository).
(ii) Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company (or all of its Subsidiaries, taken as a whole) is a party, any sale or transfer of all or substantially all of its assets, or any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by email to the Holder at its last email address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding the Company or any of the Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice except as may otherwise be expressly set forth herein.
(g) Voluntary Adjustment By Company. Subject to the rules and regulations of the Trading Market, the Company may at any time during the term of this Warrant reduce the then current Exercise Price to any amount and for any period of time deemed appropriate by the board of directors of the Company.
(h) Minimum Exercise Price. Notwithstanding anything to the contrary in this Section 3, in no event will the Exercise Price of this Warrant be adjusted to an amount that is less than the Minimum Exercise price.
Section 4. Transfer of Warrant.
(a) Transferability. This Warrant and all rights hereunder are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with a written assignment of this Warrant substantially in the form attached hereto duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant to the Company within three (3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning this Warrant in full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
(b) New Warrants. If this Warrant is not held in global form through DTC (or any successor depositary), this Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the initial issuance date of this Warrant and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
(c) Warrant Register. The Warrant Agent (or to the extent this Warrant is not held in global form through DTC (or any successor depositary), the Company) shall register this Warrant, upon records to be maintained by the Warrant Agent (or to the extent this Warrant is not held in global form through DTC (or any successor depositary), the Company) for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company and the Warrant Agent may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
Section 5. Miscellaneous.
(a) No Rights as Stockholder Until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly set forth in Section 3. Without limiting any rights of a Holder to receive Warrant Shares on a “cashless exercise” pursuant to Section 2(c) or to receive cash payments pursuant to Section 2(d)(i) and Section 2(d)(iv) herein, in no event shall the Company be required to net cash settle an exercise of this Warrant.
(b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
(c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day or Trading Day, then such action may be taken or such right may be exercised on the next succeeding Business Day or Trading Day, respectively.
(d) Authorized Shares. The Company covenants that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue). Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
(e) Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law. If either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant, the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding. Notwithstanding the foregoing, nothing in this paragraph shall limit or restrict the federal district court in which a Holder may bring a claim under the federal securities laws.
(f) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
(g) Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder including, without limitation, any Notice of Exercise, shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier service, addressed to the Company, at 712 Vista Blvd. #305, Waconia, Minnesota 55387, Attention: Sue Horvath, email address: shorvath@panbela.com, with a copy (for informational purposes only) to Faegre Drinker Biddle & Reath LLP, 2200 Wells Fargo Center 90 S. Seventh Street, Minneapolis, Minnesota 55402, Attention: Joshua L. Coburn, e-mail address: joshua.colburn@faegredrinker.com, or such other email address or address as the Company may specify for such purposes by notice to the Holders. Any and all notices or other communications or deliveries to be provided by the Company hereunder shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier service addressed to each Holder at the e-mail address or address of such Holder appearing on the books of the Company. Any notice or other communication or deliveries hereunder shall be deemed given and effective on the earliest of (i) the time of transmission, if such notice or communication is delivered via e-mail at the e-mail address set forth in this Section prior to 5:30 p.m. (New York City time) on any date, (ii) the next Trading Day after the time of transmission, if such notice or communication is delivered via e-mail at the e-mail address set forth in this Section on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (iii) the second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon actual receipt by the party to whom such notice is required to be given. To the extent that any notice provided hereunder constitutes, or contains, material, non-public information regarding the Company or any Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K.
(h) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
(i) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
(j) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company, on the one hand, and the Holder or the beneficial owner of this Warrant, on the other hand.
(k) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
(l) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
(m) Warrant Agency Agreement. If this Warrant is held in global form through DTC (or any successor depositary), this Warrant is issued subject to the Warrant Agency Agreement. To the extent any provision of this Warrant conflicts with the express provisions of the Warrant Agency Agreement, the provisions of this Warrant shall govern and be controlling.
********************
(Signature Page Follows)
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| |
PANBELA THERAPEUTICS, INC.
|
|
| |
|
|
|
| |
|
|
|
| |
By:
|
|
|
| |
Name:
|
|
|
| |
Title:
|
|
|
NOTICE OF EXERCISE
TO: PANBELA THERAPEUTICS, INC.
|
(1)
|
The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
|
|
(2)
|
Payment shall take the form of (check applicable box):
|
☐ in lawful money of the United States; or
☐ if permitted the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(c), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(c).
(3) Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below: The Warrant Shares shall be delivered to the following DWAC Account Number:
[SIGNATURE OF HOLDER]
|
Name of Investing Entity:
|
|
Signature of Authorized Signatory of Investing
Name of Authorized
Title of Authorized
ASSIGNMENT FORM
(To assign the foregoing Warrant, execute this form and supply required information. Do not use this form to purchase shares.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced thereby are hereby assigned to
|
Name:
|
|
|
| |
|
(Please Print)
|
|
Address:
|
|
|
| |
|
(Please Print)
|
|
Phone Number:
|
|
|
|
Email Address:
|
|
|
|
Dated:
|
|
|
|
Holder’s Signature:
|
|
|
|
|
Holder’s Address:
|
|
|
|
Exhibit 5.1
|
|
faegredrinker.com |
| |
|
| |
Faegre Drinker Biddle & Reath LLP |
| |
2200 Wells Fargo Center |
| |
90 South Seventh Street |
| |
Minneapolis, Minnesota 55402 |
| |
+1 612 766 7000 main |
| |
+1 612 766 1600 fax |
January 25, 2024
Panbela Therapeutics, Inc.
712 Vista Boulevard #305
Waconia, MN 55387
Ladies and Gentlemen:
We have acted as counsel to Panbela Therapeutics, Inc., a Delaware corporation (the “Company”), in connection with the registration of the offer and sale by the Company of up to a maximum aggregate offering price of up to $92,968,200, consisting of (a) shares (the “Common Stock Shares”) of the Company’s common stock, par value $0.001 per share (the “Common Stock”), or pre-funded warrants to purchase shares of Common Stock (the “Pre-Funded Warrants”), (b) Class E warrants to purchase shares of Common Stock (the “Class E Common Warrants”), (c) Class F warrants to purchase shares of Common Stock (the “Class F Common Warrants” and, together with the Class E Common Warrants and the Pre-Funded Warrants, the “Warrants”) and (d) shares of Common Stock issuable from time to time upon the exercise of the Warrants (the “Warrant Shares” and, together with the Warrants and the Common Stock Shares, the “Securities”), in each case pursuant to the Registration Statement on Form S-1, as amended from time to time (the “Registration Statement”), filed on the date hereof by the Company with the Securities and Exchange Commission (the “Commission”) under the Securities Act of 1933, as amended (the “Act”), including a related prospectus filed with the Registration Statement (the “Prospectus”). The Securities are to be sold by the Company as described in the Registration Statement and the Prospectus.
This opinion letter is being delivered in accordance with the requirements of Item 601(b)(5) of Regulation S‑K.
In this capacity, we have examined originals or copies, certified or otherwise identified to our satisfaction, of such documents, corporate records and other instruments as we have deemed necessary for the purposes of this opinion, including (i) the Registration Statement and Prospectus, (ii) the form of Placement Agency Agreement by and between Roth Capital Partners, LLC and the Company, filed as Exhibit 10.30 to the Registration Statement (the “Placement Agreement”), (iii) the form of Securities Purchase Agreement between the Company and each purchaser to be identified on the signature pages thereto, filed as Exhibit 10.29 to the Registration Statement (the “Purchase Agreement”), (iv) the form of Class E Common Warrant, the form of Class F Common Warrant and the form of Pre-Funded Warrant filed as Exhibit 4.18, Exhibit 4.19 and Exhibit 4.17, respectively, to the Registration Statement, (v) the form of Warrant Agency Agreement filed as Exhibit 4.16 to the Registration Statement (the “Warrant Agreement”), (vi) the Company’s Restated Certificate of Incorporation, as amended by that Certificate of Amendment, filed as Exhibit 3.1 and Exhibit 3.2, respectively, to the Registration Statement, (vii) the Company’s Amended and Restated Bylaws, as amended to date, in the form filed as Exhibit 3.3 to the Registration Statement, and (viii) the corporate proceedings of the Company taken to date with respect to the Securities. In addition, we have assumed that the Company’s Board of Directors or its Pricing Committee has taken action to set the sale price of the Common Stock Shares and the Warrants and the exercise price of the Warrants. We have also examined such authorities of law as we have deemed relevant as a basis for our opinions hereinafter set forth.
In rendering the opinions set forth below, we have assumed, without investigation, (i) the legal capacity of each natural person signing any of the documents and corporate records examined by us, (ii) the genuineness of all signatures, including electronic signatures, appearing upon the documents submitted to us for review, (iii) the authenticity of documents submitted to us as originals, (iv) the conformity to authentic original documents of documents submitted to us as copies, (v) the truth, accuracy and completeness of all corporate records and other documents and information made available to us by the Company, (vi) the absence of any undisclosed modifications to the agreements and instruments reviewed by us, and (viii) that each party to the Placement Agreement, the Purchase Agreement and the Warrant Agreement (other than the Company) will have duly authorized, executed and delivered such agreements or instruments and complied with all legal requirements pertaining to its status as such status relates to the right to enforce such agreements or instruments against the Company and will have satisfied those legal requirements applicable to it to the extent necessary to make such agreements or instruments enforceable against it. We have not independently established or verified any facts relevant to the opinions expressed herein, but have relied upon certificates, statements and representations of officers and other representatives of the Company, public officials and others as to factual matters.
Based upon and subject to the foregoing qualifications, assumptions and limitations and the further limitations set forth below, we are of the opinion that:
1. Upon payment therefor and issuance and delivery thereof in accordance with the Placement Agreement and the Purchase Agreement, including the book entry registration and issuance thereof by the Company’s transfer agent and registrar, the Common Stock Shares will be validly issued, fully paid and non-assessable.
2. Provided that (a) the Warrants have been duly executed and delivered by the Company to the purchasers thereof against payment therefor in accordance with and as provided in resolutions adopted by the Company’s Board of Directors or its Pricing Committee and the Placement Agreement, the Purchase Agreement, the Registration Statement and the Prospectus, the Warrants will be valid and binding obligations of the Company, except as the same may be limited by applicable bankruptcy, insolvency, voidable transactions, fraudulent conveyance, fraudulent transfer, reorganization, moratorium, assignment for the benefit of creditors and other laws now or hereafter in effect relating to or affecting creditors’ rights generally and equitable principles of general applicability (regardless of whether considered in a proceeding in equity or at law).
3. Following (i) execution and delivery by the Company of the Warrants pursuant to the terms of the Placement Agreement and the Purchase Agreement, (ii) receipt by the Company of the consideration for the Warrants specified in the resolutions of the Company’s Board of Directors or its Pricing Committee, and (iii) exercise of the Warrants pursuant to their terms, receipt by the Company of any exercise price for the Warrant Shares as specified in the Warrants and issuance by the Company of the Warrant Shares thereunder, including the book entry registration and issuance thereof by the Company’s transfer agent and registrar, the Warrant Shares will be validly issued, fully paid, and nonassessable.
Without limiting any other qualifications set forth herein, the opinion expressed herein regarding the enforceability of the Warrants is subject to the effect of generally applicable laws that (i) provide for the enforcement of oral waivers or modifications where a material change of position in reliance thereon has occurred or provide that a course of performance may operate as a waiver; (ii) limit the availability of a remedy under certain circumstances where another remedy has been elected; (iii) limit the enforceability of provisions releasing, exculpating or exempting a party from, or requiring indemnification of or contribution to a party for, liability for its own action or inaction, to the extent the action or inaction involves negligence, recklessness, willful misconduct or unlawful conduct or insofar as such provisions otherwise contravene public policy; (iv) may, where less than all of a contract may be unenforceable, limit the enforceability of the balance of the contract to circumstances in which the unenforceable portion is not an essential part of the agreed exchange; (v) govern and afford judicial discretion regarding the determination of damages and entitlement to attorneys’ fees and other costs; (vi) may permit a party who has materially failed to render or offer performance required by a contract to cure that failure unless either permitting a cure would unreasonably hinder the aggrieved party from making substitute arrangements for performance or it is important under the circumstances to the aggrieved party that performance occur by the date stated in the contract; (vii) may limit the enforceability of provisions for the payment of premiums upon mandatory prepayment to the extent any such payment constitutes, or is deemed to constitute, a penalty or forfeiture; (viii) may require mitigation of damages; (ix) provide a time limitation after which a remedy may not be enforced (i.e., statutes of limitation); and (x) limit the enforceability of provisions of instruments or agreements that purport to require waiver of the obligations of good faith, fair dealing, diligence and reasonableness.
We express no opinion as to the enforceability or effect in the Warrants of (i) any agreement to submit to the jurisdiction of any particular court or other governmental authority (either as to personal jurisdiction or subject matter jurisdiction), any provision restricting access to courts (including, without limitation, agreements to arbitrate disputes), any waivers of the right to jury trial, any waivers of service of process requirements that would otherwise be applicable, any agreement that a judgment rendered by a court in one jurisdiction may be enforced in another jurisdiction, or any provision otherwise affecting the jurisdiction or venue of courts; (ii) any provision waiving legal, statutory or equitable defenses or other procedural, judicial or substantive rights; or (iii) any provision that authorizes one party to act as attorney-in-fact for another party.
