00-0000000 false 0001805890 0001805890 2024-01-04 2024-01-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 4, 2024
FUSION PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Canada |
|
001-39344 |
|
Not Applicable |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
|
| 270 Longwood Road South |
| Hamilton, Ontario, Canada, L8P 0A6 |
| (Address of principal executive offices, including zip code) |
(289) 799-0891
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trade
Symbol(s) |
|
Name of each exchange
on which registered |
| Common shares, no par value per share |
|
FUSN |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On January 4, 2024, Fusion Pharmaceuticals Inc. (the “Company”) issued a press release announcing, among other things, clinical and manufacturing updates and an updated cash runway. A copy of the press release is attached to this Current Report on Form 8-K as Exhibit 99.1 and is incorporated by reference into this Item 7.01.
The Company from time to time presents or distributes to the investment community, at various industry and other conferences, slide presentations to provide updates and summaries of its business. A copy of the Company’s current corporate slide presentation is attached to this Current Report on Form 8-K as Exhibit 99.2 and is incorporated by reference into this Item 7.01. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.2.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such filing.
Updated Cash Runway
On January 4, 2024, the Company announced that, based on its cash, cash equivalents and investments of $207.3 million as of September 30, 2023, proceeds of approximately $65 million pursuant to Company’s at-the-market equity offering program with Jefferies LLC since September 30, 2023, an anticipated borrowing of $15 million under the Company’s loan and security agreement with Oxford Finance LLC and its current operating plan, the Company estimates that it will now be able to fund its operating expenses and capital expenditure requirements into the fourth quarter of 2025.
FPI-2265 Phase 2/3 Development Plan in mCRPC
The Company announced today that it has aligned with the U.S. Food and Drug Administration (“FDA”) on its submitted Phase 2/3 protocol for FPI-2265, a targeted alpha therapy (“TAT”) targeting prostate specific membrane antigen (“PSMA”) for the treatment of patients with metastatic castration-resistant prostate cancer (“mCRPC”) with progressive disease. The updated development plan includes a Phase 2 dose optimization lead-in, expected to complete enrollment by the end of 2024, and a Phase 3 registrational trial expected to begin in 2025.
The Phase 2 portion of the development protocol is designed to evaluate the safety and efficacy of FPI-2265 across three dosing regimens in approximately 60 patients with mCRPC with progressive disease after 177Lu-based PSMA radioligand therapy, such as PLUVICTO™. Based on literature and TATCIST data reported to date, 100kBq/kg administered every 8 weeks is known to be a safe and active dose regimen. In order to further optimize the benefit/risk ratio of FPI-2265, Fusion will explore alternate regimens with higher dosing frequency while keeping cumulative dose and duration of treatment the same. Additional regimens to be evaluated will include a dose of 50 kBq/kg every 4 weeks and 75 kBq/kg every 6 weeks. The primary endpoints are safety and the proportion of patients with ≥ 50% decline in PSA level with key secondary endpoints of objective response rate (“ORR”) and radiographic progression free survival (“rPFS”). The Phase 2 trial is expected to initiate in the second quarter of 2024 with enrollment completed by year-end. The Company will seek to hold an End of Phase 2 meeting with the FDA to determine the recommended Phase 3 dosing regimen based on analysis of the Phase 2 data.
The Phase 3 portion of the trial is designed to be a registration-enabling global study evaluating the efficacy and safety of FPI-2265 compared with standard of care in approximately 550 patients with mCRPC with progressive disease who have previously been treated with a 177Lu-based PSMA radiotherapy. The primary endpoint will evaluate rPFS. Key secondary endpoints include PFS, ORR, OS, PSA50 and duration of response. The Company plans to initiate the Phase 3 trial in 2025.
As previously disclosed, in February 2023, Fusion acquired an IND for the ongoing Phase 2 clinical trial (the “TATCIST” trial) evaluating FPI-2265 (225Ac-PSMA I&T). The TATCIST trial was designed to evaluate patients with mCRPC with progressive disease, including patients who are naïve to PSMA-targeted radiopharmaceuticals and those who have been pre-treated with 177Lu-based PSMA radiopharmaceutical therapy. Fusion intends to report data from approximately 25 to 30 patients in April 2024 and then prioritize enrollment in the new Phase 2/3 trial.
The Company is also pursuing the opportunity to potentially move the therapy candidate into earlier lines of treatment with combinations of FPI-2265 and olaparib. Fusion expects to initiate a combination trial in the first half of this year.
Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking statements”, including but not limited to statements regarding the Company’s cash runway, development plans for FPI-2265 and expected timing for clinical updates. Forward-looking statements can generally be identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “project,” “potential,” “seek,” “should,” “think,” “will,” “would” and similar expressions, or they may use future dates. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from any future results, performance or achievements expressed or implied by such statements. These and other risks which may impact management’s expectations are described in greater detail under the heading “Risk Factors” in the Company’s quarterly report on Form 10-Q for the quarter ended September 30, 2023, as filed with the Securities and Exchange Commission (the “SEC”), and in any subsequent periodic or current report that the Company files with the SEC. All forward-looking statements reflect the Company’s estimates only as of the date of this Current Report (unless another date is indicated) and should not be relied upon as reflecting the Company’s views, expectations or beliefs at any date subsequent to the date of this Current Report. While the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so, even if the Company’s estimates change.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Fusion Pharmaceuticals Inc. |
|
|
|
|
| Date: January 4, 2024 |
|
|
|
By: |
|
/s/ Maria Stahl |
|
|
|
|
|
|
Maria Stahl |
|
|
|
|
|
|
Chief Legal Officer |
Exhibit 99.1

Fusion Pharmaceuticals Announces Clinical Program and Manufacturing Updates
- Aligned with the FDA on submitted protocol for
225Ac-PSMA (FPI-2265) Phase 2/3 registrational program
for patients with metastatic castration-resistant prostate cancer (mCRPC)
- Achieved target enrollment in ongoing TATCIST trial evaluating FPI-2265; Interim Phase 2
data expected to be presented in April 2024
- FPI-1434 shows promising
safety profile and early evidence of antitumor activity at 25 kBq/kg dose level
-
Fusion’s state-of-the-art manufacturing facility now fully operational with first clinical dose
produced
(Hamilton, ON & Boston) – January 4, 2024 – Fusion Pharmaceuticals Inc. (Nasdaq:
FUSN), a clinical-stage oncology company focused on developing next-generation radiopharmaceuticals as precision medicines, today announced significant progress with its FPI-2265 development program, an update
on FPI-1434 Phase 1 Cohort 2 data and the production of the first clinical doses at the Company’s proprietary manufacturing facility.
“We begin 2024 with strong momentum, given a potential registration-enabling path for FPI-2265, encouraging
results in our FPI-1434 program, including first signs of antitumor activity, and a fully operational TAT manufacturing facility that has already begun to produce clinical doses for our actinium-based PSMA
lead program,” said Chief Executive Officer John Valliant, Ph.D.
“We achieved alignment with the U.S. Food and Drug Administration (FDA) on a
protocol and development plan for FPI-2265, providing our team with a potential path to registration and positioning FPI-2265 to be the first actinium-based PSMA
targeting radioligand therapy to market, if approved. Given the significant and growing market for PLUVICTO™, we believe that FPI-2265 will address an
important unmet need for patients who progress on or after lutetium-based therapy.”
FPI-2265 Phase 2/3
Development Plan in mCRPC
The Company announced today that it has aligned with the FDA on its submitted Phase 2/3 protocol for FPI-2265, a targeted alpha therapy (TAT) targeting prostate specific membrane antigen (PSMA) for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) with progressive disease. The
updated development plan includes a Phase 2 dose optimization lead-in, expected to complete enrollment by the end of 2024, and a Phase 3 registrational trial expected to begin in 2025.
The Phase 2 portion of the protocol is designed to evaluate the safety and efficacy of FPI-2265 across three dosing
regimens in approximately 60 patients with mCRPC with progressive disease after 177Lu-based PSMA radioligand therapy, such as PLUVICTO™. Based on literature and TATCIST data reported to

