false
0000715446
0000715446
2024-11-08
2024-11-08
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or Section 15(d) of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): November 8, 2024
ANIXA
BIOSCIENCES, INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
001-37492 |
|
11-2622630 |
(State
or other jurisdiction
of
incorporation) |
|
(Commission
File
Number) |
|
(IRS
Employer
Identification
No.) |
3150
Almaden Expressway, Suite 250
San
Jose, CA |
|
95118 |
| (Address
of principal executive offices) |
|
(Zip
Code) |
Registrant’s
telephone number, including area code: (408) 708-9808
(Former
name or former address, if changed since last report)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation to the registrant under
any of the following provisions:
| ☐ | Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | |
| ☐ | Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | |
| ☐ | Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | |
| ☐ | Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common
Stock, par value $0.01 per share |
|
ANIX |
|
The
NASDAQ Stock Market LLC |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item
7.01. Regulation FD Disclosure.
On
November 8, 2024, Anixa Biosciences, Inc. (“we,” “us,” “our,” or the “Company”) issued
a press release announcing that the Company and The Cleveland Clinic Foundation (“Cleveland Clinic”) presented new, updated
positive data for the Phase 1 study of its breast cancer vaccine. The press release, which is furnished as Exhibit 99.1 hereto, was issued
following a presentation made by Dr. Emily Rhoades, FDA/IND Trial Program Manager at Cleveland Clinic. Furnished hereto as Exhibit 99.2
is the poster utilized by Dr. Rhoades for the presentation.
Statements
that are not historical fact may be considered forward-looking statements within the meaning of the Private Securities Litigation Reform
Act of 1995. Forward-looking statements are not statements of historical facts, but rather reflect our current expectations concerning
future events and results. We generally use the words “believes,” “expects,” “intends,” “plans,”
“anticipates,” “likely,” “will” and similar expressions to identify forward-looking statements. Such
forward-looking statements, including those concerning our clinical trials, involve risks, uncertainties and other factors, some of which
are beyond our control, which may cause our actual results, performance or achievements, or industry results, to be materially different
from any future results, performance, or achievements expressed or implied by such forward-looking statements. These risks, uncertainties
and factors include, but are not limited to, those factors set forth in “Item 1A - Risk Factors” and other sections of our
most recent Annual Report on Form 10-K as well as in our Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. We undertake
no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise,
except as required by law. You are cautioned not to unduly rely on such forward-looking statements when evaluating the information presented
in this Current Report.
Item
9.01. Financial Statements and Exhibits
(d)
Exhibits
The
following exhibits are filed with this Current Report on Form 8-K:
| Exhibit
No. |
|
Description |
| |
|
|
| 99.1 |
|
Press Release |
| 99.2 |
|
Presentation
|
| 104 |
|
Cover
Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
