Form 8-K - Current report
22 8월 2023 - 5:50AM
Edgar (US Regulatory)
0001035354
false
0001035354
2023-08-21
2023-08-21
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
August 21, 2023
Eloxx Pharmaceuticals, Inc.
(Exact name of registrant as specified
in its charter)
| Delaware |
|
001-31326 |
|
84-1368850 |
|
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
|
480
Arsenal Way, Suite 130, Watertown, MA |
|
02451 |
| (Address of principal executive offices) |
|
(Zip Code) |
(Registrant’s telephone number,
including area code): (781) 577-5300
N/A
(Former name or former address, if changed
since last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered
pursuant to Section 12(b) of the Act:
| Title
of each class |
Trading
Symbol(s) |
Name of each exchange on which
registered |
| Common Stock, $0.01 par value per share |
ELOX |
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01 |
Regulation FD Disclosure. |
On August 21, 2023, Eloxx Pharmaceuticals, Inc.
(the “Company”) posted an updated corporate presentation within the “Investors” section of the Company’s
website, which is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 of this Current
Report on Form 8-K (including Exhibit 99.1 hereto) shall not be deemed “filed” for purposes of Section 18 of the Securities
Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section, nor shall it be deemed to be incorporated by
reference into any filing of the Company under the Securities Act of 1933, as amended, except as expressly set forth by specific reference
in such filing.
| Item 9.01 |
Financial Statements and Exhibits. |
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| Date: August 21, 2023 |
ELOXX PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Sumit Aggarwal |
| |
Name: Sumit Aggarwal |
| |
Title: President and Chief Executive Officer |
Exhibit 99.1

| RARE Thinking for RARE Solutions
Small Molecule Gene Therapy
August 2023 |

| /
2
Forward-looking statements
This presentation contains forward-looking statements, which are generally statements that are not historical facts.
Forward-looking statements can be identified by the words "expects," "anticipates," "believes," "intends," "estimates,"
"plans," "will," "outlook" and similar expressions. Forward-looking statements are based on management's current plans,
estimates, assumptions and projections, and speak only as of the date they are made. We undertake no obligation to
update any forward-looking statement in light of new information or future events, except as otherwise required by law.
Forward-looking statements involve inherent risks and uncertainties, most of which are difficult to predict and are generally
beyond our control. Actual results or outcomes may differ materially from those implied by the forward-looking statements
as a result of the impact of a number of factors, including: the development of the Company’s readthrough technology; the
approval of the Company’s patent applications; the Company’s ability to successfully defend its intellectual property or
obtain necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s
research and development programs and collaborations; the Company’s ability to obtain applicable regulatory approvals for
its current and future product candidates; the acceptance by the market of the Company’s products should they receive
regulatory approval; the timing and success of the Company’s preliminary studies, preclinical research, clinical trials, and
related regulatory filings; the ability of the Company to consummate additional financings as needed; the impact of global
health concerns, such as the COVID-19 global pandemic, on our ability to continue our clinical and preclinical programs
and otherwise operate our business effectively; including successfully integrating the combined companies; as well as
those discussed in more detail in our Annual Report on Form 10-K and our other reports filed with the Securities and
Exchange Commission. |
| /
3
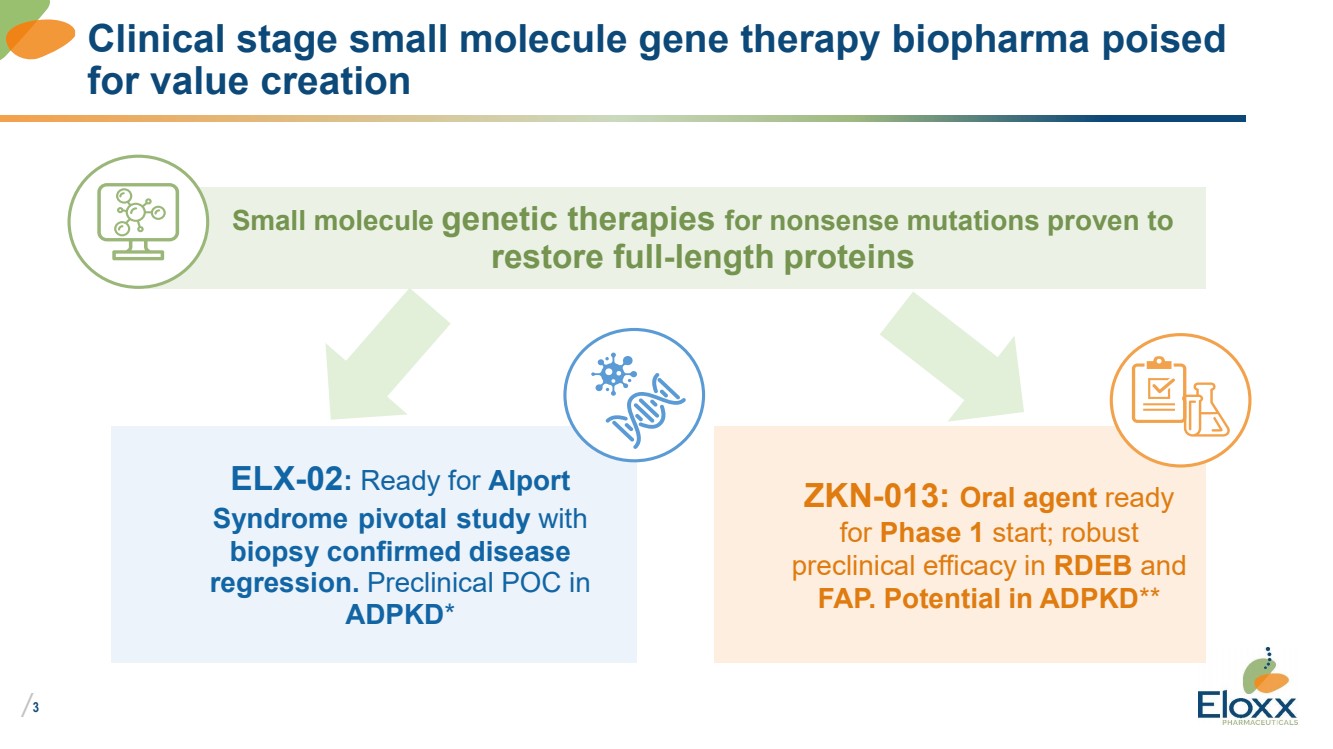
Clinical stage small molecule gene therapy biopharma poised
for value creation
Small molecule genetic therapies for nonsense mutations proven to
restore full-length proteins
ELX-02: Ready for Alport
Syndrome pivotal study with
biopsy confirmed disease
regression. Preclinical POC in
ADPKD*
ZKN-013: Oral agent ready
for Phase 1 start; robust
preclinical efficacy in RDEB and
FAP. Potential in ADPKD** |
| /
4
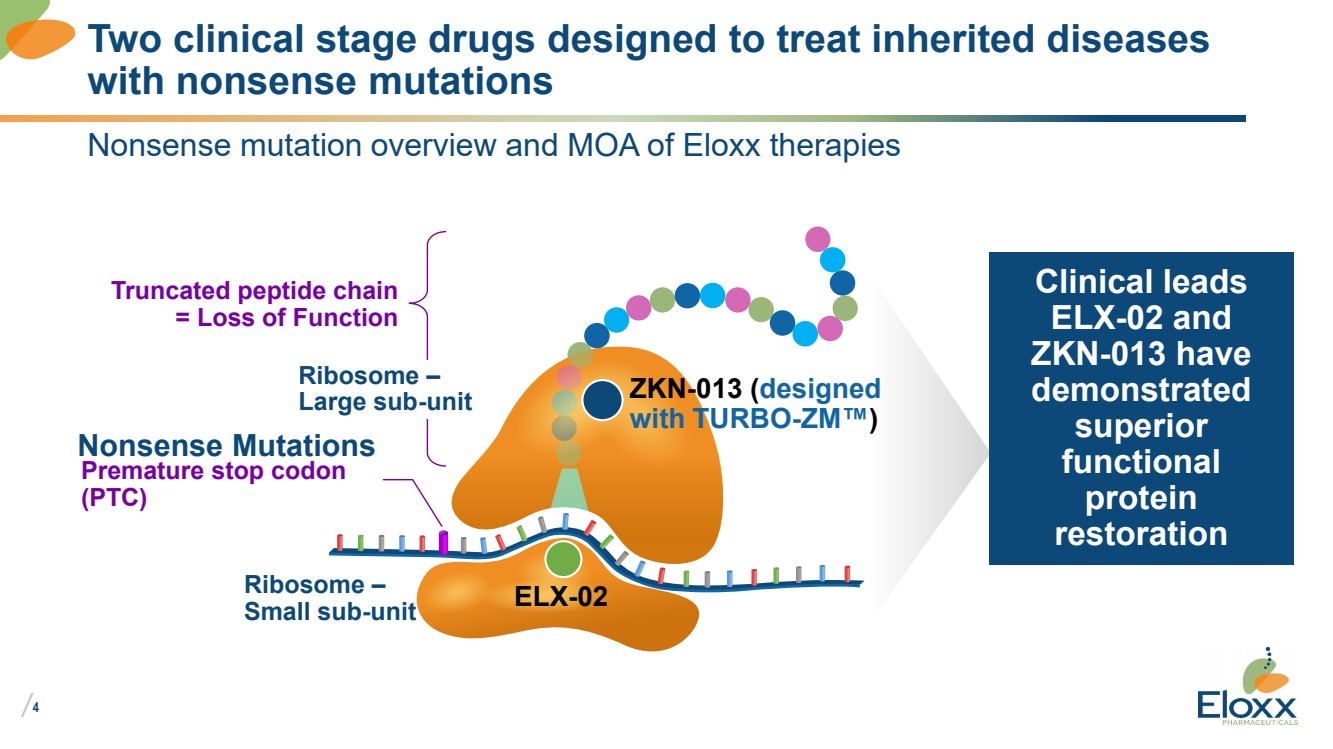
Nonsense mutation overview and MOA of Eloxx therapies
Two clinical stage drugs designed to treat inherited diseases
with nonsense mutations
Truncated peptide chain
= Loss of Function
Premature stop codon
(PTC)
Nonsense Mutations
Ribosome –
Small sub-unit
Clinical leads
ELX-02 and
ZKN-013 have
demonstrated
superior
functional
protein
restoration
ELX-02
ZKN-013 (designed
with TURBO-ZM™)
Ribosome –
Large sub-unit |
| /
5
1Data adapted from: J Med Chem. 2012 Dec 13;55(23):10630-43.1;
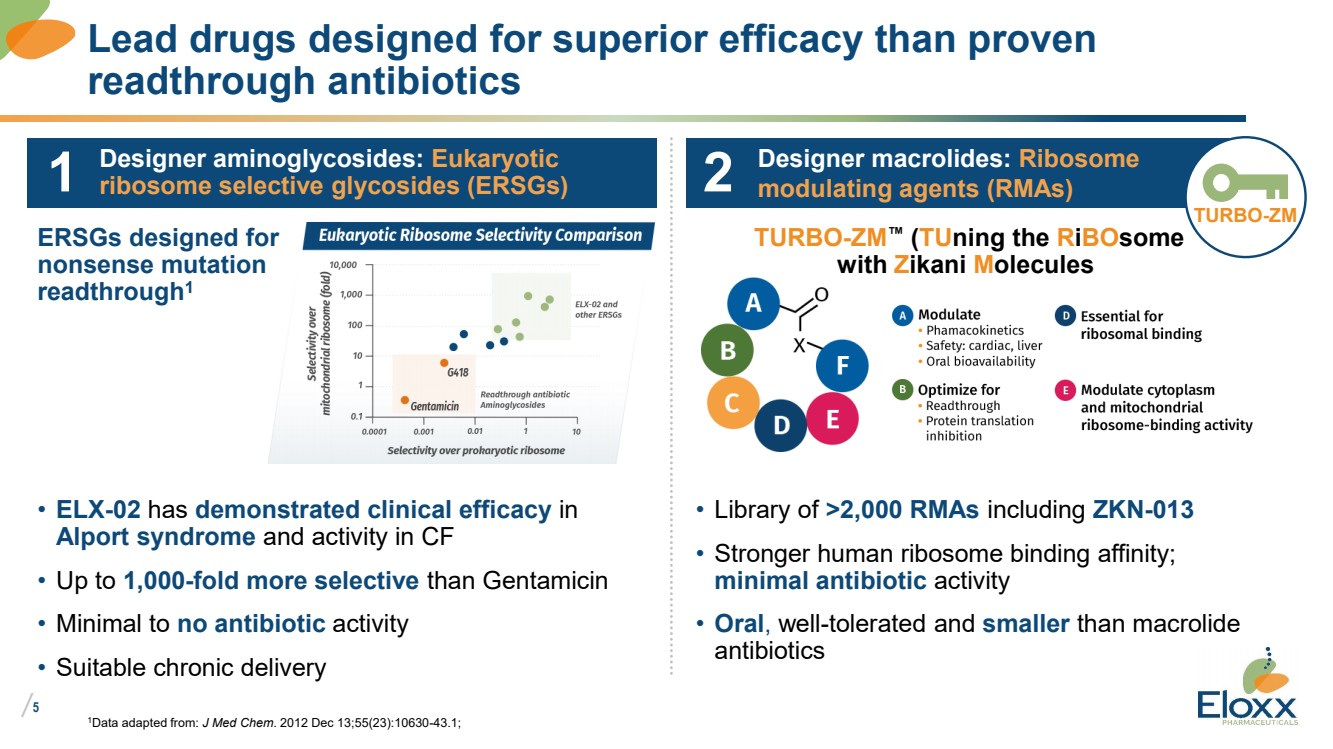
Lead drugs designed for superior efficacy than proven
readthrough antibiotics
Designer aminoglycosides: Eukaryotic
ribosome selective glycosides (ERSGs)
Designer macrolides: Ribosome
modulating agents (RMAs)
ERSGs designed for
nonsense mutation
readthrough1
TURBO-ZM
TURBO-ZM™ (TUning the RiBOsome
with Zikani Molecules
1
• ELX-02 has demonstrated clinical efficacy in
Alport syndrome and activity in CF
• Up to 1,000-fold more selective than Gentamicin
• Minimal to no antibiotic activity
• Suitable chronic delivery
• Library of >2,000 RMAs including ZKN-013
• Stronger human ribosome binding affinity;
minimal antibiotic activity
• Oral, well-tolerated and smaller than macrolide
antibiotics
2 |
| /
6
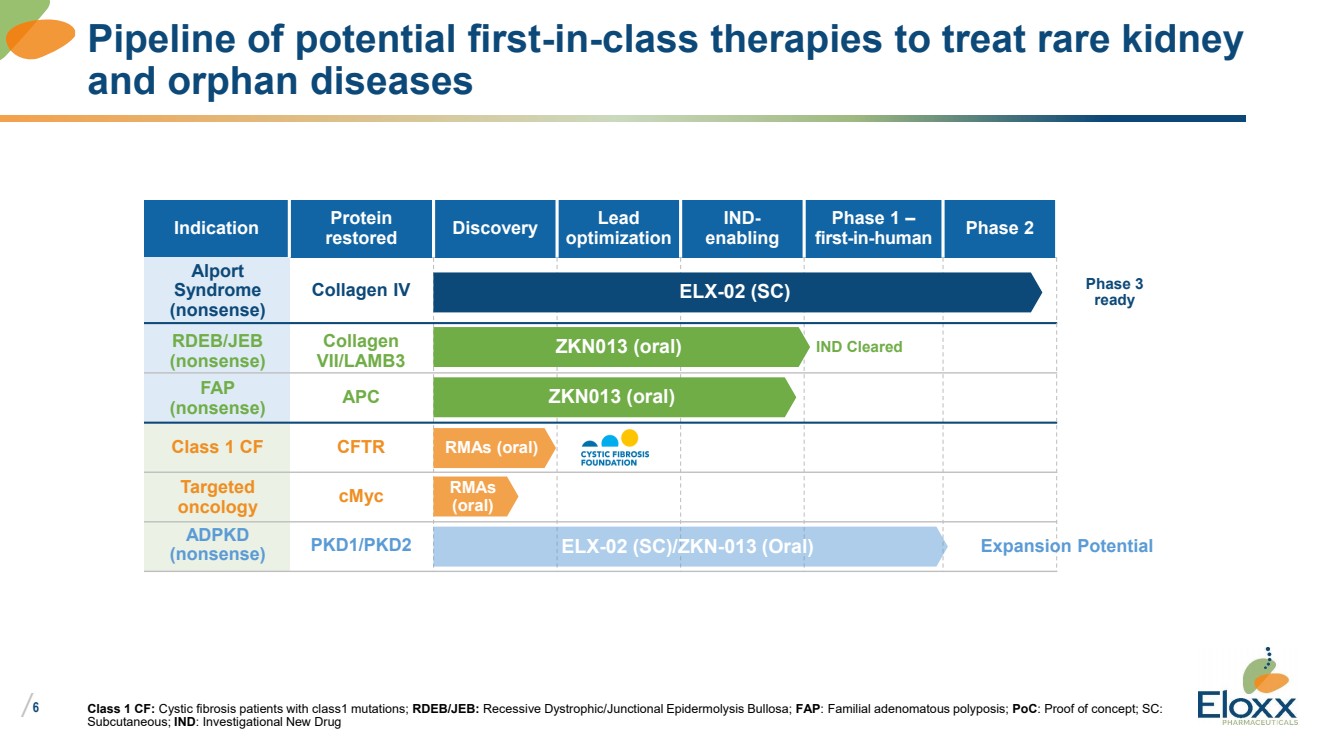
Indication Protein
restored Discovery Lead
optimization
IND-enabling
Phase 1 –
first-in-human Phase 2
Class 1 CF: Cystic fibrosis patients with class1 mutations; RDEB/JEB: Recessive Dystrophic/Junctional Epidermolysis Bullosa; FAP: Familial adenomatous polyposis; PoC: Proof of concept; SC:
Subcutaneous; IND: Investigational New Drug
Pipeline of potential first-in-class therapies to treat rare kidney
and orphan diseases
ELX-02 (SC)
ZKN013 (oral)
ZKN013 (oral)
Alport
Syndrome
(nonsense)
RDEB/JEB
(nonsense)
FAP
(nonsense)
Collagen IV
Collagen
VII/LAMB3
APC
Class 1 CF CFTR RMAs (oral)
Targeted
oncology cMyc RMAs
(oral)
IND Cleared
ELX-02 (SC)/ZKN-013 (Oral) ADPKD
(nonsense) PKD1/PKD2 Expansion Potential
Phase 3
ready |

| / 7
ELX
-02: Alport
Syndrome and
ADPKD with
Nonsense
Mutations |
| /
8
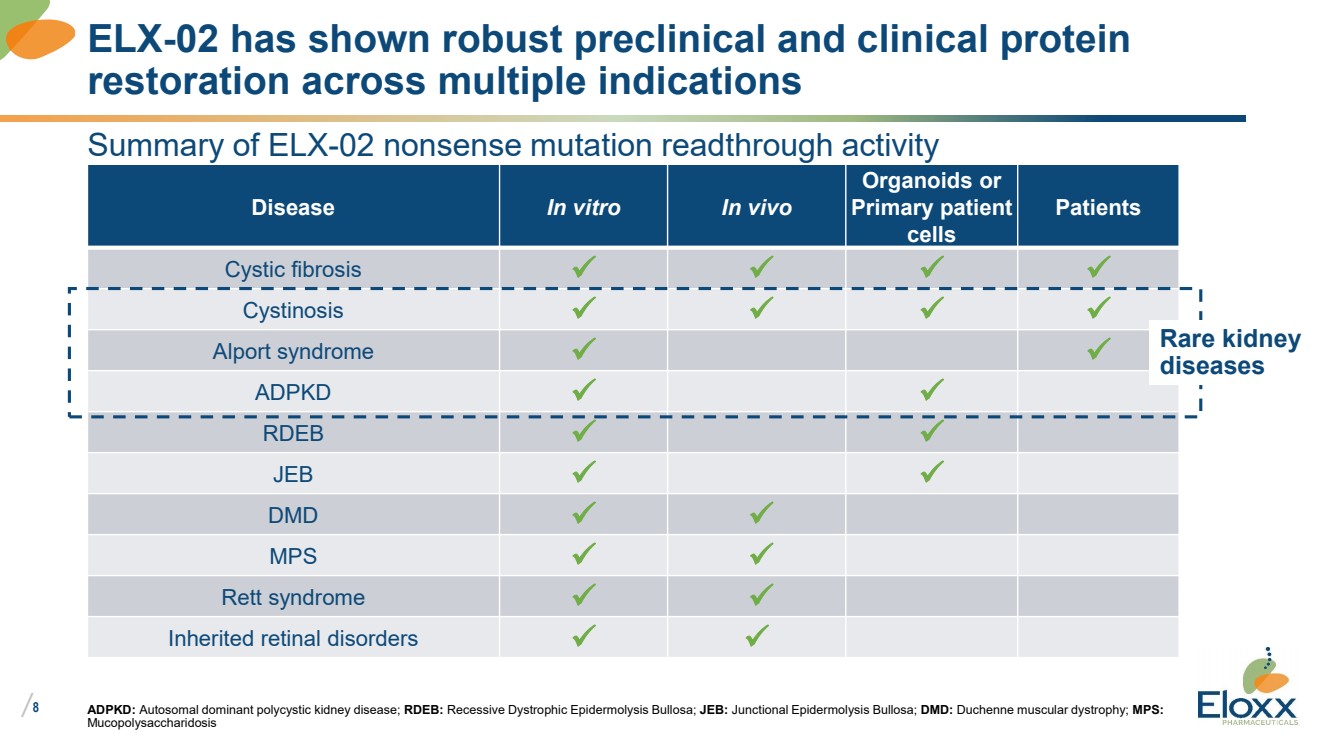
Summary of ELX-02 nonsense mutation readthrough activity
Disease In vitro In vivo
Organoids or
Primary patient
cells
Patients
Cystic fibrosis ✓ ✓ ✓ ✓
Cystinosis ✓ ✓ ✓ ✓
Alport syndrome ✓ ✓
ADPKD ✓ ✓
RDEB ✓ ✓
JEB ✓ ✓
DMD ✓ ✓
MPS ✓ ✓
Rett syndrome ✓ ✓
Inherited retinal disorders ✓ ✓
ADPKD: Autosomal dominant polycystic kidney disease; RDEB: Recessive Dystrophic Epidermolysis Bullosa; JEB: Junctional Epidermolysis Bullosa; DMD: Duchenne muscular dystrophy; MPS:
Mucopolysaccharidosis
ELX-02 has shown robust preclinical and clinical protein
restoration across multiple indications
Rare kidney
diseases |
| /
9
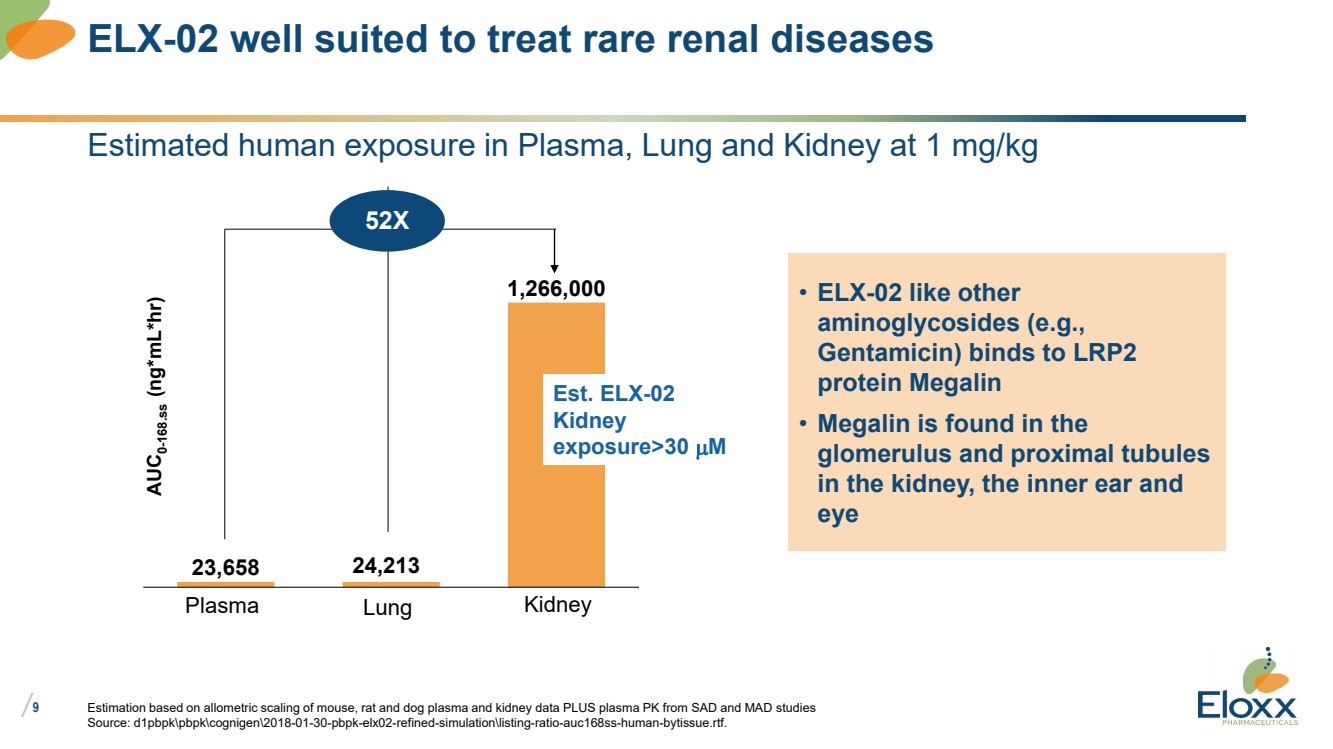
Estimated human exposure in Plasma, Lung and Kidney at 1 mg/kg
Estimation based on allometric scaling of mouse, rat and dog plasma and kidney data PLUS plasma PK from SAD and MAD studies
Source: d1pbpkpbpkcognigen2018-01-30-pbpk-elx02-refined-simulationlisting-ratio-auc168ss-human-bytissue.rtf.