date, 100 kBq/kg administered every 8 weeks is known to be a safe and active dose regimen. In order to
further optimize the benefit/risk ratio of FPI-2265, Fusion will explore alternate regimens with higher dosing frequency while keeping cumulative dose and total duration of treatment the same. Additional
regimens to be evaluated will include a dose of 50 kBq/kg every 4 weeks and 75 kBq/kg every 6 weeks. The primary endpoints are safety and the proportion of patients with ≥ 50% decline in PSA level with key secondary endpoints of objective
response rate (ORR) and radiographic progression free survival (rPFS). The Phase 2 trial is expected to initiate in the second quarter of 2024 with enrollment completed by year-end. The Company will seek to
hold an End of Phase 2 meeting with the FDA to determine the recommended Phase 3 dosing regimen based on analysis of the Phase 2 data.
“We believe
evaluating dosing regimens that deliver the same total dose over the same duration of treatment in the Phase 2 portion of the study allows us to optimize our Phase 3 clinical trial dose in alignment with FDA guidance and determine the best potential
regimen of FPI-2265,” said Chief Medical Officer, Dmitri Bobilev, M.D.
The Phase 3 portion of the trial is
designed to be a registration-enabling global trial evaluating the efficacy and safety of FPI-2265 compared with standard of care in approximately 550 patients with mCRPC with progressive disease who have
previously been treated with a 177Lu-based PSMA radiotherapy. The primary endpoint will evaluate rPFS. Key secondary endpoints include PFS, ORR, OS, PSA50 and duration of response. The Company plans to initiate the Phase 3 trial in 2025.
In February
2023, Fusion acquired an IND for the ongoing Phase 2 clinical trial (the TATCIST trial) evaluating FPI-2265
(225Ac-PSMA I&T). The TATCIST trial was designed to evaluate patients with mCRPC with progressive disease, including patients who are naïve to
PSMA-targeted radiopharmaceuticals and those who have been pre-treated with 177Lu-based PSMA radiopharmaceutical
therapy. Fusion intends to report data from approximately 25 to 30 patients in April 2024 and then prioritize enrollment in the new Phase 2/3 trial.
The
Company is also pursuing the opportunity to potentially move the therapy candidate into earlier lines of treatment with combinations of FPI-2265 and olaparib. Fusion expects to initiate a combination trial in
the first half of this year.
FPI-1434 Cohort 2 Data & Next Steps
Fusion announced today encouraging early findings from Cohort 2 in the ongoing FPI-1434 Phase 1 clinical trial. No dose
limiting toxicities (DLTs) were observed to date in the 25 kBq/kg dose cohort. Two out of three patients completed the DLT period, and one pancreatic cancer patient discontinued treatment due to disease progression.
One heavily treated patient with Ewing sarcoma showed evidence of anti-tumor activity after a single 25 kBq/kg dose of
FPI-1434. The second patient received four cycles of therapy and showed stable disease as best response. FPI-1434 was well tolerated, with no DLTs and transient Grade 1
or less thrombocytopenia at the 25 kBq/kg dose level.

The Company plans to complete and further evaluate results from Cohort 2 and hold a Safety Review Committee
(SRC) meeting to evaluate the emerging data. Fusion plans to share more details on the data and the FPI-1434 development program in mid-2024.
Dr. Valliant continued, “We continue to believe alpha emitters represent the evolution of the toxin used in antibody-drug conjugates (ADCs) and hold
the potential to improve the potency of naked antibodies. There is significant untapped potential to use the precision targeting of antibodies to deliver the potent payload of actinium directly to tumor cells. While early, we are encouraged by the
results showing good safety and evidence of antitumor activity at low doses of FPI-1434.”
In June 2023,
Fusion reported results from three patients at 15 kBq/kg in Cohort 1 of the cold/hot dosing regimen. In Cohort 1, cold/hot dosing was observed to be generally well tolerated with no treatment-related serious adverse events (SAEs) or dose DLTs. Pre-administration of cold antibody demonstrated improved tumor uptake while also reducing hematological toxicity observed in the hot only dosing arm. Two heavily pre-treated
patients from the cold/hot dosing arm received three and five cycles of treatment, with both achieving durable stable disease as their best response.
Manufacturing Facility Update
Fusion reported today that
it has completed validation of its state-of-the-art good manufacturing practice (GMP) manufacturing facility and produced the
first clinical dose of a TAT.
Dr. Valliant continued, “The initiation of production at our own facility, and the diversification afforded by
our external partnerships, positions us for execution on our multiple clinical programs. We built Fusion on a foundation of end-to-end manufacturing expertise, including
experience with global radiopharmaceutical logistics and distribution. We also now have one of the first in-house TAT manufacturing facilities with access to a generator technology that allows for convenient
onsite production of actinium-225, providing us with additional capacity and flexibility in our manufacturing programs.”
Fusion’s facility, which has clinical and commercial scale manufacturing capabilities, is designed to support the Company’s growing pipeline of TATs
and expected to be capable of producing up to 100,000 doses per year. Doses produced out of Fusion’s manufacturing facility are expected to support FPI-2265 manufacturing and are expected to be expanded
to include Fusion’s other proprietary and partnered programs.
Financials
On a pro forma basis as of September 30, 2023, Fusion’s cash, cash equivalents and investments were approximately $287 million, after
taking into account subsequent proceeds of approximately $65 million from sales under the Company’s at-the-market equity offering program and an expected
$15 million draw down under the Company’s existing debt facility. Fusion expects its cash, cash equivalents and investments will now be sufficient to fund operating expenses and capital expenditure requirements into the fourth quarter of
2025.

About Fusion
Fusion Pharmaceuticals is a clinical-stage oncology company focused on developing next-generation radiopharmaceuticals as precision medicines. Fusion connects
alpha particle emitting isotopes to various targeting molecules in order to selectively deliver the alpha emitting payloads to tumors. Fusion’s clinical portfolio includes: FPI-2265 targeting prostate
specific membrane antigen (PSMA) for metastatic castration resistant prostate cancer currently in a Phase 2 trial; FPI-1434 targeting insulin-like growth factor 1 receptor currently in a Phase 1 clinical
trial; and FPI-2059, a small molecule targeting neurotensin receptor 1 (NTSR1), currently in a Phase 1 trial. In addition to a robust proprietary pipeline, Fusion has a collaboration with AstraZeneca to
jointly develop novel targeted alpha therapies (TATs) and combination programs between Fusion’s TATs and AstraZeneca’s DNA Damage Response Inhibitors (DDRis) and immuno-oncology agents. The Company received IND clearance for FPI-2068, the first novel TAT under the collaboration, which targets EGFR-cMET. Fusion has also entered into a collaboration with Merck to evaluate FPI-1434 in combination
with Merck’s KEYTRUDA® (pembrolizumab) in patients with solid tumors expressing IGF-1R. Fusion leases a current Good Manufacturing Practice (GMP)
compliant radiopharmaceutical manufacturing facility designed to support manufacturing of the Company’s growing pipeline of TATs on the McMaster University campus in Hamilton, Ontario. To support Fusion’s growing pipeline of TATs, the
Company has signed strategic actinium supply agreements with Niowave, Inc. and BWXT Medical.
Forward-Looking Statements
This press release contains “forward-looking statements” for purposes of the safe harbor provisions of The Private Securities Litigation Reform Act
of 1995, including but not limited to the statements regarding the future business and financial performance of Fusion Pharmaceuticals Inc. (the “Company”). For this purpose, any statements contained herein that are not statements of
historical fact may be deemed forward-looking statements. Without limiting the foregoing, the words “expect,” “plans,” “anticipates,” “intends,” “will,” and similar expressions are also intended to
identify forward-looking statements, as are expressed or implied statements with respect to the Company’s financial condition, liquidity, and potential drug candidates, including any expressed or implied statements regarding the successful
development of FPI-2265 or FPI-1434. Actual results may differ materially from those indicated by such forward-looking statements as a result of risks and uncertainties,
including but not limited to the following: there can be no guarantees that the Company will advance any clinical product candidate or other component of its potential pipeline in or to the clinic, to the regulatory process or to commercialization;
management’s expectations could be affected by unexpected patient recruitment delays or regulatory actions or delays; uncertainties relating to, or unsuccessful results of, clinical trials, including additional data relating to the ongoing
clinical trials evaluating its product candidates; the Company’s ability to obtain additional funding required to conduct its research, development and commercialization activities; changes in the Company’s business plan or objectives;
competition in general; the Company’s ability to obtain, maintain and enforce patent and other intellectual property protection for its product candidates and its discoveries; and the Company partners’ ability to advance any technology
relating to actinium-225 to development. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause

actual results to be materially different from any future results, performance or achievements expressed or
implied by such statements. These and other risks which may impact management’s expectations are described in greater detail under the heading “Risk Factors” in the Company’s quarterly report on Form 10-Q for the period ended September 30, 2023, as filed with the Securities and Exchange Commission (the “SEC”) and in any subsequent periodic or current report that the Company files with the SEC. All
forward-looking statements reflect the Company’s estimates only as of the date of this release (unless another date is indicated) and should not be relied upon as reflecting the Company’s views, expectations, or beliefs at any date
subsequent to the date of this release. While Fusion may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so, even if the Company’s estimates change.
Contacts:
Fusion
Amanda Cray
Senior Director of Investor Relations &
Corporate Communications
617-967-0207
cray@fusionpharma.com

Corporate Presentation January 2024
Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Exhibit 99.2