Dated:
November 8, 2024
| |
ANIXA BIOSCIENCES, INC. |
| |
|
|
| |
By: |
/s/ Michael J. Catelani |
| |
Name: |
Michael J. Catelani |
| |
Title: |
President, Chief Operating Officer and Chief Financial Officer |
Exhibit
99.1

Anixa
Biosciences and Cleveland Clinic Present New Updated Positive Data from Phase 1 Study of Breast Cancer Vaccine at the 39th Society for
Immunotherapy of Cancer (SITC) Annual Meeting
| ● Data
continues positive trend as additional patients are enrolled in 3 cohorts |
| ● Vaccine
was safe and well tolerated by participants in all 3 cohorts |
| ● Protocol
defined immune responses were exhibited in over 70% of patients |
| ● A
Phase 2 study evaluating the vaccine in the neoadjuvant setting is planned to commence in 2025 |
SAN
JOSE, Calif., November 8, 2024 — Anixa Biosciences, Inc. (“Anixa” or the “Company”) (NASDAQ: ANIX), a biotechnology
company focused on the treatment and prevention of cancer, today announced a presentation of new, updated positive data from the Phase
1 clinical trial of its breast cancer vaccine (NCT04674306) at the Society for Immunotherapy of Cancer (SITC) 39th Annual Meeting, being
held in Houston, Texas. The trial is being conducted in collaboration with Cleveland Clinic with funding by a grant from the U.S. Department
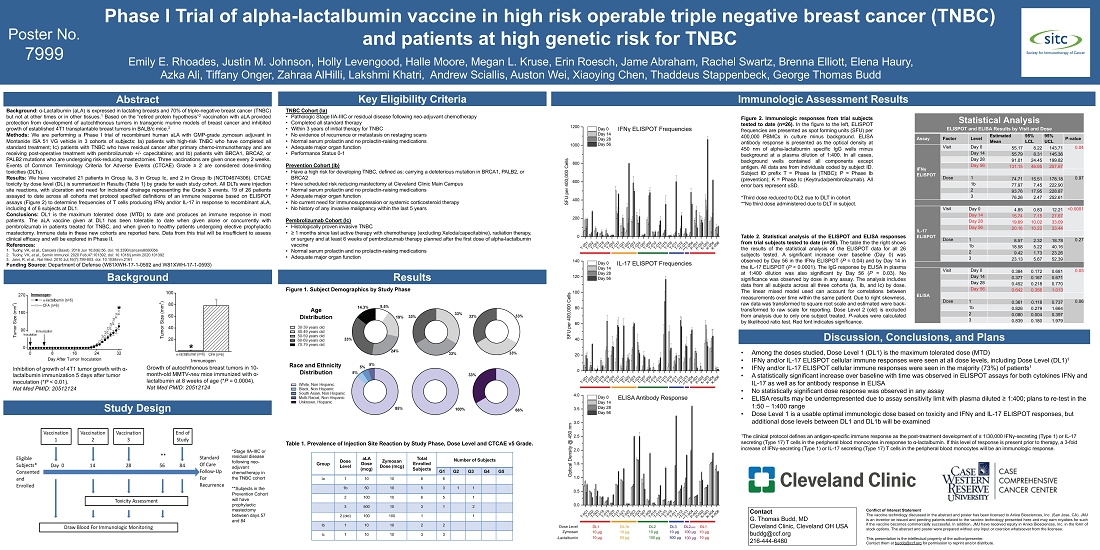
of Defense. The presentation, titled “Phase I Trial of alpha-lactalbumin vaccine in high-risk operable triple negative breast cancer
(TNBC) and patients at high genetic risk for TNBC,” was presented by Dr. Emily Rhoades, FDA/IND Trial Program Manager at Cleveland
Clinic.
“Triple
negative breast cancer is the form of the disease for which we have the least effective treatments,” said G. Thomas Budd, M.D.
of Cleveland Clinic’s Cancer Institute and principal investigator of the Phase 1 study. “Long term, we are hoping that this
can be a true preventive vaccine that would be administered to individuals who are cancer-free to prevent them from developing this highly
aggressive disease.”
“We
are pleased with the data we have observed in this clinical trial. The data continues to exceed our expectations. As we near completion
of the Phase 1 trial, with the very positive data to date, we are planning a Phase 2 study which is expected to commence in 2025,”
stated Dr. Amit Kumar, Chairman and CEO of Anixa Biosciences. “We want to thank all of the participants in this trial and also
the extensive group of scientists and physicians (19 are listed as co-authors of the SITC presentation) who have worked on this study,
along with the numerous additional personnel including nurses, pharmacists, phlebotomists and others who have provided support.”
The
investigational vaccine is based on decades of groundbreaking pre-clinical research led by the late Vincent Tuohy, Ph.D., who was the
Mort and Iris November Distinguished Chair in Innovative Breast Cancer Research at Cleveland Clinic’s
Lerner Research Institute. Dr. Tuohy’s research led to the development of this investigational vaccine. The study is based
on Dr. Tuohy’s research that showed that activating the immune system against α-lactalbumin was safe and effective in preventing
breast tumors in mice. The research, originally published in Nature Medicine, was funded in part by philanthropic gifts
to Cleveland Clinic from more than 20,000 people over the last 12 years.
