
1 Corporate & Clinical Overview November 2023

2 Forward Looking Statements This presentation contains forward-looking statements of Lumos Pharma, Inc. that involve substantial risks and uncertainties. All such statements contained in this presentation are forward- looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. This law that, in part, gives us the opportunity to share our outlook for the future without fear of litigation if it turns out our predictions were not correct. We are passionate about our business - including LUM-201 and the potential it may have to help patients in the clinic. This passion feeds our optimism that our efforts will be successful and bring about meaningful change for patients. Please keep in mind that actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make. We have attempted to identify forward-looking statements by using words such as “projected,” "upcoming," "will," “would,” "plan," “intend,” "anticipate," "approximate," "expect," “potential,” “imminent,” and similar references to future periods or the negative of these terms. Not all forward-looking statements contain these identifying words. Examples of forward-looking statements include, among others, statements we make regarding the plan to have an end-of-phase 2 meeting with the FDA in the first half of 2024 and the anticipated initiation of a Phase 3 program in the second half of 2024, our Phase 2 data providing a clear path to Phase 3 in PGHD, that PEMs enrich trials for patients likely to respond to LUM-201, the expected benefits to LUM-201, and any other statements other than statements of historical fact. We wish we were able to predict the future with 100% accuracy, but that just is not possible. Our forward-looking statements are neither historical facts nor assurances of future performance. You should not rely on any of these forward-looking statements and, to help you make your own risk determinations, we have provided an extensive discussion of risks that could cause actual results to differ materially from our forward-looking statements including risks related to the continued analysis of data from our LUM-201 Trials, the timing and outcome of our future interactions with regulatory authorities including our end of Phase 2 meeting with the FDA, the timing and ability of Lumos to raise additional equity capital as needed to fund our Phase 3 Trial, our ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the ability to structure our Phase 3 trial in an effective and timely manner, any statements regarding potential enrollment timelines, the ability to successfully develop our LUM-201 product candidate, the effects of pandemics, other widespread health problems or military conflicts including the Ukraine-Russia conflict and the Middle East conflict and other risks that could cause actual results to differ materially from those matters expressed in or implied by such forward-looking statements including information in the "Risk Factors" section and elsewhere in Lumos Pharma’s Quarterly Report on Form 10-Q for the period ended September 30, 2023, as well as other reports filed with the SEC including our subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. All of these documents are available on our website. Before making any decisions concerning our stock, you should read and understand those documents. We anticipate that subsequent events and developments will cause our views to change. We may choose to update these forward-looking statements at some point in the future, however, we disclaim any obligation to do so. As a result, you should not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. 11.14.2023

3 Overview Lead asset targeting children with growth disorders Novel Oral Rare Disease Asset Pipeline in a Product Solid Financial Position • Novel oral therapeutic asset, LUM-201, for growth hormone deficiency (GHD) disorders • LUM-201 acts within natural endocrine pathway, differentiated from injectable therapies • Worldwide injectable market for GHD disorders is $3.4 billion, excluding China* • Market for Pediatric GHD (PGHD), initial oral LUM-201 indication, is $1.2 billion* • Cash balance of $42.7 million as of close of 3Q 2023 • Cash runway through 3Q 2024 Late-stage Trials in PGHD • Topline data from two Phase 2 OraGrowtH Trials in PGHD met all endpoints • Growth on 1.6 mg/kg LUM-201 in line with historical benchmarks and expectations • Ph 2 data provided preliminary validation of PEMs to identify likely LUM-201 responders** PGHD = Pediatric Growth Hormone Deficiency * USA, Germany, France, Italy, Spain, UK, Japan (Grandview Research, Growth Hormone Market Forecast, 2019). China GHD market estimated at $1 billion. ** PEM (Predictive Enrichment Marker) strategy consists of screening for PEM+ PGHD patients = Baseline IGF-1 > 30 ng/ml & Peak stimulation GH ≥ 5 ng/ml from single oral dose of LUM-201 Potential for 1st oral therapeutic to disrupt injectable market for GHD Program Advancement • End-of-Phase 2 meeting with FDA anticipated 1H 2024 to review Phase 3 program • Initiation of Phase 3 trial anticipated 2H 2024

4 Richard Hawkins Chairman & CEO Developed Growth Hormone (GH) Receptor Antagonist for Acromegaly at Sensus (sold to Pfizer). Built one of the first contract recombinant protein manufacturing facilities (Covance Biotechnology). Founder of Pharmaco, a pioneer in the contract research organization sector (merged with PPD). John McKew, PhD President & Chief Scientific Officer Prior VP of Research at aTyr Pharma – led team advancing protein-based therapeutics for rare diseases. Former Scientific Director, NIH - National Center for Advancing Translational Science (NCATS) and Therapeutics for Rare and Neglected Diseases (TRND). Lori Lawley, CPA Chief Financial Officer Former SVP, Finance and Controller at Lumos Pharma. Previously, SVP, Finance and Member of the Office of the CEO of NewLink Genetics. Prior to that, Senior Manager in Assurance Services at Ernst and Young. Aaron Schuchart, MBA Chief Business Officer Former Chief Business Officer of Aeglea BioTherapeutics. Former leadership roles in Business Development, Strategy, and Finance at Coherus Biosciences, Novartis Diagnostics/Grifols, and Amgen. Pisit “Duke” Pitukcheewanont, MD SVP Global Clinical Development and Medical Affairs Pediatric endocrinologist and Professor, Clinical Pediatrics, Keck School of Medicine, USC. President, Human Growth Foundation. Former VP Medical Affairs and VP Global Medical Ambassador & Medical Education at Ascendis Pharma; project: long-acting TransCon GH. Former Advisory Board member at Pfizer, Ipsen, Alexion, Ultragenyx, Pharmacia, Serono, others. Peter Clayton, MD, PhD Senior Medical Advisor and CSAB Member Professor of Child Health and Paediatric Endocrinology, University of Manchester. Prior member of Councils of GH Research Society, Society for Endocrinology UK, and European Society of Paediatric Endocrinology. Served as Chair of ESPE Corporate Liaison Board. Authored over 300 publications on clinical and scientific aspects of paediatric endocrinology. Michael Thorner, MB, BS, DSC VP Endocrine Sciences Endocrinologist. Former Chairman of Dept of Medicine, Chief of Division of Endocrinology & Metabolism, Director Clinical Research Center at University of Virginia. Led research group investigating GH secretion regulation. Discovered GH releasing hormone. Instrumental in early studies of LUM-201 (MK-0677). Pioneered use of dopamine agonist drugs for prolactin secreting pituitary tumors. Management and Advisors – Significant Clinical Development and Commercial Experience