Forward-Looking Statements This
presentation contains express or implied forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements that are based on our management's belief and assumptions and on
information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future operational or financial performance,
and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these
forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as "may," "should," "expects," "intends," "plans," "anticipates," "believes," "estimates," "predicts," "potential," "continue," or the
negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other facts, which
are, in some cases, beyond our control and which could materially affect results. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from
those implied or projected by the forward-looking statements. These risks and uncertainties include, but are not limited to, uncertainties inherent in clinical trials and the drug development process, including that the results of early clinical
trials may not be predictive of the successes of later clinical trials, the process of designing and conducting preclinical and clinical trials, the regulatory approval processes, the timing of regulatory filings, the challenges associated with
manufacturing drug products, Fusion’s ability to successfully establish, protect and defend its intellectual property, Fusion’s ability to access actinium-225, and other matters that could affect the sufficiency of existing cash to fund
operations. For a discussion of other risks and uncertainties, and other important factors, see the section entitled “Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, as well as
other risks detailed in the Company’s subsequent filings with the Securities and Exchange Commission. You should read this presentation and the documents that we reference in this presentation completely and with the understanding that our
actual future results may be materially different from any future results expressed or implied by these forward-looking statements. The forward-looking statements in this presentation represent our views as of the date of this presentation. We
anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent
required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. This presentation also contains estimates, projections and other
information concerning our industry, our business and the markets for our product candidates. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual
events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market, and other data from our own internal estimates and
research as well as from reports, research, surveys, studies, and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. While we are not aware of any
misstatements regarding any third-party information presented in this presentation, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties and are subject to change based
on various factors. Market data and industry information used throughout this presentation are based on management’s knowledge of the industry and the good faith estimates of management. We also relied, to the extent available, upon
management’s review of independent industry surveys and publications and other publicly available information prepared by a number of third party sources. All of the market data and industry information used in this presentation involves a
number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Although we believe that these sources are reliable, we cannot guarantee the accuracy or completeness of this information, and we have not
independently verified this information. While we believe the estimated market position, market opportunity and market size information included in this presentation are generally reliable, such information, which is derived in part from
management’s estimates and beliefs, is inherently uncertain and imprecise. No representations or warranties are made by the Company or any of its affiliates as to the accuracy of any such statements or projections. Projections, assumptions and
estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described above. These and other factors
could cause results to differ materially from those expressed in our estimates and beliefs and in the estimates prepared by independent parties. Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved
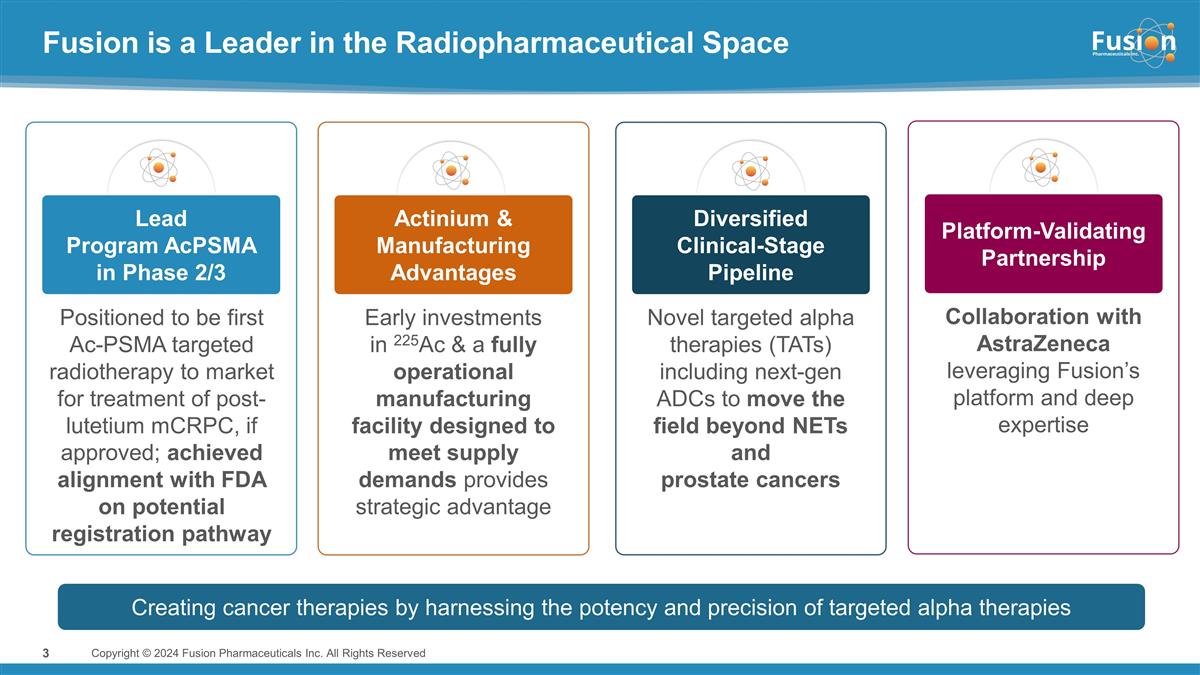
Fusion is a Leader in the
Radiopharmaceutical Space Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved 3 Positioned to be first Ac-PSMA targeted radiotherapy to market for treatment of post-lutetium mCRPC, if approved; achieved alignment with FDA on
potential registration pathway Lead Program AcPSMA in Phase 2/3 Actinium & Manufacturing Advantages Platform-Validating Partnership Diversified Clinical-Stage Pipeline Early investments in 225Ac & a fully operational manufacturing facility
designed to meet supply demands provides strategic advantage Collaboration with AstraZeneca leveraging Fusion’s platform and deep expertise Novel targeted alpha therapies (TATs) including next-gen ADCs to move the field beyond NETs and
prostate cancers Creating cancer therapies by harnessing the potency and precision of targeted alpha therapies