With respect to our opinion regarding the Warrant Shares, we express no opinion to the extent that, notwithstanding the Company’s current reservation of the maximum number of Warrant Shares as of the date hereof, future issuances of securities of the Company, including the Warrant Shares, and/or antidilution adjustments to outstanding securities of the Company, including the Warrants, may cause the Warrants to be exercisable for more shares of Common Stock than the number that then remain authorized but unissued. Further, we have assumed the Exercise Price (as defined in the Warrants) will not be adjusted to an amount below the par value per share of the Warrant Shares.
This opinion is limited to the General Corporation Law of the State of Delaware and, solely with respect to opinion paragraph 2, the laws of the State of New York. We express no opinion as to any other matters, including without limitation any matters relating to the securities or blue sky laws of any jurisdiction or any rules or regulations thereunder, and no opinion may be inferred or implied beyond that expressly stated herein. In addition, we express no opinion as to whether, or the extent to which, the laws of any particular jurisdiction apply to the subject matter hereof, including, without limitation, the enforceability of the governing law provision contained in the Warrants.
We hereby consent to the filing of this opinion letter with the Commission as Exhibit 5.1 to the Registration Statement. We also consent to the reference to our firm under the caption “Legal Matters” in the Prospectus. In giving this consent, we do not hereby admit that we come within the categories of persons whose consent is required under Section 7 of the Act or under the rules and regulations of the Commission issued thereunder.
This opinion letter is given as of the date hereof, and we assume no responsibility for updating this opinion letter or the opinions or statements set forth herein to take into account any event, action, interpretation or change in facts or law occurring subsequent to the date hereof that may affect the validity of any of such opinions or statements.
| |
Very truly yours, |
| |
|
| |
FAEGRE DRINKER BIDDLE & REATH LLP |
| |
|
| |
/s/ Faegre Drinker Biddle & Reath LLP |
Exhibit 10.30
SECURITIES PURCHASE AGREEMENT
This Securities Purchase Agreement (this “Agreement”) is dated as of , 2024, between Panbela Therapeutics, Inc., a Delaware corporation (the “Company”), and each purchaser identified on the signature pages hereto (each, including its successors and assigns, a “Purchaser” and collectively the “Purchasers”).
WHEREAS, subject to the terms and conditions set forth in this Agreement and pursuant to an effective registration statement under the Securities Act (as defined below), the Company desires to issue and sell to each Purchaser, and each Purchaser, severally and not jointly, desires to purchase from the Company, securities of the Company as more fully described in this Agreement.
NOW, THEREFORE, IN CONSIDERATION of the mutual covenants contained in this Agreement, and for other good and valuable consideration the receipt and adequacy of which are hereby acknowledged, the Company and each Purchaser agree as follows:
ARTICLE I.
DEFINITIONS
1.1 Definitions. In addition to the terms defined elsewhere in this Agreement, for all purposes of this Agreement, the following terms have the meanings set forth in this Section 1.1:
“Acquiring Person” shall have the meaning ascribed to such term in Section 4.5.
“Action” shall have the meaning ascribed to such term in Section 3.1(j).
“Affiliate” means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with a Person as such terms are used in and construed under Rule 405 under the Securities Act.
“Board of Directors” means the board of directors of the Company.
“Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”, “non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The City of New York are generally open for use by customers on such day.
“Class E Common Warrants” means, collectively, the Common Stock purchase warrants delivered to the Purchasers at the Closing in accordance with Section 2.2(a) hereof, which Common Warrants shall be exercisable immediately and have a term of exercise equal to five (5) years, in the form of Exhibit A attached hereto.
“Class E Common Warrant Shares” means the shares of Common Stock issuable upon exercise of the Class E Common Warrants.
“Class F Common Warrants” means, collectively, the Common Stock purchase warrants delivered to the Purchasers at the Closing in accordance with Section 2.2(a) hereof, which Common Warrants shall be exercisable immediately and have a term of exercise equal to five (5) years, in the form of Exhibit B attached hereto.
“Class F Common Warrant Shares” means the shares of Common Stock issuable upon exercise of the Class F Common Warrants.
“Closing” means the closing of the purchase and sale of the Securities pursuant to Section 2.1.
“Closing Date” means the Trading Day on which all of the Transaction Documents have been executed and delivered by the applicable parties thereto, and all conditions precedent to (i) the Purchasers’ obligations to pay the Subscription Amount and (ii) the Company’s obligations to deliver the Securities, in each case, have been satisfied or waived, but in no event later than the second (2nd) Trading Day following the date hereof.
“Commission” means the United States Securities and Exchange Commission.
“Common Stock” means the common stock of the Company, par value $0.001 per share, and any other class of securities into which such securities may hereafter be reclassified or changed.
“Common Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle the holder thereof to acquire at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Common Warrants” means, collectively, the Class E Common Warrants and the Class F Common Warrants.
“Common Warrant Shares” means, collectively, the Class E Common Warrant Shares and the Class F Common Warrant Shares.
“Company Counsel” means Faegre Drinker Biddle & Reath LLP, with offices located at 90 South Seventh Street, 2200 Wells Fargo Center, Minneapolis, Minnesota 55402-3901.
“Disclosure Schedules” means the Disclosure Schedules of the Company delivered concurrently herewith.
“Disclosure Time” means, (i) if this Agreement is signed on a day that is not a Trading Day or after 9:00 a.m. (New York City time) and before midnight (New York City time) on any Trading Day, 9:01 a.m. (New York City time) on the Trading Day immediately following the date hereof, unless otherwise instructed as to an earlier time by the Placement Agent, and (ii) if this Agreement is signed between midnight (New York City time) and 9:00 a.m. (New York City time) on any Trading Day, no later than 9:01 a.m. (New York City time) on the date hereof, unless otherwise instructed as to an earlier time by the Placement Agent.
“Evaluation Date” shall have the meaning ascribed to such term in Section 3.1(s).
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Exempt Issuance” means the issuance of (a) shares of Common Stock, options, restricted stock units or other equity-based awards to employees, officers or directors of the Company or its subsidiaries pursuant to any compensation plan duly adopted for such purpose, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose for services rendered to the Company, (b) securities upon the exercise or exchange of or conversion of any Securities issued hereunder and/or other securities exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of this Agreement, provided that such securities have not been amended since the date of this Agreement to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock splits or combinations) or to extend the term of such securities, and (c) securities issued pursuant to acquisitions or strategic transactions (including, without limitation, joint venture, co-marketing, co-development or other collaboration agreements) approved by a majority of the disinterested directors of the Company, provided that such securities are issued as “restricted securities” (as defined in Rule 144) and carry no registration rights that require or permit the filing of any registration statement in connection therewith during the prohibition period in Section 4.11(a) herein, and provided that any such issuance shall only be to a Person (or to the equityholders of a Person) which is, itself or through its subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of the Company and shall provide to the Company additional benefits in addition to the investment of funds, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities.
“FCPA” means the Foreign Corrupt Practices Act of 1977, as amended.
“FDA” shall have the meaning ascribed to such term in Section 3.1(hh).
“FDCA” shall have the meaning ascribed to such term in Section 3.1(hh).
“GAAP” shall have the meaning ascribed to such term in Section 3.1(h).
“Indebtedness” shall have the meaning ascribed to such term in Section 3.1(aa).
“Intellectual Property Rights” shall have the meaning ascribed to such term in Section 3.1(p).
“Liens” means a lien, charge, pledge, security interest, encumbrance, right of first refusal, preemptive right or other restriction.
“Lock-Up Agreement” means the Lock-Up Agreement, dated as of the date hereof, by and among the Company and the directors, officers and 5% stockholders of the Company, in the form of Exhibit B attached hereto.
“Material Adverse Effect” shall have the meaning assigned to such term in Section 3.1(b).
“Material Permits” shall have the meaning ascribed to such term in Section 3.1(n).
“PC” means Pryor Cashman LLP, with offices located at 7 Times Square, New York, New York 10036.
“Per Share Purchase Price” equals $ , subject to adjustment for reverse and forward stock splits, stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement, provided that the purchase price per Pre-Funded Warrant shall be the Per Share Purchase Price minus $0.001.
“Person” means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Placement Agent” means Roth Capital Partners, LLC.
“Pre-Funded Warrant” means, collectively, the Pre-Funded Common Stock purchase warrants delivered to the Purchasers at the Closing in accordance with Section 2.2(a) hereof, which Pre-Funded Warrants shall be exercisable immediately and shall expire when exercised in full, in the form of Exhibit C attached hereto.
“Pre-Funded Warrant Shares” means the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants.
“Preliminary Prospectus” means any preliminary prospectus included in the Registration Statement, as originally filed or as part of any amendment thereto, or filed with the Commission pursuant to Rule 424(a) of the rules and regulations of the Commission under the Securities Act.
“Proceeding” means an action, claim, suit, investigation or proceeding (including, without limitation, an informal investigation or partial proceeding, such as a deposition), whether commenced or, to the Company’s knowledge, threatened.
“Product” shall have the meaning ascribed to such term in Section 3.1(hh).
“Prospectus” means the final pricing prospectus filed for the Registration Statement.
“Purchaser Party” shall have the meaning ascribed to such term in Section 4.8.
“Registration Statement” means the effective registration statement with the Commission (File No. 333-276367) which registers the sale of the Shares, the Warrants and the Warrant Shares to the Purchasers, and includes any Rule 462(b) Registration Statement.
“Required Approvals” shall have the meaning ascribed to such term in Section 3.1(e).
“Rule 144” means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
“Rule 424” means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
“Rule 462(b) Registration Statement” means any registration statement prepared by the Company registering additional Securities, which was filed with the Commission on or prior to the date hereof and became automatically effective pursuant to Rule 462(b) promulgated by the Commission pursuant to the Securities Act, if applicable.
“SEC Reports” shall have the meaning ascribed to such term in Section 3.1(h).
“Securities” means the Shares, the Warrants and the Warrant Shares.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Shares” means the shares of Common Stock issued or issuable to each Purchaser pursuant to this Agreement.
“Short Sales” means all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act (but shall not be deemed to include locating and/or borrowing shares of Common Stock).
“Subscription Amount” means, as to each Purchaser, the aggregate amount to be paid for Shares and Warrants purchased hereunder as specified below such Purchaser’s name on the signature page of this Agreement and next to the heading “Subscription Amount,” in United States dollars and in immediately available funds (minus, if applicable, a Purchaser’s aggregate exercise price of the Pre-Funded Warrants, which amounts shall be paid as and when such Pre-Funded Warrants are exercised for cash).
“Subsidiary” means any subsidiary of the Company as set forth in the SEC Reports, and shall, where applicable, also include any direct or indirect subsidiary of the Company formed or acquired after the date hereof.
“Trading Day” means a day on which the principal Trading Market is open for trading.
“Trading Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market or the New York Stock Exchange (or any successors to any of the foregoing).
“Transaction Documents” means this Agreement, the Lock-Up Agreement, the Warrants, all exhibits and schedules thereto and hereto and any other documents or agreements executed in connection with the transactions contemplated hereunder.
“Transfer Agent” means VStock Transfer, the current transfer agent of the Company, with a mailing address of 18 Lafayette Place, Woodmere, New York 11598, and any successor transfer agent of the Company.
“Variable Rate Transaction” shall have the meaning ascribed to such term in Section 4.11(b).
“Warrants” means, collectively, the Common Warrants and the Pre-Funded Warrants.
“Warrant Shares” means, collectively, the Common Warrant Shares and the Pre-Funded Warrant Shares.
ARTICLE II.
