Redx Pharma Lung Disease Treatment Receives US FDA Orphan Drug Designation
21 8월 2023 - 4:17PM
Dow Jones News
By Michael Susin
Redx Pharma's lung disease treatment RCX007 has received orphan
drug designation from the U.S. Food and Drug Administration for the
potential treatment of idiopathic pulmonary fibrosis, the company
said Monday.
The clinical-stage biotechnology company said the designation
provides various development and commercial incentives, including
market exclusivity, in order to address unmet demand.
The treatment is currently in a Phase 2 clinical study for IPF,
with topline data expected to be released in the first quarter of
2024.
Write to Michael Susin at michael.susin@wsj.com
(END) Dow Jones Newswires
August 21, 2023 03:02 ET (07:02 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
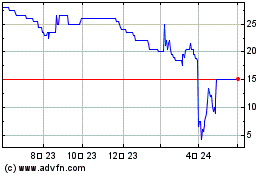
Redx Pharma (LSE:REDX)
과거 데이터 주식 차트
부터 4월(4) 2024 으로 5월(5) 2024
Redx Pharma (LSE:REDX)
과거 데이터 주식 차트
부터 5월(5) 2023 으로 5월(5) 2024