The
vaccine was developed at Cleveland Clinic and licensed to Anixa Biosciences. Cleveland Clinic is entitled to royalties and other commercialization
revenues from the Company.
“It
was Dr. Tuohy’s hope that this vaccine would demonstrate the potential of immunization as a new way to control breast cancer, and
that a similar approach could someday be applied to other types of malignancy,” said Dr. Budd.
Description
of the Breast Cancer Vaccine
The
vaccine targets a lactation protein, α-lactalbumin, which is only expressed in the breast when a woman is lactating but not at
other times in her life or in other tissues. However, when a woman develops breast cancer, including TNBC or other types of breast cancer,
many of the malignant cells will express α-lactalbumin. Activating the immune system, through vaccination, to direct cytotoxic
T cells to the tumor cell expressing this protein may provide preemptive immune protection against emerging breast tumors that express
α-lactalbumin.
Initial
Phase 1 data was presented at the San Antonio Breast Cancer Symposium in December 2023. The synopsis below summarizes the additional
findings which were presented today at the SITC 39th Annual Meeting.
Presentation
Summary
The trial is recruiting patients into three cohorts. Below is a description of each cohort as well as a summary of
the key results and conclusions to date.
Cohort
1a participants: The patients enrolled are women who, within the previous three years, have completed standard of care (SOC) treatment,
including surgery, for TNBC, the most lethal type of breast cancer. The study is evaluating the safety and tolerability of the vaccine,
characterizing immune responses, and identifying a maximum tolerated dose (MTD).
Key
Results: All three goals noted above have been achieved, in a group of 21 patients in this cohort. While the MTD has been successfully
identified, additional dosages are being evaluated to confirm the MTD. In all patients at the current MTD, the vaccine was safe, producing
no flu-like symptoms such as fever and myalgias, no abnormal clinical laboratory tests, or other observed adverse side effects. The only
notable side effect was injection site irritation. The majority of patients exhibited protocol defined immune responses of α-lactalbumin
specific T cell induced interferon gamma and interleukin-17.
Cohort
1b participants: The patients enrolled are women who carry mutations in their BRCA1, BRCA2, or PALB2 genes that place them at high
risk of developing breast cancer, which is frequently TNBC. These women have chosen to have prophylactic mastectomies to reduce their
risk of breast cancer. These participants were vaccinated prior to their surgeries, after which they were monitored for safety and immune
responses. Immunohistochemistry (IHC) analysis will be performed on their resected breast tissue to evaluate their healthy breast tissue
to determine if there are micro-foci of lactational cells, inflammation in the area of those foci and the presence of micro-tumors.
Key
Results: Three women have been enrolled in this cohort to date. The safety and tolerability of the vaccine were similar to that in
Cohort 1a. Enrollment of additional patients in this cohort is ongoing. The IHC analysis is ongoing and will be presented in a future
scientific presentation.
Cohort
1c participants: The patients enrolled in this group are women diagnosed with TNBC who have completed SOC, including surgery, and
are receiving pembrolizumab (Keytruda) in the adjuvant, post-surgery setting. Since Keytruda, a checkpoint inhibitor, is already a powerful
immunotherapy with its own side effect profile, one of the primary goals of this cohort is to evaluate whether the administration of
the vaccine in combination with Keytruda causes intolerable side effects. Immune responses are also being monitored in these participants.
Key
Results: Three women have been enrolled in this cohort to date. Most notably, there were no major adverse side effects when the combination
of vaccine and Keytruda were administered. As with the patients in cohorts 1a and 1b, the primary adverse side effect was injection site
irritation. One patient exhibited a Grade 3 adverse event, which was a greater amount of irritation at one injection site. This patient
had been diagnosed with breast cancer while she was pregnant, and she had recently lactated when the vaccine was administered. The trial
protocol is being amended to provide for a six-month delay after lactation before a patient can be vaccinated. Similar to the patients
enrolled in cohort 1a, the participants in this trial also exhibited antigen-specific T cell immune responses as hoped. Now that antigen-specific
T cell responses have been confirmed in women receiving Keytruda and the vaccine, with no major side effects, the data provide the confidence
to plan a Phase 2 study in the neoadjuvant setting with newly diagnosed breast cancer patients.