5 LUM-201 Program Pipeline Study Pre-Clinical Phase 1 Phase 2 Phase 3 Status LUM-201 (Ibutamoren) in Moderate PGHD* Dose-finding trial Phase 2 Topline Data: Primary and secondary endpoints met (Nov 2023) Long-term extension Long-term extension study for OraGrowtH Trials: Ongoing enrollment of patients from Phase 2 trials PK/PD trial Phase 2 Topline Data: Primary and secondary endpoints met (Nov 2023) Switch trial Switch trial evaluating LUM-201 in subjects from rhGH arm of OraGrowtH210 Trial: Ongoing LUM-201 in NAFLD** Phase 2 pilot trial Pilot trial initiated by Mass Gen Hospital (MGH) evaluating LUM-201 in NAFLD: Enrolling Lumos Pharma is evaluating additional indications for LUM-201 for Phase 2 studies * PGHD Pediatric Growth Hormone Deficiency **NAFLD Non-Alcoholic Fatty Liver Disease ***Trial supported by prior data evaluating rhGH in NAFLD: (ENDO 2022) JES, Volume 6, Issue Supplement_1, November-December 2022, Page A525, and JES, June 2023. MGH pilot trial***
6 Pediatric Growth Hormone Deficiency (PGHD) – Conversion from Injection to Oral 1 GlobalData EpiCast Report for Growth Hormone Deficiency Epidemiology forecast to 2026 2 Rosenfeld 2008 Endocrine Practice 3 Cutfield 2011 PLOS ONE Inadequate secretion of growth hormone during childhood • Majority of cases are moderate • Slower physical growth • Negative effect on metabolic processes • Incidence ≈ 1:35001 Injectable therapies are only options • Daily, subcutaneous injections of recombinant human growth hormone (rhGH) represent standard of care • Weekly rhGH injections are entering the market Standard treatment is ~2,500 daily injections over multi-year period • Injections can be painful and burdensome • Missed doses lead to suboptimal growth2,3 • Initial market research supports oral therapy vs weekly injections What is PGHD? Current Treatment Unmet Need An established market is now primed for the first oral alternative
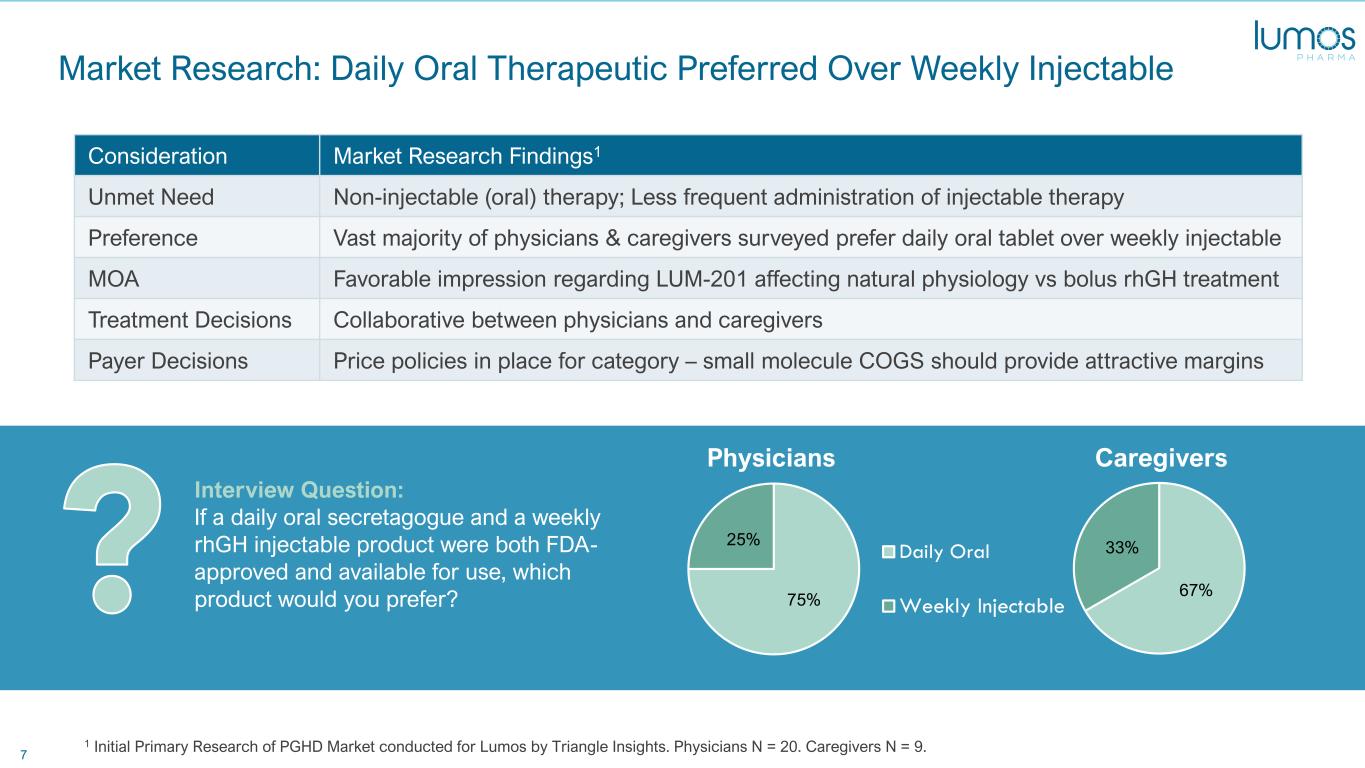
7 Market Research: Daily Oral Therapeutic Preferred Over Weekly Injectable 1 Initial Primary Research of PGHD Market conducted for Lumos by Triangle Insights. Physicians N = 20. Caregivers N = 9. 75% 25% Interview Question: If a daily oral secretagogue and a weekly rhGH injectable product were both FDA- approved and available for use, which product would you prefer? 67% 33%Daily Oral Weekly Injectable Physicians Caregivers Consideration Market Research Findings1 Unmet Need Non-injectable (oral) therapy; Less frequent administration of injectable therapy Preference Vast majority of physicians & caregivers surveyed prefer daily oral tablet over weekly injectable MOA Favorable impression regarding LUM-201 affecting natural physiology vs bolus rhGH treatment Treatment Decisions Collaborative between physicians and caregivers Payer Decisions Price policies in place for category – small molecule COGS should provide attractive margins

8 LUM-201 Stimulates Natural Growth Hormone Secretion • LUM-201 is an oral GH secretagogue* • Acts on specific receptors in hypothalamus and pituitary to stimulate release of GH1 • Increases the amplitude of natural pulsatile GH secretion, 2,3 normalizing GH levels after 6 months on therapy4 • LUM-201 stimulated GH release regulated by natural GH/IGF-1 feedback mechanisms • Differentiated mechanism versus exogenous injection of recombinant human growth hormone (rhGH) products LUM-201 mimics natural release of growth hormone (GH) Different from injections of synthetic GH 1 Howard 1996 Science 2 Nass 2008 Ann Intern Med 3 Chapman 1997 J Clin Endocrinol Metab 4 Supported by Lumos Pharma Topline Phase 2 Data * GH secretagogue = molecule that stimulates the secretion of growth hormone (GH)
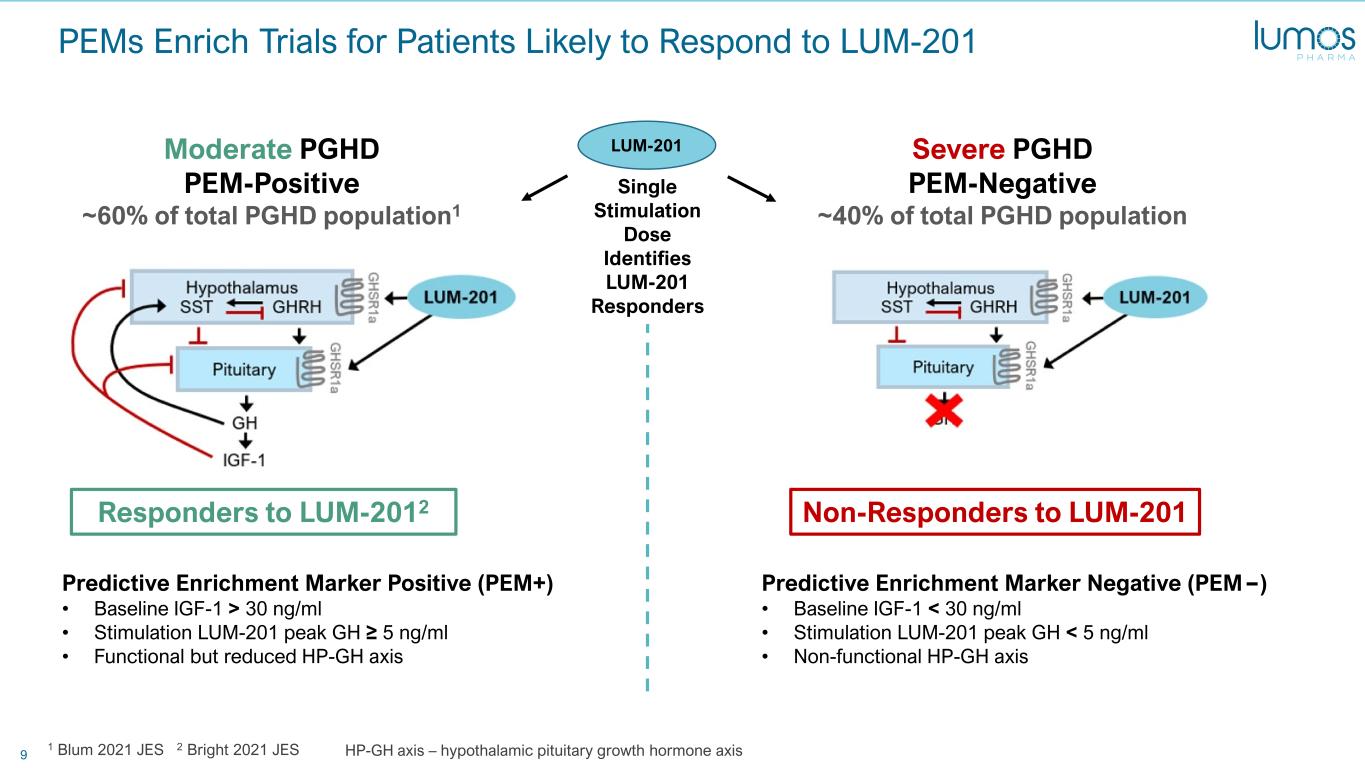
9 PEMs Enrich Trials for Patients Likely to Respond to LUM-201 Responders to LUM-2012 Non-Responders to LUM-201 Moderate PGHD PEM-Positive ~60% of total PGHD population1 Severe PGHD PEM-Negative ~40% of total PGHD population Predictive Enrichment Marker Positive (PEM+) • Baseline IGF-1 > 30 ng/ml • Stimulation LUM-201 peak GH ≥ 5 ng/ml • Functional but reduced HP-GH axis Predictive Enrichment Marker Negative (PEM ) • Baseline IGF-1 < 30 ng/ml • Stimulation LUM-201 peak GH < 5 ng/ml • Non-functional HP-GH axis Single Stimulation Dose Identifies LUM-201 Responders 1 Blum 2021 JES 2 Bright 2021 JES HP-GH axis – hypothalamic pituitary growth hormone axis LUM-201
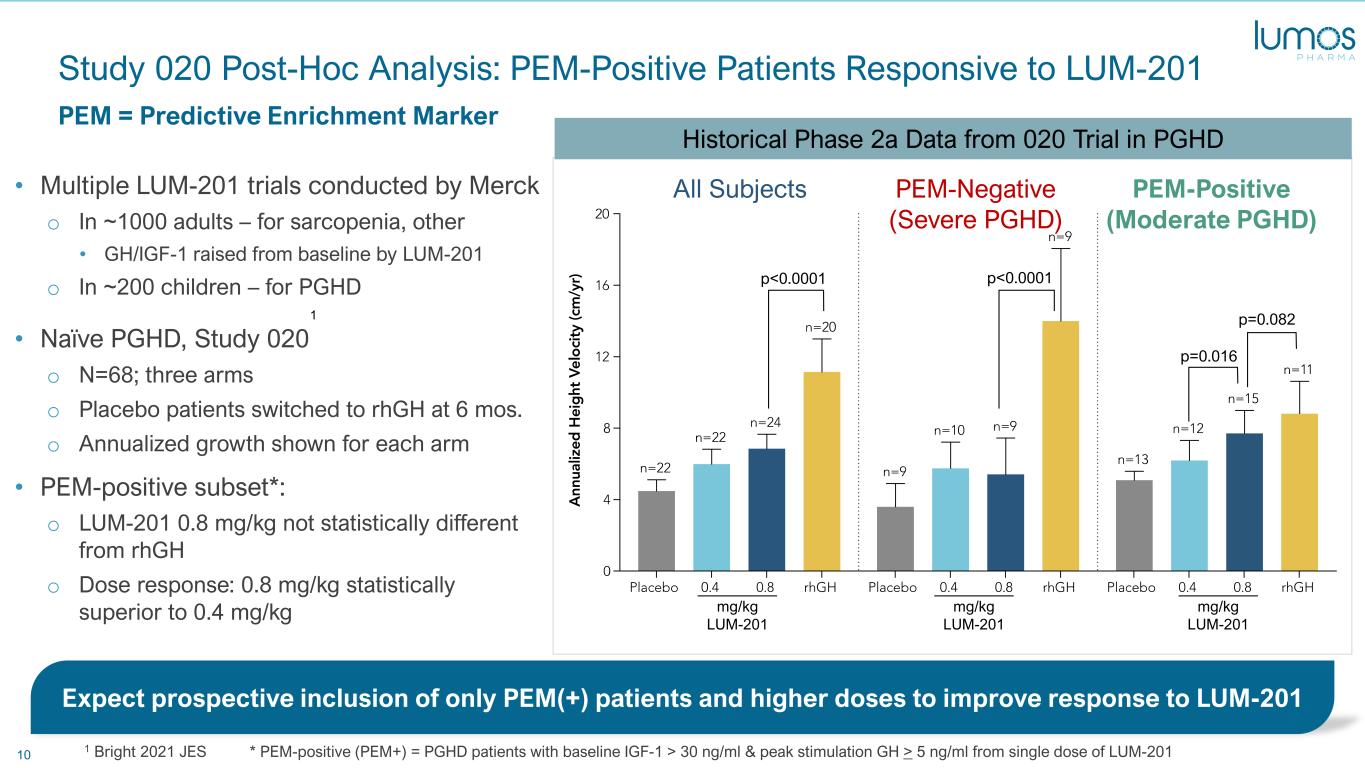
10 Study 020 Post-Hoc Analysis: PEM-Positive Patients Responsive to LUM-201 • Multiple LUM-201 trials conducted by Merck o In ~1000 adults – for sarcopenia, other • GH/IGF-1 raised from baseline by LUM-201 o In ~200 children – for PGHD • Naïve PGHD, Study 020 o N=68; three arms o Placebo patients switched to rhGH at 6 mos. o Annualized growth shown for each arm • PEM-positive subset*: o LUM-201 0.8 mg/kg not statistically different from rhGH o Dose response: 0.8 mg/kg statistically superior to 0.4 mg/kg PEM = Predictive Enrichment Marker 1 Bright 2021 JES * PEM-positive (PEM+) = PGHD patients with baseline IGF-1 > 30 ng/ml & peak stimulation GH > 5 ng/ml from single dose of LUM-201 All Subjects PEM-Negative (Severe PGHD) PEM-Positive (Moderate PGHD) mg/kg LUM-201 mg/kg LUM-201 mg/kg LUM-201 p<0.0001 p<0.0001 p=0.082 p=0.016 Expect prospective inclusion of only PEM(+) patients and higher doses to improve response to LUM-201 1 Historical Phase 2a Data from 020 Trial in PGHD
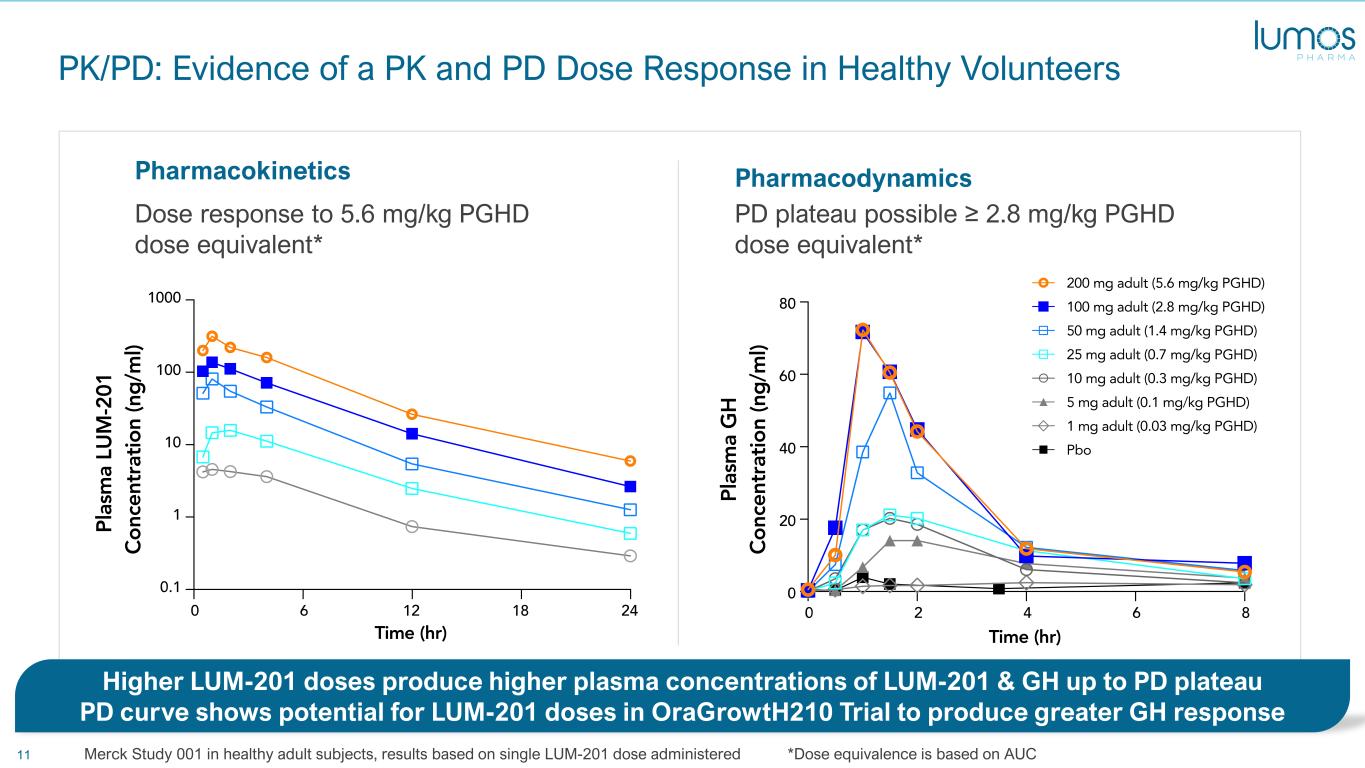
11 PK/PD: Evidence of a PK and PD Dose Response in Healthy Volunteers PharmacodynamicsPharmacokinetics Dose response to 5.6 mg/kg PGHD dose equivalent* PD plateau possible ≥ 2.8 mg/kg PGHD dose equivalent* Higher LUM-201 doses produce higher plasma concentrations of LUM-201 & GH up to PD plateau PD curve shows potential for LUM-201 doses in OraGrowtH210 Trial to produce greater GH response Merck Study 001 in healthy adult subjects, results based on single LUM-201 dose administered *Dose equivalence is based on AUC
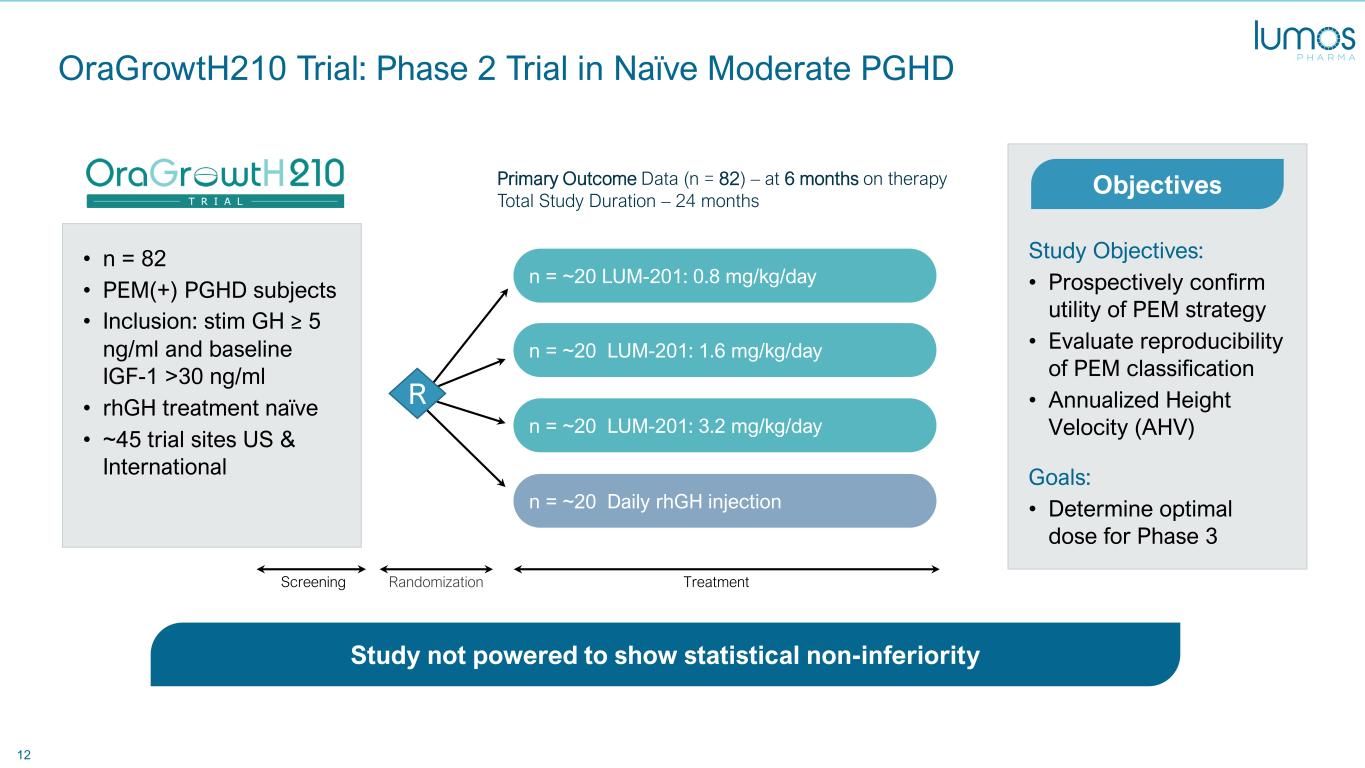
12 OraGrowtH210 Trial: Phase 2 Trial in Naïve Moderate PGHD n = ~20 Daily rhGH injection n = ~20 LUM-201: 3.2 mg/kg/day n = ~20 LUM-201: 1.6 mg/kg/day n = ~20 LUM-201: 0.8 mg/kg/day • n = 82 • PEM(+) PGHD subjects • Inclusion: stim GH ≥ 5 ng/ml and baseline IGF-1 >30 ng/ml • rhGH treatment naïve • ~45 trial sites US & International Study Objectives: • Prospectively confirm utility of PEM strategy • Evaluate reproducibility of PEM classification • Annualized Height Velocity (AHV) Goals: • Determine optimal dose for Phase 3 Primary Outcome Data (n = 82) – at 6 months on therapy Total Study Duration – 24 months Objectives TreatmentRandomizationScreening R Study not powered to show statistical non-inferiorityStudy n t powered to show statistical -inferiority