PURCHASE AND SALE
2.1 Closing. On the Closing Date, upon the terms and subject to the conditions set forth herein, substantially concurrent with the execution and delivery of this Agreement by the parties hereto, the Company agrees to sell, and the Purchasers, severally and not jointly, agree to purchase, up to an aggregate of $ of Shares and Common Warrants; provided, however, that, to the extent that a Purchaser determines, in its sole discretion, that such Purchaser (together with such Purchaser’s Affiliates, and any Person acting as a group together with such Purchaser or any of such Purchaser’s Affiliates) would beneficially own in excess of the Beneficial Ownership Limitation, or as such Purchaser may otherwise choose, in lieu of purchasing Shares such Purchaser may elect to purchase Pre-Funded Warrants in lieu of Shares in such manner to result in the same aggregate purchase price being paid by such Purchaser to the Company. The “Beneficial Ownership Limitation” shall be 4.99% (or, at the election of the Purchaser at Closing, 9.99%) of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of the Securities on the Closing Date. Each Purchaser’s Subscription Amount as set forth on the signature page hereto executed by such Purchaser shall be made available for “Delivery Versus Payment” (“DVP”) settlement with the Company or its designee. The Company shall deliver to each Purchaser its respective Shares and Common Warrants and/or Pre-Funded Warrants (as applicable to such Purchaser) and Common Warrants as determined pursuant to Section 2.2(a), and the Company and each Purchaser shall deliver the other items set forth in Section 2.2 deliverable at the Closing. Upon satisfaction of the covenants and conditions set forth in Sections 2.2 and 2.3, the Closing shall occur at the offices of PC or such other location as the parties shall mutually agree take place remotely by electronic transfer of the Closing documentation. Unless otherwise directed by the Placement Agent, settlement of the Shares shall occur via DVP (i.e., on the Closing Date, the Company shall issue the Shares registered in the Purchasers’ names and addresses and released by the Transfer Agent directly to the account(s) at the Placement Agent identified by each Purchaser; upon receipt of such Shares, the Placement Agent shall promptly electronically deliver such Shares to the applicable Purchaser, and payment therefor shall be made by the Placement Agent (or its clearing firm) by wire transfer to the Company). To the extent that a Purchaser’s beneficial ownership of the Shares would otherwise be deemed to exceed the Beneficial Ownership Maximum, such Purchaser’s Subscription Amount shall automatically be reduced as necessary in order to comply with this paragraph. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise (as defined in the Pre-Funded Warrants) delivered on or prior to 12:00 p.m. (New York City time) on the Closing Date, which may be delivered at any time after the time of execution of the this Agreement, the Company agrees to deliver the Warrant Shares subject to such notice(s) by 4:00 p.m. (New York City time) on the Closing Date and the Closing Date shall be the Warrant Share Delivery Date (as defined in the Pre-Funded Warrants) for purposes hereunder. Notwithstanding anything herein to the contrary, if at any time on or after the time of execution of this Agreement by the Company and an applicable Purchaser, through, and including the time immediately prior to the Closing (the “Pre-Settlement Period”), such Purchaser sells to any Person all, or any portion, of the Shares to be issued hereunder to such Purchaser at the Closing (collectively, the “Pre-Settlement Shares”), such Purchaser shall, automatically hereunder (without any additional required actions by such Purchaser or the Company), be deemed to be unconditionally bound to purchase, such Pre-Settlement Shares to such Purchaser at the Closing, and the Company shall be deemed unconditionally bound to sell, such Pre-Settlement Shares to such Purchaser at the Closing; provided, that the Company shall not be required to deliver any Pre-Settlement Shares to such Purchaser prior to the Company’s receipt of the purchase price of such Pre-Settlement Shares hereunder; and provided further that the Company hereby acknowledges and agrees that the forgoing shall not constitute a representation or covenant by such Purchaser as to whether or not during the Pre-Settlement Period such Purchaser shall sell any shares of Common Stock to any Person and that any such decision to sell any shares of Common Stock by such Purchaser shall solely be made at the time such Purchaser elects to effect any such sale, if any.
2.2 Deliveries.
(a) On or prior to the Closing Date (except as indicated below), the Company shall deliver or cause to be delivered to each Purchaser the following:
(i) this Agreement duly executed by the Company;
(ii) a legal opinion of Company Counsel, in a form reasonably acceptable to the Placement Agent and the Purchasers;
(iii) subject to the seventh sentence of Section 2.1, the Company shall have provided each Purchaser with the Company’s wire instructions, on Company letterhead and executed by the Chief Executive Officer or Chief Financial Officer;
(iv) subject to the seventh sentence of Section 2.1, a copy of the irrevocable instructions to the Transfer Agent instructing the Transfer Agent to deliver on an expedited basis via The Depository Trust Company Deposit or Withdrawal at Custodian system (“DWAC”) Shares equal to such Purchaser’s Subscription Amount divided by the Per Share Purchase Price, registered in the name of such Purchaser;
(v) a Class E Common Warrant registered in the name of such Purchaser to purchase up to a number of shares of Common Stock equal to 25% of such Purchaser’s Shares plus Pre-Funded Warrant Shares initially issuable upon exercise of the Pre-Funded Warrants, if applicable, with an exercise price equal to $ , subject to adjustment therein;
(vi) a Class F Common Warrant registered in the name of such Purchaser to purchase up to a number of shares of Common Stock equal to 175% of such Purchaser’s Shares plus Pre-Funded Warrant Shares initially issuable upon exercise of the Pre-Funded Warrants, if applicable, with an exercise price equal to $ , subject to adjustment therein;
(vii) for each Purchaser of Pre-Funded Warrants pursuant to Section 2.1, a Pre-Funded Warrant registered in the name of such Purchaser to purchase up to a number of shares of Common Stock equal to the portion of such Purchaser’s Subscription Amount applicable to Pre-Funded Warrant divided by the Per Share Purchase Price minus $0.001, with an exercise price equal to $0.001, subject to adjustment therein;
(viii) on the date hereof, the duly executed Lock-Up Agreements; and
(ix) the Preliminary Prospectus and the Prospectus (which may be delivered in accordance with Rule 172 under the Securities Act).
(b) On or prior to the Closing Date, each Purchaser shall deliver or cause to be delivered to the Company the following:
(i) this Agreement duly executed by such Purchaser; and
(ii) such Purchaser’s Subscription Amount, which shall be made available for “Delivery Versus Payment” settlement with the Company or its designee.
2.3 Closing Conditions.
(a) The obligations of the Company hereunder in connection with the Closing are subject to the following conditions being met:
(i) the accuracy in all material respects (or, to the extent representations or warranties are qualified by materiality or Material Adverse Effect, in all respects) on the Closing Date of the representations and warranties of the Purchasers contained herein (unless as of a specific date therein in which case they shall be accurate as of such date);
(ii) all obligations, covenants and agreements of each Purchaser required to be performed at or prior to the Closing Date shall have been performed in all material respects; and
(iii) the delivery by each Purchaser of the items set forth in Section 2.2(b) of this Agreement.
(b) The respective obligations of the Purchasers hereunder in connection with the Closing are subject to the following conditions being met:
(i) the accuracy in all material respects (or, to the extent representations or warranties are qualified by materiality or Material Adverse Effect, in all respects) when made and on the Closing Date of the representations and warranties of the Company contained herein (unless as of a specific date therein in which case they shall be accurate as of such date);
(ii) all obligations, covenants and agreements of the Company required to be performed at or prior to the Closing Date shall have been performed in all material respects;
(iii) the delivery by the Company of the items set forth in Section 2.2(a) of this Agreement;
(iv) there shall have been no Material Adverse Effect with respect to the Company since the date hereof; and
(v) from the date hereof to the Closing Date, trading in the Common Stock shall not have been suspended by the Commission or the Company’s principal Trading Market, and, at any time prior to the Closing Date, trading in securities generally as reported by Bloomberg L.P. shall not have been suspended or limited, or minimum prices shall not have been established on securities whose trades are reported by such service, or on any Trading Market, nor shall a banking moratorium have been declared either by the United States or New York State authorities nor shall there have occurred any material outbreak or escalation of hostilities or other national or international calamity of such magnitude in its effect on, or any material adverse change in, any financial market which, in each case, in the reasonable judgment of such Purchaser, makes it impracticable or inadvisable to purchase the Securities at the Closing.
ARTICLE III.
REPRESENTATIONS AND WARRANTIES
3.1 Representations and Warranties of the Company. Except as set forth in the Disclosure Schedules, which Disclosure Schedules shall be deemed a part hereof and shall qualify any representation or otherwise made herein to the extent of the disclosure contained in the corresponding section of the Disclosure Schedules, the Company hereby makes the following representations and warranties to each Purchaser:
(a) Subsidiaries. All of the direct and indirect subsidiaries of the Company are set forth in the SEC Reports. The Company owns, directly or indirectly, all of the capital stock or other equity interests of each Subsidiary free and clear of any Liens, and all of the issued and outstanding shares of capital stock of each Subsidiary are validly issued and are fully paid, non-assessable and free of preemptive and similar rights to subscribe for or purchase securities.
(b) Organization and Qualification. The Company and each of the Subsidiaries is an entity duly incorporated or otherwise organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization, with the requisite power and authority to own and use its properties and assets and to carry on its business as currently conducted. Neither the Company nor any Subsidiary is in violation nor default of any of the provisions of its respective certificate or articles of incorporation, bylaws or other organizational or charter documents. Each of the Company and the Subsidiaries is duly qualified to conduct business and is in good standing as a foreign corporation or other entity in each jurisdiction in which the nature of the business conducted or property owned by it makes such qualification necessary, except where the failure to be so qualified or in good standing, as the case may be, could not have or reasonably be expected to result in: (i) a material adverse effect on the legality, validity or enforceability of any Transaction Document, (ii) a material adverse effect on the results of operations, assets, business, prospects or condition (financial or otherwise) of the Company and the Subsidiaries, taken as a whole, or (iii) a material adverse effect on the Company’s ability to perform in any material respect on a timely basis its obligations under any Transaction Document (any of (i), (ii) or (iii), a “Material Adverse Effect”) and, to the Company’s knowledge, no Proceeding has been instituted in any such jurisdiction revoking, limiting or curtailing or seeking to revoke, limit or curtail such power and authority or qualification.
(c) Authorization; Enforcement. The Company has the requisite corporate power and authority to enter into and to consummate the transactions contemplated by this Agreement and each of the other Transaction Documents to which the Company is a party and otherwise to carry out its obligations hereunder and thereunder. The execution and delivery of this Agreement and each of the other Transaction Documents by the Company and the consummation by it of the transactions contemplated hereby and thereby have been duly authorized by all necessary action on the part of the Company and no further action is required by the Company, the Board of Directors or the Company’s stockholders in connection herewith or therewith other than in connection with the Required Approvals. This Agreement and each other Transaction Document to which the Company is a party has been (or upon delivery will have been) duly executed by the Company and, when delivered in accordance with the terms hereof and thereof, will constitute the valid and binding obligation of the Company enforceable against the Company in accordance with its terms, except (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by applicable law.
(d) No Conflicts. The execution, delivery and performance by the Company of this Agreement and the other Transaction Documents to which it is a party, the issuance and sale of the Securities and the consummation by it of the transactions contemplated hereby and thereby do not and will not (i) conflict with or violate any provision of the Company’s or any Subsidiary’s certificate or articles of incorporation, bylaws or other organizational or charter documents, or (ii) conflict with, or constitute a default (or an event that with notice or lapse of time or both would become a default) under, result in the creation of any Lien upon any of the properties or assets of the Company or any Subsidiary, or give to others any rights of termination, amendment, anti-dilution or similar adjustments, acceleration or cancellation (with or without notice, lapse of time or both) of, any agreement, credit facility, debt or other instrument (evidencing a Company or Subsidiary debt or otherwise) or other understanding to which the Company or any Subsidiary is a party or by which any property or asset of the Company or any Subsidiary is bound or affected, or (iii) subject to the Required Approvals, conflict with or result in a violation of any law, rule, regulation, order, judgment, injunction, decree or other restriction of any court or governmental authority to which the Company or a Subsidiary is subject (including federal and state securities laws and regulations), or by which any property or asset of the Company or a Subsidiary is bound or affected; except in the case of each of clauses (ii) and (iii), such as could not have or reasonably be expected to result in a Material Adverse Effect.
(e) Filings, Consents and Approvals. The Company is not required to obtain any consent, waiver, authorization or order of, give any notice to, or make any filing or registration with, any court or other federal, state, local or other governmental authority or other Person in connection with the execution, delivery and performance by the Company of the Transaction Documents, other than: (i) the filings required pursuant to Section 4.4 of this Agreement, (ii) the filing with the Commission of the Prospectus, (iii) notice(s) to each applicable Trading Market for the listing of the Shares and Warrant Shares for trading thereon, and (iv) such filings as are required to be made under applicable state securities laws (collectively, the “Required Approvals”).
(f) Issuance of the Securities; Registration. The Securities are duly authorized and, when issued and paid for in accordance with the applicable Transaction Documents, will be duly and validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company other than restrictions on transfer provided for in the Transaction Documents. The Warrant Shares, when issued in accordance with the terms of the Warrants, will be validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company other than restrictions on transfer provided for in the Transaction Documents. The Company has reserved from its duly authorized capital stock the maximum number of shares of Common Stock issuable pursuant to this Agreement and the Warrants as of the date hereof. The Company has prepared and filed the Registration Statement in conformity with the requirements of the Securities Act, which became effective on , 2024 (the “Effective Date”), including the Prospectus, and such amendments and supplements thereto as may have been required to the date of this Agreement. The Registration Statement is effective under the Securities Act and no stop order preventing or suspending the effectiveness of the Registration Statement or suspending or preventing the use of the Preliminary Prospectus or the Prospectus has been issued by the Commission and no proceedings for that purpose have been instituted or, to the knowledge of the Company, are threatened by the Commission. The Company, if required by the rules and regulations of the Commission, shall file the Prospectus with the Commission pursuant to Rule 424(b). At the time the Registration Statement and any amendments thereto became effective, at the date of this Agreement and at the Closing Date, the Registration Statement and any amendments thereto conformed and will conform in all material respects to the requirements of the Securities Act and did not and will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein not misleading; and the Preliminary Prospectus and the Prospectus and any amendments or supplements thereto, at the time the Preliminary Prospectus or the Prospectus, as applicable, or any amendment or supplement thereto was issued and at the Closing Date, conformed and will conform in all material respects to the requirements of the Securities Act and did not and will not contain an untrue statement of a material fact or omit to state a material fact necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading.
