UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of October 2023 (Report No. 5)
Commission file number: 001-38041
SCISPARC LTD.
(Translation of registrant’s name into English)
20 Raul Wallenberg Street, Tower A,
Tel Aviv 6971916 Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
CONTENTS
On October 18, 2023, SciSparc
Ltd. made available a presentation on its website. A copy of the presentation is attached hereto as Exhibit 99.1.
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
SciSparc Ltd. |
| |
|
|
| Date: October 18, 2023 |
By: |
/s/ Oz Adler |
| |
Name: |
Oz Adler |
| |
Title: |
Chief Executive Officer
and Chief Financial Officer |
3
Exhibit 99.1

CORPORATE PRESENTATION Nasdaq: SPRC October 2023

This presentation of SciSparc Ltd . (the “ Company ” or “ SciSparc ” ) contains “ forward - looking statements ” within the meaning of the Private Securities Litigation Reform Act and other securities laws . Words such as “ expects, ” “ anticipates, ” “ intends, ” “ plans, ” “ believes, ” “ seeks, ” “ estimates ” and similar expressions or variations of such words are intended to identify forward - looking statements . For example, the Company is using forward - looking statements when it discusses statements relating to : its objectives, plans, and strategies ; the expected timing of trials ; its product pipeline ; the research, development, and use of its platform technologies, products and product candidates ; its three programs in advanced clinical trials ; its pending patent applications in Europe, China and Japan ; the prospective growth markets in Tourette syndrome, status epilepticus, Alzheimer ’ s disease, autism spectrum disorder and pain and inflammation ; the ongoing study and full or partial results in the different phases of certain pre - clinical and clinical trials for alcohol use disorder and the aforementioned conditions ; the prospective benefits from its collaboration with Clearmind Medicine Inc . ( “ Clearmind ” or “ Clearmind Medicine ” ) ; and all statements (other than statements of historical facts) that address activities, events, or developments that the Company intends, expects, projects, believes, or anticipates will or may occur in the future . Forward - � looking statements are not historical facts, and are based upon management ’ s current expectations, beliefs and projections, many of which, by their nature, are inherently uncertain . Such expectations, beliefs and projections are expressed in good faith . However, there can be no assurance that management ’ s expectations, beliefs and projections will be achieved and actual results may differ materially from what is expressed in or indicated by the forward - looking statements . Forward - looking statements are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in the forward - looking statements . In addition, historical results of scientific research and clinical and preclinical trials do not guarantee that the conclusions of future research or trials will suggest identical or even similar conclusions . For a more detailed description of the risks and uncertainties affecting the Company, reference is made to the Company ’ s reports filed from time to time with the Securities and Exchange Commission including, but not limited to, the risks detailed in the Company ’ s annual report on Form 20 - F for the year ended December 31 , 20 22 . Forward - � looking statements speak only as of the date the statements are made . The Company assumes no obligation to update forward - looking statements to reflect actual results, subsequent events or circumstances, changes in assumptions or changes in other factors affecting forward - looking information except to the extent required by applicable securities laws . If the Company does update one or more forward - looking statements, no inference should be drawn that the Company will make additional updates with respect thereto or with respect to other forward - looking statements . The information contained herein does not constitute a prospectus or other offering document nor does it constitute or form part of any invitation or offer to sell, or any solicitation of any invitation or offer to purchase or subscribe for, any securities of the Company, nor shall the information or any part of it or the fact of its distribution form the basis of, or be relied on in connection with, any action, contract, commitment or relating thereto or to the securities of the Company . SAFE HARBOR STATEMENT �

SciSparc NASDAQ: SPRC � • 3 programs in advanced clinical trials • Cash and cash equivalents as of June 30, 2023: $2.08 million • Recently raised gross funds of $6.3 million in the equity capital markets • Consumer supplement activity, Wellution TM , generated approximately $2 million in revenues for the first half of 2023 • Collaboration in the psychedelic field • Strengthened IP portfolio: New patents granted - U.S., Japan, Europe and Australia
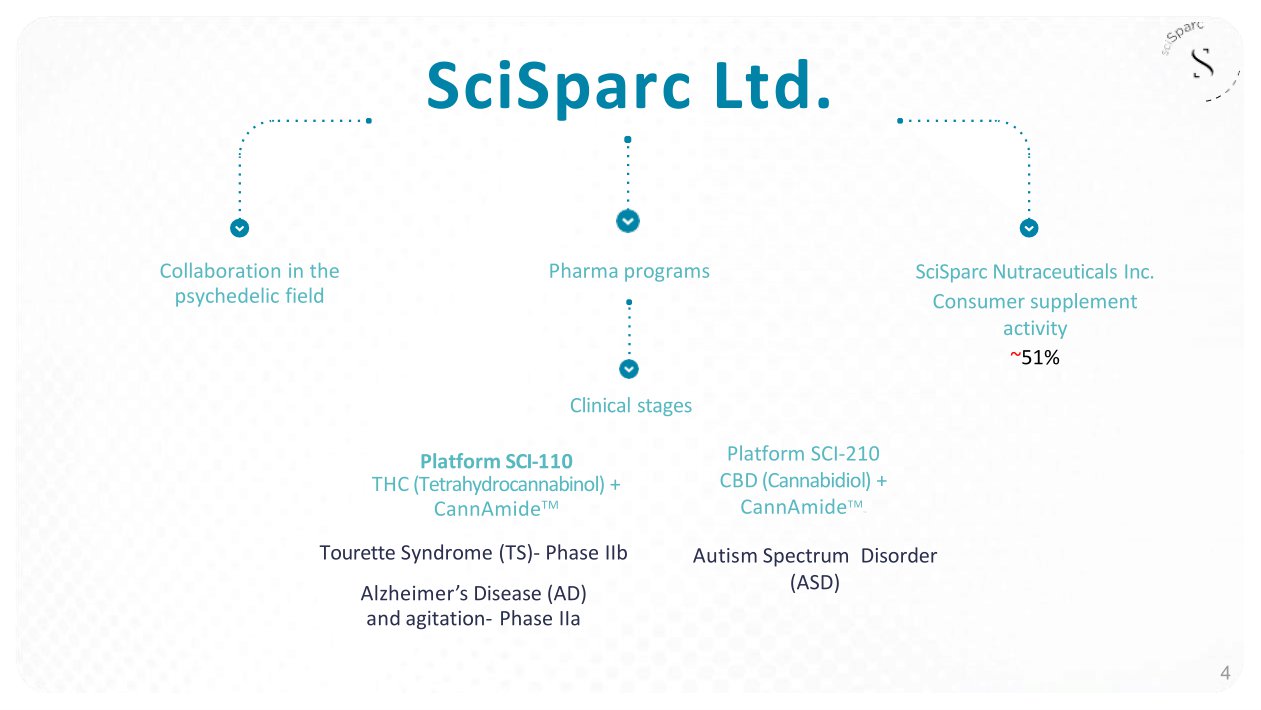
Pharma programs Autism Spectrum � Disorder (ASD) Tourette Syndrome (TS) - Phase IIb Alzheime r ’ s Diseas e (AD) and agitation - Phase IIa SciSparc Nutraceuticals Inc. Consumer supplement activity ~ 51 % Platform SCI - 210 CBD (Cannabidiol) + CannAmide TM TM � SciSparc Ltd. Clinical stages Platform SCI - 110 THC (Tetrahydrocannabinol) + CannAmide TM Collaboration in the psychedelic field

Ow n s t r ong IP portfolio 9 patent families ϭϱ granted patents ( ϳ in the U.S.) � Additional pending patent applications ( Europe, China Japan and more) INTELLECTUAL PROPERTY

Utilizing the Endocannabinoid system to affect the central nervous system (CNS) Combining CannAmide TM * with Different cannabinoids Formulation Ideal solution Reducing cannabinoids doses Maintaining therapeutic efficacy Increasing safety OUR METHODOLOGY * CannAmide TM - SciSparc proprietary formulation based on active pharmaceutical ingredient Palmitoyletanolamide �

Status Epilepticus The global epilepsy market size would grow from $ ϭϬ .6 B in 20 Ϯϭ to $ 14.9 B In 2031 ** � Pain and Inflammation The global chronic pain treatment market was estimated at US$ 81.36 billion in 2021 and is expected to surpass a valuation of US$ 191.61 billion by 2030 ***** Alzheimer ’ s Disease (AD) The global Alzheimer ’ s Disease therapeutics market size was valued at USD 4.05 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 19.99 % from 2023 to 2030 **** Tourette Syndrome (TS) Tourette Syndrome drugs market size is expected to grow by USD 598.85 million during 2022 - 2027 * Autism Spectrum Disorder (ASD) The global autism spectrum disorder therapeutics market is projected to grow from $ 2.01 billion in 2023 to $ 3.42 billion by 2030 , at a CAGR of 7.9 %*** MARKET OPPORTUNITY *https://www.technavio.com/report/tourettes - syndrome - drugs - market - analysis#:~:text=The% 20 global% 20 Tourettes% 20 syndrome% 20 drugs,government% 20 and% 20 non% 2 Dgovernment% 20 organizations ** https://www.transparencymarketresearch.com/epilepsy - therapeutics - market.html *** https://www.fortunebusinessinsights.com/industry - reports/autism - spectrum - disorder - therapeutics - market - 101207 **** https://www.grandviewresearch.com/industry - analysis/alzheimers - therapeutics - market ***** https://www.growthplusreports.com/report/chronic - pain - treatment - market/ 8030

2023 ACHIEVEMENTS Initiated our clinical trial with SCI - 210 in pediatrics with Autism Spectrum Disorder at the Soroka Medical Center Announced initiation of randomized Phase IIb clinical trial of our proprietary SCI - 110 for Tourette Syndrome Granted an exclusive license for the sales of CannAmide TM on the Amazon Marketplace in Canada to SciSparc Nutraceuticals Announced successful final Phase IIa Results, meeting end points of its Phase IIa Alzheimer ’ s Disease patients with agitation trial. Topline results using the Company's SCI - 110 met the trial ’ s primary and secondary end points, demonstrating high safety profile and reduced agitation Drug discovery Joint Venture MitoCareX Bio successfully developed its core algorithm to allow suitable modelling of human mitochondrial carriers to accelerate its cancer drug discovery program Acquired Wellution TM , Top Seller of American Food Supplements and Cosmetics Brand on Amazon �
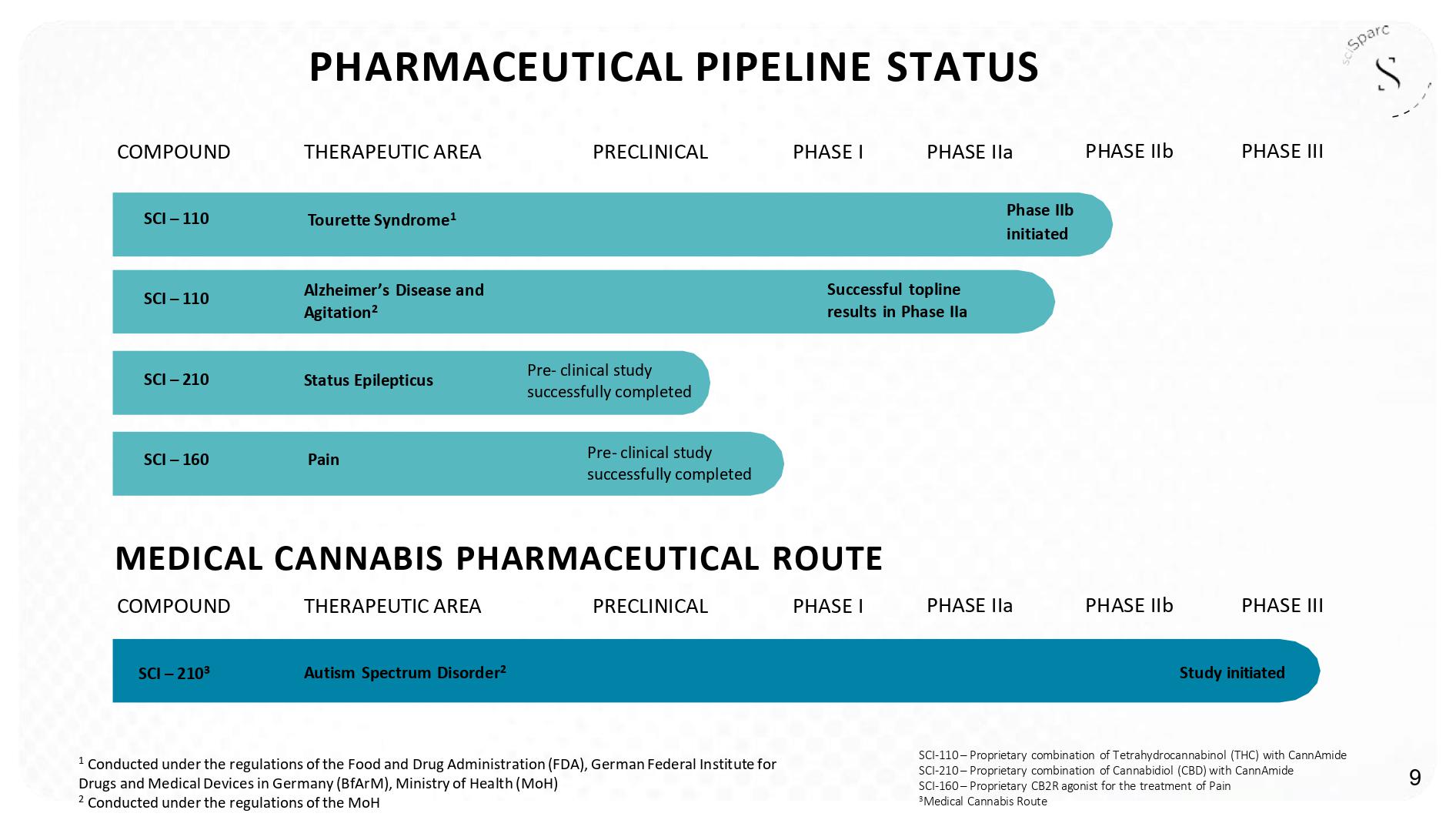
PHASE IIa C OMPOUN D THERAPEUTI C AR E A PR E CLINICA L PHASE I PHASE IIa PHASE IIb PHASE IIb PHASE III PHASE III SCI - 110 – Proprietary combination of T etrahydrocannabinol (THC) with CannAmide SCI - 210 – Proprietary combination of Cannabidiol (CBD) with CannAmide SCI - 160 – Proprietary CB 2 R agonist for the treatment of Pain *Medical Cannabis Route T ou r e t t e S ynd r ome * ���� Phase II b initiated Alzheimer ’ s Disease and Agitation ΎΎ Successful topline results in Phase IIa Status Epilepticus Pre - clinical study successfully completed Pain SCI – 110 SCI – 110 SCI – 210 SCI – 160 PHARMACEUTICAL PIPELINE STATUS Autism Spectrum Disorder ΎΎ Study initiated SCI – 210 MEDICAL CANNABIS PHARMACEUTICAL ROUTE C OMPOUN D THERAPEUTI C AR E A PR E CLINICA L PHASE I � Pre - clinical study successfully completed * Conducted under the regulations of the Food and Drug Administration (FDA), Federal Institute for Drugs and Medical Devices in Germany ( BfArM ), Ministry of Health (MoH) ** Conducted under the regulations of the MoH ** Conducted under the regulations of the MoH