ELX-02 well suited to treat rare renal diseases
23,658
1,266,000
AUC0-168.ss (ng*mL*hr)
Plasma Lung Kidney
24,213
52X
• ELX-02 like other
aminoglycosides (e.g.,
Gentamicin) binds to LRP2
protein Megalin
• Megalin is found in the
glomerulus and proximal tubules
in the kidney, the inner ear and
eye
Est. ELX-02
Kidney
exposure>30 mM |
| /
10
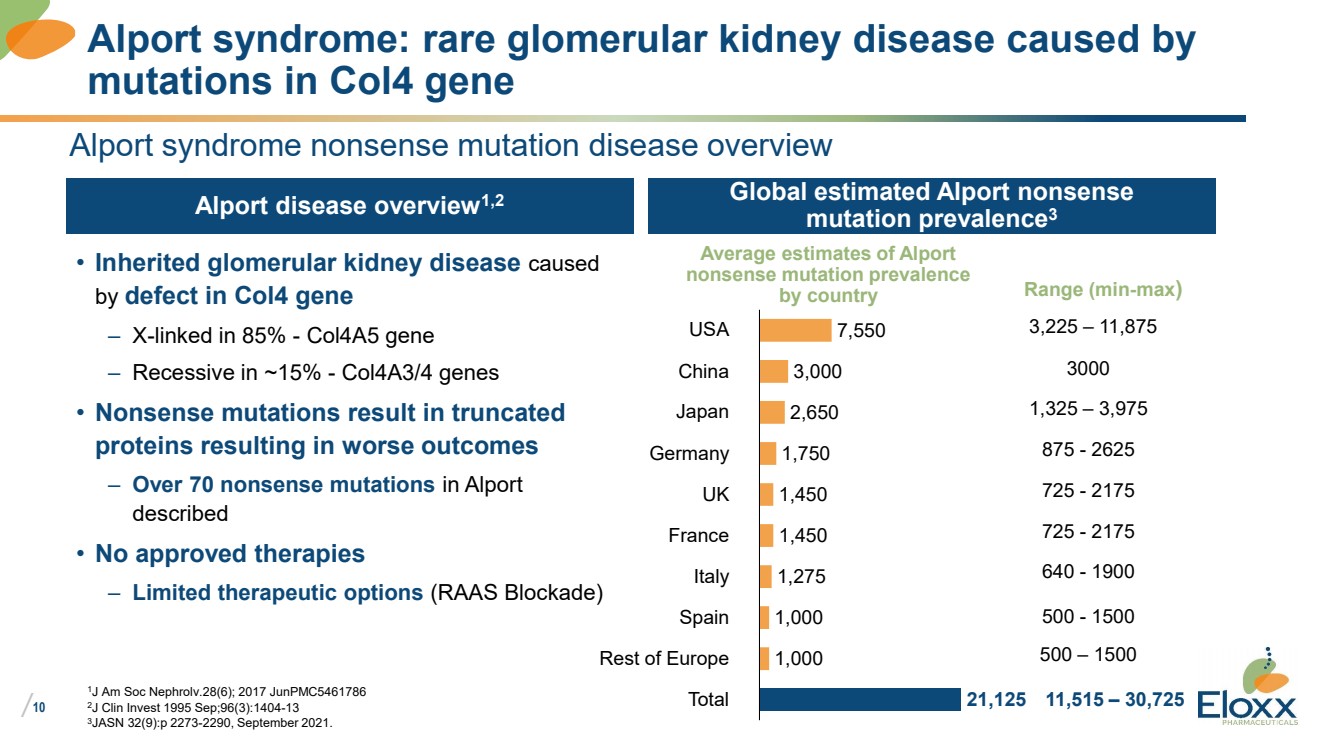
• Inherited glomerular kidney disease caused
by defect in Col4 gene
– X-linked in 85% - Col4A5 gene
– Recessive in ~15% - Col4A3/4 genes
• Nonsense mutations result in truncated
proteins resulting in worse outcomes
– Over 70 nonsense mutations in Alport
described
• No approved therapies
– Limited therapeutic options (RAAS Blockade)
Alport syndrome nonsense mutation disease overview
Alport disease overview1,2 Global estimated Alport nonsense
mutation prevalence3
1J Am Soc Nephrolv.28(6); 2017 JunPMC5461786
2J Clin Invest 1995 Sep;96(3):1404-13
3JASN 32(9):p 2273-2290, September 2021.
Alport syndrome: rare glomerular kidney disease caused by
mutations in Col4 gene
7,550
3,000
2,650
1,750
1,450
1,450
1,275
1,000
1,000
21,125
USA
China
Japan
Germany
UK
France
Italy
Spain
Rest of Europe
Total
Average estimates of Alport
nonsense mutation prevalence
by country
3,225 – 11,875
1,325 – 3,975
875 - 2625
725 - 2175
725 - 2175
640 - 1900
500 - 1500
500 – 1500
11,515 – 30,725
3000
Range (min-max) |
| /
11
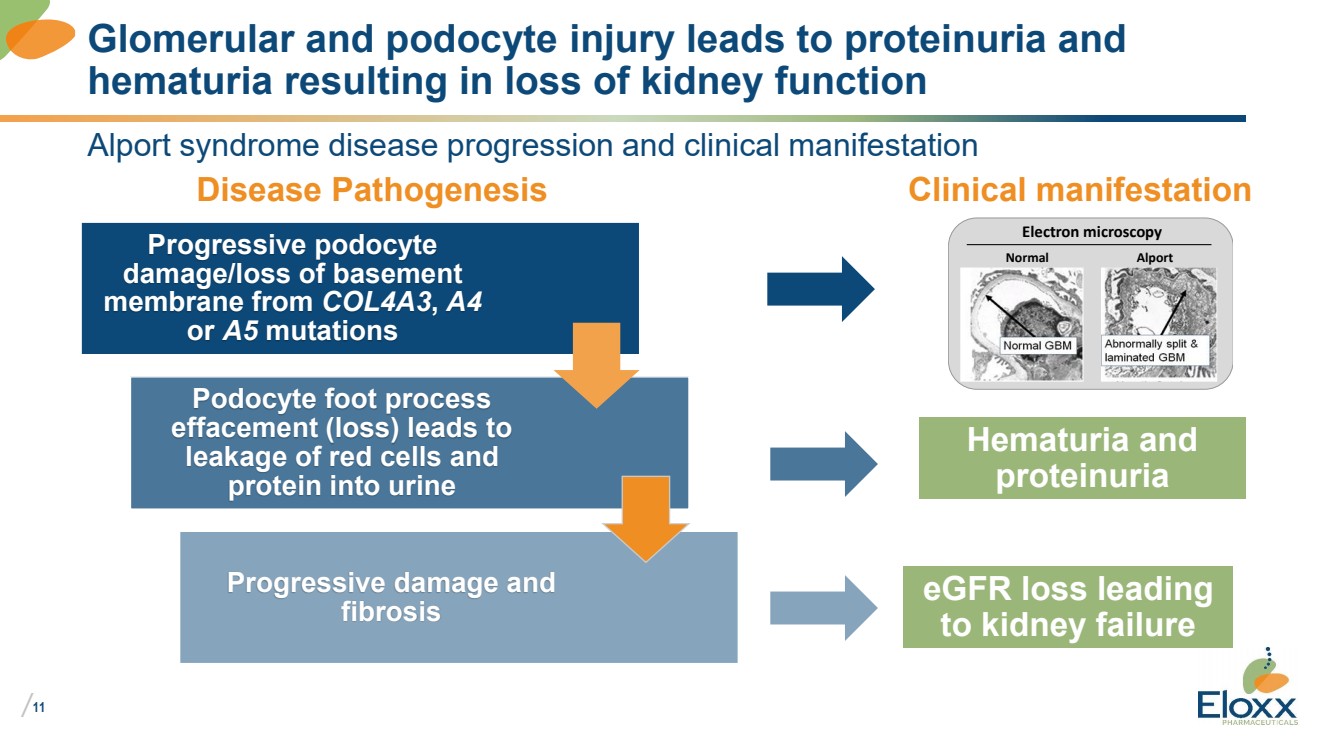
Alport syndrome disease progression and clinical manifestation
Glomerular and podocyte injury leads to proteinuria and
hematuria resulting in loss of kidney function
Progressive podocyte
damage/loss of basement
membrane from COL4A3, A4
or A5 mutations
Podocyte foot process
effacement (loss) leads to
leakage of red cells and
protein into urine
Progressive damage and
fibrosis
Hematuria and
proteinuria
eGFR loss leading
to kidney failure
Disease Pathogenesis Clinical manifestation
Normal Alport
Electron microscopy |
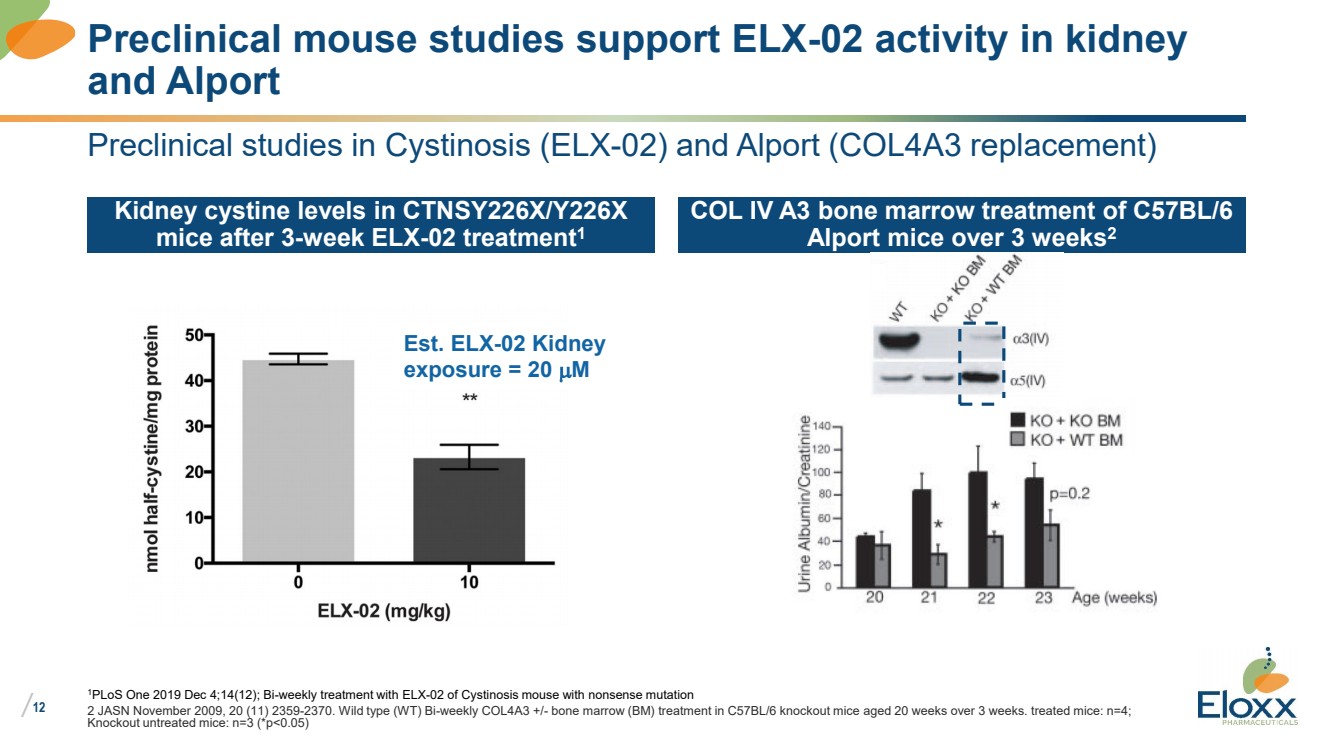
| /
12
Preclinical studies in Cystinosis (ELX-02) and Alport (COL4A3 replacement)
Kidney cystine levels in CTNSY226X/Y226X
mice after 3-week ELX-02 treatment1
COL IV A3 bone marrow treatment of C57BL/6
Alport mice over 3 weeks2
1PLoS One 2019 Dec 4;14(12); Bi-weekly treatment with ELX-02 of Cystinosis mouse with nonsense mutation
2 JASN November 2009, 20 (11) 2359-2370. Wild type (WT) Bi-weekly COL4A3 +/- bone marrow (BM) treatment in C57BL/6 knockout mice aged 20 weeks over 3 weeks. treated mice: n=4;
Knockout untreated mice: n=3 (*p<0.05)
Preclinical mouse studies support ELX-02 activity in kidney
and Alport
Est. ELX-02 Kidney
exposure = 20 mM |
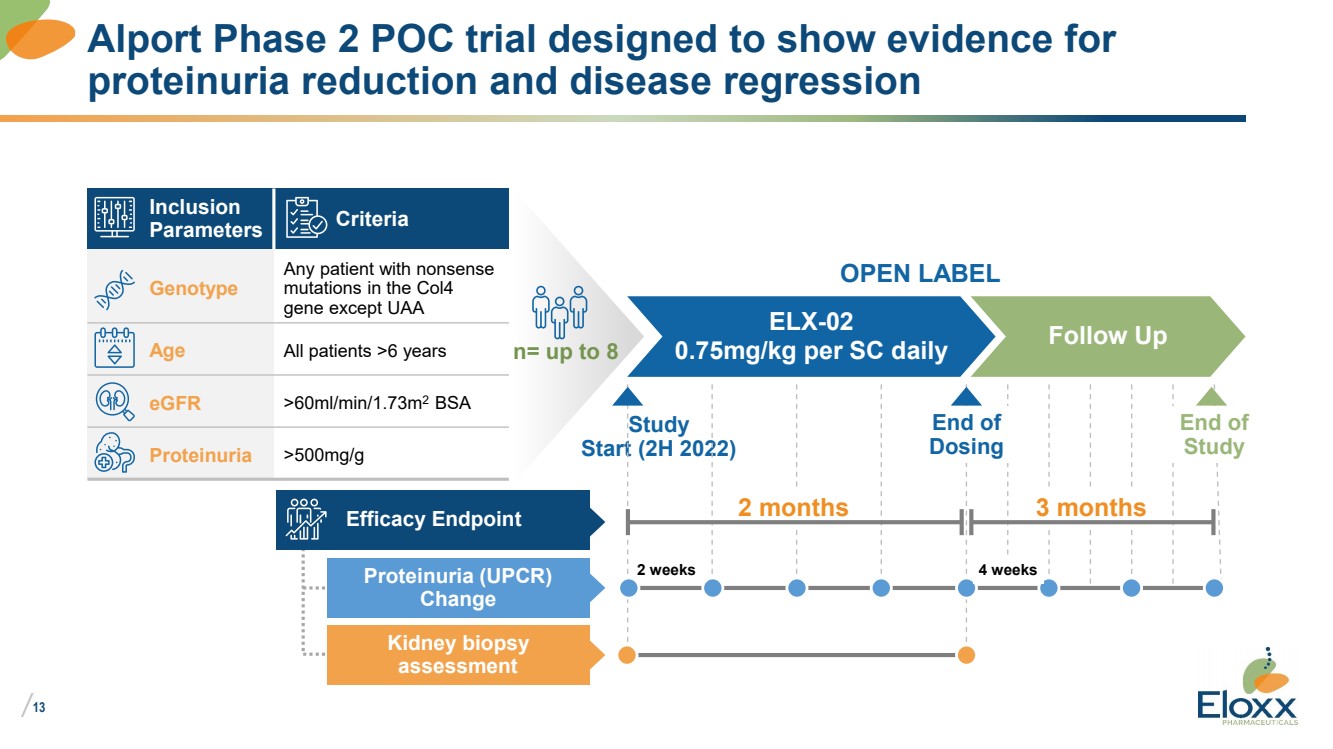
| /
13
Study
Start (2H 2022)
Alport Phase 2 POC trial designed to show evidence for
proteinuria reduction and disease regression
Inclusion
Parameters Criteria
Genotype
Any patient with nonsense
mutations in the Col4
gene except UAA
Age All patients >6 years
eGFR >60ml/min/1.73m2 BSA
Proteinuria >500mg/g
n= up to 8
ELX-02
0.75mg/kg per SC daily Follow Up
OPEN LABEL
End of
Study
Efficacy Endpoint
Proteinuria (UPCR)
Change
Kidney biopsy
assessment
2 months
End of
Dosing
3 months
2 weeks 4 weeks |
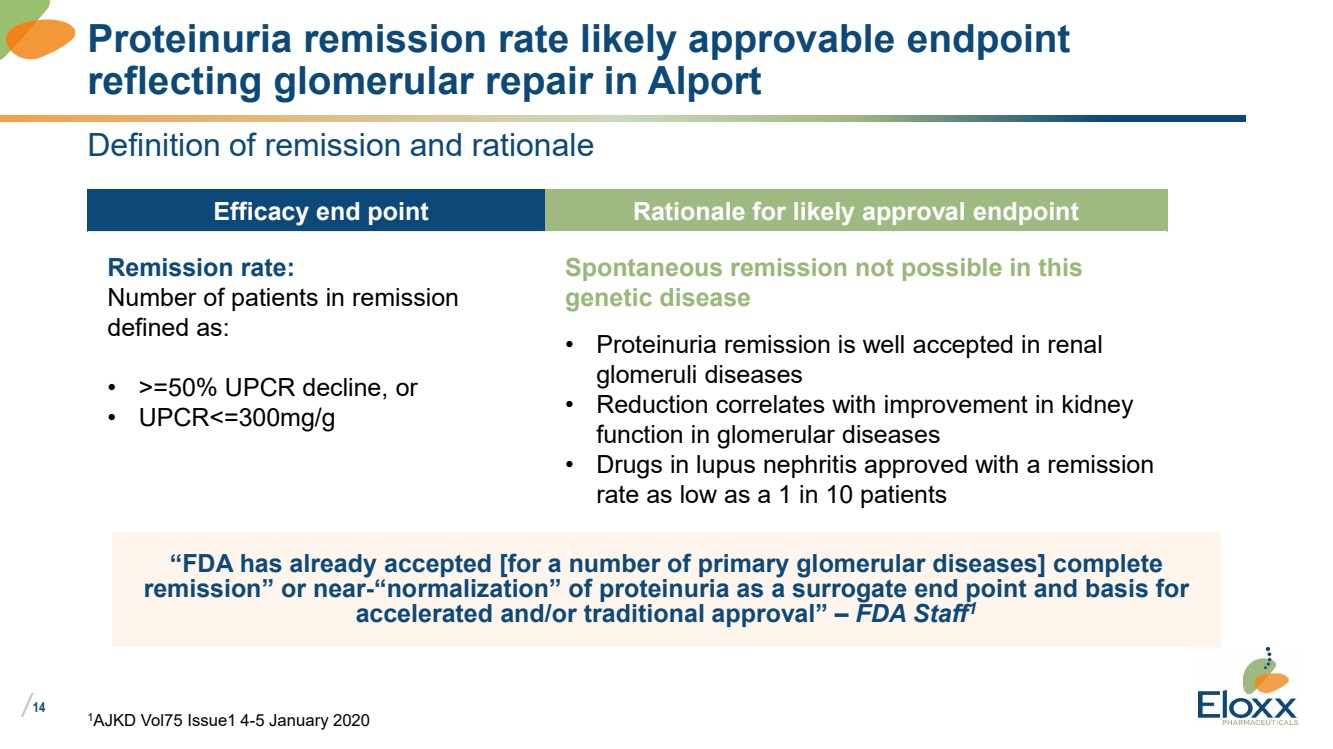
| /
14
1AJKD Vol75 Issue1 4-5 January 2020
Proteinuria remission rate likely approvable endpoint
reflecting glomerular repair in Alport
Definition of remission and rationale
“FDA has already accepted [for a number of primary glomerular diseases] complete
remission” or near-“normalization” of proteinuria as a surrogate end point and basis for
accelerated and/or traditional approval” – FDA Staff1
Efficacy end point Rationale for likely approval endpoint
Remission rate:
Number of patients in remission
defined as:
• >=50% UPCR decline, or
• UPCR<=300mg/g
Spontaneous remission not possible in this
genetic disease
• Proteinuria remission is well accepted in renal
glomeruli diseases
• Reduction correlates with improvement in kidney
function in glomerular diseases
• Drugs in lupus nephritis approved with a remission