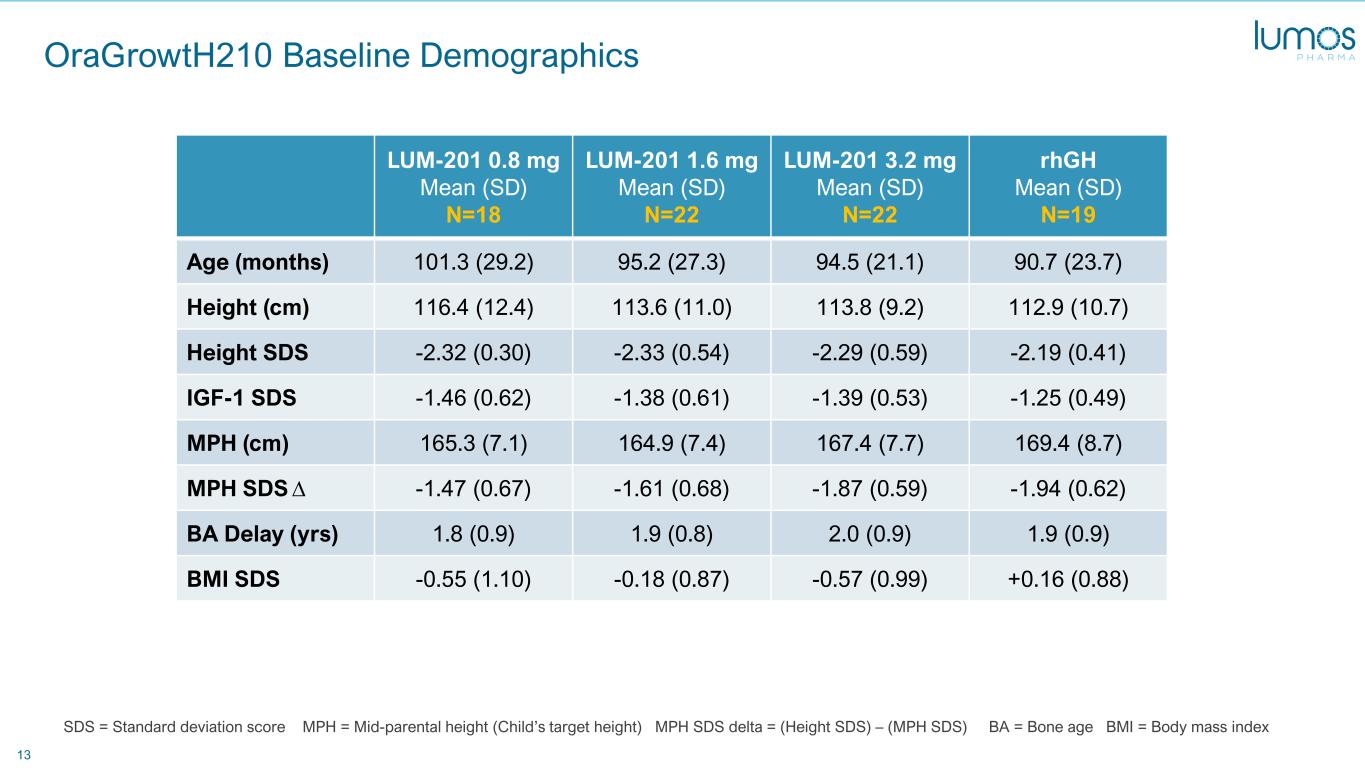
13 OraGrowtH210 Baseline Demographics LUM-201 0.8 mg Mean (SD) N=18 LUM-201 1.6 mg Mean (SD) N=22 LUM-201 3.2 mg Mean (SD) N=22 rhGH Mean (SD) N=19 Age (months) 101.3 (29.2) 95.2 (27.3) 94.5 (21.1) 90.7 (23.7) Height (cm) 116.4 (12.4) 113.6 (11.0) 113.8 (9.2) 112.9 (10.7) Height SDS -2.32 (0.30) -2.33 (0.54) -2.29 (0.59) -2.19 (0.41) IGF-1 SDS -1.46 (0.62) -1.38 (0.61) -1.39 (0.53) -1.25 (0.49) MPH (cm) 165.3 (7.1) 164.9 (7.4) 167.4 (7.7) 169.4 (8.7) MPH SDS ∆ -1.47 (0.67) -1.61 (0.68) -1.87 (0.59) -1.94 (0.62) BA Delay (yrs) 1.8 (0.9) 1.9 (0.8) 2.0 (0.9) 1.9 (0.9) BMI SDS -0.55 (1.10) -0.18 (0.87) -0.57 (0.99) +0.16 (0.88) SDS = Standard deviation score MPH = Mid-parental height (Child’s target height) MPH SDS delta = (Height SDS) – (MPH SDS) BA = Bone age BMI = Body mass index

14 • PEM test ensures patients enrolled in the study are capable of secreting GH in response to a single-dose of LUM-201 • PEM-positive criteria: o PGHD patients with baseline IGF-1 >30 ng/ml o Peak stimulated GH ≥ 5 ng/ml after a single 0.8 mg/kg dose of LUM-201 no enrich 0.8 PEM 1.6 PEM 3.2 PEM 0 50 100 Application of PEM enriched responder population % o f p op ul at io n responder non-responder 50/50 distribution 70/30 enrichment goal OraGrowtH210 Met Primary Statistical Objective: PEM enriches the responder population Highlights Enrichment strategy demonstrated that >70% of PEM+ subjects met pre- specified target growth in 1.6 and 3.2 mg/kg/day cohorts
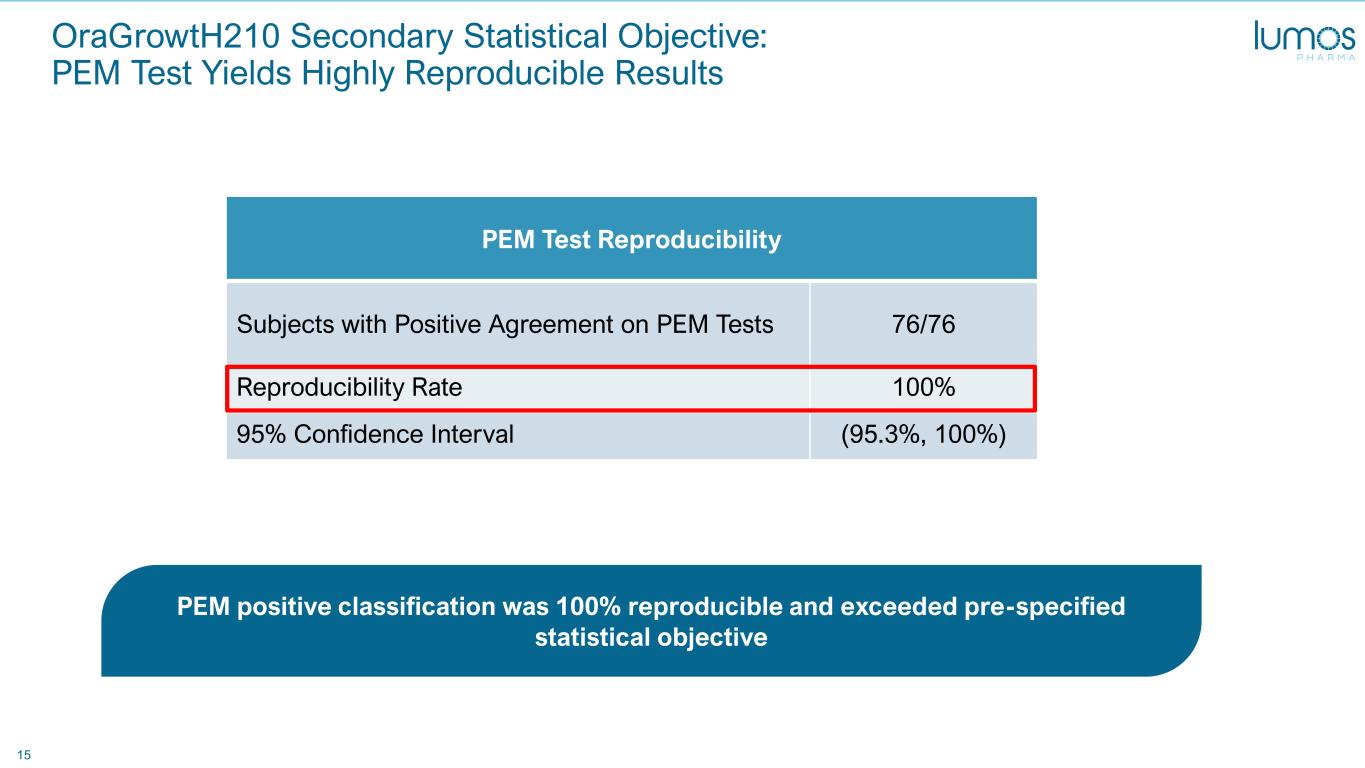
15 PEM Test Reproducibility Subjects with Positive Agreement on PEM Tests 76/76 Reproducibility Rate 100% 95% Confidence Interval (95.3%, 100%) OraGrowtH210 Secondary Statistical Objective: PEM Test Yields Highly Reproducible Results PEM positive classification was 100% reproducible and exceeded pre-specified statistical objective
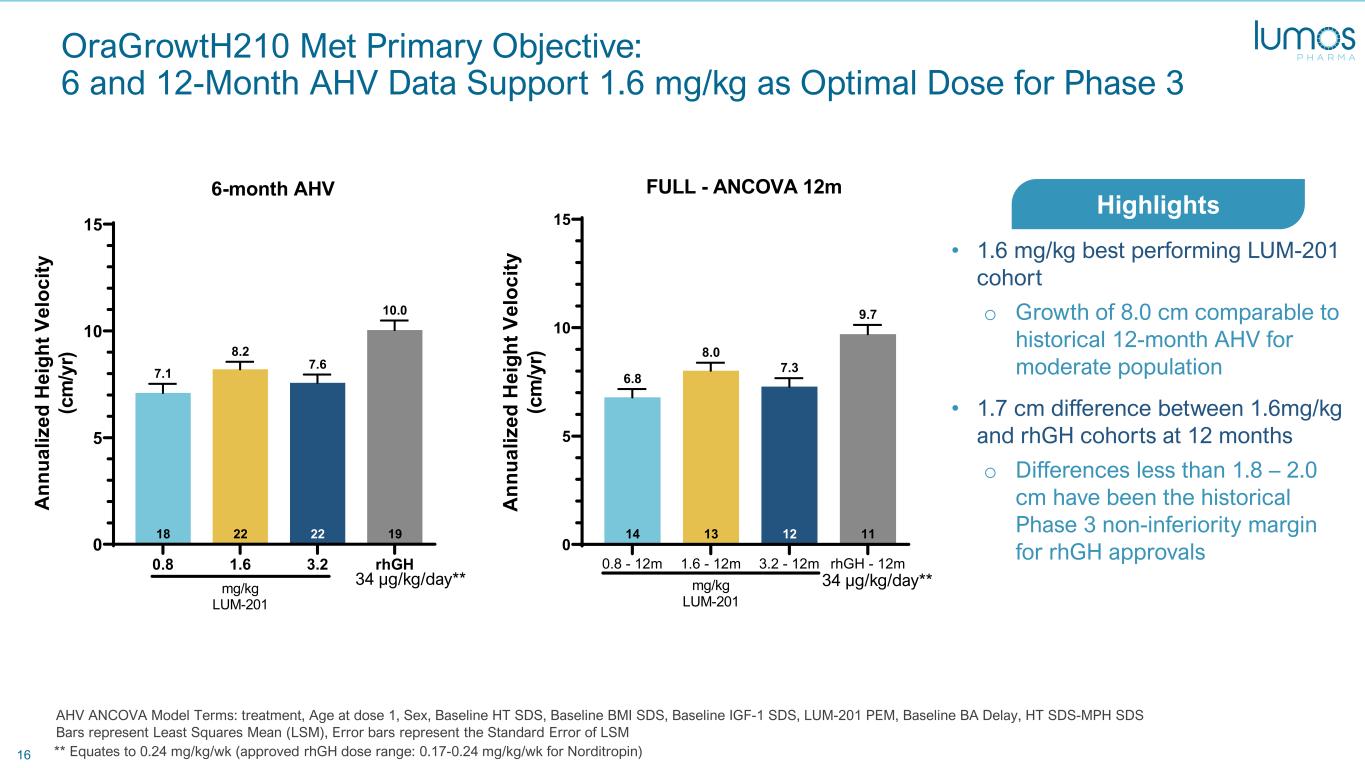
16 OraGrowtH210 Met Primary Objective: 6 and 12-Month AHV Data Support 1.6 mg/kg as Optimal Dose for Phase 3 AHV ANCOVA Model Terms: treatment, Age at dose 1, Sex, Baseline HT SDS, Baseline BMI SDS, Baseline IGF-1 SDS, LUM-201 PEM, Baseline BA Delay, HT SDS-MPH SDS Bars represent Least Squares Mean (LSM), Error bars represent the Standard Error of LSM 0.8 1.6 3.2 rhGH 0 5 10 15 19 10.0 22 7.6 22 8.2 18 7.1 6-month AHV A nn ua liz ed H ei gh t V el oc ity (c m /y r) mg/kg LUM-201 34 μg/kg/day** ** Equates to 0.24 mg/kg/wk (approved rhGH dose range: 0.17-0.24 mg/kg/wk for Norditropin) 0.8 - 12m 1.6 - 12m 3.2 - 12m rhGH - 12m 0 5 10 15 11 9.7 12 7.3 13 8.0 14 6.8 FULL - ANCOVA 12m A nn ua liz ed H ei gh t V el oc ity (c m /y r) mg/kg LUM-201 34 μg/kg/day** • 1.6 mg/kg best performing LUM-201 cohort o Growth of 8.0 cm comparable to historical 12-month AHV for moderate population • 1.7 cm difference between 1.6mg/kg and rhGH cohorts at 12 months o Differences less than 1.8 – 2.0 cm have been the historical Phase 3 non-inferiority margin for rhGH approvals Highlights
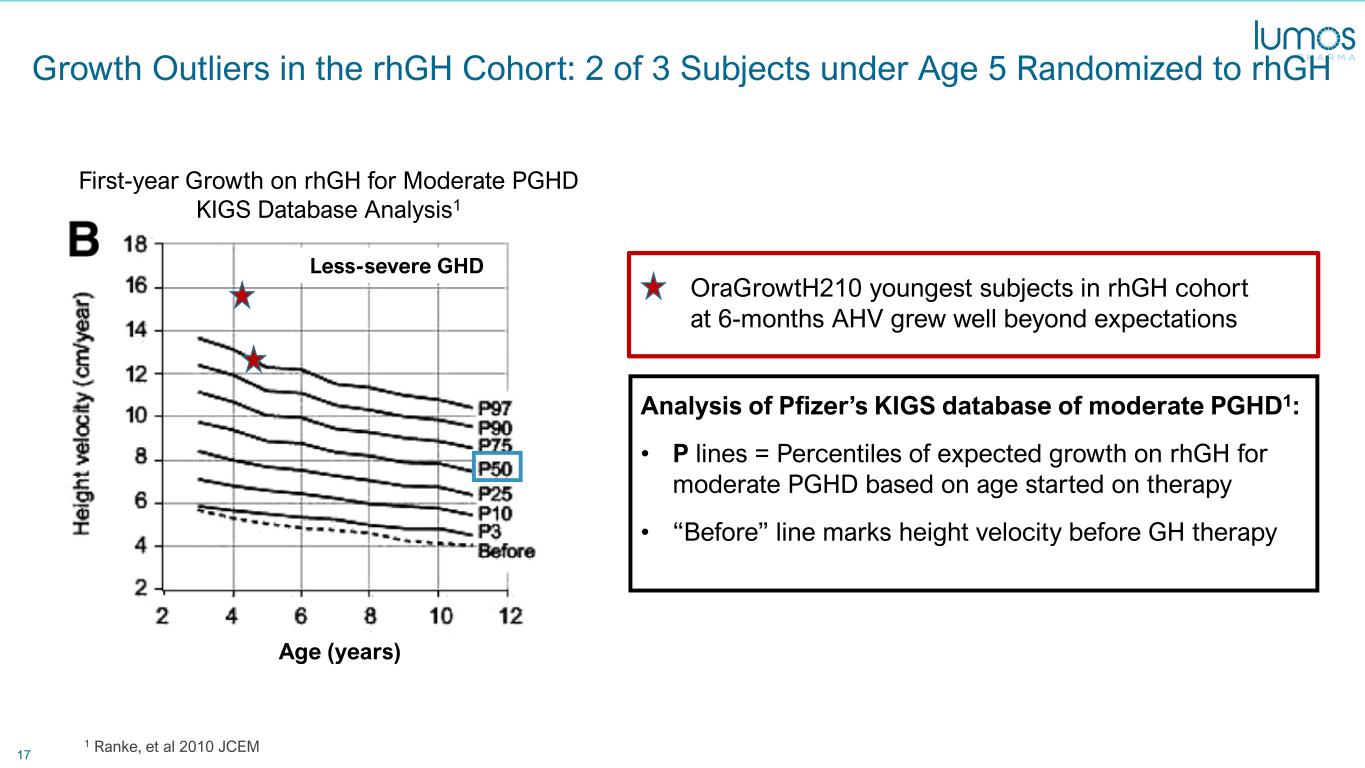
17 Age (years) Growth Outliers in the rhGH Cohort: 2 of 3 Subjects under Age 5 Randomized to rhGH 1 Ranke, et al 2010 JCEM OraGrowtH210 youngest subjects in rhGH cohort at 6-months AHV grew well beyond expectations Analysis of Pfizer’s KIGS database of moderate PGHD1: • P lines = Percentiles of expected growth on rhGH for moderate PGHD based on age started on therapy • “Before” line marks height velocity before GH therapy First-year Growth on rhGH for Moderate PGHD KIGS Database Analysis1 Less-severe GHD
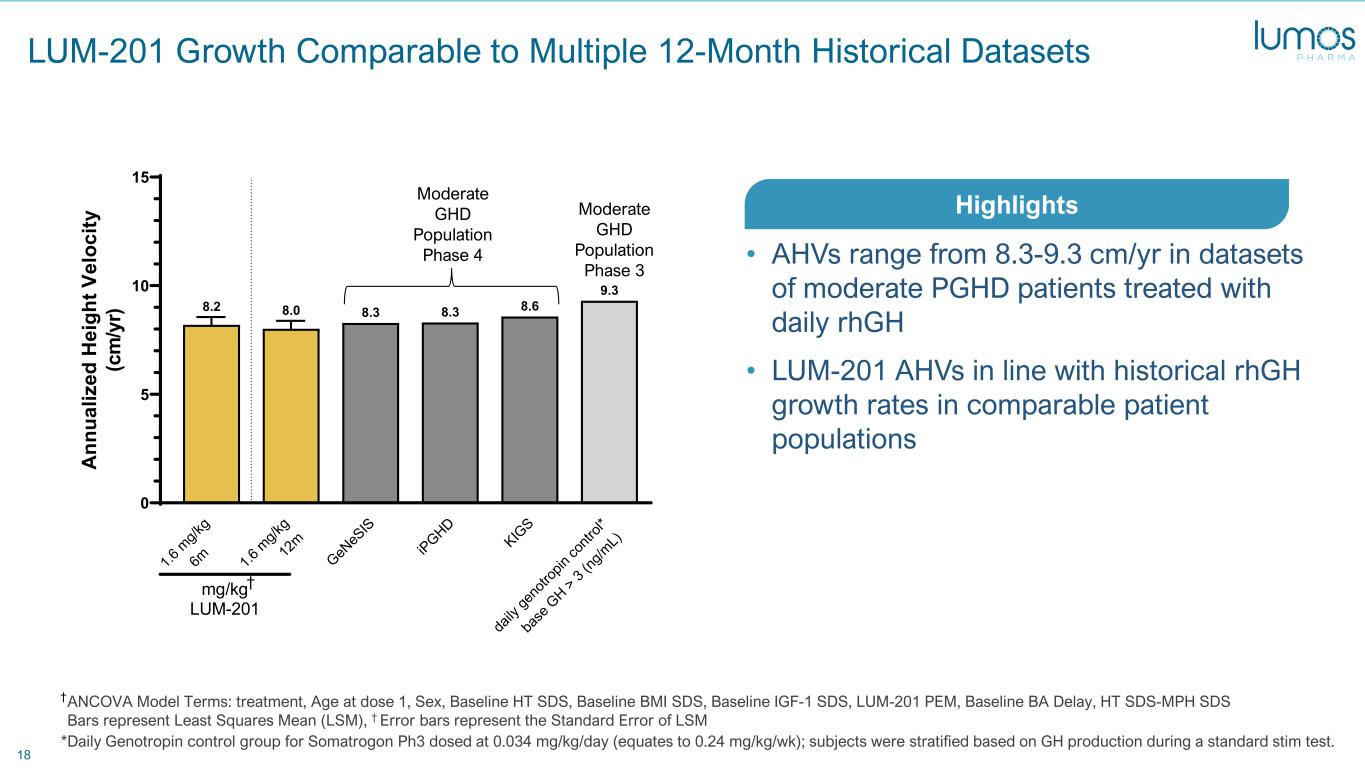
18 LUM-201 Growth Comparable to Multiple 12-Month Historical Datasets *Daily Genotropin control group for Somatrogon Ph3 dosed at 0.034 mg/kg/day (equates to 0.24 mg/kg/wk); subjects were stratified based on GH production during a standard stim test. 1.6 m g/k g 6m 1.6 m g/k g 12 m GeN eS IS iPGHD KIG S da ily ge no tro pin co ntr ol* ba se G H > 3 (ng /m L) 0 5 10 15 9.3 8.68.38.38.08.2 12m ANCOVA vs contemporaries A nn ua liz ed H ei gh t V el oc ity (c m /y r) mg/kg LUM-201 Moderate GHD Population Phase 4 Moderate GHD Population Phase 3 • AHVs range from 8.3-9.3 cm/yr in datasets of moderate PGHD patients treated with daily rhGH • LUM-201 AHVs in line with historical rhGH growth rates in comparable patient populations Highlights † ANCOVA Model Terms: treatment, Age at dose 1, Sex, Baseline HT SDS, Baseline BMI SDS, Baseline IGF-1 SDS, LUM-201 PEM, Baseline BA Delay, HT SDS-MPH SDS Bars represent Least Squares Mean (LSM), † Error bars represent the Standard Error of LSM †
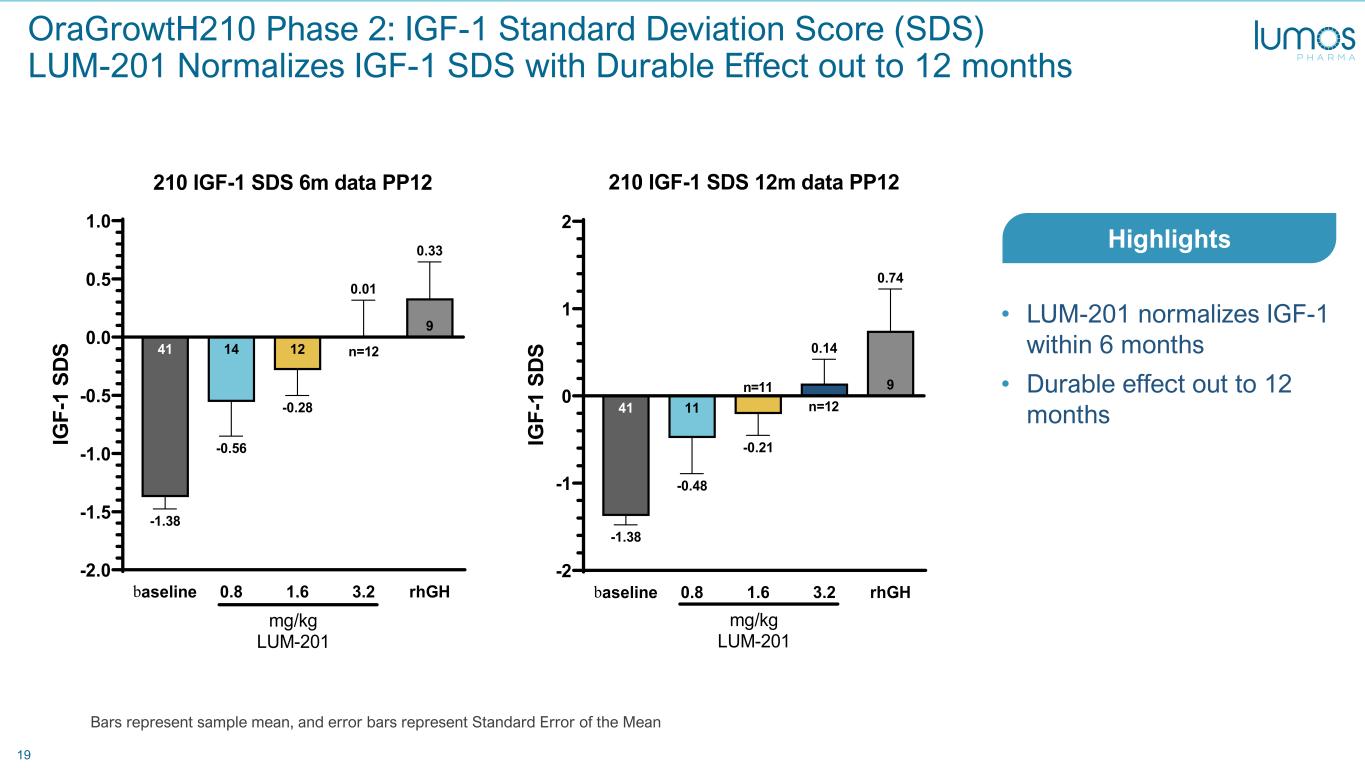
19 OraGrowtH210 Phase 2: IGF-1 Standard Deviation Score (SDS) LUM-201 Normalizes IGF-1 SDS with Durable Effect out to 12 months baseline 0.8 1.6 3.2 rhGH -2 -1 0 1 2 9 0.74 0.14 -0.21 11 -0.48 41 -1.38 210 IGF-1 SDS 12m data PP12 IG F- 1 SD S mg/kg LUM-201 n=11 n=12 • LUM-201 normalizes IGF-1 within 6 months • Durable effect out to 12 months Bars represent sample mean, and error bars represent Standard Error of the Mean Highlights baseline 0.8 1.6 3.2 rhGH -2.0 -1.5 -1.0 -0.5 0.0 0.5 1.0 9 0.33 0.01 12 -0.28 14 -0.56 41 -1.38 210 IGF-1 SDS 6m data PP12 IG F- 1 SD S mg/kg LUM-201 n=12
20 All primary and secondary endpoints met LUM-201 AHV’s consistent with pre-specified targets from historical benchmarks in moderate PGHD population AHV delta for LUM-201 1.6 mg/kg from comparator daily rhGH arm at 6- and 12-months is within the non-inferiority margin (difference less than 1.8 to 2.0 cm) typically used in Phase 3 pivotal trials for rhGH approvals LUM-201 normalizes IGF-1 SDS within 6 months on treatment Investigational product safety profile remains clean after >1,300 patients treated to date1 Phase 2 results support advancing to Phase 3 with final design to be confirmed following EOP2 FDA meeting, anticipated in 1H 2024 OraGrowtH210 Summary 1 Includes adult and pediatric subjects from prior Merck studies EOP2 = End of Phase 2
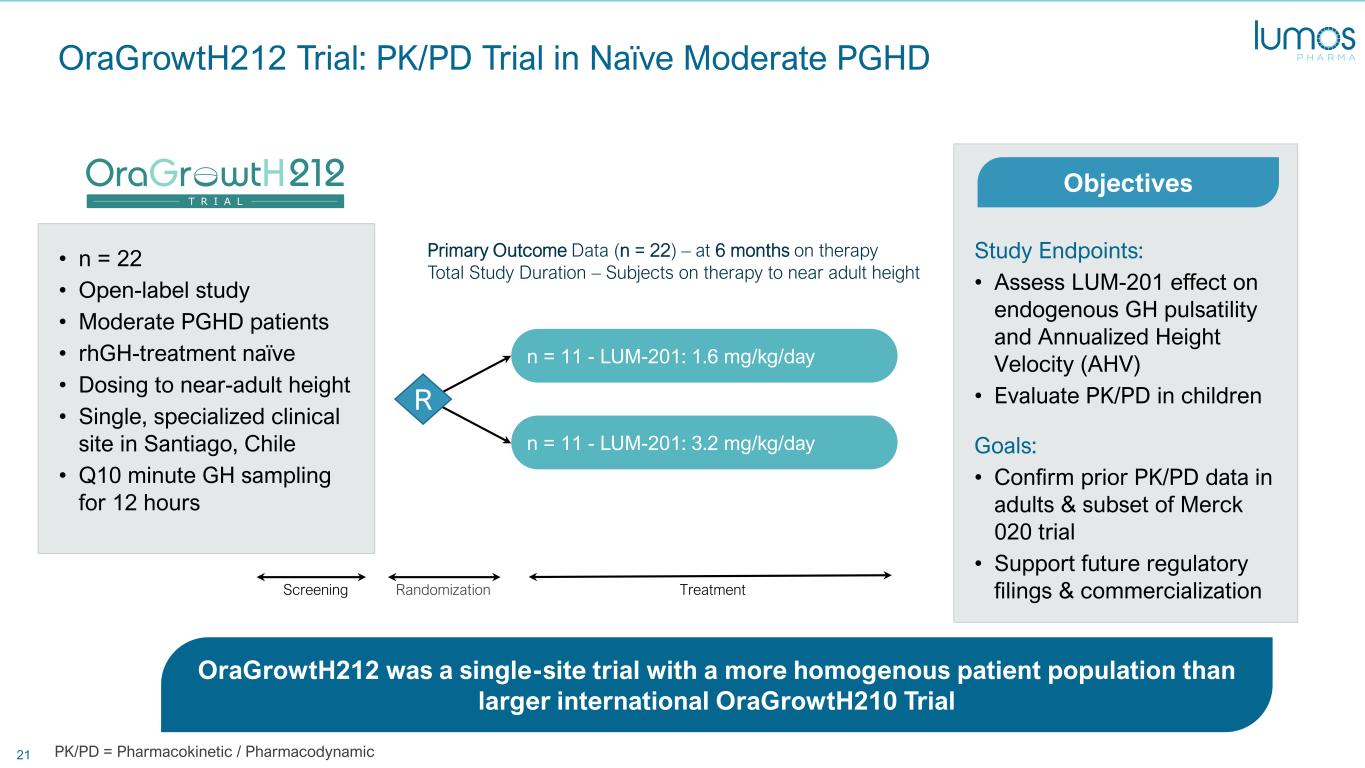
21 • n = 22 • Open-label study • Moderate PGHD patients • rhGH-treatment naïve • Dosing to near-adult height • Single, specialized clinical site in Santiago, Chile • Q10 minute GH sampling for 12 hours OraGrowtH212 Trial: PK/PD Trial in Naïve Moderate PGHD Study Endpoints: • Assess LUM-201 effect on endogenous GH pulsatility and Annualized Height Velocity (AHV) • Evaluate PK/PD in children Goals: • Confirm prior PK/PD data in adults & subset of Merck 020 trial • Support future regulatory filings & commercialization OraGrowtH212 was a single-site trial with a more homogenous patient population than larger international OraGrowtH210 Trial Objectives n = 11 - LUM-201: 3.2 mg/kg/day n = 11 - LUM-201: 1.6 mg/kg/day R TreatmentRandomizationScreening Primary Outcome Data (n = 22) – at 6 months on therapy Total Study Duration – Subjects on therapy to near adult height PK/PD = Pharmacokinetic / Pharmacodynamic
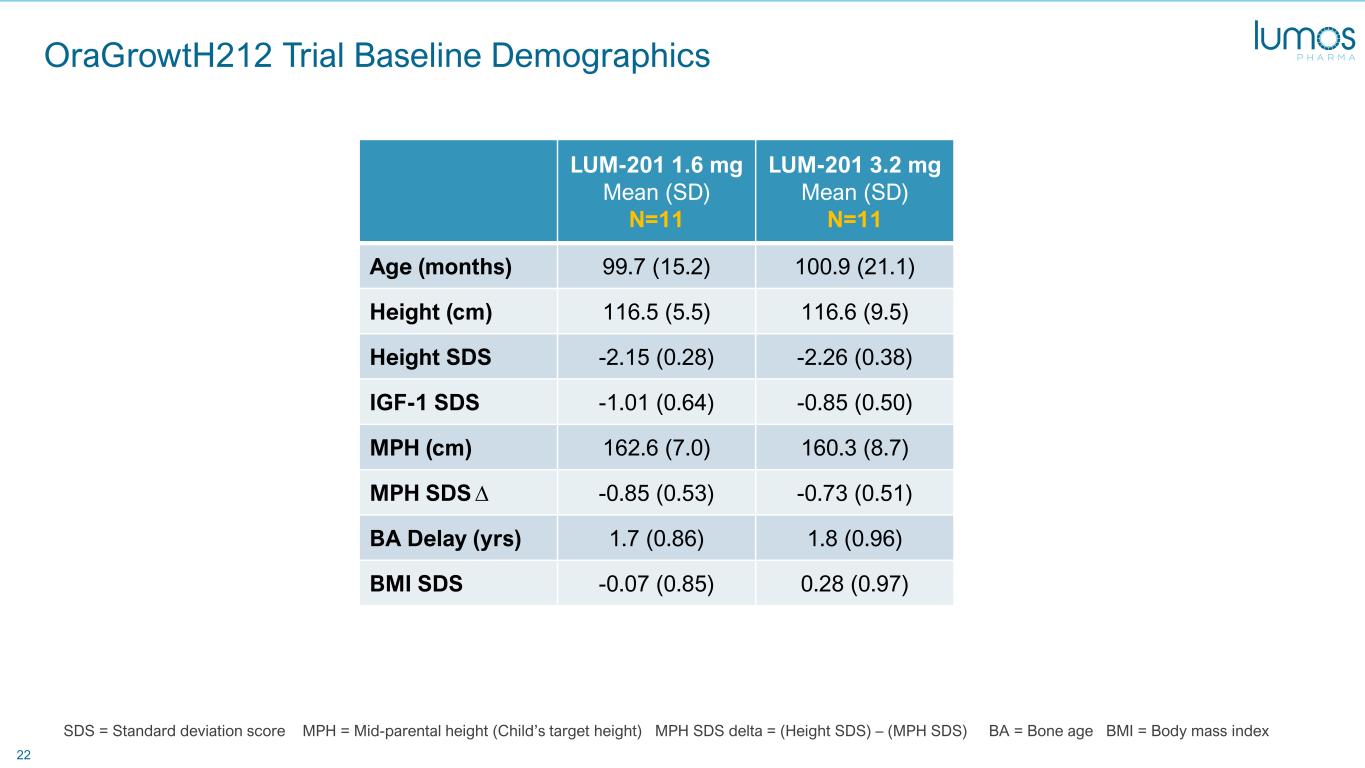
