false
0001763950
0001763950
2023-11-08
2023-11-08
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): November 8, 2023
Lantern
Pharma Inc.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
001-39318 |
|
46-3973463 |
(State
or Other Jurisdiction
of
Incorporation) |
|
(Commission
File
Number) |
|
(IRS
Employer
Identification
No.) |
1920
McKinney Avenue, 7th Floor
Dallas,
Texas |
|
75201 |
| (Address
of Principal Executive Offices) |
|
(Zip
Code) |
(972)
277-1136
(Registrant’s
telephone number, including area code)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act: Common Stock
| Title
of each class |
|
Trading
Symbol |
|
Name
of each exchange on which registered |
| Common
Stock, $0.0001 par value |
|
LTRN |
|
The
Nasdaq Stock Market |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☒
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item
2.02 Results of Operations and Financial Condition.
On
November 8, 2023, Lantern Pharma Inc. (the “Company”) will issue a press release announcing its financial results for the
third quarter ended September 30, 2023. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and
is incorporated herein by reference.
The
information in this Item 2.02, including Exhibit 99.1 hereto, shall not be deemed “filed” for purposes of Section 18 of the
Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section,
nor shall it be deemed incorporated by reference in any filings under the Securities Act of 1933, as amended, or the Exchange Act, regardless
of any general incorporation language in such filings, unless expressly incorporated by specific reference in such filing.
Item
7.01 Regulation FD Disclosure.
On
November 8, 2023, the Company will utilize a presentation to assist with the Company’s discussions during a conference call and
live webinar hosted by the Company to discuss financial and operating results for the third quarter ended September 30, 2023. A copy
of the presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
The
information in this Item 7.01, including Exhibit 99.2 hereto, shall not be deemed “filed” for purposes of Section 18 of the
Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section,
nor shall it be deemed incorporated by reference in any filings under the Securities Act of 1933, as amended, or the Exchange Act, regardless
of any general incorporation language in such filings, unless expressly incorporated by specific reference in such filing.
Item
9.01 Financial Statements and Exhibits.
(d)
Exhibits.
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
Lantern
Pharma Inc., |
| |
A
Delaware Corporation |
| |
|
| Dated:
November 8, 2023 |
By: |
/s/
David R. Margrave |
| |
|
David
R. Margrave, Chief Financial Officer |
Exhibit
99.1

Lantern
Pharma Reports Third Quarter 2023 Financial Results and Operational Highlights
| |
● |
Received
IND clearance from FDA to initiate Phase 1 clinical trial for LP-284, a first-in-human trial for advanced, refractory non-Hodgkin’s
lymphomas (NHL). |
| |
|
|
| |
● |
Dosed
initial patient in Phase 1 with LP-184, a clinical trial for multiple advanced solid tumors that are refractory to standard-of-care
therapies. |
| |
|
|
| |
● |
Progressed
Phase 2 LP-300 Harmonic™ clinical trial towards enrollment in East Asian countries where 30-35+% of all lung cancer
cases occur in never-smokers with NSCLC; continued expansion of additional clinical trial sites in the US and increased focus on
recruitment activity with advocacy groups. |
| |
|
|
| |
● |
Developed
initial proof-of-concept and preclinical evidence for a novel cryptophycin-based ADC (antibody-drug conjugate); initial data
is planned to be shared in January 2024. |
| |
|
|
| |
● |
Furthered
development of Lantern’s AI platform, RADR®, to include modules for the streamlined development of ADCs
and the prediction of drug combinations with existing approved checkpoint inhibitors. |
| |
|
|
| |
● |
Approximately
$45 million in cash, cash equivalents, and marketable securities as of September 30, 2023, is anticipated to provide a cash
runway into at least Q3 of 2025. |
| |
|
|
| |
● |
The
conference call and webcast are scheduled for today, Wednesday, at 4:30 p.m. ET / 1:30 p.m. PT. |
DALLAS—(Business
Wire)—Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (“AI”) company developing targeted and transformative
cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical-stage
drug programs, today announced operational highlights and financial results for the third quarter ended September 30, 2023.