rate as low as a 1 in 10 patients |
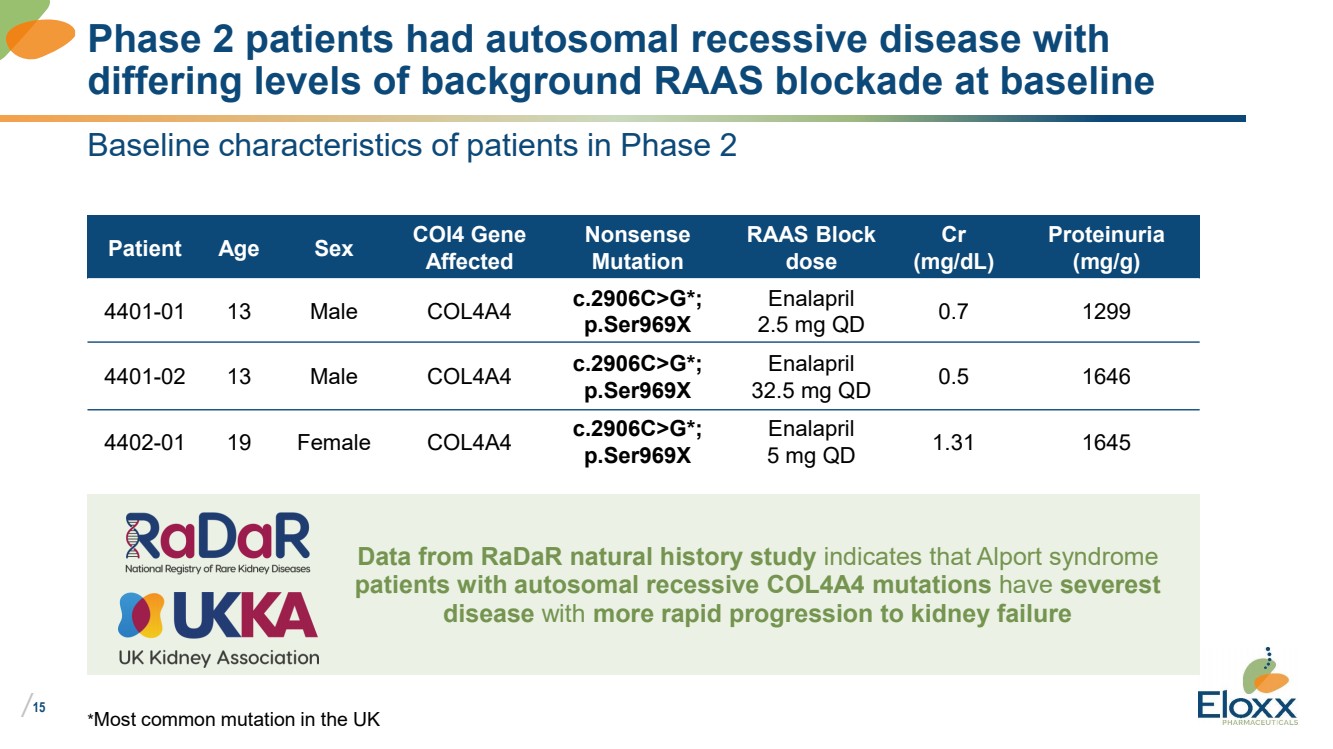
| /
15
Baseline characteristics of patients in Phase 2
Patient Age Sex COl4 Gene
Affected
Nonsense
Mutation
RAAS Block
dose
Cr
(mg/dL)
Proteinuria
(mg/g)
4401-01 13 Male COL4A4 c.2906C>G*;
p.Ser969X
Enalapril
2.5 mg QD 0.7 1299
4401-02 13 Male COL4A4 c.2906C>G*;
p.Ser969X
Enalapril
32.5 mg QD 0.5 1646
4402-01 19 Female COL4A4 c.2906C>G*;
p.Ser969X
Enalapril
5 mg QD 1.31 1645
*Most common mutation in the UK
Phase 2 patients had autosomal recessive disease with
differing levels of background RAAS blockade at baseline
Data from RaDaR natural history study indicates that Alport syndrome
patients with autosomal recessive COL4A4 mutations have severest
disease with more rapid progression to kidney failure |
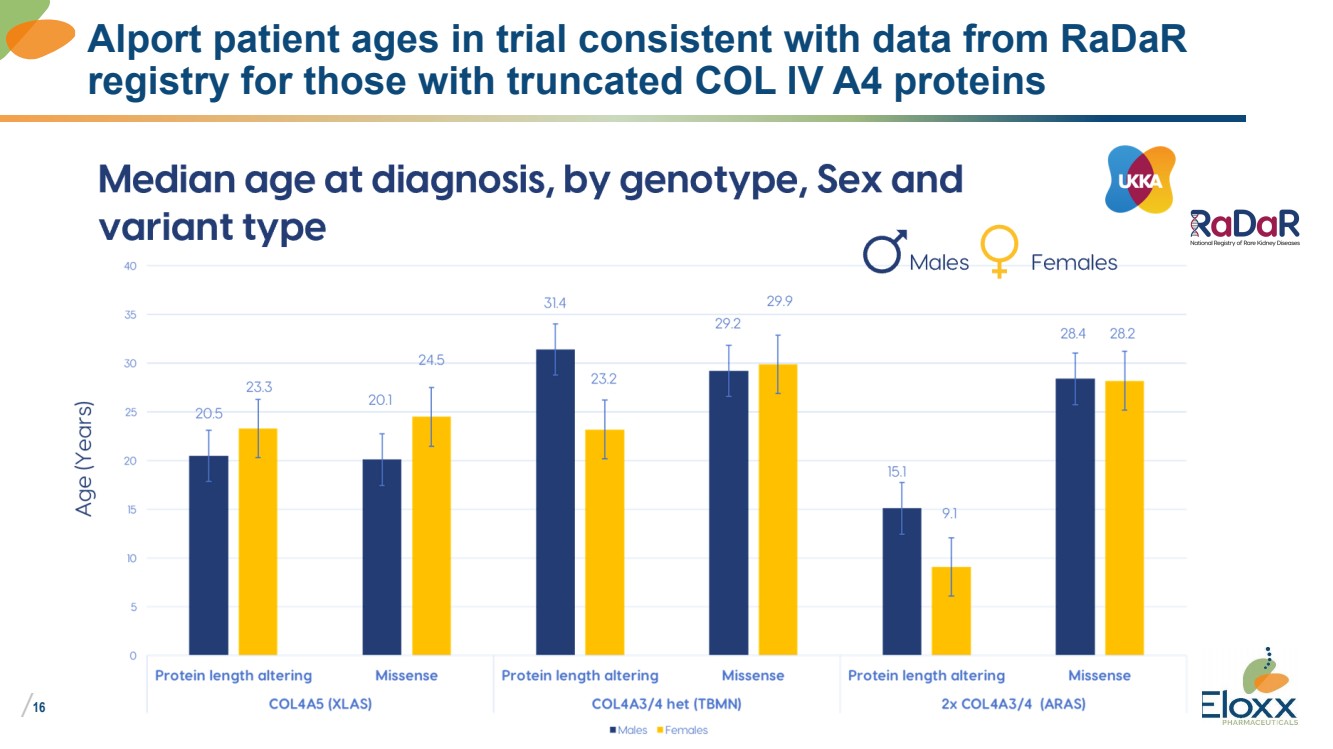
| /
16
Alport patient ages in trial consistent with data from RaDaR
registry for those with truncated COL IV A4 proteins |
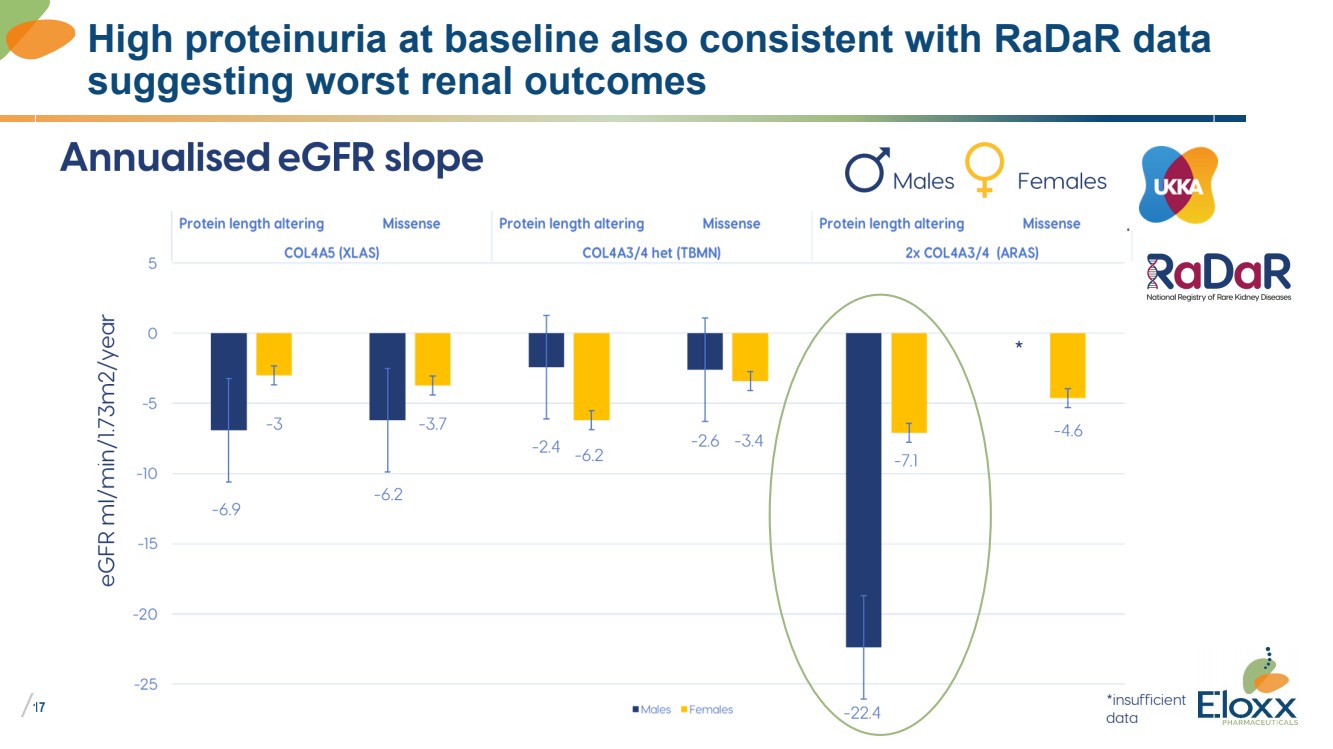
| /
17
High proteinuria at baseline also consistent with RaDaR data
suggesting worst renal outcomes |
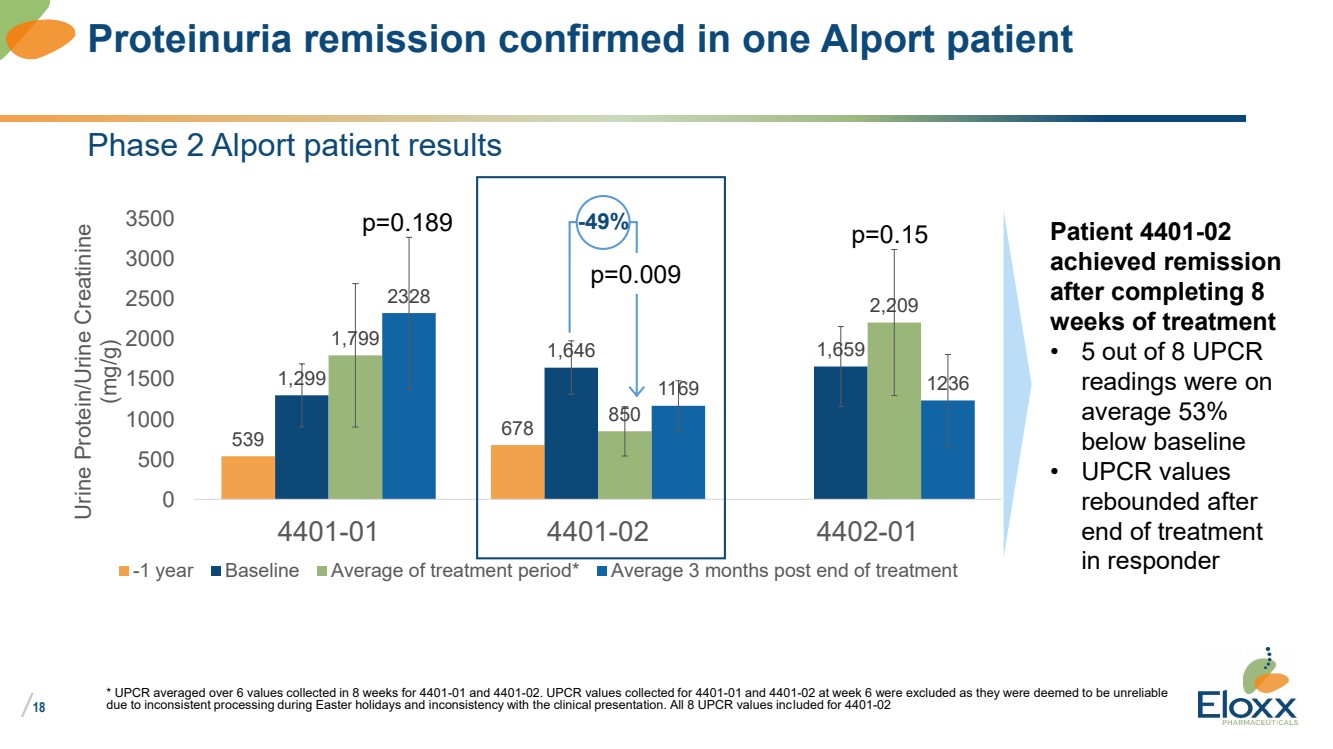
| /
18
Phase 2 Alport patient results
539 678
1,299
1,646 1,659 1,799
850
2,209 2328
1169 1236
0
500
1000
1500
2000
2500
3000
3500
4401-01 4401-02 4402-01
Urine Protein/Urine Creatinine
(mg/g)
-1 year Baseline Average of treatment period* Average 3 months post end of treatment
* UPCR averaged over 6 values collected in 8 weeks for 4401-01 and 4401-02. UPCR values collected for 4401-01 and 4401-02 at week 6 were excluded as they were deemed to be unreliable
due to inconsistent processing during Easter holidays and inconsistency with the clinical presentation. All 8 UPCR values included for 4401-02
Proteinuria remission confirmed in one Alport patient
p=0.189 -49%
p=0.009
Patient 4401-02
achieved remission
after completing 8
weeks of treatment
• 5 out of 8 UPCR
readings were on
average 53%
below baseline
• UPCR values
rebounded after
end of treatment
in responder
p=0.15 |
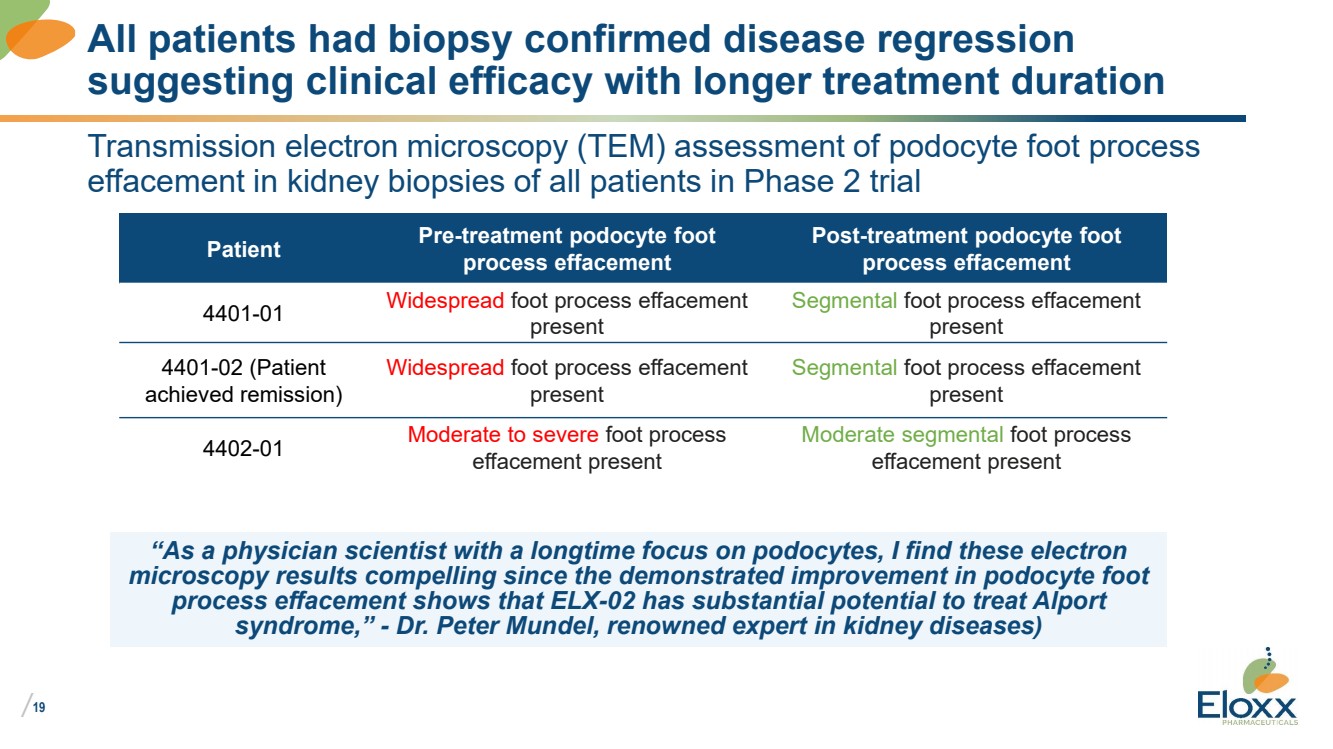
| /
19
Transmission electron microscopy (TEM) assessment of podocyte foot process
effacement in kidney biopsies of all patients in Phase 2 trial
Patient Pre-treatment podocyte foot
process effacement
Post-treatment podocyte foot
process effacement
4401-01 Widespread foot process effacement
present
Segmental foot process effacement
present
4401-02 (Patient
achieved remission)
Widespread foot process effacement
present
Segmental foot process effacement
present
4402-01 Moderate to severe foot process
effacement present
Moderate segmental foot process
effacement present
All patients had biopsy confirmed disease regression
suggesting clinical efficacy with longer treatment duration
“As a physician scientist with a longtime focus on podocytes, I find these electron
microscopy results compelling since the demonstrated improvement in podocyte foot
process effacement shows that ELX-02 has substantial potential to treat Alport
syndrome,” - Dr. Peter Mundel, renowned expert in kidney diseases) |
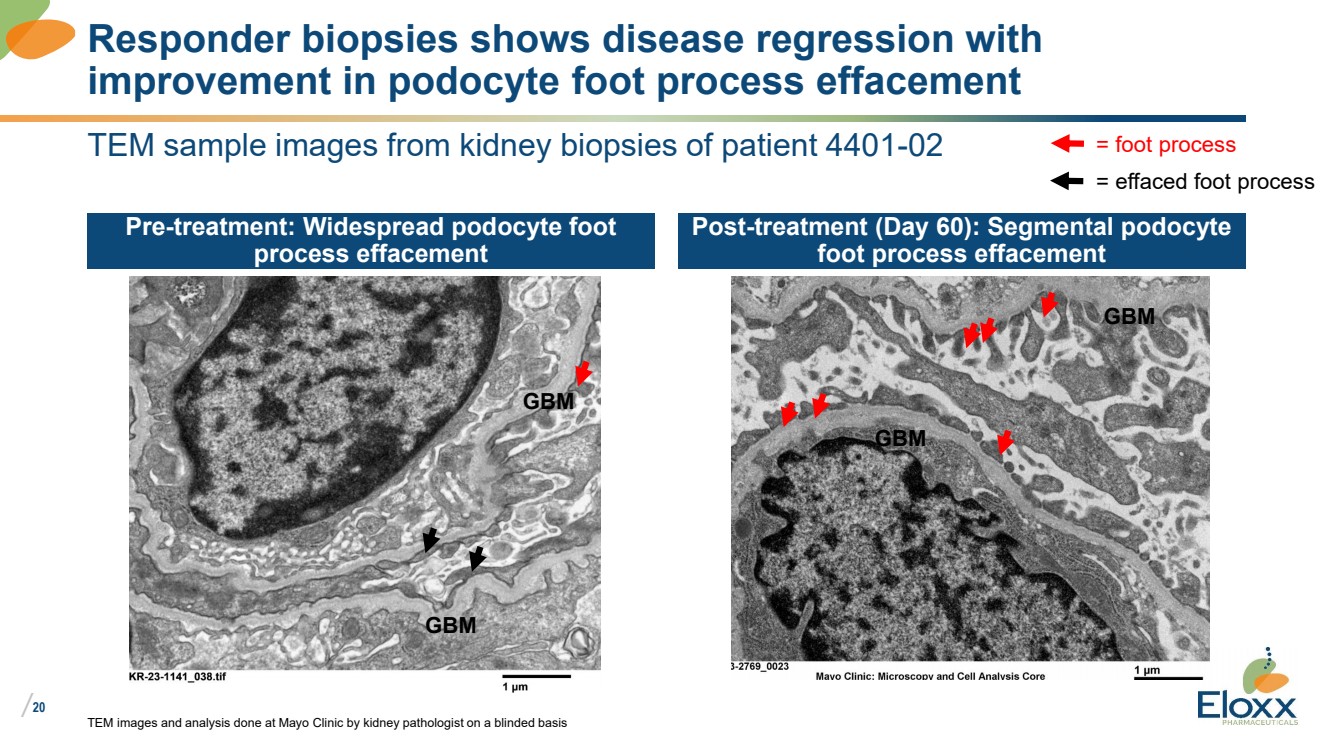
| /
20
TEM sample images from kidney biopsies of patient 4401-02
Pre-treatment: Widespread podocyte foot
process effacement
Post-treatment (Day 60): Segmental podocyte
foot process effacement
TEM images and analysis done at Mayo Clinic by kidney pathologist on a blinded basis
Responder biopsies shows disease regression with
improvement in podocyte foot process effacement
GBM
GBM
GBM
GBM
= foot process
= effaced foot process |
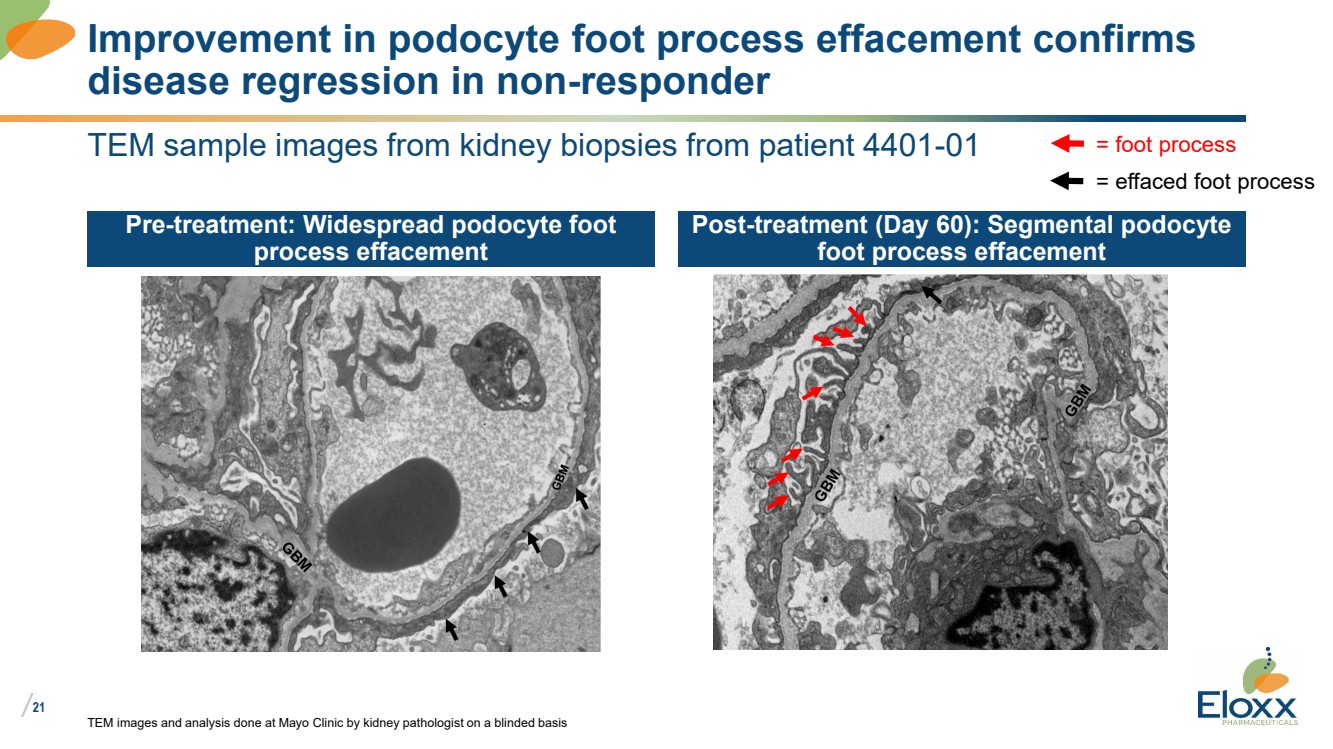
| /
21
TEM sample images from kidney biopsies from patient 4401-01
Pre-treatment: Widespread podocyte foot
process effacement
Post-treatment (Day 60): Segmental podocyte
foot process effacement
TEM images and analysis done at Mayo Clinic by kidney pathologist on a blinded basis
Improvement in podocyte foot process effacement confirms
disease regression in non-responder
= foot process
= effaced foot process |
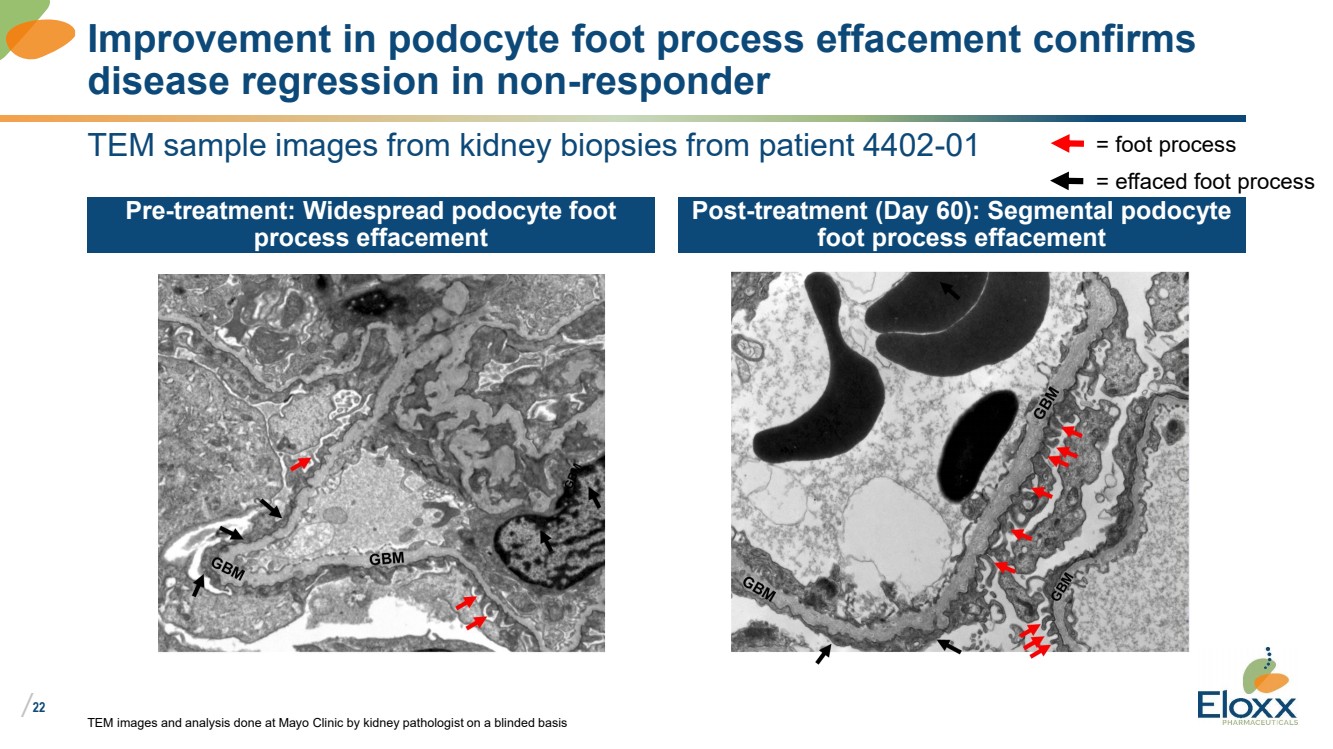
| /
22