SCI - 110 for T OURE T T E S YND R OM E (TS) PHASE IIA Led by Child Study Center at Yale University About : TS is a movement and neurobehavioral disorder characterized by motor and vocal tics and is highly linked with co - morbidities As the currently used medications are managing only a small number of disease symptoms with limited efficacy and questionable safety, there is a clear unmet medical need for the management of TS Results from our Phase II A c linical trial conducted in Yale University : An average tic reduction of 21 % across the entire sample with almost 40 % of the patients experienc ing greater than 25 % in tic reduction as defined by YGTSS - TTS (a clinician - rated instrument considered as the gold standard for assessing tics in patients with Tourette's Syndrome) Th e medi ca tio n w a s gene r all y w ell - t ole r at e d b y subjects 12 out of the 16 subjects elected to continue into a 24 - week extension phase of the trial ��

SCI - 110 for TS PHASE IIB • Objective: to evaluate the efficacy, safety and tolerability of the Company ’ s proprietary SCI - 110 in a randomized, double - blind, placebo controlled, cross - over study • Three leading medical centers: Yale Child Study Center at Yale University, Hannover Medical School, Hannover, Germany and Tel - Aviv Sourasky Medical Center, Tel - Aviv, Israel • 1:1 ratio randomization to receive either SCI - 110 or SCI - 110 matched placebo (i.e., THC active, CannAmide TM placebo), Design: 12 weeks treatment, washout period of 2 weeks, crossover for another 12 weeks • Primary efficacy: change in YGTSS - R - TTS 1 as a continuous endpoint at week 12 and week 26 of the double - blind phase compared to baseline. Primary safety: absolute and relative frequencies of Serious Adverse Events (SAEs) for the whole population and separately for SCI - 110 and placebo groups ��

SCI - 110 for ALZHEIMER ’ S � DISEASE (AD) & AGITATION Phase IIA Objective: to evaluate the safety, tolerability and efficacy trend of SCI - 110 in an open label study in patients with Alzheimer Disease (AD) and Agitation • P ositive final results meeting primary and secondary endpoints at Phase IIa study in AD patients with agitation - SCI - 110 administration was found to be safe at all tested doses. SCI - 110 treatment was also found to reduce agitation - 75 % of patients did not need to use additional medication to control agitation and 75 % of patients experienced increased appetite • Clinical site: Sophie & Abraham Stuchynski Israeli Alzheimer's Medical Center Israel • 20 patients were treated with SCI - 110 in a daily dose of up to 12.5 mg THC+ 800 mg PEA • Primary end point: safety and tolerability of SCI - 110 in AD patients with agitation. Main efficacy trend: The ability SCI - 110 to ameliorate agitation in patients with AD as measured by the Cohen Mansfield Agitation Inventory (CMAI) ��

• Study objective: to evaluate the safety, tolerability and efficacy of SCI - 210 in children with ASD in a randomized, double - blind, placebo controlled with cross - over study • Study site: the Clinical Research Center and Negev Autism Center and Soroka University Medical Center, Be'er - Sheva, Israel • Study design: A 20 - week, randomized double - blind placebo - controlled with cross - over clinical trial of 60 children • 30 participants for SCI - 210 , or SCI - 210 placebo (CBD active, CannAmide TM placebo) for 20 weeks, Washout for 2 weeks, Crossed over for additional 20 weeks SCI - 210 FOR AUTISM SPECTRUM DISORDER (ASD) Phase III* About: ASD is a condition related to brain development that impacts how a person perceives and socializes with others, causing problems in social interaction and communication. T he term "spectrum" in autism spectrum disorder refers to the wide range of symptoms and severity *Medical Cannabis Route �� • Three primary efficacy end points: The Aberrant Behavior Checklist - Community (ABC - C) parent questionnaire; The Clinical Global Impressions - Improvement (CGI - I) performed by a clinician; The effective therapeutic dose • Safety end point: Tolerability and adverse effects