“Lantern
had a very productive and efficient third quarter where the team made excellent and continued progress across our lead clinical programs,
launched a new program into the clinic, and accelerated our efforts to ensure that our AI platform for cancer drug development, RADR®,
maintains its industry-leading position. We now have three active clinical programs that we are confident will make significant strides
in Q4 and throughout 2024 – with multiple readouts expected during 2024. In addition, we continued to maintain a financially disciplined
operation that will allow us to achieve milestones in both our drug programs and our AI platform over the next several years. Our RADR®
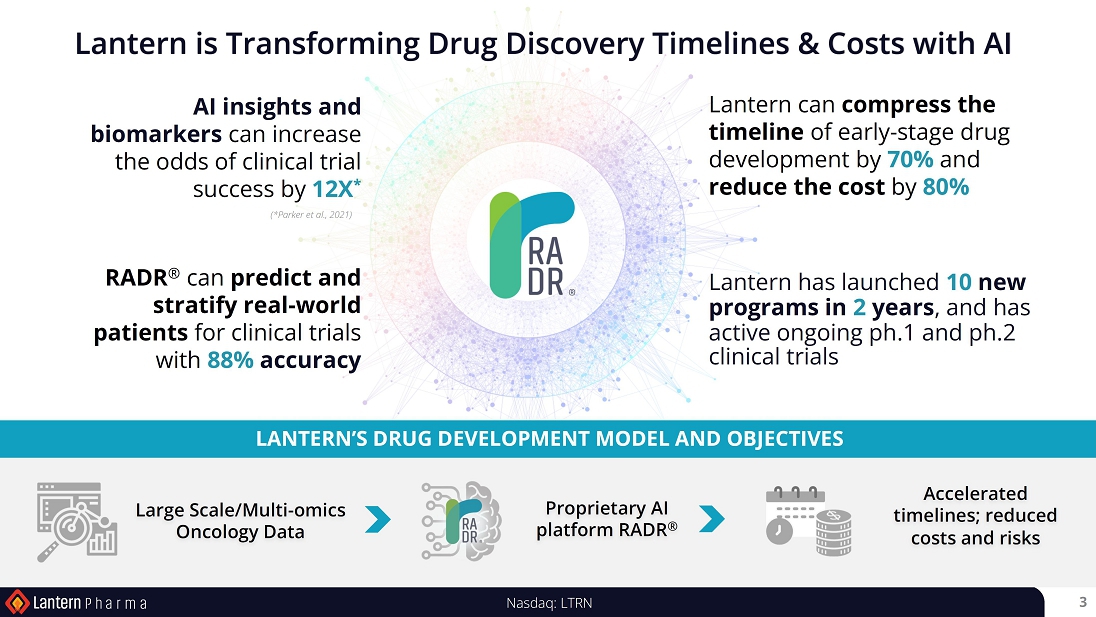
AI platform is revolutionizing the way we understand and predict drug-cancer interactions, enabling us to advance our newly developed
drug programs from initial AI insights to first-in-human clinical trials in 2 to 3 years and at a cost of roughly $1 to 2.5 million per
program - a milestone unheard of in the realm of oncology drug discovery,” said Panna Sharma, CEO of Lantern Pharma.
Sharma
continued, “This past quarter we launched another first-in-human, Phase 1 program, with LP-284, a synthetically lethal small-molecule,
in refractory NHL where there is significant patient need for improved therapies. Therapies that can work with proven monotherapy efficacy
and in combination with existing standard-of-care agents are critically needed in cancers where relapse from existing treatments can
be a dire consequence. Computational and AI-driven approaches are increasing their ability to predict meaningful and clinically relevant
combination regimens for cancer, and our team continues to increase the value of our platform in this regard while helping to also de-risk
and sharpen the focus of our existing clinical drug candidates. Our leadership in the innovative use of AI and machine learning to transform
costs and timelines in the development of precision oncology therapies should yield significant returns for investors and patients as
our industry matures and adopts an AI-centric approach to drug development.”
Highlights
of AI-Powered Pipeline:
| ● | LP-284
– Launched the first-in-human Phase 1 clinical trial with LP-284 targeting recurrent
non-Hodgkin’s lymphomas (NHL). LP-284 has shown nanomolar potency across multiple in
vitro and in vivo studies, including mantle cell lymphoma (MCL), double hit lymphoma (DHL),
and other advanced NHL cancer subtypes with DNA damage response deficiencies, notably those
with compromised functioning of the ataxia-telangiectasia mutated (ATM) gene due to mutations
or deletions. |
In
xenograft PDX models of high-grade B cell lymphomas (HGBL), LP-284 showed synergistic and significantly enhanced anti-cancer activity
when used in combination with rituximab. In in-vivo PDX models, the combined synergy of rituximab with LP-284 was 63% more effective
in destroying HGBL tumors—93% tumor growth inhibition with both rituximab and LP-284 versus 57% tumor growth inhibition with rituximab
alone. Rituximab is a standard-of-care approved therapy used in a wide range of B-cell cancers and non-Hodgkin’s lymphomas. Lantern
plans to release additional details and data on this set of results with LP-284 in this setting in the coming month.
