UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of July 2023
Commission file number: 001-39957
NLS PHARMACEUTICS LTD.
(Translation of registrant’s name into English)
The Circle 6
8058 Zurich, Switzerland
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form
40-F ☐
CONTENTS
Ordinary Shareholders’
Meeting Results
On June 30, 2023, NLS Pharmaceutics
Ltd., or the Registrant, convened an ordinary shareholders’ meeting, or the Meeting. At the Meeting, a quorum was present, and the
shareholders of the Registrant approved all agenda items as originally proposed.
Appointment of New Directors
On June 30, 2023, the Registrant
announced that Mrs. Audrey Greenberg and Mr. Anthony Walsh have been appointed as directors, effective as of June 30, 2023.
Ms. Greenberg is the Executive
Managing Director and board member of the Discovery Labs Center for Breakthrough Medicines, an integrated life science innovation hub,
a position she has held since 2019. Ms. Greenberg is also a member of the Board of Directors for New York Mortgage Trust (NASDAQ: NYMT), a position she has held since 2021. Ms. Greenberg
received her Bachelor of Science in Business Administration in accounting and finance from the University of Arizona and her Master’s
in Business Administration from the Wharton School of the University of Pennsylvania.
Mr. Walsh is the Chief
Experience Officer of Stealth Biotech, a position he has held since 2022. Mr. Walsh has also served as the Managing Director of Lyfe
Capital, a position he held from 2021 until 2022. Mr. Walsh received his Bachelor of Arts in Biochemistry from Trinity College,
Dublin, and a Ph.D. in Biophysics from Oxford University.
Press Releases
On June 30, 2023, the Registrant
issued a press release titled: “NLS Pharmaceutics Releases the Results of its Annual General Meeting.” A copy of this press
release is furnished herewith as Exhibit 99.1. In addition, on June 30, 2023, the Registrant issued a press release titled: “NLS
Pharmaceutics Company Update and Webcast Today Postponed.” A copy of this press release is furnished herewith as Exhibit 99.2.
On July 3, 2023, the Registrant
issued a press release titled: “NLS Pharmaceutics to Proceed with Phase 3 Clinical Program (AMAZE) for Mazindol ER for the Treatment
of Narcolepsy Following FDA Approval and IRB Approval of the Full Study Protocol.” A copy of this press release is furnished herewith
as Exhibit 99.3.
This
Report of Foreign Private Issuer on Form 6-K is incorporated by reference into the Registrant’s Registration Statements on Form
F-3 (File No. 333-262489, and 333-268690 and 333-269220), filed
with the Securities and Exchange Commission, to be a part thereof from the date on which this report is submitted, to the extent not superseded
by documents or reports subsequently filed or furnished.
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
NLS Pharmaceutics Ltd. |
| |
|
|
| Date: July 3, 2023 |
By: |
/s/ Alexander Zwyer |
| |
|
Name: |
Alexander Zwyer |
| |
|
Title: |
Chief Executive Officer |
2
Exhibit 99.1

NLS Pharmaceutics Releases the Results from
its Annual General Meeting
Zürich, Switzerland,
June 30, 2023 – NLS Pharmaceutics Ltd. (Nasdaq: NLSP, NLSPW) (“NLS” or the “Company”), a Swiss clinical-stage
biopharmaceutical company focused on the discovery and development of innovative therapies for patients with rare and complex central
nervous system disorders, today announced that the NLS Shareholders approved all of the Board of Directors’ proposals for the 2023
Annual General Meeting (AGM) that took place in Zürich, Switzerland today. This included the election of Audrey Greenberg and Dr.
Anthony Walsh to the Board of Directors.
No shareholders or their
proxies attended the AGM in person and 64% percent of the shares entitled to votes were represented. The Board of Directors received the
highest voting approval in the Company’s history of 99.5% of votes cast in favor of the proposals.
“I am very grateful
for the continuous support of our shareholders and specifically the overarching endorsement of our strategic development plan as we move
into a new phase of NLS and commence the Phase 3 program for Mazindol ER,” commented Ronald Hafner, Chairman of the Board of Directors.
Audrey Greenberg, Co-Founder
and Chief Business Officer, Center for Breakthrough Medicines, and Anthony Walsh, PhD, Chief Business Officer of Ability Biologics, were
both elected to the NLS Pharmaceutics Board of Directors, broadening the board membership to include U.S. repesentation. The Chairman
and all other members of the Board of Directors were re-elected for a term of office until the end of the next Annual General Meeting.
Shareholders also elected the members of the Compensation Committee for a one-year term.
Shareholders approved
the financial statements, the compensation report, and the balance sheet results of the Company for the fiscal year 2022. Shareholders
also approved the total compensation budgets for NLS Pharmaceutics’ Board of Directors and Executive Management for the financial
year 2024. PricewaterhouseCoopers AG was re-elected as NLS’ independent auditors for
another term lasting until the next Annual General Meeting.
All information on the
AGM can be found on the company website here: https://nlspharma.com/investors/agm-2023/.
About NLS Pharmaceutics
Ltd.
NLS Pharmaceutics Ltd.