22 OraGrowtH212 Trial Baseline Demographics LUM-201 1.6 mg Mean (SD) N=11 LUM-201 3.2 mg Mean (SD) N=11 Age (months) 99.7 (15.2) 100.9 (21.1) Height (cm) 116.5 (5.5) 116.6 (9.5) Height SDS -2.15 (0.28) -2.26 (0.38) IGF-1 SDS -1.01 (0.64) -0.85 (0.50) MPH (cm) 162.6 (7.0) 160.3 (8.7) MPH SDS ∆ -0.85 (0.53) -0.73 (0.51) BA Delay (yrs) 1.7 (0.86) 1.8 (0.96) BMI SDS -0.07 (0.85) 0.28 (0.97) SDS = Standard deviation score MPH = Mid-parental height (Child’s target height) MPH SDS delta = (Height SDS) – (MPH SDS) BA = Bone age BMI = Body mass index
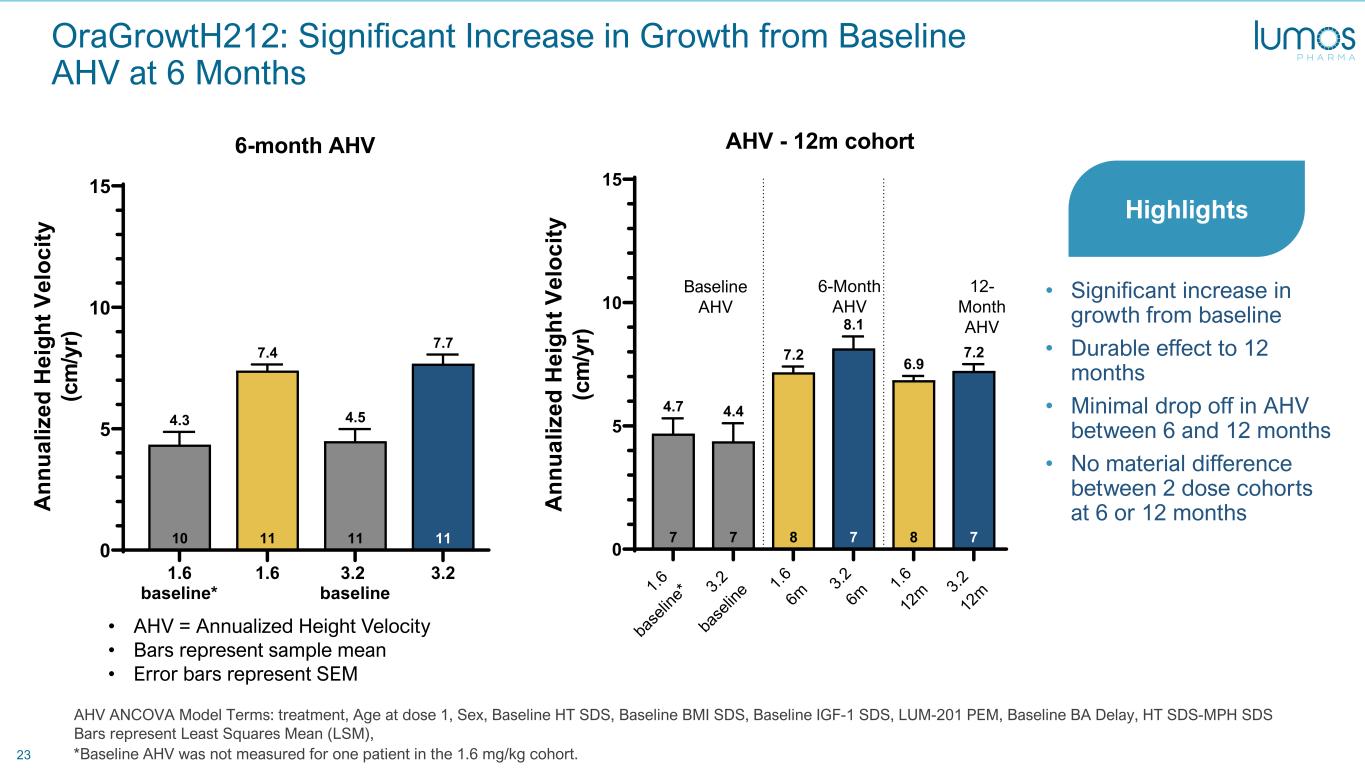
23 OraGrowtH212: Significant Increase in Growth from Baseline AHV at 6 Months • AHV = Annualized Height Velocity • Bars represent sample mean • Error bars represent SEM 1.6 baseline* 1.6 3.2 baseline 3.2 0 5 10 15 11 7.7 11 4.5 11 7.4 10 4.3 6-month AHV A nn ua liz ed H ei gh t V el oc ity (c m /y r) *Baseline AHV was not measured for one patient in the 1.6 mg/kg cohort. AHV ANCOVA Model Terms: treatment, Age at dose 1, Sex, Baseline HT SDS, Baseline BMI SDS, Baseline IGF-1 SDS, LUM-201 PEM, Baseline BA Delay, HT SDS-MPH SDS Bars represent Least Squares Mean (LSM), 1.6 ba se lin e* 3.2 ba se lin e 1.6 6m 3.2 6m 1.6 12 m 3.2 12 m 0 5 10 15 7 7.2 8 6.9 7 8.1 8 7.2 7 4.4 7 4.7 AHV - 12m cohort A nn ua liz ed H ei gh t V el oc ity (c m /y r) 6-Month AHV 12- Month AHV Baseline AHV • Significant increase in growth from baseline • Durable effect to 12 months • Minimal drop off in AHV between 6 and 12 months • No material difference between 2 dose cohorts at 6 or 12 months Highlights
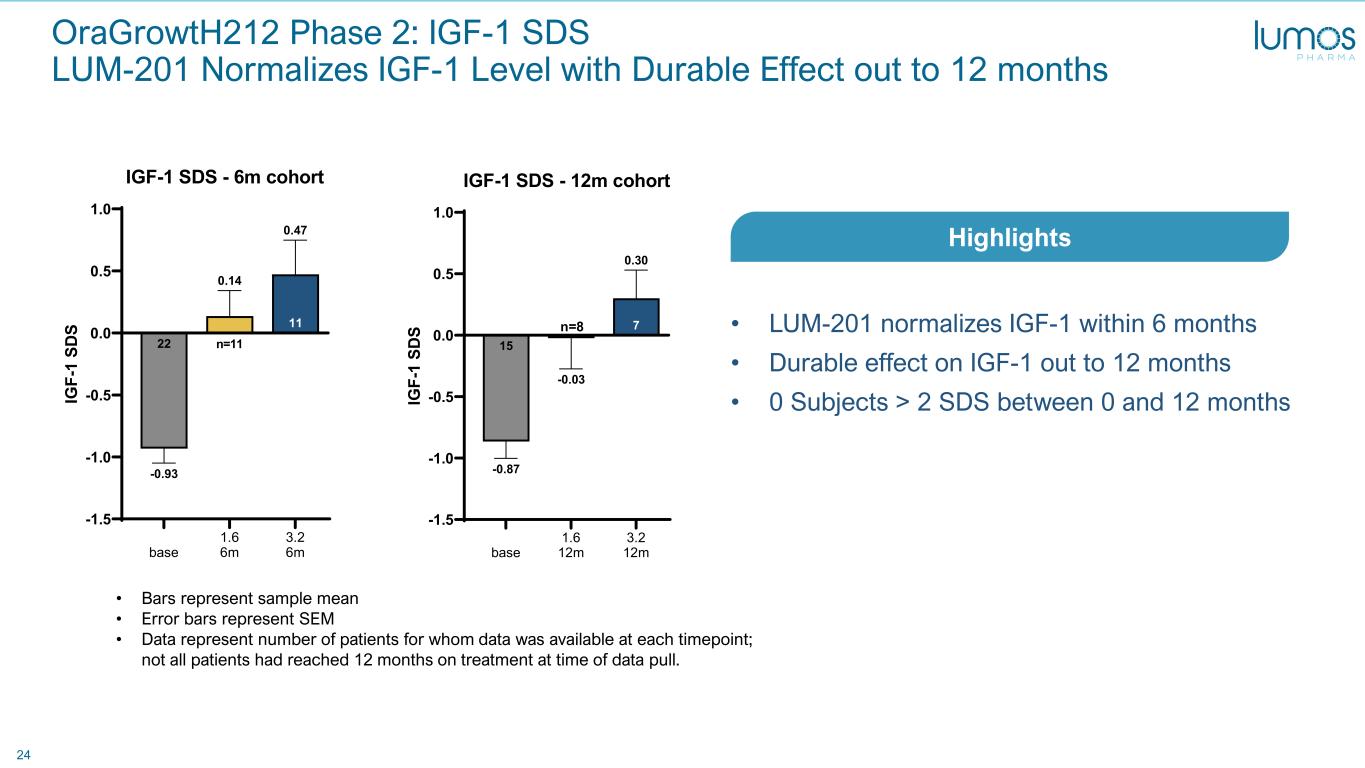
24 base 1.6 6m 3.2 6m -1.5 -1.0 -0.5 0.0 0.5 1.0 11 0.47 0.14 22 -0.93 IGF-1 SDS - 6m cohort IG F- 1 SD S n=11 base 1.6 12m 3.2 12m -1.5 -1.0 -0.5 0.0 0.5 1.0 7 0.30 -0.03 15 -0.87 IGF-1 SDS - 12m cohort IG F- 1 SD S n=8 • Bars represent sample mean • Error bars represent SEM • Data represent number of patients for whom data was available at each timepoint; not all patients had reached 12 months on treatment at time of data pull. • LUM-201 normalizes IGF-1 within 6 months • Durable effect on IGF-1 out to 12 months • 0 Subjects > 2 SDS between 0 and 12 months Highlights OraGrowtH212 Phase 2: IGF-1 SDS LUM-201 Normalizes IGF-1 Level with Durable Effect out to 12 months
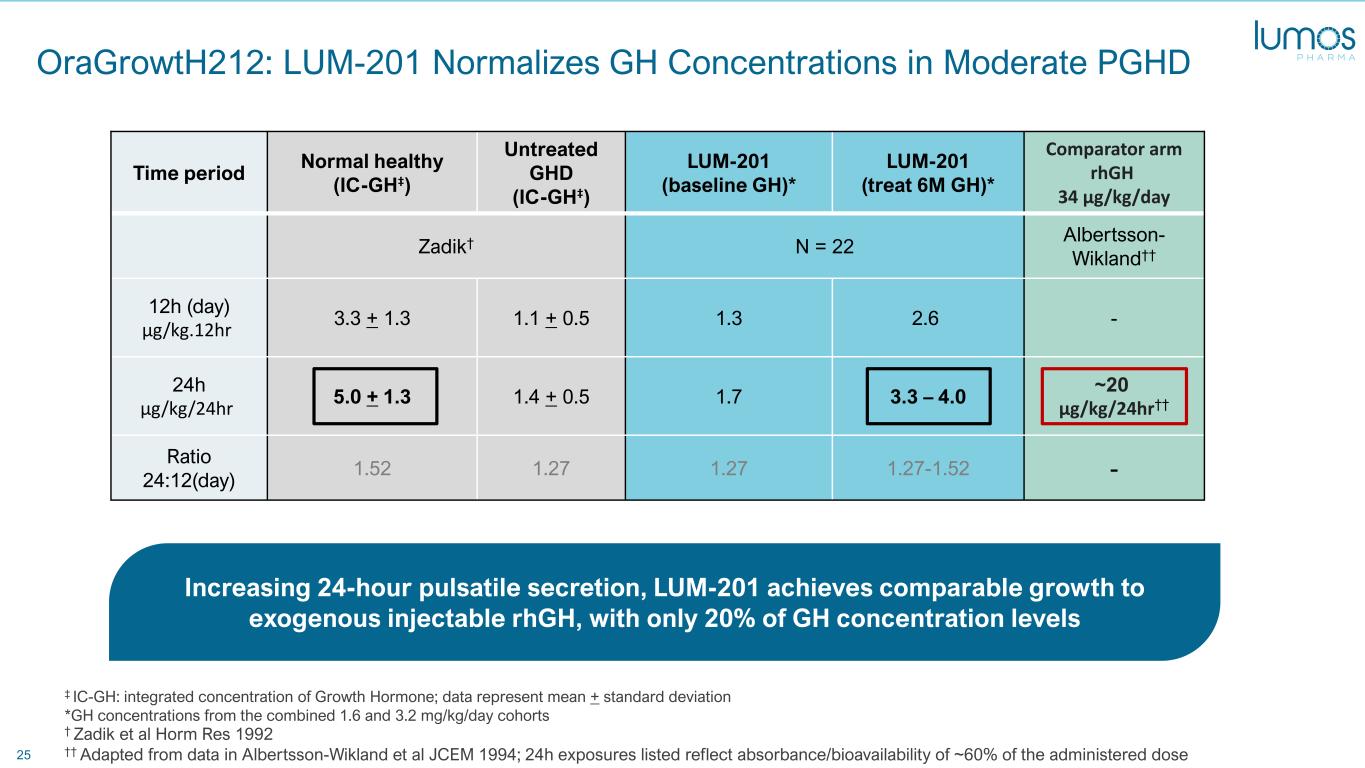
25 Time period Normal healthy (IC-GH‡) Untreated GHD (IC-GH‡) LUM-201 (baseline GH)* LUM-201 (treat 6M GH)* Comparator arm rhGH 34 µg/kg/day Zadik† N = 22 Albertsson- Wikland†† 12h (day) µg/kg.12hr 3.3 + 1.3 1.1 + 0.5 1.3 2.6 - 24h µg/kg/24hr 5.0 + 1.3 1.4 + 0.5 1.7 3.3 – 4.0 ~20 µg/kg/24hr†† Ratio 24:12(day) 1.52 1.27 1.27 1.27-1.52 - ‡ IC-GH: integrated concentration of Growth Hormone; data represent mean + standard deviation *GH concentrations from the combined 1.6 and 3.2 mg/kg/day cohorts OraGrowtH212: LUM-201 Normalizes GH Concentrations in Moderate PGHD † Zadik et al Horm Res 1992 †† Adapted from data in Albertsson-Wikland et al JCEM 1994; 24h exposures listed reflect absorbance/bioavailability of ~60% of the administered dose Increasing 24-hour pulsatile secretion, LUM-201 achieves comparable growth to exogenous injectable rhGH, with only 20% of GH concentration levels
26 OraGrowtH212 Summary All primary and secondary endpoints met Increased 6- and 12-month AHV meaningfully from baseline LUM-201 normalized IGF-1 SDS values within 6 months of treatment with durable effect LUM-201 stimulates an increase in pulsatile secretion of GH approximating normal physiologic levels Increasing 24-hour pulsatile secretion, LUM-201 achieves comparable growth to daily exogenous injectable rhGH, with only 20% of GH concentration levels
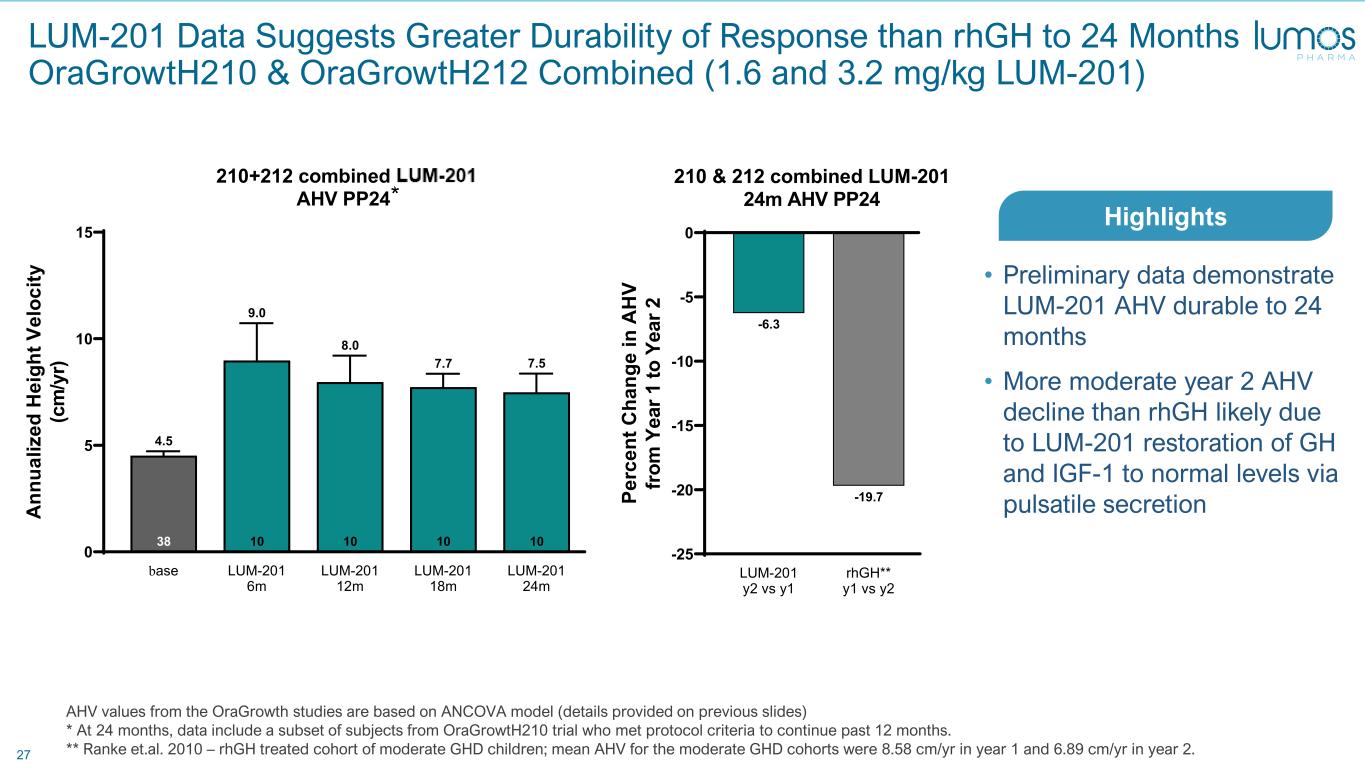
27 AHV values from the OraGrowth studies are based on ANCOVA model (details provided on previous slides) * At 24 months, data include a subset of subjects from OraGrowtH210 trial who met protocol criteria to continue past 12 months. ** Ranke et.al. 2010 – rhGH treated cohort of moderate GHD children; mean AHV for the moderate GHD cohorts were 8.58 cm/yr in year 1 and 6.89 cm/yr in year 2. base LUM-201 6m LUM-201 12m LUM-201 18m LUM-201 24m 0 5 10 15 10 7.5 10 7.7 10 8.0 10 9.0 38 4.5 210+212 combined LUM201 AHV PP24 A nn ua liz ed H ei gh t V el oc ity (c m /y r) LUM-201 y2 vs y1 rhGH** y1 vs y2 -25 -20 -15 -10 -5 0 -19.7 -6.3 210 & 212 combined LUM-201 24m AHV PP24 Pe rc en t C ha ng e in A H V fro m Y ea r 1 to Y ea r 2 LUM-201 Data Suggests Greater Durability of Response than rhGH to 24 Months OraGrowtH210 & OraGrowtH212 Combined (1.6 and 3.2 mg/kg LUM-201) • Preliminary data demonstrate LUM-201 AHV durable to 24 months • More moderate year 2 AHV decline than rhGH likely due to LUM-201 restoration of GH and IGF-1 to normal levels via pulsatile secretion Highlights *
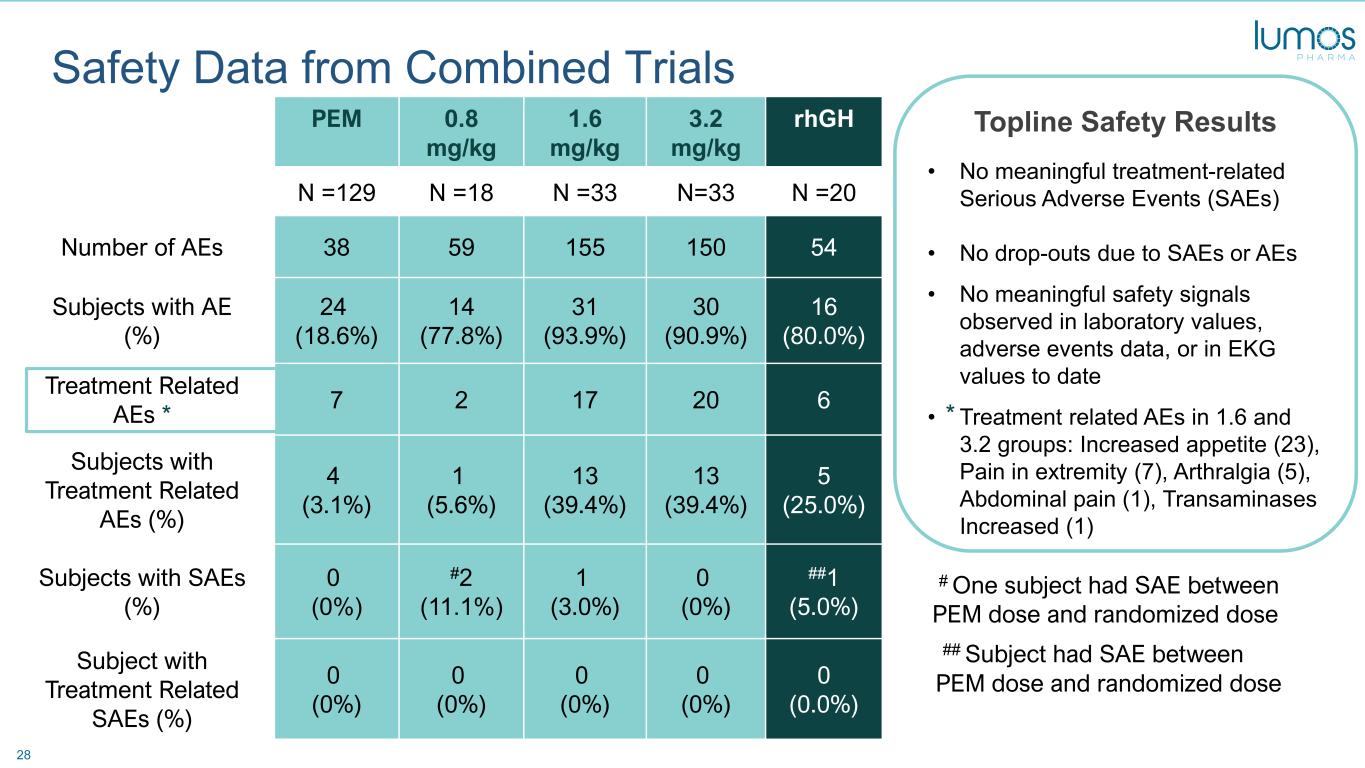
28 PEM 0.8 mg/kg 1.6 mg/kg 3.2 mg/kg rhGH N =129 N =18 N =33 N=33 N =20 Number of AEs 38 59 155 150 54 Subjects with AE (%) 24 (18.6%) 14 (77.8%) 31 (93.9%) 30 (90.9%) 16 (80.0%) Treatment Related AEs * 7 2 17 20 6 Subjects with Treatment Related AEs (%) 4 (3.1%) 1 (5.6%) 13 (39.4%) 13 (39.4%) 5 (25.0%) Subjects with SAEs (%) 0 (0%) #2 (11.1%) 1 (3.0%) 0 (0%) ##1 (5.0%) Subject with Treatment Related SAEs (%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0.0%) Safety Data from Combined Trials Topline Safety Results • No meaningful treatment-related Serious Adverse Events (SAEs) • No drop-outs due to SAEs or AEs • No meaningful safety signals observed in laboratory values, adverse events data, or in EKG values to date • Treatment related AEs in 1.6 and 3.2 groups: Increased appetite (23), Pain in extremity (7), Arthralgia (5), Abdominal pain (1), Transaminases Increased (1) * * # One subject had SAE between PEM dose and randomized dose ## Subject had SAE between PEM dose and randomized dose

29 Lumos Pharma Financial Information as of September 30, 2023 Values in USD Cash, equivalents & short-term investments $42.7M Debt $0 Shares Outstanding 7.9M Cash Use for 4Q 2023 ~ $9.0-$10.0M Fiscal Year End December 31 LUMO Cash, cash equivalents, & short-term investments to support operations through 3Q 2024, inclusive of activities related to advancing the PGHD program into Phase 3
30 Recap Summary and Next Steps o Met all primary and secondary endpoints o LUM-201 increases pulsatility, restores GH secretion and normalizes IGF-1 o LUM-201 promotes growth comparable to rhGH with only 20% of GH concentration levels o AHV* delta between optimal 1.6 mg/kg LUM-201 dose and rhGH comparator arm at 6 and 12-months was within historical Phase 3 non-inferiority margins OraGrowtH210 and OraGrowtH212 Phase 2 Clinical Trials o Plan to request End-of-Phase 2 meeting with FDA and conduct in 1H 2024 o Anticipate initiating Phase 3 program in 2H 2024 o Phase 3 enrollment is estimated to span 12-18 months from first patient dosed** Considerations for Phase 3 in PGHD * AHV = Least Squares Mean Annualized Height Velocity, AHV values from the OraGrowtH studies are based on ANCOVA model (details provided on previous slides) ** This estimated enrollment timeline is based on recent peer PGHD registrational trial enrollment timelines and the absence of simultaneous PGHD trials during our planned enrollment period. This enrollment timeline is subject to change dependent upon the EOP2 meeting with the FDA and other factors.