Nearly
all MCL, DHL, and HGBL patients relapse from the current standard-of-care agents and there is an urgent and unmet need for novel improved
therapeutic options for these patients. In the US and Europe, MCL, DHL, and HGBLs are diagnosed in 16,000-20,000 patients each year and
have an estimated annual market potential of over USD 3+ billion.
| ● | LP-184
– Dosed the first patient in Phase 1A clinical trial – a first-in-human Phase
1 basket trial across multiple solid tumor indications that are advanced and refractory to
existing standard-of-care therapies. The trial is anticipated to enroll patients that have
relapsed/refractory advanced solid tumors, such as pancreatic cancer, glioblastoma (GBM),
brain metastases (brain mets.), lung, triple-negative breast cancer, and multiple other solid
tumor types with DNA damage response deficiencies. Lantern expects to continue Phase 1 enrollment
throughout the remainder of 2023 and the first half of 2024 across a growing number of US
clinical trial sites, including Fox Chase Cancer Center and Johns Hopkins Medicine. |
The
dosage and safety data obtained in the Phase 1 trial will be used to advance the central nervous system (CNS) indications for a future
Phase 2 trial to be sponsored by Lantern’s wholly-owned subsidiary, Starlight Therapeutics. Globally, the aggregate annual market
potential of LP-184’s target indications is estimated to be approximately $11+ billion, consisting of $5+ billion for CNS cancers
and $6+ billion for solid tumors.
| ● | LP-300
– Activated additional sites in the US which will increase the potential for dosing
additional patients in the Phase 2 Harmonic™ trial during 2023. The Harmonic trial
is assessing the effect of LP-300 in combination with standard-of-care chemotherapy in never-smoker
patients with relapsed non-small cell lung cancer (NSCLC). In addition to the dosed patients,
more than two dozen potential patients have been pre-screened and are being monitored for
possible enrollment during Q4 across 10 clinical sites in the US. The Company is also actively
advancing the Harmonic™ clinical trial to countries in Asia that are known to have
a significantly higher incidence of never-smokers with NSCLC – Taiwan, Japan, and South
Korea. In these countries, the incidence of never-smokers with NSCLC is double or higher
than that of patients in the US. |
Dr.
Joseph Treat MD of Fox Chase Cancer Center has been appointed the lead principal investigator of the Harmonic™ study. Dr. Treat
is a leading expert in lung malignancies, including NSCLC in never smokers, and has dedicated his career, since 1991, to serving patients
with lung cancer.
Globally,
never-smokers with NSCLC are a growing population of patients and do not respond well to PD-1/PD-L1-based therapies, leaving them with
reduced treatment options. In the US, there are approximately 20,000-40,000 never-smokers with NSCLC diagnosed annually, representing
an estimated US annual market potential of $1.5 billion and a global estimated annual market potential of over $2.6 billion. Additional
information on the Harmonic™ trial can be found at the Harmonic™ website and clinicaltrials.gov.
RADR®
Platform Growth and Development:
| ● | RADR®
continues to advance in size, scope, and capabilities and is also progressing towards
becoming a standard for AI-driven drug development in oncology – for both early-stage
development and later-stage patient biomarker and combination therapy identification. RADR®
has now surpassed 36 billion oncology-focused datapoints and is projected to reach
over 50 billion datapoints by the end of 2023. The scope of RADR®’s
data has broadened with a strategic focus on additional classes of compounds, including antibodies,
checkpoint inhibitors, and DNA-damaging agents. Additionally, data from clinical studies
such as those being obtained from liquid biopsy, and data from preclinical combination studies
that aim to define drug interaction and optimal dosage are being incorporated into the datapoints
and data sets powering RADR®. |
| ● | These
datapoints, the associated advancements in automation, along with algorithms and code comprise
a functional module and have advanced RADR®’s drug development capabilities.