(Nasdaq: NLSP) is a global development-stage biopharmaceutical company, working with a network of world-class partners and internationally
recognized scientists, focused on the discovery and development of innovative therapies for patients with rare and complex central nervous
system disorders who have unmet medical needs. Headquartered in Switzerland and founded in 2015, NLS is led by an experienced management
team with a track record of developing and commercializing product candidates. For more information, please visit www.nlspharma.com.
For additional information:
Marianne Lambertson (investors & media)
NLS Pharmaceutics Ltd.
+1 239.682.8500
ml@nls-pharma.com
www.nlspharmaceutics.com
###
Exhibit 99.2

NLS to Webcast its
Event on Thursday, July 6, 2023, at 11:00 am ET
ZURICH, SWITERLAND
/ ACCESSWIRE / June 30, 2023 / NLS Pharmaceutics Ltd. (NASDAQ:NLSP)(NASDAQ:NLSPW) (“NLS” or the “Company”),
a Swiss clinical-stage biopharmaceutical company focused on the discovery and development of innovative therapies for patients with rare
and complex central nervous system disorders, today announced it will postpone its webcast previously scheduled for today due to important
developments impacting the timing of the company update. The webcast will now take place on Thursday, July 6, 2023, at 11:00AM ET. Members
of the NLS Leadership Team will discuss the global strategic research and development progress and platform, including:
| ● | Feedback from the SLEEP 2023 APSS Conference |
| ● | Initiation of the Phase 3 program AMAZE for Mazindol ER |
| ● | Pipeline Goals for 2023/2024 |
Webcast Information
The event will be held
July 6, 2023, at 11:00 am ET and will include a video stream on the Investors section of the Company’s website found here or
at the SummitCast event page found here. A replay will be available on NLS’ website within 48 hours after the event.
About NLS Pharmaceutics Ltd.
NLS Pharmaceutics Ltd.
(Nasdaq:NLSP) is a global development-stage biopharmaceutical company, working with a network of world-class partners and internationally
recognized scientists, focused on the discovery and development of innovative therapies for patients with rare and complex central nervous
system disorders who have unmet medical needs. Headquartered in Switzerland and founded in 2015, NLS is led by an experienced management
team with a track record of developing and commercializing product candidates. For more information, please visit www.nlspharma.com.
For additional information:
Marianne Lambertson (investors & media)
NLS Pharmaceutics Ltd.
+1 239.682.8500
ml@nls-pharma.com
www.nlspharma.com
SOURCE: NLS Pharmaceutics AG
Exhibit 99.3

NLS Pharmaceutics to Proceed with Phase 3 Clinical
Program (AMAZE) for
Mazindol ER for the Treatment of Narcolepsy Following FDA Review and IRB Approval of the Full Study Protocol
Zürich, Switzerland,
July 3, 2023 – NLS Pharmaceutics Ltd. (Nasdaq: NLSP, NLSPW) (“NLS” or the “Company”), a Swiss clinical-stage
biopharmaceutical company focused on the discovery and development of innovative therapies for patients with rare and complex central
nervous system disorders, today announced that the U.S. Food and Drug Administration (FDA) has reviewed the full protocol for the NLS-1031
study, part of the Phase 3 program for Mazindol ER, called AMAZE. In addition, the Company is pleased to announce that the Phase 3 clinical
trial protocol to evaluate the safety and efficacy of Mazindol ER in patients with narcolepsy type 1 received approval from the independent
Institutional Review Board (“IRB”). The AMAZE Program will encompass two almost-identical double-blind Phase 3 studies (N=50
each) investigating Mazindol ER versus placebo in adult patients with narcolepsy commencing this summer at multiple sites exclusively
in the U.S.
Based on the FDA’s recommendations,
both Phase 3 trials will measure the weekly cataplexy episodes as the primary endpoint over 8 weeks of treatment and excessive daytime
sleepiness as a secondary objective using the Patient-Reported Outcomes Measurement Information System (PROMIS-SRI) and the Epworth Sleepiness
Scale (ESS).
“In addition to
IRB approval of the Phase 3 study protocol for AMAZE obtained last week, with this regulatory milestone acheived, we can recruit U.S.
clinical sites quickly and efficiently, allowing us to move forward with providing Mazindol ER to patients with narcolepsy type 1,”
commented George Apostal, MD, MS, Chief Medical Officer of NLS.
Patients who complete
these studies will be offered participation in a 12-month open-label extension (OLE) study To be eligible for enrollment into the OLE
study, patients must be at least 18 years of age and have been diagnosed with narcolepsy with cataplexy.
Alex Zwyer, Chief Executive
Officer of NLS, said, “We are pleased with the FDA’s review of the Phase 3 protocol and now expect to move quickly to begin
enrolling patients in the AMAZE program in centers across the U.S. in the coming days.”