31 Investment Thesis Lead asset targeting children with growth disorders Attractive Market Opportunity Novel Asset with Unique MOA Solid Financial Position • Daily oral expected to be well received in GH markets • Market research supports rapid conversation to oral and potential expansion opportunities* • Novel MOA takes advantage of natural physiology • Orphan Drug Designation in US/EU and issued patents in major markets • Cash balance of $42.7 million as of close of 3Q 2023 • Cash runway through 3Q 2024 Clear Proof of Concept • PEM strategy de-risks patient selection, identifying likely LUM-201 responders** • Phase 2 trials met all primary and secondary endpoints • Consistent PK/PD and attractive safety profile to date in > 1,300 subjects studied * Initial Primary Research of PGHD Market conducted for Lumos by Triangle Insights ** PEM (Predictive Enrichment Marker) strategy consists of screening for PEM+ PGHD patients = Baseline IGF-1 > 30 ng/ml & Peak stimulation GH ≥ 5 ng/ml from single oral dose of LUM-201 Potential for 1st oral therapeutic to disrupt injectable market for GHD Focused Execution • End-of-Phase 2 meeting with FDA anticipated 1H 2024 to review Phase 3 program • Initiation of Phase 3 trial anticipated 2H 2024 REVISED: Aaron Summary Slide w/ Lisa’s edits

Additional Analyses of Phase 2 Data
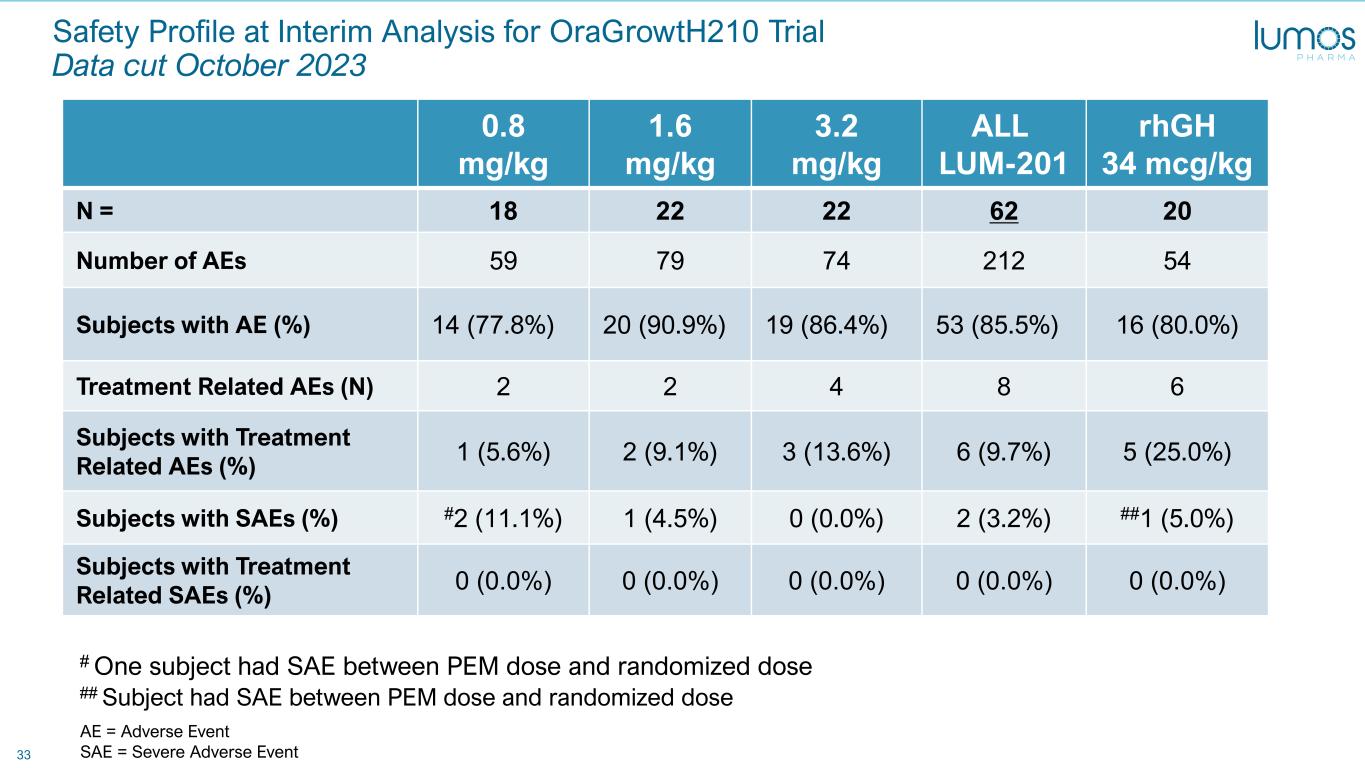
33 Safety Profile at Interim Analysis for OraGrowtH210 Trial Data cut October 2023 0.8 mg/kg 1.6 mg/kg 3.2 mg/kg ALL LUM-201 rhGH 34 mcg/kg N = 18 22 22 62 20 Number of AEs 59 79 74 212 54 Subjects with AE (%) 14 (77.8%) 20 (90.9%) 19 (86.4%) 53 (85.5%) 16 (80.0%) Treatment Related AEs (N) 2 2 4 8 6 Subjects with Treatment Related AEs (%) 1 (5.6%) 2 (9.1%) 3 (13.6%) 6 (9.7%) 5 (25.0%) Subjects with SAEs (%) #2 (11.1%) 1 (4.5%) 0 (0.0%) 2 (3.2%) ##1 (5.0%) Subjects with Treatment Related SAEs (%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) # One subject had SAE between PEM dose and randomized dose ## Subject had SAE between PEM dose and randomized dose AE = Adverse Event SAE = Severe Adverse Event
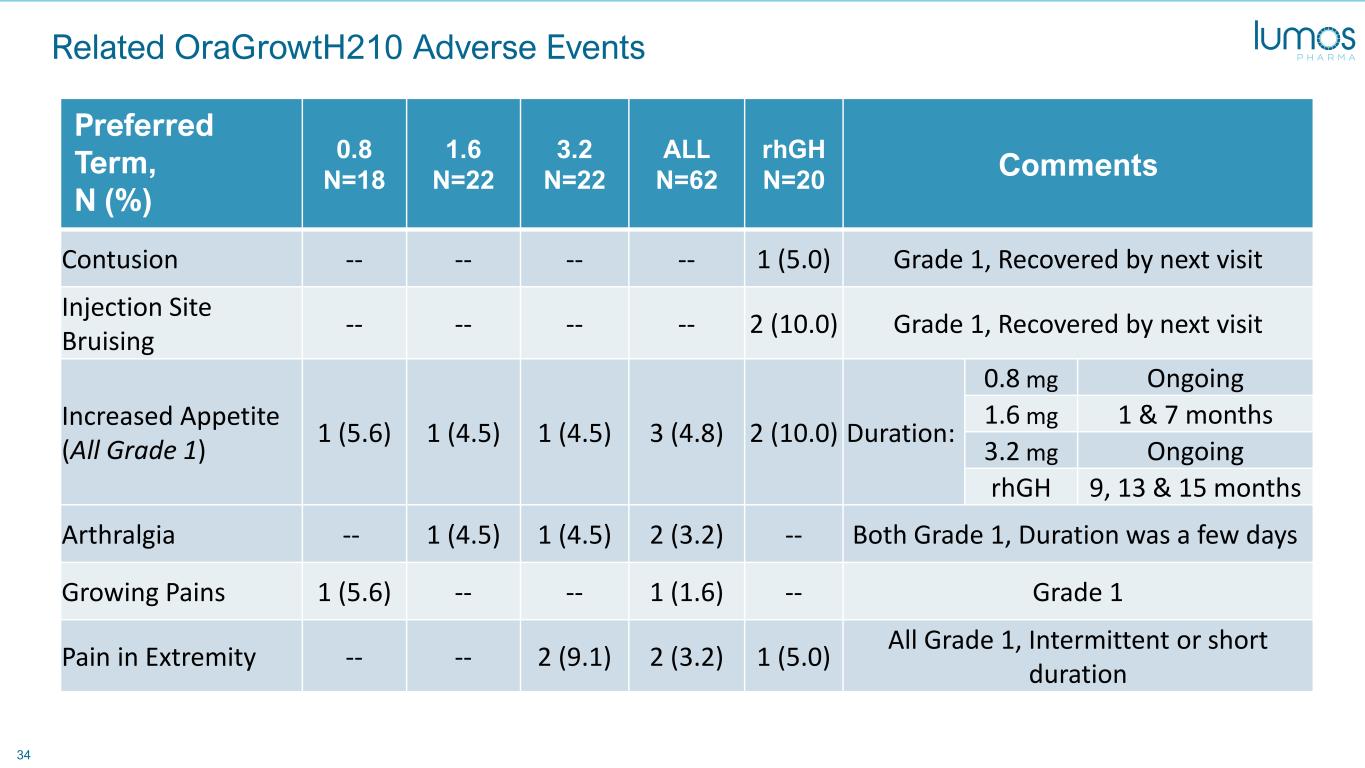
34 Related OraGrowtH210 Adverse Events Preferred Term, N (%) 0.8 N=18 1.6 N=22 3.2 N=22 ALL N=62 rhGH N=20 Comments Contusion -- -- -- -- 1 (5.0) Grade 1, Recovered by next visit Injection Site Bruising -- -- -- -- 2 (10.0) Grade 1, Recovered by next visit Increased Appetite (All Grade 1) 1 (5.6) 1 (4.5) 1 (4.5) 3 (4.8) 2 (10.0) Duration: 0.8 mg Ongoing 1.6 mg 1 & 7 months 3.2 mg Ongoing rhGH 9, 13 & 15 months Arthralgia -- 1 (4.5) 1 (4.5) 2 (3.2) -- Both Grade 1, Duration was a few days Growing Pains 1 (5.6) -- -- 1 (1.6) -- Grade 1 Pain in Extremity -- -- 2 (9.1) 2 (3.2) 1 (5.0) All Grade 1, Intermittent or short duration
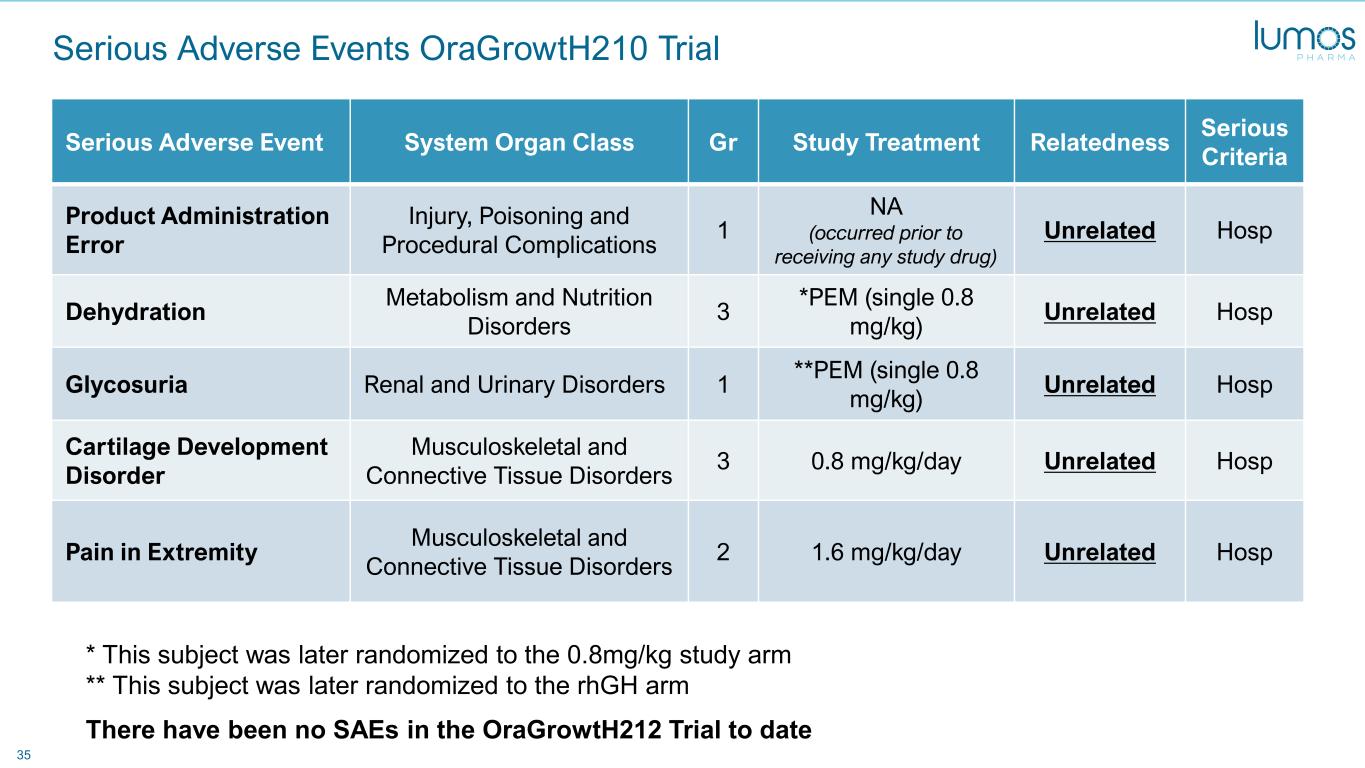
35 Serious Adverse Events OraGrowtH210 Trial Serious Adverse Event System Organ Class Gr Study Treatment Relatedness Serious Criteria Product Administration Error Injury, Poisoning and Procedural Complications 1 NA (occurred prior to receiving any study drug) Unrelated Hosp Dehydration Metabolism and Nutrition Disorders 3 *PEM (single 0.8 mg/kg) Unrelated Hosp Glycosuria Renal and Urinary Disorders 1 **PEM (single 0.8 mg/kg) Unrelated Hosp Cartilage Development Disorder Musculoskeletal and Connective Tissue Disorders 3 0.8 mg/kg/day Unrelated Hosp Pain in Extremity Musculoskeletal and Connective Tissue Disorders 2 1.6 mg/kg/day Unrelated Hosp * This subject was later randomized to the 0.8mg/kg study arm ** This subject was later randomized to the rhGH arm There have been no SAEs in the OraGrowtH212 Trial to date
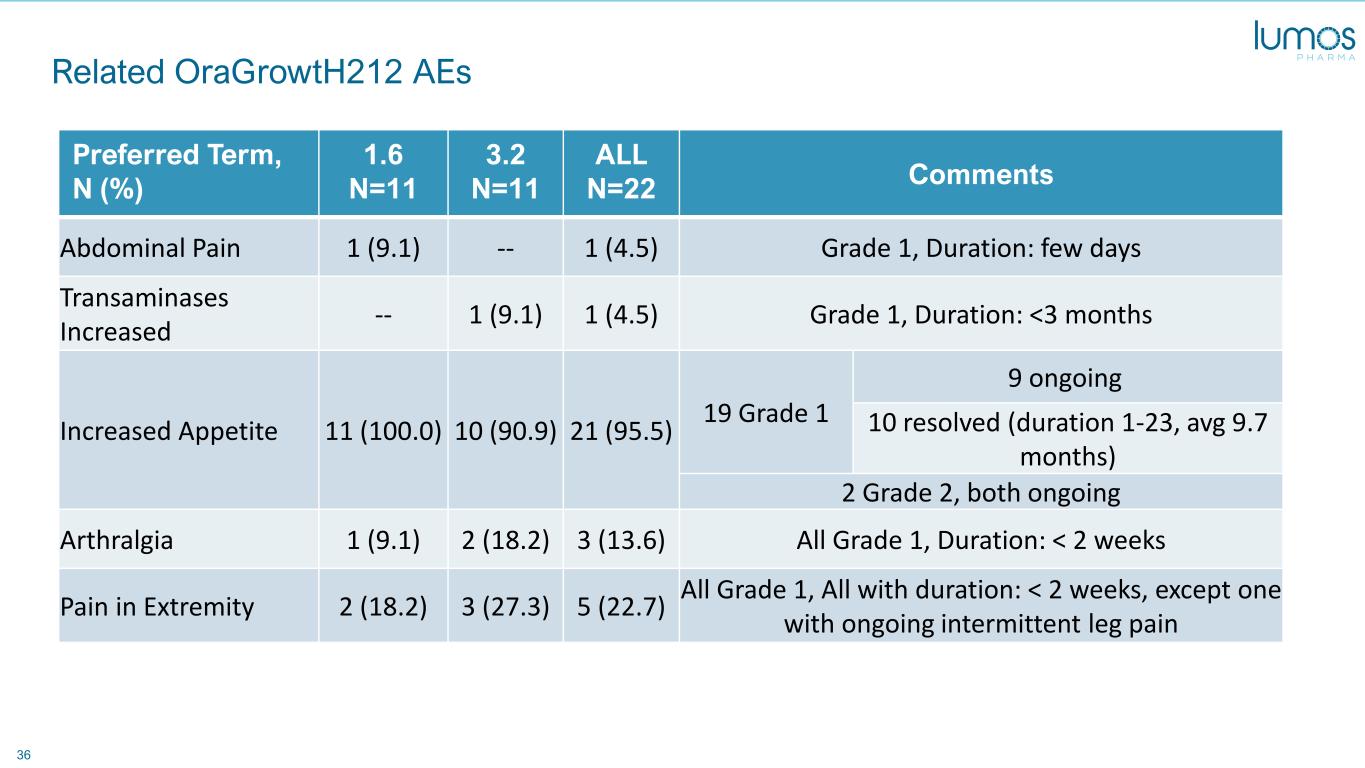
36 Related OraGrowtH212 AEs Preferred Term, N (%) 1.6 N=11 3.2 N=11 ALL N=22 Comments Abdominal Pain 1 (9.1) -- 1 (4.5) Grade 1, Duration: few days Transaminases Increased -- 1 (9.1) 1 (4.5) Grade 1, Duration: <3 months Increased Appetite 11 (100.0) 10 (90.9) 21 (95.5) 19 Grade 1 9 ongoing 10 resolved (duration 1-23, avg 9.7 months) 2 Grade 2, both ongoing Arthralgia 1 (9.1) 2 (18.2) 3 (13.6) All Grade 1, Duration: < 2 weeks Pain in Extremity 2 (18.2) 3 (27.3) 5 (22.7) All Grade 1, All with duration: < 2 weeks, except one with ongoing intermittent leg pain
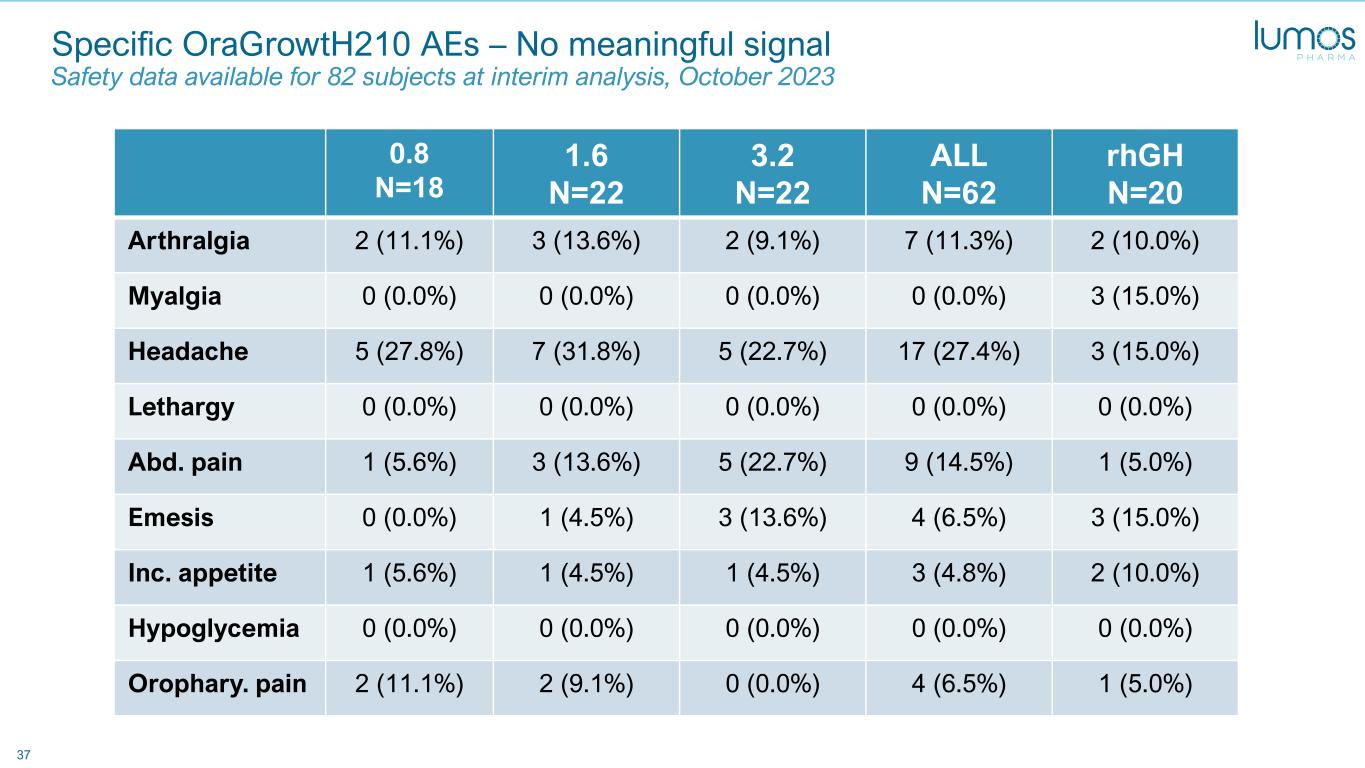
37 Specific OraGrowtH210 AEs – No meaningful signal Safety data available for 82 subjects at interim analysis, October 2023 0.8 N=18 1.6 N=22 3.2 N=22 ALL N=62 rhGH N=20 Arthralgia 2 (11.1%) 3 (13.6%) 2 (9.1%) 7 (11.3%) 2 (10.0%) Myalgia 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 3 (15.0%) Headache 5 (27.8%) 7 (31.8%) 5 (22.7%) 17 (27.4%) 3 (15.0%) Lethargy 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) Abd. pain 1 (5.6%) 3 (13.6%) 5 (22.7%) 9 (14.5%) 1 (5.0%) Emesis 0 (0.0%) 1 (4.5%) 3 (13.6%) 4 (6.5%) 3 (15.0%) Inc. appetite 1 (5.6%) 1 (4.5%) 1 (4.5%) 3 (4.8%) 2 (10.0%) Hypoglycemia 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) Orophary. pain 2 (11.1%) 2 (9.1%) 0 (0.0%) 4 (6.5%) 1 (5.0%)