(g) Capitalization. The capitalization of the Company as of the date hereof is as set forth on Schedule 3.1(g), which Schedule 3.1(g) shall also include the number of shares of Common Stock owned beneficially, and of record, by Affiliates of the Company as of the date hereof. Except for the transactions contemplated by the Sales Agreement between the Company and Roth Capital Partners, LLC, the Company has not issued any capital stock since the filing of its most recently filed periodic report under the Exchange Act, other than pursuant to the exercise of employee stock options or settlement of restricted stock units, the issuance of equity-based awards pursuant to the Company’s equity compensation plans and pursuant to the conversion and/or exercise of Common Stock Equivalents outstanding as of the filing date of the most recently filed periodic report under the Exchange Act. No Person has any right of first refusal, preemptive right, right of participation, or any similar right to participate in the transactions contemplated by the Transaction Documents. Except as a result of the purchase and sale of the Securities and as set forth on Schedule 3.1(g), there are no outstanding options, warrants, scrip rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities, rights or obligations convertible into or exercisable or exchangeable for, or giving any Person any right to subscribe for or acquire, any shares of Common Stock or the capital stock of any Subsidiary, or contracts, commitments, understandings or arrangements by which the Company or any Subsidiary is or may become bound to issue additional shares of Common Stock or Common Stock Equivalents or the capital stock of any Subsidiary. The issuance and sale of the Securities will not obligate the Company or any Subsidiary to issue shares of Common Stock or other securities to any Person (other than the Purchasers). There are no outstanding securities or instruments of the Company or any Subsidiary with any provision that adjusts the exercise, conversion, exchange or reset price of such security or instrument upon an issuance of securities by the Company or any Subsidiary. There are no outstanding securities or instruments of the Company or any Subsidiary that contain any redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any Subsidiary is or may become bound to redeem a security of the Company or such Subsidiary. The Company does not have any stock appreciation rights or “phantom stock” plans or agreements or any similar plan or agreement. All of the outstanding shares of capital stock of the Company are duly authorized, validly issued, fully paid and nonassessable, have been issued in compliance with all federal and state securities laws, and none of such outstanding shares was issued in violation of any preemptive rights or similar rights to subscribe for or purchase securities. No further approval or authorization of any stockholder, the Board of Directors or others is required for the issuance and sale of the Securities. There are no stockholders agreements, voting agreements or other similar agreements with respect to the Company’s capital stock to which the Company is a party or, to the knowledge of the Company, between or among any of the Company’s stockholders.
(h) SEC Reports; Financial Statements. The Company has filed all reports, schedules, forms, statements and other documents required to be filed by the Company under the Securities Act and the Exchange Act, including pursuant to Section 13(a) or 15(d) thereof, for the two years preceding the date hereof (or such shorter period as the Company was required by law or regulation to file such material) (the foregoing materials, including the exhibits thereto and documents incorporated by reference therein, together with the Preliminary Prospectus and the Prospectus, being collectively referred to herein as the “SEC Reports”) on a timely basis or has received a valid extension of such time of filing and has filed any such SEC Reports prior to the expiration of any such extension, except as could not have or reasonably be expected to result in a Material Adverse Effect. As of their respective dates, the SEC Reports complied in all material respects with the requirements of the Securities Act and the Exchange Act, as applicable, and none of the SEC Reports, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. The financial statements of the Company included in the SEC Reports comply in all material respects with applicable accounting requirements and the rules and regulations of the Commission with respect thereto as in effect at the time of filing. Such financial statements have been prepared in accordance with United States generally accepted accounting principles applied on a consistent basis during the periods involved (“GAAP”), except as may be otherwise specified in such financial statements or the notes thereto and except that unaudited financial statements may not contain all footnotes required by GAAP, and fairly present in all material respects the financial position of the Company and its consolidated Subsidiaries as of and for the dates thereof and the results of operations and cash flows for the periods then ended, subject, in the case of unaudited statements, to normal, immaterial, year-end audit adjustments.
(i) Material Changes; Undisclosed Events, Liabilities or Developments. Since the date of the latest audited financial statements included within the SEC Reports, except as set forth on Schedule 3.1(i), (i) there has been no event, occurrence or development that has had or that could reasonably be expected to result in a Material Adverse Effect, (ii) the Company has not incurred any material liabilities (contingent or otherwise) other than (A) trade payables and accrued expenses incurred in the ordinary course of business consistent with past practice and (B) liabilities not required to be reflected in the Company’s financial statements pursuant to GAAP or disclosed in filings made with the Commission, (iii) the Company has not altered its method of accounting, (iv) the Company has not declared or made any dividend or distribution of cash or other property to its stockholders or purchased, redeemed or made any agreements to purchase or redeem any shares of its capital stock and (v) the Company has not issued any equity securities to any officer, director or Affiliate, except pursuant to existing Company stock option plans. The Company does not have pending before the Commission any request for confidential treatment of information. Except for the issuance of the Securities contemplated by this Agreement, no event, liability, fact, circumstance, occurrence or development has occurred or exists or is reasonably expected to occur or exist with respect to the Company or its Subsidiaries or their respective businesses, prospects, properties, operations, assets or financial condition that would be required to be disclosed by the Company under applicable securities laws at the time this representation is made or deemed made that has not been publicly disclosed at least 1 Trading Day prior to the date that this representation is made.
(j) Litigation. There is no action, suit, inquiry, notice of violation, proceeding or investigation pending or, to the knowledge of the Company, threatened against or affecting the Company, any Subsidiary or any of their respective properties before or by any court, arbitrator, governmental or administrative agency or regulatory authority (federal, state, county, local or foreign) (collectively, an “Action”) which (i) adversely affects or challenges the legality, validity or enforceability of any of the Transaction Documents or the Securities or (ii) could, if there were an unfavorable decision, have or reasonably be expected to result in a Material Adverse Effect. Neither the Company nor any Subsidiary, nor any director or officer thereof, is or has been the subject of any Action involving a claim of violation of or liability under federal or state securities laws or a claim of breach of fiduciary duty. There has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the Commission involving the Company or any current or former director or officer of the Company. The Commission has not issued any stop order or other order suspending the effectiveness of any registration statement filed by the Company or any Subsidiary under the Exchange Act or the Securities Act.
(k) Labor Relations. No labor dispute exists or, to the knowledge of the Company, is imminent with respect to any of the employees of the Company, which could reasonably be expected to result in a Material Adverse Effect. None of the Company’s or its Subsidiaries’ employees is a member of a union that relates to such employee’s relationship with the Company or such Subsidiary, and neither the Company nor any of its Subsidiaries is a party to a collective bargaining agreement, and the Company and its Subsidiaries believe that their relationships with their employees are good. To the knowledge of the Company, no executive officer of the Company or any Subsidiary, is, or is now expected to be, in violation of any material term of any employment contract, confidentiality, disclosure or proprietary information agreement or non-competition agreement, or any other contract or agreement or any restrictive covenant in favor of any third party, and the continued employment of each such executive officer does not subject the Company or any of its Subsidiaries to any liability with respect to any of the foregoing matters. The Company and its Subsidiaries are in compliance with all U.S. federal, state, local and foreign laws and regulations relating to employment and employment practices, terms and conditions of employment and wages and hours, except where the failure to be in compliance could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect.
(l) Compliance. Neither the Company nor any Subsidiary: (i) is in default under or in violation of (and no event has occurred that has not been waived that, with notice or lapse of time or both, would result in a default by the Company or any Subsidiary under), nor has the Company or any Subsidiary received notice of a claim that it is in default under or that it is in violation of, any indenture, loan or credit agreement or any other agreement or instrument to which it is a party or by which it or any of its properties is bound (whether or not such default or violation has been waived), (ii) is in violation of any judgment, decree or order of any court, arbitrator or other governmental authority or (iii) is or has been in violation of any statute, rule, ordinance or regulation of any governmental authority, including without limitation all foreign, federal, state and local laws relating to taxes, environmental protection, occupational health and safety, product quality and safety and employment and labor matters, except in each case as could not have or reasonably be expected to result in a Material Adverse Effect.
(m) Environmental Laws. The Company and its Subsidiaries (i) are in compliance with all federal, state, local and foreign laws relating to pollution or protection of human health or the environment (including ambient air, surface water, groundwater, land surface or subsurface strata), including laws relating to emissions, discharges, releases or threatened releases of chemicals, pollutants, contaminants, or toxic or hazardous substances or wastes (collectively, “Hazardous Materials”) into the environment, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials, as well as all authorizations, codes, decrees, demands, or demand letters, injunctions, judgments, licenses, notices or notice letters, orders, permits, plans or regulations, issued, entered, promulgated or approved thereunder (“Environmental Laws”); (ii) have received all permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses; and (iii) are in compliance with all terms and conditions of any such permit, license or approval where in each of clause (i), (ii) and (iii), the failure to so comply could be reasonably expected to have, individually or in the aggregate, a Material Adverse Effect.
(n) Regulatory Permits. The Company and the Subsidiaries possess all certificates, authorizations and permits issued by the appropriate federal, state, local or foreign regulatory authorities necessary to conduct their respective businesses as described in the SEC Reports, except where the failure to possess such permits could not reasonably be expected to result in a Material Adverse Effect (“Material Permits”), and neither the Company nor any Subsidiary has received any notice of proceedings relating to the revocation or modification of any Material Permit.
(o) Title to Assets. The Company and the Subsidiaries have good and marketable title in fee simple to, or have valid and marketable rights to lease or otherwise use, all real property and all personal property owned by them that is material to the business of the Company and the Subsidiaries, in each case free and clear of all Liens, except for (i) Liens as do not materially affect the value of such property and do not materially interfere with the use made and proposed to be made of such property by the Company and the Subsidiaries and (ii) Liens for the payment of federal, state or other taxes, for which appropriate reserves have been made therefor in accordance with GAAP and, the payment of which is neither delinquent nor subject to penalties. Any real property and facilities held under lease by the Company and the Subsidiaries are held by them under valid, subsisting and enforceable leases with which the Company and the Subsidiaries are in compliance.
(p) Intellectual Property. The Company and the Subsidiaries have, or have rights to use, all patents, patent applications, trademarks, trademark applications, service marks, trade names, trade secrets, inventions, copyrights, licenses and other intellectual property rights in connection with their respective businesses as described in the SEC Reports and which the failure to do so could have a Material Adverse Effect (collectively, the “Intellectual Property Rights”). Neither the Company nor any Subsidiary has received a notice (written or otherwise) that any of, the Intellectual Property Rights has expired, terminated or been abandoned, or is expected to expire or terminate or be abandoned, within two (2) years from the date of this Agreement. Neither the Company nor any Subsidiary has received, since the date of the latest audited financial statements included within the SEC Reports, a written notice of a claim or otherwise has any knowledge that the operation of their respective businesses violate or infringe upon the intellectual property rights of any Person, except as could not have or reasonably be expected to have a Material Adverse Effect. To the knowledge of the Company, all such Intellectual Property Rights are enforceable and there is no existing infringement by another Person of any of the Intellectual Property Rights. The Company and its Subsidiaries have taken reasonable security measures to protect the secrecy, confidentiality and value of all of their intellectual properties, except where failure to do so could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect.
(q) Insurance. The Company and the Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as are prudent and customary in the businesses in which the Company and the Subsidiaries are engaged, including, but not limited to, directors and officers insurance coverage at least equal to the aggregate Subscription Amount. Neither the Company nor any Subsidiary has any reason to believe that it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business without a significant increase in cost.
(r) Transactions With Affiliates and Employees. Except as set forth on Schedule 3.1(r), none of the officers or directors of the Company or any Subsidiary and, to the knowledge of the Company, none of the employees of the Company or any Subsidiary is presently a party to any transaction with the Company or any Subsidiary (other than for services as employees, officers and directors), including any contract, agreement or other arrangement providing for the furnishing of services to or by, providing for rental of real or personal property to or from, providing for the borrowing of money from or lending of money to or otherwise requiring payments to or from any officer, director or such employee or, to the knowledge of the Company, any entity in which any officer, director, or any such employee has a substantial interest or is an officer, director, trustee, stockholder, member or partner, in each case in excess of $120,000 other than for (i) payment of salary, bonus or consulting fees for services rendered, (ii) reimbursement for expenses incurred on behalf of the Company and (iii) other employee benefits, including equity-based award agreements under any equity compensation plans of the Company.