TEM sample images from kidney biopsies from patient 4402-01
Pre-treatment: Widespread podocyte foot
process effacement
Post-treatment (Day 60): Segmental podocyte
foot process effacement
TEM images and analysis done at Mayo Clinic by kidney pathologist on a blinded basis
Improvement in podocyte foot process effacement confirms
disease regression in non-responder
= foot process
= effaced foot process |
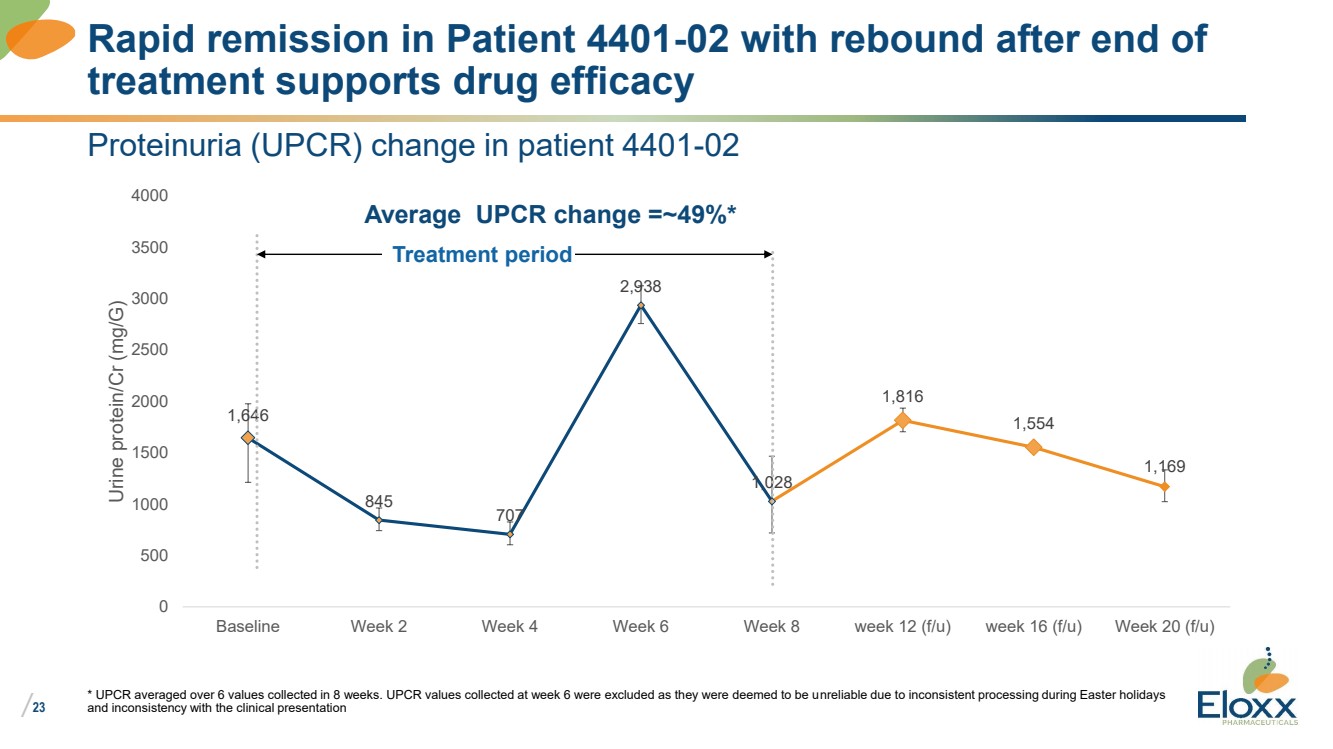
| /
23
Proteinuria (UPCR) change in patient 4401-02
1,646
845
707
2,938
1,028
1,816
1,554
1,169
0
500
1000
1500
2000
2500
3000
3500
4000
Baseline Week 2 Week 4 Week 6 Week 8 week 12 (f/u) week 16 (f/u) Week 20 (f/u)
Urine protein/Cr (mg/G)
* UPCR averaged over 6 values collected in 8 weeks. UPCR values collected at week 6 were excluded as they were deemed to be unreliable due to inconsistent processing during Easter holidays
and inconsistency with the clinical presentation
Rapid remission in Patient 4401-02 with rebound after end of
treatment supports drug efficacy
Treatment period
Average UPCR change =~49%* |

| /
24
Cumulative ELX-02 safety experience across all clinical studies
*Patient had an undisclosed history of tinnitus
SAE: Serious adverse events
Robust safety experience for advancing to longer treatment
duration in pivotal study
No ELX-02 related SAEs in Phase 1 and 2 studies at doses up to 7.5 mg/kg in 148
subjects with no nephrotoxicity
ELX-02 was well tolerated up to 1.5 mg/kg dose across Phase 2 patients (n=34)
– Combination therapy in CF trials at 1.5 mg/kg showed drug related
discontinuations
• 2 patients discontinued due to injection site reactions (mild to moderate)
• 1 patient withdrew from trial due to injection burden prior to dosing
• 1 patient with tinnitus*
– No drug related discontinuations in Alport Phase 2 trial at 0.75mg/kg |
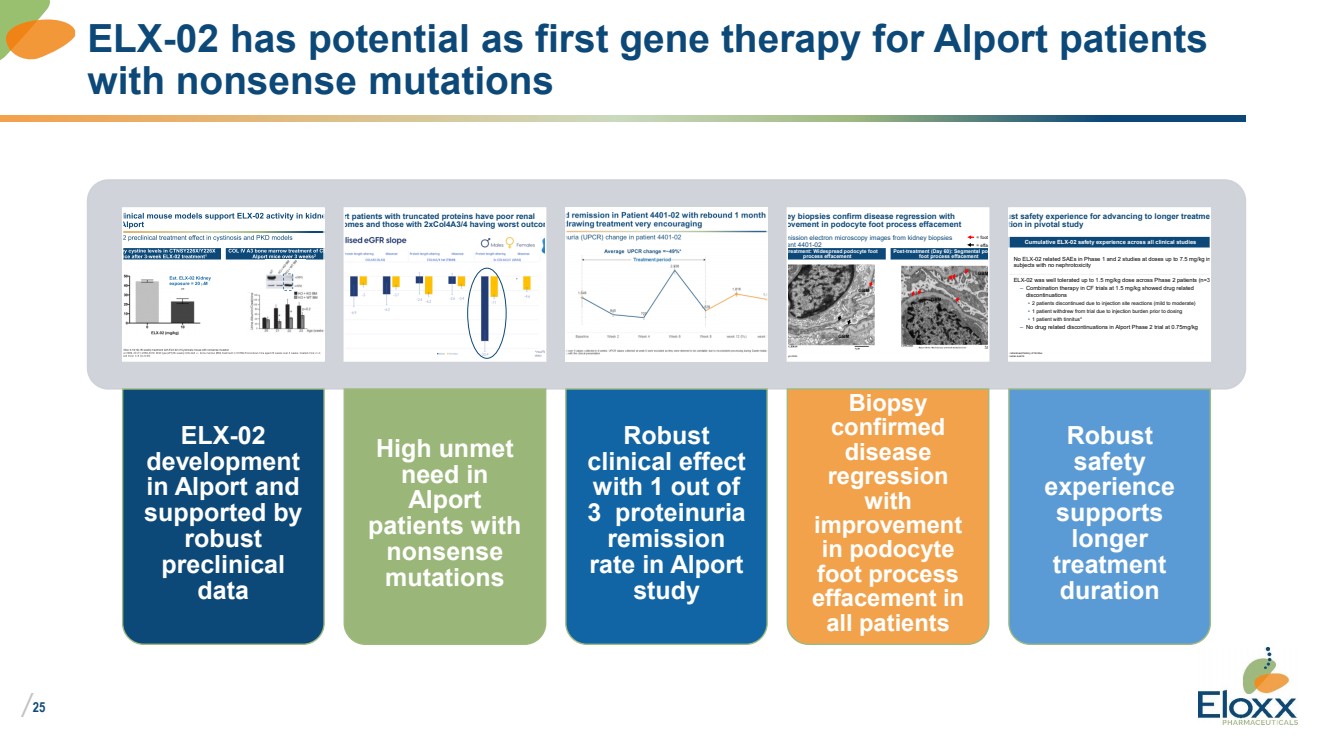
| /
25
ELX-02 has potential as first gene therapy for Alport patients
with nonsense mutations
ELX-02
development
in Alport and
supported by
robust
preclinical
data
/
24
Alport patients with truncated proteins have poor renal outcomes and those with 2xCol4A3/4 having worst outcomes
High unmet
need in
Alport
patients with
nonsense
mutations
Robust
clinical effect
with 1 out of
3 proteinuria
remission
rate in Alport
study
/
25
Transmission electron microscopy images from kidney biopsies of patient 4401-02
Pre-treatment: Widespread podocyte foot process effacement Post-treatment (Day 60): Segmental podocyte
foot process effacement
Performed at Mayo clinic
Kidney biopsies confirm disease regression with
improvement in podocyte foot process effacement
GBM
GBM
GBM
GBM
= foot process = effaced foot process
Biopsy
confirmed
disease
regression
with
improvement
in podocyte
foot process
effacement in
all patients
/
24
Cumulative ELX-02 safety experience across all clinical studies
*Patient had an undisclosed history of tinnitus SAE: Serious adverse events
Robust safety experience for advancing to longer treatment duration in pivotal study
No ELX-02 related SAEs in Phase 1 and 2 studies at doses up to 7.5 mg/kg in 148
subjects with no nephrotoxicity
ELX-02 was well tolerated up to 1.5 mg/kg dose across Phase 2 patients (n=34) – Combination therapy in CF trials at 1.5 mg/kg showed drug related
discontinuations
• 2 patients discontinued due to injection site reactions (mild to moderate)
• 1 patient withdrew from trial due to injection burden prior to dosing
• 1 patient with tinnitus* – No drug related discontinuations in Alport Phase 2 trial at 0.75mg/kg
Robust
safety
experience
supports
longer
treatment
duration |
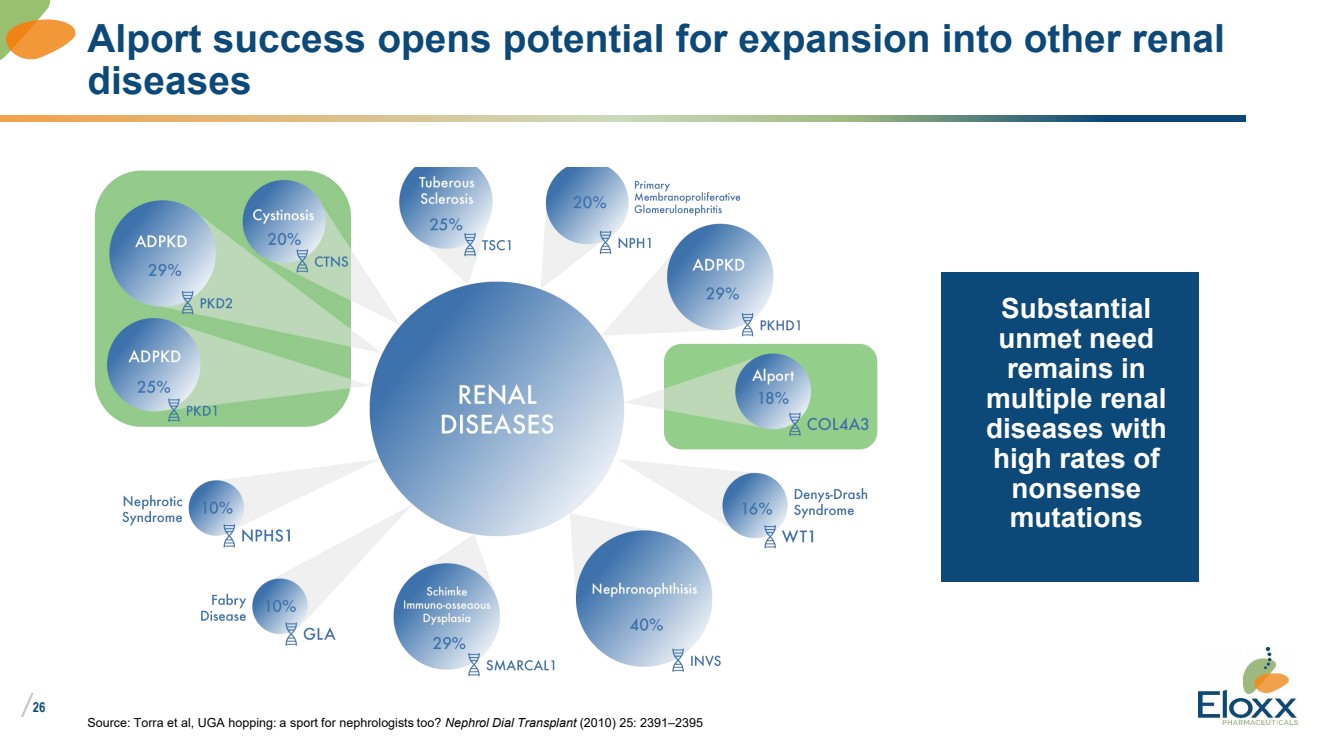
| /
26
Source: Torra et al, UGA hopping: a sport for nephrologists too? Nephrol Dial Transplant (2010) 25: 2391–2395
Alport success opens potential for expansion into other renal
diseases
Substantial
unmet need
remains in
multiple renal
diseases with
high rates of
nonsense
mutations |
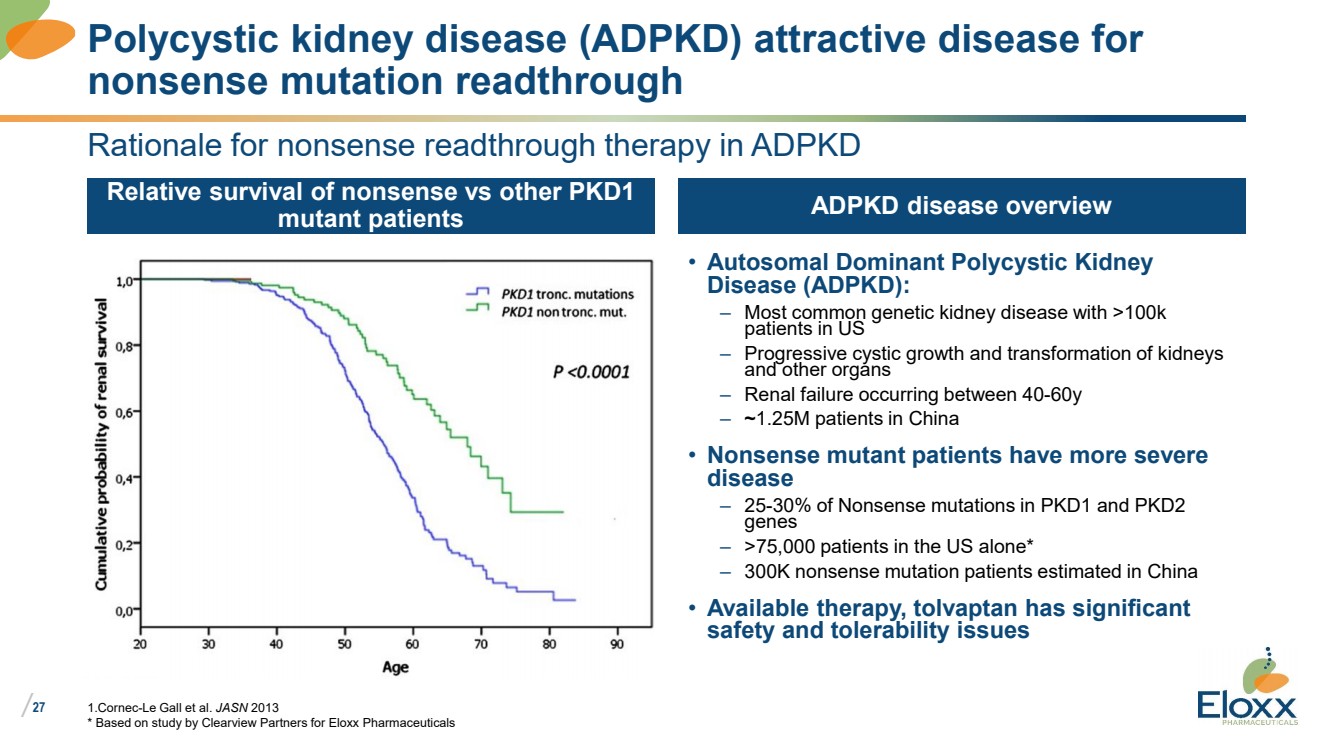
| /
27
Rationale for nonsense readthrough therapy in ADPKD
Relative survival of nonsense vs other PKD1
mutant patients