38 Laboratory Shifts: No meaningful signal 82 subjects 0.8 mg/kg N=18 1.6 mg/kg N=22 3.2 mg/kg N=22 ALL N=62 rhGH N=20 ALT Nl to high 2/17 (11.8%) 5/22 (22.7%) 4/22 (18.2%) 11/61 (18%) 7/20 (35%) AST Nl to high 3/14 (21.4%) 4/21 (19%) 5/22 (22.7%) 12/57 (21.1%) 6/20 (30%) Bicarb Nl to high 0/18 (0%) 0/22 (0.0%) 1/22 (4.5%) 1/62 (1.6%) 0/20 (0%) Bicarb Nl to low 8/18 (44.4%) 6/22 (27.3%) 8/22 (36.4%) 22/62 (35.5%) 5/20 (25%) Bilirubin Nl to high* 4/18 (22.2%) 4/22 (18.2%) 4/22 (18.2%) 12/62 (19.4%) 2/20 (10%) Calcium Nl to low 1/18 (5.6%) 2/21 (9.5%) 4/22 (18.2%) 7/61 (11.5%) 2/20 (10%) Calcium Nl to high 0/18 (0%) 2/22 (9.1%) 0/22 (0.0%) 2/61 (3.3%) 0/20 (0%) Creatinine Nl to low 2/18 (11.1%) 3/22 (13.6%) 2/22 (9.1%) 7/62 (11.3%) 2/20 (10%) GGT Nl to high 2/17 (11.8%) 6/22 (27.3%) 8/22 (36.4%) 16/61 (26.2%) 1/20 (5%) For the shift to study visit, the denominator is the number of subjects with a non-missing value for the given parameter at baseline and the visit. Baseline is defined as the latest results obtained prior to the first dose of study drug. * Bilirubin Q2 laboratory normal range high values are lower than most laboratories
39 Laboratory Shifts 0.8 mg/kg N=18 1.6 mg/kg N=22 3.2 mg/kg N=22 ALL N=62 rhGH N=20 Urea nitro Nl to low 4/18 (22.2%) 4/21 (19%) 7/22 (31.8%) 15/61 (24.6%) 7/20 (35%) Urea nitro Nl to high 1/18 (5.6%) 0/22 (0%) 1/22 (4.5%) 2/62 (3.2%) 0/20 (0%) Basophils Nl to high 7/17 (41.2%) 12/22 (54.5%) 10/21 (47.6%) 29/60 (48.3%) 4/20 (20%) Eosinophils Nl to high 2/17 (11.8%) 4/22 (18.2%) 3/21 (14.3%) 9/60 (15%) 5/20 (25%) Hematocrit Nl to low 2/18 (11.1%) 0/22 (0.0%) 2/22 (9.1%) 4/61 (6.6%) 0/20 (0%) Hematocrit Nl to high 1/17 (5.9%) 1/22 (4.5%) 2/22 (9.1%) 4/61(6.6%) 0/20 (0%) Hemoglob. Nl to low 4/18 (22.2%) 2/22 (9.1%) 5/22 (22.7%) 11/62 (17.7%) 0/20 (0%) Lymphoc. Nl to low 3/17 (17.6%) 0/21 (0.0%) 1/21 (4.8%) 4/59 (6.8 %) 1/20 (5%) Lymphoc. Nl to high 0/17 (0.0%) 0/22 (0.0%) 2/21 (9.5%) 2/60 (3.3%) 0/20 (0%)
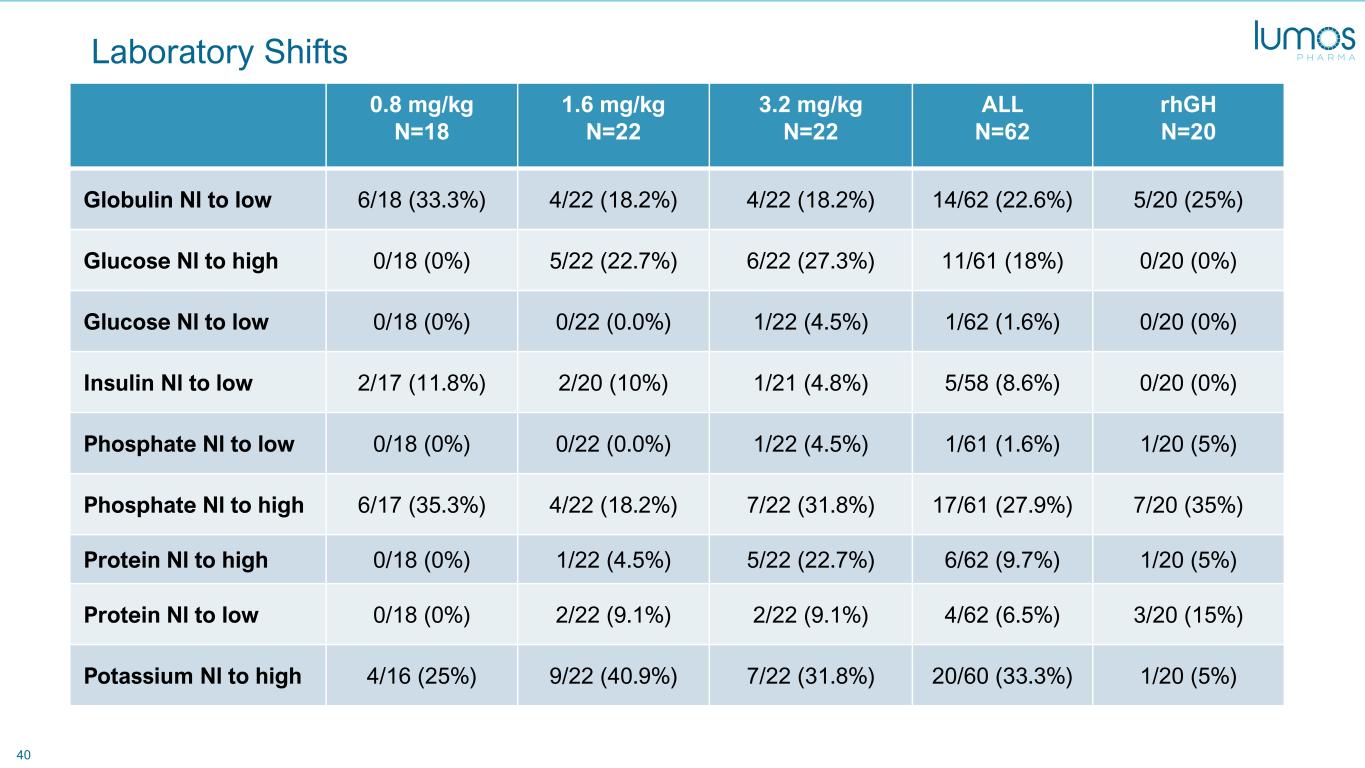
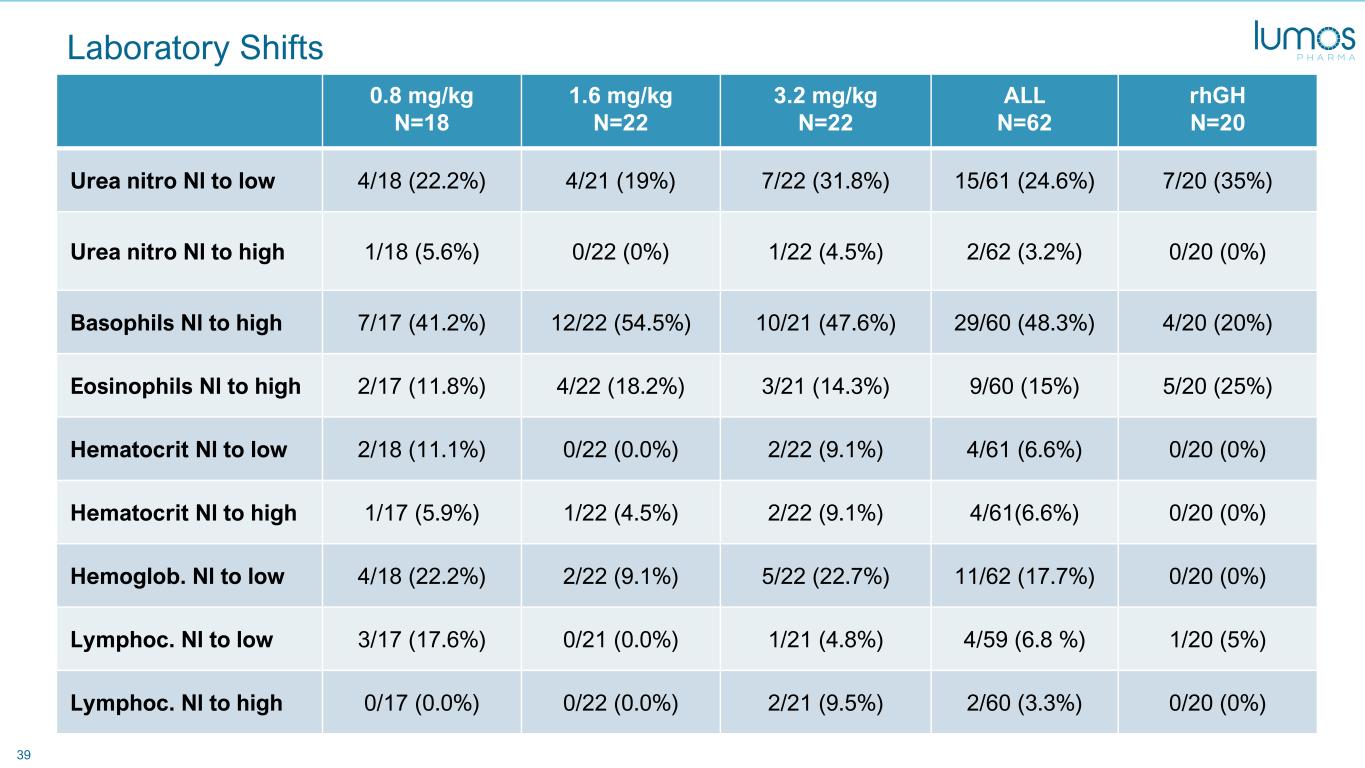
40 Laboratory Shifts 0.8 mg/kg N=18 1.6 mg/kg N=22 3.2 mg/kg N=22 ALL N=62 rhGH N=20 Globulin Nl to low 6/18 (33.3%) 4/22 (18.2%) 4/22 (18.2%) 14/62 (22.6%) 5/20 (25%) Glucose Nl to high 0/18 (0%) 5/22 (22.7%) 6/22 (27.3%) 11/61 (18%) 0/20 (0%) Glucose Nl to low 0/18 (0%) 0/22 (0.0%) 1/22 (4.5%) 1/62 (1.6%) 0/20 (0%) Insulin Nl to low 2/17 (11.8%) 2/20 (10%) 1/21 (4.8%) 5/58 (8.6%) 0/20 (0%) Phosphate Nl to low 0/18 (0%) 0/22 (0.0%) 1/22 (4.5%) 1/61 (1.6%) 1/20 (5%) Phosphate Nl to high 6/17 (35.3%) 4/22 (18.2%) 7/22 (31.8%) 17/61 (27.9%) 7/20 (35%) Protein Nl to high 0/18 (0%) 1/22 (4.5%) 5/22 (22.7%) 6/62 (9.7%) 1/20 (5%) Protein Nl to low 0/18 (0%) 2/22 (9.1%) 2/22 (9.1%) 4/62 (6.5%) 3/20 (15%) Potassium Nl to high 4/16 (25%) 9/22 (40.9%) 7/22 (31.8%) 20/60 (33.3%) 1/20 (5%)
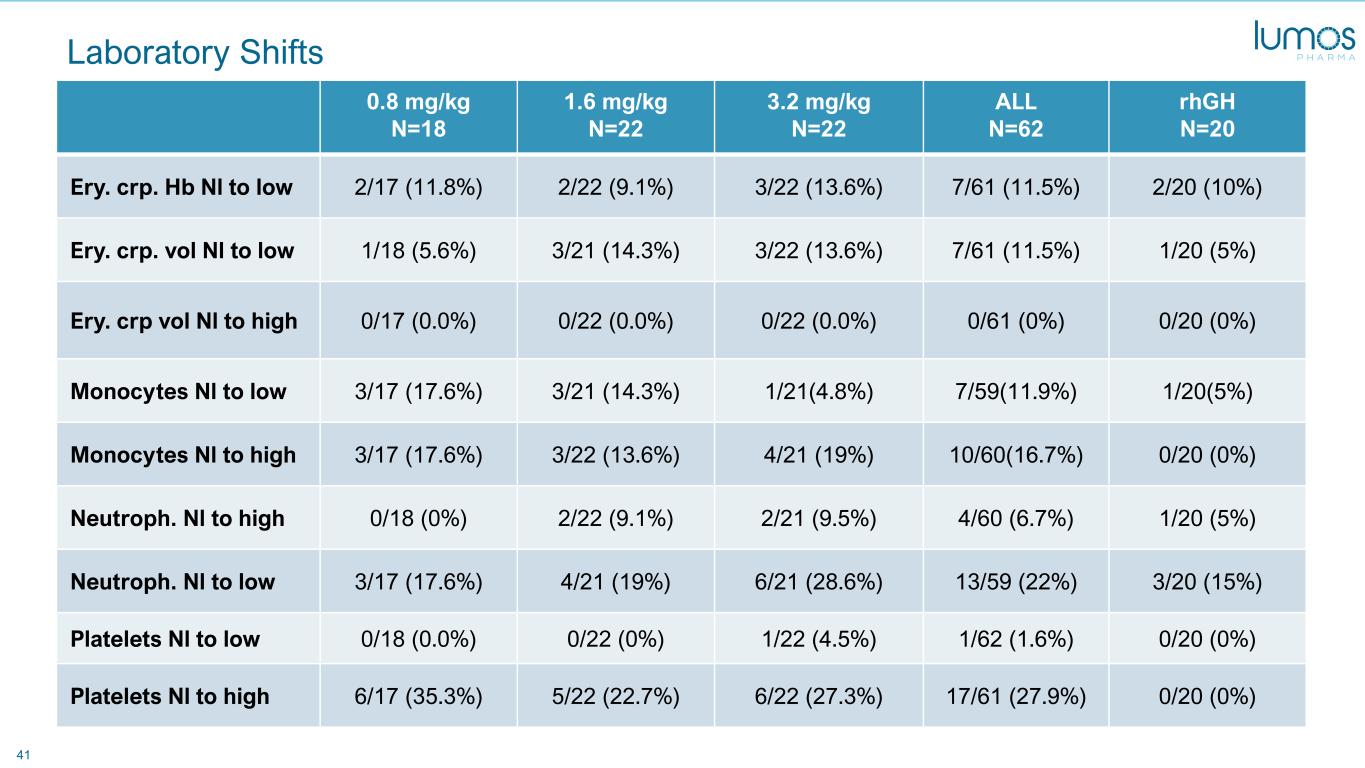
41 Laboratory Shifts 0.8 mg/kg N=18 1.6 mg/kg N=22 3.2 mg/kg N=22 ALL N=62 rhGH N=20 Ery. crp. Hb Nl to low 2/17 (11.8%) 2/22 (9.1%) 3/22 (13.6%) 7/61 (11.5%) 2/20 (10%) Ery. crp. vol Nl to low 1/18 (5.6%) 3/21 (14.3%) 3/22 (13.6%) 7/61 (11.5%) 1/20 (5%) Ery. crp vol Nl to high 0/17 (0.0%) 0/22 (0.0%) 0/22 (0.0%) 0/61 (0%) 0/20 (0%) Monocytes Nl to low 3/17 (17.6%) 3/21 (14.3%) 1/21(4.8%) 7/59(11.9%) 1/20(5%) Monocytes Nl to high 3/17 (17.6%) 3/22 (13.6%) 4/21 (19%) 10/60(16.7%) 0/20 (0%) Neutroph. Nl to high 0/18 (0%) 2/22 (9.1%) 2/21 (9.5%) 4/60 (6.7%) 1/20 (5%) Neutroph. Nl to low 3/17 (17.6%) 4/21 (19%) 6/21 (28.6%) 13/59 (22%) 3/20 (15%) Platelets Nl to low 0/18 (0.0%) 0/22 (0%) 1/22 (4.5%) 1/62 (1.6%) 0/20 (0%) Platelets Nl to high 6/17 (35.3%) 5/22 (22.7%) 6/22 (27.3%) 17/61 (27.9%) 0/20 (0%)
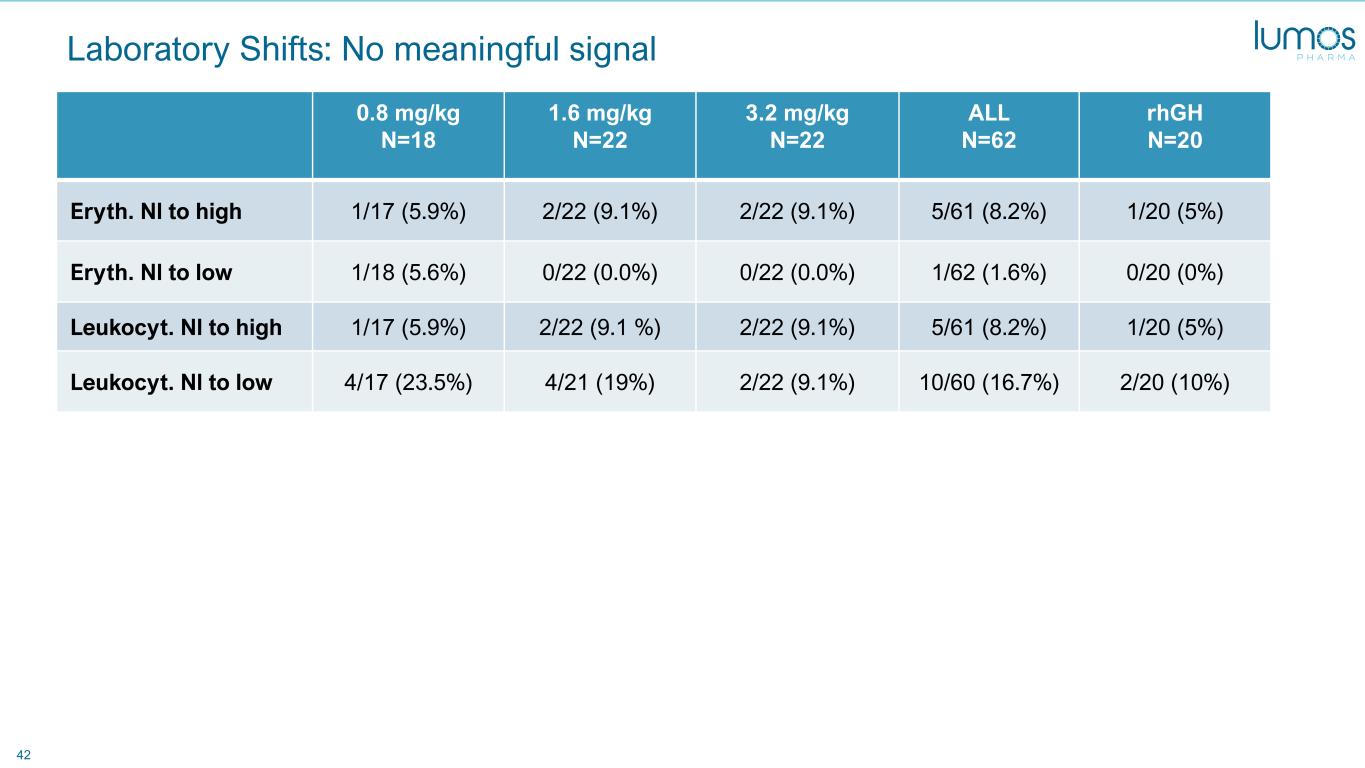
42 Laboratory Shifts: No meaningful signal 0.8 mg/kg N=18 1.6 mg/kg N=22 3.2 mg/kg N=22 ALL N=62 rhGH N=20 Eryth. Nl to high 1/17 (5.9%) 2/22 (9.1%) 2/22 (9.1%) 5/61 (8.2%) 1/20 (5%) Eryth. Nl to low 1/18 (5.6%) 0/22 (0.0%) 0/22 (0.0%) 1/62 (1.6%) 0/20 (0%) Leukocyt. Nl to high 1/17 (5.9%) 2/22 (9.1 %) 2/22 (9.1%) 5/61 (8.2%) 1/20 (5%) Leukocyt. Nl to low 4/17 (23.5%) 4/21 (19%) 2/22 (9.1%) 10/60 (16.7%) 2/20 (10%)

Supplementary Materials
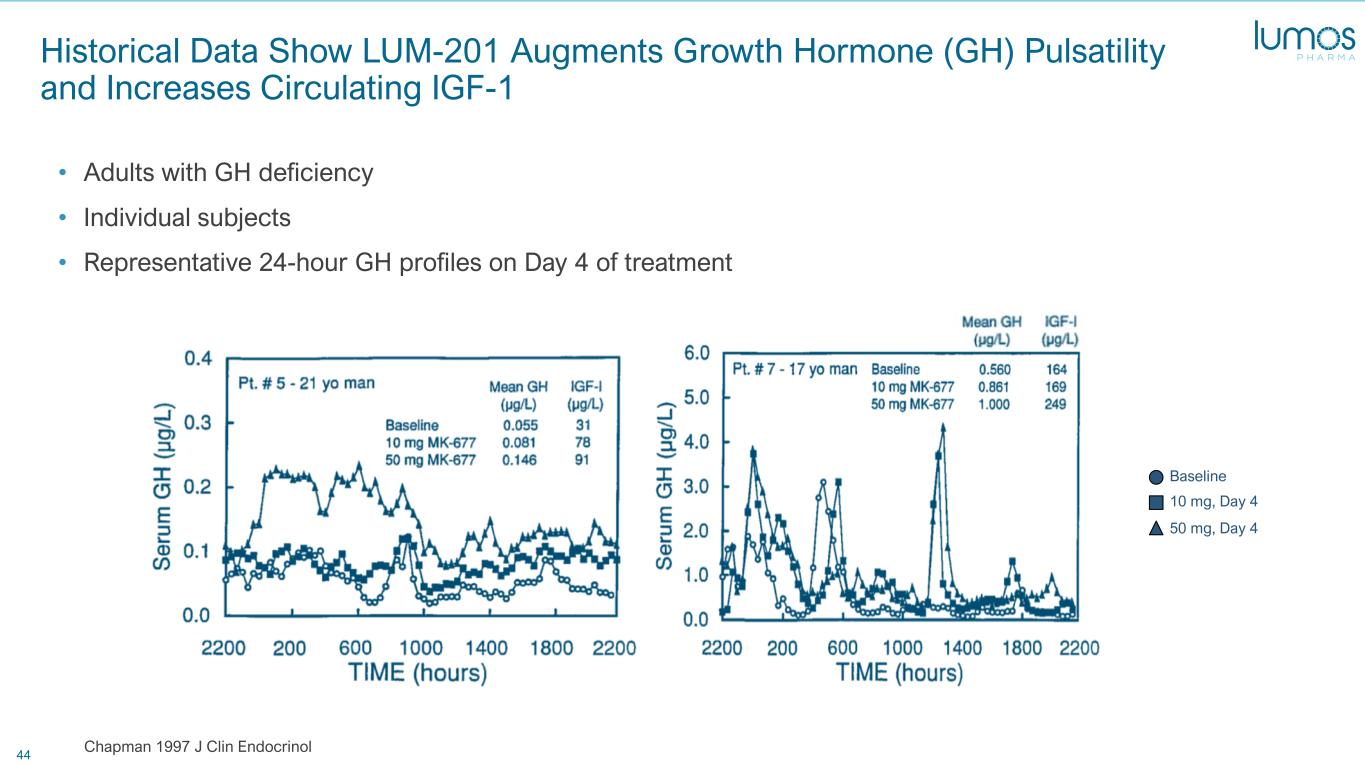
44 Historical Data Show LUM-201 Augments Growth Hormone (GH) Pulsatility and Increases Circulating IGF-1 • Adults with GH deficiency • Individual subjects • Representative 24-hour GH profiles on Day 4 of treatment Chapman 1997 J Clin Endocrinol Baseline 10 mg, Day 4 50 mg, Day 4

45 Historical Data Demonstrate Differentiated MOA of LUM-201 vs rhGH LUM-201 Augments Growth Hormone (GH) Pulsatility in GHD Adults • Adults with GH deficiency • LUM-201 augments endogenous GH pulses • rhGH is administered as single, daily bolus doses 1 Adapted, Chapman 1997 J Clin Endocrinol 2 Janssen 1999 Br J Clin Pharmacol (Genotropin) 24h GH profile following oral LUM-201 administration in an adult with GH deficiency1 24h PK profile following subcutaneous rhGH injection in adults with GH deficiency2 0.6 IU 1.8 IU 1.2 IU G H (m U I-1 ) Potential to achieve non-inferior growth from smaller GH AUC via LUM-201 pulsatile delivery vs rhGH bolus administration
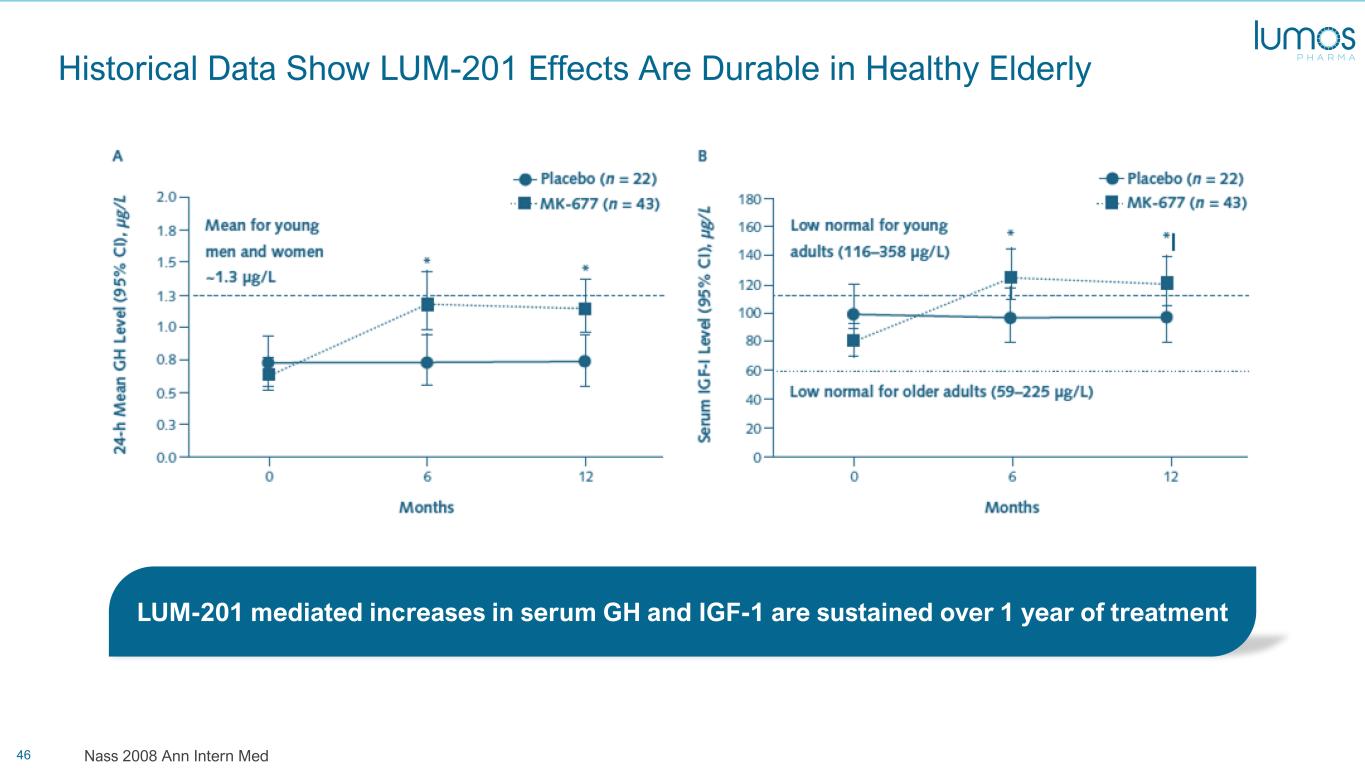
46 Historical Data Show LUM-201 Effects Are Durable in Healthy Elderly Nass 2008 Ann Intern Med LUM-201 mediated increases in serum GH and IGF-1 are sustained over 1 year of treatment