Lead Program: FPI-2265 Copyright
© 2024 Fusion Pharmaceuticals Inc. All Rights Reserved
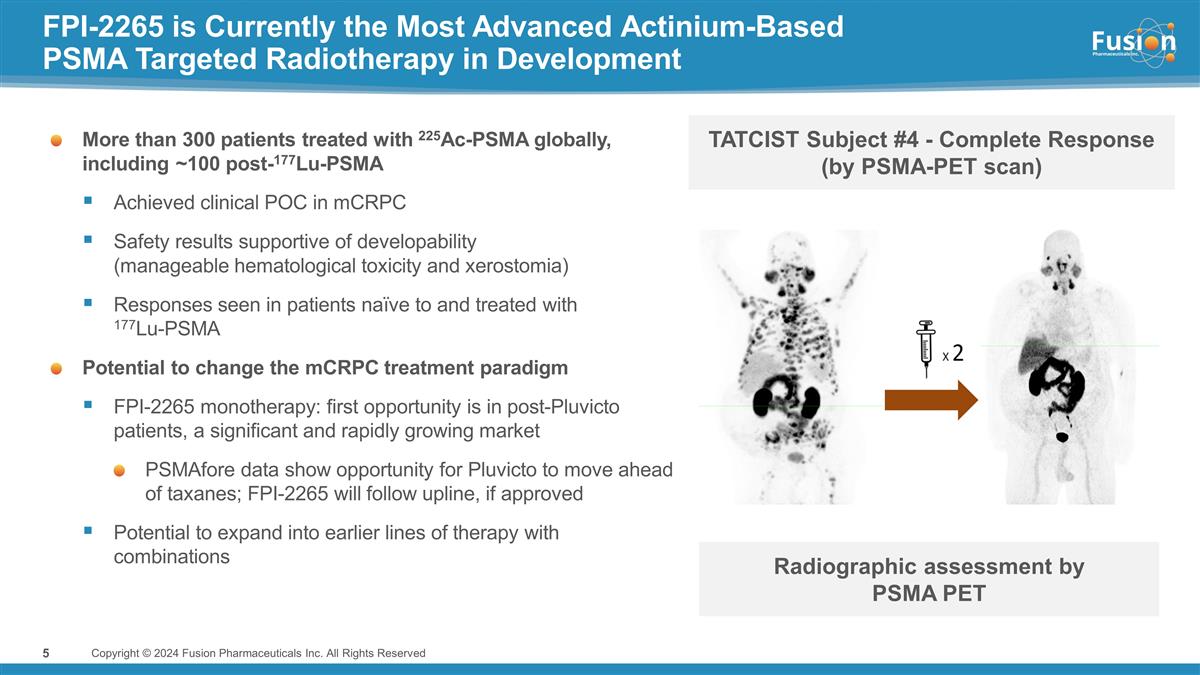
FPI-2265 is Currently the Most
Advanced Actinium-Based PSMA Targeted Radiotherapy in Development More than 300 patients treated with 225Ac-PSMA globally, including ~100 post-177Lu-PSMA Achieved clinical POC in mCRPC Safety results supportive of developability (manageable
hematological toxicity and xerostomia) Responses seen in patients naïve to and treated with 177Lu-PSMA Potential to change the mCRPC treatment paradigm FPI-2265 monotherapy: first opportunity is in post-Pluvicto patients, a significant and
rapidly growing market PSMAfore data show opportunity for Pluvicto to move ahead of taxanes; FPI-2265 will follow upline, if approved Potential to expand into earlier lines of therapy with combinations Copyright © 2024 Fusion Pharmaceuticals
Inc. All Rights Reserved 5 TATCIST Subject #4 - Complete Response (by PSMA-PET scan) Radiographic assessment by PSMA PET
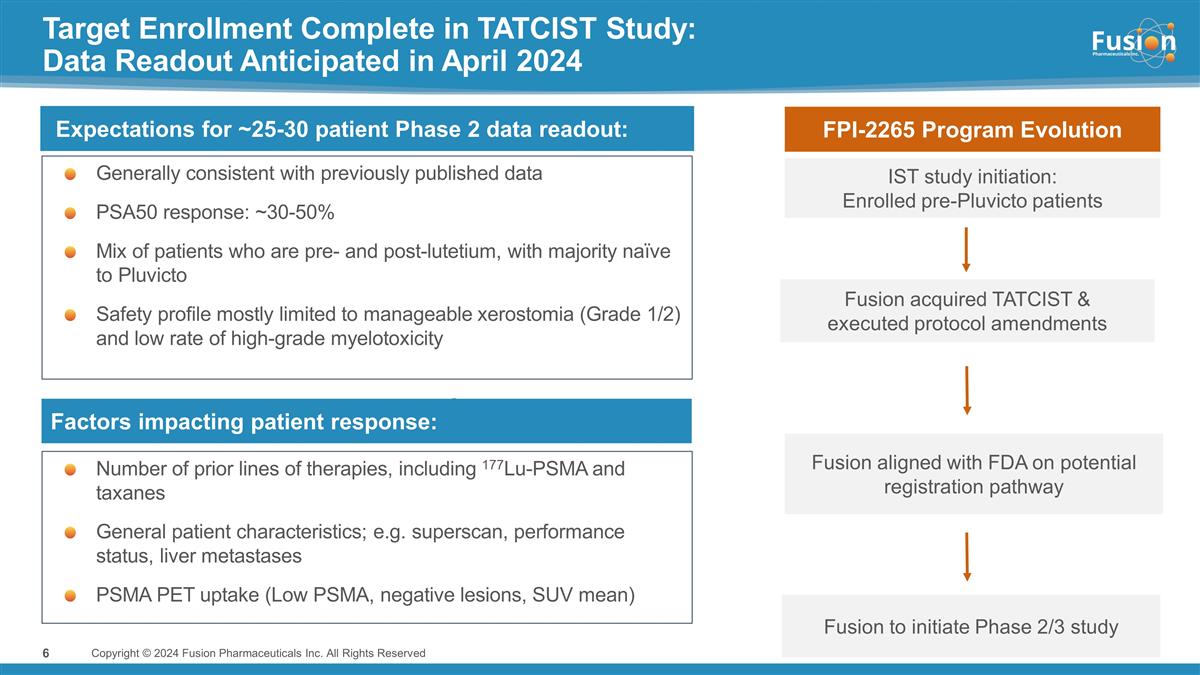
Target Enrollment Complete in TATCIST
Study: Data Readout Anticipated in April 2024 Generally consistent with previously published data PSA50 response: ~30-50% Mix of patients who are pre- and post-lutetium, with majority naïve to Pluvicto Safety profile mostly limited to
manageable xerostomia (Grade 1/2) and low rate of high-grade myelotoxicity Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Expectations for ~25-30 patient Phase 2 data readout: IST study initiation: Enrolled pre-Pluvicto
patients Fusion acquired TATCIST & executed protocol amendments Fusion aligned with FDA on potential registration pathway Factors impacting patient response: FPI-2265 Program Evolution Number of prior lines of therapies, including 177Lu-PSMA and
taxanes General patient characteristics; e.g. superscan, performance status, liver metastases PSMA PET uptake (Low PSMA, negative lesions, SUV mean) Fusion to initiate Phase 2/3 study
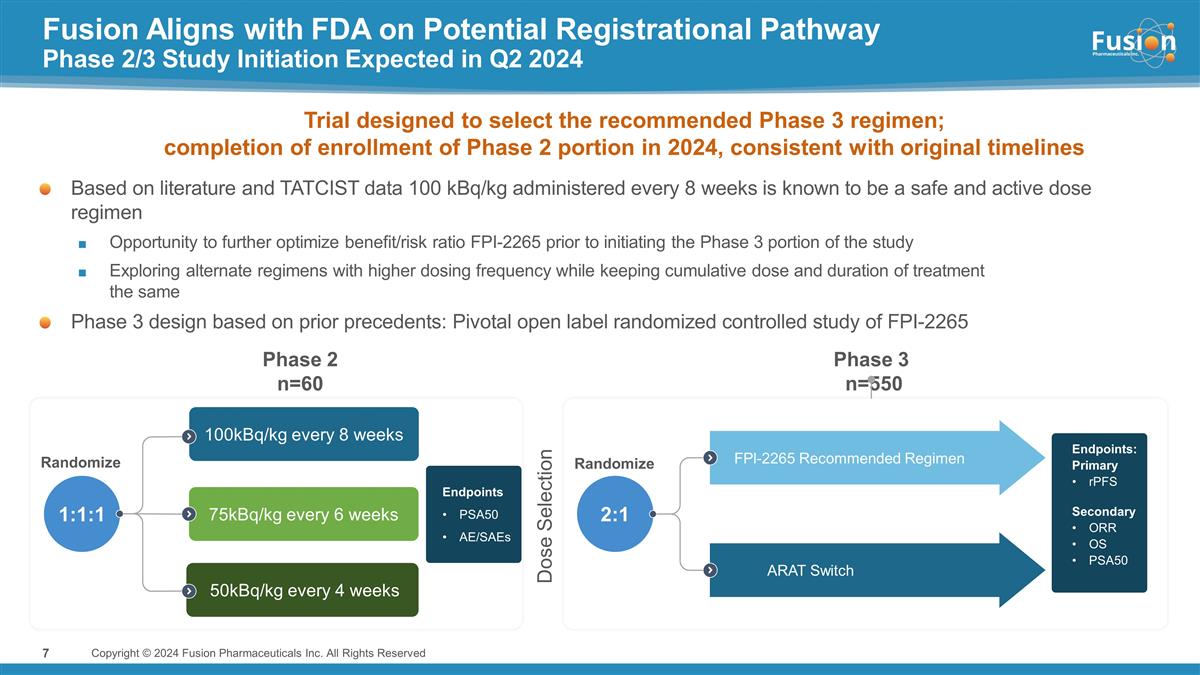
Fusion Aligns with FDA on Potential
Registrational Pathway Phase 2/3 Study Initiation Expected in Q2 2024 Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved 2:1 50kBq/kg every 4 weeks 75kBq/kg every 6 weeks 100kBq/kg every 8 weeks FPl-2265 Recommended Regimen ARAT