• Autosomal Dominant Polycystic Kidney
Disease (ADPKD):
– Most common genetic kidney disease with >100k
patients in US
– Progressive cystic growth and transformation of kidneys
and other organs
– Renal failure occurring between 40-60y
– ~1.25M patients in China
• Nonsense mutant patients have more severe
disease
– 25-30% of Nonsense mutations in PKD1 and PKD2
genes
– >75,000 patients in the US alone*
– 300K nonsense mutation patients estimated in China
• Available therapy, tolvaptan has significant
safety and tolerability issues
ADPKD disease overview
1.Cornec-Le Gall et al. JASN 2013
* Based on study by Clearview Partners for Eloxx Pharmaceuticals
Polycystic kidney disease (ADPKD) attractive disease for
nonsense mutation readthrough |
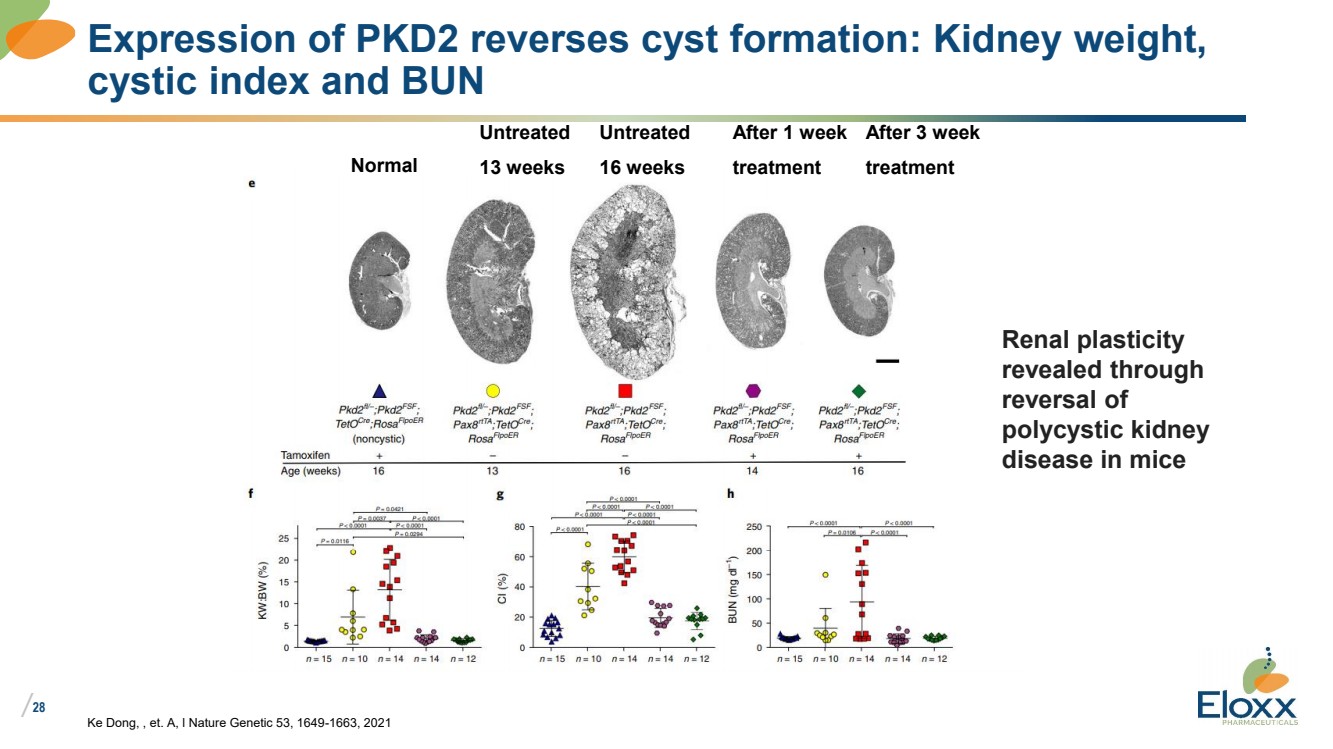
| /
28
Ke Dong, , et. A, l Nature Genetic 53, 1649-1663, 2021
Expression of PKD2 reverses cyst formation: Kidney weight,
cystic index and BUN
Normal
Untreated
13 weeks
Untreated
16 weeks
After 1 week
treatment
After 3 week
treatment
Renal plasticity
revealed through
reversal of
polycystic kidney
disease in mice |
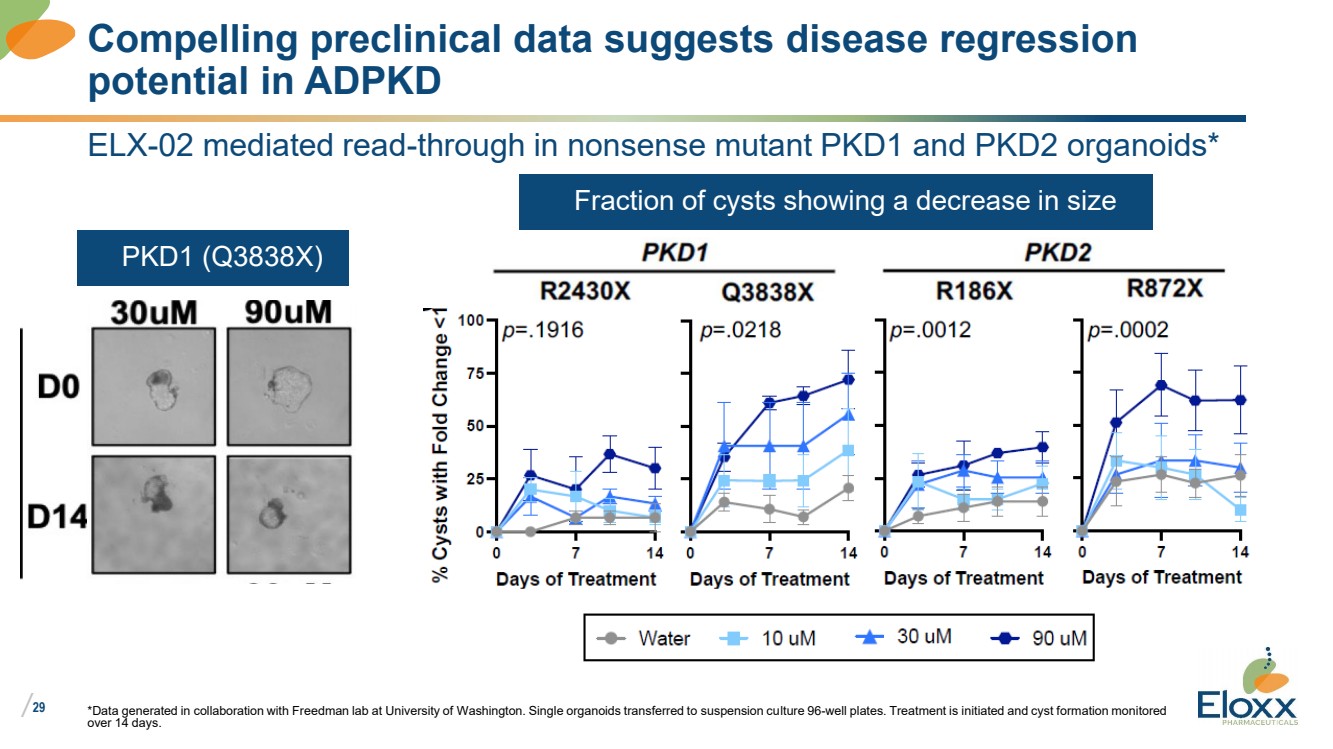
| /
29
ELX-02 mediated read-through in nonsense mutant PKD1 and PKD2 organoids*
*Data generated in collaboration with Freedman lab at University of Washington. Single organoids transferred to suspension culture 96-well plates. Treatment is initiated and cyst formation monitored
over 14 days.
Compelling preclinical data suggests disease regression
potential in ADPKD
• PKD1 (Q3838X)
• Fraction of cysts showing a decrease in size |

| /
30
ZKN-013: RDEB
and FAP |
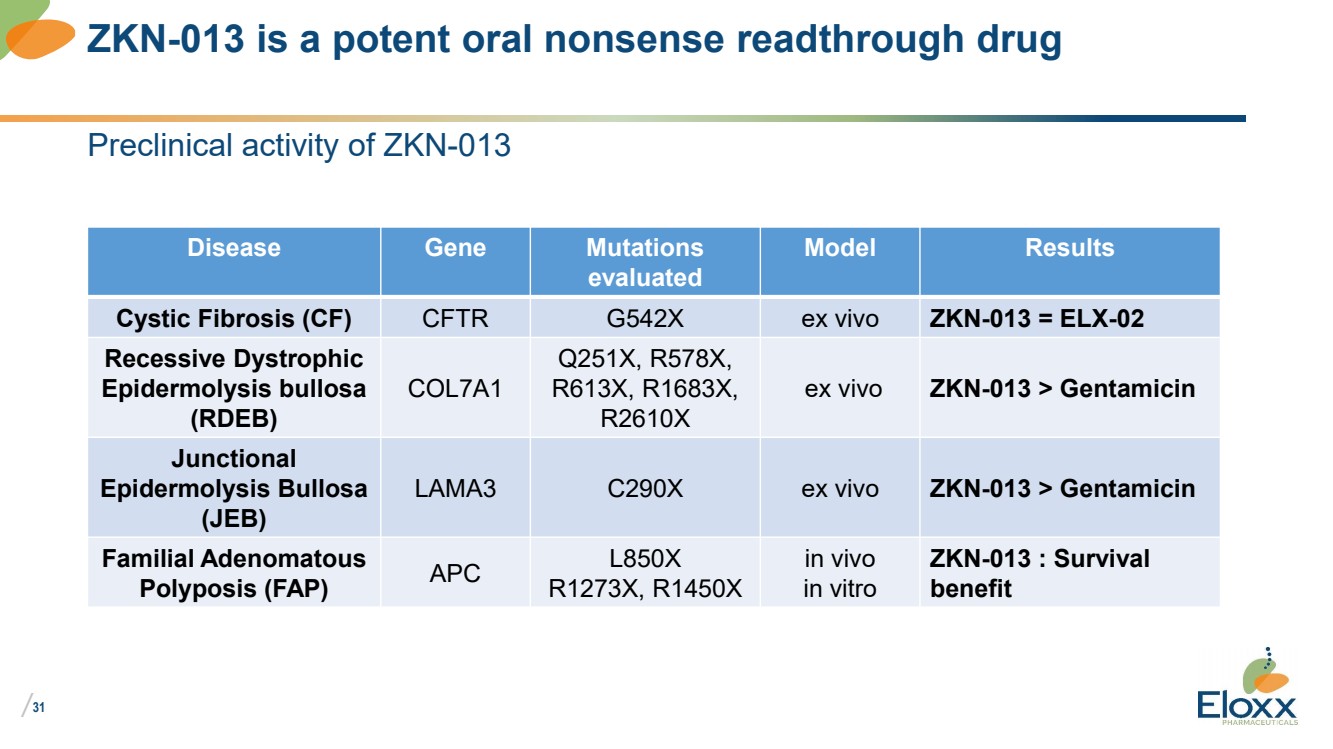
| /
31
Preclinical activity of ZKN-013
Disease Gene Mutations
evaluated
Model Results
Cystic Fibrosis (CF) CFTR G542X ex vivo ZKN-013 = ELX-02
Recessive Dystrophic
Epidermolysis bullosa
(RDEB)
COL7A1
Q251X, R578X,
R613X, R1683X,
R2610X
ex vivo ZKN-013 > Gentamicin
Junctional
Epidermolysis Bullosa
(JEB)
LAMA3 C290X ex vivo ZKN-013 > Gentamicin
Familial Adenomatous
Polyposis (FAP) APC L850X
R1273X, R1450X
in vivo
in vitro
ZKN-013 : Survival
benefit
ZKN-013 is a potent oral nonsense readthrough drug |
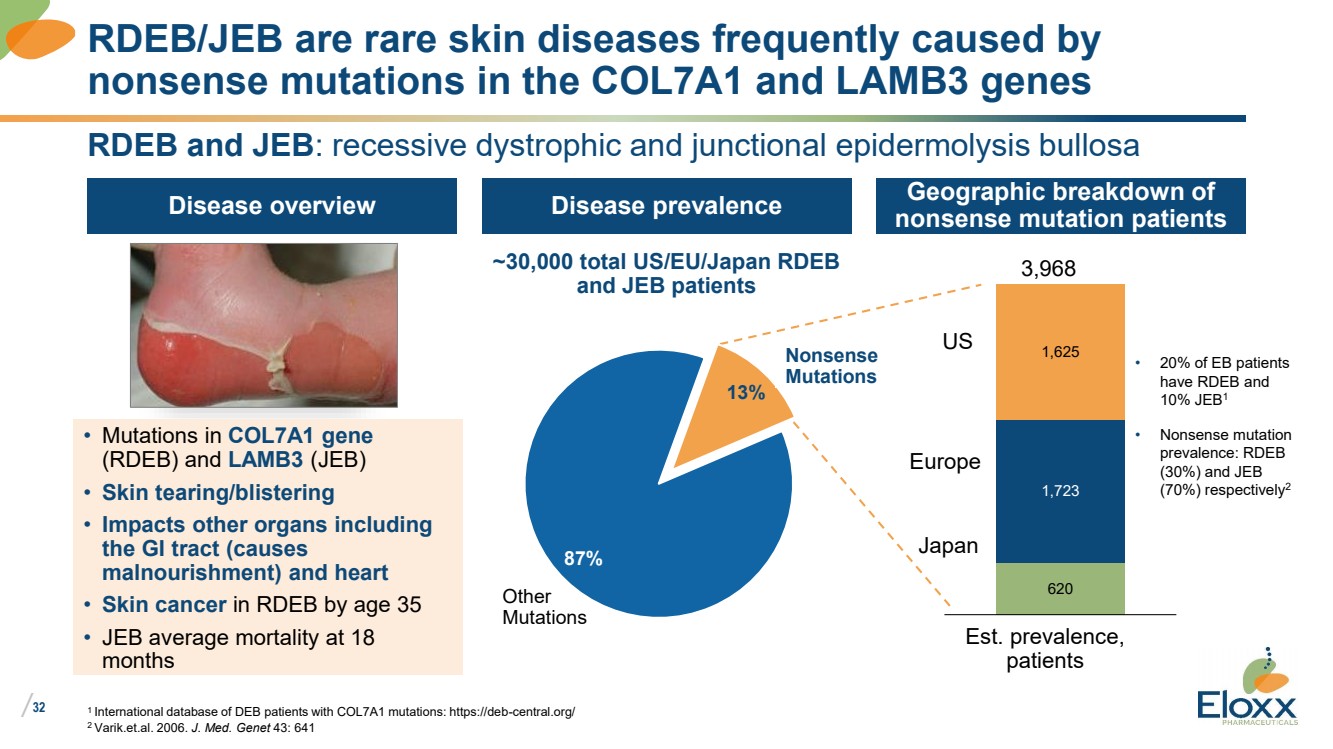
| /
32
RDEB and JEB: recessive dystrophic and junctional epidermolysis bullosa
Disease overview
1 International database of DEB patients with COL7A1 mutations: https://deb-central.org/
2 Varik.et.al. 2006. J. Med. Genet 43: 641
RDEB/JEB are rare skin diseases frequently caused by
nonsense mutations in the COL7A1 and LAMB3 genes
Disease prevalence Geographic breakdown of
nonsense mutation patients
• Mutations in COL7A1 gene
(RDEB) and LAMB3 (JEB)
• Skin tearing/blistering
• Impacts other organs including
the GI tract (causes
malnourishment) and heart
• Skin cancer in RDEB by age 35
• JEB average mortality at 18
months
13%
87%
Other
Mutations
Nonsense
Mutations
~30,000 total US/EU/Japan RDEB
and JEB patients
620
1,723
1,625
Japan
Est. prevalence,
patients
US
Europe
3,968
• 20% of EB patients
have RDEB and
10% JEB1
• Nonsense mutation
prevalence: RDEB
(30%) and JEB
(70%) respectively2 |
| /
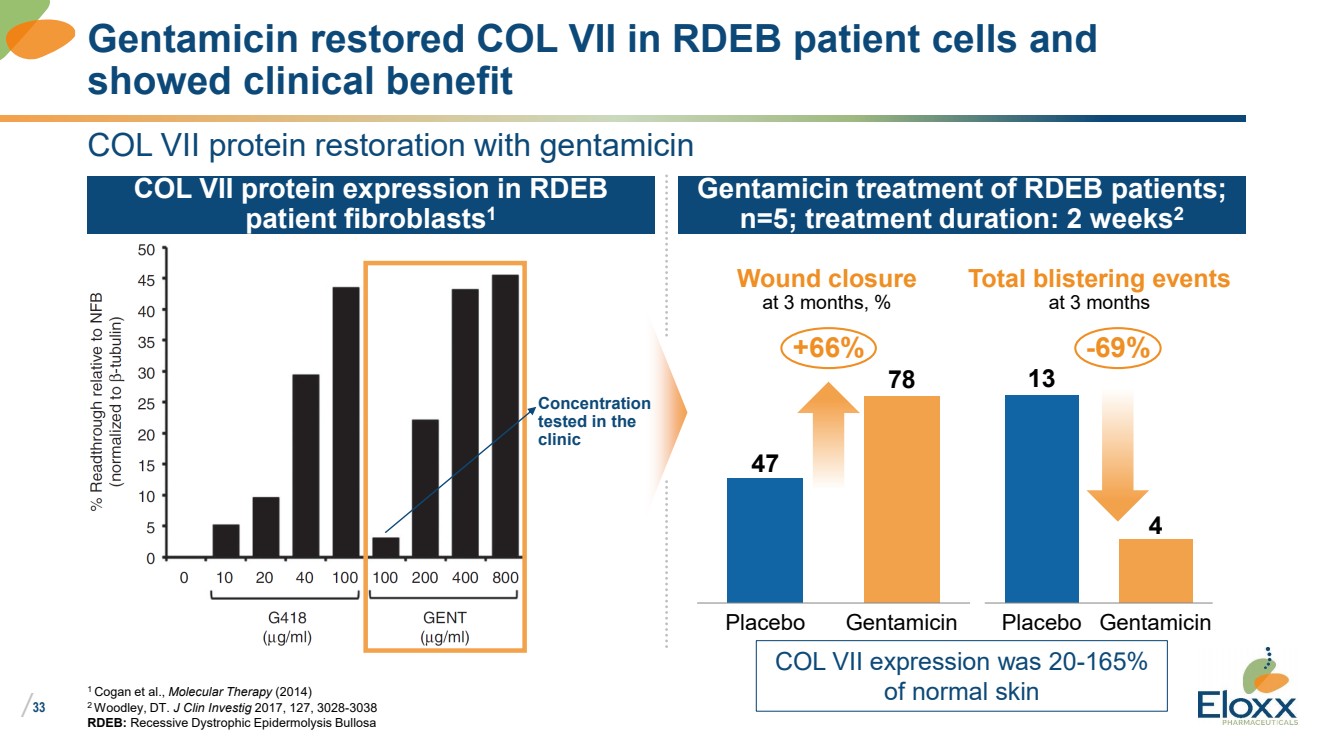
33
COL VII protein restoration with gentamicin
1 Cogan et al., Molecular Therapy (2014)
2 Woodley, DT. J Clin Investig 2017, 127, 3028-3038
RDEB: Recessive Dystrophic Epidermolysis Bullosa
Gentamicin restored COL VII in RDEB patient cells and
showed clinical benefit
Total blistering events
at 3 months
13
4
Placebo Gentamicin
47
78
Placebo Gentamicin
Gentamicin treatment of RDEB patients;
n=5; treatment duration: 2 weeks2
COL VII protein expression in RDEB
patient fibroblasts1
+66%
Wound closure
at 3 months, %
-69%
Concentration
tested in the
clinic
COL VII expression was 20-165%
of normal skin |
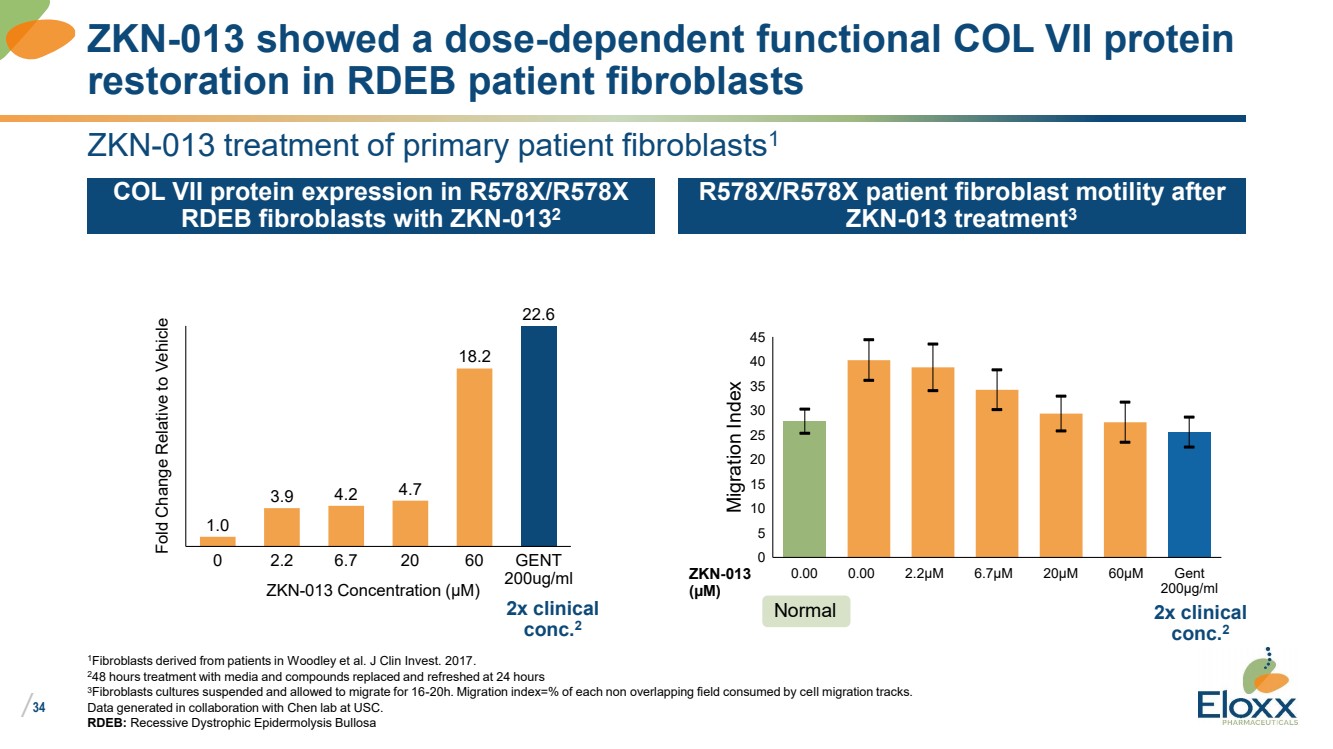
| /
34
ZKN-013 treatment of primary patient fibroblasts1
COL VII protein expression in R578X/R578X
RDEB fibroblasts with ZKN-0132
R578X/R578X patient fibroblast motility after
ZKN-013 treatment3
1Fibroblasts derived from patients in Woodley et al. J Clin Invest. 2017.
