
Cannabix's MSBS Marijuana
Breathalyzer Technology Advances Quantification of THC in
Breath
Vancouver, British
Columbia, Canada -- October 3, 2023 -- InvestorsHub NewsWire --
Cannabix Technologies Inc. (CSE:
BLO) (OTC
PINK: BLOZF) (the "Company or Cannabix") developer of marijuana
and alcohol breath testing devices is pleased to report that its
Mass Spectrometer Breath Sampler ("MSBS") technology along with the
Breath Collection Unit ("BCU") have been used to quantify delta-9
THC levels from smoking and edibles in humans. This emerging
capability is a significant milestone and a major leap forward in
the arena of drug testing. Virtually all levels of law enforcement,
government, and industry have an interest in quantifying THC levels
in breath in an efficient and reliable manner which will enable the
ability to correlate breath concentrations with established blood
levels.
Cannabix has been
focused on developing the MSBS as a simple effective way to test
for recent use of marijuana and to confirm presence of delta-9 THC
in breath using gold-standard mass spectrometry (MS). Over recent
months, the Company has developed the capability to quantify
delta-9 THC in breath samples using Cannabix's proprietary
technology.
Highlights and Updates
-
The ability to
efficiently and reliably quantify THC in breath has been a
long-standing goal of researchers and industry and holds
potentially wide raging benefits to society.
-
Cannabix scientists
have quantified delta-9 THC in human breath samples using
proprietary hardware and methods.
-
Cannabix scientists are
using deuterated delta-9 THC (THC-D3)
as an internal standard to generate a calibration curve for THC
quantification in breath samples (ng/cartridge). Currently, a limit
of detection and limit of quantification have been achieved with
human subjects in the low picogram range. This allows detection of
THC from smoking and edibles up to 4+ hours after
consumption.
-
Experiments at
Cannabix's lab have been conducted using the BCU for sample
collection and MSBS front-end hardware interfaced with
a Thermo TSQ Quantum Ultra triple-quadrupole mass spectrometer for
sample analyzation.
-
In late August, it was reported that the U.S. Department of Health
and Human Services has recommended to the Drug Enforcement
Administration that marijuana be reclassified as a lower-risk,
Schedule III controlled substance, from its current Schedule I
status. If the reclassification is accepted, it can be reasonably
expected that even more cannabis-based pharmaceutical and
recreational products will be available to the public which further
increases the need for THC testing technology for public safety
purposes. (1) (2)
Importance of Quantification – how much is too
much?
Efficient and
reliable quantifying of delta-9 THC levels in breath has been a
long-standing goal of researchers and industry alike, to build
consensus for standardization, public safety, and the legal system.
This is particularly important in regions where cannabis is
legalized for both medical and recreational use where clear
guidelines are urgently needed. The quantification of delta-9 THC
in breath presents an opportunity to help establish a legal limit
for THC in breath, a limit which has not yet been achieved.
Cannabix's hardware (MSBS & BCU) coupled to gold standard MS is
now advancing this capability.
Quantification method using Cannabix
Hardware
Over recent months,
Cannabix scientists have been focussing on sample quantification
results using THC-D3 internal standard
after subjects have smoked or consumed marijuana edibles.
Concentrations per
sample cartridge are calculated using pre-determined calibration
curve which are generated from cartridges with known amounts of
THC-D3
using the MS Breath Sampler front-end hardware. The curve was
generated based on the ratio between the Area Under the Curve (AUC)
of the main fragments (193m/z for THC and 196m/z for
THC-D3).
The Level of Detection (LOD) and Quantification (LOQ) were
calculated in accordance with the ICH guidelines (3).
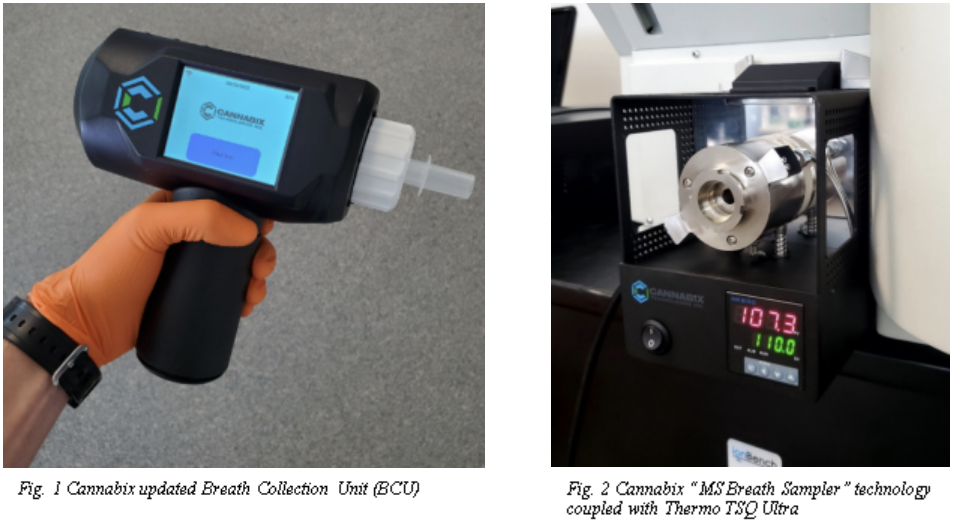
MSBS Technology and BCU
The Company's
handheld Breath Collection Unit ("BCU", Figure 1) and mass
spectrometer coupled laboratory "MS Breath Sampler" (Figure 2) are
being used together to provide a new method for drug detection that
complements gold-standard mass spectrometry with measurements
taking under 2 minutes to acquire. This equipment significantly
simplifies laboratory analysis methods, reduces sample turnaround
times (thus minimizing operating costs), while maintaining
sensitive, precise results.