Switch 1:1:1 Randomize Randomize Phase 2 n=60 Phase 3 n=550 Dose Selection Based on literature and TATCIST data 100 kBq/kg administered every 8 weeks is known to be a safe and active dose regimen Opportunity to further optimize benefit/risk ratio
FPI-2265 prior to initiating the Phase 3 portion of the study Exploring alternate regimens with higher dosing frequency while keeping cumulative dose and duration of treatment the same Phase 3 design based on prior precedents: Pivotal open label
randomized controlled study of FPI-2265 Trial designed to select the recommended Phase 3 regimen; completion of enrollment of Phase 2 portion in 2024, consistent with original timelines Endpoints: Primary rPFS Secondary ORR OS PSA50 Endpoints PSA50
AE/SAEs
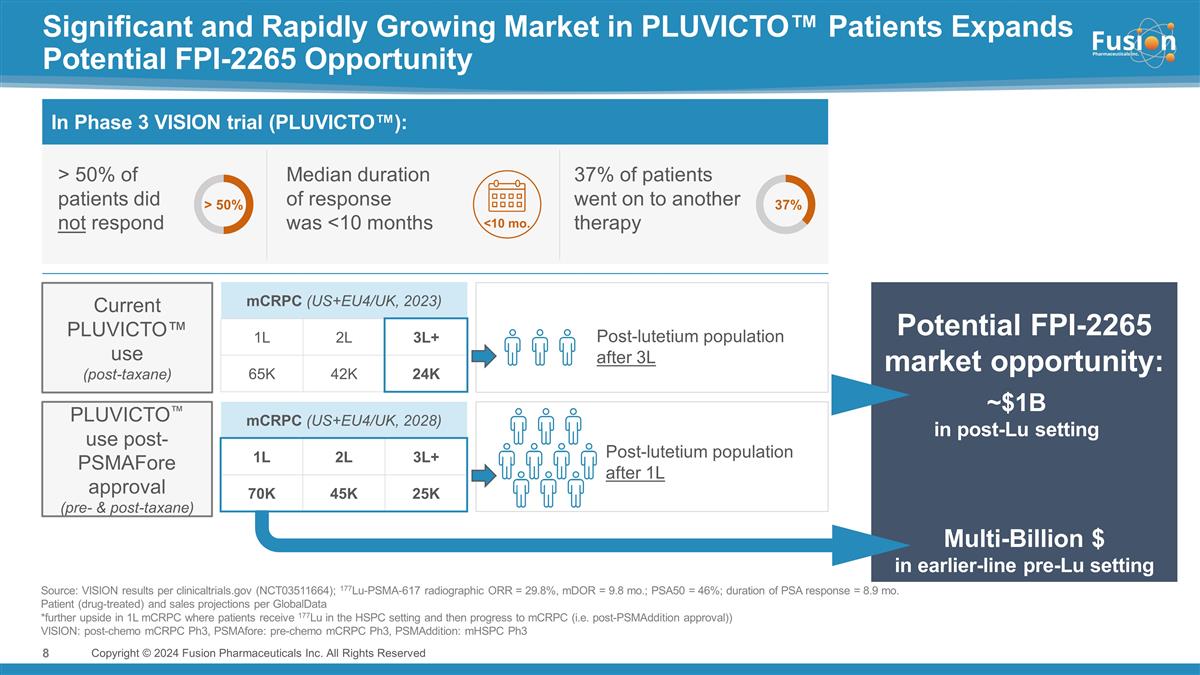
Potential FPI-2265 market opportunity:
Significant and Rapidly Growing Market in PLUVICTO™ Patients Expands Potential FPI-2265 Opportunity Source: VISION results per clinicaltrials.gov (NCT03511664); 177Lu-PSMA-617 radiographic ORR = 29.8%, mDOR = 9.8 mo.; PSA50 = 46%; duration of
PSA response = 8.9 mo. Patient (drug-treated) and sales projections per GlobalData *further upside in 1L mCRPC where patients receive 177Lu in the HSPC setting and then progress to mCRPC (i.e. post-PSMAddition approval)) VISION: post-chemo mCRPC
Ph3, PSMAfore: pre-chemo mCRPC Ph3, PSMAddition: mHSPC Ph3 Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Current PLUVICTO™ use (post-taxane) mCRPC (US+EU4/UK, 2023) 1L 2L 3L+ 65K 42K 24K PLUVICTO™ use post-
PSMAFore approval (pre- & post-taxane) mCRPC (US+EU4/UK, 2028) 1L 2L 3L+ 70K 45K 25K > 50% of patients did not respond Median duration of response was <10 months 37% of patients went on to another therapy > 50% 37% <10 mo. In Phase 3
VISION trial (PLUVICTO™): Post-lutetium population after 3L Post-lutetium population after 1L Multi-Billion $ in earlier-line pre-Lu setting ~$1B in post-Lu setting
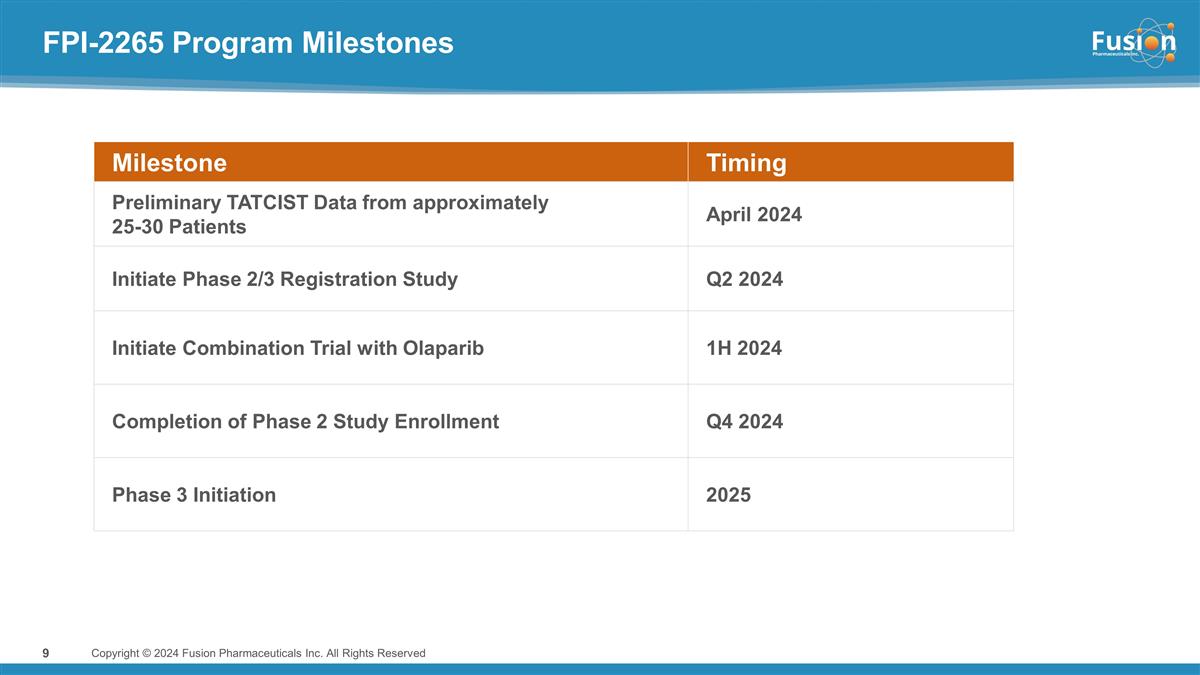
FPI-2265 Program Milestones Copyright
© 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Milestone Timing Preliminary TATCIST Data from approximately 25-30 Patients April 2024 Initiate Phase 2/3 Registration Study Q2 2024 Initiate Combination Trial with Olaparib 1H 2024
Completion of Phase 2 Study Enrollment Q4 2024 Phase 3 Initiation 2025