(s) Sarbanes-Oxley; Internal Accounting Controls. The Company and the Subsidiaries are in compliance in all material respects with any and all applicable requirements of the Sarbanes-Oxley Act of 2002, as amended, that are effective as of the date hereof, and any and all applicable rules and regulations promulgated by the Commission thereunder that are effective as of the date hereof and as of the Closing Date. Except as disclosed in the SEC Reports, the Company and the Subsidiaries maintain a system of internal accounting controls sufficient to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset accountability, (iii) access to assets is permitted only in accordance with management’s general or specific authorization, and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences. Except as disclosed in the SEC Reports, the Company and the Subsidiaries have established disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the Company and the Subsidiaries and designed such disclosure controls and procedures to ensure that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Commission’s rules and forms. The Company’s certifying officers have evaluated the effectiveness of the disclosure controls and procedures of the Company and the Subsidiaries as of the end of the period covered by the most recently filed periodic report under the Exchange Act (such date, the “Evaluation Date”). The Company presented in its most recently filed periodic report under the Exchange Act the conclusions of the certifying officers about the effectiveness of the disclosure controls and procedures based on their evaluations as of the Evaluation Date. Since the Evaluation Date, there have been no changes in the internal control over financial reporting (as such term is defined in the Exchange Act) of the Company and its Subsidiaries that have materially affected, or is reasonably likely to materially affect, the internal control over financial reporting of the Company and its Subsidiaries.
(t) Certain Fees. Except as set forth in the Prospectus, no brokerage or finder’s fees or commissions are or will be payable by the Company or any Subsidiary to any broker, financial advisor or consultant, finder, placement agent, investment banker, bank or other Person with respect to the transactions contemplated by the Transaction Documents. The Purchasers shall have no obligation with respect to any fees or with respect to any claims made by or on behalf of other Persons for fees of a type contemplated in this Section that may be due in connection with the transactions contemplated by the Transaction Documents.
(u) Investment Company. The Company is not, and is not an Affiliate of, and immediately after receipt of payment for the Securities, will not be or be an Affiliate of, an “investment company” within the meaning of the Investment Company Act of 1940, as amended. The Company shall conduct its business in a manner so that it will not become an “investment company” subject to registration under the Investment Company Act of 1940, as amended.
(v) Registration Rights. Except as set forth on Schedule 3.1(v), no Person has any right to cause the Company or any Subsidiary to effect the registration under the Securities Act of any securities of the Company or any Subsidiary.
(w) Listing and Maintenance Requirements. The Common Stock is registered pursuant to Section 12(b) of the Exchange Act, and the Company has taken no action designed to, or which to its knowledge is likely to have the effect of, terminating the registration of the Common Stock under the Exchange Act nor has the Company received any notification that the Commission is contemplating terminating such registration. Except as set forth in the SEC Reports, (i) the Company has not, in the 12 months preceding the date hereof, received notice from any Trading Market on which the Common Stock is or has been listed or quoted to the effect that the Company is not in compliance with the listing or maintenance requirements of such Trading Market, and (ii) following the Closing hereunder, the Company has no reason to believe that it will not in the foreseeable future continue to be, in compliance with all such listing and maintenance requirements. The Common Stock is currently eligible for electronic transfer through the Depository Trust Company or another established clearing corporation and the Company is current in payment of the fees to the Depository Trust Company (or such other established clearing corporation) in connection with such electronic transfer.
(x) Application of Takeover Protections. The Company and the Board of Directors have taken all necessary action, if any, in order to render inapplicable any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or other similar anti-takeover provision under the Company’s certificate of incorporation (or similar charter documents) or the laws of its state of incorporation that is or could become applicable to the Purchasers as a result of the Purchasers and the Company fulfilling their obligations or exercising their rights under the Transaction Documents, including without limitation as a result of the Company’s issuance of the Securities and the Purchasers’ ownership of the Securities.
(y) Disclosure. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, the Company confirms that neither it nor any other Person acting on its behalf has provided any of the Purchasers or their agents or counsel with any information that it believes constitutes or might constitute material, non-public information which is not otherwise disclosed in the Preliminary Prospectus or the Prospectus. The Company understands and confirms that the Purchasers will rely on the foregoing representation in effecting transactions in securities of the Company. All of the disclosure furnished by or on behalf of the Company to the Purchasers regarding the Company and its Subsidiaries, their respective businesses and the transactions contemplated hereby, including the Disclosure Schedules to this Agreement, is true and correct and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. The press releases disseminated by the Company during the twelve months preceding the date of this Agreement taken as a whole do not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made and when made, not misleading. The Company acknowledges and agrees that no Purchaser makes or has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 3.2 hereof.
(z) No Integrated Offering. Assuming the accuracy of the Purchasers’ representations and warranties set forth in Section 3.2, neither the Company, nor any of its Affiliates, nor any Person acting on its or their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to be integrated with prior offerings by the Company for purposes of any applicable shareholder approval provisions of any Trading Market on which any of the securities of the Company are listed or designated.
(aa) Solvency. Based on the consolidated financial condition of the Company as of the Closing Date, after giving effect to the receipt by the Company of the proceeds from the sale of the Securities hereunder, (i) the fair saleable value of the Company’s assets exceeds the amount that will be required to be paid on or in respect of the Company’s existing debts and other liabilities (including known contingent liabilities) as they mature, (ii) the Company’s assets do not constitute unreasonably small capital to carry on its business as now conducted and as proposed to be conducted including its capital needs taking into account the particular capital requirements of the business conducted by the Company, consolidated and projected capital requirements and capital availability thereof, and (iii) the current cash flow of the Company, together with the proceeds the Company would receive, were it to liquidate all of its assets, after taking into account all anticipated uses of the cash, would be sufficient to pay all amounts on or in respect of its liabilities when such amounts are required to be paid. The Company does not intend to incur debts beyond its ability to pay such debts as they mature (taking into account the timing and amounts of cash to be payable on or in respect of its debt). The Company has no knowledge of any facts or circumstances which lead it to believe that it will file for reorganization or liquidation under the bankruptcy or reorganization laws of any jurisdiction within one year from the Closing Date. Schedule 3.1(aa) sets forth as of the date hereof all outstanding secured and unsecured Indebtedness of the Company or any Subsidiary, or for which the Company or any Subsidiary has commitments. For the purposes of this Agreement, “Indebtedness” means (x) any liabilities for borrowed money or amounts owed in excess of $50,000 (other than trade accounts payable incurred in the ordinary course of business), (y) all guaranties, endorsements and other contingent obligations in respect of indebtedness of others, whether or not the same are or should be reflected in the Company’s consolidated balance sheet (or the notes thereto), except guaranties by endorsement of negotiable instruments for deposit or collection or similar transactions in the ordinary course of business; and (z) the present value of any lease payments in excess of $50,000 due under leases required to be capitalized in accordance with GAAP. Neither the Company nor any Subsidiary is in default with respect to any Indebtedness.
(bb) Tax Status. Except for matters that would not, individually or in the aggregate, have or reasonably be expected to result in a Material Adverse Effect, the Company and its Subsidiaries each (i) has made or filed, or secured all extensions for the filing of, all applicable United States federal, state and local income and all foreign income and franchise tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations and (iii) has set aside on its books provision reasonably adequate for the payment of all material taxes for periods subsequent to the periods to which such returns, reports or declarations apply. There are no unpaid taxes in any material amount claimed to be due by the taxing authority of any jurisdiction, and the officers of the Company or of any Subsidiary know of no basis for any such claim.
(cc) Foreign Corrupt Practices. Neither the Company nor any Subsidiary, nor to the knowledge of the Company or any Subsidiary, any agent or other person acting on behalf of the Company or any Subsidiary, has (i) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (iii) failed to disclose fully any contribution made by the Company or any Subsidiary (or made by any person acting on its behalf of which the Company is aware) which is in violation of law, or (iv) violated in any material respect any provision of FCPA.
(dd) Accountants. The Company’s independent registered public accounting firm is Cherry Bekaert LLP. To the knowledge and belief of the Company, such accounting firm (i) is a registered public accounting firm as required by the Exchange Act and (ii) will express its opinion with respect to the financial statements to be included in the Company’s Annual Report for the fiscal year ending December 31, 2023.
(ee) Acknowledgment Regarding Purchasers’ Purchase of Securities. The Company acknowledges and agrees that each of the Purchasers is acting solely in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated thereby. The Company further acknowledges that no Purchaser is acting as a financial advisor or fiduciary of the Company (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated thereby and any advice given by any Purchaser or any of their respective representatives or agents in connection with the Transaction Documents and the transactions contemplated thereby is merely incidental to the Purchasers’ purchase of the Securities. The Company further represents to each Purchaser that the Company’s decision to enter into this Agreement and the other Transaction Documents has been based solely on the independent evaluation of the transactions contemplated hereby by the Company and its representatives.
(ff) Acknowledgment Regarding Purchaser’s Trading Activity. Anything in this Agreement or elsewhere herein to the contrary notwithstanding (except for Sections 3.2(f) and 4.13 hereof), it is understood and acknowledged by the Company that: (i) in this Agreement, none of the Purchasers has been asked by the Company to agree, nor has any Purchaser agreed, to desist from purchasing or selling, long and/or short, securities of the Company, or “derivative” securities based on securities issued by the Company or to hold the Securities for any specified term; (ii) past or future open market or other transactions by any Purchaser, specifically including, without limitation, Short Sales or “derivative” transactions, before or after the closing of this or future private placement transactions, may negatively impact the market price of the Company’s publicly-traded securities; (iii) any Purchaser, and counter-parties in “derivative” transactions to which any such Purchaser is a party, directly or indirectly, presently may have a “short” position in the Common Stock, and (iv) each Purchaser shall not be deemed to have any affiliation with or control over any arm’s length counter-party in any “derivative” transaction. The Company further understands and acknowledges that (y) one or more Purchasers may engage in hedging activities at various times during the period that the Securities are outstanding, including, without limitation, during the periods that the value of the Warrant Shares deliverable with respect to Securities are being determined, and (z) such hedging activities (if any) could reduce the value of the existing stockholders’ equity interests in the Company at and after the time that the hedging activities are being conducted. The Company acknowledges that such aforementioned hedging activities do not constitute a breach of any of the Transaction Documents.
(gg) Regulation M Compliance. The Company has not, and to its knowledge no one acting on its behalf has, (i) taken, directly or indirectly, any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company to facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or paid any compensation for soliciting purchases of, any of the Securities, or (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities of the Company, other than, in the case of clauses (ii) and (iii), compensation paid to the Placement Agent in connection with the placement of the Securities.
(hh) FDA. As to each product subject to the jurisdiction of the U.S. Food and Drug Administration (“FDA”) under the Federal Food, Drug and Cosmetic Act, as amended, and the regulations thereunder (“FDCA”) that is manufactured, packaged, labeled, tested, distributed, sold, and/or marketed by the Company or any of its Subsidiaries (each such product, a “Product”), such Product is being manufactured, packaged, labeled, tested, distributed, sold and/or marketed by the Company in compliance with all applicable requirements under FDCA and similar laws, rules and regulations relating to registration, investigational use, premarket clearance, licensure, or application approval, good manufacturing practices, good laboratory practices, good clinical practices, product listing, quotas, labeling, advertising, record keeping and filing of reports, except where the failure to be in compliance would not have a Material Adverse Effect. There is no pending, completed or, to the Company’s knowledge, threatened, action (including any lawsuit, arbitration, or legal or administrative or regulatory proceeding, charge, complaint, or investigation) against the Company or any of its Subsidiaries, and none of the Company or any of its Subsidiaries has received any notice, warning letter or other written communication from the FDA or any other governmental entity, which (i) contests the premarket clearance, licensure, registration, or approval of, the uses of, the distribution of, the manufacturing or packaging of, the testing of, the sale of, or the labeling and promotion of any Product, (ii) withdraws its approval of, requests the recall, suspension, or seizure of, or withdraws or orders the withdrawal of advertising or sales promotional materials relating to, any Product, (iii) imposes a clinical hold on any clinical investigation by the Company or any of its Subsidiaries, (iv) enjoins production at any facility of the Company or any of its Subsidiaries, (v) enters or proposes to enter into a consent decree of permanent injunction with the Company or any of its Subsidiaries, or (vi) otherwise alleges any violation of any laws, rules or regulations by the Company or any of its Subsidiaries, and which, either individually or in the aggregate, would have a Material Adverse Effect. The properties, business and operations of the Company have been and are being conducted in all material respects in accordance with all applicable laws, rules and regulations of the FDA. The Company has not been informed by the FDA that the FDA will prohibit the marketing, sale, license or use in the United States of any product proposed to be developed, produced or marketed by the Company nor has the FDA expressed any concern as to approving or clearing for marketing any product being developed or proposed to be developed by the Company.
(ii) Cybersecurity. (i)(x) There has been no security breach or other compromise of or relating to any of the Company’s or any Subsidiary’s information technology and computer systems, networks, hardware, software, data (including the data of its respective customers, employees, suppliers, vendors and any third party data maintained by or on behalf of it), equipment or technology (collectively, “IT Systems and Data”) and (y) the Company and the Subsidiaries have not been notified of, and have no knowledge of any event or condition that would reasonably be expected to result in, any security breach or other compromise to its IT Systems and Data; (ii) the Company and the Subsidiaries are presently in compliance with all applicable laws or statutes and all judgments, orders, rules and regulations of any court or arbitrator or governmental or regulatory authority, internal policies and contractual obligations relating to the privacy and security of IT Systems and Data and to the protection of such IT Systems and Data from unauthorized use, access, misappropriation or modification, except as would not, individually or in the aggregate, have a Material Adverse Effect; (iii) the Company and the Subsidiaries have implemented and maintained commercially reasonable safeguards to maintain and protect its material confidential information and the integrity, continuous operation, redundancy and security of all IT Systems and Data; and (iv) the Company and the Subsidiaries have implemented backup and disaster recovery technology consistent with industry standards and practices.
