Filed by Baird Medical Investment Holdings
Limited and ExcelFin Acquisition Corp.
Pursuant to Rule 425 under the Securities
Act of 1933
and deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: ExcelFin Acquisition Corp.
Commission File No.: 001-40933
Date: August 1, 2023
Baird Medical’s microwave ablation needle approved for thyroid
nodules
BioWorld
July 31, 2023
By Doris Yu
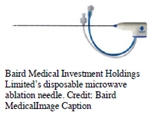
Baird Medical Investment Holdings Ltd.’s disposable microwave ablation
(MWA) needle was approved by China’s National Medical Products Administration for treatment of benign thyroid nodules, which
will help the company build a portfolio of products for thyroid diseases.
Guangzhou-based Baird Medical expects this approval will simplify sales processes
and streamline the admission procedure for its MWA needles.
The company also plans to expand the specifications and models of MWA needles,
which helps customize its sales strategy for different disease types.
“Our MWA products
offer surgeons and doctors a compelling alternative for the treatment of benign thyroid nodules,” Zhitao Wu, vice president
and head of legal at Baird Medical, told BioWorld.
“It provides several distinct advantages over other treatment options,
including shorter operation times, faster patient recovery, reduced procedure costs and fewer postsurgery complications,”
Wu added. “In comparison to traditional approaches such as open surgery, radiofrequency ablation and cryoablation, these
advantages make it an attractive choice for patients seeking a minimally invasive and efficient treatment approach that yields
improved outcomes.”
Over the past two decades, minimally invasive ablation technology for thyroid
nodules has gained significant recognition due to its many potential applications. Thyroid nodule ablation technology has continuously
advanced during that time with better safety and effectiveness.
Compared to surgical procedures, thyroid ablation technology offers advantages
such as precise local anaesthesia and real-time ultrasound-guided tumour ablation. It also reduces damage to surrounding tissues,
avoids surgical scars and effectively preserves thyroid function post-surgery.
| |
|
A flexible technology |
 |
|
Founded in 2012, Baird Medical develops MWA medical devices to provide minimally
invasive treatment solutions for the treatment of benign and malignant tumours, including thyroid nodules, liver cancer, lung cancer
and breast lumps. The company claims that they are the first to obtain a Class III medical devices registration certificate for
MWA medical devices specifically indicated for thyroid nodules in China.
Its MWA treatment increases operational efficiency by offering shorter observation
times and hospital stay periods. In addition, MWA treatment requires shorter operation times and has a relatively lower risk compared
to open surgery. |
Apart from the MWA needle, Baird Medical is in the process of applying for additional
medical device registration certificates for its MWA medical devices specifically indicated for pulmonary nodules and other conditions.
Its portfolio of MWA medical devices positions Baird Medical as one of the largest
providers in China of MWA medical devices to treat thyroid nodules and breast lumps by sales revenue and by volume of sales of
MWA needles, according to Frost & Sullivan. In 2021, it had a 40% market share and competed with companies like Nanjing Eco
Medical Technology Co. Ltd and Micro-Tech (Nanjing) Co. Ltd. as well as global players like Medtronic plc and Johnson & Johnson.
Building on a strong reputation in China
“Baird has earned a strong reputation in MWA treatment for thyroid nodules
through active participation in medical conferences and collaborations with relevant departments and hospitals in Mainland China,”
said Wu. “We believe surgeons and doctors from the ultrasound department highly regard Baird’s products for meeting key requirements,
such as maintaining high stability during operations especially concerning precise power and temperature control, with no reported
incidents of needle breakage.”
Wu added that Baird Medical strategically focuses on penetrating top-tier hospitals,
influential doctors, and well-known surgeons, as well as expanding its market presence to lower-tier regions and hospitals.
Based on its partnerships with more than 100 distributors in China, the company
has established an extensive distribution network, allowing Baird Medical to penetrate more than 430 hospitals in the country.
In 2022, in excess of 60% of its revenues came from direct sales to hospitals with higher margins, and the company posted net income
of $13 million. The company expects to generate $18 million in projected net income, and $45 million in projected revenue in 2023.
In June 2023, Baird Medical announced plans to go public on the Nasdaq through
a proposed business combination with Excelfin Acquisition Corp.
“Baird Medical has a goal to expand into the U.S., Europe and Southeast
Asia within the next three years,” said Wu. “The business combination with Excelfin will provide resources to support
our expansion and growth, so that we may provide our medical solutions to patients around the world. This supports our vision of
transforming healthcare through advanced, minimally invasive treatments. Being a publicly traded company enhances our visibility
to our key stakeholders.”
MWA is a minimally invasive treatment technique that denaturises and coagulates
the protein of tumour cells with extreme heat generated by microwave energy. With the advantages of being safe, minimally invasive
and easy to operate with a rapid recovery and low complication rate for patients, MWA treatments have been applied to a range of
benign and malignant tumours.