248 hours treatment with media and compounds replaced and refreshed at 24 hours
3Fibroblasts cultures suspended and allowed to migrate for 16-20h. Migration index=% of each non overlapping field consumed by cell migration tracks.
Data generated in collaboration with Chen lab at USC.
RDEB: Recessive Dystrophic Epidermolysis Bullosa
ZKN-013 showed a dose-dependent functional COL VII protein
restoration in RDEB patient fibroblasts
1.0
3.9 4.2 4.7
18.2
22.6
Fold Change Relative to Vehicle
0 2.2 6.7 20 60 GENT
200ug/ml ZKN-013 Concentration (µM)
30
0
5
25
10
15
35
20
40
45
0.00
Migration Index
0.00 2.2µM 6.7µM 20µM 60µM Gent
200µg/ml
Normal
ZKN-013
(µM)
2x clinical
conc.2
2x clinical
conc.2 |
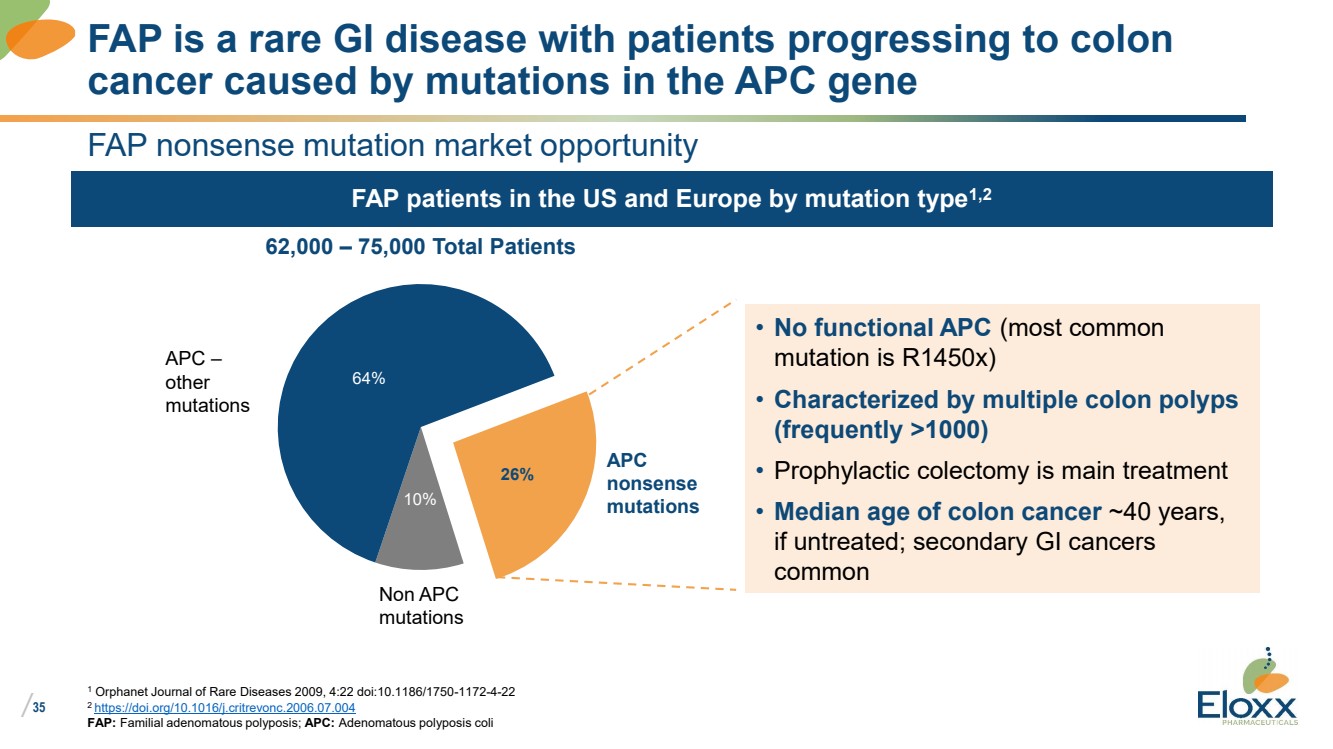
| /
35
FAP patients in the US and Europe by mutation type1,2
FAP nonsense mutation market opportunity
1 Orphanet Journal of Rare Diseases 2009, 4:22 doi:10.1186/1750-1172-4-22
2 https://doi.org/10.1016/j.critrevonc.2006.07.004
FAP: Familial adenomatous polyposis; APC: Adenomatous polyposis coli
FAP is a rare GI disease with patients progressing to colon
cancer caused by mutations in the APC gene
• No functional APC (most common
mutation is R1450x)
• Characterized by multiple colon polyps
(frequently >1000)
• Prophylactic colectomy is main treatment
• Median age of colon cancer ~40 years,
if untreated; secondary GI cancers
common
62,000 – 75,000 Total Patients
26%
10%
64%
APC
nonsense
mutations
APC –
other
mutations
Non APC
mutations |
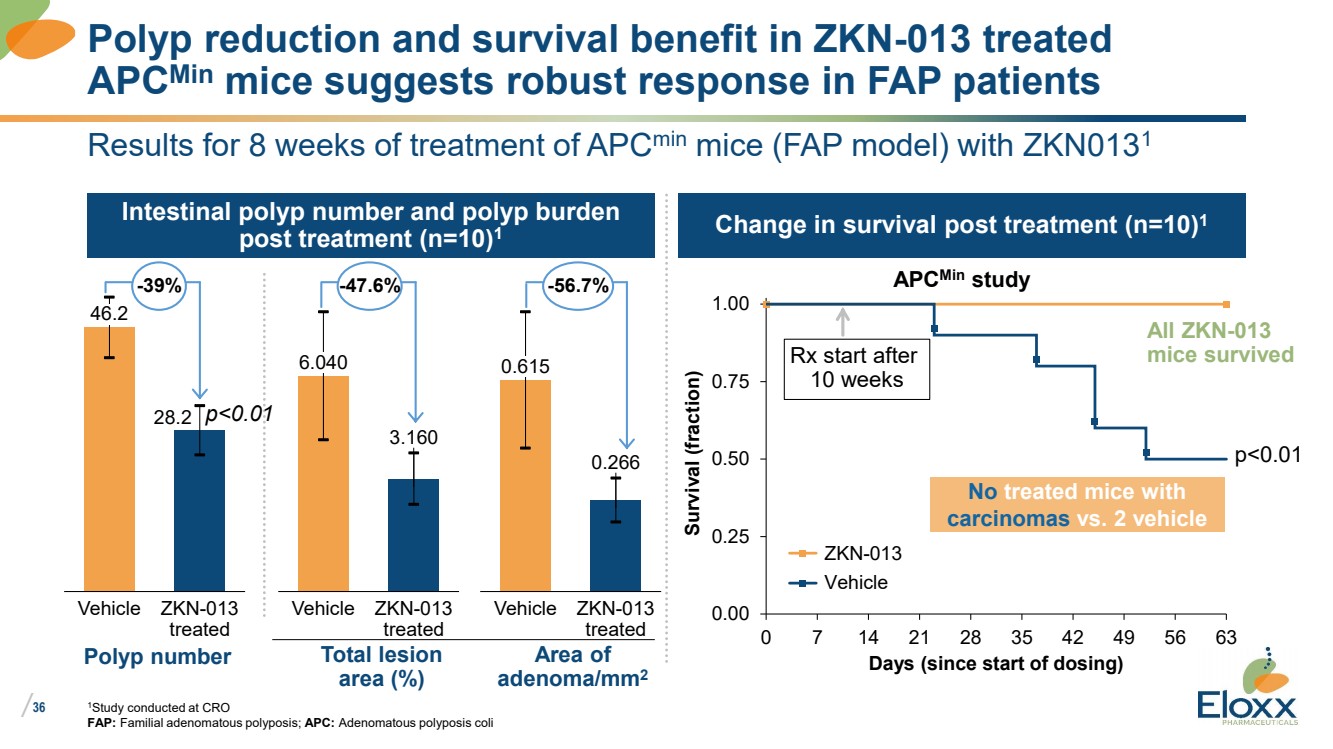
| /
36
Results for 8 weeks of treatment of APCmin mice (FAP model) with ZKN0131
1Study conducted at CRO
FAP: Familial adenomatous polyposis; APC: Adenomatous polyposis coli
Change in survival post treatment (n=10)1
Intestinal polyp number and polyp burden
post treatment (n=10)1
0.00
0.25
0.50
0.75
1.00
0 7 14 21 28 35 42 49 56 63
Survival (fraction)
Days (since start of dosing)
ZKN-013
Vehicle
APCMin study
p<0.01
Rx start after
10 weeks
All ZKN-013
mice survived
Polyp reduction and survival benefit in ZKN-013 treated
APCMin mice suggests robust response in FAP patients
No treated mice with
carcinomas vs. 2 vehicle
Area of
adenoma/mm2
Polyp number
0.266
Vehicle ZKN-013
treated
0.615
28.2
Vehicle
46.2
ZKN-013
treated
-39% -56.7%
3.160
Vehicle ZKN-013
treated
6.040
Total lesion
area (%)
-47.6%
p<0.01 |
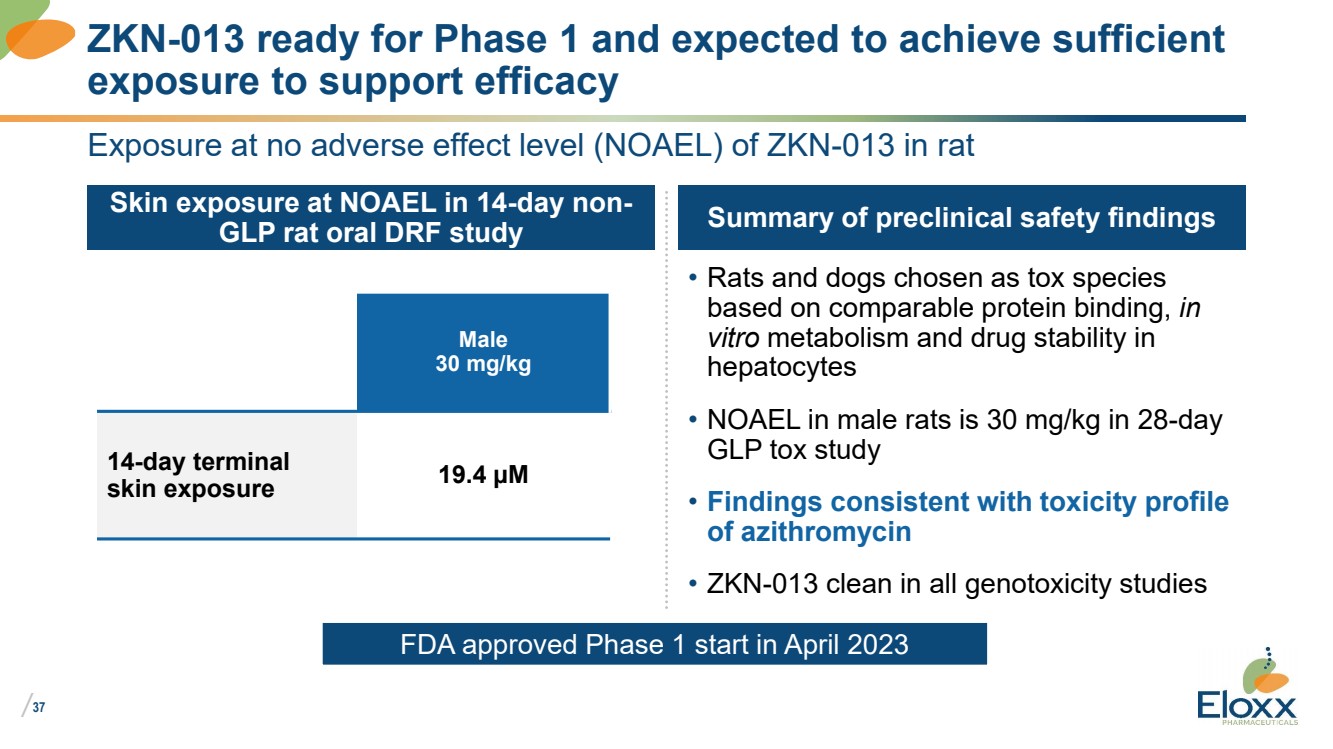
| /
37
Exposure at no adverse effect level (NOAEL) of ZKN-013 in rat
Skin exposure at NOAEL in 14-day non-GLP rat oral DRF study Summary of preclinical safety findings
• Rats and dogs chosen as tox species
based on comparable protein binding, in
vitro metabolism and drug stability in
hepatocytes
• NOAEL in male rats is 30 mg/kg in 28-day
GLP tox study
• Findings consistent with toxicity profile
of azithromycin
• ZKN-013 clean in all genotoxicity studies
ZKN-013 ready for Phase 1 and expected to achieve sufficient
exposure to support efficacy
Male
30 mg/kg
14-day terminal
skin exposure 19.4 µM
FDA approved Phase 1 start in April 2023 |
| /
38
Value creation
potential |
| /
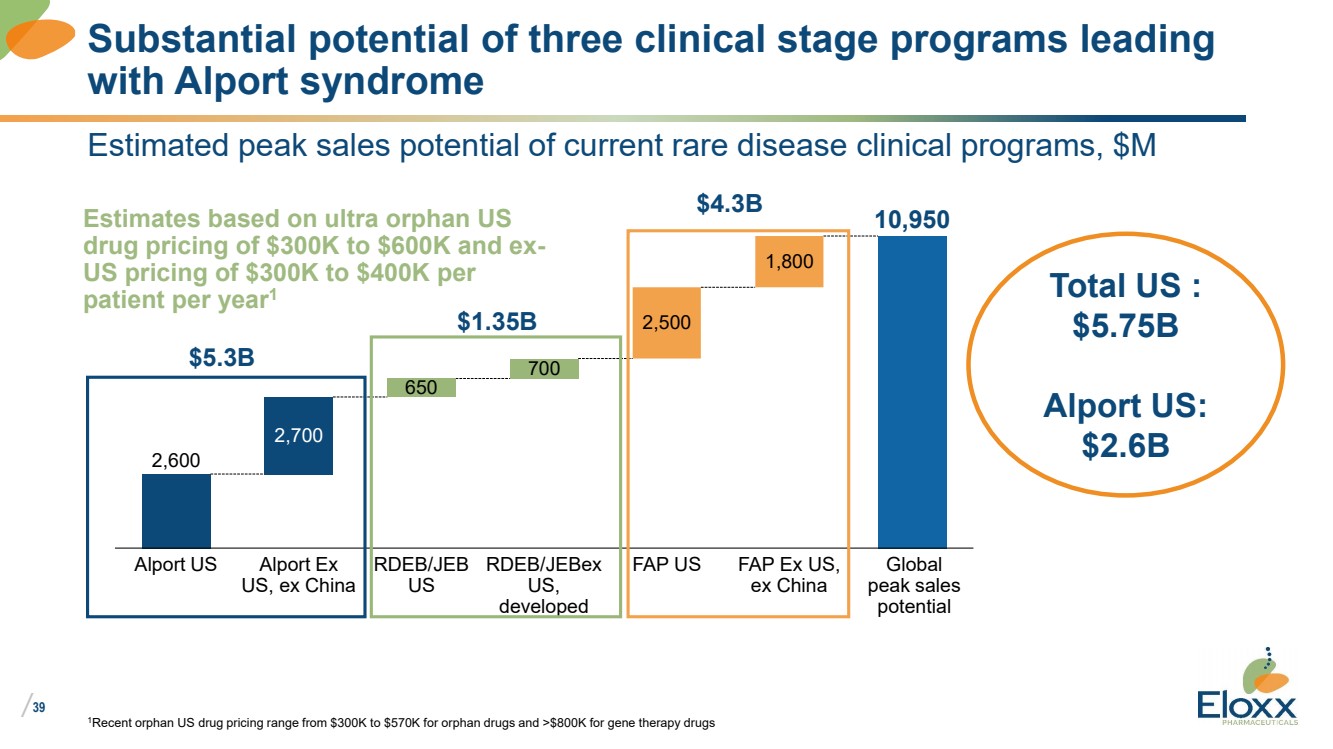
39
Estimated peak sales potential of current rare disease clinical programs, $M
1Recent orphan US drug pricing range from $300K to $570K for orphan drugs and >$800K for gene therapy drugs
Substantial potential of three clinical stage programs leading
with Alport syndrome
2,600
10,950
2,700
650
700
2,500
1,800
Alport US Alport Ex
US, ex China
RDEB/JEB
US
RDEB/JEBex
US,
developed
FAP US FAP Ex US,
ex China
Global
peak sales
potential
Estimates based on ultra orphan US
drug pricing of $300K to $600K and ex-US pricing of $300K to $400K per
patient per year1 Total US :
$5.75B
Alport US:
$2.6B
$5.3B
$1.35B
$4.3B |
| /
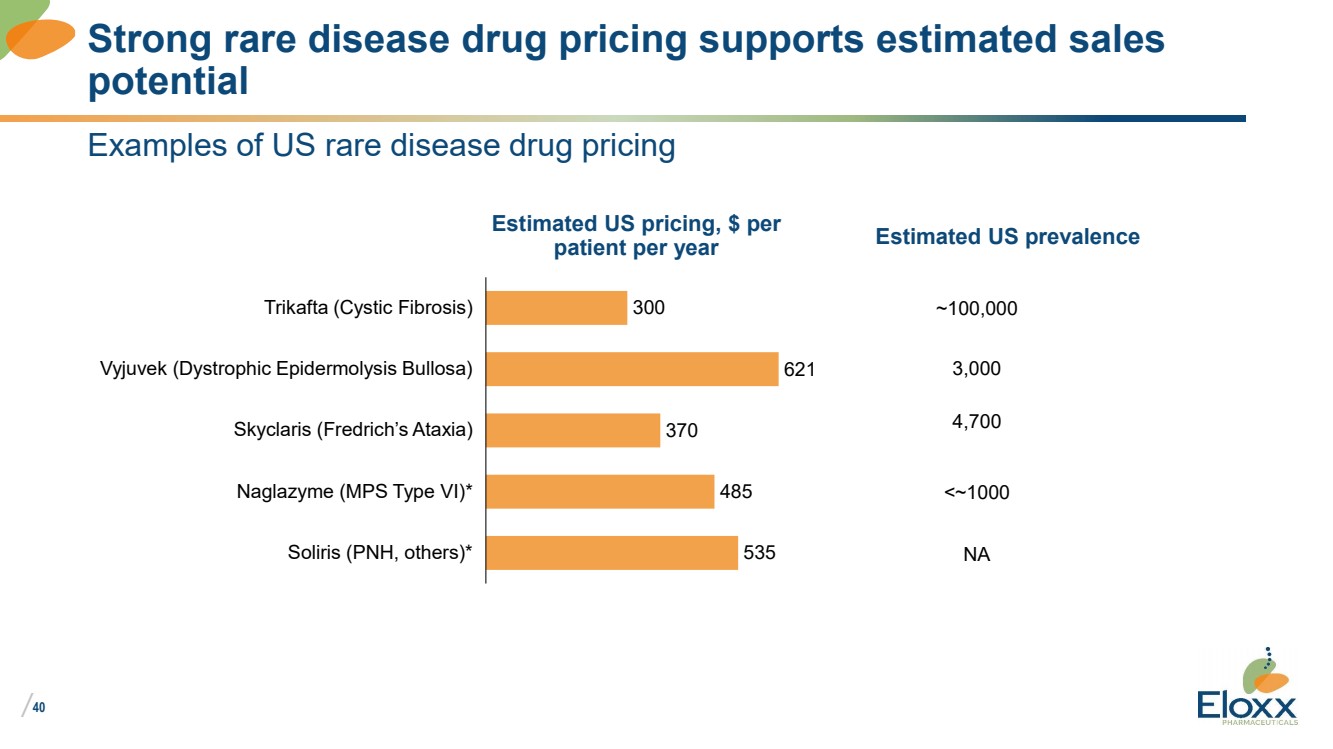
40
Examples of US rare disease drug pricing
Strong rare disease drug pricing supports estimated sales