Key modules that are being advanced are those for 1) predicting patient responses and identifying
optimal combination regimens for immuno-oncology (IO) drugs such as immune checkpoint inhibitors,
2) predicting the BBB permeability, with 89% to 92% accuracy, of any compound at a scale
and speed that allows the analysis of tens of thousands of compounds a day, and 3) accelerating
the design and development of drug-conjugate templates for next-generation antibody-drug
conjugates (ADCs) that have increased potential for improved safety and efficacy. These 3
additional modules exemplify the type of rapid and meaningful progress the RADR®
platform is expected to make over the next several quarters as it aims to improve the
speed and reduce the costs and risks associated with creating cancer medicines. |
Starlight
Therapeutics:
| ● | In
Q1 2023, Lantern formed a wholly-owned subsidiary, Starlight Therapeutics Inc. (“Starlight”),
for the clinical development of drug candidate LP-184’s central nervous system (CNS)
and brain cancer indications – including GBM, brain mets., and several rare pediatric
CNS cancers. Starlight will refer to the molecule LP-184, as it is developed in CNS indications,
as “STAR-001”. |
| ● | Lantern
expects to recruit additional management focused on Starlight operations during Q4, 2023.
Lantern has also begun discussions with leading clinicians and key opinion leaders at CNS-focused
cancer centers to serve as clinical trial sites for planned upcoming clinical trials in adult
and pediatric CNS cancers. |
Additional
Operational Highlights:
| ● | During
the 3rd quarter of 2023, Lantern filed 4 new patent applications for LP-184 and LP-284 relating
to breast, liver, and blood cancers and an additional application directed to lyophilized
formulations of these molecules. |
| ● | New
data and scientific findings along with AI platform updates to be presented at several upcoming
conferences: |
SNO
(Society for Neuro-Oncology) 28th Annual Meeting and Education Day in Vancouver, Canada
| ➢ | Date:
November 17, 2023, 10:55a-11:05a PST |
| ➢ | Presentation
Title: LP-184, an MGMT-agnostic small molecule, has potent synergy with Spironolactone
to effectively inhibit orthotopic GBM xenograft tumors |
| ➢ | Presenter:
Dr. John Laterra (clinician-scientist collaborator from Johns Hopkins Medicine
& Kennedy Krieger Institute) |
Bengaluru
Tech Summit 23 in Bengaluru, India
| ➢ | Date:
December 1, 2023, 12p-12:50p IST |
| ➢ | Presentation
Topic: Biotech Future Forward – Pharma 4.0 & How AI is changing the playing
field in Biopharma |
| ➢ | Presenter:
Panna Sharma (President & CEO) |
5th
Annual CNS Drug Delivery Summit in Boston, MA
| ➢ | Date:
December 5, 2023 at 1:30p EST |
| ➢ | Presentation
Topic: Leveraging AI & Machine Learning to Accelerate the Development of CNS &
Brain Cancer Molecules |
| ➢ | Presenter:
Kishor Bhatia, Ph.D. (CSO) |
Third
Quarter 2023 Financial Overview:
| ● | Balance
Sheet: Cash, cash equivalents, and marketable securities were approximately $44.9 million
as of September 30, 2023, compared to approximately $55.2 million as of December 31, 2022.
The quarterly cash burn rate continues to reflect our capital-efficient, collaborator-centered
business model. |
| ● | R&D
Expenses: Research and development expenses were approximately $2.2 million for the quarter
ended September 30, 2023, compared to approximately $0.7 million for the quarter ended September
30, 2022. R&D expenses for the 3rd quarter of 2022 were significantly reduced,
by $0.9 million, due to a one-time payment received from a service provider to resolve a
difference of views regarding the service agreement. |
| ● | G&A
Expenses: General and administrative expenses were approximately $1.3 million for the
quarter ended September 30, 2023, compared to approximately $1.4 million for the quarter
ended September 30, 2022. |
| ● | Net
Loss: Net loss was approximately $3.2 million (or $0.29 per share) for the quarter ended
September 30, 2023, compared to a net loss of approximately $2.3 million (or $0.21 per share)
for the quarter ended September 30, 2022. |
Earnings
Call and Webinar Details:
Lantern
will host its third quarter 2023 earnings call and webinar today, November 8, 2023, at 4:30 p.m. ET.