MSBS compared to legacy LCMS
methods
The MSBS is a novel
method for efficient collection of analytes of low volatility from
human breath utilizing liquid secondary adsorption (LSA) technique.
The novel LSA concept has successfully demonstrated efficient
capturing and releasing of THC using the breath aerosol as a
carrier of solid and viscous liquid particle analytes as well as a
secondary adsorbent to prevent sample loss from surface contact
deposition.
Legacy conventional
quantifying methods rely on the use of complex time consuming
liquid chromatography mass spectrometry (LCMS) research based
methods which require multiple time consuming preparation steps
(e.g., solvent extraction and preconcentration) that are plagued
with sample loss. This type of analysis is complex and can take
hours to perform. Using the Cannabix equipment the results of a
breath sample can be processed within 2 minutes without any sample
preparation, preconcentration, or derivatisation steps resulting in
an efficient and simple workflow.
The Company also
reports that BCU hardware pilot in Warren County, PA has now been
re-directed to another unrelated pilot site. The Company was
informed that due to the small size of the county, and limited
number of testing opportunities at roadside, the hardware would be
of better use at a different site.
Stock Option
Grant
The Company is
granting a total of 2,675,000 incentive stock options to directors
and consultants of the Company. 2,100,000 options will be
exercisable at $0.35 per share for five years. 575,000 stock
options will be exercisable at $0.45 per share for 2 years, and
subject to vesting provisions.
Readers should
note, although the Company has achieved proof of concept prototype
for its BCU and MS Breath Sampler ("technology"), the testing
method and technology are still in the preapproval stage and
accordingly the Company is not currently making any express or
implied claims that the technology will proceed to commercial use.
Furthermore, the method and technology used to quantify delta-9 THC
in breath samples are in the preliminary testing phase and will
require additional validation.
(1)
https://www.dentons.com/en/insights/alerts/2023/august/30/hhs-recommends-reclassifying-cannabis-to-a-schedule-iii-drug
(2)
https://www.washingtonpost.com/health/2023/08/30/hhs-recommends-marijuana-reclassified/
(3)
International Conference on
Harmonisation. Q2 (R1): Validation of analytical procedures: text
and methodology. International Conference on Harmonization, Geneva,
2005, p. 1–13.
About Cannabix
Technologies Inc.
Cannabix
Technologies Inc. is a developer of marijuana and alcohol
breathalyzer technologies for law enforcement, workplaces and
laboratories. Cannabix is working to develop delta-9 THC and
alcohol screening devices. Delta-9 THC is the psychoactive
component of marijuana that causes impairment. Breath testing for
delta-9 THC would allow employers and law enforcement
to identify recent marijuana use. Cannabix is the developer of
contactless breath alcohol
detection devices for employers and a host of other
settings.
We seek Safe
Harbor.
On behalf of the Board of Directors
"Rav Mlait"
CEO
Cannabix
Technologies Inc.
For further
information, contact the Company at info@cannabixtechnologies.com
The CSE has not reviewed and does not accept
responsibility for the adequacy or accuracy of this
release.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking information that
involves various risks and uncertainties regarding future events.
Such forward-looking information can include without limitation
statements based on current expectations involving a number of
risks and uncertainties and are not guarantees of future
performance of the Company, such as final development of a
commercial or prototype product(s), successful trial or pilot of
company technologies, no assurance that commercial sales of any
kind actually materialize; no assurance the Company will have
sufficient funds to complete product development. There are
numerous risks and uncertainties that could cause actual results
and the Company's plans and objectives to differ materially from
those expressed in the forward-looking information, including: (i)
adverse market conditions; (ii) risks regarding protection of
proprietary technology; (iii) the ability of the Company to
complete financings; (iv) the ability of the Company to develop and
market its future product; and (v) risks regarding government
regulation, managing and maintaining growth, the effect of adverse
publicity, litigation, competition and other factors which may be
identified from time to time in the Company's public announcements
and filings. There is no assurance that its development of
breathalyzer technologies will provide any benefit to the Company,
and no assurance that any proposed new products will be built, will
be successful in beta testing or clinical trials. The is no
assurance that the Company will enter into any partnerships to
advance any of its corporate initiatives or technologies. There is
no assurance that any "patent pending" or "provisional patents"
technologies licensed by the Company or owned by the Company will
receive patent status by regulatory authorities. The Company
is not currently selling commercial breathalyzers. Actual results
and future events could differ materially from those anticipated in
such information. These and all subsequent written and oral
forward-looking information are based on estimates and opinions of
management on the dates they are made and are expressly qualified
in their entirety by this notice. Except as required by law, the
Company does not intend to update these forward-looking
statements.
501-3292 Production Way,
Burnaby, B.C., V5A 4R4
Phone: (604)
551-7831
Fax: 604-676-2767
info@cannabixtechnologies.com
cannabixtechnologies.com
Cannabix Technologies (CSE:BLO)
과거 데이터 주식 차트
부터 11월(11) 2024 으로 12월(12) 2024

Cannabix Technologies (CSE:BLO)
과거 데이터 주식 차트
부터 12월(12) 2023 으로 12월(12) 2024