Fore more information
on the AMAZE Program, please visit https://amaze.nlspharma.com/
NLS previously reported
on the Phase 2 study results in narcolepsy in which Mazindol ER met all primary and secondary endpoints. Patients treated with Mazindol
ER in the randomized Phase 2 trial showed continued improvement after rolling over into the OLE study and patients treated with placebo
in the randomized Phase 2 trial and who subsequently received Mazindol ER in the OLE study showed similar efficacy with the Mazindol ER-treated
patients in the randomized trial. Data from the Phase 2 studies were presented in early June at SLEEP 2023, the annual meeting of the
American Academy of Sleep Medicine (AASM) and the Sleep Research Society (SRS). A recording of the Phase 2 data presentation can be found
here: https://nlspharma.com/news/nls-satellite-symposium/
An IRB operates under
FDA regulations and is an FDA registered constituted group that has been formally designated to review and monitor biomedical research
involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications (to secure approval),
or disapprove research. The purpose of IRB review is to assure, both in advance and by periodic review, that appropriate steps are taken
to protect the rights and welfare of humans participating as subjects in the research. To accomplish this purpose, IRBs use a group process
to review research protocols and related materials (e.g., informed consent documents and investigator brochures) to ensure the protection
of the rights and welfare of human subjects of research.
About NLS Pharmaceutics
Ltd.
NLS Pharmaceutics Ltd.
(Nasdaq: NLSP) is a global development-stage biopharmaceutical company, working with a network of world-class partners and internationally
recognized scientists, focused on the discovery and development of innovative therapies for patients with rare and complex central nervous
system disorders, who have unmet medical needs. Headquartered in Switzerland and founded in 2015, NLS is led by an experienced management
team with a track record of developing and commercializing product candidates. For more information, please visit www.nlspharma.com.
About Mazindol ER
The Company’s lead product
candidate, Mazindol ER, is a proprietary extended-release formulation of mazindol and is being developed for the treatment of narcolepsy,
and potentially other sleep-wake disorders such as Idiopathic Hypersomnia (IH), for which NLS recently obtained Orphan Disease Designation
(ODD) from the FDA and the European Medicines Agency (EMA). Mazindol is a triple monoamine reuptake inhibitor and partial Orexin-2 Receptor
agonist, which was used for many years to treat patients diagnosed with narcolepsy in compassionate use programs. A Phase 2 multi-center
U.S. clinical study evaluating Mazindol ER in adult patients suffering from narcolepsy met its primary endpoint with high statistical
significance and demonstrated a favorable safety and tolerability profile. NLS also successfully completed a Phase 2 study in the U.S.
evaluating Mazindol Controlled-Release in adult patients suffering from Attention Deficity/Hyperacitvity Disorder. The study met all primary
and secondary endpoints and was well-tolerated. Mazindol ER has received Orphan Drug Designations both in the U.S. and in Europe for
the treatment of narcolepsy. Up to 1/3 of narcoleptic patients are also diagnosed with ADHD.
Safe Harbor Statement
This press release contains expressed or implied
forward-looking statements pursuant to U.S. Federal securities laws. For example, NLS is using forward-looking statements when it discusses
the potential benefits of Mazindol ER, and the timing and the expected format of the AMAZE Phase 3 clinical program. These forward-looking
statements and their implications are based on the current expectations of the management of NLS only and are subject to a number of factors
and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following
factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes
in technology and market requirements; NLS may encounter delays or obstacles in launching and/or successfully completing its clinical
trials; NLS’ products may not be approved by regulatory agencies, NLS’ technology may not be validated as it progresses further
and its methods may not be accepted by the scientific community; NLS may be unable to retain or attract key employees whose knowledge
is essential to the development of its products; unforeseen scientific difficulties may develop with NLS’ process; NLS’ products
may wind up being more expensive than it anticipates; results in the laboratory may not translate to equally good results in real clinical
settings; results of preclinical studies may not correlate with the results of human clinical trials; NLS’ patents may not be sufficient;
NLS’ products may harm recipients; changes in legislation may adversely impact NLS; inability to timely develop and introduce new
technologies, products and applications; and loss of market share and pressure on pricing resulting from competition, which could cause
the actual results or performance of NLS to differ materially from those contemplated in such forward-looking statements. Except as otherwise
required by law, NLS undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events
or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks
and uncertainties affecting NLS is contained under the heading “Risk Factors” in NLS’ annual report on Form 20-F for
the year ended December 31, 2022 filed with the Securities and Exchange Commission (SEC), which is available on the SEC’s website,
www.sec.gov, and in subsequent filings made by NLS with the SEC.
For additional information:
Marianne Lambertson (investors & media)
NLS Pharmaceutics Ltd.
+1 239.682.8500
ml@nls-pharma.com
www.nlspharmaceutics.com
###
3
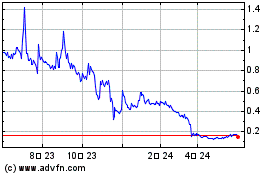
NLS Pharmaceutics (NASDAQ:NLSP)
과거 데이터 주식 차트
부터 1월(1) 2025 으로 2월(2) 2025
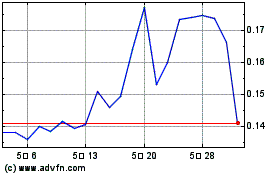
NLS Pharmaceutics (NASDAQ:NLSP)
과거 데이터 주식 차트
부터 2월(2) 2024 으로 2월(2) 2025