***
Additional Information and Where to Find It
In connection with the proposed transaction,
Baird Medical intends to file with the SEC a registration statement on Form F-4, which will include a preliminary proxy statement/prospectus
and other relevant documents, which will be both the proxy statement to be distributed to ExcelFin’s stockholders in connection
with ExcelFin’s solicitation of proxies for the vote by ExcelFin’s stockholders with respect to the proposed business
combination and other matters as may be described in the Registration Statement, as well as the prospectus relating to the offer
and sale of the securities of Baird Medical to be issued in connection with the business combination. STOCKHOLDERS OF EXCELFIN
ARE URGED TO READ THE PROXY STATEMENT/PROSPECTUS (INCLUDING ANY AMENDMENTS OR SUPPLEMENTS THERETO AND ANY DOCUMENTS INCORPORATED
BY REFERENCE THEREIN) AND OTHER RELEVANT DOCUMENTS IN CONNECTION WITH THE PROPOSED TRANSACTION THAT BAIRD MEDICAL AND EXCELFIN
WILL FILE WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION
ABOUT THE PROPOSED TRANSACTION AND THE PARTIES TO THE PROPOSED TRANSACTION. Stockholders and investors will be able to obtain free
copies of the proxy statement/prospectus and other relevant materials (when they become available) and other documents filed by
Baird Medical and ExcelFin at the SEC’s website at www.sec.gov. Copies of the proxy statement/prospectus (when they become
available) and the filings that will be incorporated by reference therein may also be obtained, without charge, on ExcelFin’s
website at www.excelfinacquisitioncorp.com or by directing a request to: ExcelFin Acquisition Corp., 473 Jackson St., Suite 300,
San Francisco, CA, 94111. The information contained on, or that may be accessed through, the websites referenced in this document
is not incorporated by reference into, and is not a part of, this document.
Participants in Solicitation
Each of ExcelFin and Baird Medical and their
respective directors, executive officers and certain employees, may be deemed, under SEC rules, to be participants in the solicitation
of proxies in respect of the proposed transaction. Information regarding ExcelFin’s directors and executive officers is available
in ExcelFin’s final prospectus dated October 22, 2021 relating to its initial public offering and in ExcelFin’s subsequent
filings with the SEC. Other information regarding Baird Medical and the other participants in the proxy solicitation and a description
of their direct and indirect interests, by security holdings or otherwise, will be contained in the proxy statement/prospectus
and other relevant materials to be filed with the SEC (when they become available). These documents can be obtained free of charge
from the sources indicated above.
No Offer or Solicitation
This communication is for informational
purposes only and not intended to and does not constitute an offer to subscribe for, buy or sell, the solicitation of an offer
to subscribe for, buy or sell or an invitation to subscribe for, buy or sell any securities or the solicitation of any vote or
approval in any jurisdiction pursuant to or in connection with the proposed transaction or otherwise, nor shall there be any sale,
issuance or transfer of securities in any jurisdiction in contravention of applicable law. No offer of securities shall be made
except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended, and otherwise
in accordance with applicable law.
Forward-Looking Statements
This communication contains “forward-looking
statements” within the meaning of the U.S. federal securities laws. Such statements include statements concerning anticipated
future events and expectations that are not historical facts. All statements other than statements of historical fact are statements
that could be deemed forward-looking statements. Forward-looking statements are typically identified by words such as “believe,”
“expect,” “anticipate,” “intend,” “target,” “estimate,” “continue,”
“positions,” “plan,” “predict,” “project,” “forecast,” “guidance,”
“goal,” “objective,” “prospects,” “possible” or “potential,” by future
conditional verbs such as “assume,” “will,” “would,” “should,” “could”
or “may,” or by variations of such words or by similar expressions or the negative thereof. Actual results may vary
materially from those expressed or implied by forward-looking statements based on a number of factors, including, without limitation:
(1) risks related to the consummation of the proposed transaction, including the risks that (a) the proposed transaction may not
be consummated within the anticipated time period, or at all, (b) ExcelFin may fail to obtain stockholder approval of the proposed
business combination, (c) the parties may fail to secure required regulatory approvals under applicable laws, and (d) other conditions
to the consummation of the proposed transaction under the business combination agreement may not be satisfied; (2) the effects
that any termination of the business combination agreement may have on ExcelFin or Baird Medical or their respective business,
including the risks that ExcelFin’s share price may decline significantly if the proposed transaction is not completed; (3)
the effects that the announcement or pendency of the proposed transaction may have on Baird Medical’s and its business, including
the risks that as a result (a) ExcelFin’s business, operating results or stock price may suffer or (b) ExcelFin’s or
Baird Medical’s current plans and operations may be disrupted; (4) the inability to recognize the anticipated benefits of
the proposed transaction; (5) unexpected costs resulting from the proposed transaction; (6) changes in general economic conditions;
(7) regulatory conditions and developments; (8) changes in applicable laws or regulations; (9) the nature, cost and outcome of
pending and future litigation and other legal proceedings, including any such proceedings related to the proposed transaction and
instituted against ExcelFin, Baird Medical and others; and (10) other risks and uncertainties indicated from time to time in the
registration and proxy statement relating to the proposed transaction, including those under “Risk Factors” therein,
and in ExcelFin’s filings with the SEC. Potential investors, shareholders and other readers are cautioned not to place undue
reliance on these forward-looking statements, which speak only as of the date on which they are made. Neither ExcelFin nor Baird
Medical assumes any obligation to publicly update any forward-looking statement after it is made, whether as a result of new information,
future events or otherwise, except as required by law.
ExcelFin Acquisition (NASDAQ:XFINU)
과거 데이터 주식 차트
부터 1월(1) 2025 으로 2월(2) 2025

ExcelFin Acquisition (NASDAQ:XFINU)
과거 데이터 주식 차트
부터 2월(2) 2024 으로 2월(2) 2025