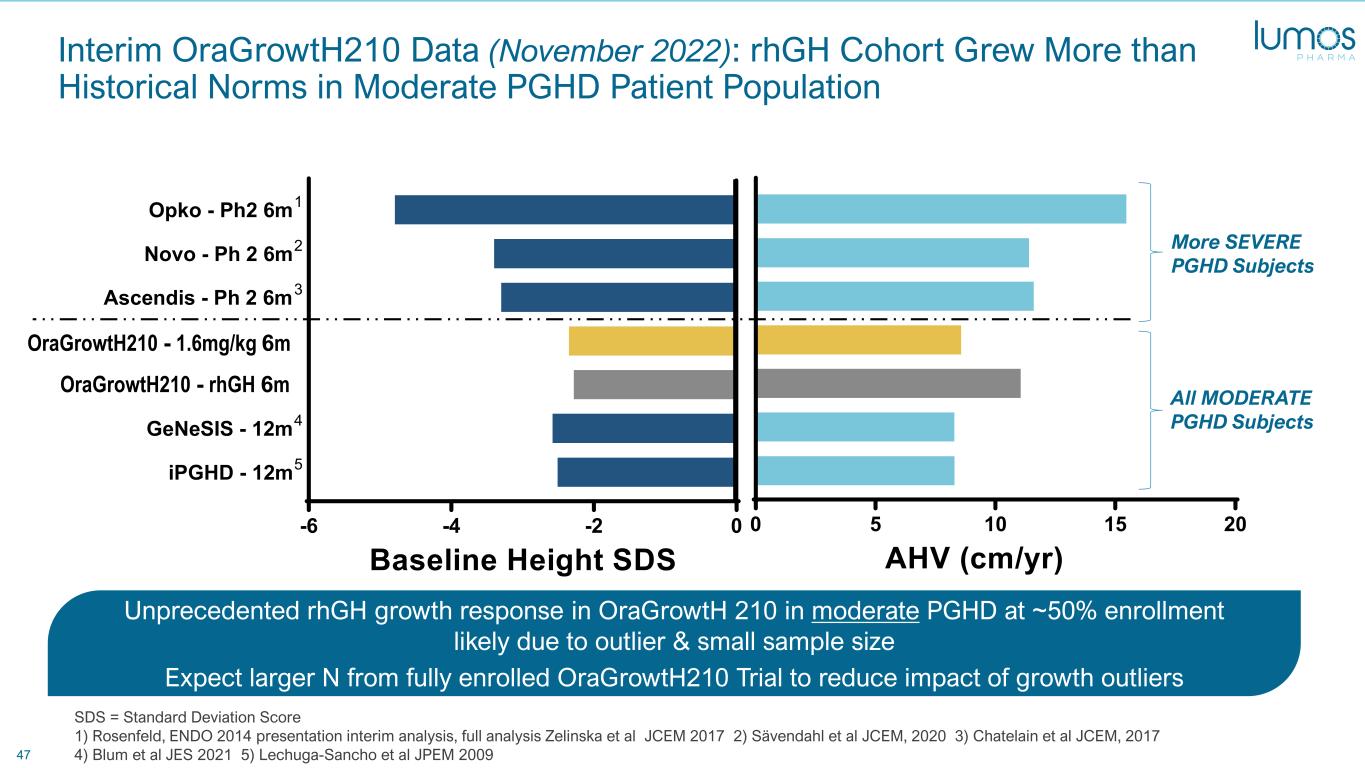
47 Interim OraGrowtH210 Data (November 2022): rhGH Cohort Grew More than Historical Norms in Moderate PGHD Patient Population Unprecedented rhGH growth response in OraGrowtH 210 in moderate PGHD at ~50% enrollment likely due to outlier & small sample size Expect larger N from fully enrolled OraGrowtH210 Trial to reduce impact of growth outliers SDS = Standard Deviation Score 1) Rosenfeld, ENDO 2014 presentation interim analysis, full analysis Zelinska et al JCEM 2017 2) Sävendahl et al JCEM, 2020 3) Chatelain et al JCEM, 2017 4) Blum et al JES 2021 5) Lechuga-Sancho et al JPEM 2009 More SEVERE PGHD Subjects All MODERATE PGHD Subjects 0 5 10 15 20 1 2 3 4 5 6 7 AHV (cm/yr) -6 -4 -2 0 iPGHD - 12m5 GeNeSIS - 12m4 OraGrowth210 - rhGH 6m OraGrowth210 - 1.6mg/kg 6m Ascendis - Ph 2 6m3 Novo - Ph 2 6m2 Opko - Ph2 6m1 Baseline Height SDS OraGro tH . g/kg 6m Ora H 0 - rhGH 6m
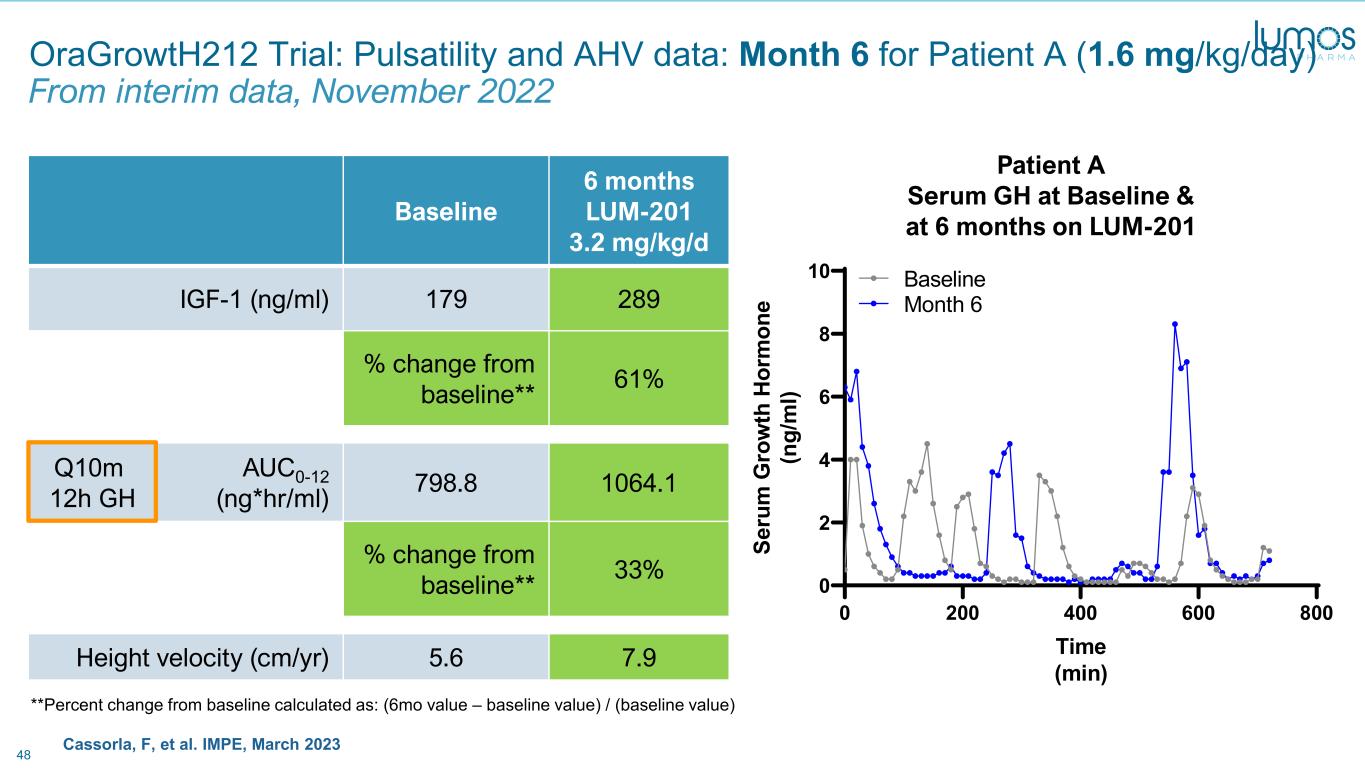
48 Cassorla, F, et al. IMPE, March 2023 OraGrowtH212 Trial: Pulsatility and AHV data: Month 6 for Patient A (1.6 mg/kg/day) From interim data, November 2022 Baseline 6 months LUM-201 3.2 mg/kg/d IGF-1 (ng/ml) 179 289 % change from baseline** 61% Q10m 12h GH AUC0-12 (ng*hr/ml) 798.8 1064.1 % change from baseline** 33% Height velocity (cm/yr) 5.6 7.9 **Percent change from baseline calculated as: (6mo value – baseline value) / (baseline value) 0 200 400 600 800 0 2 4 6 8 10 Patient A Time (min) Se ru m G ro w th H or m on e (n g/ m l) Baseline Month 6 Patient A Serum GH at Baseline & at 6 months on LUM-201
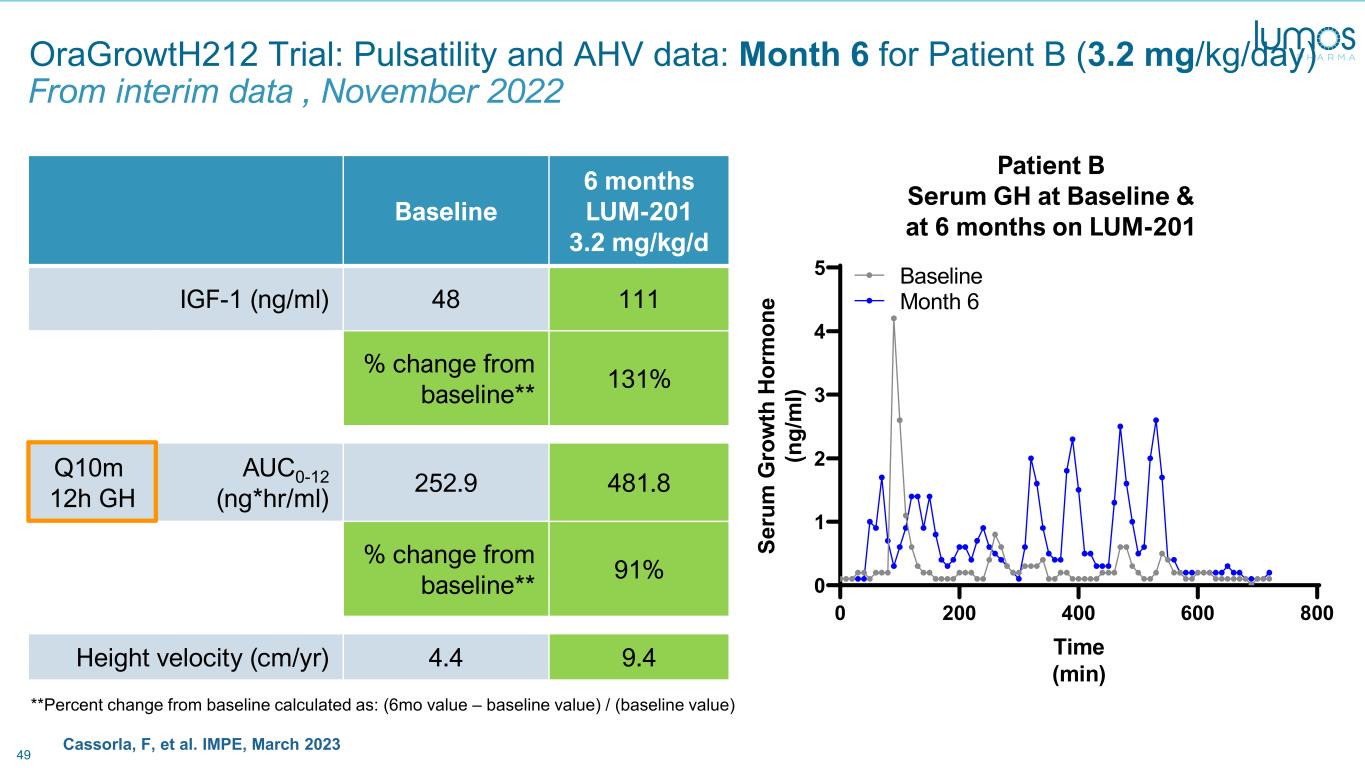
49 Cassorla, F, et al. IMPE, March 2023 0 200 400 600 800 0 1 2 3 4 5 Patient B Time (min) Se ru m G ro w th H or m on e (n g/ m l) Baseline Month 6 Patient B Serum GH at Baseline & at 6 months on LUM-201 OraGrowtH212 Trial: Pulsatility and AHV data: Month 6 for Patient B (3.2 mg/kg/day) From interim data , November 2022 Baseline 6 months LUM-201 3.2 mg/kg/d IGF-1 (ng/ml) 48 111 % change from baseline** 131% Q10m 12h GH AUC0-12 (ng*hr/ml) 252.9 481.8 % change from baseline** 91% Height velocity (cm/yr) 4.4 9.4 **Percent change from baseline calculated as: (6mo value – baseline value) / (baseline value)
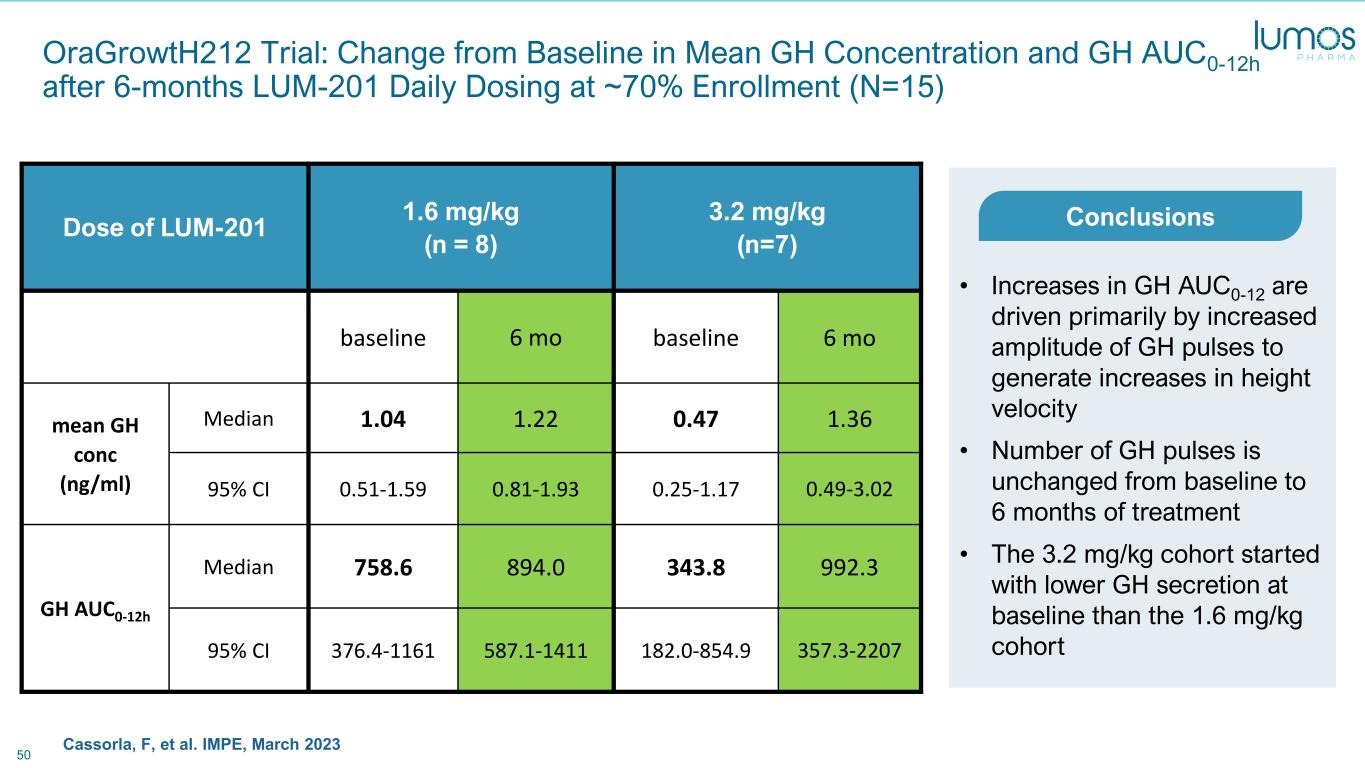
50 OraGrowtH212 Trial: Change from Baseline in Mean GH Concentration and GH AUC0-12h after 6-months LUM-201 Daily Dosing at ~70% Enrollment (N=15) Dose of LUM-201 1.6 mg/kg (n = 8) 3.2 mg/kg (n=7) baseline 6 mo baseline 6 mo mean GH conc (ng/ml) Median 1.04 1.22 0.47 1.36 95% CI 0.51-1.59 0.81-1.93 0.25-1.17 0.49-3.02 GH AUC0-12h Median 758.6 894.0 343.8 992.3 95% CI 376.4-1161 587.1-1411 182.0-854.9 357.3-2207 • Increases in GH AUC0-12 are driven primarily by increased amplitude of GH pulses to generate increases in height velocity • Number of GH pulses is unchanged from baseline to 6 months of treatment • The 3.2 mg/kg cohort started with lower GH secretion at baseline than the 1.6 mg/kg cohort Conclusions Cassorla, F, et al. IMPE, March 2023
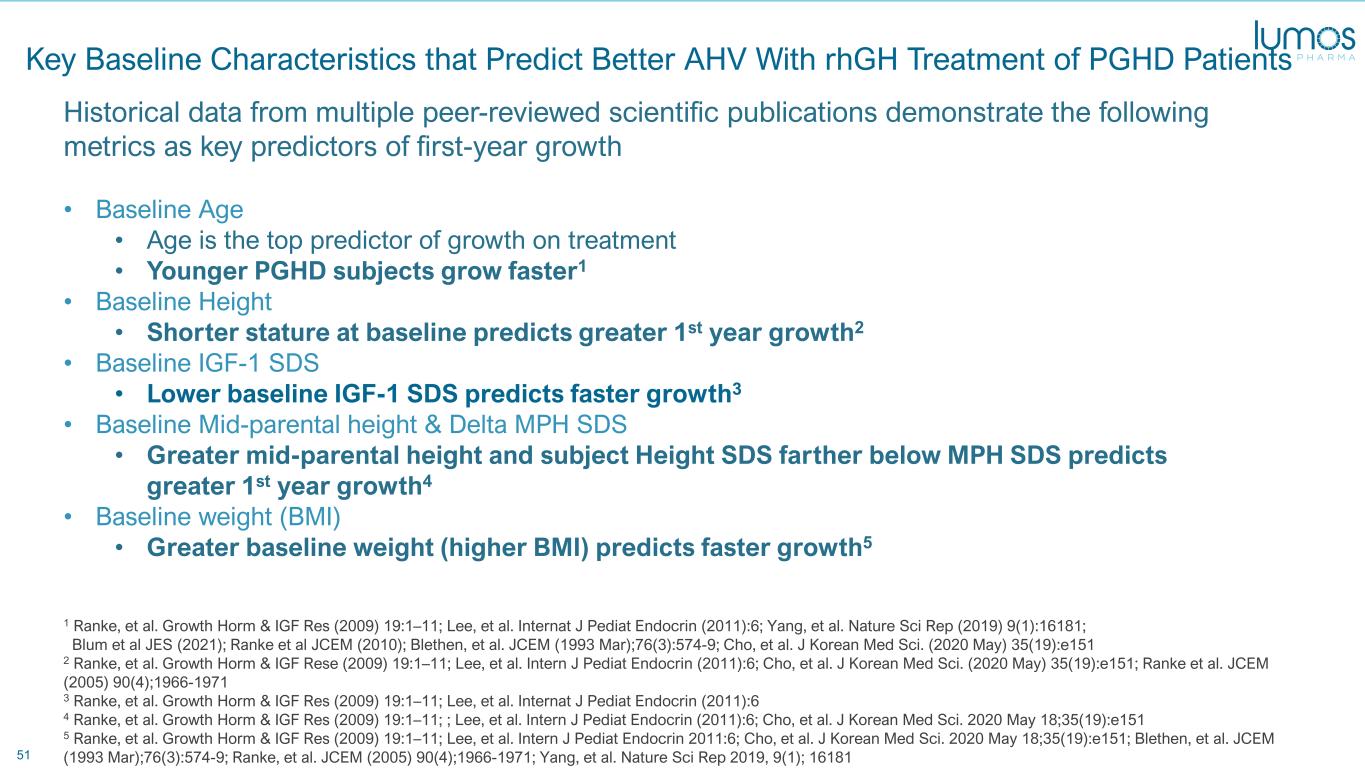
51 Key Baseline Characteristics that Predict Better AHV With rhGH Treatment of PGHD Patients Historical data from multiple peer-reviewed scientific publications demonstrate the following metrics as key predictors of first-year growth • Baseline Age • Age is the top predictor of growth on treatment • Younger PGHD subjects grow faster1 • Baseline Height • Shorter stature at baseline predicts greater 1st year growth2 • Baseline IGF-1 SDS • Lower baseline IGF-1 SDS predicts faster growth3 • Baseline Mid-parental height & Delta MPH SDS • Greater mid-parental height and subject Height SDS farther below MPH SDS predicts greater 1st year growth4 • Baseline weight (BMI) • Greater baseline weight (higher BMI) predicts faster growth5 1 Ranke, et al. Growth Horm & IGF Res (2009) 19:1–11; Lee, et al. Internat J Pediat Endocrin (2011):6; Yang, et al. Nature Sci Rep (2019) 9(1):16181; Blum et al JES (2021); Ranke et al JCEM (2010); Blethen, et al. JCEM (1993 Mar);76(3):574-9; Cho, et al. J Korean Med Sci. (2020 May) 35(19):e151 2 Ranke, et al. Growth Horm & IGF Rese (2009) 19:1–11; Lee, et al. Intern J Pediat Endocrin (2011):6; Cho, et al. J Korean Med Sci. (2020 May) 35(19):e151; Ranke et al. JCEM (2005) 90(4);1966-1971 3 Ranke, et al. Growth Horm & IGF Res (2009) 19:1–11; Lee, et al. Internat J Pediat Endocrin (2011):6 4 Ranke, et al. Growth Horm & IGF Res (2009) 19:1–11; ; Lee, et al. Intern J Pediat Endocrin (2011):6; Cho, et al. J Korean Med Sci. 2020 May 18;35(19):e151 5 Ranke, et al. Growth Horm & IGF Res (2009) 19:1–11; Lee, et al. Intern J Pediat Endocrin 2011:6; Cho, et al. J Korean Med Sci. 2020 May 18;35(19):e151; Blethen, et al. JCEM (1993 Mar);76(3):574-9; Ranke, et al. JCEM (2005) 90(4);1966-1971; Yang, et al. Nature Sci Rep 2019, 9(1); 16181
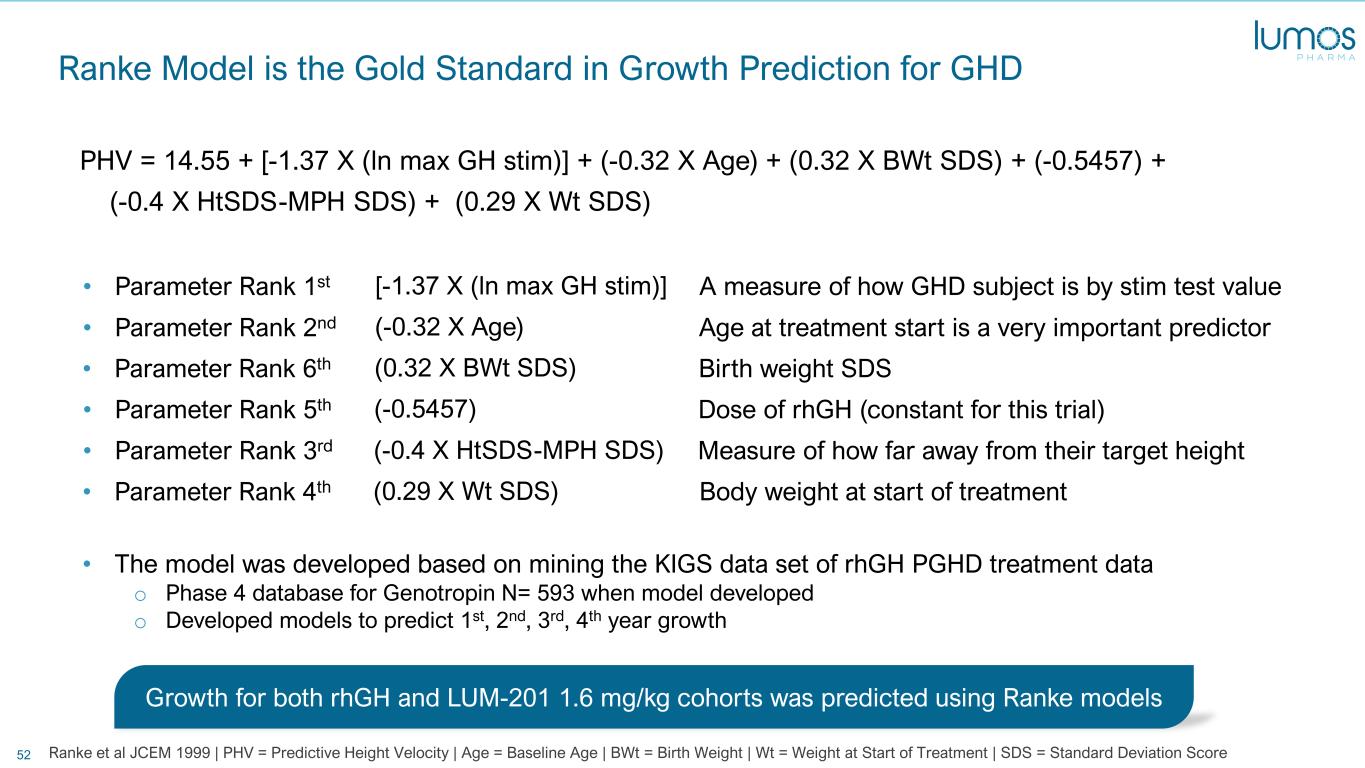