potential
300
621
370
485
535
Trikafta (Cystic Fibrosis)
Vyjuvek (Dystrophic Epidermolysis Bullosa)
Skyclaris (Fredrich’s Ataxia)
Naglazyme (MPS Type VI)*
Soliris (PNH, others)*
Estimated US pricing, $ per
patient per year
~100,000
Estimated US prevalence
4,700
3,000
<~1000
NA |
| /
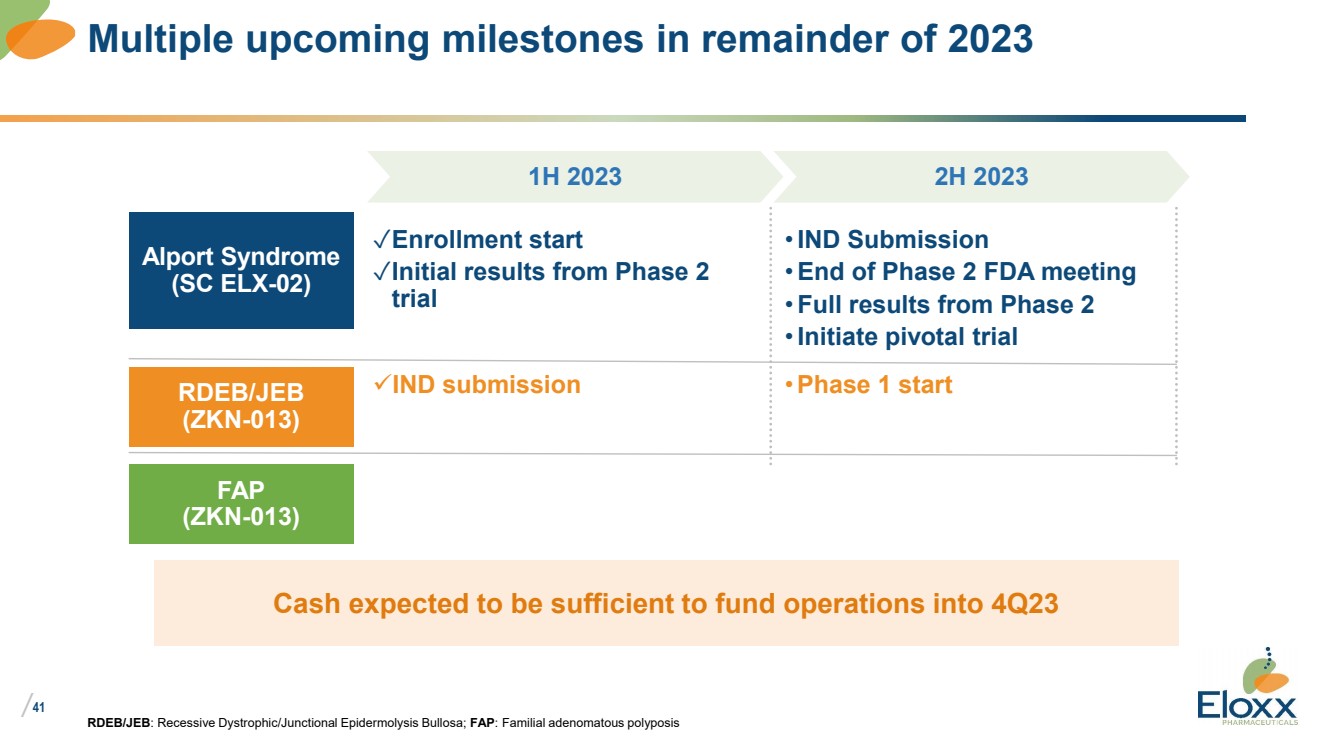
41
•IND Submission
•End of Phase 2 FDA meeting
• Full results from Phase 2
•Initiate pivotal trial
RDEB/JEB: Recessive Dystrophic/Junctional Epidermolysis Bullosa; FAP: Familial adenomatous polyposis
Multiple upcoming milestones in remainder of 2023
1H 2023 2H 2023
Alport Syndrome
(SC ELX-02)
✓Enrollment start
✓Initial results from Phase 2
trial
RDEB/JEB
(ZKN-013)
✓IND submission •Phase 1 start
FAP
(ZKN-013)
Cash expected to be sufficient to fund operations into 4Q23 |
| /
42
Clinical stage small molecule gene therapy biopharma poised
for value creation
Small molecule genetic therapies for nonsense mutations proven to
restore full-length proteins
ELX-02: Ready for Alport
Syndrome pivotal study with
biopsy confirmed disease
regression. Preclinical POC in
ADPKD*
ZKN-013: Oral agent ready
for Phase 1 start; robust
preclinical efficacy in RDEB and
FAP. Potential in ADPKD** |

| /
43 |
| /
44
Appendix |
| /
45
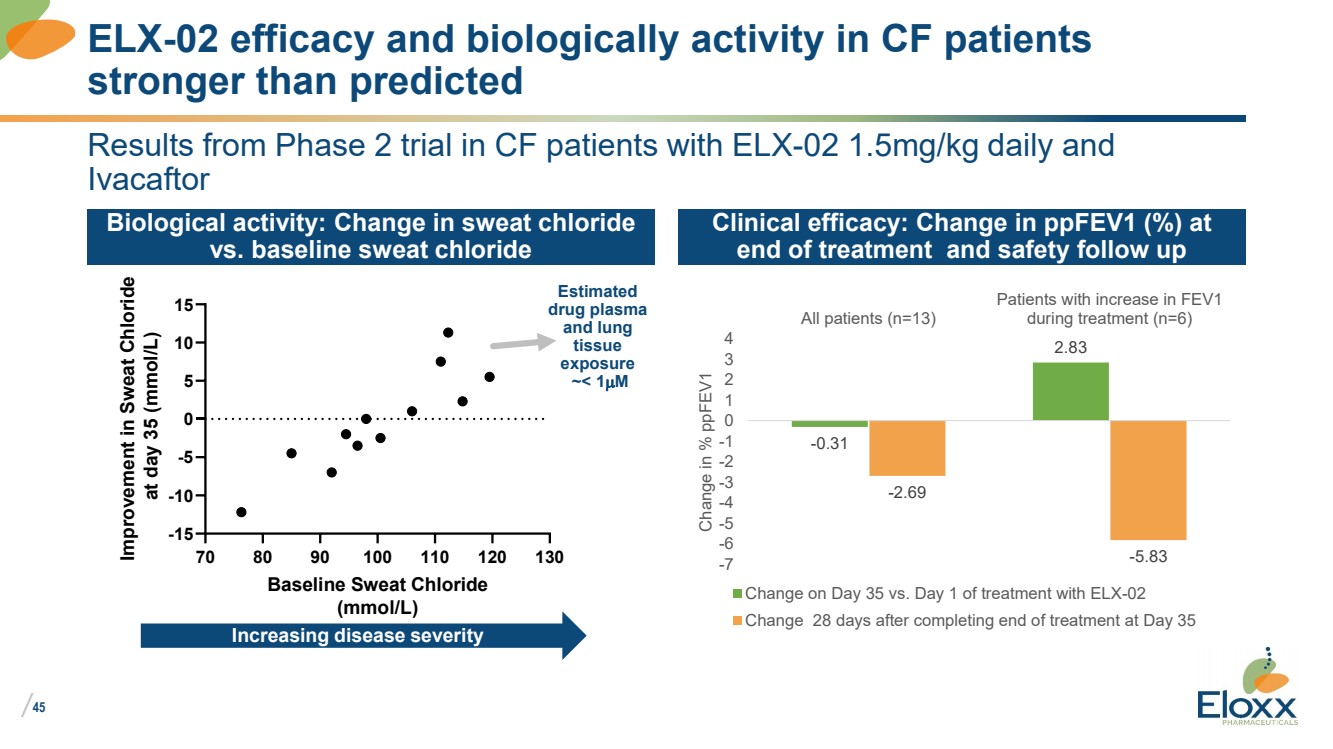
Results from Phase 2 trial in CF patients with ELX-02 1.5mg/kg daily and
Ivacaftor
Biological activity: Change in sweat chloride
vs. baseline sweat chloride
Clinical efficacy: Change in ppFEV1 (%) at
end of treatment and safety follow up
ELX-02 efficacy and biologically activity in CF patients
stronger than predicted
70 80 90 100 110 120 130
-15
-10
-5
0
5
10
15
Baseline Sweat Chloride
(mmol/L)
Improvement in Sweat Chloride
at day 35 (mmol/L)
-0.31
2.83
-2.69
-5.83 -7
-6
-5
-4
-3
-2
-1
0
1
2
3
4
All patients (n=13)
Patients with increase in FEV1
during treatment (n=6)
Change in % ppFEV1
Change on Day 35 vs. Day 1 of treatment with ELX-02
Change 28 days after completing end of treatment at Day 35
Estimated
drug plasma
and lung
tissue
exposure
~< 1mM
Increasing disease severity |
| /
46
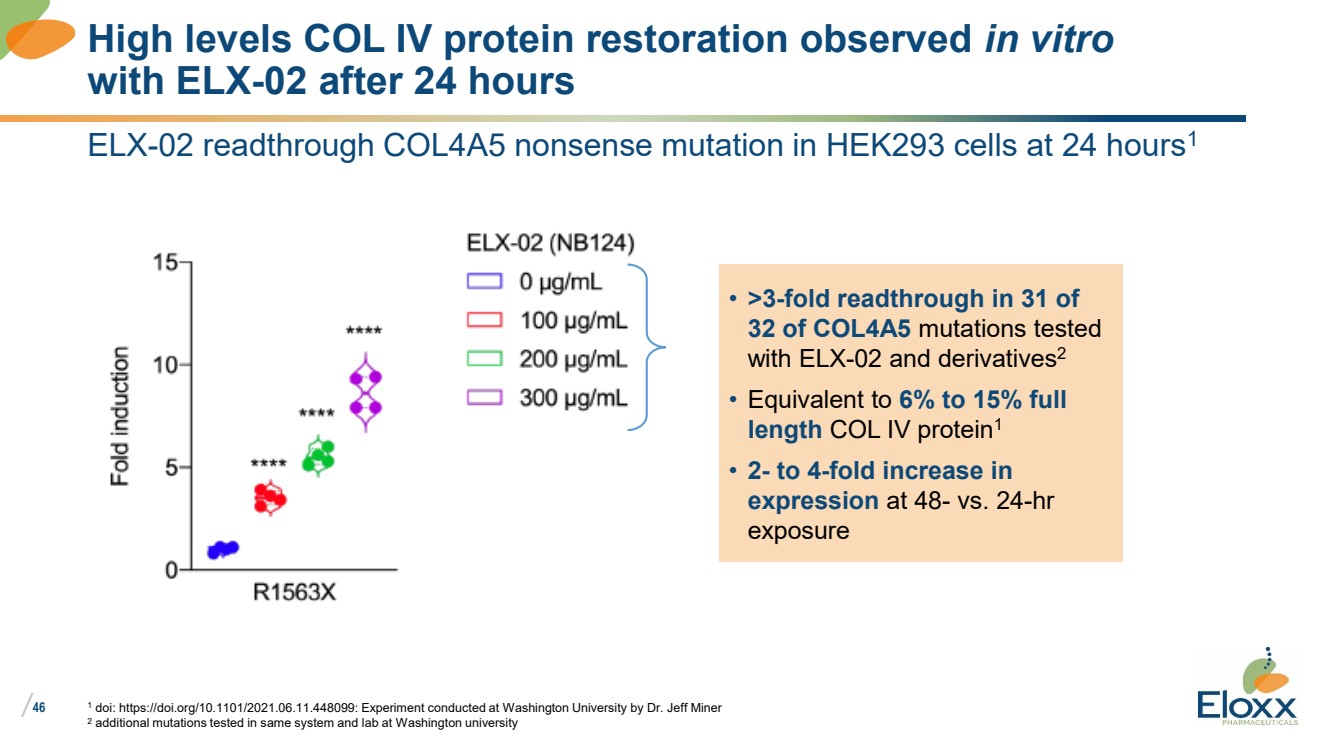
ELX-02 readthrough COL4A5 nonsense mutation in HEK293 cells at 24 hours1
1 doi: https://doi.org/10.1101/2021.06.11.448099: Experiment conducted at Washington University by Dr. Jeff Miner
2 additional mutations tested in same system and lab at Washington university
High levels COL IV protein restoration observed in vitro
with ELX-02 after 24 hours
• >3-fold readthrough in 31 of
32 of COL4A5 mutations tested
with ELX-02 and derivatives2
• Equivalent to 6% to 15% full
length COL IV protein1
• 2- to 4-fold increase in
expression at 48- vs. 24-hr
exposure |
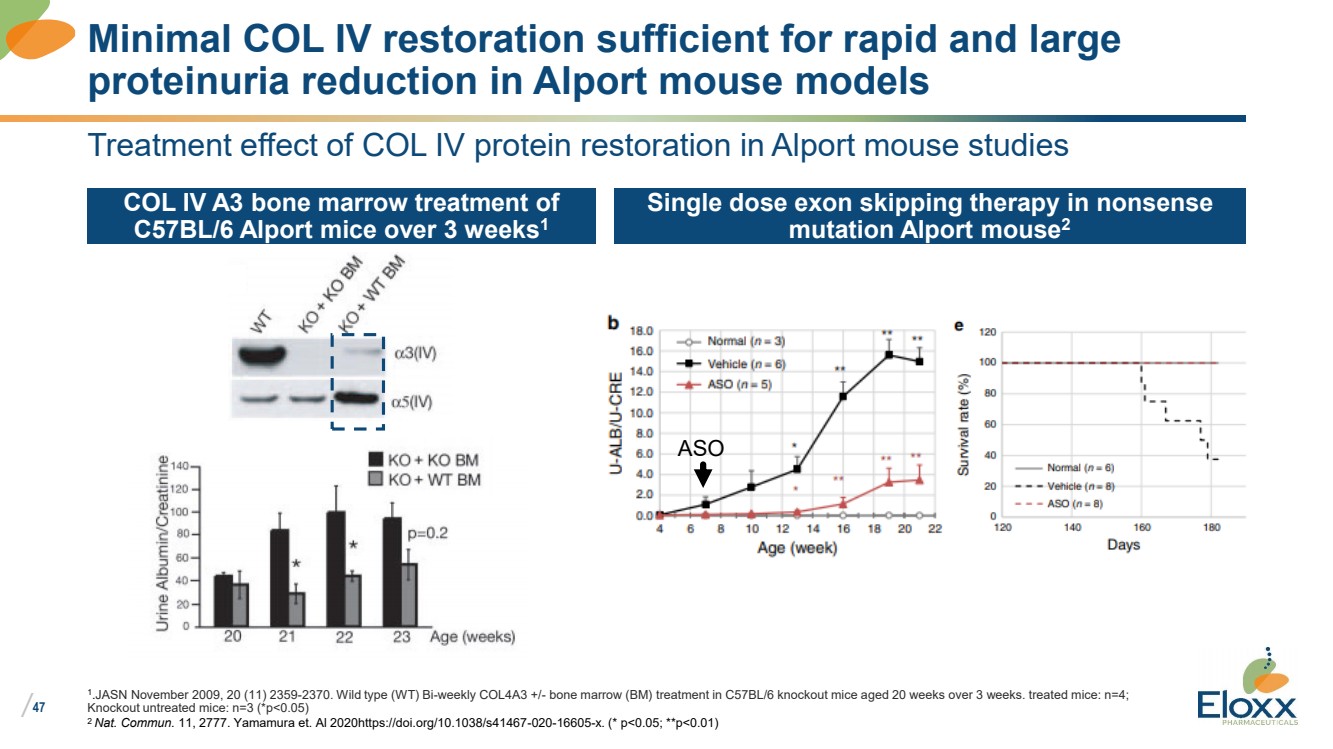
| /
47
Treatment effect of COL IV protein restoration in Alport mouse studies
COL IV A3 bone marrow treatment of
C57BL/6 Alport mice over 3 weeks1
Single dose exon skipping therapy in nonsense
mutation Alport mouse2
1
..JASN November 2009, 20 (11) 2359-2370. Wild type (WT) Bi-weekly COL4A3 +/- bone marrow (BM) treatment in C57BL/6 knockout mice aged 20 weeks over 3 weeks. treated mice: n=4;
Knockout untreated mice: n=3 (*p<0.05)
2 Nat. Commun. 11, 2777. Yamamura et. Al 2020https://doi.org/10.1038/s41467-020-16605-x. (* p<0.05; **p<0.01)
Minimal COL IV restoration sufficient for rapid and large
proteinuria reduction in Alport mouse models
ASO |
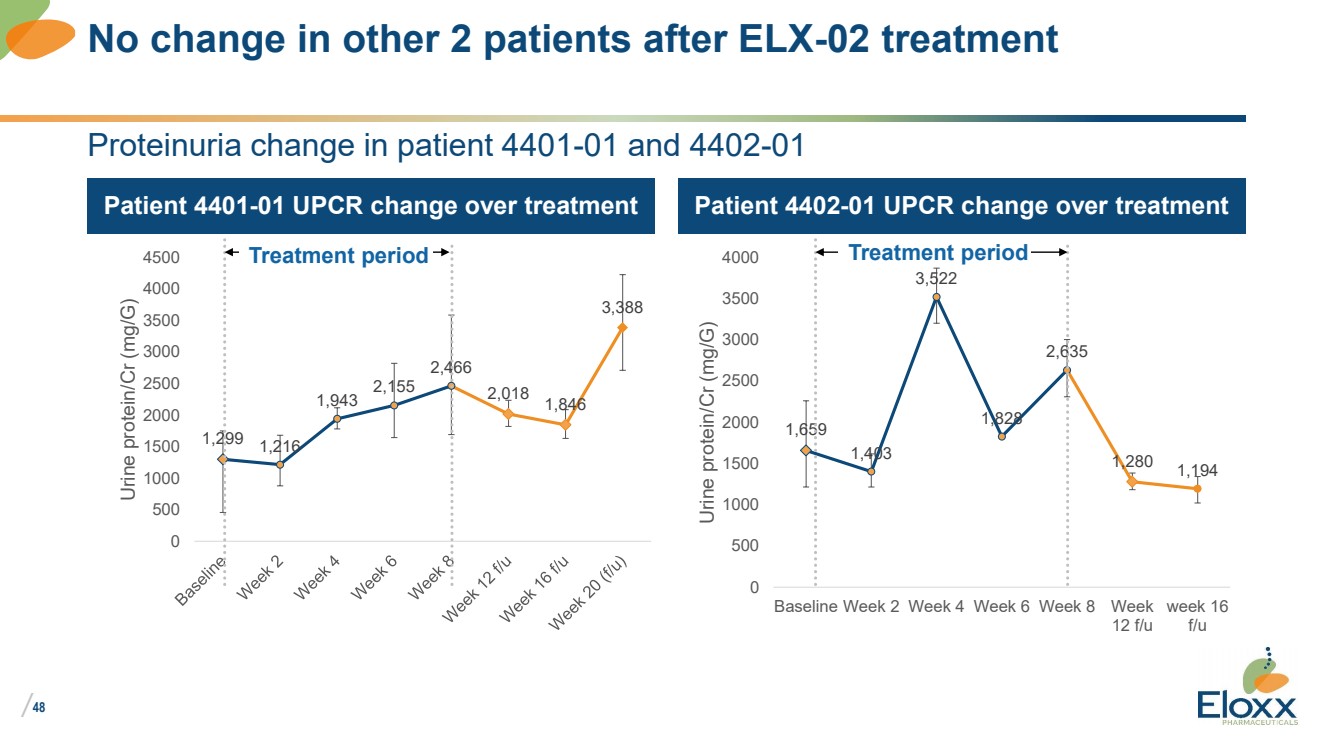
| /
48
Proteinuria change in patient 4401-01 and 4402-01
Patient 4401-01 UPCR change over treatment Patient 4402-01 UPCR change over treatment
1,659
1,403
3,522
1,828
2,635
1,280 1,194
0
500
1000
1500
2000
2500
3000
3500
4000
Baseline Week 2 Week 4 Week 6 Week 8 Week
12 f/u
week 16
f/u
Urine protein/Cr (mg/G)
No change in other 2 patients after ELX-02 treatment
1,299 1,216
1,943
2,155
2,466
2,018 1,846
3,388
0
500
1000
1500
2000
2500