Psychedelics collaboration with Clearmind Medicine

SCISPARC - CLEARMIND COLLABORATION SciSparc ’ s CannAmide Ρ and Clearmind ’ s MEAI The collaboration includes examination of the benefit of integrating the core technologies of each company for the development of innovative psychedelic - centric drug candidates targeting addiction treatment and mental health - related diseases CannAmide Ρ proprietary Palmitoylethanolamide ( PEA) formulation MEAI innovative psychedelic molecule
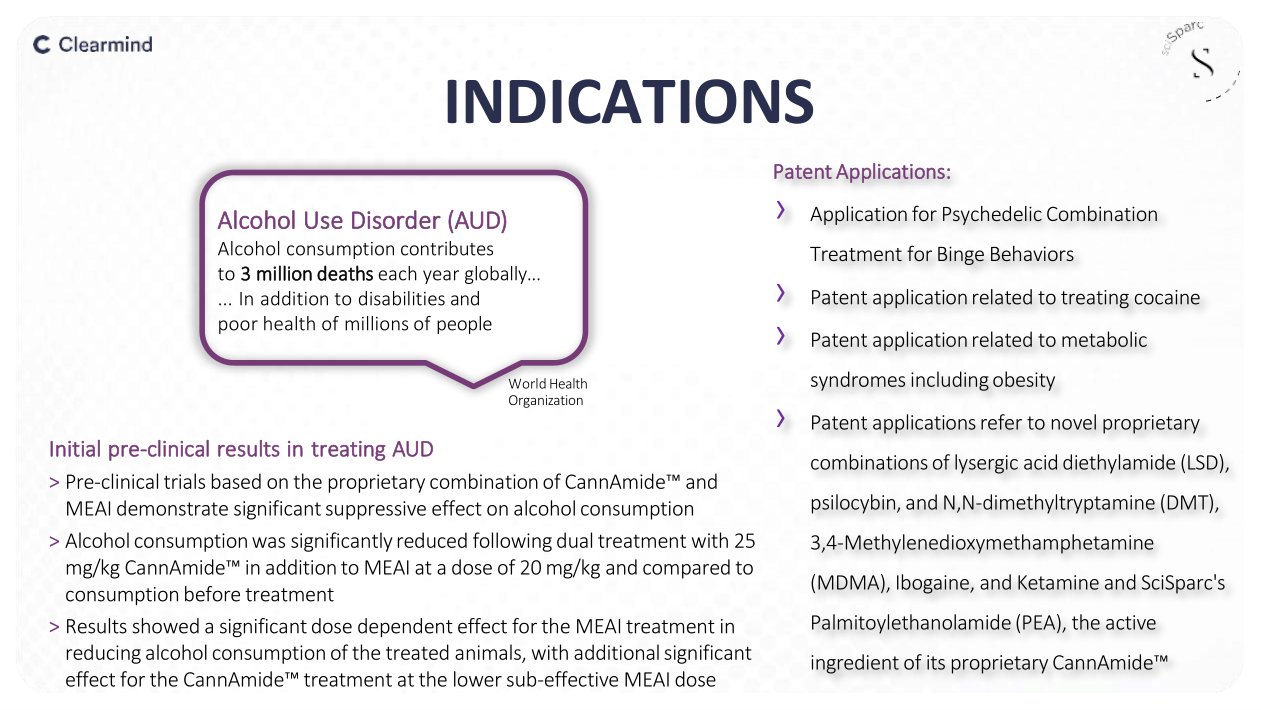
INDICATIONS Alcohol Use Disorder (AUD) Alcohol consumption contributes to 3 million deaths each year globally... ... In addition to disabilities and poor health of millions of people World Health Organization Initial pre - clinical results in treating AUD > Pre - clinical trials based on the proprietary combination of CannAmide Ρ and MEAI demonstrate significant suppressive effect on alcohol consumption > Alcohol consumption was significantly reduced following dual treatment with 25 mg/kg CannAmide Ρ in addition to MEAI at a dose of 20 mg/kg and compared to consumption before treatment > Results showed a significant dose dependent effect for the MEAI treatment in reducing alcohol consumption of the treated animals, with additional significant effect for the CannAmide Ρ treatment at the lower sub - effective MEAI dose Patent A pplications: › Application for Psychedelic Combination Treatment for Binge Behaviors › Patent application related to treating cocaine › Patent application related to metabolic syndromes including obesity › Patent applications refer to novel proprietary combinations of lysergic acid diethylamide (LSD), psilocybin, and N,N - dimethyltryptamine (DMT), 3,4 - Methylenedioxymethamphetamine (MDMA), Ibogaine, and Ketamine and SciSparc's Palmitoylethanolamide (PEA), the active ingredient of its proprietary CannAmide Ρ

Top Seller of American Food Supplements and Cosmetics Brand on Amazon. com

Wellution Ρ SciSparc Nutraceuticals Inc., a majority - owned subsidiary of SciSparc Ltd. (~ 51 %) , holds the Amazon Marketplace account, American food supplements and cosmetics brand and trademark of Wellution Wellution Ρ is strong health and beauty brand selling on Amazon US which was established in 2019 Wellution offers 8 variations of natural hemp candy supplements, creams and gels under two parent ASINs on Amazon US that are differentiated by their hemp oil potency The leading parent ASIN, that was launched in 2019 , has received over 26 , 500 reviews and since its launch has consistently been ranked as the # 1 best seller in its category on Amazon In total, the Brand has over 40 , 000 product reviews, a majority of which are 4 and 5 - star reviews .