Actinium Supply & Manufacturing
Capabilities Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved
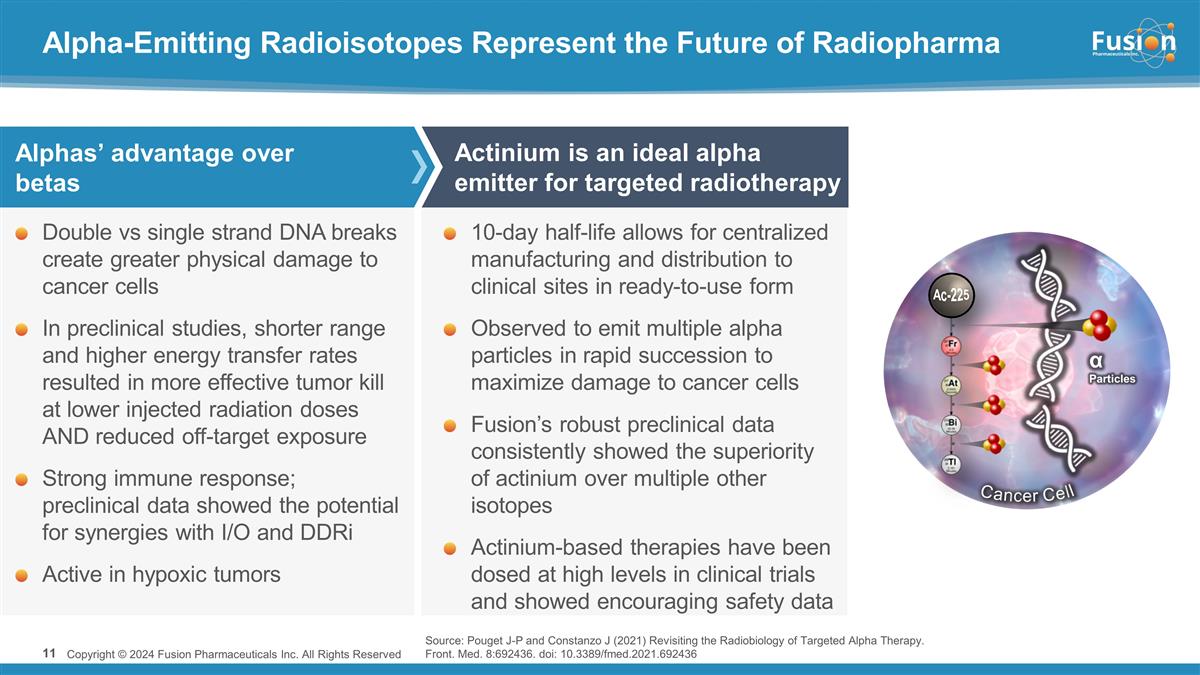
Alpha-Emitting Radioisotopes
Represent the Future of Radiopharma Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Double vs single strand DNA breaks create greater physical damage to cancer cells In preclinical studies, shorter range and higher energy
transfer rates resulted in more effective tumor kill at lower injected radiation doses AND reduced off-target exposure Strong immune response; preclinical data showed the potential for synergies with I/O and DDRi Active in hypoxic tumors 10-day
half-life allows for centralized manufacturing and distribution to clinical sites in ready-to-use form Observed to emit multiple alpha particles in rapid succession to maximize damage to cancer cells Fusion’s robust preclinical data
consistently showed the superiority of actinium over multiple other isotopes Actinium-based therapies have been dosed at high levels in clinical trials and showed encouraging safety data Alphas’ advantage over betas Actinium is an ideal alpha
emitter for targeted radiotherapy α Particles Cancer Cell Ac-225 Source: Pouget J-P and Constanzo J (2021) Revisiting the Radiobiology of Targeted Alpha Therapy. Front. Med. 8:692436. doi: 10.3389/fmed.2021.692436
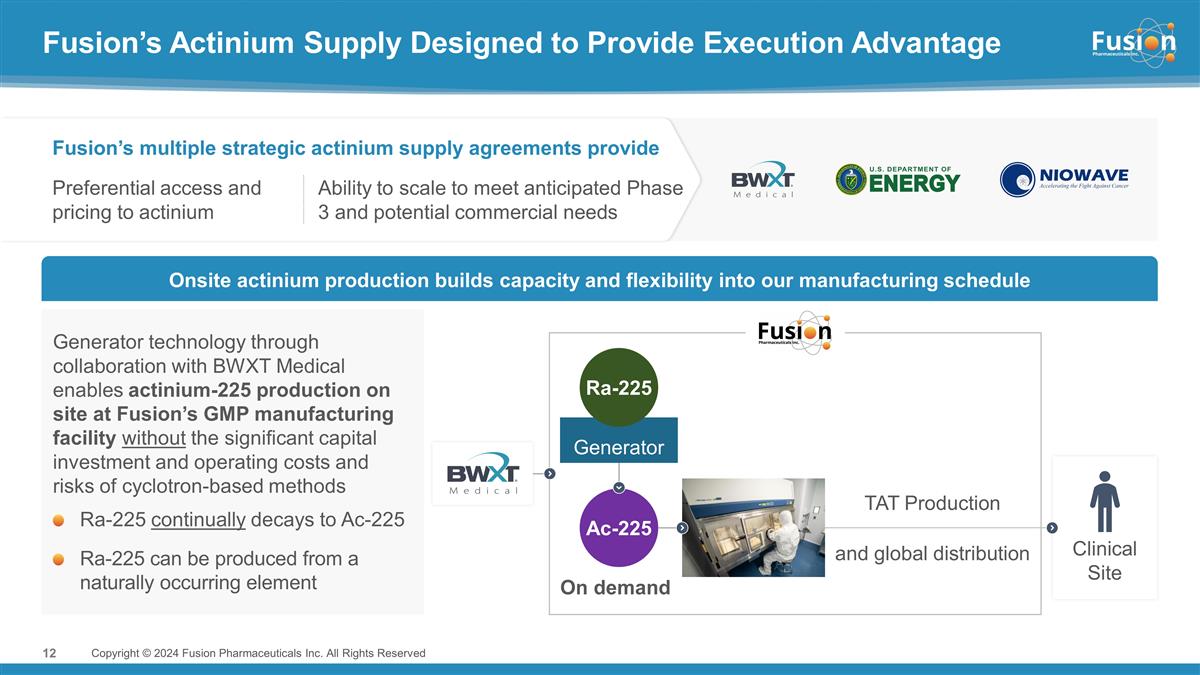
Fusion’s Actinium Supply
Designed to Provide Execution Advantage Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Clinical Site Ac-225 Generator On demand Ra-225 Fusion’s multiple strategic actinium supply agreements provide Preferential access
and pricing to actinium Ability to scale to meet anticipated Phase 3 and potential commercial needs Onsite actinium production builds capacity and flexibility into our manufacturing schedule and global distribution TAT Production Generator
technology through collaboration with BWXT Medical enables actinium-225 production on site at Fusion’s GMP manufacturing facility without the significant capital investment and operating costs and risks of cyclotron-based methods Ra-225
continually decays to Ac-225 Ra-225 can be produced from a naturally occurring element