3000
3500
4000
4500
Urine protein/Cr (mg/G)
Treatment period Treatment period |
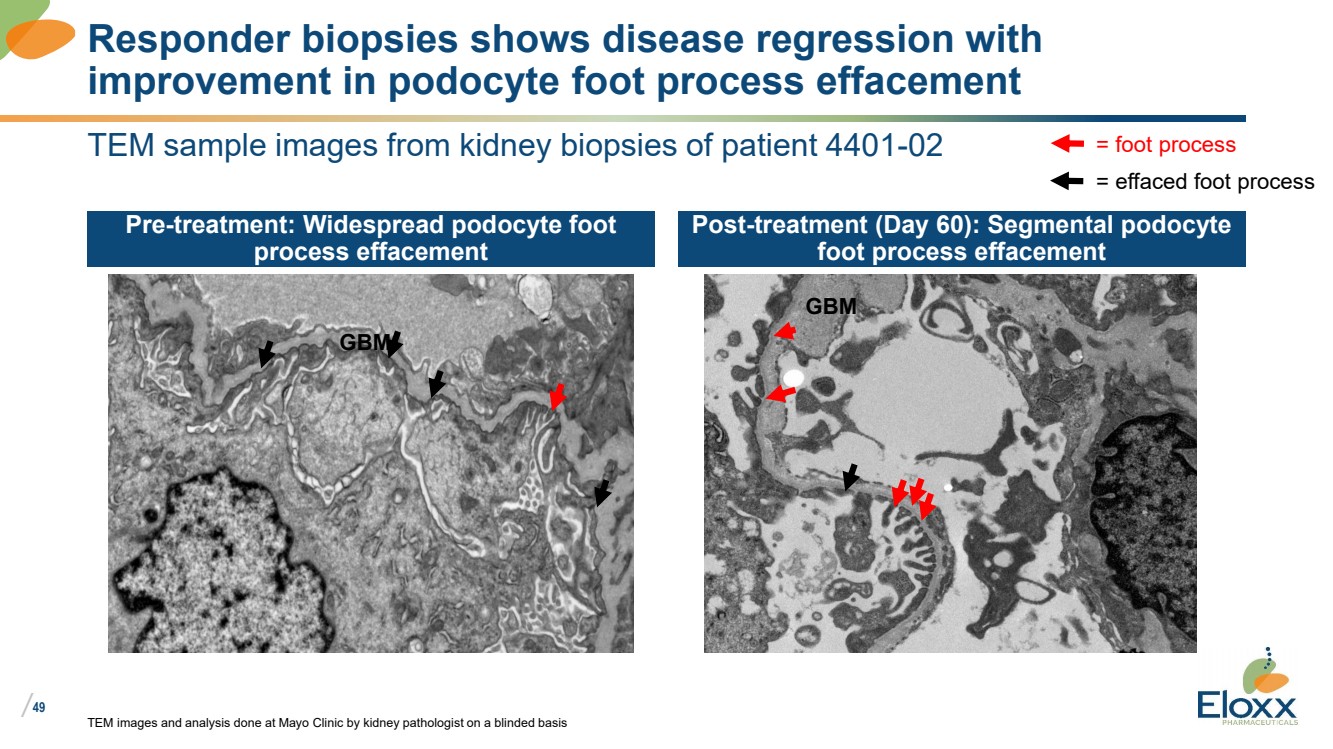
| /
49
TEM sample images from kidney biopsies of patient 4401-02
Pre-treatment: Widespread podocyte foot
process effacement
Post-treatment (Day 60): Segmental podocyte
foot process effacement
TEM images and analysis done at Mayo Clinic by kidney pathologist on a blinded basis
Responder biopsies shows disease regression with
improvement in podocyte foot process effacement
= foot process
= effaced foot process
GBM
GBM |
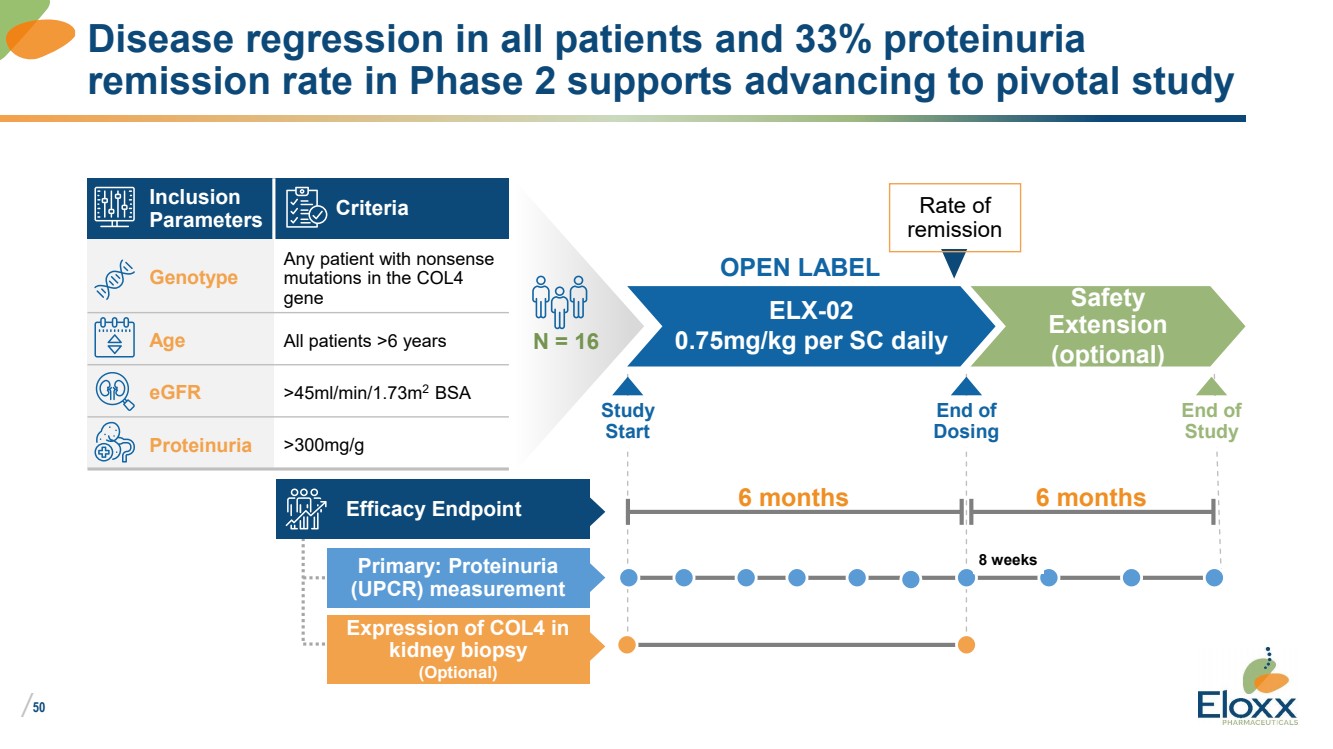
| /
50
Disease regression in all patients and 33% proteinuria
remission rate in Phase 2 supports advancing to pivotal study
Inclusion
Parameters Criteria
Genotype
Any patient with nonsense
mutations in the COL4
gene
Age All patients >6 years
eGFR >45ml/min/1.73m2 BSA
Proteinuria >300mg/g
N = 16
ELX-02
0.75mg/kg per SC daily
Safety
Extension
(optional)
OPEN LABEL
End of
Study
Efficacy Endpoint
Primary: Proteinuria
(UPCR) measurement
Expression of COL4 in
kidney biopsy
(Optional)
6 months
Study
Start
End of
Dosing
6 months
8 weeks
Rate of
remission |
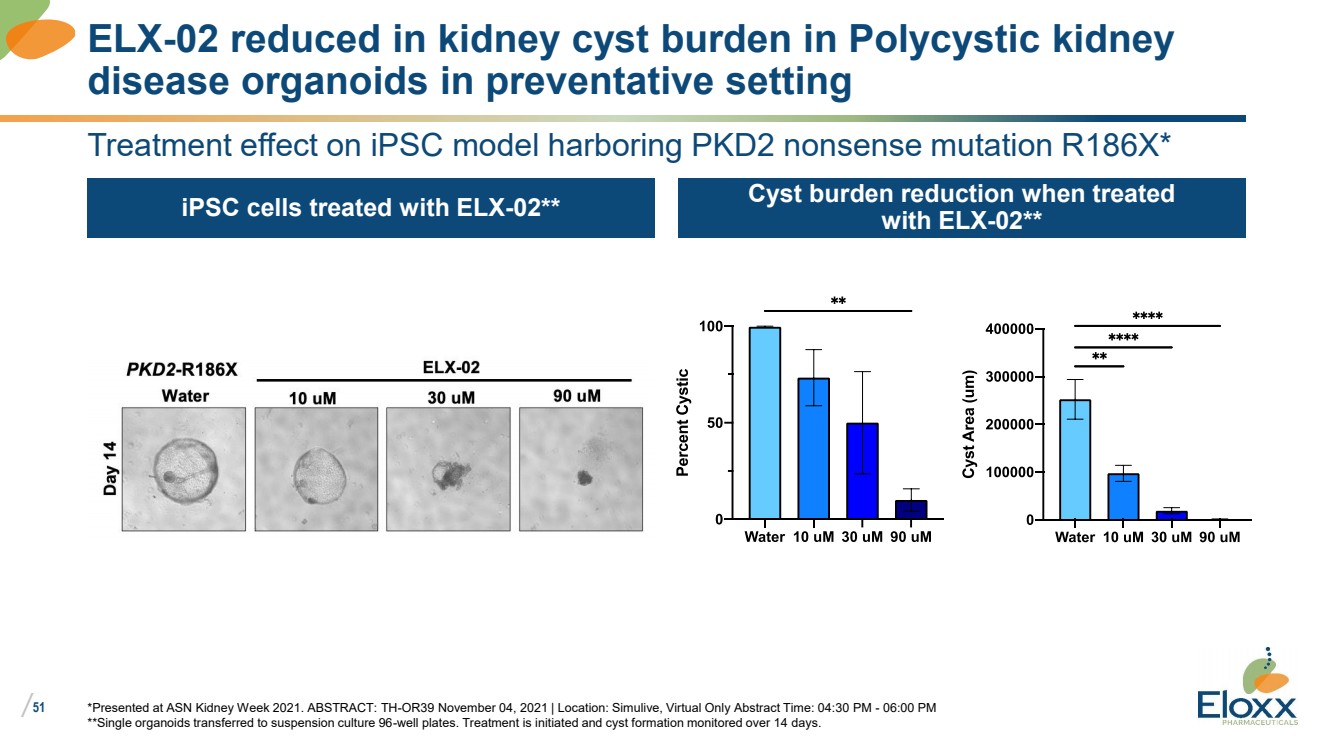
| /
51
Treatment effect on iPSC model harboring PKD2 nonsense mutation R186X*
iPSC cells treated with ELX-02** Cyst burden reduction when treated
with ELX-02**
*Presented at ASN Kidney Week 2021. ABSTRACT: TH-OR39 November 04, 2021 | Location: Simulive, Virtual Only Abstract Time: 04:30 PM - 06:00 PM
**Single organoids transferred to suspension culture 96-well plates. Treatment is initiated and cyst formation monitored over 14 days.
ELX-02 reduced in kidney cyst burden in Polycystic kidney
disease organoids in preventative setting |
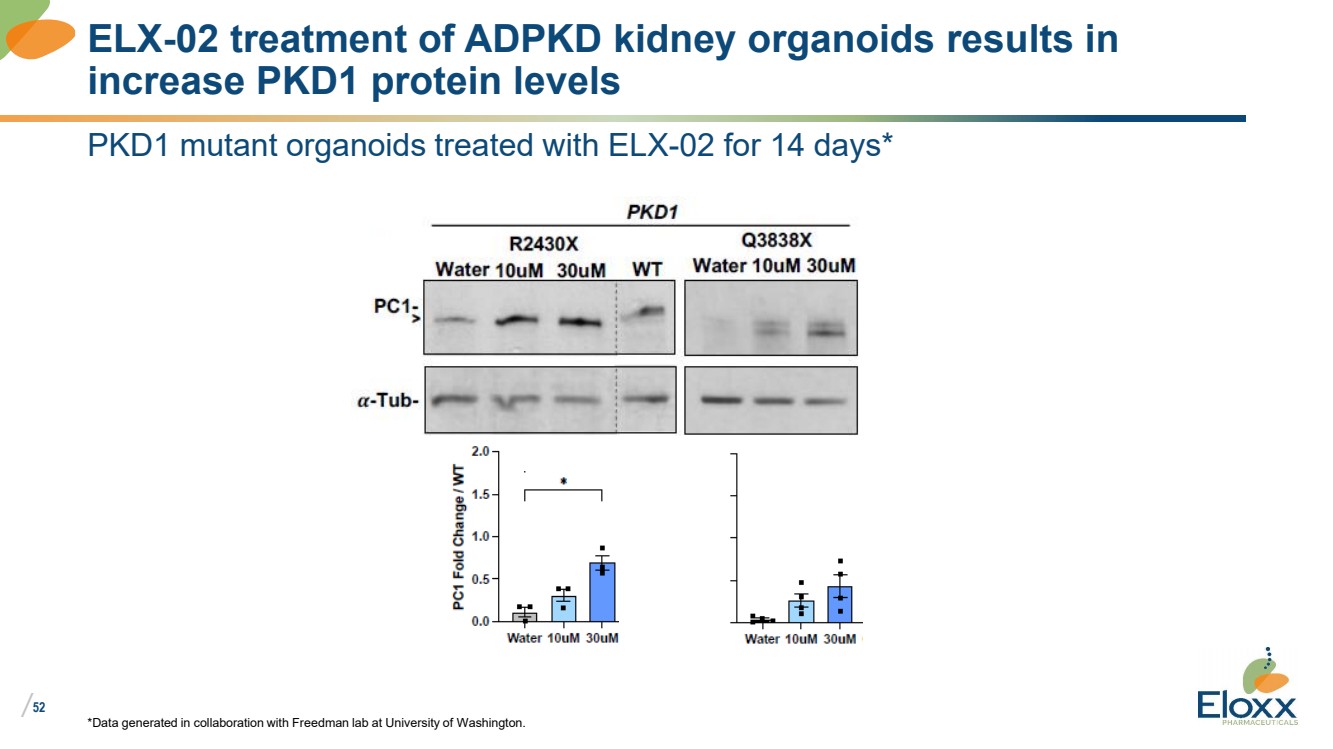
| /
52
PKD1 mutant organoids treated with ELX-02 for 14 days*
*Data generated in collaboration with Freedman lab at University of Washington.
ELX-02 treatment of ADPKD kidney organoids results in
increase PKD1 protein levels |
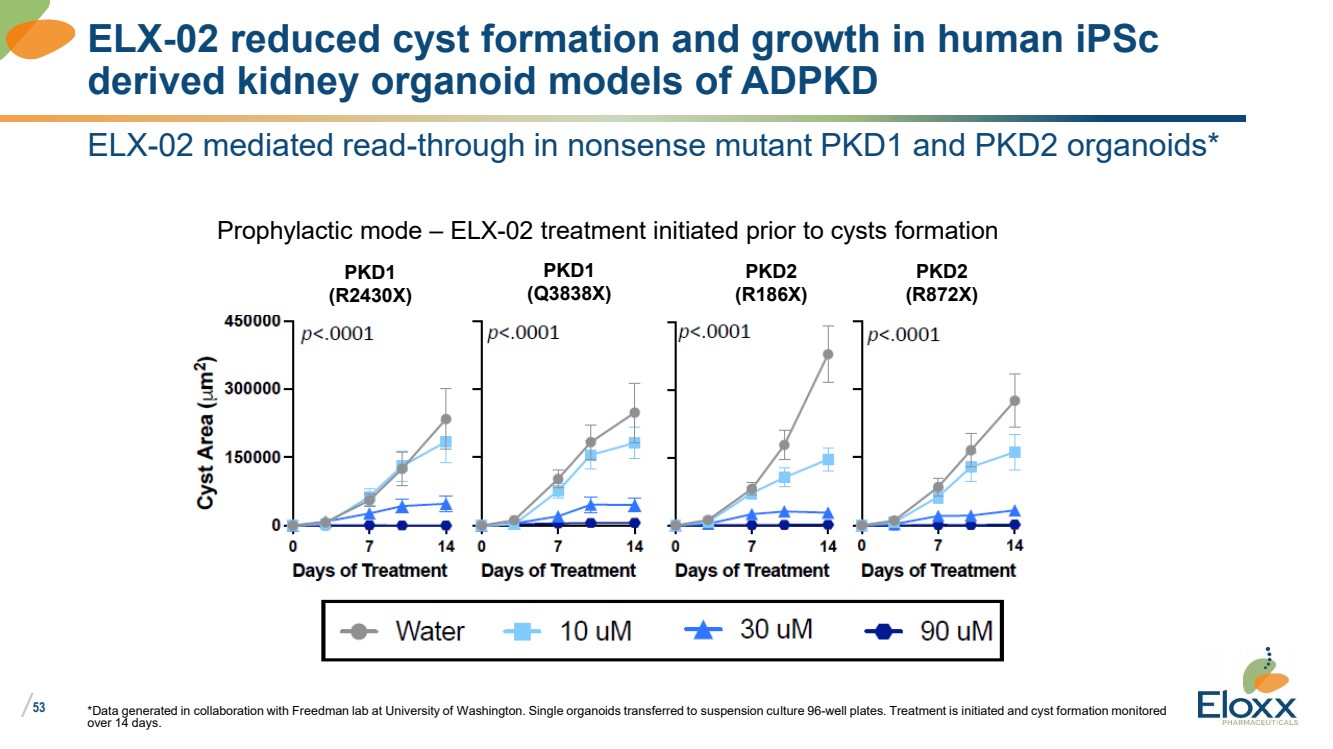
| /
53
ELX-02 mediated read-through in nonsense mutant PKD1 and PKD2 organoids*
*Data generated in collaboration with Freedman lab at University of Washington. Single organoids transferred to suspension culture 96-well plates. Treatment is initiated and cyst formation monitored
over 14 days.
ELX-02 reduced cyst formation and growth in human iPSc
derived kidney organoid models of ADPKD
PKD1
(R2430X)
PKD1
(Q3838X)
PKD2
(R186X)
PKD2
(R872X)
Prophylactic mode – ELX-02 treatment initiated prior to cysts formation |
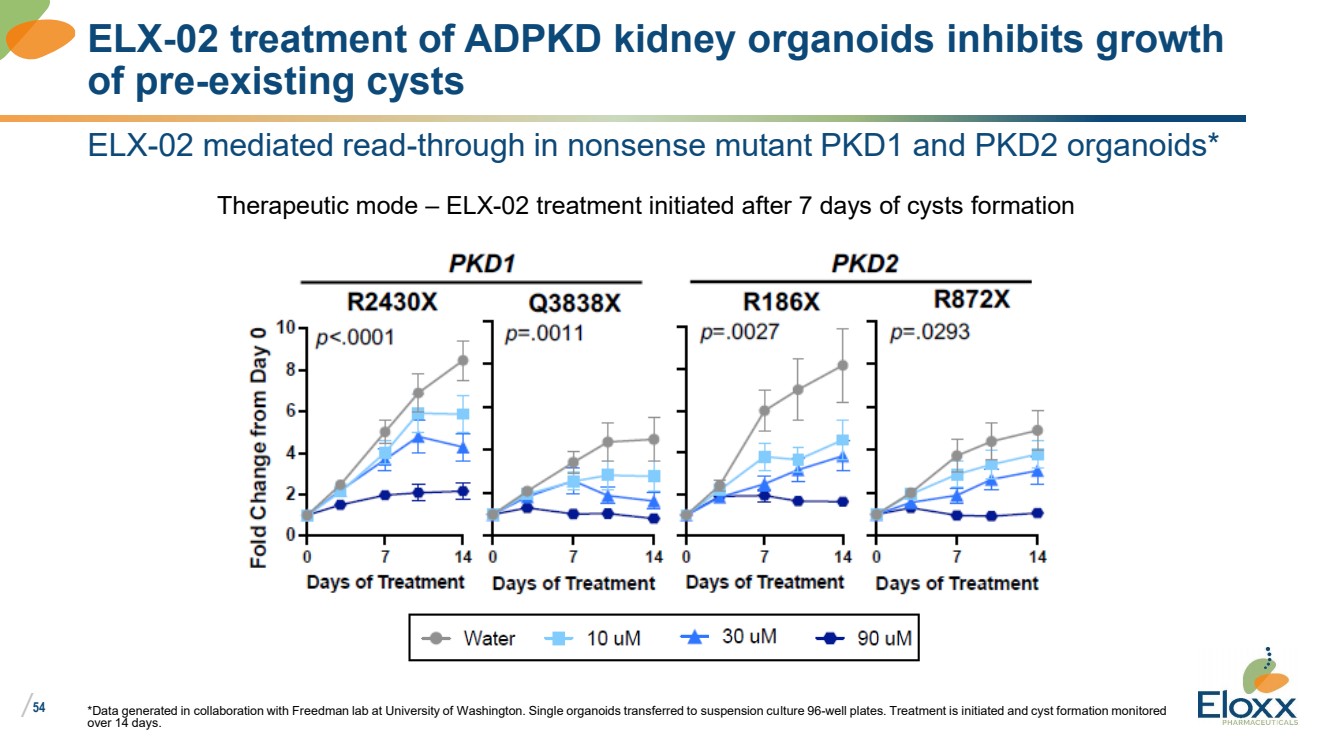
| /
54
ELX-02 mediated read-through in nonsense mutant PKD1 and PKD2 organoids*
*Data generated in collaboration with Freedman lab at University of Washington. Single organoids transferred to suspension culture 96-well plates. Treatment is initiated and cyst formation monitored
over 14 days.
ELX-02 treatment of ADPKD kidney organoids inhibits growth
of pre-existing cysts
Therapeutic mode – ELX-02 treatment initiated after 7 days of cysts formation |
Cover
|
Aug. 21, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Aug. 21, 2023
|
| Entity File Number |
001-31326
|
| Entity Registrant Name |
Eloxx Pharmaceuticals, Inc.
|
| Entity Central Index Key |
0001035354
|
| Entity Tax Identification Number |
84-1368850
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
480
Arsenal Way, Suite 130
|
| Entity Address, City or Town |
Watertown
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02451
|
| City Area Code |
781
|
| Local Phone Number |
577-5300
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.01 par value per share
|
| Trading Symbol |
ELOX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Eloxx Pharmaceuticals (NASDAQ:ELOX)
과거 데이터 주식 차트
부터 3월(3) 2025 으로 4월(4) 2025

Eloxx Pharmaceuticals (NASDAQ:ELOX)
과거 데이터 주식 차트
부터 4월(4) 2024 으로 4월(4) 2025