O z Adler C E O , C F O Mr. Adler has experience in a wide variety of managerial, financial, tax and accounting practices. From 2012 to 2017 , Mr. Adler was employed as a certified public accountant at Kost Forer Gabbay & Kasierer , a member of Ernst & Young Global. Mr. Adler holds a B.A. degree in Accounting and Business management from the College of Management, Israel. Adi Zuloff - Shani, PhD CTO Dr. Zuloff - Shani is a Research & Development professional with overall experience of about 20 years in the bio - tech and healthcare industry. Dr. Zuloff - Shani has brought two products from bench to market; an immuno - cell - based product and a food - supplement and is currently leading the development of several pharmaceutical products designated to the U.S., EU, and Israeli markets. Dr. Zul off - Shani has extensive experience in research and development, manufacturing, clinical, and regulatory affairs. Dr. Zuloff - Shani holds a Ph.D. in human biology and immunology from Bar - Ilan University, Israel. Amitay Weiss Chairman Mr. Weiss serves as chairman of the board of directors of P.L.T Financial Services Ltd., as chairman of the board of director s o f Matomy Media Group Ltd. and as an external director of Cofix Group Ltd. In 2016 , Mr. Weiss founded Amitay Weiss Management Ltd. and now serves as its chief executive officer. Itschak Shrem President & Director Mr . Shrem has more than 40 years of experience in financial markets and venture capital . In 1991 , Mr . Shrem founded Dovrat Shrem Ltd . , an investment banking, management and technology company . Prior to that, he spent 15 years at Clal Israel Ltd . , where he served in various capacities, including chief operating officer, and was responsible for capital markets and insurance businesses . In 1993 , Mr . Shrem founded Pitango Venture Capital Fund (formerly, Polaris) and served as a partner of Pitango Funds I, II and III . He has been the Managing Director of Yaad Consulting 1995 Ltd . since 1995 . Mr . Shrem currently serves on the board of directors of Rail Visions Ltd . Previously, Mr . Shrem served on the board of Tel - Aviv Sourasky Medical Center, the Weizmann Institute Eden Spring Ltd . , Nano Dimension Ltd . , Ormat Industries Ltd . , Retalix Ltd . and as chairman of Sphera Funds Management Ltd . Mr . Shrem holds a B . A . in Economics and Accounting from Bar - Ilan University and an M . B . A . from Tel - Aviv University . SCISPARC ’ S MANAGEMENT TEAM ϮϬ
SCISPARC SCIENTIFIC ADVISORY BOARD Award winning scientists Ϯϭ Prof. James Leckman A child Psychiatrist at Yale University S erved as Director of the Child Study Center at Yale for over two decades A prominent international expert in the field of research and treatment of Tourette Syndrome Prof. Kirsten Muller - Vahl Professor of Psychiatry the Hannover Medical School, Germany R ecognized as the leading researcher in the field of cannabinoid use in treatment � of Tourette Syndrome S erved as a member of the scientific advisory board of the German Tourette Syndrome Association Dr. Michael Bloch Associate training director of the Child Study Center's Solnit Integrated Program, Yale School of Medicine N oted researcher on the study of Tourette Syndrome, obsessive - compulsive disorder and trichotillomania Dr. Daniele Piomelli The Editor - in - Chief of Cannabis and Cannabinoid Research S erves as Louise Turner Arnold Chair in Neurosciences Professor of Anatomy and Neurobiology, Pharmacology, and Biological Chemistry at University of California, � Irvine Prof. Joseph Tam, DMD, PhD Head of Metabolic Disorders Research Hebrew University, Jerusalem A world leader in the field of Cannabis based solutions and is the Director of the Multidisciplinary Center on Cannabinoid s in Israel
SUMMARY Proprietary Technology with cutting - edge drug combinations addressing large global unmet medical needs Two Phase 2 Assets addressing multi - billion markets and a Pending Phase 3 * Clinical Trial Positive final results meeting primary and secondary endpoints at Phase IIa study in AD patients Key strategic partners, including leading universities, medical centers and KOLs Revenue streamline from sales of Wellution products on the Amazon platform *Medical Cannabis Route ��

Thank You Investor Contact: IR@scisparc.com Tel: + 972 - 3 - 6167055 https://scisparc.com/ ��
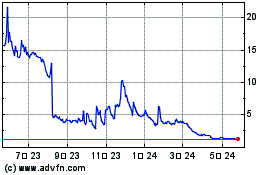
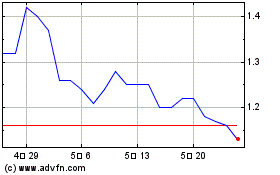
SciSparc (NASDAQ:SPRC)
과거 데이터 주식 차트
부터 4월(4) 2024 으로 5월(5) 2024
SciSparc (NASDAQ:SPRC)
과거 데이터 주식 차트
부터 5월(5) 2023 으로 5월(5) 2024