| ● | https://us06web.zoom.us/webinar/register/8716986910268/WN_9BISSepwSbeLD4x9Wgi_eA#/registration |
| ● | Related
presentation materials will be accessible at: https://ir.lanternpharma.com |
| ● | A
replay of the third quarter earnings call and webinar will be available at https://ir.lanternpharma.com |
About
Lantern Pharma:
Lantern
Pharma (NASDAQ: LTRN) is an AI company transforming the cost, pace, and timeline of oncology drug discovery and development. Our proprietary
AI and machine learning (ML) platform, RADR®, leverages over 36 billion oncology-focused data points and a library of 200+ advanced
ML algorithms to help solve billion-dollar, real-world problems in oncology drug development. By harnessing the power of AI and with
input from world-class scientific advisors and collaborators, we have accelerated the development of our growing pipeline of therapies
that span multiple cancer indications, including both solid tumors and blood cancers and an antibody-drug conjugate (ADC) program. On
average, our newly developed drug programs have been advanced from initial AI insights to first-in-human clinical trials in 2-3 years
and at approximately $1.0 - 2.5 million per program.
Our
lead development programs include a Phase 2 clinical program and multiple Phase 1 clinical trials. We have also established a wholly-owned
subsidiary, Starlight Therapeutics, to focus exclusively on the clinical execution of our promising therapies for CNS and brain cancers,
many of which have no effective treatment options. Our AI-driven pipeline of innovative product candidates is estimated to have a combined
annual market potential of over $15 billion USD and have the potential to provide life-changing therapies to hundreds of thousands of
cancer patients across the world.
Contact:
Investor
Relations
ir@lanternpharma.com
Please
find more information at:
| ➢ | Website:
www.lanternpharma.com |
| ➢ | LinkedIn:
https://www.linkedin.com/company/lanternpharma/ |
| ➢ | Newsletter
– The Spark: Sign-up here |
Forward-looking
Statements:
This
press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section
21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating
to: future events or our future financial performance; the potential advantages of our RADR® platform in identifying drug
candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the development of
our drug candidates and antibody drug conjugate (ADC) development program; estimates regarding the development timing for our drug candidates
and ADC development program; expectations and estimates regarding clinical trial timing and patient enrollment; our research and development
efforts of our internal drug discovery programs and the utilization of our RADR® platform to streamline the drug development
process; our intention to leverage artificial intelligence, machine learning and biomarker data to streamline and transform the pace,
risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug candidate;
estimates regarding patient populations, potential markets and potential market sizes; sales estimates for our drug candidates and our
plans to discover and develop drug candidates and to maximize their commercial potential by advancing such drug candidates ourselves
or in collaboration with others. Any statements that are not statements of historical fact (including, without limitation, statements
that use words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,”
“expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,”
“predict,” “project,” “target,” “model,” “objective,” “aim,”
“upcoming,” “should,” “will,” “would,” or the negative of these words or other similar
expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results
to differ materially from those indicated by the forward-looking statements, such as (i) the risk that our research and the research
of our collaborators may not be successful, (ii) the risk that none of our product candidates has received FDA marketing approval, and
we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates,
(iii) the risk that no drug product based on our proprietary RADR® AI platform has received FDA marketing approval or
otherwise been incorporated into a commercial product, and (iv) those other factors set forth in the Risk Factors section in our Annual
Report on Form 10-K for the year ended December 31, 2022, filed with the Securities and Exchange Commission on March 20, 2023. You may
access our Annual Report on Form 10-K for the year ended December 31, 2022 under the investor SEC filings tab of our website at www.lanternpharma.com
or on the SEC’s website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking
statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements
will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this
press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update
any forward-looking statements to conform the statement to actual results or changes in our expectations.
Lantern
Pharma Disclosure Channels to Disseminate Information:
Lantern
Pharma’s investors and others should note that we announce material information to the public about our company and its technologies,
clinical developments, licensing matters and other matters through a variety of means, including Lantern Pharma’s website, press
releases, SEC filings, digital newsletters, and social media, in order to achieve broad, non-exclusionary distribution of information
to the public. We encourage our investors and others to review the information we make public in the locations above as such information
could be deemed to be material information. Please note that this list may be updated from time to time.
Exhibit
99.2



















v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Lantern Pharma (NASDAQ:LTRN)
과거 데이터 주식 차트
부터 12월(12) 2024 으로 1월(1) 2025

Lantern Pharma (NASDAQ:LTRN)
과거 데이터 주식 차트
부터 1월(1) 2024 으로 1월(1) 2025