(jj) Stock Option Plans. Each stock option granted by the Company under the Company’s equity compensation plan was granted (i) in accordance with the terms of the Company’s equity compensation plan and (ii) with an exercise price at least equal to the fair market value of the Common Stock on the date such stock option would be considered granted under GAAP and applicable law. No stock option granted under the Company’s equity compensation plan has been backdated. The Company has not knowingly granted, and there is no and has been no Company policy or practice to knowingly grant, stock options prior to, or otherwise knowingly coordinate the grant of stock options with, the release or other public announcement of material information regarding the Company or its Subsidiaries or their financial results or prospects.
(kk) Office of Foreign Assets Control. Neither the Company nor any Subsidiary nor, to the Company’s knowledge, any director, officer, agent, employee or affiliate of the Company or any Subsidiary is currently subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department (“OFAC”).
(ll) U.S. Real Property Holding Corporation. The Company is not and has never been a U.S. real property holding corporation within the meaning of Section 897 of the Internal Revenue Code of 1986, as amended, and the Company shall so certify upon Purchaser’s request.
(mm) Bank Holding Company Act. Neither the Company nor any of its Subsidiaries or Affiliates is subject to the Bank Holding Company Act of 1956, as amended (the “BHCA”), and to regulation by the Board of Governors of the Federal Reserve System (the “Federal Reserve”). Neither the Company nor any of its Subsidiaries or Affiliates owns or controls, directly or indirectly, five percent (5%) or more of the outstanding shares of any class of voting securities or twenty-five percent (25%) or more of the total equity of a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve. Neither the Company nor any of its Subsidiaries or Affiliates exercises a controlling influence over the management or policies of a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve.
(nn) Money Laundering. The operations of the Company and its Subsidiaries are and have been conducted at all times in material compliance with applicable financial record-keeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, applicable money laundering statutes and applicable rules and regulations thereunder (collectively, the “Money Laundering Laws”), and no Action or Proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company or any Subsidiary with respect to the Money Laundering Laws is pending or, to the knowledge of the Company or any Subsidiary, threatened.
3.2 Representations and Warranties of the Purchasers. Each Purchaser, for itself and for no other Purchaser, hereby represents and warrants as of the date hereof and as of the Closing Date to the Company as follows (unless as of a specific date therein, in which case they shall be accurate as of such date):
(a) Organization; Authority. Such Purchaser is either an individual or an entity duly incorporated or formed, validly existing and in good standing under the laws of the jurisdiction of its incorporation or formation with full right, corporate, partnership, limited liability company or similar power and authority to enter into and to consummate the transactions contemplated by the Transaction Documents and otherwise to carry out its obligations hereunder and thereunder. The execution and delivery of the Transaction Documents and performance by such Purchaser of the transactions contemplated by the Transaction Documents have been duly authorized by all necessary corporate, partnership, limited liability company or similar action, as applicable, on the part of such Purchaser. Each Transaction Document to which it is a party has been duly executed by such Purchaser, and when delivered by such Purchaser in accordance with the terms hereof, will constitute the valid and legally binding obligation of such Purchaser, enforceable against it in accordance with its terms, except: (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by applicable law.
(b) Understandings or Arrangements. Such Purchaser is acquiring the Securities as principal for its own account and has no direct or indirect arrangement or understandings with any other persons to distribute or regarding the distribution of such Securities (this representation and warranty not limiting such Purchaser’s right to sell the Securities pursuant to the Registration Statement or otherwise in compliance with applicable federal and state securities laws). Such Purchaser is acquiring the Securities hereunder in the ordinary course of its business.
(c) Purchaser Status. At the time such Purchaser was offered the Securities, it was, and as of the date hereof it is, and on each date on which it exercises any Warrants, it will be an “accredited investor” as defined in Rule 501(a)(1), (a)(2), (a)(3), (a)(7), (a)(8), (a)(9), (a)(12), or (a)(13) under the Securities Act.
(d) Experience of Such Purchaser. Such Purchaser, either alone or together with its representatives, has such knowledge, sophistication and experience in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the Securities, and has so evaluated the merits and risks of such investment. Such Purchaser is able to bear the economic risk of an investment in the Securities and, at the present time, is able to afford a complete loss of such investment.
(e) Access to Information. Such Purchaser acknowledges that it has had the opportunity to review the Transaction Documents (including all exhibits and schedules thereto) and the SEC Reports and has been afforded, (i) the opportunity to ask such questions as it has deemed necessary of, and to receive answers from, representatives of the Company concerning the terms and conditions of the offering of the Securities and the merits and risks of investing in the Securities; (ii) access to information about the Company and its financial condition, results of operations, business, properties, management and prospects sufficient to enable it to evaluate its investment; and (iii) the opportunity to obtain such additional information that the Company possesses or can acquire without unreasonable effort or expense that is necessary to make an informed investment decision with respect to the investment. Such Purchaser acknowledges and agrees that neither the Placement Agent nor any Affiliate of the Placement Agent has provided such Purchaser with any information or advice with respect to the Securities nor is such information or advice necessary or desired. Neither the Placement Agent nor any Affiliate has made or makes any representation as to the Company or the quality of the Securities and the Placement Agent and any Affiliate may have acquired non-public information with respect to the Company which such Purchaser agrees need not be provided to it. In connection with the issuance of the Securities to such Purchaser, neither the Placement Agent nor any of its Affiliates has acted as a financial advisor or fiduciary to such Purchaser.
(f) Certain Transactions and Confidentiality. Other than consummating the transactions contemplated hereunder, such Purchaser has not, nor has any Person acting on behalf of or pursuant to any understanding with such Purchaser, directly or indirectly executed any purchases or sales, including Short Sales, of the securities of the Company during the period commencing as of the time of the definitive pricing of the transactions contemplated hereunder and ending immediately prior to the execution hereof. Notwithstanding the foregoing, in the case of a Purchaser that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Purchaser’s assets and the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions of such Purchaser’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Securities covered by this Agreement. Other than to other Persons party to this Agreement or to such Purchaser’s representatives, including, without limitation, its officers, directors, partners, legal and other advisors, employees, agents and Affiliates, such Purchaser has maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction). Notwithstanding the foregoing, for the avoidance of doubt, nothing contained herein shall constitute a representation or warranty, or preclude any actions, with respect to locating or borrowing shares in order to effect Short Sales or similar transactions in the future.
The Company acknowledges and agrees that the representations contained in this Section 3.2 shall not modify, amend or affect such Purchaser’s right to rely on the Company’s representations and warranties contained in this Agreement or any representations and warranties contained in any other Transaction Document or any other document or instrument executed and/or delivered in connection with this Agreement or the consummation of the transactions contemplated hereby. Notwithstanding the foregoing, for the avoidance of doubt, nothing contained herein shall constitute a representation or warranty, or preclude any actions, with respect to locating or borrowing shares in order to effect Short Sales or similar transactions in the future.
ARTICLE IV.
OTHER AGREEMENTS OF THE PARTIES
4.1 Warrant Shares. If all or any portion of a Warrant is exercised at a time when there is an effective registration statement to cover the issuance or resale of the Warrant Shares or if the Warrant is exercised via cashless exercise, the Warrant Shares issued pursuant to any such exercise shall be issued free of all legends. If at any time following the date hereof the Registration Statement (or any subsequent registration statement registering the sale or resale of the Warrant Shares) is not effective or is not otherwise available for the sale or resale of the Warrant Shares, the Company shall immediately notify the holders of the Warrants in writing that such registration statement is not then effective and thereafter shall promptly notify such holders when the registration statement is effective again and available for the sale or resale of the Warrant Shares (it being understood and agreed that the foregoing shall not limit the ability of the Company to issue, or any Purchaser to sell, any of the Warrant Shares in compliance with applicable federal and state securities laws). The Company shall use best efforts to keep a registration statement (including the Registration Statement) registering the issuance or resale of the Warrant Shares effective during the term of the Warrants.
4.2 Furnishing of Information. Until the earliest of the time that (i) no Purchaser owns Securities or (ii) the Warrants have expired, the Company covenants to timely file (or obtain extensions in respect thereof and file within the applicable grace period) all reports required to be filed by the Company after the date hereof pursuant to the Exchange Act even if the Company is not then subject to the reporting requirements of the Exchange Act.
4.3 Integration. The Company shall not sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2 of the Securities Act) that would be integrated with the offer or sale of the Securities for purposes of the rules and regulations of any Trading Market such that it would require shareholder approval prior to the closing of such other transaction unless shareholder approval is obtained before the issuances subject to such shareholder approval have occurred.
4.4 Securities Laws Disclosure; Publicity. The Company shall (a) by the Disclosure Time, issue a press release disclosing the material terms of the transactions contemplated hereby, and (b) file a Current Report on Form 8-K, including the Transaction Documents as exhibits thereto, with the Commission within the time required by the Exchange Act. From and after the issuance of such press release, the Company represents to the Purchasers that it shall have publicly disclosed all material, non-public information delivered to any of the Purchasers by the Company or any of its Subsidiaries, or any of their respective officers, directors, employees, Affiliates or agents, including, without limitation, the Placement Agent, in connection with the transactions contemplated by the Transaction Documents. In addition, effective upon the issuance of such press release, the Company acknowledges and agrees that any and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any of its Subsidiaries or any of their respective officers, directors, agents, employees, Affiliates or agents, including, without limitation, the Placement Agent, on the one hand, and any of the Purchasers or any of their Affiliates on the other hand, shall terminate and be of no further force or effect. The Company understands and confirms that each Purchaser shall be relying on the foregoing covenant in effecting transactions in securities of the Company. The Company and each Purchaser shall consult with each other in issuing any other press releases with respect to the transactions contemplated hereby, and neither the Company nor any Purchaser shall issue any such press release nor otherwise make any such public statement without the prior consent of the Company, with respect to any press release of any Purchaser, or without the prior consent of each Purchaser, with respect to any press release of the Company, which consent shall not unreasonably be withheld or delayed, except if such disclosure is required by law, in which case the disclosing party shall promptly provide the other party with prior notice of such public statement or communication. Notwithstanding the foregoing, the Company shall not publicly disclose the name of any Purchaser, or include the name of any Purchaser in any filing with the Commission or any regulatory agency or Trading Market, without the prior written consent of such Purchaser, except (a) as required by federal securities law in connection with the filing of final Transaction Documents with the Commission and (b) to the extent such disclosure is required by law or Trading Market regulations, in which case the Company shall provide the Purchasers with prior notice of such disclosure permitted under this clause (b) and reasonably cooperate with such Purchaser regarding such disclosure.
4.5 Shareholder Rights Plan. No claim will be made or enforced by the Company or, with the consent of the Company, any other Person, that any Purchaser is an “Acquiring Person” under any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or similar anti-takeover plan or arrangement in effect or hereafter adopted by the Company, or that any Purchaser could be deemed to trigger the provisions of any such plan or arrangement, by virtue of receiving Securities under the Transaction Documents or under any other agreement between the Company and the Purchasers.
4.6 Non-Public Information. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, which shall be disclosed pursuant to Section 4.4, the Company covenants and agrees that neither it, nor any other Person acting on its behalf will provide any Purchaser or its agents or counsel with any information that constitutes, or the Company reasonably believes constitutes, material non-public information, unless prior thereto such Purchaser shall have consented in writing to the receipt of such information and agreed in writing with the Company to keep such information confidential. The Company understands and confirms that each Purchaser shall be relying on the foregoing covenant in effecting transactions in securities of the Company. To the extent that the Company, any of its Subsidiaries, or any of their respective officers, directors, agents, employees or Affiliates delivers any material, non-public information to a Purchaser without such Purchaser’s consent, the Company hereby covenants and agrees that such Purchaser shall not have any duty of confidentiality to the Company, any of its Subsidiaries, or any of their respective officers, directors, employees, Affiliates or agents, including, without limitation, the Placement Agent, or a duty to the Company, any of its Subsidiaries or any of their respective officers, directors, employees, Affiliates or agents, including, without limitation, the Placement Agent, not to trade on the basis of, such material, non-public information, provided that the Purchaser shall remain subject to applicable law. To the extent that any notice provided pursuant to any Transaction Document constitutes, or contains, material, non-public information regarding the Company or any Subsidiaries, the Company shall simultaneously with the delivery of such notice file such notice with the Commission pursuant to a Current Report on Form 8-K. The Company understands and confirms that each Purchaser shall be relying on the foregoing covenant in effecting transactions in securities of the Company.
4.7 Use of Proceeds. Except as set forth in the Preliminary Prospectus and the Prospectus, the Company shall use the net proceeds from the sale of the Securities hereunder for working capital purposes and shall not use such proceeds: (a) for the satisfaction of any portion of the Company’s debt (other than payment of trade payables in the ordinary course of the Company’s business and prior practices), (b) for the redemption of any Common Stock or Common Stock Equivalents, (c) for the settlement of any outstanding litigation, or (d) in violation of FCPA or OFAC regulations.