52 Ranke Model is the Gold Standard in Growth Prediction for GHD PHV = 14.55 + [-1.37 X (ln max GH stim)] + (-0.32 X Age) + (0.32 X BWt SDS) + (-0.5457) + (-0.4 X HtSDS-MPH SDS) + (0.29 X Wt SDS) [-1.37 X (ln max GH stim)]• Parameter Rank 1st A measure of how GHD subject is by stim test value (-0.32 X Age)• Parameter Rank 2nd Age at treatment start is a very important predictor (0.32 X BWt SDS)• Parameter Rank 6th Birth weight SDS (-0.5457)• Parameter Rank 5th Dose of rhGH (constant for this trial) (-0.4 X HtSDS-MPH SDS)• Parameter Rank 3rd Measure of how far away from their target height (0.29 X Wt SDS)• Parameter Rank 4th Body weight at start of treatment • The model was developed based on mining the KIGS data set of rhGH PGHD treatment data o Phase 4 database for Genotropin N= 593 when model developed o Developed models to predict 1st, 2nd, 3rd, 4th year growth Growth for both rhGH and LUM-201 1.6 mg/kg cohorts was predicted using Ranke models Ranke et al JCEM 1999 | PHV = Predictive Height Velocity | Age = Baseline Age | BWt = Birth Weight | Wt = Weight at Start of Treatment | SDS = Standard Deviation Score
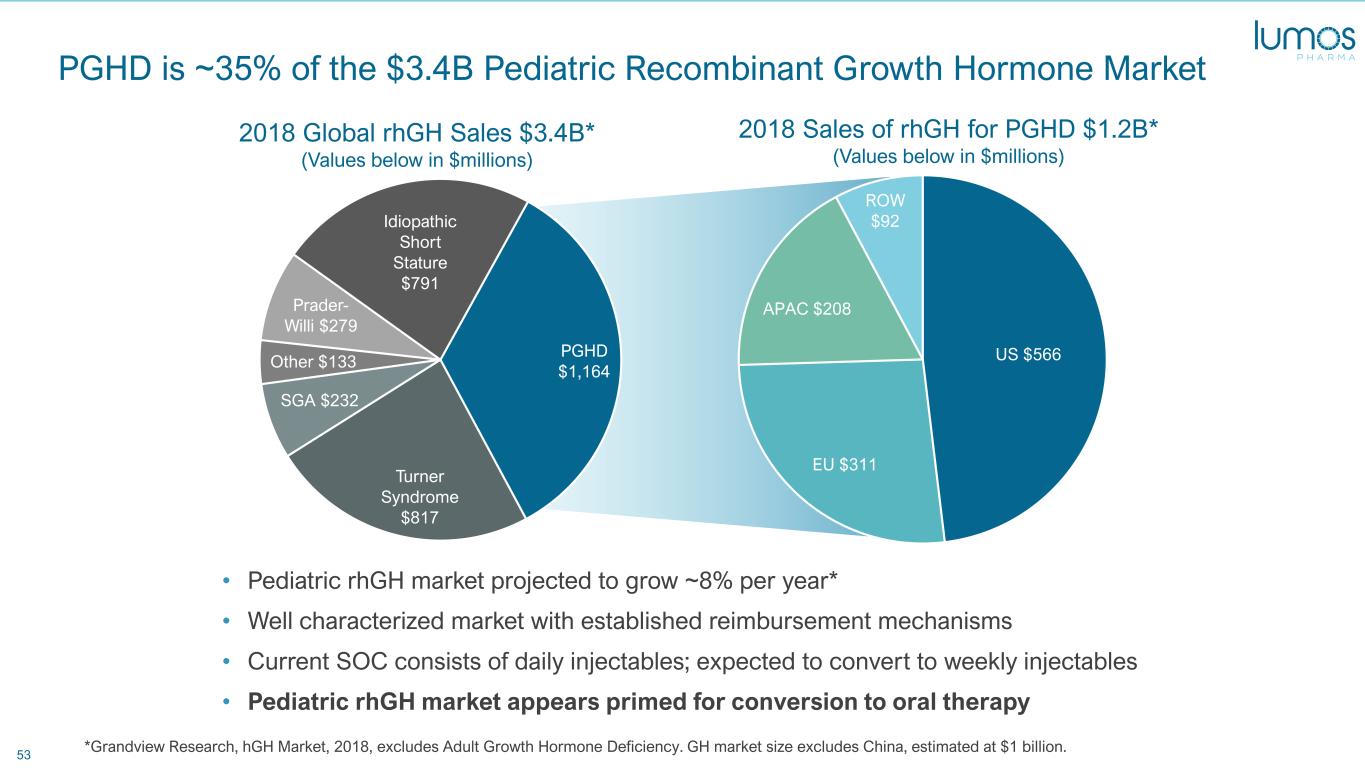
53 PGHD is ~35% of the $3.4B Pediatric Recombinant Growth Hormone Market • Pediatric rhGH market projected to grow ~8% per year* • Well characterized market with established reimbursement mechanisms • Current SOC consists of daily injectables; expected to convert to weekly injectables • Pediatric rhGH market appears primed for conversion to oral therapy *Grandview Research, hGH Market, 2018, excludes Adult Growth Hormone Deficiency. GH market size excludes China, estimated at $1 billion. PGHD $1,164 Turner Syndrome $817 SGA $232 Other $133 Prader- Willi $279 Idiopathic Short Stature $791 US $566 EU $311 APAC $208 ROW $92 2018 Global rhGH Sales $3.4B* (Values below in $millions) 2018 Sales of rhGH for PGHD $1.2B* (Values below in $millions)
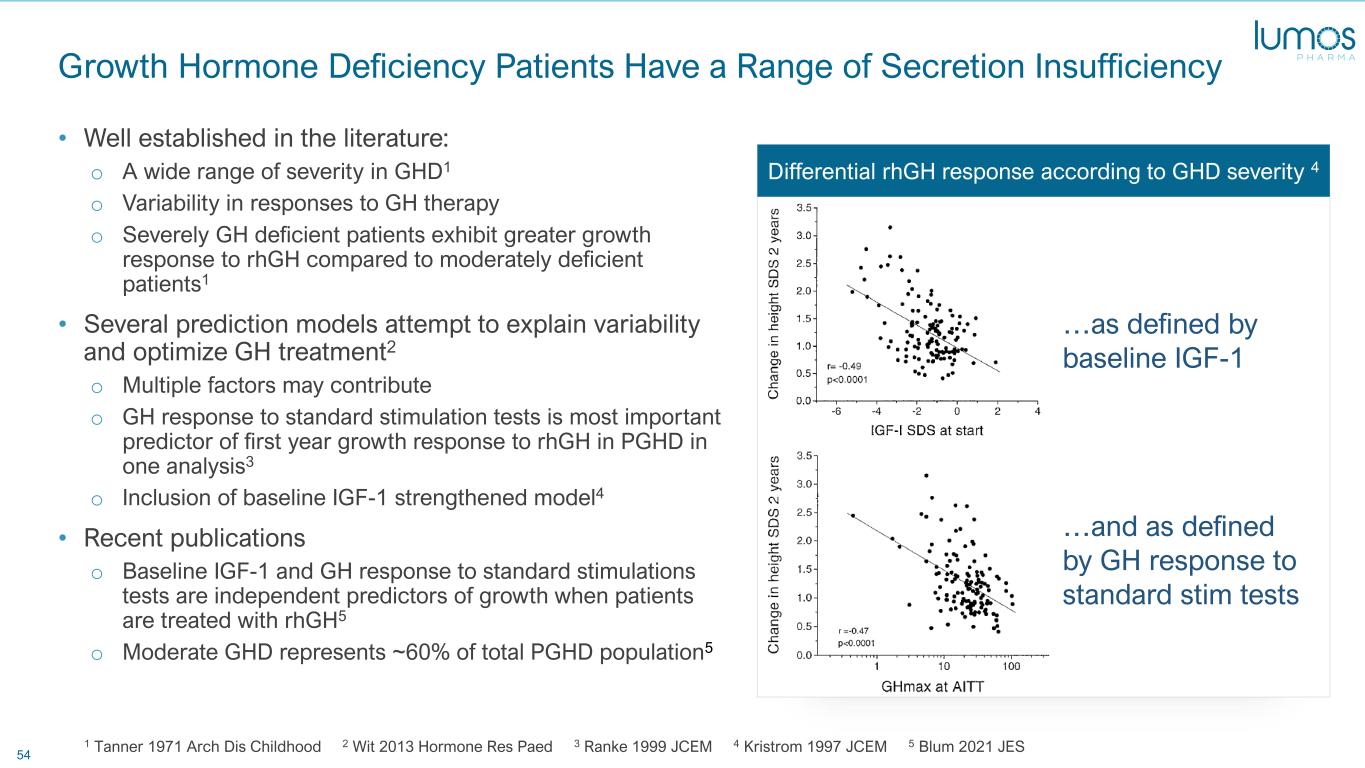
54 Growth Hormone Deficiency Patients Have a Range of Secretion Insufficiency • Well established in the literature: o A wide range of severity in GHD1 o Variability in responses to GH therapy o Severely GH deficient patients exhibit greater growth response to rhGH compared to moderately deficient patients1 • Several prediction models attempt to explain variability and optimize GH treatment2 o Multiple factors may contribute o GH response to standard stimulation tests is most important predictor of first year growth response to rhGH in PGHD in one analysis3 o Inclusion of baseline IGF-1 strengthened model4 • Recent publications o Baseline IGF-1 and GH response to standard stimulations tests are independent predictors of growth when patients are treated with rhGH5 o Moderate GHD represents ~60% of total PGHD population5 1 Tanner 1971 Arch Dis Childhood 2 Wit 2013 Hormone Res Paed 3 Ranke 1999 JCEM 4 Kristrom 1997 JCEM 5 Blum 2021 JES …as defined by baseline IGF-1 …and as defined by GH response to standard stim tests Differential rhGH response according to GHD severity 4
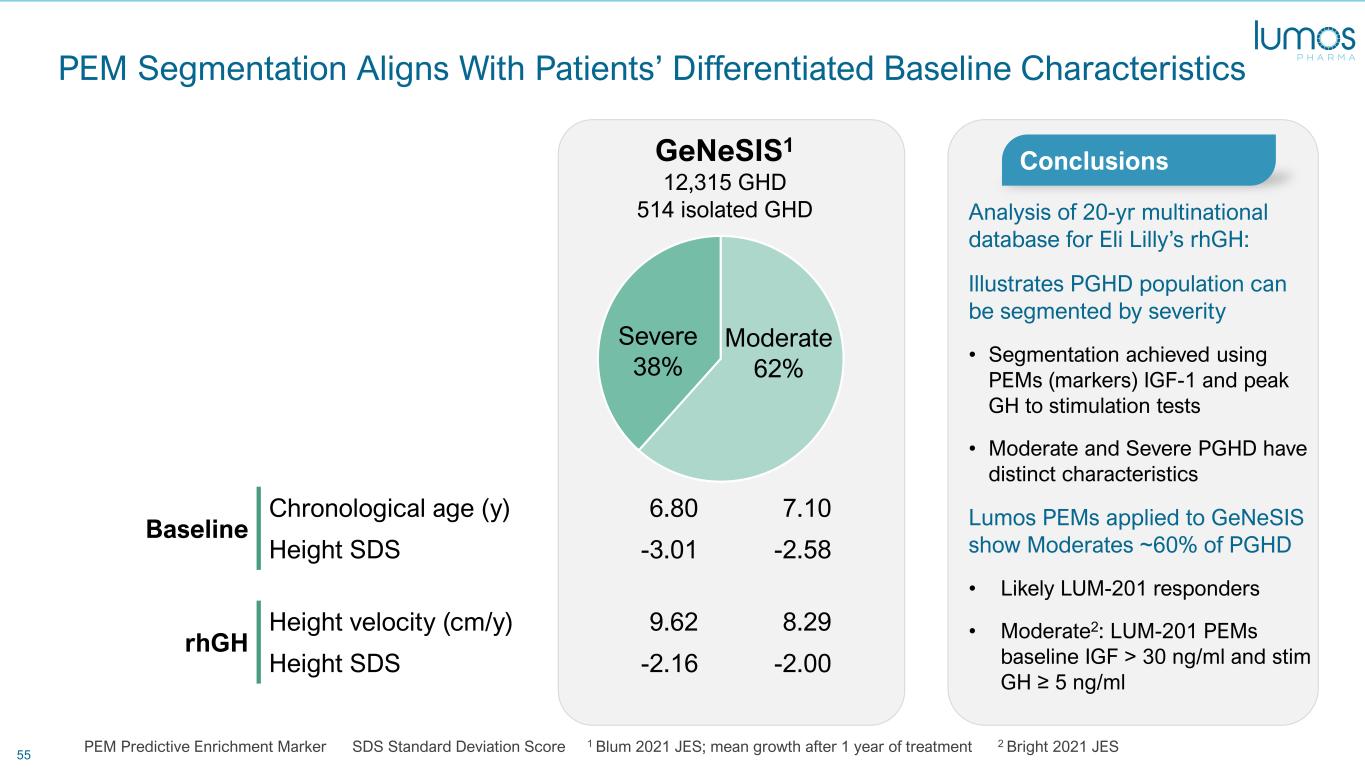
55 PEM Segmentation Aligns With Patients’ Differentiated Baseline Characteristics PEM Predictive Enrichment Marker SDS Standard Deviation Score 1 Blum 2021 JES; mean growth after 1 year of treatment 2 Bright 2021 JES Severe 38% Moderate 62% GeNeSIS1 12,315 GHD 514 isolated GHD Baseline Chronological age (y) 6.80 7.10 Height SDS -3.01 -2.58 rhGH Height velocity (cm/y) 9.62 8.29 Height SDS -2.16 -2.00 Analysis of 20-yr multinational database for Eli Lilly’s rhGH: Illustrates PGHD population can be segmented by severity • Segmentation achieved using PEMs (markers) IGF-1 and peak GH to stimulation tests • Moderate and Severe PGHD have distinct characteristics Lumos PEMs applied to GeNeSIS show Moderates ~60% of PGHD • Likely LUM-201 responders • Moderate2: LUM-201 PEMs baseline IGF > 30 ng/ml and stim GH ≥ 5 ng/ml Conclusions
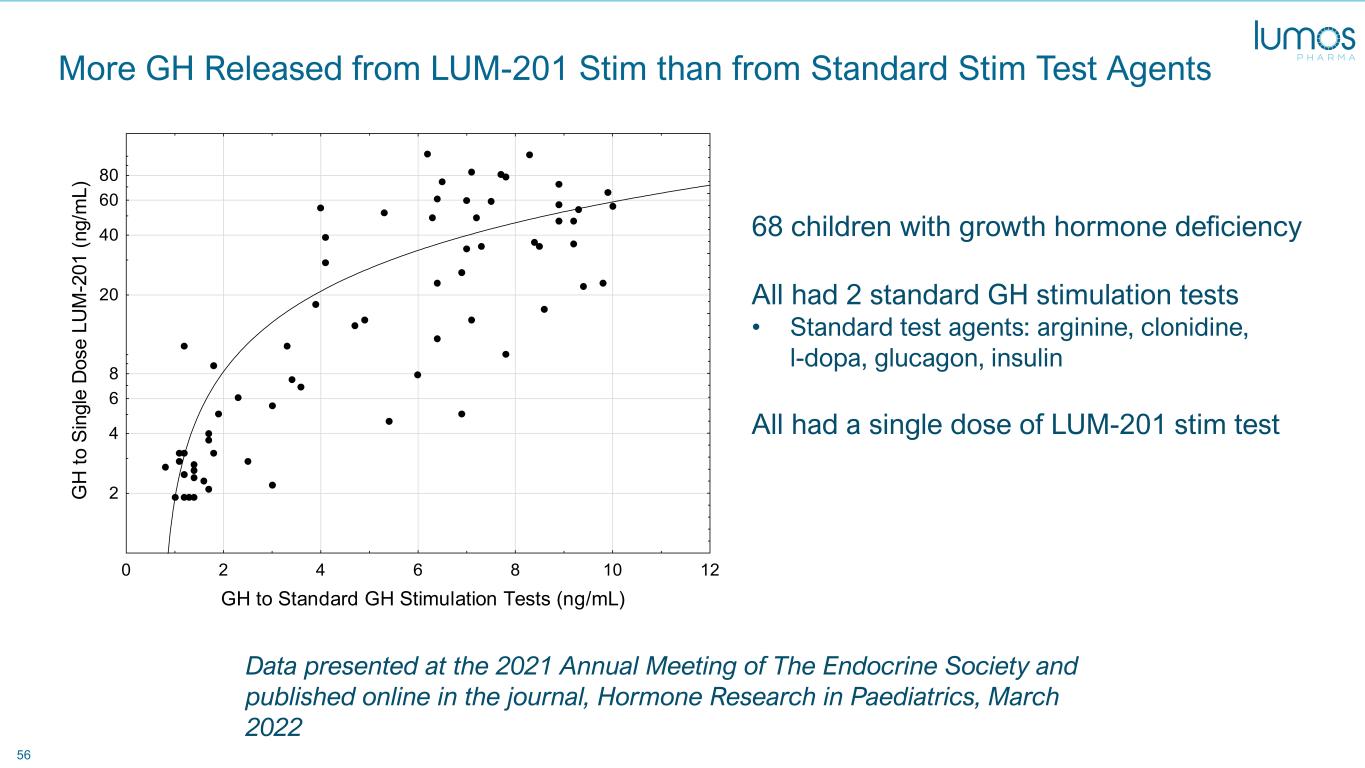
56 More GH Released from LUM-201 Stim than from Standard Stim Test Agents 0 2 4 6 8 10 12 GH to Standard GH Stimulation Tests (ng/mL) 2 4 6 8 20 40 60 80 G H to S in gl e D os e LU M -2 01 (n g/ m L) 68 children with growth hormone deficiency All had 2 standard GH stimulation tests • Standard test agents: arginine, clonidine, l-dopa, glucagon, insulin All had a single dose of LUM-201 stim test Data presented at the 2021 Annual Meeting of The Endocrine Society and published online in the journal, Hormone Research in Paediatrics, March 2022
57 • n = 10 • Adult NAFLD subjects with relative GH/IGF-1 deficiency • Open-label • Single-site pilot study • 6-month dosing Study of Oral LUM-201 in Non-Alcoholic Fatty Liver Disease (NAFLD) Mass General Investigator-Initiated Phase 2 Pilot Trial Primary Objective: • Determine changes in intra- hepatic lipid content, inflammation, and potentially fibrosis resulting from LUM-201 induced GH augmentation compared to historical placebo-treated controls Massachusetts General Hospital (MGH) initiated pilot study of oral LUM-201 in NAFLD: Enrollment ongoing Objectives n = 10 – LUM-201 at dose level of 25 mg/day Study Duration – 6 months MGH Initiated Phase 2 Pilot Trial# Currently enrolling subjects # Principal Investigator: Laura Dichtel, MD, Assistant Professor, Massachusetts General Hospital Trial supported by prior data evaluating rhGH in NAFLD: (ENDO 2022) JES, Volume 6, Issue Supplement_1, November-December 2022, Page A525