“Since
the trial results to date have been very positive, the planned Phase 2 trial will enroll newly diagnosed breast cancer patients undergoing
neoadjuvant treatment. Patients will be randomized in a one-to-one ratio, to receive either the standard of care, as defined by NCCN
guidelines, alone or the vaccine plus standard of care. The important endpoints in this study will include characterization of T and
B cell immune responses and repertoires, pathologic complete response and safety. Utilizing the vaccine in this type of setting will
enable us to determine the effect within months for individual patients. The presence of a control group will allow us to determine efficacy
in this setting. Assuming the trial data continues to be positive, such a trial may enable a quicker route to a strategic relationship
with a large pharmaceutical partner for commercialization,” stated Dr. Kumar.
The
poster presented at SITC can be viewed at https://ir.anixa.com/events.
For
more information and eligibility requirements visit clinicaltrials.gov.
About
Anixa Biosciences, Inc.
Anixa
is a clinical-stage biotechnology company focused on the treatment and prevention of cancer. Anixa’s therapeutic portfolio consists
of an ovarian cancer immunotherapy program being developed in collaboration with Moffitt Cancer Center, which uses a novel type of CAR-T,
known as chimeric endocrine receptor-T cell (CER-T) technology. The Company’s vaccine portfolio includes vaccines being developed
in collaboration with Cleveland Clinic to treat and prevent breast cancer and ovarian cancer, as well as additional cancer vaccines to
address many intractable cancers, including high incidence malignancies in lung, colon, and prostate. These vaccine technologies focus
on immunizing against “retired” proteins that have been found to be expressed in certain forms of cancer. Anixa’s unique
business model of partnering with world-renowned research institutions on all stages of development allows the Company to continually
examine emerging technologies in complementary fields for further development and commercialization. To learn more, visit www.anixa.com
or follow Anixa on Twitter, LinkedIn, Facebook and YouTube.
Forward-Looking
Statements
Statements
that are not historical fact may be considered forward-looking statements within the meaning of the Private Securities Litigation Reform
Act of 1995. Forward-looking statements are not statements of historical facts, but rather reflect Anixa’s current expectations
concerning future events and results. We generally use the words “believes,” “expects,” “intends,”
“plans,” “anticipates,” “likely,” “will” and similar expressions to identify forward-looking
statements. Such forward-looking statements, including those concerning our expectations, involve risks, uncertainties and other factors,
some of which are beyond our control, which may cause our actual results, performance or achievements, or industry results, to be materially
different from any future results, performance, or achievements expressed or implied by such forward-looking statements. These risks,
uncertainties and factors include, but are not limited to, those factors set forth in “Item 1A - Risk Factors” and other
sections of our most recent Annual Report on Form 10-K as well as in our Quarterly Reports on Form 10-Q and Current Reports on Form 8-K.
We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise, except as required by law. You are cautioned not to unduly rely on such forward-looking statements when evaluating
the information presented in this press release.
Contact:
Mike
Catelani
President, COO & CFO
mcatelani@anixa.com
408-708-9808
Exhibit 99.2
v3.24.3
Cover
|
Nov. 08, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Nov. 08, 2024
|
| Entity File Number |
001-37492
|
| Entity Registrant Name |
ANIXA
BIOSCIENCES, INC.
|
| Entity Central Index Key |
0000715446
|
| Entity Tax Identification Number |
11-2622630
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
3150
Almaden Expressway
|
| Entity Address, Address Line Two |
Suite 250
|
| Entity Address, City or Town |
San
Jose
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
95118
|
| City Area Code |
(408)
|
| Local Phone Number |
708-9808
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common
Stock, par value $0.01 per share
|
| Trading Symbol |
ANIX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Anixa Biosciences (NASDAQ:ANIX)
과거 데이터 주식 차트
부터 10월(10) 2024 으로 11월(11) 2024
Anixa Biosciences (NASDAQ:ANIX)
과거 데이터 주식 차트
부터 11월(11) 2023 으로 11월(11) 2024