4.8 Indemnification of Purchasers. Subject to the provisions of this Section 4.8, the Company will indemnify and hold each Purchaser and its directors, officers, shareholders, members, partners, employees and agents (and any other Persons with a functionally equivalent role of a Person holding such titles notwithstanding a lack of such title or any other title), each Person who controls such Purchaser (within the meaning of Section 15 of the Securities Act and Section 20 of the Exchange Act), and the directors, officers, shareholders, agents, members, partners or employees (and any other Persons with a functionally equivalent role of a Person holding such titles notwithstanding a lack of such title or any other title) of such controlling persons (each, a “Purchaser Party”) harmless from any and all losses, liabilities, obligations, claims, contingencies, damages, costs and expenses, including all judgments, amounts paid in settlements, court costs and reasonable attorneys’ fees and costs of investigation that any such Purchaser Party may suffer or incur as a result of or relating to (a) any breach of any of the representations, warranties, covenants or agreements made by the Company in this Agreement or in the other Transaction Documents or (b) any action instituted against the Purchaser Parties in any capacity, or any of them or their respective Affiliates, by any stockholder of the Company who is not an Affiliate of such Purchaser Party, with respect to any of the transactions contemplated by the Transaction Documents (unless such action is solely based upon a material breach of such Purchaser Party’s representations, warranties or covenants under the Transaction Documents or any agreements or understandings such Purchaser Party may have with any such stockholder or any violations by such Purchaser Party of state or federal securities laws or any conduct by such Purchaser Party which is finally judicially determined to constitute fraud, gross negligence or willful misconduct). If any action shall be brought against any Purchaser Party in respect of which indemnity may be sought pursuant to this Agreement, such Purchaser Party shall promptly notify the Company in writing, and the Company shall have the right to assume the defense thereof with counsel of its own choosing reasonably acceptable to the Purchaser Party. Any Purchaser Party shall have the right to employ separate counsel in any such action and participate in the defense thereof, but the fees and expenses of such counsel shall be at the expense of such Purchaser Party except to the extent that (i) the employment thereof has been specifically authorized by the Company in writing, (ii) the Company has failed after a reasonable period of time to assume such defense and to employ counsel or (iii) in such action there is, in the reasonable opinion of counsel, a material conflict on any material issue between the position of the Company and the position of such Purchaser Party, in which case the Company shall be responsible for the reasonable fees and expenses of no more than one such separate counsel. The Company will not be liable to any Purchaser Party under this Agreement (y) for any settlement by a Purchaser Party effected without the Company’s prior written consent, which shall not be unreasonably withheld or delayed; or (z) to the extent, but only to the extent that a loss, claim, damage or liability is attributable to any Purchaser Party’s breach of any of the representations, warranties, covenants or agreements made by such Purchaser Party in this Agreement or in the other Transaction Documents. The indemnification required by this Section 4.8 shall be made by periodic payments of the amount thereof during the course of the investigation or defense, as and when bills are received or are incurred. The indemnity agreements contained herein shall be in addition to any cause of action or similar right of any Purchaser Party against the Company or others and any liabilities the Company may be subject to pursuant to law.
4.9 Reservation of Common Stock. As of the date hereof, the Company has reserved and the Company shall continue to reserve and keep available at all times, free of preemptive rights, a sufficient number of shares of Common Stock for the purpose of enabling the Company to issue Shares pursuant to this Agreement and Warrant Shares pursuant to any exercise of the Warrants.
4.10 Listing of Common Stock. The Company hereby agrees to use best efforts to maintain the listing or quotation of the Common Stock on the Trading Market on which it is currently listed, and concurrently with the Closing, the Company shall have provided all required notices regarding the listing or quotation of all of the Shares and Warrant Shares on such Trading Market. The Company further agrees, if the Company applies to have the Common Stock traded on any other Trading Market, it will then include in such application all of the Shares and Warrant Shares, and will take such other action as is necessary to cause all of the Shares and Warrant Shares to be listed or quoted on such other Trading Market as promptly as possible. The Company will then take all reasonable action necessary to continue the listing and trading of its Common Stock on a Trading Market and will comply in all respects with the Company’s reporting, filing and other obligations under the bylaws or rules of the Trading Market. The Company agrees to take all reasonable action to maintain the eligibility of the Common Stock for electronic transfer through the Depository Trust Company or another established clearing corporation, including, without limitation, by timely payment of fees to the Depository Trust Company or such other established clearing corporation in connection with such electronic transfer.
4.11 Subsequent Equity Sales.
(a) From the date hereof until ninety (90) days after the Closing Date, neither the Company nor any Subsidiary shall (i) issue, enter into any agreement to issue or announce the issuance or proposed issuance of any shares of Common Stock or Common Stock Equivalents or (ii) file any registration statement or amendment or supplement thereto, other than the Prospectus or filing a registration statement on Form S-8 in connection with any employee benefit plan.
(b) From the date hereof until one-hundred eighty (180) days after the Closing Date, the Company shall be prohibited from effecting or entering into an agreement to effect any issuance by the Company or any of its Subsidiaries of Common Stock or Common Stock Equivalents (or a combination of units thereof) involving a Variable Rate Transaction. “Variable Rate Transaction” means a transaction in which the Company (i) issues or sells any debt or equity securities that are convertible into, exchangeable or exercisable for, or include the right to receive additional shares of Common Stock either (A) at a conversion price, exercise price or exchange rate or other price that is based upon and/or varies with the trading prices of or quotations for the shares of Common Stock at any time after the initial issuance of such debt or equity securities, or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such debt or equity security or upon the occurrence of specified or contingent events directly or indirectly related to the business of the Company or the market for the Common Stock or (ii) enters into, or effects a transaction under, any agreement, including, but not limited to, an equity line of credit or an “at-the-market offering”, whereby the Company may issue securities at a future determined price. Any Purchaser shall be entitled to obtain injunctive relief against the Company to preclude any such issuance, which remedy shall be in addition to any right to collect damages.
(c) Notwithstanding the foregoing, this Section 4.11 shall not apply in respect of (A) an Exempt Issuance, except that no Variable Rate Transaction shall be an Exempt Issuance or (B) after the date that is ninety (90) days after the Closing Date, the transactions contemplated by the Sales Agreement between the Company and Roth Capital Partners, LLC.
4.12 Equal Treatment of Purchasers. No consideration (including any modification of this Agreement) shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of the Transaction Documents unless the same consideration is also offered to all of the parties to the Transaction Documents. For clarification purposes, this provision constitutes a separate right granted to each Purchaser by the Company and negotiated separately by each Purchaser, and is intended for the Company to treat the Purchasers as a class and shall not in any way be construed as the Purchasers acting in concert or as a group with respect to the purchase, disposition or voting of Securities or otherwise.
4.13 Certain Transactions and Confidentiality. Each Purchaser, severally and not jointly with the other Purchasers, covenants that neither it nor any Affiliate acting on its behalf or pursuant to any understanding with it will execute any purchases or sales, including Short Sales of any of the Company’s securities during the period commencing with the execution of this Agreement and ending at such time that the transactions contemplated by this Agreement are first publicly announced pursuant to the initial press release as described in Section 4.4. Each Purchaser, severally and not jointly with the other Purchasers, covenants that until such time as the transactions contemplated by this Agreement are publicly disclosed by the Company pursuant to the initial press release as described in Section 4.4, such Purchaser will maintain the confidentiality of the existence and terms of this transaction and the information included in the Disclosure Schedules (other than as disclosed to its legal and other representatives). Notwithstanding the foregoing and notwithstanding anything contained in this Agreement to the contrary, the Company expressly acknowledges and agrees that (i) no Purchaser makes any representation, warranty or covenant hereby that it will not engage in effecting transactions in any securities of the Company after the time that the transactions contemplated by this Agreement are first publicly announced pursuant to the initial press release as described in Section 4.4, (ii) no Purchaser shall be restricted or prohibited from effecting any transactions in any securities of the Company in accordance with applicable securities laws from and after the time that the transactions contemplated by this Agreement are first publicly announced pursuant to the initial press release as described in Section 4.4 and (iii) no Purchaser shall have any duty of confidentiality or duty not to trade in the securities of the Company to the Company, any of its Subsidiaries, or any of their respective officers, directors, employees, Affiliates, or agent, including, without limitation, the Placement Agent, after the issuance of the initial press release as described in Section 4.4. Notwithstanding the foregoing, in the case of a Purchaser that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Purchaser’s assets and the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions of such Purchaser’s assets, the covenant set forth above shall only apply with respect to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Securities covered by this Agreement.
4.14 Exercise Procedures. The form of Notice of Exercise included in the Warrants set forth the totality of the procedures required of the Purchasers in order to exercise the Warrants. No additional legal opinion, other information or instructions shall be required of the Purchasers to exercise their Warrants. Without limiting the preceding sentences, no ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise form be required in order to exercise the Warrants. The Company shall honor exercises of the Warrants and shall deliver Warrant Shares in accordance with the terms, conditions and time periods set forth in the Transaction Documents.
4.15 Lock-Up Agreements. The Company shall not amend, modify, waive or terminate any provision of any of the Lock-Up Agreements except to extend the term of the lock-up period and shall enforce the provisions of each Lock-Up Agreement in accordance with its terms. If any party to a Lock-Up Agreement breaches any provision of a Lock-Up Agreement, the Company shall promptly use its best efforts to seek specific performance of the terms of such Lock-Up Agreement.
4.16 Restrictions on Offers and Sales of the Securities in Canada. Each of the Company and the Purchaser acknowledge and agree that none of the Securities that may be issued to the Purchaser under this Agreement have been or will be qualified for distribution in any Province or Territory of Canada. The Purchaser covenants and agrees that it shall offer or sell any Securities that may be issued to the Purchaser pursuant to this Agreement only in transactions executed on a Trading Market through a U.S. registered broker-dealer that is located in the United States.
ARTICLE V.
MISCELLANEOUS
5.1 Termination. This Agreement may be terminated by any Purchaser, as to such Purchaser’s obligations hereunder only and without any effect whatsoever on the obligations between the Company and the other Purchasers, by written notice to the other parties, if the Closing has not been consummated on or before the fifth (5th) Trading Day following the date hereof; provided, however, that no such termination will affect the right of any party to sue for any breach by any other party (or parties).
5.2 Fees and Expenses. Except as expressly set forth in the Transaction Documents to the contrary, each party shall pay the fees and expenses of its advisers, counsel, accountants and other experts, if any, and all other expenses incurred by such party incident to the negotiation, preparation, execution, delivery and performance of this Agreement. The Company shall pay all Transfer Agent fees (including, without limitation, any fees required for same-day processing of any instruction letter delivered by the Company and any exercise notice delivered by a Purchaser), stamp taxes and other taxes and duties levied in connection with the delivery of any Securities to the Purchasers.
5.3 Entire Agreement. The Transaction Documents, together with the exhibits and schedules thereto, the Preliminary Prospectus and the Prospectus, contain the entire understanding of the parties with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, oral or written, with respect to such matters, which the parties acknowledge have been merged into such documents, exhibits and schedules.
5.4 Notices. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall be deemed given and effective on the earliest of: (a) the time of transmission, if such notice or communication is delivered via email attachment at the email address as set forth on the signature pages attached hereto at or prior to 5:30 p.m. (New York City time) on a Trading Day, (b) the next Trading Day after the time of transmission, if such notice or communication is delivered via email attachment at the email address as set forth on the signature pages attached hereto on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (c) the second (2nd) Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service or (d) upon actual receipt by the party to whom such notice is required to be given. The address for such notices and communications shall be as set forth on the signature pages attached hereto. To the extent that any notice provided pursuant to any Transaction Document constitutes, or contains, material, non-public information regarding the Company or any of its Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K.
5.5 Amendments; Waivers. No provision of this Agreement may be waived, modified, supplemented or amended except in a written instrument signed, in the case of an amendment, by the Company and Purchasers which purchased at least 50.1% in interest of the Shares based on the initial Subscription Amounts hereunder (or, prior to the Closing, the Company and each Purchaser) or, in the case of a waiver, by the party against whom enforcement of any such waived provision is sought, provided that if any amendment, modification or waiver disproportionately and adversely impacts a Purchaser (or group of Purchasers), the consent of such disproportionately impacted Purchaser (or group of Purchasers) shall also be required. No waiver of any default with respect to any provision, condition or requirement of this Agreement shall be deemed to be a continuing waiver in the future or a waiver of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any party to exercise any right hereunder in any manner impair the exercise of any such right. Any proposed amendment or waiver that disproportionately, materially and adversely affects the rights and obligations of any Purchaser relative to the comparable rights and obligations of the other Purchasers shall require the prior written consent of such adversely affected Purchaser. Any amendment effected in accordance with this Section 5.5 shall be binding upon each Purchaser and holder of Securities and the Company.
5.6 Headings. The headings herein are for convenience only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any of the provisions hereof.