Fusion is Built on a Foundation of
Radiopharmaceutical Manufacturing Expertise Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Internal GMP manufacturing is fully operational: FPI-2265 (225AcPSMA) doses being shipped Clinical & potential commercial supply
capabilities Capacity designed to produce 100,000+ doses annually Located in an established transportation hub for medical isotopes with decades of experience shipping products globally

Moving the Field Beyond Prostate
and NETs: Fusion’s Clinical-Stage Pipeline Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved
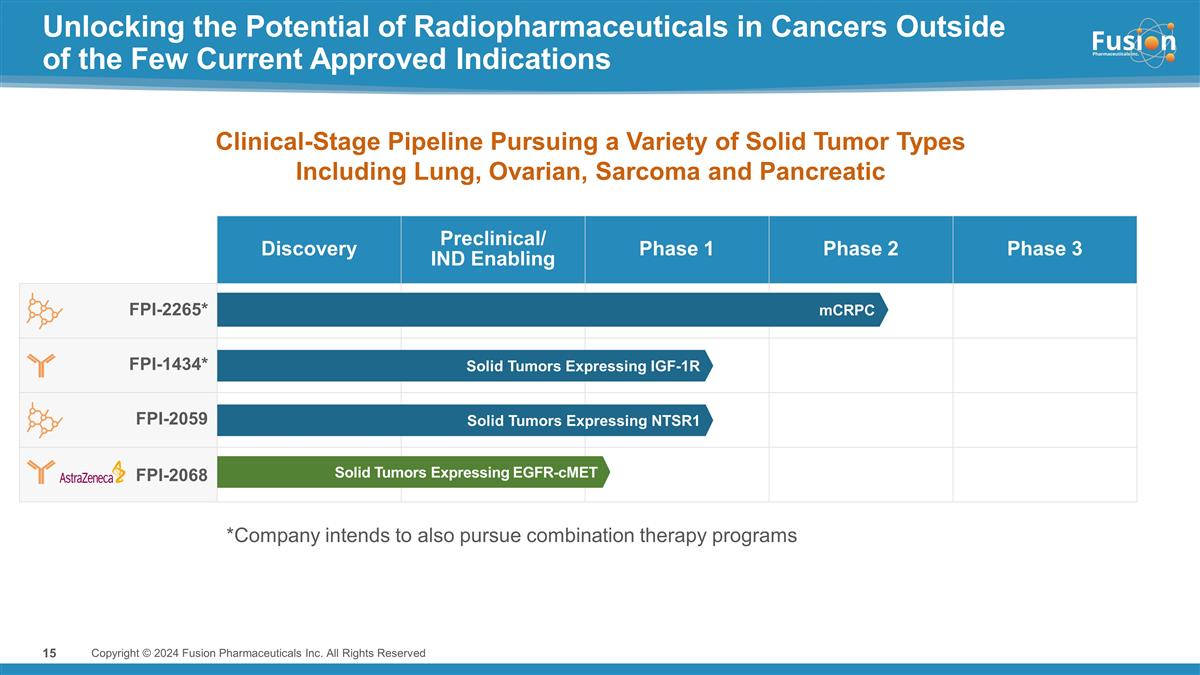
Unlocking the Potential of
Radiopharmaceuticals in Cancers Outside of the Few Current Approved Indications Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Discovery Preclinical/ IND Enabling Phase 1 Phase 2 Phase 3 FPI-2265* FPI-1434* FPI-2059 FPI-2068
Solid Tumors Expressing IGF-1R Solid Tumors Expressing NTSR1 Solid Tumors Expressing EGFR-cMET mCRPC Clinical-Stage Pipeline Pursuing a Variety of Solid Tumor Types Including Lung, Ovarian, Sarcoma and Pancreatic *Company intends to also pursue
combination therapy programs
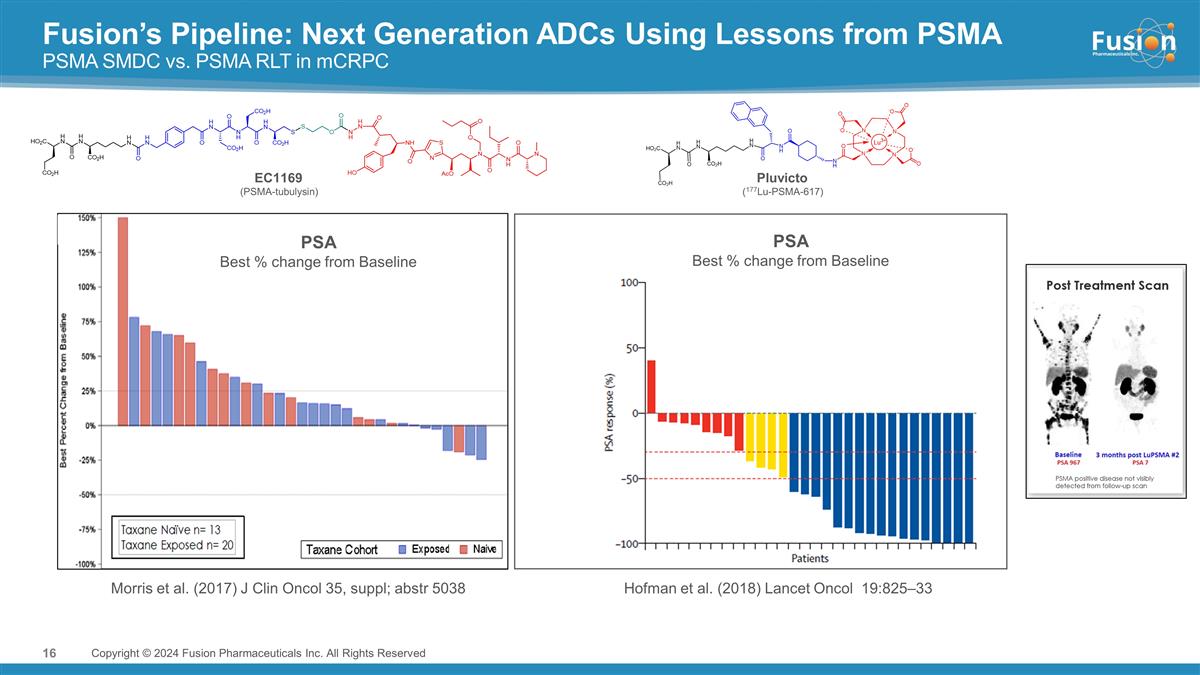
Fusion’s Pipeline: Next
Generation ADCs Using Lessons from PSMA PSMA SMDC vs. PSMA RLT in mCRPC Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Morris et al. (2017) J Clin Oncol 35, suppl; abstr 5038 PSA Best % change from Baseline EC1169
(PSMA-tubulysin) Pluvicto (177Lu-PSMA-617) Hofman et al. (2018) Lancet Oncol 19:825–33 PSA Best % change from Baseline
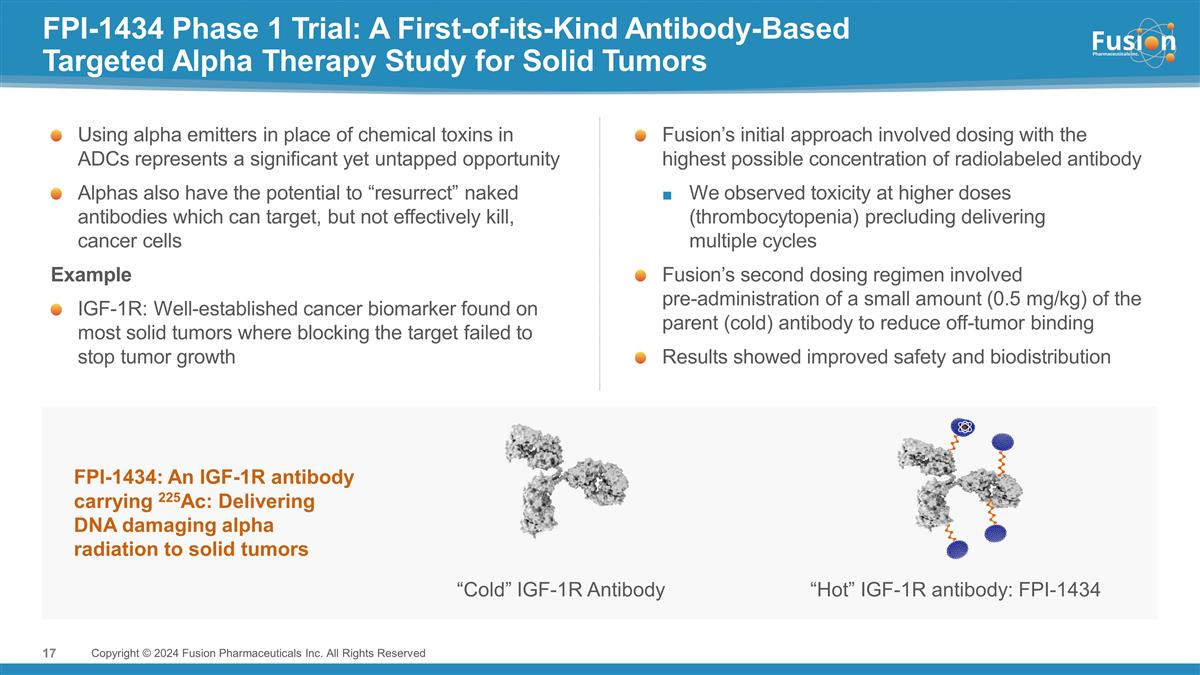
FPI-1434 Phase 1 Trial: A
First-of-its-Kind Antibody-Based Targeted Alpha Therapy Study for Solid Tumors Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved “Cold” IGF-1R Antibody “Hot” IGF-1R antibody: FPI-1434 FPI-1434: An IGF-1R
antibody carrying 225Ac: Delivering DNA damaging alpha radiation to solid tumors Fusion’s initial approach involved dosing with the highest possible concentration of radiolabeled antibody We observed toxicity at higher doses (thrombocytopenia)
precluding delivering multiple cycles Fusion’s second dosing regimen involved pre-administration of a small amount (0.5 mg/kg) of the parent (cold) antibody to reduce off-tumor binding Results showed improved safety and biodistribution Using
alpha emitters in place of chemical toxins in ADCs represents a significant yet untapped opportunity Alphas also have the potential to “resurrect” naked antibodies which can target, but not effectively kill, cancer cells Example IGF-1R:
Well-established cancer biomarker found on most solid tumors where blocking the target failed to stop tumor growth
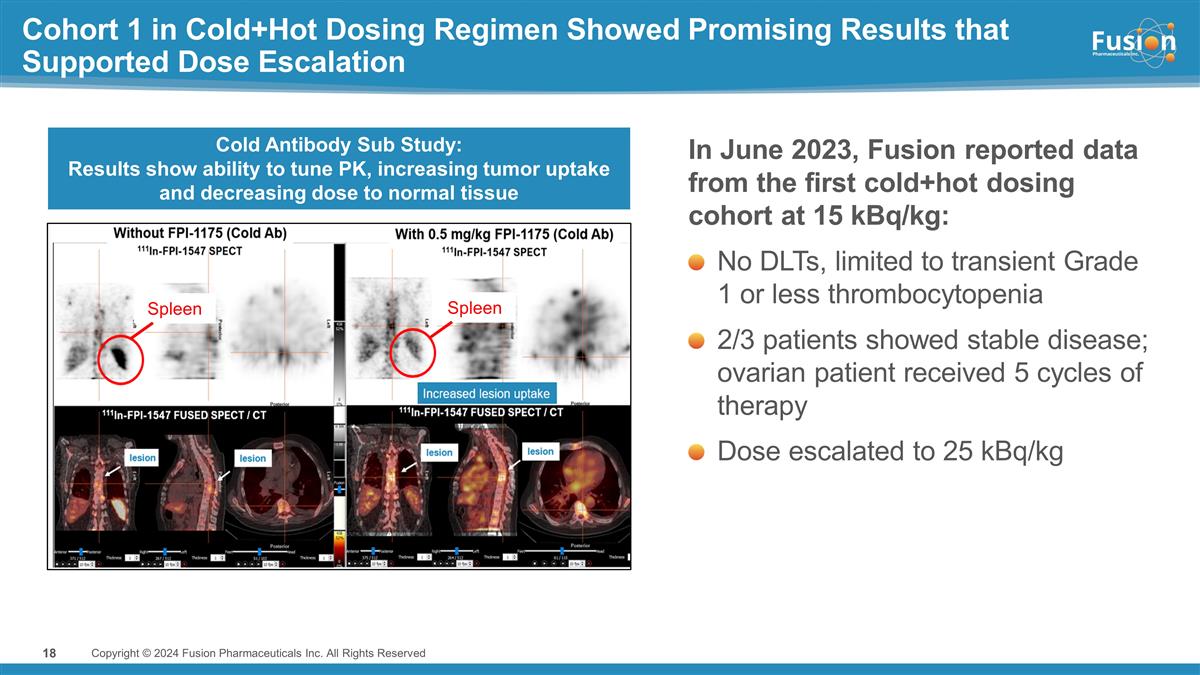
Cohort 1 in Cold+Hot Dosing Regimen
Showed Promising Results that Supported Dose Escalation In June 2023, Fusion reported data from the first cold+hot dosing cohort at 15 kBq/kg: No DLTs, limited to transient Grade 1 or less thrombocytopenia 2/3 patients showed stable disease; ovarian
patient received 5 cycles of therapy Dose escalated to 25 kBq/kg 18 Cold Antibody Sub Study: Results show ability to tune PK, increasing tumor uptake and decreasing dose to normal tissue Spleen Spleen Copyright © 2024 Fusion Pharmaceuticals
Inc. All Rights Reserved

Encouraging Safety and Early
Signals of Antitumor Activity at 25 kBq/kg Safety: 2 out of 3 patients completed DLT period No DLTs, limited to transient Grade 1 or less thrombocytopenia (comparable to 15 kBq/kg) Activity: One patient (chordoma) showed stable disease; received 4
cycles of therapy One patient (Ewing sarcoma) showed lesion shrinkage after a single dose Heavily pre-treated Ewing sarcoma patient, including chemotherapy combined with ganitumab (an IGF-1R antibody) and mixed response after most recent
nivolumab/ipilimumab treatment Plan to share more detailed FPI-1434 data and next steps around mid-year Baseline PET-CT 09/07/2023 Follow up PET-CT 12/21/2023 Surgical clip Encouraging early results show ability to target solid tumors with
antibody-based TATs to create “next-generation ADCs” Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved

Fusion’s Early-Stage Pipeline
Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved
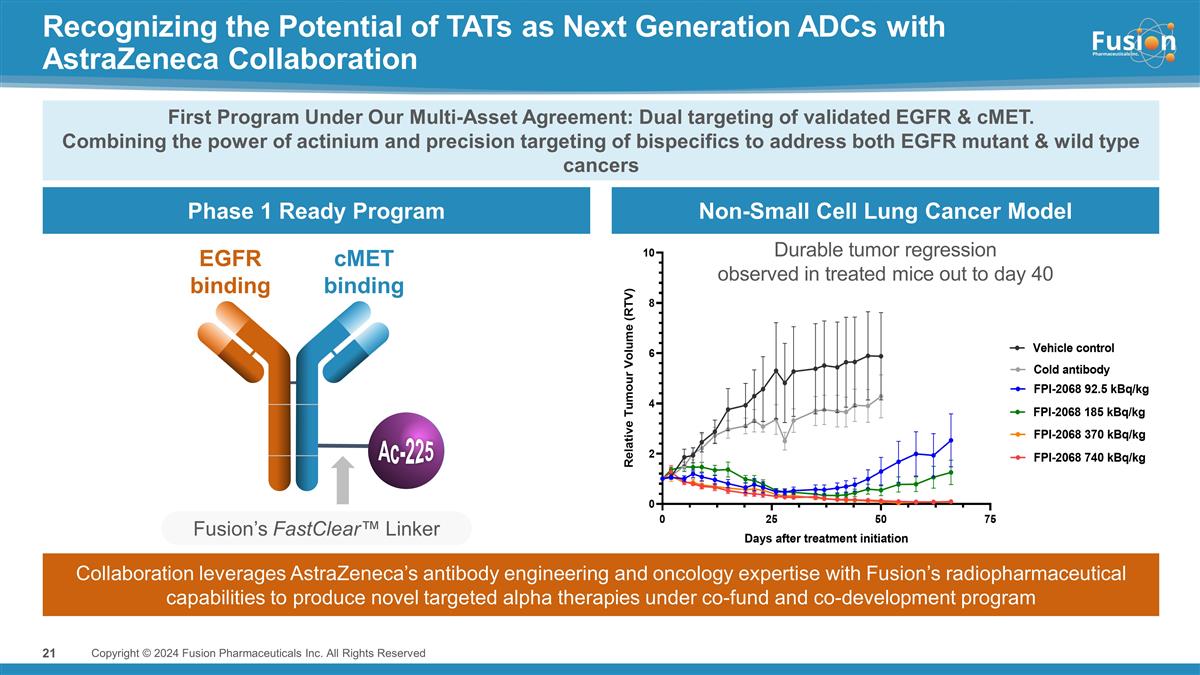
Phase 1 Ready Program Non-Small
Cell Lung Cancer Model Recognizing the Potential of TATs as Next Generation ADCs with AstraZeneca Collaboration Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Fusion’s FastClear™ Linker EGFR binding cMET binding
Ac-225 First Program Under Our Multi-Asset Agreement: Dual targeting of validated EGFR & cMET. Combining the power of actinium and precision targeting of bispecifics to address both EGFR mutant & wild type cancers Durable tumor regression
observed in treated mice out to day 40 Collaboration leverages AstraZeneca’s antibody engineering and oncology expertise with Fusion’s radiopharmaceutical capabilities to produce novel targeted alpha therapies under co-fund and
co-development program
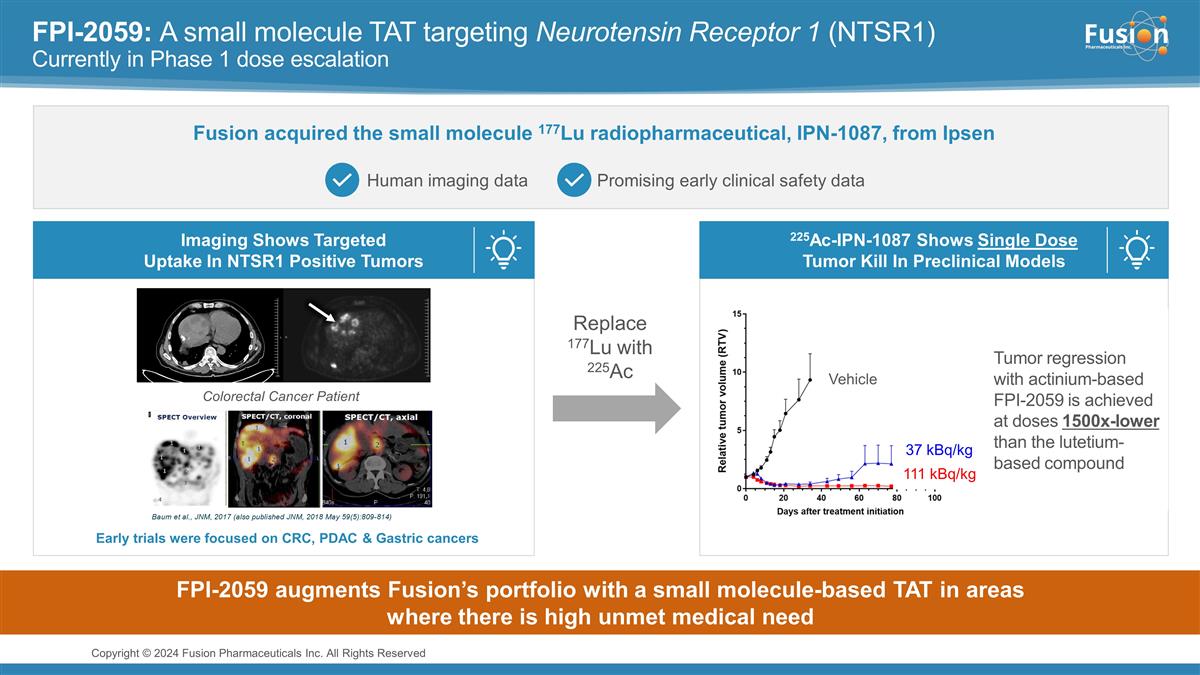
FPI-2059: A small molecule TAT
targeting Neurotensin Receptor 1 (NTSR1) Currently in Phase 1 dose escalation Fusion acquired the small molecule 177Lu radiopharmaceutical, IPN-1087, from Ipsen Human imaging data Promising early clinical safety data 225Ac-IPN-1087 Shows Single Dose
Tumor Kill In Preclinical Models Imaging Shows Targeted Uptake In NTSR1 Positive Tumors Replace 177Lu with 225Ac Colorectal Cancer Patient Early trials were focused on CRC, PDAC & Gastric cancers Vehicle 37 kBq/kg 111 kBq/kg Tumor regression
with actinium-based FPI-2059 is achieved at doses 1500x-lower than the lutetium-based compound FPI-2059 augments Fusion’s portfolio with a small molecule-based TAT in areas where there is high unmet medical need Copyright © 2024 Fusion
Pharmaceuticals Inc. All Rights Reserved

Fusion’s Radiopharmaceutical
& Oncology Drug Development Expertise Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved CHIEF OF STAFF Cara Ferreira, PhD PRESIDENT & CBO Mohit Rawat CLO Maria Stahl, JD CSO Chris Leamon, PhD CEO John Valliant, PhD CTO
Eric Burak, PhD CFO John Crowley, CPA CMO Dmitri Bobilev, MD Fusion’s team has deep radiopharmaceutical & oncology expertise: Created and operated a global radiopharmaceutical manufacturer Developed multiple radiopharmaceuticals, including
targeted alpha therapies Developed a PSMA-targeted radiopharmaceutical acquired by Novartis Lead R&D at Nordion, a global leader in medical isotope and radiopharmaceutical production Lead commercial portfolio within Novartis Oncology; multiple
commercial launches in various disease areas

Unaudited Financials and 2024
Milestones Unaudited Financials Extended Cash Runway ~$287M* Pro Forma Cash, Cash Equivalents and Investments Cash to Fund Operations extended into Q4 2025 Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved *Pro forma 9/30/23
cash, cash equivalents and investments include subsequent proceeds from ATM sales of $65 million and expected draw down of $15 million tranche available under Oxford debt facility Milestone Timing FPI-2265: Preliminary TATCIST Data from ~25-30
Patients April 2024 FPI-2265: Initiate Phase 2/3 Study Q2 2024 FPI-1434: Detailed FPI-1434 Cohort 2 data and next steps Mid-2024 FPI-2265: Initiate Combination Trial with Olaparib 1H 2024 FPI-2265: Completion of Phase 2 Study Enrollment Q4
2024
Fusion is a Leader in the
Radiopharmaceutical Space Copyright © 2024 Fusion Pharmaceuticals Inc. All Rights Reserved 25 Achieved alignment with FDA on potential registration pathway Lead Program AcPSMA in Phase 2/3 Actinium & Manufacturing Advantages Creating
Next-Generation ADCs GMP radiopharma manufacturing facility fully operational and producing and shipping clinical doses Early evidence that antibodies can precisely and safely deliver an active dose of actinium to solid tumors

www.FusionPharma.com Copyright
© 2024 Fusion Pharmaceuticals Inc. All Rights Reserved Thank You
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Fusion Pharmaceuticals (NASDAQ:FUSN)
과거 데이터 주식 차트
부터 4월(4) 2024 으로 5월(5) 2024

Fusion Pharmaceuticals (NASDAQ:FUSN)
과거 데이터 주식 차트
부터 5월(5) 2023 으로 5월(5) 2024