5.7 Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their successors and permitted assigns. The Company may not assign this Agreement or any rights or obligations hereunder without the prior written consent of each Purchaser (other than by merger). Any Purchaser may assign any or all of its rights under this Agreement to any Person to whom such Purchaser assigns or transfers any Securities, provided that such transferee agrees in writing to be bound, with respect to the transferred Securities, by the provisions of the Transaction Documents that apply to the “Purchasers.”
5.8 No Third-Party Beneficiaries. The Placement Agent shall be the third party beneficiary of the representations and warranties of the Company in Section 3.1 and the representations and warranties of the Purchasers in Section 3.2. This Agreement is intended for the benefit of the parties hereto and their respective successors and permitted assigns and is not for the benefit of, nor may any provision hereof be enforced by, any other Person, except as otherwise set forth in Section 4.8 and this Section 5.8.
5.9 Governing Law. All questions concerning the construction, validity, enforcement and interpretation of the Transaction Documents shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all legal Proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement and any other Transaction Documents (whether brought against a party hereto or its respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein (including with respect to the enforcement of any of the Transaction Documents), and hereby irrevocably waives, and agrees not to assert in any Action or Proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such Action or Proceeding is improper or is an inconvenient venue for such Proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any such Action or Proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law. If any party shall commence an Action or Proceeding to enforce any provisions of the Transaction Documents, then, in addition to the obligations of the Company under Section 4.8, the prevailing party in such Action or Proceeding shall be reimbursed by the non-prevailing party for its reasonable attorneys’ fees and other costs and expenses incurred with the investigation, preparation and prosecution of such Action or Proceeding.
5.10 Survival. The representations and warranties contained herein shall survive the Closing and the delivery of the Securities.
5.11 Execution. This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to each other party, it being understood that the parties need not sign the same counterpart. In the event that any signature is delivered by e-mail delivery of a “.pdf” format data file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such “.pdf” signature page were an original thereof.
5.12 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
5.13 Rescission and Withdrawal Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) any of the other Transaction Documents, whenever any Purchaser exercises a right, election, demand or option under a Transaction Document and the Company does not timely perform its related obligations within the periods therein provided, then such Purchaser may rescind or withdraw, in its sole discretion from time to time upon written notice to the Company, any relevant notice, demand or election in whole or in part without prejudice to its future actions and rights; provided, however, that, in the case of a rescission of an exercise of a Warrant, the applicable Purchaser shall be required to return any shares of Common Stock subject to any such rescinded exercise notice concurrently with the return to such Purchaser of the aggregate exercise price paid to the Company for such shares and the restoration of such Purchaser’s right to acquire such shares pursuant to such Purchaser’s Warrant (including, issuance of a replacement warrant certificate evidencing such restored right).
5.14 Replacement of Securities. If any certificate or instrument evidencing any Securities is mutilated, lost, stolen or destroyed, the Company shall issue or cause to be issued in exchange and substitution for and upon cancellation thereof (in the case of mutilation), or in lieu of and substitution therefor, a new certificate or instrument, but only upon receipt of evidence reasonably satisfactory to the Company of such loss, theft or destruction. The applicant for a new certificate or instrument under such circumstances shall also pay any reasonable third-party costs (including customary indemnity) associated with the issuance of such replacement Securities.
5.15 Remedies. In addition to being entitled to exercise all rights provided herein or granted by law, including recovery of damages, each of the Purchasers and the Company will be entitled to specific performance under the Transaction Documents. The parties agree that monetary damages may not be adequate compensation for any loss incurred by reason of any breach of obligations contained in the Transaction Documents and hereby agree to waive and not to assert in any Action for specific performance of any such obligation the defense that a remedy at law would be adequate.
5.16 Payment Set Aside. To the extent that the Company makes a payment or payments to any Purchaser pursuant to any Transaction Document or a Purchaser enforces or exercises its rights thereunder, and such payment or payments or the proceeds of such enforcement or exercise or any part thereof are subsequently invalidated, declared to be fraudulent or preferential, set aside, recovered from, disgorged by or are required to be refunded, repaid or otherwise restored to the Company, a trustee, receiver or any other Person under any law (including, without limitation, any bankruptcy law, state or federal law, common law or equitable cause of action), then to the extent of any such restoration the obligation or part thereof originally intended to be satisfied shall be revived and continued in full force and effect as if such payment had not been made or such enforcement or setoff had not occurred.
5.17 Independent Nature of Purchasers’ Obligations and Rights. The obligations of each Purchaser under any Transaction Document are several and not joint with the obligations of any other Purchaser, and no Purchaser shall be responsible in any way for the performance or non-performance of the obligations of any other Purchaser under any Transaction Document. Nothing contained herein or in any other Transaction Document, and no action taken by any Purchaser pursuant hereto or thereto, shall be deemed to constitute the Purchasers as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Purchasers are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by the Transaction Documents. Each Purchaser shall be entitled to independently protect and enforce its rights including, without limitation, the rights arising out of this Agreement or out of the other Transaction Documents, and it shall not be necessary for any other Purchaser to be joined as an additional party in any Proceeding for such purpose. Each Purchaser has been represented by its own separate legal counsel in its review and negotiation of the Transaction Documents. For reasons of administrative convenience only, each Purchaser and its respective counsel have chosen to communicate with the Company through PC. PC does not represent any of the Purchasers and only represents the Placement Agent. The Company has elected to provide all Purchasers with the same terms and Transaction Documents for the convenience of the Company and not because it was required or requested to do so by any of the Purchasers. It is expressly understood and agreed that each provision contained in this Agreement and in each other Transaction Document is between the Company and a Purchaser, solely, and not between the Company and the Purchasers collectively and not between and among the Purchasers.
5.18 Liquidated Damages. The Company’s obligations to pay any partial liquidated damages or other amounts owing under the Transaction Documents is a continuing obligation of the Company and shall not terminate until all unpaid partial liquidated damages and other amounts have been paid notwithstanding the fact that the instrument or security pursuant to which such partial liquidated damages or other amounts are due and payable shall have been canceled.
5.19 Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the next succeeding Business Day.
5.20 Construction. The parties agree that each of them and/or their respective counsel have reviewed and had an opportunity to revise the Transaction Documents and, therefore, the normal rule of construction to the effect that any ambiguities are to be resolved against the drafting party shall not be employed in the interpretation of the Transaction Documents or any amendments thereto. In addition, each and every reference to share prices and shares of Common Stock in any Transaction Document shall be subject to adjustment for reverse and forward stock splits, stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement.
5.21 WAIVER OF JURY TRIAL. IN ANY ACTION, SUIT, OR PROCEEDING IN ANY JURISDICTION BROUGHT BY ANY PARTY AGAINST ANY OTHER PARTY, THE PARTIES EACH KNOWINGLY AND INTENTIONALLY, TO THE GREATEST EXTENT PERMITTED BY APPLICABLE LAW, HEREBY ABSOLUTELY, UNCONDITIONALLY, IRREVOCABLY AND EXPRESSLY WAIVES FOREVER TRIAL BY JURY.
(Signature Pages Follow)
IN WITNESS WHEREOF, the parties hereto have caused this Securities Purchase Agreement to be duly executed by their respective authorized signatories as of the date first indicated above.
|
PANBELA THERAPEUTICS, INC.
|
|
Address for Notice:
|
|
By:
|
|
|
|
| |
Name:
|
|
E-Mail:
|
| |
Title:
|
|
|
| |
|
|
|
|
With a copy to (which shall not constitute notice):
|
|
|
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK
SIGNATURE PAGE FOR PURCHASER FOLLOWS]
[PURCHASER SIGNATURE PAGES TO PBLA SECURITIES PURCHASE AGREEMENT]
IN WITNESS WHEREOF, the undersigned have caused this Securities Purchase Agreement to be duly executed by their respective authorized signatories as of the date first indicated above.
|
Signature of Authorized Signatory of Purchaser:
|
|
|
Name of Authorized Signatory:
|
|
|
Title of Authorized Signatory:
|
|
|
Email Address of Authorized Signatory:
|
|
|
Address for Notice to Purchaser:
|
|
Address for Delivery of Securities to Purchaser (if not same as address for notice):
Subscription Amount: $_________________
Shares: _________________
Pre-Funded Warrant Shares: ___________ Beneficial Ownership Blocker ☐ 4.99% or ☐ 9.99%
Class E Common Warrant Shares: __________________ Beneficial Ownership Blocker ☐ 4.99% or ☐ 9.99%
Class F Common Warrant Shares: __________________ Beneficial Ownership Blocker ☐ 4.99% or ☐ 9.99%
EIN Number: ____________________
☐ Notwithstanding anything contained in this Agreement to the contrary, by checking this box (i) the obligations of the above-signed to purchase the securities set forth in this Agreement to be purchased from the Company by the above-signed, and the obligations of the Company to sell such securities to the above-signed, shall be unconditional and all conditions to Closing shall be disregarded, (ii) the Closing shall occur on the second (2nd) Trading Day following the date of this Agreement and (iii) any condition to Closing contemplated by this Agreement (but prior to being disregarded by clause (i) above) that required delivery by the Company or the above-signed of any agreement, instrument, certificate or the like or purchase price (as applicable) shall no longer be a condition and shall instead be an unconditional obligation of the Company or the above-signed (as applicable) to deliver such agreement, instrument, certificate or the like or purchase price (as applicable) to such other party on the Closing Date.
[SIGNATURE PAGES CONTINUE]
Exhibit 107
Calculation of Filing Fee Tables
Form S-1
(Form Type)
Panbela Therapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward Securities
| |
Security Type
|
|
Security Class Title
|
|
Fee Calculation
Rule
|
|
Amount
Registered
|
|
Proposed
Maximum
Offering Price
Per Unit
|
|
Maximum
Aggregate
Offering Price
(1)
|
|
Fee Rate
|
|
Amount of Registration Fee
|
|
Fees to be Paid
|
Equity
|
|
Common stock, $0.001 par value per share(2)
|
|
Rule 457(o)
|
|
|
|
|
|
$30,160,000(3)
|
|
0.00014760
|
|
$4,452
|
| |
Equity
|
|
Class E common stock purchase warrants(4)
|
|
Other
|
|
|
|
|
|
|
|
|
|
|
| |
Equity
|
|
Common stock, $0.001 par value per share, underlying Class E common stock purchase warrants
|
|
Rule 457(o)
|
|
|
|
|
|
$10,028,200
|
|
0.00014760
|
|
$1,481
|
| |
Equity
|
|
Class F common stock purchase warrants(4)
|
|
Other
|
|
|
|
|
|
|
|
|
|
|
| |
Equity
|
|
Common stock, $0.001 par value per share, underlying Class F common stock purchase warrants
|
|
Rule 457(o)
|
|
|
|
|
|
$52,780,000
|
|
0.00014760
|
|
$7,791
|
| |
Equity
|
|
Pre-funded warrants(4)
|
|
Other
|
|
|
|
|
|
|
|
|
|
|
| |
Equity
|
|
Common stock, $0.001 par value per share, underlying pre-funded warrants
|
|
Rule 457(o)
|
|
|
|
|
|
–(3)
|
|
0.00014760
|
|
–
|
| |
Total Offering Amounts
|
|
|
|
|
|
$92,968,200
|
|
|
|
$13,724
|
| |
Total Fees Previously Paid(5)
|
|
|
|
|
|
|
|
|
|
$8,856
|
| |
Total Fee Offsets
|
|
|
|
|
|
|
|
|
|
–
|
| |
Net Fee Due
|
|
|
|
|
|
|
|
|
|
$4,868 |
(1) Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) of the Securities Act of 1933, as amended (the “Securities Act”).
(2) Pursuant to Rule 416 under the Securities Act, the shares of common stock registered hereby also include an indeterminate number of additional shares of common stock as may from time to time become issuable by reason of stock split, stock dividends, recapitalizations, or other similar transactions.
(3) The proposed maximum aggregate offering price of the common stock will be reduced on a dollar-for-dollar basis based on the offering price of any pre-funded warrants issued in the offering, and the proposed maximum aggregate offering price of the pre-funded warrants to be issued in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any common stock issued in the offering. Accordingly, the proposed maximum aggregate offering price of the common stock, common warrants and pre-funded warrants (including the common stock issuable upon exercise of the pre-funded warrants), if any, is $30,160,000.
(4) No fee pursuant to Rule 457(g) of the Securities Act.
(5) A registration fee of $8,856 was previously paid in connection with the initial filing of the Registration Statement. Accordingly, the remaining $4,868 is being paid with this Amendment No. 2. The maximum aggregate offering price of shares of common stock underlying Class E common stock purchase warrants has been reduced and the maximum aggregate offering price of shares of common stock underlying Class F common stock purchase warrants has been increased to reflect the proposed reallocation of warrant coverage. The resulting full balances are reflected as Fees to be Paid notwithstanding the prior payment of a portion of the fee.
Panbela Therapeutics (NASDAQ:PBLA)
과거 데이터 주식 차트
부터 4월(4) 2024 으로 5월(5) 2024

Panbela Therapeutics (NASDAQ:PBLA)
과거 데이터 주식 차트
부터 5월(5) 2023 으로 5월(5) 2024
